Abstract
Humans are constantly exposed to exogenous chemicals throughout their life, which can lead to a multitude of negative health impacts. Advanced materials can play a key role in preventing or mitigating these impacts through a wide variety of applications. The tunable properties of hydrogels and hydrogel nanocomposites (e.g., swelling behavior, biocompatibility, stimuli responsiveness, functionality, etc.) have deemed them ideal platforms for removal of environmental contaminants, detoxification, and reduction of body burden from exogenous chemical exposures for prevention of disease initiation, and advanced treatment of chronic diseases, including cancer, diabetes, and cardiovascular disease. In this review, three main junctures where the use of hydrogel and hydrogel nanocomposite materials can intervene to positively impact human health are highlighted: 1) preventing exposures to environmental contaminants, 2) prophylactic treatments to prevent chronic disease initiation, and 3) treating chronic diseases after they have developed.
Keywords: chronic diseases, hydrogel nanocomposites, hydrogels, remediations, therapeutics
Graphical Abstract
Humans are constantly exposed to environmental contaminants, which lead to a multitude of negative health impacts. Hydrogels and hydrogel nanocomposites can play a key role in preventing or mitigating these impacts through a wide variety of ways, including: (1) preventing exposures to environmental contaminants; (2) prophylactic treatments to prevent chronic disease initiation; (3) treating chronic diseases after they have developed.
1. Introduction
Advanced materials can prevent or mitigate negative health impacts to humans from exposure to exogenous chemical throughout their life. In recent years, it has become apparent that genetics cannot explain the majority of diseases, acknowledging that exposures to contaminants can lead to adverse health effects in life.[1–3] The World Health Organization (WHO) estimates that environmental risk factors cause over 24% of human disease globally and are responsible for 13 million deaths each year worldwide.[4–6] Here, environmental risks to health are defined as “all the external physical, chemical, biological, and work-related factors that affect a person’s health, excluding factors in the natural environment that cannot be modified.”[6,7] It is estimated over 80% of all major diseases have significant links to environmental exposures, and certain age groups are at higher risk for these diseases, such as children under 5 years and adults aged 50–75 years.[8] Moreover, the costs associated with preventable environmental chemical exposures are believed to exceed 10% of the global gross domestic products worldwide.[9,10]
Environmental risk factors are wide-ranging and include exposure to hazardous chemicals in our air, water, soil, and food. Many environmental contaminants are present at a concentration higher than normal because of their anthropogenic nature and have detrimental effects on wildlife and the ecosystem.[11,12] These contaminants can be inorganic or organic compounds (i.e., pesticides, solvents, pharmaceuticals, metals, plastics, etc.), and can have short-term effects on humans and the environment, as well as long-term effects caused by low-level chronic exposures associated with adverse health effects. These early life exposures to chemical contaminants have been directly associated to elevated risks of developing several diseases later in life, such as asthma and neurodevelopmental conditions.[13] These exposures are also considered major contributors to the development of obesity and chronic diseases, specifically cancer in genetically susceptible individuals. Recent studies indicate significant association between contaminant exposures in air, food, and water to cardiovascular diseases and diabetes.[13,14] Furthermore, through recent advances in epigenetic studies, it is now known that the development of chronic diseases is often triggered by an environmental exposure in predisposed individuals, with surmounting evidence for noncommunicable diseases originating during fetal development and early childhood.[14–17]
For example, since its discovery lead has been widely used in a variety of products like paint, cosmetics, gasoline, batteries, ammunition, pipes, and plumbing, among others. It enters the environment from any of its past or current uses and can remain there indefinitely. Plants can absorb lead through their leaves or take it up from polluted soil and/or water, thereby causing inhibition of ATP production, DNA damage by over production of reactive oxygen species (ROS), inhibition of seed germination, reduced chlorophyll production, and stunted growth.[18,19] In humans and other species, lead is also toxic upon exposure through inhalation, absorption, ingestion, and/or bioaccumulation in food, and has adverse reproductive, respiratory, and neurological effects, among others.[20] Chronic lead exposure, like many other environmental contaminants, affects the human body differently depending on the age of those exposed. During developmental stages, prolonged exposure to lead can lead to learning disabilities and motor alteration, and bioaccumulation can also cause development of high blood pressure, kidney disease, diabetes, and other comorbidities.[21–23] Human exposure to environmental contaminants is an issue of great concern given the ubiquity of these pollutants and the variety of exposure routes that exist. As such, an effective response requires multilevel risk management to identify the most relevant sources, mitigation of their environmental persistence, lessening the burden of disease in humans, and proper treatment of affected individuals (Figure 1).
Figure 1.

Hydrogels and hydrogel nanocomposites interventions to 1) prevent exposures to environmental contaminants, 2) prevent chronic disease initiation via prophylactic treatments, and 3) treat diseases after they have developed.
