Abstract
There are abundant natural diterpenoids in the plants of the genus Daphne from the Thymelaeaceae family, featuring a 5/7/6-tricyclic ring system and usually with an orthoester group. So far, a total of 135 diterpenoids has been isolated from the species of the genus Daphne, which could be further classified into three main types according to the substitution pattern of ring A and oxygen-containing functions at ring B. A variety of studies have demonstrated that these compounds exert a wide range of bioactivities both in vitro and in vivo including anticancer, anti-inflammatory, anti-HIV, antifertility, neurotrophic, and cholesterol-lowering effects, which is reviewed herein. Meanwhile, the fascinating structure–activity relationship is also concluded in this review in the hope of providing an easy access to available information for the synthesis and optimization of efficient drugs.
Keywords: Daphne, diterpenoid, bioactivities
1. Introduction
The genus Daphne Linn., with its ca. 95 species, is the most diverse genus in the Thymelaeaceae family [1]. Some of the species from the genus Daphne have been applied for a long history in traditional treatments for aches, rheumatism, inflammation, and abortion in Asia, Africa, and Europe [2]. Yet, none of the principles of these bioactivities had been identified until daphnetoxin was isolated as a major toxic principle from commercial “mezeron” bark which was made from Daphne mezereum L. and other Daphne species in 1970. Subsequently, an increasing number of diterpenoids have been discovered from Daphne species.
The diterpenoids are believed to be representative components of the genus Daphne, and the genus Daphne itself also acts as an important role in the discovery of phytochemical and bioactive properties of diterpenoids. The archetype of one class, daphnetoxin, was first isolated from D. mezereum, which was named after the genus Daphne. Then, daphnetoxin and its analogues have been collectively known as the daphnetoxin class. Similarly, genkwanine A from D. genkwa is the archetypical diterpenoid of genkwanines.
The diterpenoids from the Daphne genus also contribute to the pharmacological study of diterpenoids and have been demonstrated to possess a variety of important biological activities including anticancer, anti-inflammatory, anti-HIV, antifertility, neurotrophic, and cholesterol-lowering effects [3], and some of them are undoubtedly efficient agents which have the potential to be developed as new drugs, such as yuanhuacine and genkwadaphnin.
The current review provides a comprehensive coverage of all natural diterpenoids in the genus Daphne. The occurrence and distribution of these diterpenoids are also discussed thoroughly, including the source species of every diterpenoid listed in a chronological order of discovery and the parts of plants which they were isolated from. Besides, detailed information on every class of diterpenoids is provided in this review. When the adequate information is given, the structure–activity relationship (SAR) is discussed.
2. Classification, Structures and Origins
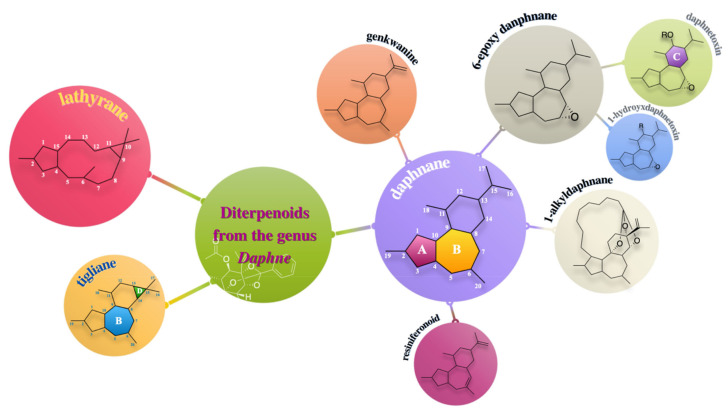
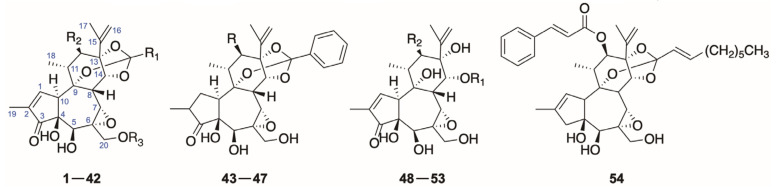
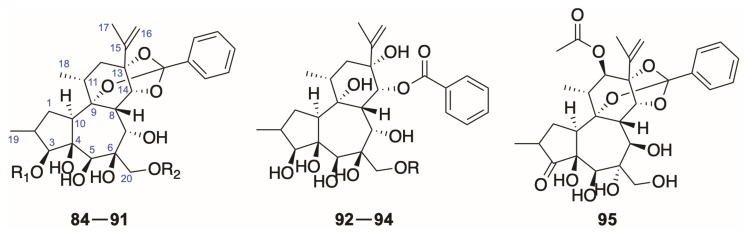
So far, there are three types of diterpenoids, daphnane, tigliane and lathyrane, isolated from species of the genus Daphne in total. Most of the natural diterpenoids occurring in the genus Daphne belong to the daphnane type featuring a 5/7/6-tricyclic ring system with polyhydroxyl groups at C-3, C-4, C-5, C-9, C-13, C-14, or C-20 and an orthoester function located at ring C, which could be further categorized into four major classes:
6-epoxy daphnane diterpenoids (1–83), sharing the characteristic of an epoxy ring at C-6 and C-7;
genkwanines (84–95), possessing a saturated ring A and a 6,7-dihydroxyl group in the ring B;
resiniferonoids (96–97), which could be viewed as 5-deoxy-6,7-double bond daphnetoxin derivatives;
1-alkyldaphnanes (98–107), featuring a saturated ring A and a macrocyclic bridge connecting C-1 in the ring A and the end of the aliphatic orthoester group (Figure 1).
Figure 1.
Classification and skeletons of diterpenoids from the genus Daphne.
For the 6-epoxy daphnane diterpenoids, they could be further subdivided into 12-hydroxydaphnetoxins (1–54) and daphnetoxins (55–83) according to the possession of an oxygen group at C-12 in the ring C [3].
Nonetheless, it is worth noticing that the existing classification system could not apply to some daphnane diterpenoids (108–123). As for compounds 108–112, the skeleton resembles the genkwanine one but possesses a 3-ketone group, a C-12 oxygenated substituents, and an additional 1,2-double bond, which makes it not accord with the definition of genkwanine. What attracts more attention is the unique 4,7 or 4,6-ether structure of neogenkwanine A-I and daphneodorin H (113–123), which is believed to never be found in any other daphnane diterpenoid [4].
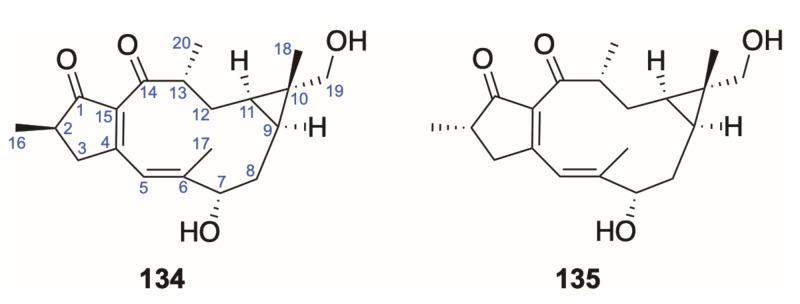
Besides daphnane diterpenoids, tigliane diterpenoids (124–133) in Daphne species, instead of a caged 9,13,14-orthoester, distinguish themselves with a cyclopropane ring D, which is regarded to be closely related to the daphnane one [5,6]. Meanwhile, two lathyranes (134 and 135) were also reported to be isolated from Daphne genkwa, featuring a characteristic 5/11/3-membered ring system.
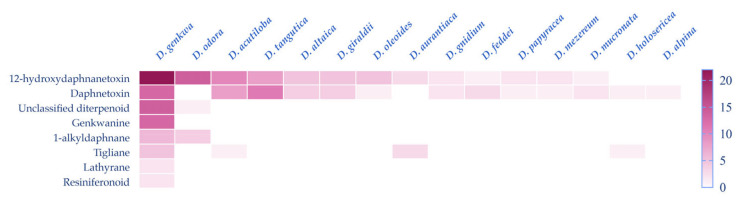
Although a large amount of diterpenoids were isolated and identified, they were reported to occur mainly in Thymelaeaceae and Euphorbiaceae and to mainly distribute in the genus Daphne, Wikstroemia and Stellera in the Thymelaeaceae family, as well as Excoecaria and Euphorbia in the Euphorbiaceae family [3]. In terms of the genus Daphne, diterpenoids are reported to be obtained from fifteen Daphne species including D. acutiloba, D. altaica, D. alpina, D. aurantiaca, D. feddei, D. genkwa, D. giraldii, D. gnidium, D. holosericea, D. mezereum, D. mucronata, D. odora, D. oleoides, D. papyracea, and D. tangutica (Scheme 1).
Scheme 1.
Distribution of diterpenoids in the species of the genus Daphne.
Diterpenes are abundant in several Daphne species including D. odora, D. acutiloba, and D. tangutica, especially in D. genkwa. It is intuitively demonstrated that diterpenoids from the Daphne genera are mainly of the 6-epoxy daphnane-type. In D. genkwa, diterpenoids of every class discussed in this review have been isolated, which may suggest a variety of diterpenoids in D. genkwa. Interestingly, it is also observed that the quantity of 12-hydrodaphnetoxins is much larger than that of daphnetoxins in D. odora while the amounts of these two types are nearly equivalent in other species, and the biogenetic mechanism behind this remains unclear.
2.1. Daphnane-Type Diterpenoids
2.1.1. 6-Epoxy Daphnane Diterpenoids
The diterpenoids from this class share the common features of a 6α,7α-epoxy and 4β,5β-dihydroxy in the seven-membered ring B. The 6-epoxy daphnane diterpenoids could be further divided into two classes, 12-hydroxydaphnetoxins (1–54) and daphnetoxins (55–83), based on the oxygenated substituent at C-12 in the ring C.
12-Hydroxydaphnetoxins
Compared to daphnetoxins, 12-hydroxydaphnetoxins have an additional oxygen group at C-12. There are more 12-hydroxydaphnanetoxins than daphnetoxins existing in the species from the Daphne genera, including 1,2-dihydro derivatives (43–47), 3-deoxy derivative (54), and the ones with a 5,20-acetonide (27 and 28). The archetypal compound of 12-hydoxydaphnanetoxins is 12-hydroxydaphnetoxin (39), which was first found as a degradation product in Lasiosiphon Burchellii Meisn. [7] (Figure 2).
Figure 2.
Structures of 12-hydroxydaphnetoxins (1–54).
The species from the Thymelaeaceae are rich sources of 12-hydroxydaphnanetoxins, especially the genus Daphne. Compounds of this class were mainly isolated from D. genkwa, D. tangutica, and D. acutiloba and these diterpenoids were abundant in the flower buds of D. genkwa, stems, roots, and especially bark of the plants (Table 1).
Table 1.
Structures and sources of 12-hydroxydaphnetoxins (1–54).
| No. | Compound (Synonym) | Chemical Structure | Source Species (Part) 1 |
|---|---|---|---|
| 1 | Acutilobin A | R1 = Ph, R2 = OCO(CH=CH)2CHOCH(CH2)2CH3, R3 = H |
D. acutiloba (stems) [8] |
| 2 | Acutilobin B | R1 = Ph, R2 = OCO(CH=CH)3CH(OH)CH2CH3, R3 = H |
D. acutiloba (stems) [8] |
| 3 | Acutilobin C | R1 = (CHCH) (CH=CH)2(CH2)2CH3, R2 = OCOCH=CHC6H3(3-OCH3) (4-OH), R3 = H |
D. acutiloba (stems) [8]; D. odora (leaves and branches) [4] |
| 4 | Acutilobin D | R1 = (CHCH) (CH=CH) (CH2)4CH3, R2 = OCOCH=CHC6H3(3-OCH3) (4-OH), R3 = H |
D. acutiloba (stems) [8]; D. odora (leaves and branches) [4] |
| 5 | Acutilobin E | R1 = Ph, R2 = OCOCH=CHC6H3(3-OCH3) (4-OH), R3 = H |
D. acutiloba (stems) [8] |
| 6 | Altadaphnan C | R1 = Ph, R2 = OCO(CHCH) (CH=CH)2(CH2)2CH3, R3 = H | D. altaica (aerial parts) [9] |
| 7 | Daphgenkin F | R1 = Ph, R2 = OCO(CH2)2CH3, R3 = H | D. genkwa (buds) [10] |
| 8 | Daphgenkin G | R1 = Ph, R2 = OCOCH(CH3)2, R3 = H | D. genkwa (buds) [10] |
| 9 | Daphnegiraldicine | R1 = Ph, R2 = OCOCH=CH(CH2)3CH3, R3 = H | D. giraldii (stem bark) [11,12] |
| 10 | Daphnegiraldidine | R1 = Ph, R2 = OCO(CH2)10CH3, R3 = H | D. giraldii (stem bark) [12,13] |
| 11 | Daphneodorin D | R1 = (CH=CH)2(CH2)4CH3, R2 = OCOCH=CHC6H4(4-OH), R3 = H |
D. odora (leaves and branches) [4] |
| 12 | Daphneodorin E | R1 = (CH=CH)3(CH2)2CH3, R2 = OCOCH=CHC6H4(4-OH), R3 = H |
D. odora (leaves and branches) [4] |
| 13 | Genkwadane D | R1 = (CH=CH)2(CH2)4CH3, R2 = OCOCH(CH3)2, R3 = H |
D. genkwa (buds) [10,14] |
| 14 | Genkwadaphin 20-palmitate | R1 = Ph, R2 = OBz, R3 = CO(CH2)14CH3 | D. oleoides (stems) [15] |
| 15 | Genkwadaphnin (Daphne factor F2, 12-benzoyloxy daphnetoxin) |
R1 = Ph, R2 = OBz, R3 = H |
D. genkwa (buds [10], flos [16], roots and stems [17], roots [18]); D. feddei (roots) [19]; D. oleoides (stems) [15]; D. aurantiaca (stem bark) [20]; D. altaica (aerial parts) [9] |
| 16 | Gnidicin | R1 = Ph, R2 = OCOCH=CH–Ph, R3 = H |
D. papyracea (NA 2) [13]; D. giraldii (stem bark) [21]; D. tangutica (root bark) [22]; D. acutiloba (stems) [8]; D. gnidium (aerial parts) [23]; D. altaica (aerial parts) [9] |
| 17 | Gnidicin 20-palmitate | R1 = Ph, R2 = OCOCH=CH–Ph, R3 = CO(CH2)14CH3 |
D. oleoides (stems) [15] |
| 18 | Gnididin | R1 = Ph, R2 = OCO(CH=CH)2(CH2)4CH3, R3 = H |
D. acutiloba (stems) [8] |
| 19 | Gnidilatidin 20-palmitate | R1 = (CH=CH)2(CH2)4CH3, R2 = OBz, R3 = CO(CH2)14CH3 |
D. oleoides (stems) [15]; D. genkwa (buds) [24] |
| 20 | Gniditrin | R1 = Ph, R2 = CO(CH=CH)3(CH2)2CH3, R3 = H |
