Abstract
Background:
Deficits in goal-directed behavior (i.e., behavior conducted to achieve a specific goal or outcome) are core to schizophrenia, difficult to treat, and associated with poor functional outcomes. Factors such as negative symptoms, effort-cost decision-making, cognition, and functional skills have all been associated with goal-directed behavior in schizophrenia as indexed by clinical interviews or laboratory-based tasks. However, little work has examined whether these factors relate to real-world goal-directed behavior in this population.
Methods:
This study aimed to fill this gap by using EMA (4 survey prompts per day for one week) to test hypotheses about symptom, effort allocation, cognitive, and functional measures associated with planned and completed goal-directed behavior in the daily lives of 63 individuals with schizophrenia.
Results:
Individuals with schizophrenia completed more goal-directed activities than they planned (t(62) = −4.01, p < 0.001). Motivation and pleasure (i.e., experiential) negative symptoms, controlling for depressive symptoms, negatively related to planned goal-directed behavior (OR = 0.92, p = 0.005). Increased effort expenditure for high probability rewards (planned: OR = 1.01, p = 0.034, completed: OR = 1.01, p = 0.034) along with performance on a daily functional skills task (planned: OR = 1.04, p = 0.002, completed: OR = 1.03, p = 0.047) negatively related to both planned and completed goal-directed activity.
Conclusions:
Our results present correlates of real-world goal-directed behavior that largely align with impaired ability to make future estimations in schizophrenia. This insight in could help identify targeted treatments for this elusive motivated behavior deficits in this population.
Keywords: schizophrenia, goal-directed behavior, motivation, negative symptoms, effort-cost decision-making, working memory, cognitive function, ecological momentary assessment, anticipation
Deficits in motivation and goal-directed behavior are core to schizophrenia. Despite their close link to daily and long-term functioning (Barch, Treadway, & Schoen, 2014a; Fervaha, Foussias, Agid, & Remington, 2014b; Foussias et al., 2011), they remain particularly challenging treatment targets (Aleman et al., 2017; Velthorst et al., 2015). Research using laboratory-based clinical or behavioral indices of goal-directed behavior has associated this deficit with factors such as negative or depressive symptoms, effort-cost decision-making, and cognitive and functional skills. However, this use of laboratory-based proxies for motivation may limit the ability to capture the real-world nature of this construct. Thus, there is a critical need to elucidate the nature and correlates of reduced motivated behavior as it appears in the daily lives of individuals with schizophrenia.
Motivation, which involves the planning and pursuit of behaviors that lead to desired outcomes (i.e., goal-directed behavior), is reduced in mental illnesses such as schizophrenia (e.g., Barch, Pagliaccio, & Luking, 2015) and has largely been associated with the diminished motivation and pleasure factor of negative symptoms (MAP negative symptoms; e.g., Horan, Kring, Gur, Reise, & Blanchard, 2011). Notably, individuals with schizophrenia can have symptoms of depression, and major depressive disorder is also associated with reduced goal-directed activity, although the magnitude of this deficit may be less than in schizophrenia (Barch et al., 2015). Previous work shows that negative symptoms account for functional impairment in schizophrenia beyond the effect of depressive symptoms (Fervaha, Foussias, Agid, & Remington, 2014a). However, it is unclear whether reductions in goal-directed behavior in schizophrenia are related to depressive symptoms independent of their relationship to negative symptoms.
Some research has suggested that decreased goal-directed behavior in schizophrenia is related to deficits in the anticipation of pleasure (i.e., hedonic deficits), but these findings are mixed (e.g., Gard, Kring, Gard, Horan, & Green, 2007; Gard et al., 2014) and it is not clear that pursuit of goal-directed activities is related to anticipation of pleasurable outcomes. For example, some studies collecting real-time reports from patients’ daily lives using Ecological Momentary Assessment (EMA) have found that individuals with schizophrenia show intact anticipatory pleasure yet still participate in fewer goal-directed activities in their daily lives (Edwards, Cella, Emsley, Tarrier, & Wykes, 2018; Gard et al., 2014). Therefore, the current work focuses on factors aside from hedonics, such as effort allocation and cognitive function, that may relate to the degree to which individuals with schizophrenia partake in goal-directed activities.
Effort-cost decision-making (ECDM), the process of making decisions about whether to expend effort for favorable outcomes, has been identified as a key mechanism of motivated behavior in schizophrenia (see Culbreth, Moran, & Barch, 2018 for a review). On behavioral ECDM tasks such as the Effort Expenditure for Reward Task (EEfRT; Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009), there is mixed literature on whether individuals with schizophrenia expend less effort than healthy controls overall, but consistent evidence that they are less willing than healthy controls to increase their effort expenditure for higher probability or higher magnitude rewards (Barch et al., 2014a; Fervaha et al., 2014b; Gold et al., 2013; Huang et al., 2016; McCarthy, Treadway, Bennett, & Blanchard, 2016; Treadway, Peterman, Zald, & Park, 2015; Treadway et al., 2015; Whitton, Merchant, & Lewandowski, 2020). Further, this difficulty using probability and value information to guide effortful decisions has been associated with MAP negative symptoms of psychosis (e.g. Gold et al., 2013) suggesting it is more related to motivation-related individual differences in schizophrenia than is effort expenditure as a whole. Very limited literature has examined factors associated with ECDM in daily life in schizophrenia, but EMA studies that have done so found that ECDM was associated with momentary survey reports of experiential negative symptoms (Moran, Culbreth, & Barch, 2017a) or motivation (at a trend-level) in this population (Culbreth, Moran, Kandala, Westbrook, & Barch, 2020). Still, EEfRT task performance has not been examined in conjunction with the frequency of planned or completed goal-directed behaviors in schizophrenia.