Advanced materials can be specifically designed with controlled physical, chemical, electrical, and biological properties to prevent environmental contaminant exposures, minimize body burden, and/or treat associated diseases at various stages. One way to mitigate environmental exposures is by treating the actual environment (i.e., air, soil, water) to remove pollutants and prevent transfer to humans. Similarly, advanced materials can be designed to sorb these contaminants in vivo to remove contaminants from the body after exposure, resulting in a detoxification effect and lowering pollutant body burden. These materials can also be used to deliver nutrients to the body, such as antioxidants, to prevent disease initiation or progression. Furthermore, when chronic diseases have already developed due to gene–environment interactions, like apolipoprotein-E4 interactions with heavy metals which have been associated with Alzheimer’s disease, the use of advanced materials has been critical in developing effective therapeutics and treatments to ameliorate human health.[24]
One such class of advanced materials is hydrogels. Hydrogels are 3D hydrophilic polymer networks that typically absorb high volumes of water (in some cases greater than 90% of their dry weight) while maintaining physical integrity.[25–28] The hydrophilic functional groups attached to the polymer backbone allow for water absorption and retention, while crosslinks between polymer chains prevent dissolution, allowing them to remain stable in aqueous environments.[25,29–31] These unique characteristics have made hydrogels attractive for a variety of applications in the medical, pharmaceutical, agricultural, food, personal care, and environmental fields.[25,27,29,32–37] In addition, hydrogels can respond to different types of external stimuli, such as pH or temperature shifts, exhibiting dramatic changes in their network structure, swelling behavior, permeability, and mechanical strength. One drawback of traditional hydrogel materials is their inability to entrap/absorb hydrophobic molecules due to their high hydrophilicity. To overcome this limitation, introduction of hydrophobic functionalities into the polymeric structure via chemical (e.g., hydrophobic polymer crosslinkers) or physical interactions (e.g., nanoparticles) has been proposed. The latter modification gives rise to hydrogel nanocomposite systems, a composite material with physically or covalently embedded nanoparticles or nanostructures within a crosslinked polymer, that can act as drug carriers, impart responsiveness to external stimuli, like to an alternating magnetic field (AMF), and increase sorption of hydrophobic contaminants, among others.[38–42] Hydrogel nanocomposites possess characteristics of the individual hydrogel components (i.e., high solvent content, high porosity/permeability, ability to absorb molecules and their controlled release) with those of the entrapped nanoparticles (i.e., electrical conductivity, mechanical reinforcement, magnetic features, catalytic activity, etc.) thereby creating unique materials with synergistic properties that cannot be achieved by either individual component alone. Hydrogel nanocomposites are highly tailored materials with desired functionalities that have expanded the potential uses of hydrogels in nanotechnology, biomedicine, and environmental applications.
While there is a plethora of reviews dedicated to the design, synthesis, and/or application of hydrogels and hydrogel nanocomposites in different fields,[43–49] the present article will provide a unique contribution to literature by highlighting research spanning three various stages where hydrogels and hydrogel nanocomposites can intervene to positively impact human health: 1) preventing exposures to environmental contaminants, 2) prophylactic treatments to prevent chronic disease initiation, and 3) treating diseases after they have developed. While there have been positive impacts from hydrogel and hydrogel nanocomposites in these application areas for quite some time, this review will primarily focus on the most recent developments in this area over the past 5 years.
2. Treating the Environment to Prevent Human Exposure to Environmental Contaminants
In this section, the application of hydrogels and hydrogel nanocomposites preventing exposures to environmental contaminants will be presented. Although traditionally studied for mainly biomedical and biological applications, hydrogels have also been developed for environmental remediation purposes. The more recent influx of research examining hydrogels and hydrogel nanocomposites for pollutant capture comes at a time when innovative and renewable materials are desperately needed to address the current state of water, soil, and air contamination from anthropogenic and natural processes. As such, facile functionalization and desirable physiochemical properties put hydrogels in the limelight for water and energy-based applications. As previously mentioned, hydrogels tend to exhibit hydrophilic behavior, thus allowing many aqueous contaminants to easily diffuse into a hydrogel-based sorbent. This type of model provides an alternative to that demonstrated by activated carbon or other traditional sorbents. In addition, hydrogel and hydrogel nanocomposites can respond to an external stimulus exhibiting change in their properties that aid in the removal of environmental contaminants. Temperature responsive polymers which exhibit a phase change at their critical solution temperature (attributed to disruption of intra- and intermolecular interactions), causing the polymer to either expand or collapse within the aqueous solvent resulting in sorption/desorption of the target contaminant, are some of the most extensively studied responsive systems.[48,50–56] This section highlights some of the most recent and interesting efforts to successfully demonstrate the applicability of hydrogels and hydrogel nanocomposites for environmental remediation applications (Table 1).
Table 1.
Hydrogel and hydrogel nanocomposites recently developed for removal or organic and inorganic pollutants from the environment and prevent human exposure to environmental contaminants.