D. tangutica (root bark [22,25], roots and stems [26]); D. odora (roots [27], leaves and branches [4]); D. papyracea (NA) [13]; D. giraldii (stem bark) [21]; D. aurantiaca (stems) [20]; D. gnidium (aerial parts) [23]; D. acutiloba (stems) [8]; D. mezereum (bark) [28,29]; D. alpina (bark) [29] |
| 21 | Isoyuanhuacine | R1 = (CH=CH) (CHCH) (CH2)4CH3, R2 = OBz, R3 = H |
D. genkwa (buds) [30,31] |
| 22 | Isoyuanhuadine | R1 = (CH=CH) (CHCH) (CH2)4CH3, R2 = OAc, R3 = H |
D. genkwa (buds) [10,14,30,31,32] |
| 23 | Kirkinine | R1 = (CH=CH)3(CH2)2CH3, R2 = OAc, R3 = H |
D. acutiloba (stems) [8] |
| 24 | Mezerein | R1 = Ph, R2 = OCO(CH=CH)2–Ph, R3 = H |
D. mezereum (seeds [33,34], barks [28]) |
| 25 | Odoracin | R1 = (CH=CH) (CHCH) (CH2)4CH3, R2 = OBz, R3 = H |
D. odora (roots) [27,35] |
| 26 | Tanguticacine | R1 = Ph, R2 = OCO(CH=CH)3(CH2)2CH3, R3 = CO(CH2)14CH3 |
D. tangutica (root bark) [12,25,36] |
| 27 | Tanguticadine | R1 = Ph, R2 = OCO(CH=CH)3(CH2)2CH3, R3 = 5,20-acetonide |
D. tangutica (NA) [13,36] |
| 28 | Tanguticafine | R1 = Ph, R2 = OCOCH=CH–Ph, R3 = 5,20-acetonide |
D. tangutica (NA) [13,36] |
| 29 | Tanguticagine | R1 = Ph, R2 = OCOCH=CH–Ph, R3 = CO(CH2)14CH3 |
D. tangutica (NA) [13,36] |
| 30 | Yuanhuacine (Gnidilatidin) | R1 = (CH=CH)2(CH2)4CH3, R2 = OBz, R3 = H |
D. genkwa (roots [37], roots and stems [38], buds [31,39], flos [40]) D. oleoides (stems) [15]; D. tangutica (stems) [41]; D. odora (leaves and branches) [4] |
| 31 | Yuanhuadine | R1 = (CH=CH)2(CH2)4CH3, R2 = OAc, R3 = H |
D. genkwa (roots and stems [38], roots [42], buds [30,31,43,44]) |
| 32 | Yuanhuafine | R1 = Ph, R2 = OAc, R3 = H | D. genkwa (buds [14,44], flowers [45,46]) |
| 33 | Yuanhuagine | R1 = (CH=CH)3(CH2)2CH3, R2 = OAc, R3 = H |
D. genkwa (roots and flowers [47], buds [10,24,31,48], roots [48], flowers [49]) |
| 34 | Yuanhuajine | R1 = (CH=CH)3(CH2)2CH3, R2 = OBz, R3 = H |
D. genkwa (buds [10,48], roots and flowers [47]; roots [48]) D. acutiloba (stems) [8]; D. tangutica (stems) [41]; D. odora (leaves and branches) [4] |
| 35 | Yuanhuamine A | R1 = (CHCH)2(CH2)4CH3, R2 = OAc, R3 = H |
D. genkwa (buds) [50] |
| 36 | Yuanhuamine B | R1 = (CH=CH)2(CH2)4CH3, R2 = OCO(CH2)3CH3, R3 = H |
D. genkwa (buds) [50] |
| 37 | Yuanhuamine C | R1 = (CH=CH)2(CH2)4CH3, R2 = OCO(CH2)4CH3, R3 = H |
D. genkwa (buds) [50] |
| 38 | Yuanhuaoate A | R1 = Ph, R2 = OCOCH2CH3, R3 = H | D. genkwa (buds) [51] |
| 39 | 12-hydroxydaphnetoxin | R1 = Ph, R2 = OH, R3 = H |
D. giraldii (roots [52], stem bark [11]); D. genkwa (buds) [10] |
| 40 | 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | R1 = (CH=CH)3(CH2)2CH3, R2 = OCOCH=CH–Ph, R3 = H |
D. odora (roots [27], leaves and branches [4]) |
| 41 | 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide | R1 = (CH=CH)2(CH2)4CH3, R2 = OCOCH=CH–Ph, R3 = H |
D. odora (roots [27], leaves and branches [4]) |
| 42 | 5β-hydroxyresiniferonol-6α,7α-epoxy-12β-acetoxy-9,13,14-ortho-2E-decenoate | R1 = (CH=CH) (CH2)5CH3, R2 = OAc, R3 = H |
D. genkwa (flowers) [46] |
| 43 | Altadaphnan A | R = OCOCH=CH—Ph | D. altaica (aerial parts) [9] |
| 44 | Altadaphnan B | R = OCO(CHCH) (CH=CH)2(CH2)2CH3 | D. altaica (aerial parts) [9] |
| 45 | Odoratrin | R = OCO(CH=CH)3(CH2)2CH3 | D. odora (roots) [27] |
| 46 | Yuanhuapine | R = OAc | D. genkwa (buds [44,53,54], flos [46,55]) |
| 47 | Yuanhuatine | R = OBz |
D. genkwa (buds [14,56], flos [55], roots and stems [38]); D. aurantiaca (stem bark) [20] |
| 48 | Daphgenkin D | R1 = H, R2 = OAc | D. genkwa (buds) [10] |
| 49 | Daphgenkin E | R1 = CO(CH=CH)3(CH2)2CH3, R2 = OAc | D. genkwa (buds) [10] |
| 50 | Daphnane-type diterpene ester-7 | R1 = CO(CH=CH)2(CH2)4CH3, R2 = OBz |
D. genkwa (buds) [57,58]; D. odora (roots) [27] |
| 51 | Daphneodorin F | R1 = CO(CH=CH)3(CH2)2CH3, R2 = OCOCH=CHC6H3(3-OCH3)(4-OH) |
D. odora (leaves and branches) [4] |
| 52 | Daphneodorin G | R1 = CO(CH=CH)2(CH2)4CH3, R2 = H, R2 = OCOCH=CH–Ph |
D. odora (leaves and branches) [4] |
| 53 | Yuanhuaoate C | R1 = CO(CH=CH)2(CH2)4CH3, R2 = OAc |
D. genkwa (buds) [10,31,51] |
| 54 | Gnidilatimonoein | D. mucronata (leaves) [59,60] |
1 The fine lines in the table are used to separate the diterpenoids with slightly different skeletons (same in the tables below). 2 For the diterpenoids with multiple sources, the source species are listed in the chronological order in which the diterpenoids were isolated from them (same in the tables below).
Daphnetoxins
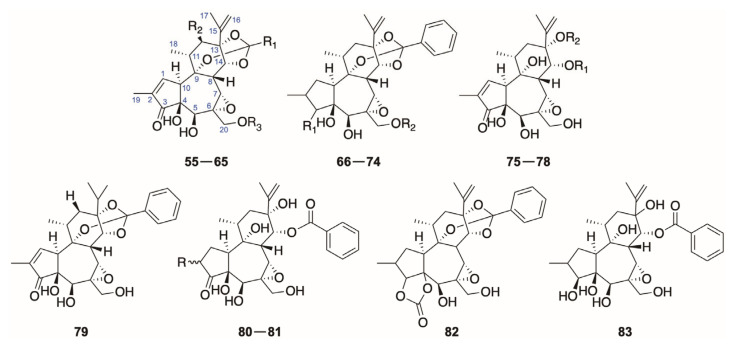
There are less compounds from the genus Daphne belong to daphnetoxins than 12-hydrodaphnetoxins including a 15,16-dihyrdo derivative tanguticahine (79). The archetype of this class is daphnetoxin (14), which was separated from the bark of D. mezereum for the first time [61] and later proved to exist also in D. papyracea [13], D. giraldii [52], D. tangutica [25], and D. acutiloba [8] in chronological order (Figure 3 and Table 2).
Figure 3.
Structures of daphnetoxins (55–83).
Table 2.
Structures and sources of daphnetoxins (55–83).
| No. | Compound (Synonym) | Chemical Structure | Source Species (Part) |
|---|---|---|---|
| 55 | Daphnegiraldifine | R1 = Ph, R2 = H, R3 = CO(CH2)14CH3 | D. giraldii (roots and stem bark) [52]; |
| 56 | Daphnetoxin | R1 = Ph, R2 = H, R3 = H |
D. mezereum (bark [28,61], seeds [33]); D. papyracea (NA) [13]; D. giraldii (roots and stem bark [52], roots and leaves [62], aerial parts [62], stems [21]); D. tangutica (root bark) [22,25]; D. acutiloba (stems) [8]; D. gnidium (stem bark [63], aerial parts [23]); D. mucronata (shoots) [64]; D. altaica (aerial parts) [9] |
| 57 | Excoecaria factor O1 | R1 = (CH=CH)3(CH2)2CH3, R2 = H, R3 = H |
D. acutiloba (stems) [8]; D. tangutica (stems) [41] |
| 58 | Excoecariatoxin | R1 = (CH=CH)2(CH2)4CH3, R2 = H, R3 = H |
D. tangutica (root bark) [22,25]; D. gnidium (aerial parts) [23]; D. altaica (aerial parts) [9] |
| 59 | Huratoxin (Daphne factor F1) | R1 = (CH=CH)2(CH2)8CH3, R2 = H, R3 = H |
D. feddei (roots) [19] |
| 60 | Simplexin | R1 = (CH2)8CH3, R2 = H, R3 = H |
D. genkwa (buds) [14]; D. holosericea (stems) [65] |
| 61 | Tanguticaline | R1 = Ph, R2 = H, R3 = CO(CH2)16CH3 |
D. tangutica (NA) [13,36] |
| 62 | Tanguticamine | R1 = Ph, R2 = H, R3 = COCH=CH(CH2)14CH3 |
D. tangutica (NA) [13,36] |
| 63 | Yuanhuahine | R1 = (CH=CH)2(CH2)4CH3, R2 = CH2CH3, R3 = H |
D. genkwa (flowers [49], buds [10,14,43]) |
| 64 | Yuanhualine | R1 = (CH=CH)2(CH2)4CH3, R2 = CH2CH2CH3, R3 = H |
D. genkwa (flowers [49], buds [10,44]) |
| 65 | 14′-ethyltetrahydrohuratoxin | R1 = (CH2)14CH3, R2 = H, R3 = H | D. acutiloba (stems) [8] |
| 66 | Acutilobin F | R1 = OCO(CH=CH)3(CH2)2CH3, R2 = H |
D. acutiloba (stems) [8]; D. genkwa (roots and stems) [38] |
| 67 | Acutilobin G | R1 = OCOCH=CH–Ph, R2 = H | D. acutiloba (stems) [8] |
| 68 | Genkwanine M | R1 = OH, R2 = Bz | D. genkwa (buds [14,54,57], flowers [66]) |
| 69 | Genkwanine N | R1 = OBz, R2 = H | D. genkwa (buds [54], flowers [55,66], roots and stems [17]) |
| 70 | Genkwanine N 20-palmitate | R1 = OBz, R2 = CO(CH2)14CH3 | D. genkwa (buds) [24] |
| 71 | Orthobenzoate 2 | R1 = OH, R2 = H | D. genkwa (buds [14,54,67], flowers [66]) |
| 72 | Wikstroemia factor M1 | R1 = OCO(CH=CH)2(CH2)4CH3, R2 = H |
D. acutiloba (stems) [8]; D. genkwa (buds [68], roots and stems [38], flowers [55]) |
| 73 | 1,2α-dihydrodaphnetoxin (Tanguticakine, Daphne factor F4) | R1 = O, R2 = H |
D. feddei (stem bark) [19]; D. oleoides (stems) [15]; D. giraldii (stems) [62]; D. genkwa (flowers) [66] |
| 74 | 1,2α-dihydro-20-palimoyldaphnetoxin (1,2-dihydrodaphnegiraldifine) | R1 =O, R2 = CO(CH2)14CH3 | D. tangutica (root bark) [13,69] |
| 75 | Daphnediraldigin | R1 = Bz, R2 = H | D. giraldii (stem bark) [70] |
| 76 | Isovesiculosin | R1 = H, R2 = CO(CH=CH)2(CH2)4CH3 | D. tangutica (root bark) [22] |
| 77 | Prohuratoxin (Wikstroelide M, Daphne factor F3) | R1 = CO(CH=CH)2(CH2)8CH3, R2 = H |
D. feddei (roots) [19]; D. acutiloba (stems) [71]; D. altaica (aerial parts) [9] |
| 78 | Vesiculosin | R1 = CO(CH=CH)2(CH2)4CH3, R2 = H |
D. tangutica (root bark) [22]; D. altaica (aerial parts) [9] |
| 79 | 15,16-dihydrodaphnetoxin (Tanguticahine) | D. tangutica (NA) [12,13,36] | |
| 80 | 1,2α-dihydro-5β-hydroxy-6α,7α-epoxy-resiniferonol-14-benzonate | R = β-CH3 | D. tangutica (root bark) [22] |
| 81 | 1,2β-dihydro-5β-hydroxy-6α,7α-epoxy-resiniferonol-14-benzoate | R = α-CH3 | D. tangutica (root bark) [22] |
| 82 | Genkwanin I | D. genkwa (buds [72], flos [66]) | |
| 83 | Genkwanine O | D. genkwa (buds [54], flos [66]) |
2.1.2. Genkwanines
The highly oxygenated diterpenoids genkwanines has a 6,7-dihydroxyl in the seven-membered ring B instead of a 6α,7α-epoxy in 6-epoxy daphnane diterpenoids or a 6,7-double bond possessed by resiniferonoids and also a saturated ring A, which could be almost viewed as 1,2-dihydro-3-hydroxy-daphnetoxin derivatives. The archetypal diterpenoid of this class is genkwanine A (84). Among these genkwanines, only genkwanine L (95) possesses a ketone function at C-3 and an oxygen-containing function at C-12 (Figure 4).
Figure 4.
Structures of genkwanines (84–95).
Up to now, all compounds of this class were reported to occur in D. genkwa, and these compounds were isolated only from the flower (mostly buds) of D. genkwa [43,54,55,57,66,73] (Table 3).
Table 3.
Structures and sources of genkwanines (84–95).
| No. | Compound (Synonym) | Chemical Structure | Source Species (Part) |
|---|---|---|---|
| 84 | Genkwanine A | R1 = H, R2 = H | D. genkwa (buds [43,57,73], flos [66]) |
| 85 | Genkwanine B | R1 = CO(CH=CH)2(CH2)4CH3, R2 = H | D. genkwa (buds) [73] |
| 86 | Genkwanine C | R1 = CO(CH=CH)3(CH2)2CH3, R2 = H | D. genkwa (buds) [73] |
| 87 | Genkwanine D | R1 = Bz, R2 = H | D. genkwa (buds) [54,57,73] |
| 88 | Genkwanine E | R1 = H, R2 = CO(CH=CH)3(CH2)2CH3 | D. genkwa (buds) [73] |
| 89 | Genkwanine F | R1 = H, R2 = CO(CH=CH)2(CH2)4CH3 | D. genkwa (buds [14,43,54,73], flowers [55]) |
| 90 | Genkwanine G | R1 = H, R2 = CO(CH=CH) (CH2)6CH3 | D. genkwa (buds) [73] |
| 91 | Genkwanine H | R1 = H, R2 = Bz | D. genkwa (buds [43,54,57,73], flowers [67]) |
| 92 | Genkwanine I | R = H | D. genkwa (buds [73,74], flowers [67]) |
| 93 | Genkwanine J | R = CO(CH=CH)2(CH2)4CH3 | D. genkwa (buds) [14,57,73,74] |
| 94 | Genkwanine K | R = Bz | D. genkwa (buds [73], flowers [66]) |
| 95 | Genkwanine L | D. genkwa (buds [73], flowers [46]) |
2.1.3. Resiniferonoids
The resiniferonoids are a group of 5-deoxy-6,7-double bond daphnetoxin derivatives, in which the A/B ring system possesses the pattern of phorbol. One of the most representative resiniferonoids, resiniferatoxin (RTX), was isolated from the dried latex of E. resinifera, showing significant transient receptor potential vanilloid 1 (TRVP1) activating activity and strong irritant effect [3,5], and compounds discovered in this class so far contain the same skeleton as RTX (Figure 5).