Cognition has also been linked to laboratory-based proxies for goal-directed activity in schizophrenia (Siddiqui et al., 2019; Sitnikova, Goff, & Kuperberg, 2009). Although much of the work conducted on this topic examines cognition globally, it is important to parse out what specific components of cognition relate to motivated behavior. Working memory, which involves short-term storage and manipulation of information that can inform behavioral goals, has been associated with motivated behavior (Heerey & Gold, 2007) and functional capacity (Gold et al., 2019). Thus, it is of particular interest whether this domain would relate to goal-directed behavior in patients’ daily lives. Laboratory-based measures of functioning, such as the University of California San Diego Performance-based Skills Assessment (UPSA; Patterson, Goldman, McKibbin, Hughs, & Jeste, 2001), have also been associated with working memory and cognition (Bowie, Reichenberg, Patterson, Heaton, & Harvey, 2006; Kraus et al., 2020; Twamley et al., 2002). Although a recent EMA study found that UPSA performance was not related to the number of daily productive behaviors (e.g. work/school, home-care) in schizophrenia, (Granholm et al., 2020), other studies have found that UPSA performance is related to real-world functioning and independence of living (Bowie et al., 2006; Mausbach et al., 2008; Twamley et al., 2002). These mixed findings indicate a need to further examine whether laboratory-based measures of functioning, such as the UPSA, relate to real-world motivated behavior.
Overall, the literature suggests that negative or depressive symptoms, ECDM, working memory, and functional capacity for everyday activities may be associated with motivated behavior in psychosis. However, most of this work examines laboratory-based proxies for motivation via clinical reports, or behavioral tasks—methods that are limited in their ability to capture real-world behavior in patients’ day-to-day lives. For example, clinical reports rely on individuals’ subjective memories of their past experiences, while behavioral tasks attempt to artificially induce scenarios that may not be externally valid to individuals’ lives. EMA, however, in allowing individuals to report on their current and planned behaviors as they arise naturally, may overcome these limitations. Although it has been suggested that EMA serves as a beneficial tool to assess functioning in schizophrenia (e.g. Granholm et al., 2020), few studies have used it to compare performance on laboratory-based proxies for motivation to real-time reports of patients’ goal-directed activity.
Aims and Hypotheses
The current study aims to build on previous EMA work on motivation in schizophrenia (Culbreth et al., 2020; Edwards et al., 2018; Gard et al., 2014; Granholm et al., 2020; Moran et al., 2017a; Strassnig, Harvey, Miller, Depp, & Granholm, 2021) and fill remaining gaps by investigating whether laboratory-based symptom or behavioral indices for motivation in schizophrenia relate to real-world frequency of planned and completed goal-directed activity. We hypothesized that: a) MAP negative symptoms would relate to reductions in both planned and completed goal-directed behavior over and above depressive symptoms; b) increased effort expenditure for conditions with high reward probability and value would be more positively related to planned and completed goal-directed behavior than overall effort expenditure; and c) both working memory and functional skills task performance would positively relate to frequency of planned and completed goal-directed activities.
Methods
Participants
Outpatients meeting DSM-IV-TR for schizophrenia or schizoaffective disorder (SCZ; total: N = 66; schizophrenia: n = 54, schizoaffective: n = 12) participated in the study (see Table 1 for demographics and the supplementary materials for recruitment information). Consistent with prior EMA research (Myin-Germeys, Nicolson, & Delespaul, 2001), participants with an EMA survey response rate below 33% (n = 3) were excluded from the analyses. Our final sample size of 63 meets or exceeds the range of ~63% of EMA studies in schizophrenia (Vachon, Viechtbauer, Rintala, & Myin-Germeys, 2019). Of note, the sample did not include a healthy control group as the study goals were to dimensionally explore symptom and behavioral correlates of goal-directed behavior within a schizophrenia population.
Table 1.
Demographic, Clinical and Behavioral Characteristics
| Mean (SD) | ||
|---|---|---|
| Characteristic | ||
| Age | 38.92 (10.54) | |
| Sex (% Female) | 39.7% | |
| Education | 12.75 (2.86) | |
| Parental Education | 14.31 (6.25) | |
| Race (%) | ||
| White | 36.5% | |
| African American | 60.32% | |
| Employment Status | ||
| Employed (part or full time) | 20.63% | |
| Unemployed | 79.37% | |
| Living Situation | ||
| Alone | 37% | |
| Family (e.g., parents, spouse, dependents) | 52% | |
| Friends/ Roommates | 10% | |
| Boarding home | <1% | |
| Clinical Ratings | ||
| CAINS MAP | 17.79 (5.44) | |
| BDI | 11.86 (10.09) | |
| Task Behavior | ||
| EEfRT Probability Increase | 19.77% (38.38) | |
| EEfRT Monetary Increase | 113.72% (133.83) | |
| EEfRT Total Hard | 68.91% (19.07) | |
| Running Span | 33.52 (17.56) | |
| UPSA-B | 73.93 (15.58) | |
Note: the values for Probability increase exclude the significant outlier
Procedure
The study involved: 1) an initial laboratory visit with a diagnostic interview and an EMA training session (i.e. review of EMA survey and protocol to ensure comprehension and ability to participate), 2) one week of the EMA protocol, and 3) a post-EMA laboratory visit with behavioral tasks and clinical measures documenting participants’ EMA-week symptoms. Participants were compensated $1.75 per EMA survey completed and $40 per laboratory visit.
EMA
EMA Protocol
Participants were provided Android-enabled smartphones and prompted to complete the EMA survey 4 times per day for one week. These pseudorandom prompts occurred approximately every 3 hours between 10:00 AM and 7:00PM. Any surveys started more than 15-minutes after the prompt were not counted. The mean response rate for the EMA surveys was 80.5%, with a standard deviation of 19.07% (including the response rates of the three excluded participants noted above). This represents high EMA compliance, as EMA study response rates in schizophrenia average approximately 70% after excluding low responders (Vachon et al., 2019).