| Pollutant category | Hydrogel | Functionality | Pollutant | Binding mechanism | Reference | |
|---|---|---|---|---|---|---|
| Hydrogels | Organic | Poly(N-[3-(dimethylamino)propyl] acrylamide, methyl chloride quaternary, DMAPAA-Q) | Amine and imine groups | PFAS and GenX | Hydrophobic and electrostatic interactions | [53] |
| Functionalized poly(ethylene glycol) diacrylate (PEGDA) | Fluorine and amine groups | PFAS and GenX | Hydrophobic and electrostatic interactions | [54] | ||
| Poly(2-acrylamido-2-methyl-1-) propanesulfonic acid (PAMPS) | Sulfonic groups | Oil-in-water emulsions; surfactants | Polymer wettability (superhydrophilicity and underwater superoleophobicity); Hydrogen bond interactions | [55] | ||
| 5′-hydrazino G4 K+ hydrogel | Bisnucleophilic 5′-hydrazines | Alpha, beta-unsaturated carbonyls | Covalent bonding through tandem aza-Michael addition and cyclization | [56] | ||
| Pullulan/polydopamine hybrid hydrogels (PPGels) | Polydopamine amine and hydroxyl groups | Cationic dyes | Electrostatic interactions | [57] | ||
| 3D-chitosan functionalized hydrogel sponges | Thiol groups | Anionic dyes | Hydrophobic and electrostatic interactions | [59] | ||
| Inorganic | Tetraethylenepentamine crosslinked chitosan oligosaccharide | Amino groups | Hexavalent chromium (Cr(VI)) | Electrostatic interactions | [60] | |
| Poly(vinyl imidazole) crosslinked chitosan | Amino, hydroxyl. And keto groups | Hexavalent chromium (Cr(VI)) and reactive black 5 dye (RB5) | Electrostatic interactions | [61] | ||
| Chitosan–ethylene glycol hydrogel | Amine and hydroxyl groups | Nitrate | Electrostatic interactions | [58] | ||
| Silk fibroin/polyethylenimine functional hydrogel | Amine groups | Heavy metals | Complexation and chelation | [62] | ||
| Disulfide-crosslinked carboxymethyl cellulose hydrogels | Thiol groups | Heavy metals | Complexation | [63] | ||
| Poly(2-acrylamindo-e-methyl-propanesulfonic acid-co-acrylic acid) superadsorbent hydrogel spheres (SAHS) | Carboxyl and sulfonic acid groups | Cationic dyes and heavy metals | Electrostatic interactions and complexation | [64] | ||
| Nanocomposite | Nanoparticle | Pollutant | Binding mechanism | Reference | ||
|
| ||||||
| Hydrogel nanocomposites | Organic | Magnetic poly(N-isopropylacrylamide)/chitosan hydrogel | Iron oxide (Fe3O4) | Sulfamethoxazole (SMZ) and bisphenol A (BPA) | Electrostatic and hydrogen bonding; hydrophobic interactions | [70] |
| Curcumin multiacrylate (CMA) and quercetin multiacrylate (QMA), magnetic nanocomposites | Iron oxide (Fe3O4) | polychlorinated biphenyls (PCB) | π–π stacking interactions | [66] | ||
| Chitosan/hematite nanocomposite hydrogel capsules (HC-H) | Hematite iron oxide (α-Fe2O3) | Congo red | Hydrophobic interactions and hydrogen bonding | [71] | ||
| Nanocomposite hydrogel with vinyl hybrid silica nanoparticles (VSNPs) | Silica | Methylene blue | Hydrogen bonding and ionic interactions | [72] | ||
| Magnetic pullulan hydrogel | Iron oxide (Fe3O4) | Tetracycline hydrochloride (TCH) | Fenton-like catalytic degradation | [74] | ||
| 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized chitin nanofibers (TOCNs) hydrogel with titanium dioxide nanoparticles (TiO2 NPs) incorporated into polyacrylamide (PAM) | Titanium oxide (TiO2) | Methyl orange | Photocatalytic degradation | [75] | ||
| Zirconium tungstate (ZrW) nanoparticle chitin-cl-poly (itaconic acid-co-acrylamide) hydrogel nanocomposite | Zirconium tungstate (ZrW) | Fast Sulphone Black (FSB) dye | Photocatalytic degradation | [76] | ||
| Inorganic | Microsized magnetic anionic hydrogel (nFeMAH) | Iron oxide (Fe3O4) | Cu(II) and Ni(II) | Ion exchange | [78] | |
| PAM/sodium montmorillonite (PAM/Na-MMT) nanocomposite | Sodium (Na) | Co2+ and Ni2+ | Ion exchange | [79] | ||
| TiO2 nanocomposite hydrogel | Titanium oxide (TiO2) | Cu2+ | – | [80] | ||
| Zirconium-loaded magnetic chitosan/ poly(vinyl alcohol) interpenetrating network (IPN) hydrogel | Zirconium | P | Innersphere complex and ligand exchange | [82] | ||
| PVA/graphene oxide (GO)–sodium alginate (SA) nanocomposite hydrogel beads | Graphene oxide | Pb2+ | Complexation | [84] | ||
| Xylan-g-/poly(acrylic acid (AA)-co-acrylamide (AM))/GO hydrogels | Graphene oxide | Pb2+ | High content of –OH and –COOH in GO improved adsorption by providing more ligands for this to occur | [85] | ||
| Carboxymethylcellulose (CMC)-g-poly N-isopropyl acrylamide (NIPAm-co-acrylic acid(AA)/Namontmorillonite (MMT) hydrogel | Carboxymethylcellulose (CMC) | Pb2+ | – | [86] | ||
2.1. Hydrogels for Treating the Environment
2.1.1. Organic Pollutant Removal
Here, a few examples of hydrogels being applied for the treatment of organic pollutants, including per- and polyfluoroalkyl substances (PFAS), pharmaceutical compounds, and various other commodity chemicals, are described. As of late, a very large area of environmental concern has been centered around PFAS in drinking water. In fact, the U.S. Environmental Protection Agency has issued a health advisory level of 70 ng L−1 on either individual or combined concentrations of the two most ubiquitous PFAS, perfluorooctanoic acid (PFOA), and perfluoro octanesulfonic acid (PFOS).[57] To address the cost and performance pitfalls that conventional sorbents exhibit when used for PFAS removal, several groups have turned their research efforts toward functionalized hydrogels.
