Abstract
Introduction
Prevalence of chronic kidney disease (CKD) varies around the world. Little is known about the discrepancy between the general population's needs and nephrology care offered. We aimed to contribute to filling this gap and propose a means to infer the number of patients needing follow-up.
Methods
All patients undergoing at least one nephrology consultation in 2019 were enrolled. We used the ratio between CKD Stages 3 and 4 reported in the literature, and considered that only 25–50% of CKD Stage 3 patients have progressive CKD, to hypothesize different scenarios to estimate the number of CKD Stage 3 patients still needing nephrology follow-up.
Results
The 1992 CKD patients were followed-up in our centre (56.93% males; age 66.71 ± 18.32 years; 16.82% Stage 1; 14.66% Stage 2; 39.46% Stage 3; 19.88% Stage 4; 7.68% Stage 5). The ratio between Stages 3 and 4 in population studies ranged from 7.72 to 51.29, being 1.98 in our centre. Hypothesizing that we followed-up 100, 70 or 50% of CKD Stage 4 patients, 528–2506 CKD Stage 3 patients in our area would need nephrology follow-up [1885–8946 per million population (p.m.p.)]. Three to 17 additional nephrologists p.m.p. would be necessary to fully cover the need for care.
Conclusions
The number of patients with CKD Stage 3 who would benefit from nephrology care is high. Considering that one patient-year of delay of dialysis could cover a nephrologist’s annual salary, interventions aimed to improve the care of advanced CKD may be economically sound.
Keywords: chronic kidney disease, CKD stages, epidemiology, healthcare policies, nephrology care
Graphical Abstract
INTRODUCTION
The epidemic of chronic kidney disease (CKD) has been variously described, defined and assessed [1–4]. Yet, CKD is a growing healthcare problem and responding to the needs of a predominantly elderly population, often affected by multiple comorbidities, remains a challenge; the prevalence of CKD is high, even when kidney function data are corrected for age, or the lowest figures are taken into account [5–7].
Many countries lack the resources to respond to patients’ needs and up to one-third of patients who start dialysis do so without appropriate follow-up and sometimes without any at all [8–13].
The French healthcare system recently launched an ambitious initiative to improve the care of patients with advanced CKD (Stages 4 and 5), establishing a reimbursement bundle designed to improve multidisciplinary care of late-stage CKD, including at least one yearly consultation with a dietician and a nurse specializing in therapeutic education [14].
Focusing on late-stage CKD is expected to improve access to dialysis, favouring, among other goals, the choice of home or out-of-hospital treatment, allowing for the timely creation of vascular access, optimizing nutrition in the pre-dialysis phase. Conversely, an effect on the progression of kidney disease can be expected in cases that receive nephrologist care earlier, ideally in Stage 3b or even before [15–18].
There is a significant gap in knowledge between large epidemiologic surveys that assessed the prevalence of CKD in the overall population and the data recorded in nephrology units. Indeed, little is known about which patients are actually followed up in different nephrology settings. Filling this information gap would help to tailor interventions to answer the unmet needs of the CKD population. A contributing factor is the fact that general hospitals often lack research facilities for data gathering and analysis, while reference and university centres are often biased by a higher prevalence of complex patients or rare diseases.
In our Department, Sarthe, central France, nephrology care is offered by two coordinated teams, thus allowing an estimation of the prevalence of CKD and needs for further care, with respect to the overall population. Given this favourable setting, we have tried to contribute to filling the knowledge gap between the overall population and nephrology care, by combining a cross-sectional analysis of patients who underwent at least one nephrology consultation in 2019 with the available data in the overall population, to build different scenarios, ultimately assessing the needs for CKD care.
MATERIALS AND METHODS
Study setting
This study was undertaken at Centre Hospitalier Le Mans (CHM), one of the largest non-university hospitals in France. CHM has a nephrology service with a network of outpatient care facilities (consultations and day-hospital) and is the only hospital in the Department of Sarthe with nephrology beds (Sarthe: 560 227 inhabitants on 1 January 2020). The hospital is situated in the main city in the department, Le Mans, which has 143 325 inhabitants.
The team consisted of seven nephrologists in 2019, all of whom have at least a half-day of outpatient consultations per week (2.5 days on average). CHM recently developed a consultation service dedicated to patients who had experienced an episode of preeclampsia, as well as a consultation service for patients with kidney stones. In 2019, 68 kidney biopsies were performed (60–80 in each of the previous 3 years); this activity may have led to selective referral of patients with glomerular diseases and vasculitides.