Figure 5.
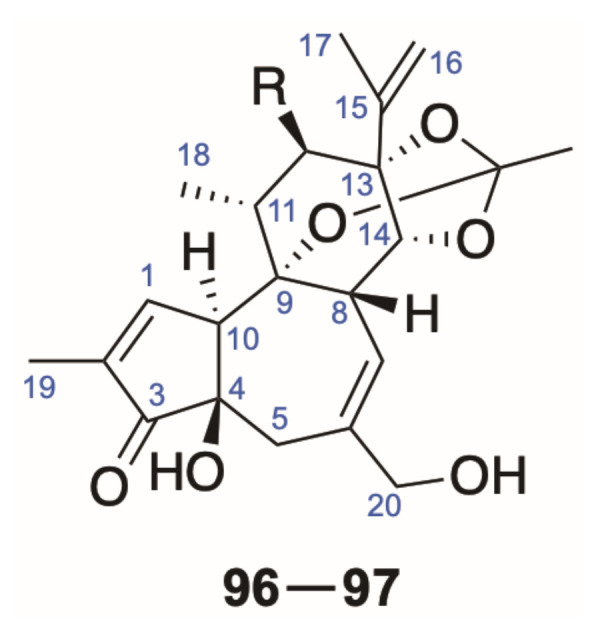
Skeleton of resiniferonoids (96–97).
Resiniferonoids have a quite narrow distribution, mostly limited to several Euphorbia genus in the Euphorbiaceae [3,5]. Two novel resiniferonoids daphneresiniferins A and B (96 and 97) obtained from the Daphne species [43] are practically identical to each other, except for the variation of oxygenated functions at C-12, which is quite rare and noteworthy (Table 4).
Table 4.
Structures and sources of resiniferonoids (96–97).
2.1.4. 1-Alkyldaphnanes
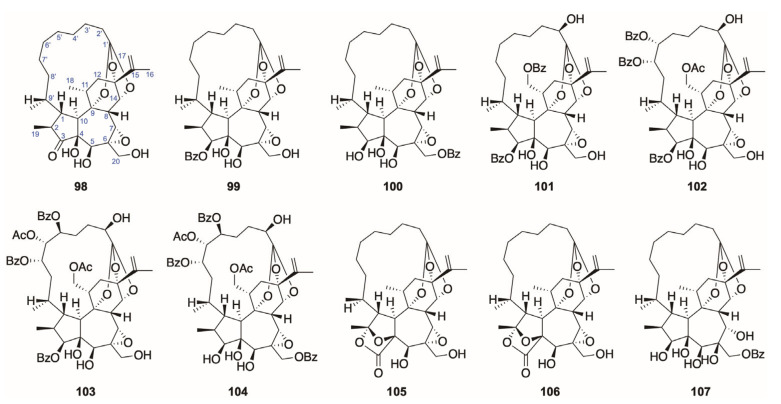
The 1-alkyldaphnanes (98–107) have an obvious feature of bridging between the C-1′ at the end of 9,13,14-orthoester group and C-1 in the ring A with a C-C bond. Meanwhile, the ring A of 1-alkyldaphnanes is usually saturated. The 1-alkyldaphnanes also has two subdivisions, 3-hydroxy and 3-acyloxy classes, depending on the oxidation degree of C-3 (Figure 6).
Figure 6.
Structures of 1-alkyldaphnanes (98–107).
The archetype of this category is gnidimacrin (101), which was obtained from Gnidia subcordata originally [75] along with its 20-palmitate, and it was also isolated from the Daphne plants. The 1-alkyldaphnanes (98–107) tend to exist mainly in the buds of D. genkwa [14,44] and branches of D. odora [76]. As compared to Daphne genera, there appeared to be more 1-alkyldaphnane diterpenoids in the leaves and roots of the plants from the Stellera [77,78,79] and Wikstroemia genera [80,81,82] (Table 5).
Table 5.
Structures and sources of 1-alkyldaphnanes (98–107).
| No. | Compound (Synonym) | Source Species (Part) |
|---|---|---|
| 98 | Wikstroelide E | D. genkwa (buds) [14] |
| 99 | Pimelea factor P2 | D. genkwa (buds) [14] |
| 100 | Genkwadane B | D. genkwa (buds) [14] |
| 101 | Gnidimacrin | D. odora (branches) [76] |
| 102 | Daphneodorin A | D. odora (branches) [76] |
| 103 | Daphneodorin B | D. odora (branches) [76] |
| 104 | Daphneodorin C | D. odora (branches) [76] |
| 105 | Pimelotide A | D. genkwa (buds) [14,44] |
| 106 | Pimelotide C | D. genkwa (buds) [14] |
| 107 | Genkwadane C | D. genkwa (buds) [14] |
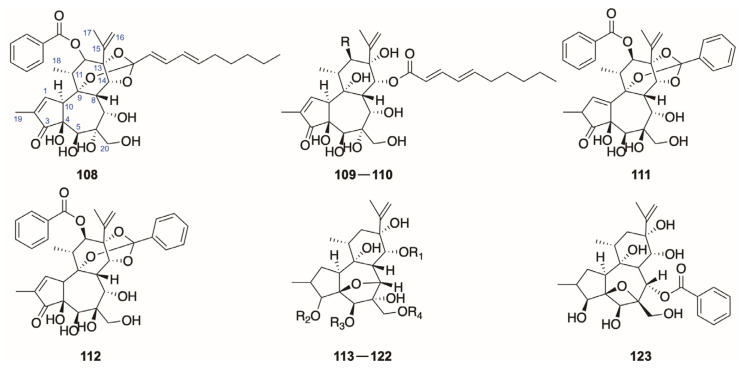
2.1.5. Other Daphnane-Type Diterpenoids
Some daphnane diterpenoids (108–123) remain uncategorized into four classes mentioned above, which could be divided into two major types. The skeleton of daphgenkin A–C (109–111) and yuanhuaoate B (112) resembles the one of genkwanines (Figure 4), but with the variations of a 3-ketone function, an oxygenated group at C-12, and a 1,2-double bond (Figure 7).
Figure 7.
Structures of other daphnane diterpenoids from the genus Daphne (108–123).
Genkwanine L (95, Figure 4), mentioned in Section 2.1.2, is classified in this review as a genkwanine diterpenoid according to earlier reviews [3,83]. It was regarded as an exception in genkwanines by some researchers [3] at that time with the possession of a 3-ketone and a 12-acetoxy, which is noteworthy. Neogenkwanine A-H, genkwanine G, daphneodorin H, and genkwanine VIII (113–122) feature a 4,7-epoxy-bridged structure (a 4,6-epoxy-bridged one in neogenkwanine I) in ring B, which is very rare in daphnane diterpenoids, yet there is no appropriate category for these diterpenoids. These diterpenoids (108–123) were obtained from the buds of D. genkwa with only the exception of daphneodorin H (113) isolated from the leaves and branches of D. odora (Table 6).
Table 6.
Structures and sources of other daphnane diterpenoids from the genus Daphne (108–123).
| No. | Compound (Synonym) | Chemical Structure | Source Species (Part) |
|---|---|---|---|
| 108 | Daphgenkin A | D. genkwa (buds) [10] | |
| 109 | Daphgenkin B | R = OBz | D. genkwa (buds) [10] |
| 110 | Daphgenkin C | R = OAc | D. genkwa (buds) [10] |
| 111 | Genkwadane A | D. genkwa (buds) [14] | |
| 112 | Yuanhuaoate B | D. genkwa (buds) [51] | |
| 113 | Daphneodorin H | R1 = H, R2 = CO(CH=CH)3(CH2)2CH3, R3 = H, R4 = Bz | D. odora (leaves and branches) [4] |
| 114 | Genkwanine VIII | R1 = Bz, R2 = H, R3 = H, R4 = Bz | D. genkwa (flowers) [44,84] |
| 115 | Neogenkwanine A | R1 = Bz, R2 = H, R3 = H, R4 = H | D. genkwa (buds) [57,85] |
| 116 | Neogenkwanine B | R1 = H, R2 = H, R3 = H, R4 = Bz | D. genkwa (buds) [57,85] |
| 117 | Neogenkwanine C | R1 = Bz, R2 = CO(CH=CH) (CHCH) (CH2)4CH3, R3 = H, R4 = H | D. genkwa (buds) [57,85] |
| 118 | Neogenkwanine D | R1 = Bz, R2 = CO(CH=CH)2(CH2)4CH3, R3 = H, R4 = H | D. genkwa (buds) [57,85] |
| 119 | Neogenkwanine E | R1 = H, R2 = CO(CH=CH)2(CH2)4CH3, R3 = H, R4 = Bz | D. genkwa (buds) [57,74,85] |
| 120 | Neogenkwanine F | R1 = H, R2 = CO(CH=CH) (CHCH) (CH2)4CH3, R3 = H, R4 = Bz | D. genkwa (buds) [57,74,85] |
| 121 | Neogenkwanine G | R1 = Bz, R2 = CO(CH=CH) (CHCH) (CH2)4CH3, R3 = Bz, R4 = Bz | D. genkwa (buds) [57] |
| 122 | Neogenkwanine I | R1 = H, R2 = H, R3 = Bz, R4 = H | D. genkwa (buds) [74,85] |
| 123 | Neogenkwanine H | D. genkwa (buds) [57] |
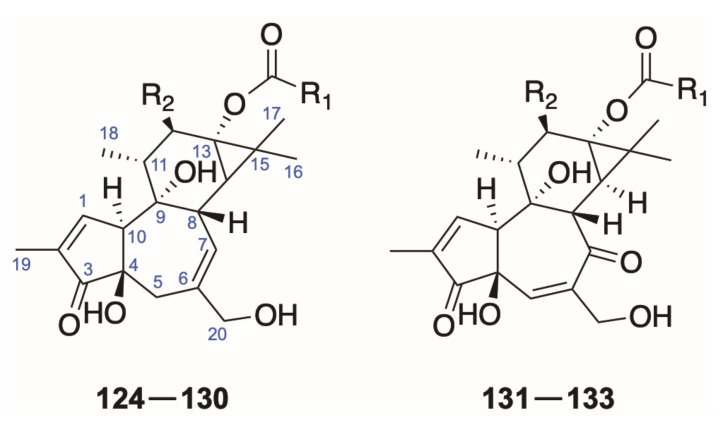
2.2. Tigliane-Type Diterpenoids
The tigliane tetracyclic diterpenoids share a closely related skeleton with daphnane ones with a highly substituted 13,14-cyclopropane ring D [6], presenting potent irritant and cocarcinogenic activities [86]. One representative and parent diterpene in this class is phorbol isolated from Croton tiglium [86]. The tigliane diterpenes widely distribute in many species from the genus Daphne [20,65,87], Pimelea [88], and Stellera [89] in the Thymelaeaceae fam, as well as Euphorbia [90,91] and Jatropha [86] in the Euphorbiaceae family. As compared to the phorbol esters (124–130), there are structural variations of a 7-ketone function and a 5,6-double bond in the ring B in the others (131–133, Figure 8 and Table 7).
Figure 8.
Structures of tigliane-type diterpenoids (124–133).
Table 7.
Structures and sources of tigliane-type diterpenoids (124–133).
| No. | Compound (Synonym) | Chemical Structure | Source Species (Part) |
|---|---|---|---|
| 124 | 12-O-benzoylphorbol-13-nonanoate | R1 = (CH2)7CH3, R2 = OBz | D. aurantiaca (stem bark) [20] |
| 125 | 12-O-benzoylphorbol-13-octanoate | R1 = (CH2)6CH3, R2 = OBz | D. aurantiaca (stem bark [20], stems [87]) |
| 126 | 12-O-decanoylphorbol-13-acetate | R1 = CH3, R2 = OCO(CH2)8CH3 | D. genkwa (buds) [85,92] |
| 127 | 12-O-n-deca-2,4,6-trienoyl-phorbol-(13)-acetate | R1 = CH3, R2 = OCO(CH=CH)3(CH2)2CH3 | D. genkwa (roots and stems) [38] |
| 128 | Prostratin Q (12-O-(2′E,4′E-decadienoyl)-4-hydroxyphorbol-13-acetyl) | R1 = CH3, R2 = OCO(CH=CH)2(CH2)4CH3 | D. genkwa (buds [31,32,85], roots and stems [38,92]) |
| 129 | Prostratin | R1 = CH3, R2 = H | D. acutiloba (stems) [8] |
| 130 | Phorbol 13-monoacetate | R1 = CH3, R2 = OH | D. aurantiaca (stems) [87] |
| 131 | 12-O-(2′E,4′E-decadienoyl)-7-oxo-5-ene-phorbol-13-acetate | R1 = CH3, R2 = OCO(CH=CH)2(CH2)4CH3 | D. genkwa (buds) [85,92] |
| 132 | Daphwanin (12-O-decanoyl-7-oxo-5-ene-phorbol-13-acetate) | R1 = CH3, R2 = OBz | D. genkwa (buds) [66,92] |
| 133 | Dapholosericin A | R1 = (CH2)6CH3, R2 = OBz | D. holosericea (stems) [65] |
2.3. Lathyrane-Type Diterpenoids
Lathyranes, named after Euphorbia lathyris, were more often found in species from the Euphorbiaceae other than those in the Thymelaeaceae, especially in the Euphorbia species [86,93]. The archetypal compound of lathyrane is 17-hydrojolkinol obtained as a derivative product of ‘ester 7′ from the seeds of E. lathyris [94]. As for the skeleton with an additional 4,15-bond possessed by 134 and 135, it was believed to be a precursor for crotofolin from Croton corlifolious [86] (Figure 9).
Figure 9.
Structures of lathyrane-type diterpenoids (134–135).
So far, these are the only two lathyrane diterpenoids reported to be isolated from the Daphne species [66] (Table 8).
Table 8.
Structures and sources of lathyrane-type diterpenoids (134–135).
3. Biological Activities
Several Daphne plants have been used as traditional medicines for the treatment of cancer, inflammation, and rheumatism in Asia, North Africa, and Europe, and some of these plants were also regarded as virulent poisons. The flower bud of D. genkwa, a Chinese traditional medicine, has been used as a diuretic, antitussive, and pesticide, and one of its synonyms is “yu-du”, which means “a fish poison” [1,2,95].
However, the poisonous principles of any Daphne species have never been identified until daphnetoxin (56) was isolated from commercial “mezeron” bark (D. mezereum, D. laureola, and D. gnidium) in 1970 and identified as a major toxic component possessing both a similar skeleton and similar sites of functions as phorbol, which was a known toxic principle in Croton species [61,96] at that time. Subsequently, mezerein (24) with the same skeleton as daphnetoxin (56) was identified as another toxic principle of D. mezereum [33].
With a considerable number of modern pharmacological and chemical studies, it was demonstrated that diterpenoids from the Daphne genus possess a wide range of pharmacological activities including anticancer, anti-inflammatory, anti-HIV, cholesterol-lowering, neurotrophic, antifertility, skin irritant, nematicidal, and pesticidal activities. Among these biological activities, the anticancer ones have received the most attention since there remains a need for both efficient and safe anticancer drugs with novel structures.