Ateia et al. have recently reported on the synthesis (Figure 2A) and application of a cationic hydrogel matrix for removal of GenX and short-chain PFAS from surface and wastewaters at environmentally relevant concentrations.[58] The poly (N-[3-(dimethylamino)propyl] acrylamide, methyl chloride quaternary, DMAPAA-Q) hydrogel was successfully demonstrated as an effective sorbent for the removal of 16 different PFAS from various water matrices and achieved maximum removal efficiencies in less than 2 h. Furthermore, the hydrogel maintained high PFAS removal over a range of pH from 4 to 9 (Figure 2B), likely due to the quaternary ammonium functional group contained within the polymer. The main removal mechanism varies depending on the type of PFAS: hydrophobic interactions facilitate binding for long chain and sulfonic PFAS; electrostatic interactions are responsible for binding of other PFAS such as short chain and carboxylic analogs. Although recyclability was demonstrated through solvent extraction, practicality is potentially limited due to associated waste and cost.
Figure 2.

A) Preparation of poly DMAPAA-Q hydrogel schematic. B) PFAS removal efficiencies for DMAPAA-Q hydrogels in varying pH solutions for GenX, ADONA, F-53B, PFBS, PFOS, PFBA, and PFOA at initial concentrations of 1000 ng L−1 of each PFAS, an adsorbent dose of 70 mg L−1, and contact time of 24 h with n = 3. Reproduced with permission.[58] Copyright 2019, Elsevier.
Huang et al. also reported the use of hydrogels for PFAS removal by exploring a bifunctional approach that equips poly(ethyleneglycol) diacrylate (PEGDA)-based sorbents with fluorinated and/or aminated moieties.[59] The approach was successful in creating hydrogels with high PFAS specificity because of dual electrostatic forces and hydrophobic interactions that occur simultaneously when placed in contaminated water. However, the aminated PEGDA exhibited the highest sorption capacity for all five PFAS examined whereas the bifunctional PEGDA showed higher capacities for PFOA and PFOS. This suggests that the dominant mechanism for removal is via electrostatic attractive forces and that sorbent PFAS capacity could be improved by increasing the zeta potential of functionalized PEGDA hydrogels. Spent sorbents were regenerated with 70% methanol containing 1% NaCl, however multiple regeneration cycles have yet to be examined. Additionally, the effect of solution pH and ion presence on binding affinity still needs to be investigated and will likely alter hydrogel PFAS affinity.
In addition to the afore mentioned water pollution issues, oil discharge from various industrial processes and day to day human activities have also proven to plague our clean water supplies. Hydrogel coated membranes have been developed as a viable option for mitigation of harmful oil spills and releases. Research conducted by Zhang et al. suggests a facile fabrication of poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (PAMPS) with a multiporous membrane substrate which achieved a high separation efficiency for oil-in-water emulsions while maintaining large permeation flux and excellent stability.[60] Selective controllable emulsion separation is possible due to the superhydrophilicity and underwater superoleophobicity of the PAMPS hydrogel combined with the microscale multiporous structure of the substrate. When an oil-in-water emulsion comes in contact with the hydrogel coated membrane, tiny oil droplets dispersed in the emulsion are retained while the water phase permeates through. Additionally, the PAMPS hydrogel coated membrane exhibited selective separation for cationic and nonionic types of surfactant stabilized emulsions via hydrogen bond interactions. Also pervasive due to both industrial and natural processes, are electrophilic a,b-unsaturated carbonyls and commodity chemicals such as acrolein (AC), methyl vinyl ketone (MVK), and methyl acrylate (MA), which are known to be cytotoxic. In order to address concerns regarding environmental exposure to these harmful compounds, Xiao and Davis have shown that a supramolecular G4·K+ hydrogel made from 5′-hydrazine 3 can remove the cytotoxic a,b-UCs, AC, and MVK, as well as the less reactive MA, from both atmospheric and aquatic environments via formation of cyclic covalent adducts.[61] Incorporation of hydrazine 3 enables the gel to react with contaminants such that a tandem aza-Michael addition and cyclization occurs to entrap AC, MVK, and MA within hydrogel.
A large portion of organic pollutant remediation centers around mitigation of textile industry waste. In fact, hydrogel-based platforms for aqueous dye removal have been prolific over the last several years. To improve adsorption efficacy, the relationship between crosslinker structure and hydrogel affinity for cationic dyes was examined by Su et al.[62] Introduction of polydopamine to pullulan to create hybrid hydrogels (PPGels) significantly enhanced affinity toward cationic dyes, equipping the gels with selective functionality for binding and removal of crystal violet and methylene blue in aqueous solution, but showed little to no affinity for azophloxine removal. Through increased addition of polydopamine content, decreased hydrogel zeta-potential was achieved, and thus the lyophilized PPGels had greater potential for removal of cationic dyes via electrostatic interactions which facilitated adsorption throughout the hydrogel structure.[62] 3D porous hydrogel sponges were synthesized using chitosan-based polymers functionalized by 11-mercaptoundecanoic acid and reportedly favored high adsorption capacity for methylene orange (MO) dye in water, with an apparent removal efficiency of greater than 90% for a wide range of MO solution concentrations. Contaminant removal mostly occurred through electrostatic interactions between anionic dye with thiol moieties present in the 3D chitosan sponges. Moreover, in vitro evaluation of 3D sponge toxicity revealed strong suitability for bio-related applications and exhibited high antibacterial activity against model hazardous pathogens with no lethality against reference commensal bacteria.[63]
2.1.2. Inorganic Pollutant Removal