A local non-profit association, ECHO, has a team of six nephrologists and has independent consultations. In both cases, follow-up is free of charge. On the basis of the recent evaluation performed for the healthcare system in France, we were able to estimate that a similar population was followed by each centre: in the period from October to December 2019, 246 CKD Stage 4 and 22 CKD Stage 5 patients were seen in consultation at ECHO and 238 CKD Stage 4 and 58 CKD Stage 5 patients at CHM. The difference as for Stage 5 is probably linked to a policy of late start of renal replacement therapy by the CHM. Since there is now no other nephrologist active in the area, the data reflect all CKD Stages 4 and 5 patients being followed in the Sarthe Department by the public (CHM) and non-profit (ECHO) services. While patients from the Sarthe Department can seek treatment in neighbouring areas (Loire, Mayenne), data from the hospital management suggest that this is balanced by patients coming to Sarthe from adjacent departments. On the basis of the previously cited data, we concluded that half of the district’s CKD patients had been referred to CHM and half to ECHO; we therefore built our analysis considering CHM as the reference centre for a population of 280 113 people (half of the 560 227 inhabitants of the Sarthe on 1 January 2020).
Characterization of patients under follow-up in the hospital centre
All patients over the age of 18 years who attended at least one consultation in 2019 in the nephrology outpatient clinics at CHM were included in the study. Kidney transplant and dialysis patients were not included in the study. Patients’ data were retrieved from their electronic medical records. These included diagnosis of the different types of CKD, which followed the usual criteria (hypertension, diabetes, availability of kidney biopsy, etc.). Demographic characteristics including age, sex and cause of kidney disease were collected. The diagnoses posed by the caregiver nephrologist were recorded and validated by the senior nephrologist (G.B.P.). A cause–effect relationship between hypertension and CKD was inferred from the patient’s medical history (usually long-lasting hypertension in the absence of other clues for a different kidney disease). Kidney function was assessed by means of the Chronic Kidney Disease Epidemiology Collaboration equation [19]. Stratification was performed as per the KDIGO guidelines, according to the presence of morphological abnormalities, urinary anomalies and renal function with at least two determinations of serum creatinine levels at least at 3 months intervals [20]. When more than one visit was present in the medical records in 2019, the most recent one was used to assess the CKD stage. The cases with one data only were considered as ‘missing stages’. Since all patients were observed in the outpatient units, the incidence of acute kidney injury (AKI) was considered as negligible, and the stage was calculated on the basis of the last available creatinine level, unless AKI was explicitly mentioned in the last clinical consultation report.
Reference literature data on the overall population
Relevant studies on the prevalence of CKD and the distribution of CKD stages in different countries were retrieved from MEDLINE, updating a systematic review published in 2016 [21]. A total of 75 studies, 30 of which were set in Europe, were selected (details available in Supplementary data, Table S1 ). Most of the studies focused on CKD Stages 3 and 4.
Data from European studies were used as a reference. The prevalence of CKD in Europe was calculated by means of the weighted arithmetic mean considering the sample size of each study.
Building the scenarios
We decided to use the ratio between CKD Stages 3 and 4 to estimate the number of expected CKD Stage 3 patients in our area. The choice of considering CKD Stage 4 patients as a reference for calculation is based upon the following assumptions: (i) there is a general agreement on the fact that these patients should be referred to a nephrology centre, and these indications are shared by the French healthcare system, which identified patients in Stages 4 and 5 as those who are entitled to receive a dedicated reimbursement bundle; (ii) specific primary care physician sensitization was implemented to promote nephrology referral; (iii) prevalence is not dependent upon the policy towards dialysis start; and (iv) data available in the general population almost always include CKD Stage 4, while Stages 1, 2 and 5 are not universally reported.
We employed the ratio between CKD Stages 3 and 4 observed in the medical literature to infer the number of patients in Stage 3 who could benefit from a nephrology consultation in our area, applying the ratio to the observed Stage 4 CKD cases, according to three scenarios:
-
(i)
we hypothesized that all CKD Stage 4 patients had received a nephrology consultation;
-
(ii)
we hypothesized that 70% of all CKD Stage 4 patients had received a nephrology consultation (considering a late referral rate of 30%);
-
(iii)
we hypothesized that only 50% of all CKD Stage 4 patients had received a nephrology consultation considering the prevalence of CKD Stage 4 with respect to the estimated population, reported in France to be 0.3% [22].
Furthermore, to tailor our assessment of unmet needs, we considered that, according to the data in the literature, only 25–50% of CKD Stage 3 patients were actually CKD progressors and, as such, needed to be followed up [23–30].
We did not include dynamic data (mortality, stage shifts) in the model, hypothesizing, on the basis of the stable incidence of renal replacement therapy in this area in France, ∼150 patients per million population (p.m.p.; Supplementary data, Table S2), having reached equilibrium between mortality (subtracting patients to the prevalent pool) and stage shifts (adding patients from Stages 2 to 3 and from Stages 3 to 4).