EMA Survey
At each EMA prompt, participants were asked to indicate their current (“right now”), past (“since the last beep”), and anticipated (“in the next two hours”-to focus on a time window with a high likelihood of occurrence before the next prompt) activities, by selecting “as many as apply” from a drop-down list (e.g., Running an Errand, Socializing). Activities endorsed as anticipated were characterized as planned behaviors, while those reported as past or current were combined to comprise completed behaviors. We categorized each activity as either goal-directed or non-goal-directed. Goal-directed activities were active behaviors, conducted to achieve a specific outcome (e.g., attending school for education, or socializing for pleasure or a social network). Non-goal-directed activities were those that tend to be completed passively (e.g., watching TV) or that are a necessary to live (e.g., eating or drinking). See Table 2 for our goal-directed behavior categorizations and reported frequencies. Of note, our grouping of goal-directed behavior aligns with that in prior EMA literature in schizophrenia (e.g., Granholm et al., 2020) and with the relevant (and highest loading) factors of the Specific Level of Functioning scale (SLOF; Schneider & Struening, 1983): personal care, activities, working skills, and interpersonal relations. Planned and completed goal-directed behavior were coded (1 versus 0) at each timepoint based on the presence of at least one reported goal-directed activity.
Table 2.
Average Reported Frequency of Daily Goal-Directed and Non-Goal-Directed Behaviors
| Activity | Percent of Timepoints Endorsed | |
|---|---|---|
| Planned | Completed | |
| Goal-Directed: | ||
| Work/ School | 7.51% | 9.04% |
| Running an Errand | 12.77% | 17.36% |
| Cleaning/Hygiene/Chores | 18.49% | 24.94% |
| Cooking | 8.13% | 11.73% |
| Exercising | 8.81% | 11.57% |
| Socializing | 29.58% | 40.56% |
| Entertainment Away from Home | 8.99 % | 9.54% |
| Therapy/Doctor’s Appointment | 3.02% | 4.84% |
| In transit | 13.22% | 18.83% |
| Non-Goal Directed: | ||
| Eating or Drinking | 38.28% | 60.79% |
| TV/Radio/Reading/Computer | 57.95% | 65.64% |
| Sleeping | 13.48% | 27.29% |
| Nothing in particular | 5.56% | 20.25% |
We focused on at least one goal-directed activity at each prompt instead of counting the total frequency of these activities for various reasons. First, since we combined current and past activities into a single “completed goal-directed activities” category, we did not want to double count extended activities that may have been reported both as past and a current behavior. Additionally, since we did not have a sense of length of engagement in each behavior, we did not want to give more weight to multiple short goal-directed activities (e.g., cleaning for 10 minutes, driving, and socializing) than a single prolonged goal-directed activity (e.g., studying productively for a few hours). We also wanted to account for possible differences in response behavior among participants. For example, if bowling with friends, one participant could indicate both “socializing” and “entertainment away from home” (two goal-directed activities), while another might simply indicate “socializing” (one goal-directed activity). As such, we wanted to avoid confounding these differences with frequency of goal-directed behavior.
Clinical and Behavioral Measures (see supplement for additional details)
Clinical Assessments
We assessed MAP negative symptoms with the Motivation and Pleasure scale of the clinician-rated Clinical Assessment Interview for Negative Symptoms (CAINS-MAP); Kring, Gur, Blanchard, Horan, & Reise, 2013), which includes sections related to motivation and frequency of pleasure for social relationships, work and school, and recreation. We used the Beck Depression Inventory—Second Edition (BDI-II; Beck, Steer, & Brown, 1996) self-report measure to assess depressive symptoms. Higher scores on both the CAINS and BDI indicate increased impairment.
Behavioral Tasks
EEfRT.
Participants completed a modified version (Barch et al., 2014b; Moran et al., 2017a) of the original EEfRT task (Treadway et al., 2009) to assess ECDM. We examined participants’ likelihood to expend high physical effort for a monetary reward overall (Total Hard), when the probability of reward receipt increased from 50% to 88% (Probability Increase), and when the reward value increased from low to high (Monetary Increase).
Running Span.
We used a Running Span Task to assess working memory as per participants’ ability to remember the last x letters (steadily increasing) from an unpredictable number of presented letters.
UPSA.
Participants also completed the UPSA-B task to measure their ability to perform functional tasks involving financial (e.g., paying bills) and communication (e.g., dialing the emergency number) skills.
Statistical Analysis
All analyses were conducted using R statistical software (v 4.0.4; R Core Team, 2021). We conducted a paired samples t-test to compare the frequencies of planned versus completed goal-directed activities by using the percentage of timepoints per individual for which each of these behavior types were endorsed.
We then conducted a series of generalized linear mixed models (GLMMs) using the lme4 package (Bates et al., 2021) and the glmer function to examine the relationships between clinical symptoms or behavioral measures (numeric predictor variables) and frequency of planned and completed goal-directed activities (binary outcome variables), nested by participant ID. To account for missing data, each of these models controlled for EMA prompt adherence (the percentage of prompts to which each person responded). Negative and depressive symptoms were added simultaneously to their models to account for the effect of the other. Except where noted, each model contained a single, fixed effect predictor of interest (a clinical symptom or behavioral measure). The underlying binomial model of the data was confirmed for each outcome variable using the DHARMa package (Hartig & Lohse, 2021). We exponentiated the coefficients of our GLMMs to compute each odds ratio (OR) and calculated the associated confidence interval (CI) by exponentiating the endpoints of the original coefficient CIs.
Additionally, we examined whether within-person variance (time of day or day of week) would affect our significant results. We added each of these variables separately as random effects (controlling for adherence) to predict planned or completed goal-directed behavior. Any significant predictors (only observed for time of day’s relation to planned goal-directed behavior) were then added to our originally significant models. Autocorrelation was also explored and found to be small (for planned goal-directed behavior) or non-existent (for completed goal-directed behavior). Notably, none of these model additions altered the results. Thus, the simpler model analyses are reported.