3.1. Anticancer Activity
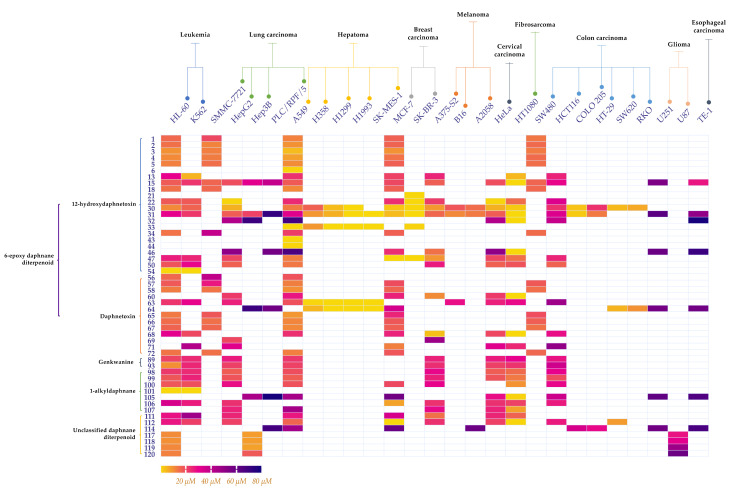
Previous studies have shown that diterpenoids from Daphne species exhibit potent anticancer activities against various types of cancers both in vitro and in vivo [2,83,97]. As for anticancer bioactivities in vitro, more than a half of the diterpenoids have been testified to exhibit cytotoxicity with IC50 values ranging from 10−6–98.46 μM (Table S1) against various cell lines, all of which belong to the daphnane type (Scheme 2).
Scheme 2.
Heat map of the IC50 values (μM) of cytotoxicity of some diterpenoids in various carcinoma cell lines in vitro.
The cytotoxicity of 51 diterpenoids against certain cell lines are intuitively shown in Scheme 2. As for the cell lines sensitive to very few diterpenoids and the diterpenoids only cytotoxic to a very limited number of cell lines, they will be additionally discussed below.
3.1.1. Structure–Activity Relationship (SAR)
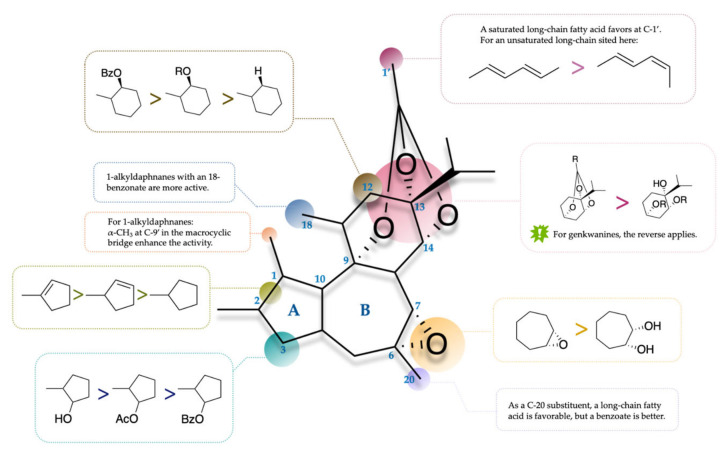
For an intuitive demonstration of the structure–anticancer activity relationship of diterpenoids from the genus Daphne, the structure and functions requirements are illustrated with a daphnane diterpenoid skeleton (Figure 10).
Figure 10.
SAR of diterpenoids as antineoplastics.
SAR in the Ring A
It is generally accepted that the 1,2-double bond could be a possible alkylating functionality in a diterpenoid and thus the reduction of it might make it a less active compound [98]. Genkwadaphnin (15), yuanhuatine (47), and yuanhuaoate B (112) share the similar structure with the only exception of an unsaturated bond in the ring A, and yuanhuatine (47) among them is the least active anticancer compound [8,14]. The site of an unsaturated carbon bond in the ring A would also have certain affect. For genkwadane A (111) and yuanhuaoate B (112), it appears that the change from the 1,10- to 1,2-double bond enhances the anticancer activity [14].
In the ring A, the variation of the oxygen-containing function at C-3 influences the anticancer activity. 12-O-benzoyl-3,5-hydroxy-6,7-epoxy-resiniferonol-9,13,14-orthobenzoate, which is a 3-hydroxyl derivative of genkwadaphnin (15), showed a promising cytotoxicity against seven carcinoma cell lines, much better than genkwadaphnin (15) itself, indicating the possibility that the hydronation of a 3-ketone favors a bioactivity [99]. However, genkwanine A (84) with a 3-hydroxyl was basically inactive against A549 cells, while its 3-acylated analogues (85–87) all possessed certain cytotoxicity with the IC50 values in the range of 0.79–8.70 μM [73], indicating that a 3-acyloxy function enhances the anticancer activity of diterpenoids with a more saturated skeleton. Genkwanine D (87) was the most potent one with a 3-benzoate group at the IC50 values of 0.79–8.00 μM [73] suggesting that a benzoate group at C-3 might favor the antineoplastic effect.
SAR in the Ring B
The 6α,7α-epoxy is a characteristic structure in 6-epoxy and 1-alkyldaphnane diterpenoids, and it was reported to help enhance the anticancer activity. Yuanhuacine (30), yuanhuadine (31), yuanhuafine (32), and yuanhuapine (46) generally showed stronger cytotoxicity against A549 cell line than genkwanine A-L (84–95) possessing a 6α,7α-dihydroxyl in the same assay [73]. Additionally, genkwadane B (100) possessed a stronger activity in seven cell lines than genkwadane C (107) [14], suggesting that the opening of 6α,7α-epoxy has a negative effect on antineoplastic activity as well.
The 20-acyloxy group in the ring B might have an important role in affecting the anticancer activity in diterpenoids. One type of 20-acylocy groups that could favor the bioactivity is a 20-palmitate ester. Yuanhuacine (30) showed no inhibitory activity against P-388 in vivo, while its 20-palmitate derivative gnidilatidin 20-palmitate (19) exhibited a strong inhibitory activity at dosages of 0.5–2.0 mg/kg/d, and the 20-plamitate derivative of gnidilatin was also observed to be more active than gnidilatin itself against P-388 [100]. This fact might also suggest the positive impact of a long-chain fatty ester on determining the anticancer activity.
Genkwanine M (68) with a 20-benzoate was demonstrated to inhibit various cell lines more significantly, especially HL-60 leukemia cells, A375-S2 melanoma cells, and HT-1080 fibrosarcoma cells, than orthobenzoate 2 (71) [14]. Similarly, genkwanine H (91) possessing a 20-benzoate exhibit much stronger cytotoxicity against P388 and A549 (IC50: 13.0 and 1.60 μM) than its analogues [58], indicating that an aromatic acyl group at C-20 might also enhance the anticancer activity.
SAR in the Ring C
The caged 9,13,14-orthoester is another characteristic structure in the daphnane-type diterpenoids in general. Genkwanines J–K (93–94) with a 9,13,14-orthoester were more cytotoxic (IC50: 4.20–42.0 μM) than genkwanine F–G (89–90, (IC50: 24.0–57.0 μM) in P-388 and A549 cells [73].
Interestingly, for a more statured skeleton, for instance, genkwanine, the caged 9,13,14-orthoester seems to reversely have a negative impact on anticancer activity. Genkwanine J (92) showed potent inhibition against P388 and A549 (IC50: 4.2 and 25.0 μM), while genkwanine F (84) were moderately active with the IC50 values of 39.0–24.0 μM [73]. More studies have further shown a more significant anticancer activity of genkwanine J (92) compared to genkwanine F (84) in different cell lines [14].
Evidence showed that the presence of a long-chain fatty could enhance the inhibitory activity against carcinoma cells. Yuanhuacine (30) and yuanhuadine (31) showed promising inhibition against HepG2 cell line with the IC50 values in the range of 5.56 to 17.06 μM, while yuanhuafine (32) showed more limited activity at the IC50 value of 42.37 μM [14].
Whether this long chain at the end of the 9,13,14 orthoester is statured or not could influence the antineoplastic activity as well. Gnidilatin was a potent antileukemia compound against P-388 in vivo; meanwhile, yuanhuacine (31) was inactive with the introduction of 2′,3′- and 4′,5′-double bonds [100]. Yuanhuagine (33) with one more unsaturated bond in the long-chain substituent in orthoester group presents weaker bioactivity (IC50: 809.4 nM) than yuanhuadine (31) and isoyuanhuadine (22, IC50: 61.6 and 83.7 nM) in SK-BR-3 [30], which indicates that the introduction of unsaturated bond in the 9,13,14 orthoester structure is unfavorable.
As for the unsaturated long-chain at C-1′, the stereochemistry matters. Yuanhuacine (30) and yuanhuadine (31) possessing a 2′E,4′E-double bond could both inhibit SK-BR-3 proliferation more significantly (IC50: 172.6 and 61.6 nM) than their conformational isomers, respectively (IC50: 217.1 and 83.7 nM) [30].
The absence of a 12-acyl group generally causes the reduction of anticancer bioactivity. Mezerein (24), a major toxic principle of D. mezereum, showed significant inhibitory activity in vivo against P-388 and L-1210 leukemia cell lines at dosage of 50 μg/kg in mice [34]. Gnidicin (16), gnididin (18), and gniditrin (20) with the closely related skeleton isolated from Gnidia lamprantha Gilg were also proved to be substantial antileukemia agents. Huratoxin (59) and simplexin (60) exhibited similar inhibitory activities against L1210 and K562 in vitro, but less active than an esterified derivative subtoxin [77]. By comparison, 12-hydroxydaphnetoxin (39) bearing a hydroxyl at C-12 in the ring C showed no antileukemia activity and esterification of it could establish the bioactivity [101]. Genkwadaphnin (15), which is in essence the benzoyl derivative of 12-hydroxydaphnin (39), was found to exert both in vitro and in vivo antileukemia activity against P-388 cells [16]. Although all the above together suggests that an acyl function at C-12 might be a prerequisite for the in vivo antileukemia bioactivity, this does not represent that a 12-acyloxyl group is necessary for antileukemia activities regarding the fact that some daphnetoxins were reported to show a quite promising inhibition against a variety of cell lines including HL-60 and K562 as well (Scheme 2).
The type of ester group at C-12 may also have an impact on the antileukemia activity. Yuanhuacine (30) showed stronger cytotoxicity against HL-60 and K562 cells (IC50: 26.81 and 16.08 μM) than yuanhuadine (31, IC50: 30.05 and 22.16 μM). Genkwadaphnin (15), with a 12-benzoate function, was also more active in antileukemia than yuanhuafine (32), as the latter showed limited inhibitory activity in HL-60 and K562 cell lines [14]. Yuanhuatine (47) was cytotoxic against human and chronic myeloid promyelocytic leukemia cell lines at the IC50 values of 17.72 and 17.54 μM [14], while yuanhuapine (46) has not been reported to possess antileukemia property. Yuanhuacine (30) and isoyuanhuacine (21) showed moderate cytotoxicity against SK-BR-3 (IC50: 172.6 and 217.1 nM), while and yuanhuadine (31) and isoyuanhuadine (22) were more active (IC50: 61.6 and 83.7 nM) [30]. These verify that a benzoyl group at C-12 might act as an important role in affecting the antileukemia activity other than an acetoxyl one.
It is noteworthy that gnidimacrin (101) is one of the most potent antileukemia agents both in vitro (IC50 in the range of 0.16–0.28 nM) to HL-60, K562, and CCRF-CEM cells [78] and in vivo at dosages of 0.02–0.03 mg/kg/d [78,79], which is also much more active than other 1-alkyldaphnanes isolated from Daphne species [57]. It is generally considered that the antineoplastic activity is related to the 18-benzoyloxy substituent at C-18 [3,102].
Interestingly, for pimelotide A (105) and pimelotide C (106), it seems that the one with α-CH3 (106) exhibits more significant antineoplastic bioactivities against a variety of cell lines including HeLa, MCF-7, HepG2, HCT116, A549, A375-S2, U937, HL-60, and K562, which may suggest that the stereochemistry of substituents is related to the anticancer activity as well [14].
3.1.2. Anticancer Activity and Involved Mechanisms
Leukemia
It is also revealed that genkwadaphnin (15) could oppose the protein and DNA synthesis of P-388 cells to exert both in vitro and in vivo antileukemia activities [103,104]. Genkwadaphnin (15, IC50: 11.8 μM) and yuanhuacine (30, IC50: 10.8 μM) were determined to suppress Bcl-2 and Bcl-XL in a dose-dependent manner to induce apoptosis in human myelocytic HL-60 cells [39].
Gnidimacrin (101) showed a significant antiproliferative effect against K562 cell lines at the IC50 of 1.2 nM by activating protein kinase C (PKC) and arresting the cell cycle at G1 phase [79].
Lung Carcinoma
Several 12-hydrodaphnetoxins including yuanhuadine (31), yuanhuadine (63), yuanhualine (64), and yuanhuagine (33) exhibited stronger antiproliferative activity against A549 cells (IC50 values in the range of 12–53 nM) than the positive control ellipticine without displaying cytotoxicity against the human normal lung epithelial cell line MRC-5, with especially yuanhuacine (30, IC50: 12 μM) presenting the most potent bioactivity [49]. Further study has indicated that yuanhuacine (30) could also be anticancer bioactive against H1993 human non-small cell lung cancer (NSCLC) cells both in vitro and in vivo by modulation of the AMPK/mTOR signaling pathway [105].
Yuanhualine (64), yuanhuadine (63), and yuanhuagine (33) showed notable inhibitory effects in drug-resistant cell lines including gemcitabine-resistant A549, gefitinib- and erlotinib-resistant H292. Further research indicated that these diterpenoids were able to arrest cell cycle in the G0/G1 and G2/M phase in A549 cells by upregulating the expression of cyclin dependent kinase inhibitors P21 and P53 as well as downregulating cell-cycle regulators, for example, c-Myc and cyclin B1/cell division cycle 2 (CDC2) complex and suppress Akt/STAT/Src signaling pathway [106]. Additionally, yuanhualine (64) was observed to have synergistic effects with certain chemotherapeutic agents (gemcitabine, gefitinib and erlotinib) in the treatment of A549 cell line [106].
Hepatoma
Wu et al. evaluated the effects of genkwadaphnin (15) on hepatocellular carcinoma (HCC) cells both in vitro and in vivo with Hep3B and PLC/PRF/5 cell lines and BALB/c nude mice, respectively, the results showed that genkwadaphnin (15) suppressed growth and invasion of HCC cells both in vitro and in vivo by blocking DHCR24-mediated cholesterol biosynthesis and lipid rafts formation [44]. Evidence also showed that yuanhuacine (30) and genkwadaphnin (15) were hepatotoxic on normal human liver cells HL-7702 in a dose- and time-dependent manner; meanwhile, the change of cell morphology and increased AST and ALT were observed as well [40].
Breast Carcinoma
Yuanhuacine (30) was found to be an active inhibitor in both MCF-7 and MDA-MB-231 cell lines, and the preliminary mechanism of strong cytotoxicity of yuanhuacine (30) against MCF-7 was investigated further by using Western blot and flow cytometry analysis; the results suggested that yuanhuacine (30) induced apoptosis via the regulation of Bcl-2, Bax, and cleavage of PARP expression in MCF-7 cells [84]. Yuanhuatine (47) was also observed to inhibit the growth of estrogen receptor alpha (ERα)-positive cells MCF-7 (IC50: 0.62 μM) significantly compared to tamoxifen (IC50: 14.43 μM) through mitochondrial dysfunction and apoptosis in ERα-positive breast cancer cells MCF-7 caused by ERα-downregulation [107]; for ERα-negative cells MDA-MB-231, either cytotoxicity or apoptosis was observed [107].