Reference data on the cost of dialysis were obtained from the literature [13, 31].
Statistical evaluation
A descriptive analysis was performed as appropriate. Data are presented as mean ± standard deviation (SD) for local continuous data or percentage for discrete variables. Continuous variables were compared by means of the analysis of variance, followed by Tukey’s multiple comparison test. A P < 0.05 was considered statistically significant.
Ethical issues
This is an observational study, involving the analysis of the clinical charts of patients who attended at least one consultation in a nephrology outpatient clinic in 2019; the anonymized database was built following the requests of the regional health council, to assess the number of cases in CKD, specifically Stages 4 and 5. The study was approved by the ethics committee at CHM on 24 September 2020.
RESULTS
Baseline data: CKD patients followed up in the hospital setting
In 2019, a total of 1992 patients underwent at least one nephrology consultation in the outpatient clinic at the CHM. Of these, 56.93% were males and average age was 66.71 ± 18.32 years (Table 1). The age distribution in the Sarthe population reflects the French one, while the majority of our patients was older (Supplementary data, Figure S1). Kidney function data were available for 1962 cases and missing, or missing confirmation, for only 30 patients (1.51% of our sample) (Table 2). The distribution across stages was as follows: CKD Stage 1, 16.82%; CKD Stage 2, 14.66%; CKD Stage 3, 39.46%; CKD Stage 4, 19.88%; and CKD Stage 5, 7.68%. Age increased significantly according to CKD stage up to Stage 4 (P < 0.0001). Interestingly, CKD Stage 4 patients were significantly older than those in CKD Stage 5 (P < 0.01). The distribution of the main diagnoses reflected the usual European prevalence of CKD. It is worth noting that the main causes of CKD were diabetes and hypertensive nephropathy. The prevalence of these two causes increased with CKD stage, reaching a peak at Stage 3. Multiple aetiologies were recorded in 14.71% of cases. Table 1 and Supplementary data, Table S3 show the main characteristics of our cohort.
Table 2.
General population: prevalence per 100 individuals of CKD, by stage (weighted arithmetic mean)
| CKD Stage 1 | CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | CKD Stage 5 | |
|---|---|---|---|---|---|
| World [20, 21, S1–S13, S15–S47, S49–S75] | 1.41 [0.30–12.80] | 1.27 [0.40–42.60] | 6.63 [0.79–32.90] | 0.39 [0.03–4.10] | 0.12 [0.03–3.00] |
| Europe [20, S46, S47, S49–S75] | 0.60 [0.30–6.00] | 3.73 [1.80–24.70] | 7.25 [0.80–32.90] | 0.50 [0.10–2.30] | 0.26 [0.10–0.60] |
Data are expressed as prevalence per 100 individuals. In brackets, the lowest and highest values in the pooled studies. Details of pooled studies are available in the Supplementary data, Table S1.
Table 1.
Characteristics of our cohort (patients followed-up in the nephrology units of the CHM)
| CKD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Stage 1 | Stage 2 | Stage 3a | Stage 3b | Stage 4 | Stage 5 | Unknown stage | |
| Number (%) | 1992 (100) | 335 (16.82) | 292 (14.66) | 300 (15.06) | 486 (24.40) | 396 (19.88) | 153 (7.68) | 30 (1.51) |
| Males, n (%) | 1134 (56.93) | 138 (41.19) | 172 (58.90) | 186 (62.00) | 288 (59.26) | 249 (62.88) | 84 (54.90) | 17 (56.67) |