Results
Planned and Completed Goal-Directed Behavior
Table 2 contains the average percent of timepoints at which each EMA activity was planned and completed. As shown in Figure 1, participants planned significantly fewer goal-directed activities than they completed t(62) = −4.01, p < 0.001). Planned and completed goal-directed behavior were significantly correlated (r = 0.82, p < 0.001). On average, participants endorsed planned goal-directed behavior at 66% of timepoints (SD= 26%) and completed goal-directed behavior at 74% of timepoints (SD = 28%).
Figure 1:

Percent of timepoints for which at least one goal-directed activity was endorsed as planned (M = 66, Med = 70, SD = 26) or completed (M = 74, Med = 85, SD = 28).
Negative and Depressive Symptom Relationships to Goal-Directed Behavior
Consistent with our hypothesis, we found that MAP negative symptoms negatively related to planned goal-directed behavior when controlling for depressive symptoms (Table 3, Figure 2a). However, in contrast to our predictions, negative symptoms only related to completed goal-directed activity at a trend level (Figure 2a). Depressive symptoms did not relate to either planned or completed goal-directed behavior when controlling for MAP negative symptoms (Table 3). Of note, exploratory analyses found that in-the-moment ratings of sadness also did not relate to either planned (OR = 0.92, p = 0.483) or completed (OR = 0.92, p = 0.899) goal-directed behavior.
Table 3.
GLMMs of Clinical and Behavioral Measure Relationships to Goal-Directed Behavior (Controlling for Adherence)
| OR [95% CI] | p | |
|---|---|---|
| Planned Goal-Directed Behavior | ||
| Model 1a: BDI Depression (controlling for CAINS MAP) | 1.02 [0.99, 1.06] | 0.268 |
| Model 2a: CAINS MAP (controlling for BDI depression) | 0.92 [0.86, 0.97] | 0.005** |
| Model 3a: EEfRT Total Hard | .33 [0.08, 1.33] | 0.119 |
| Model 4a: EEfRT Probability Increase | 1.01 [1.00, 1.02] | 0.034* |
| Model 5a: EEfRT Monetary Increase | 1.00 [0.99, 1.00] | 0.662 |
| Model 6a: Working Memory | 1.01 [0.99, 1.04] | 0.306 |
| Model 7a: UPSA | 1.04 [1.01, 1.06] | 0.002* |
| Completed Goal-Directed Behavior | ||
| Model 1b: BDI Depression (controlling for CAINS MAP) | 1.02 [0.97, 1.07] | 0.55 |
| Model 2b: CAINS MAP (controlling for BDI depression) | 0.93 [0.85, 1.01] | 0.079* |
| Model 3b: EEfRT Total Hard | 1.22 [0.23–6.53] | 0.813 |
| Model 4b: EEfRT Probability Increase | 1.01 [1.00, 1.03] | 0.034* |
| Model 5b: EEfRT Monetary Increase | 1.00 [0.98, 1.03] | 0.911 |
| Model 6b: Working Memory | 1.00 [0.97, 1.04] | 0.796 |
| Model 7b: UPSA | 1.03 [1.00, 1.07] | 0.047* |
Note: Adherence, was not significant in any of the models (OR range: 1.00 –1.01, p-value range: 0.250–0.68); Probability Increase analyses conducted excluding significant outlier (see results section for more details)
Figure 2a:
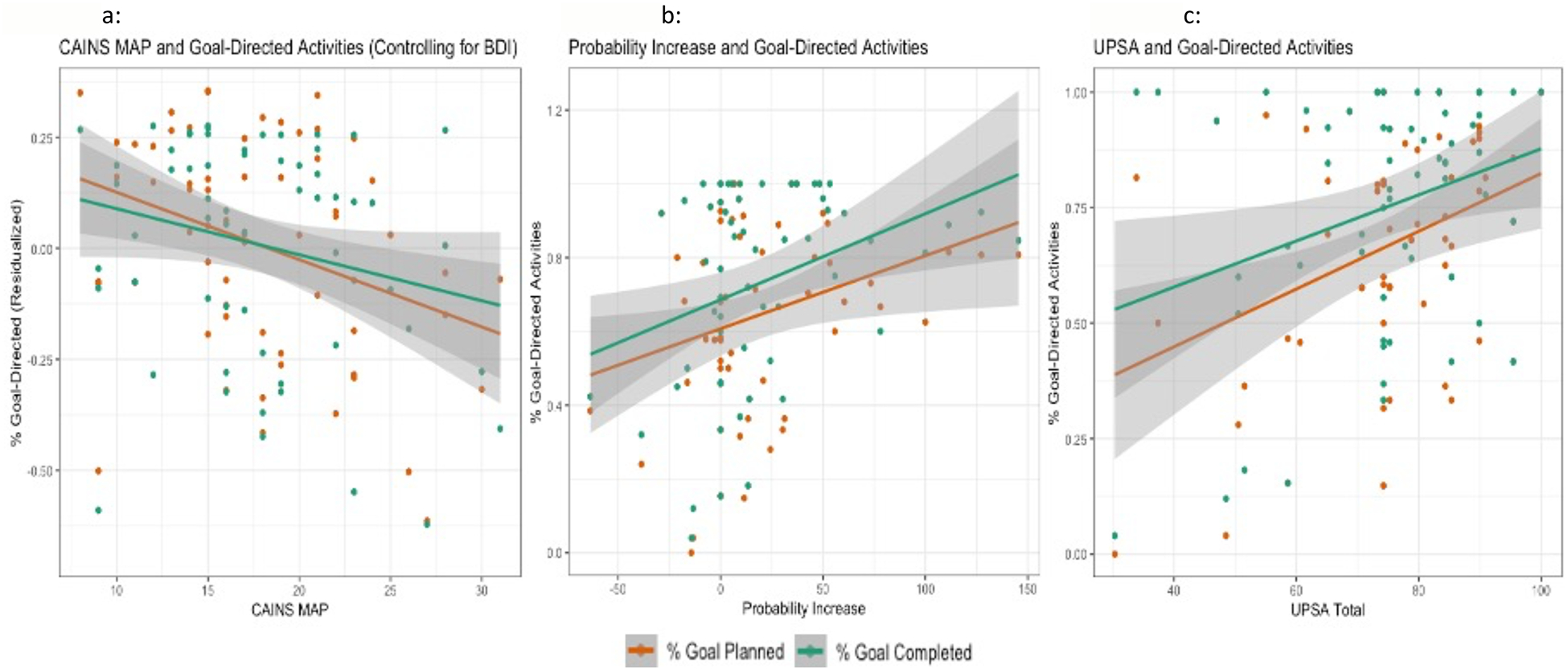
Plot of CAINS MAP scores and Goal-Directed Activities with 95% confidence bands. Of note- the y axis reflects the residualized values of BDI and Goal-Directed behavior in order to control for that variable.