Melanoma
Yuanhuacine (30), Yuanhuatine (47), and genkwanine M (68) isolated from the buds of D. genkwa displayed pronounced inhibitory bioactivity against the human melanoma cell line A375-S2 at the IC50 levels of 8.72, 9.31, and 3.62 μM, respectively [14]. Yuanhuacine (30) and yuanhuadine (31) were obtained from the dichloromethane fraction and showed potent cytotoxicity to melanoma B16 as well as A2058 cell lines, while genkwanine C (86) and genkwanine VIII (118) were selectively active in the A2058 cells [84]. It was verified that yuanhuacine (30), yuanhuadine (63), genkwadaphnin (15), genkwanine A (84), genkwanine F (89), genkwanine H (91), and daphneresiniferin A (96) and B (97) could inhibit the α-MSH-induced melanin production in B16 melanoma cells remarkably with the IC50 in the range of 0.57–9.0 μM in comparison with the positive control arbutin (IC50: 140 μM) and kojic acid (IC50: 39 μM), especially yuanhuadine (31, IC50: 0.06 μM) with the striking inhibitory activity [43]. Among these compounds, a resiniferonoid daphneresiniferin B (97) showed obvious cytotoxicity against B16 cells at the IC50 level of 6.6 μM [43], which indicated that it probably affected melanin production simply with its high cytotoxicity. Genkwadaphnin (15) was also investigated to exert apoptosis-triggering effect in squamous cell carcinoma (SCC) cells in a JNK-dependent manner [108].
Besides, the antiproliferative activity of the ethyl acetate and aqueous extract from the leaves of D. gnidium was observed in B16-F0 and B16-F10 cell lines inducing G2/M cell cycle arrest and the ethyl acetate extract was also capable of enhancing melanogenesis stimulation activity in a concentration-dependent manner in B16-F10 cells [109]; furthermore, the aqueous extract of D. gnidium exerted in vitro and in vivo antimelanoma effects on B16-F10 by activating natural killer (NK) cell and cytotoxic T lymphocyte (CTL) [110], hopefully these findings might lead to the discovery of more potential compounds affecting the melanogenesis and cell cycle of melanoma cells.
Fibrosarcoma
The alcohol–water extract of the aerial parts of D. mucronata was evaluated to possess anticancer property both in vitro and in vivo and its mechanism, similar to the natural anticancer drug Taxol, was probably related to the downregulation of human tumor necrosis factor alpha receptors (TNF-αR) [111]; the probable principle of anticancer activity, gnidilatimonoein (54), was isolated from D. mucronata afterwards and showed a strong antiproliferation effect on WEHI-164 by mediating the progress of DNA synthesis [60].
Colon Carcinoma
The mechanism of anticancer effect of genkwadaphnin (15) was revealed when it was found that it enhanced the p21 expression and simultaneously suppressed the c-Myc expression in a PRDM1-denpendent manner to arrest the cell-cycle progression in the human colon cancer SW620 cell line [112].
Yuanhuacine (30) and yuanhuadine (31) were revealed to exhibit more significant inhibitory effects on the proliferation of the COLO250 (IC50: 2–3 μM) than HT-29 cell line (IC50: 13–23 μM) [84], suggesting the selectivity of the antineoplastic activity. A further study indicated that yuanhuacine (30) inhibited the HCT116 cell line by upregulating p21 expression and transcription via a p53 protein independent cascade [113]. Daphgenkin A (108), along with yuanhuacine (30) and yuanhuadine (31) obtained from the petroleum ether extract from D. genkwa, showed definite cytotoxic effects on both SW620 and RKO cell lines with the respective IC50 value of 3.0 and 6.5 μM; then, the results of further research revealed that daphgenkin A (108) inhibited SW620 cell proliferation by stalling the cell cycle at G0/G1 phase, causing cell death by apoptosis as well as inducing cell cycle arrest via regulating the PI3K/Akt/mTOR signaling pathway [10].
Gastric Carcinoma
Yuanhuacine (30), yuanhuadine (31), yuanhuatine (47), genkwanine F (89), genkwanine N (69), and wikstroemia factor M1 (72) obtained from D. genkwa were moderately cytotoxic with IC50 levels in the range of 25.61 to 27.32 μM against the human gastric carcinoma MGC-803 cell line [68]. Furthermore, yuanhuacine (30, IC50: 17 μM) and yuanhuadine (31, IC50: 16 μM) were detected to oppose the proliferation of human gastric adenocarcinoma AGS cell lines, along with genkwanine VIII (118, IC50: 12 μM) [84].
Others
Yuanhuacine (30) and yuanhuajine (7) presented more obvious inhibitory activity against DNA topoisomerase I (DNA topo I) at the IC50 levels of 40.0 and 38.3 μM than a known topo I inhibitor hydroxycamptothecin (hCPT, IC50: 48.0 μM), and further study with the prepared derivatives suggested that less electron-withdrawing groups at C-5, C-12, and C-20 facilitated the combination between the compounds and DNA topo I [47].
Yuanhuacine (30) was also reported to be cytotoxic against two bladder cancer cell lines UMUC3 (IC50: 1.89 μM) and T24T (IC50: 1.83 μM), and it functioned in T24T cells by inducing a G2/M phase arrest significantly via modulation of Sp1 protein expression [113].
The human lymphoma cell line U937 has been reported to be moderately suppressed by daphnane-type diterpene ester-7 (50), yuanhuacine (30), and yuanhuatine (47) with the IC50 levels of 11.62–12.35 μM [14], while gnidilatimonoein (54) showed a stronger cytotoxicity (IC50: 1 μM) [59].
The chloroform extract from D. altaica was also found to significantly suppress the proliferation of esophageal squamous carcinoma Eca-109 cell line at the IC50 level of 10.6 μM and in a dose-dependent manner [114], and the ethyl acetate extract of D. altaica was reported to function by inducing apoptosis and cell cycle arrest in the S phase in the Eca-109 cell line via the PPARγ-mediated pathway [115]. However, whether its anticancer effect is related to diterpenes or not requires detailed studies.
3.2. Anti-HIV Activity
Both daphnane- and tigliane-type diterpenoids from Daphne species were demonstrated to possess an anti-HIV activity even stronger than some anti-HIV agents such as 3′-azido-3′-deoxythymidine (AZT), and structure-activity relationship in anti-HIV bioactivity is quite similar to that in the anticancer one (Figure 10).
3.2.1. Structure–Activity Relationship (SAR)
The presence of a 9,13,14-orthoester in the ring C is also favorable for anti-HIV activity as diterpenoids with an orthoester, for instance, daphneodorins D–E (11–12), presented stronger anti-HIV activity than daphneodorins F–H (51–52, 113) without one (EC50 > 25 nM) [4]. Yuanhuamine A (35) and its isomer, isoyuanhuadine (22), showed promising anti-HIV activity (EC50 < 0.9 nM), while yuanhuaoate C (53) without a 9,13,14-orthoester merely exhibited moderated activity [116], suggesting that the orthoester motif enhances the anti-HIV effect.
The substituent at C-12 in the ring C acts as an important role in anti-HIV activity as well. A tigliane diterpenoid 12-O-benzoylphorbol-13-octanoate (125) from D. aurantiaca showed definite anti-HIV-1 activity against C8166 cell line with EC50 value of 0.282 nM and SI value of 65177.305, while phorbol 13-monoacetate (130) possessing a 12-hydroxyl showed limited activity, which suggests an acyl group at C-12 might favor anti-HIV-1 bioactivity [87].
Daphneodorins A–B (102–103) presented more potent activity in inhibiting HIV-1 replication in MT4 cell line (EC50: 0.16 and 0.25 nM, respectively) than daphneodorin C (EC50: 2.9 nM) suggesting that a 20-benzoyloxy reduces the anti-HIV activity [76].
3.2.2. Anti-HIV Activity and Involved Mechanism
Prostratin (129) is known as a potent anti-HIV agent [117] and its mechanism is the protection of CD4+ cells by downregulation of the HIV receptor CD4 and co-receptors and the interaction with PKC to stimulate viral replication in infected cells [6]. Wikstroelide E (98), a HIV-latency-reversing compound that is strikingly 2500-fold more potent that prostratin (129), functioned by regulating various signaling pathways including the MAPK, PI3K-Akt, JAK-Stat, TNF, and NF-κB ones [116].
Acutilobins A–G (1–5, 66–67, EC50: 0.32–1.50 nM), genkwanine N (69, EC50: 0.17 nM), genkwadaphnin (15, EC50: 1.94 nM), kirkinine (23, EC50: 5.63 nM), excoecariatoxin (58, EC50: 5.64 nM), and 14′-ethyltetrahydrohuratoxin (65, EC50: 0.52 nM) were obtained from D. acutiloba, all of which exhibited strong anti-HIV-1 bioactivity, especially genkwanine N (69, EC50: 0.17 nM and SI: 187,010) [8].
Based on the discovery of the antiretroviral activity of the dichloromethane extract of D. gnidium without displaying cytotoxicity, daphnetoxin (56), gnidicin (16), gniditrin (20), and excoecariatoxin (58) were determined to be the principles of anti-HIV bioactivity according to the HPLC-based profiling; meanwhile, a more detailed study showed that daphnetoxin (56) selectively interfered with the expression of two key cell-surface factors CXCR4 and CCR5 for HIV-1 entry [106].
It is worth noting that wikstroelide M (77) inhibited not only HIV-1 but HIV-2 strains in a concentration-dependent manner with high SI values and low cytotoxicity, and its mechanism might be related to the inhibition of HIV-1 reverse transcription and integrase nuclear translocation [71].
3.3. Anti-Inflammatory Activity
Genkwadaphnin (15), gniditrin (20), and yuanhuatine (47) showed significant inhibitory effect against LSP-induced nitric oxide (NO) production in RAW 264.7 macrophages with the IC50 values of 0.03–0.07 μM, especially 12-O-benzoylphorbol-13-nonanoate (124) and 12-O-benzoylphorbol-13-octanoate (85) with the IC50 values of 0.01 μM could be potential therapeutic agents for inflammation [20]. Daphwanin (132, IC50: 7.2 μM), a tigliane-type diterpenoid derived from D. genkwa, and orthobenzoate 2 (64, IC50: 5.4 μM) showed stronger inhibitory activity than aminoguanidine (IC50: 17.4 μM) on NO production in RAW 264.7 cells [67].
As for another inflammatory inhibitor genkwadaphnin (15), the mechanism includes the activation of PKD1/NF-κB signaling to induce CD44 expression in a time- and concentration-dependent manner and thus promoting the migration of K562 cells, resulting in an innate immune response [39]. Genkwadaphnin (15) was also observed to restore exhausted LCMV-specific CD4+ and CD8+ T cells by downregulating negative regulatory molecule Tim-3 [118].
It is reported that diterpenes showed anti-inflammatory efficacy sorted from highest to lowest as follows: prostratin Q (128), genkwadaphnin (15), isoyuanhuacine (21), yuanhuacine (30), yuanhuaoate C (53), yuanhuagine (33), isoyuanhuadine (22), and yuanhuadine (31) in LPS-induced RAW264.7 cells by downregulating the overexpression of IL-6, IL-1β, and TNF-α as well as decreasing NO production as the results of principal component analysis (PCA) and hierarchical cluster analysis (HCA) indicated, among these, prostratin Q (128) also showed bioactivity on VEGF, MMP-3, and ICAM, which has the potential to be developed as a novel drug for rheumatoid arthritis treatment [31].
3.4. Cholesterol-Lowering Activity
Gniditrin (20) and daphnetoxin (56) extracted from D. giraldii were found to present potent cholesterol-lowering activity in vitro at the EC50 levels of 0.59 and 4.3 μM, respectively, by up-regulating the low-density lipoprotein receptor (LDLR) level and consequently promoting LDLR expression [119]. Gniditrin (20) had a lower EC50 for activating LDLR-promoter than that of daphnetoxin (56), which might indicate that the acyl group at C-12 might improve the cholesterol-lowering bioactivity as well.
3.5. Neurotrophic Activity
Yuanhuacine (30) and genkwanine N (69) significantly enhanced the function of the orphan nuclear receptor Nurr1 at a concentration of 0.3 μM and inhibited LPS-induced neuroinflammation in vitro as well as improved behavioral deficits in a hydroxydopamine (6-OHDA) -induced rat model of Parkinson’s disease [17].
Dapholosericin A (133), a tigliane diterpene from the EtOAc extract of D. holosericea, was discovered to be a moderate acetylcholinesterase (AChE) inhibitor at a concentration of 100 μM, which suggested its potential usage in the mediation of Alzheimer’s disease [65].
Genkwalathins A (134) and B (135), two lathyrane-type diterpenes isolated from the chloroform extract from D. genkwa, were demonstrated to inhibit LPS-induced NO production in microglial BV-2 cells moderately (IC50: 43.08–46.77 μM) with nearly non-cytotoxicity, while yuanhuapine (46), yuanhuatine (47), genkwanine M (68), genkwanine N (69), yuanhuafine (32), and genkwadaphnin (15) showed much stronger anti-neuroinflammatory effect with the IC50 value below 0.44 μM, affecting cell viability slightly (17.5%–32.5% cell death at 10 μM) [66].
3.6. Antifertility Activity
The flowers of D. genkwa are also used as a traditional Chinese herbal remedy for abortion. Both yuanhuacine (30) and yuanhuadine (31) have already been used clinically as labor-induced drugs at the per capita dose of 70–80 and 60 μg, respectively [42]. Yuanhuatine (47) [56] and tanguticacine (26) [25] also showed antifertility activity in Rhesus monkeys at the dosage levels of 50 and 300 μg/monkey, respectively. Further experiments suggested that the hydroxyl group at C-15 and C-20 in diterpenoid orthoesters favored antifertility activity and esterification of the hydroxyl group at C-20 by long-chain fatty acid would decrease the toxicity of the diterpenoids [120], and the underlying mechanism was preliminarily established that these diterpenoids induced the release of endogenous prostaglandin by damaging decidual cells [13].
3.7. Skin Irritant, Nematicidal, and Piscicidal Activity
Many naturally occurring daphnane and tigliane diterpenoids, including huratoxin (59), mezerein (24), simplexin (60), and pimelea factor P2 (99), are highly skin irritant and toxic, thus considered as toxic principles in Daphne species [86,121]. The SAR involved is that a 12-acyloxy group favors irritancy [19].
Mezerein (24) was isolated from the seeds of D. mezereum and verified to be skin irritant just as daphnetoxin (56) [33]. Daphnegiraldifine (55), daphnetoxin (56), and 12-hydroxydaphnetoxin (39) obtained from D. giraldii were discovered to exert skin irritant activity [52]. Huratoxin (59), genkwadaphnin (15), prohuratoxin (77), and 1,2α-dihydrodaphnetoxin (73) were isolated from D. feddei, all of which have been determined to be skin irritant, and Huratoxin (59) exhibited the highest irritant activity among these four compounds [19].
It has been discovered that the benzene extract from the roots of D. odora was nematicidal to Aphelenchoides besseyi; then, further study showed that odoracin (25) had nematicidal activity towards A. besseyi was 100% (5 ppm) after 5 days [35]. Daphnetoxin (56) was reported to be a major poisonous component in D. mezereum [61]. Odoracin (25), along with odoratrin (45), gniditrin (20), 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E)-decadienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide (41) and 12-O-(E)-cinnamoyl-9,13,14-ortho-(2E,4E,6E)-decatrienylidyne-5β,12β-dihydroxyresiniferonol-6α,7α-oxide (40), was found to have ornithine decarboxylase (ODC)-inducing activity in mouse skin, which correlated with a tumor-promoting effect; these compounds were simultaneously determined to be piscicidal [27].
4. Discussion
Back to 1970, the very first diterpenoid from Daphne species, daphnetoxin (56), was isolated as a toxic principle [61]. A total of 135 diterpenoids have been discovered from the genus Daphne after decades. These diterpenoids could be classified as daphnane-, tigliane-, and lathyrane-types according to the oxygen-containing functions and substitution pattern, and daphnane-type could be subdivided into 6-epoxy, genkwanine, 1-alkyldaphnane, and resiniferonoid. Within more and more novel diterpenoids isolated from Daphne genera, some of them could not fall into any classification. For example, genkwanine L (95) with a 3-ketone and 12-acetyl group does not completely satisfy the definition of genkwanines. As for compound 108–123, one or more suitable classifications have not been established yet.