| Age, years, mean ± SD | 66.71 ± 18.32 | 42.08 ± 14.78 | 62.09 ± 14.22 | 69.52 ± 13.02 | 75.58 ± 12.21 | 77.04 ± 12.68 | 72.03 ± 15.88 | 51.63 ± 16.70 |
| CKD cause, n (%) | ||||||||
| Nephroangiosclerosis/ hypertensive nephropathy | 470 (23.59) | 3 (0.90) | 37 (12.67) | 90 (30.00) | 184 (37.86) | 117 (29.55) | 37 (24.18) | 2 (6.67) |
| Diabetes mellitus | 354 (17.77) | 18 (5.37) | 37 (12.67) | 48 (16.00) | 92 (18.93) | 118 (29.80) | 40 (26.14) | 1 (3.33) |
| Renal stones | 306 (15.36) | 121 (36.12) | 75 (25.68) | 36 (12.00) | 42 (8.64) | 16 (4.04) | 4 (2.61) | 12 (40.00) |
| Multifactorial | 293 (14.71) | 13 (3.88) | 40 (13.70) | 61 (20.33) | 85 (17.49) | 64 (16.16) | 29 (18.95) | 1 (3.33) |
| CAKUT/obstructive/ systemic disease/solitary kidney | 148 (7.44) | 19 (5.67) | 31 (10.62) | 22 (7.34) | 24 (5.21) | 34 (8.59) | 16 (10.46) | 2 (6.66) |
| Glomerulonephritis | 139 (6.98) | 39 (11.64) | 23 (7.88) | 18 (6.00) | 28 (5.76) | 19 (4.80) | 11 (7.19) | 1 (3.33) |
| ADPKD | 75 (3.77) | 14 (4.18) | 13 (4.45) | 8 (2.67) | 15 (3.09) | 14 (3.54) | 10 (6.54) | 1 (3.33) |
| Post-preeclampsia | 68 (3.41) | 62 (18.51) | 4 (1.37) | 0 (0.00) | 1 (0.21) | 0 (0.00) | 0 (0.00) | 1 (3.33) |
| Isolated urinary abnormalities | 27 (1.36) | 12 (3.58) | 11 (3.77) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 4 (13.33) |
| Post-AKI | 23 (1.15) | 0 (0.00) | 4 (1.37) | 7 (2.33) | 5 (1.03) | 6 (1.52) | 1 (0.65) | 0 (0.00) |
| Other/not known | 89 (4.47) | 34 (10.15) | 17 (5.82) | 10 (3.34) | 10 (2.06) | 8 (2.02) | 5 (3.26) | 5 (16.67) |
ADPKD, autosomal dominant polycystic kidney disease; CAKUT, congenital anomalies of the kidneys and of the urinary tract.
Comparison with prevalence data reported in the general population
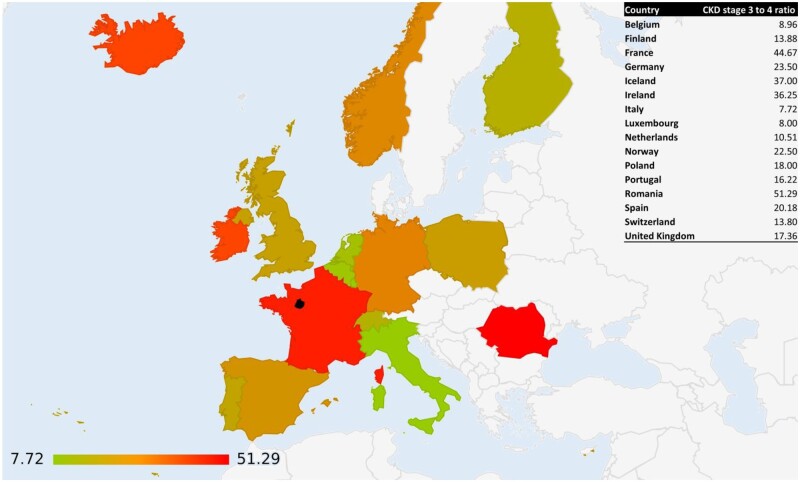
A graphical visualization of the European prevalence data of CKD in the general population is shown in Figures 1 and 2. Supplementary data, Figure S2 shows the ratio between the prevalence of CKD Stages 3 and 4 around the world.
FIGURE 1:
CKD Stage 4 prevalence in Europe. Prevalence is expressed on a colour scale; the black area represents the Sarthe region.
FIGURE 2:
CKD Stages 3–4 ratio in Europe. Prevalence is expressed on a colour scale; the black area represents the Sarthe region.
Table 2 summarizes the prevalence of CKD in different stages, in the general population, as reported in the recent literature; the wide range shows the heterogeneity of the data. The lowest and highest prevalence recorded in the papers (min–max) are reported in square brackets.
Table 3 shows the ratio between the prevalence of CKD Stage 4 (reference) and the other CKD stages in the general population. While the absolute prevalence range spans by >50 times, for example, Stage 3 CKD from ˂1% to >30%, or in Stage 4, from 0.03% to >4%, the ratio between Stages 3 and 4 is more stable, and is overall between 15 and 25 (Tables 2 and 3).
Table 3.
CKD Stages 3–4 ratio in the general population in Europe, with respect to the ratio observed in the hospital setting (CHM)
| Stage 1 to 4 ratio | Stage 2 to 4 ratio | Stage 3 to 4 ratio | Stage 5 to 4 ratio | |
|---|---|---|---|---|
| World | 3.62 | 3.26 | 17.00 | 0.31 |
| Europe | 1.20 | 7.46 | 14.50 | 0.52 |
| EU country with the lowest CKD prevalence (Finland) | NA | NA | 13.88 | NA |
| EU country with the highest CKD prevalence (Germany) | 0.21 | 3.07 | 23.50 | NA |
| CHM: ratio observed between cases on follow-up | 0.85 | 0.74 | 1.98 | 0.39 |
EU, European Union.