The majority of inorganic pollutant treatment research focuses on removal of heavy metals, which largely contribute to water contamination and pose quite a challenge for remediation efforts. There have been some very important applications for hydrogel sorbents, such as the removal of hexavalent chromium (Cr(VI)). Chitosan has been a very popular option for hydrogel functionalization and two different research groups reported on chitosan crosslinked hydrogels that show successful adsorption of Cr(VI). A tetraethylenepentamine crosslinked chitosan oligosaccharide hydrogel synthesized by Mei et al. showed excellent thermal stability and mechanical strength with high capacity and fast kinetics for Cr(VI), even at low concentrations, which is an important consideration for highly toxic metals such as this one.[64] In acidic conditions, adsorption is promoted via electrostatic attractions between the negatively charged Cr(VI) species (HCrO4−) and positively charged amino groups in the hydrogel. At pH > 7, however, the amount of protonated amino groups decreases and hydroxyl ions start to compete with CrO4− for the active binding sites, which greatly diminishes hydrogel adsorption capacity.[64]
Midya et al. reported successful removal of Cr(VI) with their poly(vinyl imidazole) crosslinked chitosan hydrogel (cl-Ch-pVI), which exhibited a higher maximum adsorption capacity than that reported by Mei et al. Additionally, cl-Ch-pVI also showed high affinity for the adsorption of organic dye reactive black 5, which reveals the potential versatility of this polymer in wastewater treatment applications.[65] Electrostatic interactions between the negatively charged adsorbate and Cr(VI)/RB5 is the major contributor for removal of these contaminants from aqueous medium.[65] As such, strongly acidic pH mediums are required for optimum contaminant removal, just as with the sorbate reported on by Mei et al. Requiring such low aqueous pH values truly limits the applicability of these materials, since lowering the pH of large quantities of wastewater can prove to be quite intensive and costly.[66] Another chitosan hydrogel, biodegradable chitosan–ethylene glycol hydrogel (CEGH) was reported as an effective, carbon-rich adsorbent for the removal of nitrate in water by Chen et al.[66] The adsorption of nitrate onto CEGH, however, was affected by pH since hydrogel amine and hydroxyl functional groups are responsible for the majority of nitrate removal via electrostatic interactions. Strongly alkaline environments reduce adsorption sites for nitrate because of competitive hydroxyl adsorption, which again, potentially limits widespread applicability of the hydrogel.
A slew of new research using hydrogels as effective sorbents for additional heavy metals has also been reported in the last few years. While Godiya et al. have focused on metal ion adsorption in aqueous environments, Hou et al. reported on capture and removal of heavy metal ions for soil remediation purposes.[67,68] A silk fibroin–polyethyleneimine (SF/PEI) composite hydrogel is not only reportedly a low-cost option for high capacity removal of numerous metal ions (Cu(II), Pb(II), Cd(II), Zn(II), Ni(II), Ag(I)), but also exhibited versatile functionalities such as catalytical activity and antibacterial properties depending on the adsorbed metal ion.[67] Primary and secondary amino groups on the SF/PEI hydrogel can form complexes with metal ions, attributing to excellent hydrogel adsorption capacity in both single metal solutions and metal mixture solutions. Adsorption capacity is suppressed, however, in the presence of organic dyes such as methylene blue (MB) due to competitive chelation between the cationic metal ions and MB on the protons of hydrogel amino groups.[67] The group suggests further functionalization steps for increased hydrogel properties in order to mitigate this effect. As a very interesting hydrogel application, dual-functional redox-responsive hydrogels based on disulfide-crosslinked carboxymethylcellulose were developed by Hou et al. for the controlled release of agrochemicals but were also reported to synchronously exhibit affinity toward heavy metal ions present in the soil after release (Figure 3).[68] When the redox-responsive hydrogels were applied to Cu2+ and Hg2+ contaminated paddy soil, loaded agrochemicals were released when the reducing agents essentially “stripped” the responsive networks. Simultaneously, the reduced hydrogels exhibited strong affinity toward heavy metal ions present in the paddy soil due to the strong complexation attraction between metal ions and thiol groups generated by the disconnected disulfide bonds in the hydrogel, thus providing a multifaceted approach to the improvement of soil quality.
Figure 3.

A) Redox-responsive behavior of carboxymethylcellulose hydrogels synthesized by Hou et al. i) reactant mixture, ii) formed hydrogel, iii) hydrogel after addition of 50 × 10−3 m DTT, and iv) regenerated gel after addition of 30% H2O2. B) Sol–gel transition schematic for redox-responsive hydrogel systems. C) Simultaneous increase in hydrogel redox potential (Eh) (solid lines) and reduction in concentration of Hg2+ (dashed lines). Agrochemical release (bars) of both GNA (red) and GBA (blue). Reproduced with permission.[68] Copyright 2018, Royal Society of Chemistry.
Another interesting bioinspired strategy is presented by Song et al. where superadsorbent hydrogel spheres are prepared from poly(2-acrylamido-2-methyl-propanesulfonic acid-co-acrylic acid) model heavy metal ions such as Pb(II)) and also show outstanding affinity toward MB.[69] Extraordinarily, the hydrogel spheres were reported to purify more than 6 L of contaminated wastewater very effectively by using only 7 mL of spheres and exhibited good recyclability, indicating great potential for wastewater treatment applications.[69]
2.2. Hydrogel Nanocomposites for Treating the Environment
Hydrogel nanocomposites have gained popularity in recent years as advanced materials for environmental contaminant remediation given their high removal efficiency, ease of handling, and cost-effectiveness.[70] Hydrogel nanocomposites are always made up of two or more materials with at least one of them being a hydrogel, where the desired properties from each individual component are combined and a composite system with enhanced properties for the desired application is obtained. Hydrogel nanocomposites have been demonstrated to exhibit higher elasticity, increased mechanical strength, large specific surface area, improved chemical/biological/electrical properties, and/or enhanced swelling/deswelling behavior that cannot be achieved by individual components on their own.[71,72] Hydrogel nanocomposites have been designed from a variety of natural or synthetic hydrogels and a wide range of nanoparticles (i.e., ceramic, metal, polymeric, carbon nanotubes, liposomes, etc.). The following section briefly discusses some of the most recent developments for hydrogel nanocomposites as environmental remediation tools.