Although the genus of Daphne embraces more than ninety species distributed in Asia, Africa, and Europe [2], the origins of these diterpenoids are limited to fifteen Daphne species and diterpenoids have been reported to occur mainly in D. genkwa. For D. mucronata, only two isolated diterpenoids but one of those, gnidilatimonoein (54), showed potent anticancer activity [59,60,122,123], which suggests the potential in the diterpenoids from the genus Daphne. The distributions of every diterpenoid in different parts of plants are available in the hope that it would be a guide for isolation and identification of diterpenoids.
Furthermore, some Daphne species have been assayed and found to possess pharmacological activities which might be closely related to diterpenoids. The methanol extract of D. malyana showed significant antimicrobial potential [124] and the identification of the antibacterial or antifungal principles has not been reported yet. D. linearifolia, of which the stem bark was used to treat inflammation and rheumatism such as some Daphne species mentioned above, showed affinity towards a promising anticancer target Hsp90 [125]. The D. cneorum extract exhibits potent antimicrobial and antioxidant activities, which makes it a possible source of new agents [126]. For D. alpina, its antioxidant and antimicrobial bioactivities have been investigated [127,128] and gniditrin (20) has been isolated from this species [29]. These suggest a prospect of further phytochemistry and pharmacological study in Daphne species.
For the structure–activity relationship (SAR) summarized in this review, despite the fact that some of the SAR remains unclear and needs to be further investigated, it showed that the pharmacological effect, to a large extent, depends on the substituents at C-3, C-12, C-20, and C-1′ and the saturation of the ring A in these molecules. The SAR study is believed to provide very useful information for the optimization and synthesis of diterpenoids and lead to the development of novel agents; however, the SAR of diterpenoids has not been completely clarified.
5. Conclusions
In this review, a total of 135 natural diterpenoids in the past decades from the plants of the genus Daphne were covered. Many of the Daphne species are bioactive and thus have been used as traditional treatments for various diseases. Both the source species and parts from which diterpenoids were isolated have been provided as detailed reference information. The natural diterpenoids from Daphne species present interesting structures with complicated stereochemistry, which are closely related to their abundant bioactivities. The biological activities and the structure–activity relationship of certain classes of diterpenoids were reviewed in the hope of providing an easier way for researchers to understand the general situation of phytochemical and pharmacological properties in diterpenoids from the genus Daphne.
Acknowledgments
This work is dedicated to the 90th anniversary of Seventh People’s Hospital of Shanghai University of Traditional Chinese Medicine.
Abbreviations
| A. besseyi | Aphelenchoides besseyi Chrisite |
| Ac | acetyl |
| AChE | acetylcholinesterase |
| AZT | 3′-azido-3′-deoxythymidine |
| Bz | benzoyl |
| D. acutiloba | Daphne acutiloba Rehd. |
| D. altaica | Daphne altaica Pall. |
| D. cneorum | Daphne cneorum L. |
| D. alpina | Daphne alpina L. |
| D. aurantiaca | Daphne aurantiaca Diels. |
| D. feddei | Daphne feddei Lévl. |
| D. genkwa | Daphne genkwa Sieb. et Zucc. |
| D. giraldii | Daphne giraldii Nitsche |
| D. gnidium | Daphne gnidium L. |
| D. holosericea | Daphne holosericea (Diels) Hamaya |
| D. linearifolia | Daphne linearifolia Hart |
| D. malyana | Daphne Malyana Blečić |
| D. mezereum | Daphne mezereum L. |
| D. mucronata | Daphne mucronata Royle |
| D. odora | Daphne odora Thunb. |
| D. oleoides | Daphne oleoides Schreb. |
| D. papyracea | Daphne papyracea Wall. ex Steud. |
| D. tangutica | Daphne tangutica Maxim. |
| D. laureola | Daphne laureola L. |
| E. resinifera | Euphorbia resinifera O. Berg |
| E. lathyris | Euphorbia lathyris L. |
| EtOAc | ethyl acetate |
| LDLR | low-density lipoprotein receptor |
| NO | nitric oxide |
| ODC | ornithine decarboxylase |
| Ph | phenyl |
| PKC | protein kinase C |
| SAR | structure–activity relationship |
| Top I | DNA topoisomerase I |
Supplementary Materials
The following are available online, Table S1: The IC50 values (μM) of cytotoxicity of some diterpenoids in various carcinoma cell lines in vitro.
Author Contributions
Conceptualization, L.L. and J.-Y.Z.; methodology, Y.-W.N. and Y.L.; formal analysis, C.-Y.Z.; investigation, Y.-W.N., K.-R.S. and Y.L.; resources, Y.-W.N. and C.-Y.Z.; data curation, Y.-W.N., L.L. and W.F.; writing—original draft preparation, Y.-W.N.; writing—review and editing, Y.-W.N.; visualization, Y.-W.N. and W.-Y.G.; supervision, L.L. and J.-Y.Z.; project administration, X.-X.Z. and W.-Y.G.; funding acquisition, K.-R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of the People’s Republic of China, grant number 81703672; the New Interdisciplinary Subjects of Pudong New District Health Committee, grant number PWXx2020-04; the Projects of Shanghai Pudong New District Academic Leaders, grant number PWRd2021-10; the Excellent Youth Medical Talents Training Program of Pudong Health Bureau of Shanghai, grant number PWRq2020-66; the Clinical Chinese Medicine Plateau Discipline Construction Project of Shanghai Pudong New District Health Committee, grant number PDZY-2018-0604; the Shanghai three-year Action Plan to further accelerate the development of Chinese Medicine, grant number ZY (2018-2020)-CCCX-2008; the Key Disciplines Group of Shanghai Pudong New District Health Committee, grant number PWZwq2017-16.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Editorial Committee of Chinese Academy of Sciences . Flora Reipublicae Popularis Sinicae. Volume 52. Science Press; Peking, China: 1999. The genus Daphne Linn; p. 331. [Google Scholar]
- 2.Moshiashvili G., Tabatadze N., Mshvildadze V. The genus Daphne: A review of its traditional uses, phytochemistry and pharmacology. Fitoterapia. 2020;143:104540. doi: 10.1016/j.fitote.2020.104540. [DOI] [PubMed] [Google Scholar]
- 3.Liao S.G., Chen H.D., Yue J.M. Plant Orthoesters. Chem. Rev. 2009;109:1092–1140. doi: 10.1021/cr0782832. [DOI] [PubMed] [Google Scholar]
- 4.Otsuki K., Li W., Miura K., Asada Y., Huang L., Chen C.H., Lee K.H., Koike K. Isolation, structural elucidation, and anti-HIV activity of daphnane diterpenoids from Daphne odora. J. Nat. Prod. 2020;83:3270–3277. doi: 10.1021/acs.jnatprod.0c00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He W.D., Cik M., Appendino G., Puyvelde L.V., Leysen J.E., De Kimpe N. Daphnane-type diterpene orthoesters and their biological activities. Mini Rev. Med. Chem. 2002;2:185–200. doi: 10.2174/1389557024605492. [DOI] [PubMed] [Google Scholar]
- 6.Tong G.H., Ding Z.W., Liu Z., Ding Y.S., Liang X., Zhang H.L., Li P.F. Total synthesis of prostratin, a bioactive tigliane diterpenoid: Access to multi-stereocenter cyclohexanes from a phenol. J. Org. Chem. 2020;2020:4813–4837. doi: 10.1021/acs.joc.0c00022. [DOI] [PubMed] [Google Scholar]
- 7.Coetzer J., Pieterse M.J. The isolation of 12-hydroxy-daphnetoxin, a degradation product of a constituent of Lasiosiphon burchellii. S. Afr. J. Chem. 1971;24:241–243. [Google Scholar]
- 8.Huang S.Z., Zhang X.J., Li X.Y., Kong L.M., Jiang H.Z., Ma Q.Y., Liu Y.Q., Hu J.M., Zheng Y.T., Li Y., et al. Daphnane-type diterpene esters with cytotoxic and anti-HIV-1 activities from Daphne acutiloba Rehd. Phytochemistry. 2012;75:99–107. doi: 10.1016/j.phytochem.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Eko-Nugroho A., Chin-Piow W., Hirasawa Y., Janar J., Kaneda T. Daphnane diterpenoids from Daphne altaica. Nat. Prod. Commun. 2016;11:1073–1075. [PubMed] [Google Scholar]
- 10.Pan R.R., Zhang C.Y., Li Y., Zhang B.B., Zhao L., Ye Y., Song Y.N., Zhang M., Tie H.Y., Zhang H., et al. Daphnane diterpenoids from Daphne genkwa inhibit PI3K/Akt/mTOR signaling and induce cell cycle arrest and apoptosis in human colon cancer cells. J. Nat. Prod. 2020;83:1238–1248. doi: 10.1021/acs.jnatprod.0c00003. [DOI] [PubMed] [Google Scholar]
- 11.Wang C.R., Huang H.Z., Han J., Chen Z.X., An B.Z. The isolation and identification of a new diterpene orthoester daphnediraldicin. J. Northwest A&F Univ. Nat. Sci. Ed. 1980;7:37–38. [Google Scholar]
- 12.Xu R.S., Gao Y.S. Recent advances in chemical studies on the active principles from plants forfertility regulation. Pure Appl. Chem. 1986;58:811–816. doi: 10.1351/pac198658050811. [DOI] [Google Scholar]
- 13.Wang C.R., Zhou B.N., Gu Z.P. The study of anti-fertility constituents from the species in the Thymelaeaceae family in China. Reprod. Contracept. 1989;9:9–11. [Google Scholar]
- 14.Li F.F., Sun Q., Hong L.L., Li L.Z., Wu Y.Y., Xia M.Y., Ikejima T., Peng Y., Song S.J. Daphnane-type diterpenes with inhibitory activities against human cancer cell lines from Daphne genkwa. Bioorg. Med. Chem. Lett. 2013;23:2500–2504. doi: 10.1016/j.bmcl.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Taninaka H., Takaishi Y., Honda G., Imakura Y., Sezik E., Yesilada E. Terpenoids and aromatic compounds from Daphne oleoides ssp. oleoides. Phytochemistry. 1999;52:1525–1529. doi: 10.1016/S0031-9422(99)00305-2. [DOI] [Google Scholar]
- 16.Kasal R., Lee K.-H., Huang H.-C. Genkwadaphnin, a potent antileukemic diterpene from Daphne genkwa. Phytochemistry. 1981;20:2592–2594. doi: 10.1016/0031-9422(81)83105-6. [DOI] [Google Scholar]
- 17.Han B.S., Kim K.S., Kim Y.J., Jung H.Y., Kang Y.M., Lee K.S., Sohn M.J., Kim C.H., Kim K.S., Kim W.G. Daphnane diterpenes from Daphne genkwa activate Nurr1 and have a neuroprotective effect in an animal model of Parkinson’s disease. J. Nat. Prod. 2016;79:1604–1609. doi: 10.1021/acs.jnatprod.6b00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Y., Su D.M., Li W.D., Cai B.C. Pharmacokinetic comparisons of six components from raw and vinegar-processed Daphne genkwa aqueous extracts following oral administration in rats by employing UHPLC-MS/MS approaches. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018;1079:34–40. doi: 10.1016/j.jchromb.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Wu D.G., Sorg B., Adolf W., Seip E.H., Hecker E. Oligo- and macrocyclic diterpenes in Thymelaeaceae and Euphorbiaceae occurring and utilized in Yunnan (Southwest China) 1. Daphnane type diterpene esters from Daphne feddei. Phytother. Res. 1991;5:163–168. [Google Scholar]
- 20.Liang S., Shen Y.H., Feng Y., Tian J.M., Liu X.H., Xiong Z., Zhang W.D. Terpenoids from Daphne aurantiaca and Their Potential Anti-inflammatory Activity. J. Nat. Prod. 2010;73:532–535. doi: 10.1021/np9005053. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G.X., Yang Y.C., Shi J.G. Study of chemical constituents in stem rind of Daphne giraldii. China J. Chin. Mater. Med. 2006;31:555–557. [PubMed] [Google Scholar]
- 22.Pan L., Zhang X.F., Deng Y., Zhou Y., Wang H., Ding L.S. Chemical constituents investigation of Daphne tangutica. Fitoterapia. 2010;81:38–41. doi: 10.1016/j.fitote.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Vidal V., Potterat O., Louvel S., Hamy F., Mojarrab M., Sanglier J.J., Klimkait T., Hamburger M. Library-based discovery and characterization of daphnane diterpenes as potent and selective HIV inhibitors in Daphne gnidium. J. Nat. Prod. 2012;75:414–419. doi: 10.1021/np200855d. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H.L., Wang R., Li J.Y., Shi Y.P. A new highly oxygenated daphnane diterpene esters from the flower buds of Daphne genkwa. Nat. Prod. Res. 2015;29:1878–1883. doi: 10.1080/14786419.2015.1009459. [DOI] [PubMed] [Google Scholar]
- 25.Wang C.R., Wang Q.L., Liu B.N., Zhou B.N., Wang S.X., Yi F.S. The study on the bioactive components isolated from Daphne tangutica I. The isolation and identification of an anti-fertility diterpene tanguticacine. Acta Chim. Sin. 1987;45:982–986. [Google Scholar]
- 26.Zhuang L.G., Seligmann O., Jurcic K., Wagner H. Constituents of Daphne tangutica. Planta Med. 1982;45:172–176. [PubMed] [Google Scholar]
- 27.Ohigashi H., Hirota M., Ohtsuka T., Koshimizu K., Fujiki H., Suganuma M., Yamaizumi Z., Sugimura T. Resiniferonol-related diterpene esters from Daphne odora Thunb. and their ornithine decarboxylase-inducing activity in mouse skin. Agric. Biol. Chem. 1982;46:2605–2608. doi: 10.1271/bbb1961.46.2605. [DOI] [Google Scholar]
- 28.Kreher B., Neszmelyi A., Wagner H. Triumbellin, a tricoumarin rhamnopyranoside from Daphne mezereum. Phytochemistry. 1990;29:3633–3637. doi: 10.1016/0031-9422(90)85290-V. [DOI] [Google Scholar]
- 29.Görick C., Melzig M.F. Gniditrin is the main diterpenoid constituent in the bark of Daphne mezereum L. Pharmazie. 2013;68:640–642. [PubMed] [Google Scholar]