Conversely, the ratio between Stages 3 and 4 in the population followed-up in our nephrology unit is much lower (1.98), thus witnessing an underrepresentation of patients in CKD Stage 3 on nephrology follow-up (Table 3).
Analysis of the scenarios
Table 4 reports the calculation of the need for nephrology care in our area. In the first scenario, based on the hypothesis that all CKD Stage 4 patients in our area are followed, we calculated that 2113 CKD Stage 3 patients in our referral area did not undergo a consultation in 2019 (Scenario 1, Table 4). Following the hypothesis that progressive CKD is found in only 25–50% of the cases, the actual need for follow-up would involve 528–1056 patients.
Table 4.
Scenarios built to assess the number of patients in CKD Stage 3 not seen by a nephrologist
| Observed and estimated CKD Stage 4 patients according to the scenarios | CKD Stage 3 patients seen in 2019 | Estimated CKD Stage 3 patients in the resident population | Estimated CKD Stage 3 patients without a nephrology consultation | CKD Stage 3 patients who should undergo a nephrology consultation in our setting |
CKD Stage 3 patients who should undergo a nephrology consultation p.m.p. |
|||
|---|---|---|---|---|---|---|---|---|
| 25% of CKD Stage 3 | 50% of CKD Stage 3 | 25% of CKD Stage 3 | 50% of CKD Stage 3 | |||||
|
Scenario 1 (100% of CKD Stage 4 patients in the area underwent a nephrology consultation) |
Observed: 396 (100%) | 786 | 2899 | 2113 | 528 | 1056 | 1885 | 3770 |
|
Scenario 2 (70% of CKD Stage 4 patients underwent a nephrology consultation) |
Estimated: 566 (70%) | 786 | 4141 | 3355 | 839 | 1678 | 2995 | 5990 |
|
Scenario 3 (50% of CKD Stage 4 patients underwent a nephrology consultation) |
Estimated: 792 (50%) | 786 | 5797 | 5011 | 1253 | 2506 | 4473 | 8946 |
Assuming, on the basis of the prevalence of urgent dialysis start and late referral in France, that only 70% of all CKD Stage 4 patients are under follow-up in our centre, the number of CKD Stage 3 patients lacking nephrological follow-up increased to 3355 (Scenario 2, Table 4).
Finally, assuming that only 50% of all CKD Stage 4 patients were seen in consultation in 2019, based on the comparison between the prevalence of CKD Stage 4 measured in France with the population of the area covered by CHM (expected prevalence: 0.3%; CKD Stage 4 patients consulted among 280 113 inhabitants: 0.14%), the number of CKD Stage 3 patients not evaluated by a nephrologist increased to 5011 (need for follow-up in 1253–2506 cases; Scenario 3, Table 4).
Moreover, according to the second and third scenario, the need for follow-up would involve ∼170 and 396 cases with CKD Stage 4, respectively. Considering a commitment to the outpatient clinic of 8 half-days/week, for 42 weeks/year and a workload of 12 patients/day, a full-time nephrologist is able to perform ∼2016 consultations per year. Considering that patients need to be seen at least three to four times a year, at least four full-time nephrologists would be needed to answer the unmet needs of CKD patients with potentially progressive CKD Stage 3 in our population. This figure should be multiplied by 3.5 to reach the need p.m.p.
DISCUSSION
There are a number of reasons why the prevalence of CKD is increasing, including longer lifespan, greater prevalence of diseases such as diabetes and hypertension, and increased awareness are factors leading to earlier diagnosis and an increased demand for specialist care. However, this increasing demand is not paralleled by an increased offer [32].
In the face of a growing number of epidemiological studies at the population level, the stratification of patients followed up in nephrology settings is not fully known. It is logical that an analysis of the discordance between these data (population versus referred cases) will help tailor interventions. Our study was performed in a favourable setting: a relatively small rural department where nephrology care is provided by two centres (public and non-profit), working in a coordinated way, thus allowing inference of observed data to the overall population.
Patients followed up reflected the characteristics of CKD patients found in the literature: the early stages of the disease mainly encompassed younger patients with congenital abnormalities, glomerulonephritis or lithiasis, while diabetes and hypertension, in older patients, accounted for the vast majority of cases from Stage 3 on (Table 2). Moreover, almost 30% of the patients referred to our outpatient clinic were in Stages 4 or 5.
Our study proposes employing the ratio between other CKD stages and Stage 4 as an indirect means of assessing the need for nephrology care.