Figure 2b: Plot of Probability Increase scores and Goal-Directed Activities with 95% confidence bands. Of note- this graph represents the data that excludes the significant Probability Increase outlier.
Figure 2c: Plot of UPSA scores and Goal-Directed activities with 95% confidence bands.
Effort-Cost Decision-Making and Goal-Directed Behavior
In line with our hypotheses, Probability Increase but not Total Hard positively related to both planned and completed goal-directed behavior (Table 3, Figure 2b). However, inconsistent with our prediction, Monetary Increase did not relate to either planned or completed goal-directed behavior (Table 3). Grubbs’ test reported a significant outlier in the output values for Probability Increase (>5 SD from the mean). Therefore, we excluded that outlier for the Probability Increase analyses.
As a follow-up, we assessed whether Probability Increase and MAP negative symptoms’ associations with goal-directed behavior would hold when they were entered simultaneously. Probability increase had a trend-level relationship with planned (OR = 1.00, p = 0.051) and completed (OR = 1.01, p = 0.051) goal-directed behavior, while negative symptoms continued to significantly relate to planned (OR = 0.92, p = 0.010) but not completed (OR = 0.94, p = 0.127) goal-directed behavior.
Cognition, Functioning, and Goal-Directed Behavior
Inconsistent with our predictions, working memory did not significantly relate to either planned or completed goal-directed behavior (Table 3). However, in line with our hypothesis, UPSA-B score related to both planned and completed goal-directed behavior (Table 3, Figure 2c). We conducted a follow-up analysis with both negative symptoms and UPSA-B to determine whether this UPSA-B significance would hold independently of negative symptoms. There was a trend-level association between UPSA-B and planned goal-directed activities (OR = 1.01, p = 0.060), but no UPSA-B association with completed goal-directed activities (OR = 1.01, p = 0.392). Negative symptoms continued to relate to planned (OR = 0.93, p = 0.016) but not completed (OR = 0.94, p = 0.123) goal-directed behavior.
Discussion
The goal of the current study was to examine correlates of real-world goal-directed behavior in the daily lives of individuals with schizophrenia. Our results showed that people with schizophrenia completed more goal-directed activities than they planned, MAP negative symptoms negatively related to planned goal-directed activity, and use of reward probability to guide effort expenditure and performance on a functional skills task positively related to both planned and completed goal-directed activity.
Individuals with schizophrenia completed more goal-directed activities than they predicted they would. In line with research that has documented impairments in future projection in schizophrenia, such as deficits in planning ability (Holt, Wolf, Funke, Weisbrod, & Kaiser, 2013; Semkovska, Bédard, Godbout, Limoge, & Stip, 2004; Seter, Giovannetti, Kessler, & Worth, 2011) or in anticipating future pleasure (e.g., Moran & Kring, 2018), this finding could reflect difficulty estimating upcoming behavioral goals. Lack of agency or passivity could also contribute to this result, as individuals with schizophrenia may not actively plan for each of their goals but be reminded in-the-moment and supported in their completion by those around them. Overall, it is possible that a large part of this motivated behavior impairment in schizophrenia may be at the level of planning.
Interestingly, these future thinking deficits in planning and anticipatory pleasure in schizophrenia have been associated with negative symptoms (Moran & Kring, 2018; Semkovska et al., 2004), which is consistent with our finding that decreased planned (but not completed) goal-directed behavior related to these symptoms. This finding that MAP negative symptoms were significantly associated with planned goal-directed behavior independent of depression (but only associated at a trend-level for completed goal-directed behavior), indicates that individuals with more severe negative symptoms may be less likely to plan future goal-directed activities and that MAP negative symptoms may be somewhat more related to the planning than completion of motivated behavior. However, this trend-level finding indicates that more research should be conducted to determine whether MAP negative symptoms also relate to the completion of goal-directed activities.
Depressive symptoms did not relate to either planned or completed goal-directed behavior independent of negative symptoms. In-the-moment sadness was also unrelated to goal-directed behavior. Although our findings differ from those of a recent EMA study that found that both overall depressive symptoms and momentary sadness were related to increased unproductive versus productive activities (Strassnig et al., 2021), they align with work suggesting that depressive symptoms in schizophrenia are not associated with daily functioning (e.g., Strassnig et al., 2015) and that MAP negative symptoms account for functional impairment in schizophrenia beyond the effect of depressive symptoms (Fervaha et al., 2014a). Overall, these findings suggest that the motivated behavior deficit in this population is more related to MAP negative symptoms than depression. However, our results do not rule out the possibility that depressed mood is related to motivated behavior in non-schizophrenia populations.