- 30.Shao Z.Y., Shang Q., Zhao X.N., Zhang J.S., Xia G.P., Bai X.X., Dong H.L., Han Y.M. Daphnane diterpene esters from flower buds of Daphne genkwa and their cytotoxic effects on cancer cells. Zhongcaoyao. 2013;44:128–132. [Google Scholar]
- 31.Wang L., Lan X.Y., Ji J., Zhang C.F., Li F., Wang C.Z., Yuan C.S. Anti-inflammatory and anti-angiogenic activities in vitro of eight diterpenes from Daphne genkwa based on hierarchical cluster and principal component analysis. J. Nat. Med. 2018;72:675–685. doi: 10.1007/s11418-018-1202-1. [DOI] [PubMed] [Google Scholar]
- 32.Xia S.X., Li L.Z., Li F.F., Peng Y., Song S.J., Gao P.Y., Tang S. Two novel diterpenes isolated from the flower buds of Daphne genkwa. Acta Chim. Sin. 2011;69:2518–2522. [Google Scholar]
- 33.Ronlán A., Wickberg B. The structure of mezerein, a major toxic principle of Daphne mezereum L. Tetrahedron Lett. 1970;11:4261–4264. doi: 10.1016/S0040-4039(00)89459-9. [DOI] [PubMed] [Google Scholar]
- 34.Kupchan S.M. Mezerein: Antileukemic principle isolated from Daphne mezereum L. Science. 1975;4177:652–653. doi: 10.1126/science.1114315. [DOI] [PubMed] [Google Scholar]
- 35.Kogiso S., Wada K., Munakata K. Odoracin, a nematicidal constituent from Daphne odora. Agric. Biol. Chem. 1976;40:2119–2120. doi: 10.1271/bbb1961.40.2119. [DOI] [Google Scholar]
- 36.Zhou B.N., Xue L., Lin L.Z., Lin L.J., Johnson M.E., Cordell G.A. NMR assignments and conformational analysis of yuanhuacin. Magn. Reson. Chem. 1993;31:194–199. doi: 10.1002/mrc.1260310208. [DOI] [Google Scholar]
- 37.Ying B.P., Wang C.R., Zhou B.N., Pan B.C., Liu J.S. The study on the bioactive components isolated from the roots of Daphne genkwa I. The isolation and identification of yuanhuacine. Acta Chim. Sin. 1977;35:103–108. [Google Scholar]
- 38.Han B.S., Minh N.V., Choi H.Y., Byun J.S., Kim W.G. Daphnane and phorbol diterpenes, anti-neuroinflammatory compounds with Nurr1 activation from the roots and stems of Daphne genkwa. Biol. Pharm. Bull. 2017;40:2205–2211. doi: 10.1248/bpb.b17-00641. [DOI] [PubMed] [Google Scholar]
- 39.Park B.Y., Min B.S., Ahn K.S., Kwon O.K., Joung H., Bae K.H., Lee H.K., Oh S.R. Daphnane diterpene esters isolated from flower buds of Daphne genkwa induce apoptosis in human myelocytic HL-60 cells and suppress tumor growth in Lewis lung carcinoma (LLC)-inoculated mouse model. J. Ethnopharmacol. 2007;111:496–503. doi: 10.1016/j.jep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y.Y., Zhang R.W., Yuan Y., Geng L.L., Zhao X., Meng X., Zhuang H.F., Bi K.S., Chen X.S. Simultaneous determination of eight active components in chloroform extracts from raw and vinegar-processed Genkwa flos using HPLC-MS and identification of the hepatotoxic ingredients with an HL-7702 cell. Anal. Methods. 2014;6:7022–7029. doi: 10.1039/C4AY01241K. [DOI] [Google Scholar]
- 41.Yin Z.Y., Cheng Y.F., Wei J.K., Luo X.K., Luo P., Liu S.N., Xu J., Chen H., Gu Q. Chemical constituents from Daphne tangutica and their cytotoxicity against nasopharyngeal carcinoma cells. Fitoterapia. 2018;130:105–111. doi: 10.1016/j.fitote.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Wang C.R., Chen Z.X., Ying B.P., Zhou B.N., Liu J.S., Pan B.C. The study on the bioactive components isolated from the roots of Daphne genkwa II. The isolation and identification of yuanhuadine, a new anti-fertility diterpenoid. Acta Chim. Sin. 1981;39:421–426. [Google Scholar]
- 43.Bang K.K., Yun C.Y., Lee C., Jin Q., Lee J.W., Jung S.H., Lee D., Lee M.K., Hong J.T., Kim Y., et al. Melanogenesis inhibitory daphnane diterpenoids from the flower buds of Daphne genkwa. Bioorg. Med. Chem. Lett. 2013;23:3334–3337. doi: 10.1016/j.bmcl.2013.03.096. [DOI] [PubMed] [Google Scholar]
- 44.Wu J., Guo L., Qiu X., Ren Y., Li F., Cui W., Song S. Genkwadaphnin inhibits growth and invasion in hepatocellular carcinoma by blocking DHCR24-mediated cholesterol biosynthesis and lipid rafts formation. Br. J. Cancer. 2020;123:1673–1685. doi: 10.1038/s41416-020-01085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C.R., Huang H.Z., Xu R.S., Dou Y.Y., Wu X.C., Li Y. The isolation and identification of a new diterpene orthoester yuanhuafine. Chin. Pharm. J. 1982;17:46. [Google Scholar]
- 46.Akhtar K., Khan S.B., Ali I. Structural determination of diterpenes from Daphne genkwa by NMR spectroscopy. Magn. Reson. Chem. 2006;44:1063–1066. doi: 10.1002/mrc.1896. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S.X. Preparation of yuanhuacine and relative daphne diterpene esters from Daphne genkwa and structure-activity relationship of potent inhibitory activity against DNA topoisomerase I. Bioorg. Med. Chem. 2006;14:3888–3895. doi: 10.1016/j.bmc.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S.X., Zhang F.H., Li X.N., Dong W.Q., Wen L.H., Wang S.S. Evaluation of Daphne genkwa diterpenes: Fingerprint and quantitative analysis by high performance liquid chromatography. Phytochem. Anal. 2007;18:91–97. doi: 10.1002/pca.953. [DOI] [PubMed] [Google Scholar]
- 49.Hong J.Y., Nam J.W., Seo E.K., Lee S.K. Daphnane diterpene esters with anti-proliferative activities against human lung cancer cells from Daphne genkwa. Chem. Pharm. Bull. 2010;58:234–237. doi: 10.1248/cpb.58.234. [DOI] [PubMed] [Google Scholar]
- 50.Zhao H.D., Lu Y., Yan M., Chen C.H., Morris-Natschke S.L., Lee K.H., Chen D.F. Rapid recognition and targeted isolation of anti-HIV daphnane diterpenes from Daphne genkwa guided by UPLC-MSn. J. Nat. Prod. 2020;83:134–141. doi: 10.1021/acs.jnatprod.9b00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng Y.M., Zuo W.J., Sun M.G., Meng H., Wang Q.H., Wang J.H., Li X. Three new daphne diterpenes from the buds of Daphne genkwa Sieb. et Zucc. processed by rice vinegar. Helv. Chim. Acta. 2009;92:1273–1281. doi: 10.1002/hlca.200800419. [DOI] [Google Scholar]
- 52.Wang C.R., An B.Z., Li S.M., Zhou B.N. The study of bioactive components isolated from Daphne odora. Acta Chim. Sin. 1987;45:993–996. [Google Scholar]
- 53.Hu H.B., Sha H., He Z.W., Wu X.C. A new diterpenoid from the flower buds of Daphne genkwa—The isolation and structure of yuanhuapine. Acta Chim. Sin. 1986;44:843–845. [Google Scholar]
- 54.Li L.Z., Gao P.Y., Peng Y., Wang L.H., Yang J.Y., Wu C.F., Zhang Y., Song S.J. Daphnane-type diterpenoids from the flower buds of Daphne genkwa. Helv. Chim. Acta. 2010;93:1172–1179. doi: 10.1002/hlca.200900359. [DOI] [Google Scholar]
- 55.Chen Y.Y., Guo J.M., Qian Y.F., Guo S., Ma C.H., Duan J.A. Toxicity of daphnane-type diterpenoids from genkwa flos and their pharmacokinetic profile in rat. Phytomedicine. 2013;21:82–89. doi: 10.1016/j.phymed.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Hu H.B., Sha H., Wang C.R., Yu D.F., Wu X.C., Yu X.G. The study of the anti-fertility components in the flower buds of Daphne genkwa—The isolation and structure of a new orthoester yuanhuatine. Acta Chim. Sin. 1985;43:460–462. [Google Scholar]
- 57.Li L.Z., Song S.J., Gao P.Y., Li F.F., Wang L.H., Liu Q.B., Huang X.X., Li D.Q., Sun Y. Neogenkwanines A-H: Daphnane-type diterpenes containing 4,7 or 4,6-ether groups from the flower bud of Daphne genkwa. RSC Adv. 2015;5:4143–4152. doi: 10.1039/C4RA13167C. [DOI] [Google Scholar]
- 58.Li L.Z., Gao P.Y., Li F.F., Huang X.X., Peng Y., Song S.J. Isolation and identification of chemical constituents of buds of Daphne genkwa Sieb. et Zucc. J. Shenyang Pham. Univ. 2010;27:699–703. [Google Scholar]
- 59.Mahdavi M., Yazdanparast R. Gnidilatimonoein from Daphne mucronata induces differentiation and apoptosis in leukemia cell lines. Arch. Pharmacal Res. 2007;30:177–181. doi: 10.1007/BF02977692. [DOI] [PubMed] [Google Scholar]
- 60.Yazdanparast R., Sadeghi H. Nucleic acid synthesis in cancerous cells under the effect of gnidilatimonoein from Daphne mucronata. Life Sci. 2004;74:1869–1876. doi: 10.1016/j.lfs.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 61.Stout G.H., Balkenhol W.G., Poling M., Hickernell G.L. Isolation and structure of daphnetoxin, the poisonous principle of Daphne species. J. Am. Chem. Soc. 1970;92:1070–1071. doi: 10.1021/ja00707a058. [DOI] [Google Scholar]
- 62.Liao S.G., Zhang B.L., Wu Y., Yue J.M. New phenolic components from Daphne giraldii. Helv. Chim. Acta. 2005;88:2873–2878. doi: 10.1002/hlca.200590230. [DOI] [Google Scholar]
- 63.Peixoto F., Carvalho M.J.M., Almeida J., Matos P.A.C. Daphnetoxin interacts with mitochondrial oxidative phosphorylation and induces membrane permeability transition in rat liver. Planta Med. 2004;70:1064–1068. doi: 10.1055/s-2004-832648. [DOI] [PubMed] [Google Scholar]
- 64.Ghanadian M., Ali Z., Khan I.A., Balachandran P., Nikahd M., Aghaei M., Mirzaei M., Sajjadi S.E. A new sesquiterpenoid from the shoots of Iranian Daphne mucronata Royle with selective inhibition of STAT3 and Smad3/4 cancer-related signaling pathways. DARU J. Pharm. Sci. 2020;28:253–262. doi: 10.1007/s40199-020-00336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma Q.Y., Chen Y.C., Huang S.Z., Kong F.D., Zhou L.M., Dai H.F., Hua Y., Zhao Y.X. Chemical constituents from the stems of Daphne holosericea (Diels) Hamaya. Chem. Biodivers. 2016;13:1469–1474. doi: 10.1002/cbdv.201600040. [DOI] [PubMed] [Google Scholar]
- 66.Minh N.V., Han B.S., Choi H.Y., Byun J., Park J.S., Kim W.G. Genkwalathins A and B, new lathyrane-type diterpenes from Daphne genkwa. Nat. Prod. Res. 2018;32:1782–1790. doi: 10.1080/14786419.2017.1402322. [DOI] [PubMed] [Google Scholar]
- 67.Li D.Y., Lee C., Jin Q., Lee J.W., Lee M.K., Hwang B.Y. A new tigliane-type diterpenoid from Daphne genkwa. Bull. Korean Chem. Soc. 2014;35:669–671. doi: 10.5012/bkcs.2014.35.2.669. [DOI] [Google Scholar]
- 68.Chen Y.Y., Duan J.A., Tang Y.P., Guo S. Cytotoxic daphnane-type diterpenoids from Daphne genkwa. Chem. Nat. Compd. 2014;50:163–164. doi: 10.1007/s10600-014-0901-4. [DOI] [Google Scholar]
- 69.Pan L., Zhang X.F., Wu H.F., Ding L.S. A new daphnane diterpene from Daphne tangutica. Chin. Chem. Lett. 2006;17:38–40. [Google Scholar]
- 70.Su J., Wu Z.J. A new daphnane-type diterpenoid from Daphne giraldii. Chem. Nat. Compd. 2014;50:285–287. doi: 10.1007/s10600-014-0932-x. [DOI] [Google Scholar]
- 71.Zhang X., Huang S.Z., Gu W.G., Yang L.M., Chen H., Zheng C.B., Zhao Y.X., Wan D.C., Zheng Y.T. Wikstroelide M potently inhibits HIV replication by targeting reverse transcriptase and integrase nuclear translocation. Chin. J. Nat. Med. 2014;12:186–193. doi: 10.1016/S1875-5364(14)60031-5. [DOI] [PubMed] [Google Scholar]
- 72.Li L.Z., Gao P.Y., Peng Y., Wang L.H., Song S.J. A novel daphnane-type dietpene from the flower buds of Daphne genkwa. Chem. Nat. Compd. 2010;46:380–382. doi: 10.1007/s10600-010-9622-5. [DOI] [Google Scholar]
- 73.Zhan Z.J., Fan C.Q., Ding J., Yue J.M. Novel diterpenoids with potent inhibitory activity against endothelium cell HMEC and cytotoxic activities from a well-known TCM plant Daphne genkwa. Bioorg. Med. Chem. 2005;13:645–655. doi: 10.1016/j.bmc.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 74.Hou X.W., Han S., Zhang Y.Y., Su H.B., Gao P.Y., Li L.Z., Song S.J. Neogenkwanine I from the flower buds of Daphne genkwa with its stereostructure confirmation using quantum calculation profiles and antitumor evaluation. Nat. Prod. Res. 2018;34:405–412. doi: 10.1080/14786419.2018.1536133. [DOI] [PubMed] [Google Scholar]
- 75.Kupchan S.M., Shizuri Y., Murae T., Sweeny J.G., Haynes H.R., Shen M.-S., Barrick J.C., Bryan R.F., Van der Helm D., Wu K.K. Gnidimacrin and gnidimacrin 20-palmitate, novel macrocyclic antileukemic diterpenoid esters from Gnidia subcordata. J. Am. Chem. Soc. 1976;98:5719–5720. doi: 10.1021/ja00434a063. [DOI] [PubMed] [Google Scholar]
- 76.Otsuki K., Li W., Asada Y., Chen C.H., Lee K.H., Koike K. Daphneodorins A–C, anti-HIV gnidimacrin related macrocyclic daphnane orthoesters from Daphne odora. Org. Lett. 2020;22:11–15. doi: 10.1021/acs.orglett.9b03539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng W.J., Fujimoto Y., Ohkawa M., Teragawa T., Xu J., Yoshida M., Ikekawa T. Antitumor principles of Stellera chamaejasme L. Chin. J. Cancer Res. 1997;9:89–95. doi: 10.1007/BF02974670. [DOI] [Google Scholar]
- 78.Yoshida M., Feng W.J., Saijo N., Ikekawa T. Antitumor activity of daphnane-type diterpene gnidimacrin isolated from Stellera chamaejasme L. Int. J. Cancer. 1996;66:268–273. doi: 10.1002/(SICI)1097-0215(19960410)66:2<268::AID-IJC22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida M., Heike Y., Ohno S., Ikekawa T., Wakasugi H. Involvement of PKC βII in anti-proliferating action of a new antitumor compound gnidimacrin. Int. J. Cancer. 2003;105:601–606. doi: 10.1002/ijc.11157. [DOI] [PubMed] [Google Scholar]
- 80.Shiryo Y., Kazuhiko K., Hiroya H., Naochika M., Fumiko A., Tatsuo Y. Diterpenoids with the daphnane skeleton from Wikstroemia retusa. Phytochemistry. 1992;32:141–143. doi: 10.1016/0031-9422(92)80120-4. [DOI] [Google Scholar]