We focused on CKD Stage 4 as a reference stage to build different projections. The choice of Stage 4 was motivated by the fact that it is clinically relevant and its prevalence is well known from the literature; it is usually progressive, albeit at different speeds and depending upon competing mortality. Furthermore, at least in France, primary care physicians have been encouraged to ask for a specialized consultation for patients with CKD Stages 4 and 5. Conversely, CKD Stage 5 prevalence is affected by policies towards dialysis initiation. The indications for nephrology work-up in CKD Stage 3 are more controversial and, in fact, Stage 3 prevalence is also dependent on the type of formula employed to estimate renal function, especially in the elderly [5, 33]. While many patients (estimated to be between 50% and 75%) have a slowly progressive or non-progressive disease, between 25% and 50% of cases would probably benefit from nephrology care to slow CKD progression and limit end-organ damage. This population is usually considered as underrepresented in nephrology outpatient services, including ours; however, studies regarding this crucial issue are lacking. We used the ratio observed in Europe (weighted arithmetic mean) between Stages 3 and 4 to infer the need for follow-up.
In the first scenario, we hypothesized that we were seeing all the cases in CKD Stage 4 in our area; in the second, that we were seeing 70% of them, on the basis of a late referral rate to dialysis of ∼30% reported in France [34, 35]; and in the third, on the basis of the CKD Stage 4 prevalence reported in our area, we hypothesized that we were seeing about half of the population [22].
Our study suggests that even in the most optimistic scenario, a high number of CKD Stage 3 patients were not enrolled in nephrology care, even considering the most conservative estimate (only 25% needing actual follow-up) (Table 4). The optimal follow-up schedule for a CKD Stage 3 patient has been estimated to be at least three times a year [15]. Accordingly, one full-time nephrologist would have to join our team to cover the needs of CKD Stage 3 patients in our area, increasing to five in the least favourable scenario. These figures should be multiplied by 3.5 to normalize to 1 million population.
The demonstration of a high demand of care, and the calculated need for a high number of specialists, is challenging and provocative. However, there are many reasons to support it: even a mild reduction in kidney function is associated with increased cardiovascular mortality and morbidity as well as the need for hospitalization [36]. Timely referral of CKD Stage 3 patients to nephrology clinics has been shown by most authors to slow the progression of the disease, improve survival, reduce the need for hospitalization and cut treatment costs [37, 38]. Indeed, late referral of CKD patients is still a concern [39, 40]. Among causes that have been associated with late nephrology care is the lack of specialists, a complaint shared by most European countries [41, 42]. It has been suggested that CKD Stage 3 patients should be followed-up by their general practitioner. However, a recent systematic review reported the concerns of primary care providers in the management of CKD. These included the challenging nature of CKD and the difficulties encountered in managing patients with multiple comorbidities, limited access to specialized nephrology services, difficulties in interpreting laboratory results, dissatisfaction with the current CKD guidelines and lack of preparation. Moreover, all these barriers were exacerbated by the limited amount of time per patient doctors are allowed [43].
The economic implications of increasing the number of specialists in a nephrology unit should take the cost of dialysis into consideration. Renal replacement therapy accounts for ∼2–5% of the global healthcare budget in countries where it is available without restrictions [44]. Moreover, the absolute expenditure for dialysis increases with the gross domestic product per capita, thus high-income countries devote a non-negligible share of their healthcare budget to it [13]. At the same time, only a minority of high-income countries invest in CKD primary or secondary prevention [13].
Most of the economic studies that have evaluated interventions to reduce the costs of CKD focused on its later stages and demonstrated a net benefit only in retarding the start of renal replacement therapy, at least in the short term [45–47].
These considerations apply to France, which has one of the lowest number of nephrologists in Europe compared with both the number of CKD patients (20 per estimated 1000 CKD patients) and the general population (2/100 000 inhabitants) [42]. Furthermore, in France, the deficit of primary care physicians is severe and the number of general practitioners per 100 000 inhabitants in the Sarthe Department is lower than the national average (118 versus 153, respectively) and studies have disclosed the lack of time French general practitioners have to devote to prevention [48, 49].
Increasing specialist care is a valid alternative, and may be economically worthwhile: the cost of 1 year of thrice weekly in-centre haemodialysis is estimated between 40 000 and 81 500 Euros, and in France the average annual pre-tax salary of a young full-time nephrologist is 55 000 Euros. Thus delaying dialysis start for one patient-year would roughly cover the salary of a full-time specialist and/or be reimbursed by consultation fees [13, 31].
Our study has several limits: the actual prevalence of CKD in our region is not known and our ratio is based on only 1 year of observation. However, the characteristics of the Sarthe region and a nephrology offer restricted to two well-coordinated teams make it unlikely that patients in the most advanced CKD stages undergoing a nephrology consultation were missed. Furthermore, we did not consider competing mortality and shifts across stages, which render the real-life picture more complex. However, our main aim was to identify the magnitude of the need for care, and to open the discussion on this neglected issue. Finally, although we are aware that the scientific community is actively debating the need to adjust the classification of CKD for age [33], in the absence of a clear consensus on the topic, we relied on the latest available guidelines for defining and classifying it.