For ECDM, we found that increased planned and completed goal-directed behavior was associated with the use of reward probability information to selectively decide when to expend effort, rather than with effort expenditure overall. This suggests that motivated behavior deficits in schizophrenia may be more related to difficulty using outcome estimations to guide choice (another potential indicator of difficulty projecting into the future to consider goals) rather than an overall unwillingness to engage in effortful activities. We did not see this same goal-directed behavior association with reward value. This could indicate that probability, rather than reward magnitude, may be more associated with future thinking. Alternatively, it is possible that greater variability in reward values (ours was ~$1.30 –$4.30) would show this association.
We also found that functional skills task performance on the UPSA-B, but not working memory, was associated with planned and completed goal-directed behavior. The null finding for working memory was at odds with our hypothesis and with a previous study that suggested this association (Heerey & Gold, 2007). However, research has strongly associated UPSA performance with cognition (Bowie et al., 2006; Kraus et al., 2020), with the suggestion that this task may serve a proxy for cognitive functioning (Keefe, Poe, Walker, & Harvey, 2006). Therefore, our findings could indicate a link between components of cognition aside from working memory and real-world motivated behavior. Interestingly, some research suggests that planning in schizophrenia relates to ability to perform daily functional skills and to cognitive measures of executive functioning but not to memory (Semkovska et al., 2004; Seter et al., 2011). Thus, it is possible that real-world motivated behavior is more related to a component of cognition such as cognitive control (the executive functioning component involved in the representation and updating of information or goals) than to working memory.
Although our finding that UPSA-B relates to real-world goal-directed behavior is in line with work suggesting it is associated with functioning or independent living (Bowie et al., 2006; Mausbach et al., 2008; Twamley et al., 2002), it is in contrast to a recent EMA study that did not find it was associated with number of real-world productive behaviors (Granholm et al., 2020). The reason for this difference is unclear, but it could relate to differences in the study samples. Our sample consisted (with the exception of one participant) of individuals living in the community, while Granholm and colleagues’ included individuals in supported living facilities. It is possible that UPSA performance is more related to functioning in a community-living sample. For example, the ability to glean important information from medical appointment letters (a task in the UPSA-B), may be more related to whether individuals living independently attend these appointments (goal-directed behavior) than to whether individuals in supported housing, who likely have assistance in this process, do so. Additionally, as UPSA-B did not continue to relate to goal-directed behavior independently of MAP negative symptoms, these negative symptoms may have a stronger relationship than UPSA-B performance to frequency of goal-directed behavior. Future research could further clarify the pathways by which functional skills versus negative symptoms versus cognition relate to real-world goal-directed behavior.
This work should be interpreted in the context of several limitations. Our study did not have a healthy control group for comparison, as our goal was to examine what factors within a schizophrenia population are associated with this documented impairment in motivated behavior. However, due to this lack of control comparison, we could not replicate the well-established findings of decreased real-world goal-directed behavior in schizophrenia (Edwards et al., 2018; Gard et al., 2014; Granholm et al., 2020), nor could we determine whether the relationships we found would extend to a non-schizophrenia sample.
In addition, there are a variety of ways in which real-world goal-directed behavior could be operationalized. We defined goal-directed behavior as activities requiring effort (beyond that to simply survive) that are associated with a positive outcome (i.e., the financial benefit that comes with attending work). Our categorizations of goal-directed and non-goal directed behavior were developed ad-hoc, with the consensus of all three authors and align with the relevant SLOF factors as described above. Still, it is possible that our categorizations of goal-directed behavior may not align with each individuals’ own interpretations of their goal-directed behavior. Therefore, there could be benefits to having participants self-report on which of their activities they perceive to be goal-directed and calculate frequency from such self-report. However, there would be limitations to such an alternative method as well, as varying definitions of goal-directed activity or lack of insight could confound findings. Definitions of unproductive (i.e., non-goal-directed) activities assessed by EMA are also still evolving (Strassnig et al., 2021).
We measured frequency by assessing the number of time-points (controlling for adherence) for which at least one goal-directed activity was endorsed. As documented in our methods, we focused on at least one goal-directed activity rather than the total number of reported goal-directed activities at each prompt since we 1) combined reports of past and current activities to comprise completed goal-directed behavior and did not want to double count a single activity that may have been endorsed in both of these sections, 2) did not measure length of engagement in each goal-directed behavior and wanted to avoid devaluing a single prolonged goal-directed activity with multiple shorter ones, and 3) did not want to conflate differences in response behavior with frequency of goal-directed activities. However, there are also limitations to this method such as likely instances in which a single, short goal-directed activity was coded the same as multiple extended goal-directed activities. Future work could benefit by assessing length of time spent on goal-directed behaviors, having participants indicate exact activities (rather than picking between categories) to assess for follow-through on specific goal-directed tasks, and distinguishing between behaviors to pursue long versus short-term goals.
Our results present correlates of real-world goal-directed behavior that largely align with impaired ability to make future estimations in schizophrenia. Future research should determine whether motivated behavior deficits in schizophrenia are largely at the level of planning, whether difficulty planning for goal-directed activities in schizophrenia also hinders their completion, and whether it does so beyond other populations. For example, if someone is unable to project into the future to plan for activities that would bring about their goals, it is possible that they would complete fewer goals as a result, even if they end up completing more goals than they predicted. Additionally, research could assess whether support from others (e.g., in-the-moment reminders) assists more in the completion than the planning of goal directed behavior. If so, interventions that target planning difficulty directly (serving almost as an external prefrontal cortex) might also increase the completion of goal-directed activities. For example, mobile interventions, that send reminder prompts to patients (e.g., via text message) have shown improvements in areas such as medication adherence and social interactions (Granholm, Ben-Zeev, Link, Bradshaw, & Holden, 2012). In this vein, it is possible that similar mobile interventions could serve as a planning tool that could assist participants in completing daily personal goals. Overall, further research into what specifically inhibits goal-directed behavior in schizophrenia in comparison to other serious forms of mental illness could clarify the nature of this deficit and contribute to long-term functional benefit for this population.