2.2.1. Organic Pollutant Removal
Environmental contamination caused by organic pollutants continues to be a significant public health concern given their potential toxicity to humans under acute or prolonged exposures. As a result, the development of technologies capable of removing organic contaminants has drawn the attention of numerous researchers in the hydrogel nanocomposites area. One of the most extensively studied is magnetic nanocomposite hydrogels because of their inherent magnetic properties, ability to adsorb and degrade pollutants via the Fenton reaction, and response to an AMF, which enables local heating and enhanced reactivity.[72–74] Zhou et al. developed a magnetic poly(N-isopropylacrylamide)/chitosan hydrogel that showed significant aqueous adsorption of both hydrophilic anionic sulfamethoxazole (SMZ) and hydrophobic uncharged bisphenol A (BPA), two emerging contaminants of concern.[70] The primary attractive forces for hydrogel binding to SMZ were hypothesized as electrostatic and hydrogen bonding interactions, whereas BPA was adsorbed via hydrophobic and hydrogen bonding interactions. Inclusion of the magnetic nanoparticles allowed for rapid separation from solution using an external magnetic field. In related work, Gutierrez et al. developed novel magnetic nanocomposite microparticles (MNMs) for the adsorption of polychlorinated biphenyls (PCBs) and their facile removal from water.[71] Two different acrylated polyphenols were incorporated, curcumin multiacrylate and quercetin multiacrylate, into the polymer hydrogel backbone to enhance affinity for PCBs. The group conducted batch binding studies for PCB 126 (Figure 4A) where MNM and pollutant contact time was 48 h, after which magnetic decantation was performed to remove the sorbent and allow for supernatant analysis of residual PCB 126. The Langmuir adsorption coefficients were obtained for all systems, including hydrogels without iron oxide nanoparticles (denoted as MP). The MNMs and MPs resulted in excellent binding for PCB 126 (Figure 4B,C) with higher affinity than that reported for activated carbon, which is considered the gold standard for organic contaminant remediation. The incorporation of the acrylated polyphenols was demonstrated to enhance adsorption of PCB 126 and was attributed to π‒π interactions between aromatic rings of the PCB and polyphenol moieties.[71]
Figure 4.
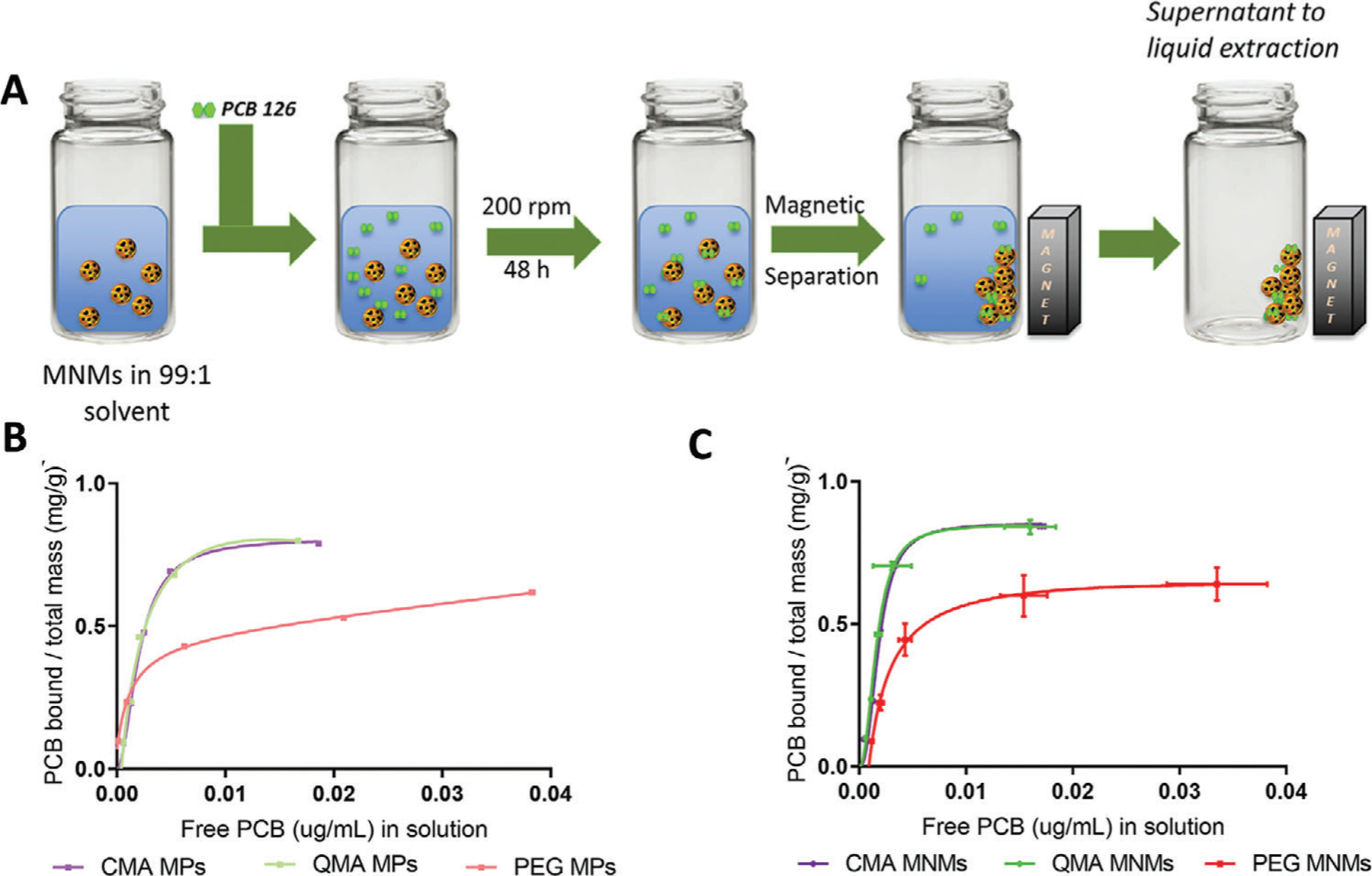
A) Schematic representation of binding studies conducted with PCB 126 in a 99:1 DI water:ethanol solvent. Room temperature adsorption isotherms for PCB 126 of the B) MNM systems and C) MP systems. PCB 126 initial concentrations from 0.003 to 0.1 ppm fitted using the Langmuir model. Reproduced with permission.[71] Copyright 2020, Wiley.