In an ideal situation, all patients with CKD should undergo an expert assessment of the potential progression of their kidney disease. In this regard, we would like to stress that even the most comprehensive scenario is likely to miss some cases with potential progression of kidney function impairment.
In conclusion, in the absence of clear epidemiological data, our study, which gathers almost 2000 patients, makes it possible to propose a simple new method to estimate the demand for early nephrology care in each setting, based upon the ratio between CKD Stages 3 and 4, which may contribute to valid healthcare decisions.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
Thanks to Susan Finnel for her careful language editing. Thanks to Valérie Giard and Cécilia Jousselin for their help in retrieving the data. We thank all the collaborators of the REIN registry, a full list of whom is available at https://www.agence-biomedecine.fr/R-E-I-N-Reseau-Epidemiologique-et-Information-en-Nephrologie?lang=fr
FUNDING
No funding was received for this study; Centre Hospitalier Le Mans covered editing and publishing expenses.
CONFLICT OF INTEREST STATEMENT
No conflict of interest for any of the authors. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. De Nicola L, Minutolo R.. Worldwide growing epidemic of CKD: fact or fiction? Kidney Int 2016; 90: 482–484 [DOI] [PubMed] [Google Scholar]
- 2. Ng JK, Li PK.. Chronic kidney disease epidemic: how do we deal with it? Nephrology 2018; 23 (Suppl 4): 116–120 [DOI] [PubMed] [Google Scholar]
- 3. Mangione F, Dal Canton A.. The epidemic of chronic kidney disease: looking at ageing and cardiovascular disease through kidney-shaped lenses. J Intern Med 2010; 268: 449–455 [DOI] [PubMed] [Google Scholar]
- 4. McCullough KP, Morgenstern H, Saran R. et al. Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol 2019; 30: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mallappallil M, Friedman EA, Delano BG. et al. Chronic kidney disease in the elderly: evaluation and management. Clin Pract (Lond) 2014; 11: 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fishbane S, Hazzan AD, Halinski C, Mathew AT.. Challenges and opportunities in late-stage chronic kidney disease. Clin Kidney J 2015; 8: 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hallan SI, Matsushita K, Sang Y. et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 2012; 308: 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blunt I, Bardsley M, Strippoli GF.. Pre-dialysis hospital use and late referrals in incident dialysis patients in England: a retrospective cohort study. Nephrol Dial Transplant 2015; 30: 124–129 [DOI] [PubMed] [Google Scholar]
- 9. Schwenger V, Morath C, Hofmann A. et al. Late referral–a major cause of poor outcome in the very elderly dialysis patient. Nephrol Dial Transplant 2006; 21: 962–967 [DOI] [PubMed] [Google Scholar]
- 10. Kessler M, Frimat L, Panescu V. et al. Impact of nephrology referral on early and midterm outcomes in ESRD: epidemiologie de l'insuffisance renale chronique terminale en Lorraine (EPIREL): results of a 2-year, prospective, community-based study. Am J Kidney Dis 2003; 42: 474–485 [DOI] [PubMed] [Google Scholar]
- 11. George C, Mogueo A, Okpechi I. et al. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Glob Health 2017; 2: e000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elshahat S, Cockwell P, Maxwell AP. et al. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS One 2020; 15: e0230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Tol A, Lameire N, Morton RL. et al. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol 2019; 14: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Combe C. Le forfait Maladie Rénale Chronique stades 4 et 5. 2019. https://www.sfndt.org/actualites/le-forfait-maladie-renale-chronique-stades-4-et-5 (21 September 2020, date last accessed)
- 15. Hirano K, Kobayashi D, Kohtani N. et al. Optimal follow-up intervals for different stages of chronic kidney disease: a prospective observational study. Clin Exp Nephrol 2019; 23: 613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Hare AM, Choi AI, Bertenthal D. et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007; 18: 2758–2765 [DOI] [PubMed] [Google Scholar]
- 17. Ku E, Johansen KL, McCulloch CE.. Time-centered approach to understanding risk factors for the progression of CKD. Clin J Am Soc Nephrol 2018; 13: 693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhong J, Yang HC, Fogo AB.. A perspective on chronic kidney disease progression. Am J Physiol Renal Physiol 2017; 312: F375–F384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 21. Hill NR, Fatoba ST, Oke JL. et al. Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stengel B, Metzger M, Froissart M. et al. Epidemiology and prognostic significance of chronic kidney disease in the elderly–the Three-City prospective cohort study. Nephrol Dial Transplant 2011; 26: 3286–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma SK, Zou H, Togtokh A. et al. Burden of CKD, proteinuria, and cardiovascular risk among Chinese, Mongolian, and Nepalese participants in the International Society of Nephrology screening programs. Am J Kidney Dis 2010; 56: 915–927 [DOI] [PubMed] [Google Scholar]