Supplementary Material
Acknowledgements
We would like to thank the participants in this study for their time on this project, and Dr. Jo Etzel for her advice on coding the goal-directed behavior data.
Financial Support
Data for this study was provided by the Cognition, emotion, motivation and reward study which was funded by the Gregory Couch Professorship held by DMB. Authors received additional funding support from NIMH grant R01-MH066031. The funding sources had no role in the design, analysis, or interpretation of the data.
Footnotes
Conflicts of Interest
The authors declare there is no conflict of interest for this study.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, & Knegtering H (2017). Treatment of negative symptoms: Where do we stand, and where do we go? Schizophrenia Research, 186, 55–62. 10.1016/j.schres.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, & Luking K (2015). Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. In Simpson EH & Balsam PD (Eds.), Behavioral Neuroscience of Motivation (pp. 411–449). Cham: Springer International Publishing. 10.1007/7854_2015_376 [DOI] [PubMed] [Google Scholar]
- Barch DM, Treadway M, & Schoen N (2014a). Effort, Anhedonia, and Function in Schizophrenia: Reduced Effort Allocation Predicts Amotivation and Functional Impairment. Journal of Abnormal Psychology, 123(2), 387–397. 10.1037/a0036299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, & Schoen N (2014b). Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. Journal of Abnormal Psychology, 123(2), 387–397. 10.1037/a0036299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker [aut B, cre Walker S, Christensen RHB, … simulate.formula), P. N. K. (shared copyright on. (2021). lme4: Linear Mixed-Effects Models using “Eigen” and S4 (Version 1.1–27.1). Retrieved from https://CRAN.R-project.org/package=lme4
- Beck A, Steer R, & Brown G (1996). Manual for the Beck Depression Inventory-II.
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, & Harvey PD (2006). Determinants of Real-World Functional Performance in Schizophrenia Subjects: Correlations With Cognition, Functional Capacity, and Symptoms. Am J Psychiatry, 8. [DOI] [PubMed] [Google Scholar]
- Culbreth AJ, Moran EK, & Barch DM (2018). Effort-cost decision-making in psychosis and depression: Could a similar behavioral deficit arise from disparate psychological and neural mechanisms? Psychological Medicine, 48(6), 889–904. 10.1017/S0033291717002525 [DOI] [PubMed] [Google Scholar]
- Culbreth Adam J., Moran EK, Kandala S, Westbrook A, & Barch DM (2020). Effort, Avolition, and Motivational Experience in Schizophrenia: Analysis of Behavioral and Neuroimaging Data With Relationships to Daily Motivational Experience. Clinical Psychological Science, 8(3), 555–568. 10.1177/2167702620901558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Cella M, Emsley R, Tarrier N, & Wykes THM (2018). Exploring the relationship between the anticipation and experience of pleasure in people with schizophrenia: An experience sampling study. Schizophrenia Research, 202, 72–79. 10.1016/j.schres.2018.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, & Remington G (2014a). Impact of primary negative symptoms on functional outcomes in schizophrenia. European Psychiatry, 29(7), 449–455. 10.1016/j.eurpsy.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, & Remington G (2014b). Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatrica Scandinavica, 130(4), 290–299. 10.1111/acps.12289 [DOI] [PubMed] [Google Scholar]
- Foussias G, Mann S, Zakzanis KK, van Reekum R, Agid O, & Remington G (2011). Prediction of longitudinal functional outcomes in schizophrenia: The impact of baseline motivational deficits. Schizophrenia Research, 132(1), 24–27. 10.1016/j.schres.2011.06.026 [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, & Green MF (2007). Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93(1), 253–260. 10.1016/j.schres.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, & Vinogradov S (2014). Do People with Schizophrenia Have Difficulty Anticipating Pleasure, Engaging in Effortful Behavior, or Both? Journal of Abnormal Psychology, 123(4), 771–782. 10.1037/abn0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Barch DM, Feuerstahler LM, Carter CS, MacDonald AW, Ragland JD, … Luck SJ (2019). Working Memory Impairment Across Psychotic disorders. Schizophrenia Bulletin, 45(4), 804–812. 10.1093/schbul/sby134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, & Frank MJ (2013). Negative Symptoms of Schizophrenia Are Associated with Abnormal Effort-Cost Computations. Biological Psychiatry, 74(2), 130–136. 10.1016/j.biopsych.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Ben-Zeev D, Link PC, Bradshaw KR, & Holden JL (2012). Mobile Assessment and Treatment for Schizophrenia (MATS): A pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophrenia Bulletin, 38(3), 414–425. psyh (2012-15724-010). 10.1093/schbul/sbr155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Holden JL, Mikhael T, Link PC, Swendsen J, Depp C, … Harvey PD (2020). What Do People With Schizophrenia Do All Day? Ecological Momentary Assessment of Real-World Functioning in Schizophrenia. Schizophrenia Bulletin, 46(2), 242–251. 10.1093/schbul/sbz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig F, & Lohse L (2021). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models (Version 0.4.3). Retrieved from https://CRAN.R-project.org/package=DHARMa
- Heerey E, & Gold J (2007). Patients With Schizophrenia Demonstrate Dissociation Between Affective Experience and Motivated Behavior. Journal of Abnormal Psychology, 116, 268–278. 10.1037/0021-843X.116.2.268 [DOI] [PubMed] [Google Scholar]
- Holt DV, Wolf J, Funke J, Weisbrod M, & Kaiser S (2013). Planning impairments in schizophrenia: Specificity, task independence and functional relevance. Schizophrenia Research, 149(1), 174–179. 10.1016/j.schres.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, & Blanchard JJ (2011). Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophrenia Research, 132(2), 140–145. 10.1016/j.schres.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yang X, Lan Y, Zhu C, Liu X, Wang Y, … Chan RCK (2016). Neural substrates of the impaired effort expenditure decision making in schizophrenia. Neuropsychology, 30(6), 685–696. 10.1037/neu0000284 [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Poe M, Walker TM, & Harvey PD (2006). The Relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to Functional Capacity and Real-world Functional Outcome. Journal of Clinical and Experimental Neuropsychology, 28(2), 260–269. 10.1080/13803390500360539 [DOI] [PubMed] [Google Scholar]