- 81.Abe F., Iwase Y., Yamauchi T., Kinjo K., Yaga S., Ishii M., Iwahana M. Minor daphnane-type diterpenoids from Wikstroemia retusa. Phytochemistry. 1998;47:833–837. doi: 10.1016/S0031-9422(97)00529-3. [DOI] [PubMed] [Google Scholar]
- 82.Abe F., Iwase Y., Yamauchi T., Kinjo K., Yaga S. Daphnane diterpenoids from the bark of Wikstroemia retusa. Phytochemistry. 1997;44:643–647. doi: 10.1016/S0031-9422(96)00602-4. [DOI] [PubMed] [Google Scholar]
- 83.Hou Z.L., Yao G.D., Song S.J. Daphnane-type diterpenes from genus Daphne and their anti-tumor activity. Zhongcaoyao. 2021;13:145–156. doi: 10.1016/j.chmed.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li S.M., Chou G.X., Hseu Y.C., Yang H.L., Kwan H., Yu Z.L. Isolation of anticancer constituents from flos genkwa (Daphne genkwa Sieb.et Zucc.) through bioassay-guided procedures. Chem. Cent. J. 2013;7:159. doi: 10.1186/1752-153X-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou X.F., Hou X.W., Li L.Z. Chemical constituents from the flower buds of Daphne genkwa (Thymelaeaceae) Biochem. Syst. Ecol. 2020;91:104055. doi: 10.1016/j.bse.2020.104055. [DOI] [Google Scholar]
- 86.Adolf W., Hecker E. Diterpenoid Irritants and Cocarcinogens in Euphorbiaceae and Thymelaeaceae: Structural Relationships in View of their Biogenesis. Isr. J. Chem. 1977;16:75–83. doi: 10.1002/ijch.197700015. [DOI] [Google Scholar]
- 87.Huang S.Z., Zhang X., Ma Q.Y., Zheng Y.T., Dai H.F., Wang Q., Zhou J., Zhao Y.X. Anti-HIV terpenoids from Daphne aurantiaca Diels. stems. RSC Adv. 2015;5:80254–80263. doi: 10.1039/C5RA17099K. [DOI] [Google Scholar]
- 88.Hayes P.Y., Chow S., Somerville M.J., Fletcher M.T., De Voss J.J. Daphnane- and tigliane-type diterpenoid esters and orthoesters from Pimelea elongata. J. Nat. Prod. 2010;73:1907–1913. doi: 10.1021/np1005746. [DOI] [PubMed] [Google Scholar]
- 89.Asada Y., Sukemori A., Watanabe T., Malla K.J., Lee K.H. Isolation, structure determination, and anti-HIV evaluation of tigliane-type diterpenes and biflavonoid from Stellera chamaejasme. J. Nat. Prod. 2013;76:852–857. doi: 10.1021/np300815t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marco J.A., Sanz-Cervera J.F., Checa J., Palomares E., Fraga B.M. Jatrophane and tigliane diterpenes from the latex of Euphorbia obtusifolia. Phytochemistry. 1999;52:479–485. doi: 10.1016/S0031-9422(99)00166-1. [DOI] [Google Scholar]
- 91.Pan L.L., Fang P.L., Zhang X.J., Ni W., Li L., Yang L.M., Chen C.X., Zheng Y.T., Li C.T., Hao X.J., et al. Tigliane-type diterpenoid glycosides from Euphorbia fischeriana. J. Nat. Prod. 2011;74:1508–1512. doi: 10.1021/np200058c. [DOI] [PubMed] [Google Scholar]
- 92.Wang R., Li J.Y., Qi H.Y., Shi Y.P. Two new tigliane diterpene esters from the flower buds of Daphne genkwa. J. Asian Nat. Prod. Res. 2013;15:502–506. doi: 10.1080/10286020.2013.786703. [DOI] [PubMed] [Google Scholar]
- 93.Marco J.A., Sanz-Cervera J.F., Yuste A. Ingenane and lathyrane diterpenes from the latex of Euphorbia canariensis. Phytochemistry. 1997;45:563–570. doi: 10.1016/S0031-9422(97)00018-6. [DOI] [Google Scholar]
- 94.Adolf W., Köhler I., Hecker E. Lathyrane type diterpene esters from Euphorbia lathyris. Phytochemistry. 1984;23:1461–1463. doi: 10.1016/S0031-9422(00)80486-0. [DOI] [Google Scholar]
- 95.Kai H., Koine T., Baba M., Okuyama T. Pharmacological effects of Daphne genkwa and Chinese medical prescription, “Jyu-So-To”. Yakugaku Zasshi. 2004;124:349–354. doi: 10.1248/yakushi.124.349. [DOI] [PubMed] [Google Scholar]
- 96.Hecker E., Bartsch H., Bresch H., Gschwendt M., Härle B., Kreibich G., Kubinyi H., Schairer H.U., Szczepanski C.V., Thielmann H.W. Structure and stereochemistry of the tetracyclic diterpene phorbol from Croton tiglium L. Tetrahedron Lett. 1967;8:3165–3170. doi: 10.1016/S0040-4039(01)89890-7. [DOI] [Google Scholar]
- 97.Jin Y.X., Shi L.L., Zhang D.P., Wei H.Y., Si Y., Ma G.X., Zhang J. A review on daphnane-type diterpenoids and their bioactive studies. Molecules. 2019;24:1842. doi: 10.3390/molecules24091842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baxter R.L., Ziegler M.F. Antileukaemic properties of 12-hydroxydaphnetoxin derivatives. Bioorg. Med. Chem. Lett. 1994;4:2649–2652. doi: 10.1016/S0960-894X(01)80689-2. [DOI] [Google Scholar]
- 99.Sadeghi H., Yazdanparast R. Isolation and structure elucidation of a new potent anti-neoplastic diterpene from Dendrostellera lessertii. Am. J. Chin. Med. 2005;33:831–837. doi: 10.1142/S0192415X05003387. [DOI] [PubMed] [Google Scholar]
- 100.Kupchan S.M., Shizuri Y., Sumner W.C., Haynes H.R., Leighton A.P., Sickles B.R. Tumor inhibitors. 117. The isolation and structural elucidation of new potent antileukemic diterpenoid esters from Gnidia species. J. Org. Chem. 1976;41:3850–3853. doi: 10.1021/jo00886a016. [DOI] [PubMed] [Google Scholar]
- 101.Kupchan S.M., Sweeny J.G., Baxter R.L., Murae T., Zimmerly V.A., Sickles B.R. Gnididin, gniditrin, gnidicin, novel potent antileukemic diterpenoid esters from Gnidia lamprantha. J. Am. Chem. Soc. 1975;97:672–673. doi: 10.1021/ja00836a051. [DOI] [PubMed] [Google Scholar]
- 102.Tyler M.I., Howden M.E.H. Antineoplastic and piscicidal 1-alkyldaphnane orthoesters from Pimelea species. J. Nat. Prod. 1985;48:440–445. doi: 10.1021/np50039a012. [DOI] [PubMed] [Google Scholar]
- 103.Hall I.H., Kasai R., Wu R.Y., Tagahara K., Lee K.H. Effects of genkwadaphnin and yuanhuacine on nucleic acid synthesis of P-388 lymphocytic leukemia cells. J. Pharm. Sci. 1982;71:1263–1267. doi: 10.1002/jps.2600711120. [DOI] [PubMed] [Google Scholar]
- 104.Liou Y.F., Hall I.H., Lee K.H. The protein synthesis inhibition by genkwadaphnin and yuanhuacine of P-388 lymphocytic leukemia cells. J. Pharm. Sci. 1982;71:1340–1344. doi: 10.1002/jps.2600711208. [DOI] [PubMed] [Google Scholar]
- 105.Kang J.I., Hong J.Y., Lee H.J., Bae S.Y., Jung C., Park H.J., Lee S.K. Anti-tumor activity of yuanhuacine by regulating AMPK/mTOR signaling pathway and actin cytoskeleton organization in non-small cell lung cancer cells. PLoS ONE. 2015;10:e0144368. doi: 10.1371/journal.pone.0144368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jo S.K., Hong J.Y., Park H.J., Lee S.K. Anticancer activity of novel daphnane diterpenoids from Daphne genkwa through cell-cycle arrest and suppression of Akt/STAT/Src signalings in human lung cancer cells. Biomol. Ther. 2012;20:513–519. doi: 10.4062/biomolther.2012.20.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y.Y., Shang X.Y., Hou X.W., Li L.Z., Wang W., Hayashi T., Zhang Y., Yao G.D., Song S.J. Yuanhuatine from Daphne genkwa selectively induces mitochondrial apoptosis in estrogen receptor α-positive breast cancer cells in vitro. Planta Med. 2019;85:1275–1286. doi: 10.1055/a-1013-1439. [DOI] [PubMed] [Google Scholar]
- 108.Li Z.J., Li X.M., Piao Y.J., Choi D.K., Kim S.J., Kim J.W., Sohn K.C., Kim C.D., Lee J.H. Genkwadaphnin induces reactive oxygen species (ROS)-mediated apoptosis of squamous cell carcinoma (SCC) cells. Biochem. Biophys. Res. Commun. 2014;450:1115–1119. doi: 10.1016/j.bbrc.2014.06.118. [DOI] [PubMed] [Google Scholar]
- 109.Chaabane F., Pinon A., Simon A., Ghedira K., Chekir-Ghedira L. Phytochemical potential of Daphne gnidium in inhibiting growth of melanoma cells and enhancing melanogenesis of B16-F0 melanoma. Cell Biochem. Funct. 2013;31:460–467. doi: 10.1002/cbf.2919. [DOI] [PubMed] [Google Scholar]
- 110.Chaabane F., Mustapha N., Mokdad-Bzeouich I., Sassi A., Kilani-Jaziri S., Franca M.G.D., Michalet S., Fathallah M., Krifa M., Ghedira K., et al. In vitro and in vivo anti-melanoma effects of Daphne gnidium aqueous extract via activation of the immune system. Tumor Biol. 2016;37:6511–6517. doi: 10.1007/s13277-015-4492-x. [DOI] [PubMed] [Google Scholar]
- 111.Hedayati M., Yazdanparast R., Fasihi H., Azizi F. Anti-tumor activity of Daphne mucronata extract and its effects on TNF-α receptors and TNF-α release in cultured human monocytes. Pharm. Biol. 2003;41:194–198. doi: 10.1076/phbi.41.3.194.15093. [DOI] [Google Scholar]
- 112.Kang H.B., Lee H.R., Jee D.J., Shin S.H., Nah S.S., Yoon S.Y., Kim J.W. PRDM1, a tumor-suppressor gene, is Iinduced by genkwadaphnin in human colon cancer SW620 cells. J. Cell. Biochem. 2016;117:172–179. doi: 10.1002/jcb.25262. [DOI] [PubMed] [Google Scholar]
- 113.Zhang R., Wang Y., Li J., Jin H., Song S., Huang C. The Chinese herb isolate yuanhuacine (YHL-14) induces G2/M arrest in human cancer cells by up-regulating p21 protein expression through an p53 protein-independent cascade. J. Biol. Chem. 2014;289:6394–6403. doi: 10.1074/jbc.M113.513960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kizaibek M., Daniar M., Li L., Upur H. Antiproliferative activity of different extracts from Daphne altaica Pall. on selected cancer cells. J. Med. Plants Res. 2011;5:3448–3452. [Google Scholar]
- 115.Kizaibek M., Wubuli A., Gu Z., Bahetjan D., Tursinbai L., Nurhamit K., Chen B., Wang J., Tahan O., Cao P. Effects of an ethyl acetate extract of Daphne altaica stem bark on the cell cycle, apoptosis and expression of PPARγ in Eca-109 human esophageal carcinoma cells. Mol. Med. Rep. 2020;22:1400–1408. doi: 10.3892/mmr.2020.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li S.F., Liang X., Wu X.K., Gao X., Zhang L.W. Discovering the mechanisms of wikstroelide E as a potential HIV-latency-reversing agent by transcriptome profiling. J. Nat. Prod. 2021;84:1022–1033. doi: 10.1021/acs.jnatprod.0c01039. [DOI] [PubMed] [Google Scholar]
- 117.Beans E.J., Fournogerakis D., Gauntlett C., Heumann L.V., Kramer R., Marsden M.D., Murray D., Chun T.W., Zack J.A., Wender P.A. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc. Natl. Acad. Sci. USA. 2013;110:11698–11703. doi: 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eo S.K., Uyangaa E., Choi J.Y., Patil A.M., Kim J.H., Kim S.B. Functional restoration of exhausted CD4(+) and CD8(+) T cells in chronic viral infection by daphnane diterpene ester derived from Daphne genkwa flos buds via negative regulatory Tim-3 molecule. Cytokine. 2014;70:38–39. [Google Scholar]
- 119.Zhang Y.L., Zhang H.Q., Hua S.N., Ma L.H., Chen C., Liu X.Y., Jiang L.Q., Yang H.M., Zhang P.C., Yu D.Q., et al. Identification of two herbal compounds with potential cholesterol-lowering activity. Biochem. Pharmacol. 2007;74:940–947. doi: 10.1016/j.bcp.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 120.Gu Z.P., Wang C.R., Liang Z.Y., Lin L.J., Lu R.F., Zhao S.X., Zhou B.N. The study of anti-fertility constituents from four species in the Thymelaeaceae family in China. Reprod. Contracept. 1989;9:48–52. [Google Scholar]
- 121.Freeman P.W., Ritchie E., Taylor W.C. The Constituents of Australian Pimelea spp. I. The Isolation and Structure of the Toxin of Pimelea simplex and P. trichostachya Form B Responsible for St. George Disease of Cattle. Aust. J. Chem. 1979;32:419–425. doi: 10.1071/CH9792495. [DOI] [Google Scholar]
- 122.Mianabadi M., Yazdanparast R. Inhibition of substrate-tumor cell adhesion under the effect of gnidilatimonoein purified from Daphne mucronata. Am. J. Chin. Med. 2004;32:369–376. doi: 10.1142/S0192415X04002028. [DOI] [PubMed] [Google Scholar]
- 123.Hedayati M., Yazdanparast R., Yeganeh M.Z., Rad L.H., Azizi F. A new diterpene extracted from Daphne mucronata, effects on human K562 and CCRF-CEM cell line. J. Cancer Ther. 2011;2:71–75. doi: 10.4236/jct.2011.21008. [DOI] [Google Scholar]
- 124.Juskovic M., Vasiljevic P., Manojlovic N., Mihailov-Krstev T., Stevanovic B. Phytochemical and antimicrobial screening of leaves and stems of balkan endemic species Daphne Malyana Blečić. Biotechnol. Biotechnol. Equip. 2012;26:3010–3015. doi: 10.5504/BBEQ.2012.0007. [DOI] [Google Scholar]
- 125.Malafronte N., Vassallo A., Dal Piaz F., Bader A., Braca A., De Tommasi N. Biflavonoids from Daphne linearifolia Hart. Phytochem. Lett. 2012;5:621–625. doi: 10.1016/j.phytol.2012.06.008. [DOI] [Google Scholar]
- 126.Manojlović N.T., Mašković P.Z., Vasiljević P.J., Jelić R.M., Jusković M.Ž., Sovrlić M., Mandić L., Radojković M. HPLC analysis, antimicrobial and antioxidant activities of Daphne cneorum L. Hem. Ind. 2012;66:709–716. doi: 10.2298/HEMIND120114029M. [DOI] [Google Scholar]
- 127.Sovrlić M., Vasiljević P., Jušković M., Mašković P., Manojlović N. Phytochemical, antioxidant and antimicrobial profiles of extracts of Daphne alpina L. (Thymelaeaceae) leaf and twig from Mt Kopaonik (Serbia) Trop. J. Pharm. Res. 2015;14:1239–1248. doi: 10.4314/tjpr.v14i7.17. [DOI] [Google Scholar]
- 128.Hong J.Y., Chung H.J., Lee H.J., Park H.J., Lee S.K. Growth inhibition of human lung cancer cells via down-regulation of epidermal growth factor receptor signaling by yuanhuadine, a daphnane diterpene from Daphne genkwa. J. Nat. Prod. 2011;74:2102–2108. doi: 10.1021/np2003512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.