In another example, hematite iron oxide nanoparticles (α-Fe2O3) were immobilized and impregnated into a chitosan gel matrix to develop chitosan/hematite hydrogel nanocomposites for the removal of Congo red (CR) from aqueous solution.[76] CR adsorption was reported to be driven by both chemisorption and physisorption attributed to hydrogen bonding between surface hydroxyl (–OH) or amine (–NH2) groups present in the hydrogel nanocomposite with electronegative residues (N, S, etc.) in CR, and a combination of hydrophobic interactions and increased surface area.[76] The strong adsorption of CR makes it a great candidate for application in removal of organic dyes from waste waters. Similarly, Chen et al. demonstrated rapid adsorption of MB and obtained a high removal rate of 90% within 40 min from water using a superadsorbent nanocomposite hydrogel that employed vinyl hybrid silica nanoparticles as the crosslinking agent. The research group stated the high adsorption capacity for MB is due to functional groups on the adsorbent (–COOH and –CONH2) interacting via hydrogen bonds or ionic interactions, depending on the ionized state of the adsorbent.[77] Another useful water-based application for hydrogel nanocomposites has been demonstrated by Umbreen et al. through investigation on uptake of three widely used pharmaceutical compounds, specifically naproxen, ibuprofen, and diclofenac from aqueous solutions.[78] The affinity between drug molecules and reduced graphene oxide-based hydrogels was ascribed primarily to electron donor acceptor like π–π and n–π interactions, hydrogen bonding, and hydrophobic interactions.[69]
Hydrogel nanocomposites have also been developed to enhance reactive degradation for treatment situations required or preferred over the sorption of environment contaminants. Cheng et al. observed a significant Fenton-like catalytic degradation of tetracycline hydrochloride (TCH) in the presence of H2O2 and the developed iron oxide nanoparticle-doped magnetic pullulan hydrogel. The degradation process was described as an adsorption–oxidation, with the iron oxide magnetic nanoparticles increasing the hydrogel catalytic activity through the addition of active sites and HO−, which demonstrates catalytic degradation of TCH.[79] In other research, Yue et al. synthesized a 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-oxidized chitin nanofibers hydrogel functionalized with titanium dioxide nanoparticles(TiO2 NPs) on its surface, which were then incorporated into a polyacrylamide (PAM) matrix obtaining TiO2-TOC-PAM10.[80] Here, the TiO2 NPs imparted the hydrogel nanocomposite photocatalytic activity, acted as a crosslinker to obtain a microporous 3D structure, and significantly increased the TiO2-TOC-PAM10 tensile and uniaxial compressive strengths. The adsorption capacity for MO removal from water was credited to the synergistic effect of the adsorption and photocatalytic degradation of the hydrogels, resulting in an outstanding removal platform capable of withstanding 10 reuse cycles without loss of performance and removing a total of 96% MO.[80] In related research, Sharma et al. fabricated a photocatalytic hydrogel nanocomposite for the removal of Fast Sulphon Black (FSB) dye from water by incorporation of zirconium tungstate (ZrW) nanoparticles into the chitin-cl-poly (itaconic acid-co-acrylamide) via microwave-induced sol–gel/copolymerization. The polymeric component of the hydrogel nanocomposites acts as an adsorbent for SFB, and since ZrW nanoparticles are supported in the polymeric matrix, the photocatalytic degradation of SFB was able to be optimized, achieving an adsorption-photocatalytic removal of 92.66% SFB under 120 min, where the photodegradation of FSB dye was hypothesized as photochemical sensitization.[81]
2.2.2. Inorganic Pollutant Removal
Heavy metals are commonly found in ground water because of anthropogenic or geological sources, and these contaminants tend to accumulate in the biosphere and living organisms (and biomagnificate).[82] To address this issue, hydrogel nanocomposites have been a prominent area of cutting-edge research and development because of their advantageous properties. Badsha et al. recently developed a hydrogel nanocomposite capable of removing Cu(II) and Ni(II) from electroplating wastewater through a pH-independent and reusable microsized magnetic anionic hydrogel (nFeMAH).[83] Upon 20 consecutive adsorption/desorption cycles, nFeMAH maintained a magnetic separationof99%ofitsstartingvalue,whereastheNiremovalefficiency dropped significantly from 92% to 75% after the first cycle, attributed primarily to an ion exchange mechanism.[83] The loss of adsorption capacity during the reusability study was attributed to metal oxide formation on the nFeMAH surface.[83] Nonetheless, the use of nFeMAH for treating electroplating wastewater still provided desirable outcomes when considering its pH independent behavior, fast adsorption, and reusability. A different approach to remove heavy metals Co2+ and Ni2+ from water was evaluated by Moreno-Sader et al. using a PAM/sodium montmorillonite (PAM/Na-MMT) nanocomposite.[84] They were able to prove that addition of sodium into the nanocomposite increased removal yield for Co2+ to 99.3% and for Ni2+ to 98.7% compared to that of PAM/MMT nanocomposite at 87.4% and 94.5%, respectively, which was attributed to the increase in ion exchange capacity after increasing the interlayer space of Na-MMT. This study shows that PAM/Na-MMT can be used as a novel adsorbent in industrial applications.