- Kraus MS, Gold JM, Barch DM, Walker TM, Chun CA, Buchanan RW, … Keefe RSE (2020). The characteristics of cognitive neuroscience tests in a schizophrenia cognition clinical trial: Psychometric properties and correlations with standard measures. Schizophrenia Research: Cognition, 19, 100161. 10.1016/j.scog.2019.100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP (2013). The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. American Journal of Psychiatry, 170(2), 165–172. 10.1176/appi.ajp.2012.12010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Bowie CR, Harvey PD, Twamley EW, Goldman SR, Jeste DV, & Patterson TL (2008). Usefulness of the UCSD performance-based skills assessment (UPSA) for predicting residential independence in patients with chronic schizophrenia. Journal of Psychiatric Research, 42(4), 320–327. 10.1016/j.jpsychires.2006.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, & Blanchard JJ (2016). Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophrenia Research, 170(2), 278–284. 10.1016/j.schres.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EK, Culbreth AJ, & Barch DM (2017a). Ecological momentary assessment of negative symptoms in schizophrenia: Relationships to effort-based decision making and reinforcement learning. Journal of Abnormal Psychology, 126(1), 96–105. 10.1037/abn0000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EK, Culbreth AJ, & Barch DM (2017b). Ecological momentary assessment of negative symptoms in schizophrenia: Relationships to effort-based decision making and reinforcement learning. Journal of Abnormal Psychology, 126(1), 96–105. 10.1037/abn0000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran EK, & Kring AM (2018). Anticipatory Emotion in Schizophrenia. Clinical Psychological Science, 6(1), 63–75. 10.1177/2167702617730877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Nicolson NA, & Delespaul PAEG (2001). The context of delusional experiences in the daily life of patients with schizophrenia. Psychological Medicine, 31(3), 489–498. 10.1017/s0033291701003646 [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, & Jeste DV (2001). UCSD Performance-Based Skills Assessment: Development of a New Measure of Everyday Functioning for Severely Mentally Ill Adults. Schizophrenia Bulletin, 27(2), 235–245. 10.1093/oxfordjournals.schbul.a006870 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A Language and Environment for Statistical Computing (Version 1.4.1106) [R Foundation for Statistical Computing; ]. Vienna, Austria. [Google Scholar]
- Semkovska M, Bédard M-A, Godbout L, Limoge F, & Stip E (2004). Assessment of executive dysfunction during activities of daily living in schizophrenia. Schizophrenia Research, 69(2), 289–300. 10.1016/j.schres.2003.07.005 [DOI] [PubMed] [Google Scholar]
- Seter C, Giovannetti T, Kessler RK, & Worth S (2011). Everyday action planning in schizophrenia. Neuropsychological Rehabilitation, 21(2), 27. 10.1080/09602011.2010.544519 [DOI] [PubMed] [Google Scholar]
- Siddiqui I, Saperia S, Fervaha G, Da Silva S, Jeffay E, Zakzanis KK, … Foussias G (2019). Goal-directed planning and action impairments in schizophrenia evaluated in a virtual environment. Schizophrenia Research, 206, 400–406. 10.1016/j.schres.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Sitnikova T, Goff D, & Kuperberg GR (2009). Neurocognitive abnormalities during comprehension of real-world goal-directed behaviors in schizophrenia. Journal of Abnormal Psychology, 118(2), 256. 10.1037/a0015619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig MT, Harvey PD, Miller ML, Depp CA, & Granholm E (2021). Real world sedentary behavior and activity levels in patients with schizophrenia and controls: An ecological momentary assessment study. Mental Health and Physical Activity, 20, 100364. 10.1016/j.mhpa.2020.100364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig MT, Raykov T, O’Gorman C, Bowie CR, Sabbag S, Durand D, … Harvey PD (2015). Determinants of different aspects of everyday outcome in schizophrenia: The roles of negative symptoms, cognition, and functional capacity. Schizophrenia Research, 165(1), 76–82. psyh (2015-43647-012). 10.1016/j.schres.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, & Zald DH (2009). Worth the ‘EEfRT’? The Effort Expenditure for Rewards Task as an Objective Measure of Motivation and Anhedonia. PLOS ONE, 4(8), e6598. 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, & Park S (2015). Impaired effort allocation in patients with schizophrenia. Schizophrenia Research, 161(2), 382–385. 10.1016/j.schres.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, … Jeste DV (2002). Generalized Cognitive Impairments, Ability to Perform Everyday Tasks, and Level of Independence in Community Living Situations of Older Patients With Psychosis. American Journal of Psychiatry, 159(12), 2013–2020. 10.1176/appi.ajp.159.12.2013 [DOI] [PubMed] [Google Scholar]
- Vachon H, Viechtbauer W, Rintala A, & Myin-Germeys I (2019). Compliance and Retention With the Experience Sampling Method Over the Continuum of Severe Mental Disorders: Meta-Analysis and Recommendations. Journal of Medical Internet Research, 21(12), e14475. 10.2196/14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst E, Koeter M, Gaag M, Nieman D, Fett A-K, Smit F, … de Haan L (2015). Adapted cognitive-behavioural therapy required for targeting negative symptoms in schizophrenia: Meta-analysis and meta-regression. Psychological Medicine, 45, 453–465. 10.1017/S0033291714001147 [DOI] [PubMed] [Google Scholar]
- Whitton AE, Merchant JT, & Lewandowski KE (2020). Dissociable mechanisms underpinning effort-cost decision-making across the psychosis spectrum. Schizophrenia Research, 224, 133–140. 10.1016/j.schres.2020.09.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