- 24. Eriksen BO, Ingebretsen OC.. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006; 69: 375–382 [DOI] [PubMed] [Google Scholar]
- 25. Baek SD, Baek CH, Kim JS. et al. Does stage III chronic kidney disease always progress to end-stage renal disease? A ten-year follow-up study. Scand J Urol Nephrol 2012; 46: 232–238 [DOI] [PubMed] [Google Scholar]
- 26. Keith DS, Nichols GA, Gullion CM. et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164: 659–663 [DOI] [PubMed] [Google Scholar]
- 27. Totoli C, Carvalho AB, Ammirati AL. et al. Associated factors related to chronic kidney disease progression in elderly patients. PLoS One 2019; 14: e0219956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Go AS, Yang J, Tan TC. et al. ; Kaiser Permanente Northern California CKD Outcomes Study. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol 2018; 19: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reichel H, Zee J, Tu C. et al. Chronic kidney disease progression and mortality risk profiles in Germany: results from the chronic kidney disease outcomes and practice patterns study. Nephrol Dial Transplant 2020; 35: 803–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stengel B, Metzger M, Combe C. et al. Risk profile, quality of life and care of patients with moderate and advanced CKD: the French CKD-REIN cohort study. Nephrol Dial Transplant 2019; 34: 277–286 [DOI] [PubMed] [Google Scholar]
- 31. Benain JP, Faller B, Briat C. et al. Coût de la prise en charge de la dialyse en France. Nephrol Ther 2007; 3: 96–106 [DOI] [PubMed] [Google Scholar]
- 32. Sharif MU, Elsayed ME, Stack AG.. The global nephrology workforce: emerging threats and potential solutions! Clin Kidney J 2016; 9: 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delanaye P, Jager KJ, Bokenkamp A. et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol 2019; 30: 1785–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michel A, Pladys A, Bayat S. et al. Deleterious effects of dialysis emergency start, insights from the French REIN registry. BMC Nephrol 2018; 19: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Padilla CM, Raffray M, Pladys A. et al. Geographic variations in the risk of emergency first dialysis for patients with end stage renal disease in the Bretagne Region, France. Int J Environ Res Public Health. 2018; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fraser SD, Roderick PJ, May CR. et al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol 2015; 16: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lonnemann G, Duttlinger J, Hohmann D. et al. Timely referral to outpatient nephrology care slows progression and reduces treatment costs of chronic kidney diseases. Kidney Int Rep 2017; 2: 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perkins RM, Bucaloiu ID, Kirchner HL. et al. GFR decline and mortality risk among patients with chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 1879–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smart NA, Titus TT.. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 2011; 124: 1073–1080.e2 [DOI] [PubMed] [Google Scholar]
- 40. Lee J, Lee JP, An JN. et al. ; Clinical Research Center for End Stage Renal Disease (CRC for ESRD) Investigators. Factors affecting the referral time to nephrologists in patients with chronic kidney disease: a prospective cohort study in Korea. Medicine (Baltimore) 2016; 95: e3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fischer MJ, Ahya SN, Gordon EJ.. Interventions to reduce late referrals to nephrologists. Am J Nephrol 2011; 33: 60–69 [DOI] [PubMed] [Google Scholar]
- 42. Bello AK, Levin A, Manns BJ. et al. ; Kidney Health for Life Initiative. Effective CKD care in European countries: challenges and opportunities for health policy. Am J Kidney Dis 2015; 65: 15–25 [DOI] [PubMed] [Google Scholar]
- 43. Neale EP, Middleton J, Lambert K.. Barriers and enablers to detection and management of chronic kidney disease in primary healthcare: a systematic review. BMC Nephrol 2020; 21: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piccoli GB, Cabiddu G, Breuer C, Jadeau C. et al. Dialysis reimbursement: what impact do different models have on clinical choices? J Clin Med 2019; 8: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu HH, Zhao S.. Savings opportunity from improved CKD care management. J Am Soc Nephrol 2018; 29: 2612–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vanholder R, Annemans L, Brown E. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 47. Smith DH, Gullion CM, Nichols G. et al. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol 2004; 15: 1300–1306 [DOI] [PubMed] [Google Scholar]
- 48.Professionnels de Santé Au 1er Janvier 2018 | Insee. https://www.insee.fr/fr/statistiques/2012677#tableau-TCRD_068_tab1_regions2016 (16 June 2020 date last accessed)
- 49. Gautier A, Fournier C, Beck F.. Pratiques et opinions des médecins généralistes en matière de prévention. Actual Dossier Santé Publique 2011; 77: 6–10 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.