Yu et al. developed a tough TiO2 nanocomposite hydrogel for heavy metal adsorption and repeatable dye degradation that is self-healing, and can be activated by ultraviolet (UV) light to efficiently adsorb heavy metal ions and degrade dye molecules.[85] The unique self-healing ability of this nanocomposite upon activation under UV light can be seen in Figure 5A, where a hydrogel was first cut into two pieces and then, when exposed to UV the two pieces rejoined. When the TiO2 nanocomposite is immersed in water containing heavy metal ions, in this case Cu2+, the contaminants are adsorbed via diffusion-binding, as seen in Figure 5B by the decrease in visible color as UV exposure time increases. The heavy metal ions bind unto the nanoparticle surface after diffusing through the hydrogel matrix, hence, in the presence of free radicals (which cause the detachment of the polymer chains from the TiO2 surface) accelerates binding by further promoting diffusion of the metal ion into the hydrogel. When there is a dye molecule present in solution, the anionic dye is adsorbed into the TiO2 nanocomposite through weak ionic bonds with the partially positive charge of the TiO2 nanoparticles, and then, degradation occurs given the in situ production of OH and O radicals at the surface of the nanoparticle. This can be clearly seen by the reduction in blue color (Figure 5C) over UV exposure time until the solution is clear again. The proposed strategy by Yu et al. harnesses the very high specific area of the TiO2 nanoparticles for in situ dye degradation preventing secondary contamination, and capable of withstanding rougher applications thanks to its durability and self-healing capability, providing a novel platform that can be used in applications beyond water treatment.
Figure 5.

A) Experimental images and polymer-network schematics to show self-healing properties. B) Schematic to show the mechanism of light-assisted adsorption of copper ions (Cu2+) on the TiO2 surface, and the image sequence of Cu2+ solution at different times of UV light exposure. C) Schematic to show the mechanism of light-assisted dye degradation, and the image sequence of the dye solution at different times of UV light exposure. Reproduced with permission.[85] Copyright 2020, MDPI.
Excess phosphorus in aquatic environments can lead to increased algae and large plant growth, and eventually to eutrophication or the production of algae blooms that produce toxins harmful to humans and other species.[86] Wan et al. designed a zirconium-loaded magnetic chitosan/poly(vinyl alcohol) interpenetrating network hydrogel to remove phosphorus from aquatic environments.[87] The sorption process follows mono-layer adsorption, suggesting the primary contributor to phosphorus sorption is Zr, with sorption mechanisms of innersphere complex and ligand exchange.
In other research, Sanchez et al. demonstrated that addition of fillers (natural and modified clay, magnetic nanoparticles, or the mesoporous carbon) into the polymeric matrix of polyvinyl alcohol (PVA) significantly improved its adsorptive capacity toward Cd2+.[83,88] Yu et al. developed a series of PVA/graphene oxide (GO)–sodium alginate (SA) nanocomposite hydrogel beads for the removal of Pb2+ from water and found that with increasing GO content, Pb2+ adsorption capacity also increased. This was attributed to an increase in pore size and a relatively loose network within the beads which led to an increase in complexation between Pb2+ and COO– on the surface of GO-SA.[89]
Further exploring the effects of GO in environmental contaminant removal, Kong et al. prepared xylan-g-/poly(acrylic acid(AA)-co-acrylamide (AM))/GO hydrogels for the adsorption of heavy metal ions.[90] The xylan-based nanocomposite hydrogel showed excellent adsorption capacity for Pb2+ when GO was added as a reinforcer. High content of –OH and –COOH in GO improved adsorption by providing more ligands for this to occur. In a different study, the removal of Cu2+ and Pb2+ ions from aqueous solutions with a carboxymethylcellulose-based nanocomposite hydrogel was examined and sorption study results indicated the removal capacities of the nanocomposite for both metal ions were found to be higher than those of the pure hydrogel.[91]
3. Prophylactic Treatment to Prevent the Development of Chronic Disease
Human and animal exposures to environmental contaminants can lead to various inflammatory diseases, and since exposures are inevitable, it is important to have prophylactic treatments to prevent the development of chronic disease. Enterosorbents have been recently studied as an alternative therapeutic approach for removing contaminants from the body to minimize body burden and prevent disease initiation or progression. The process of enterosorption refers to the treatment through oral ingestion of a natural or synthetic material that travels through the gastrointestinal (GI) tract and sorb substances present in it, without interacting or metabolizing within the gut, and therefore are removed through natural excretion from the body.[92,93] These sorbents include activated carbons, clays, silicon-based hydrogels, and nanocomposites, among others, and can prevent or treat acute and chronic exposures.[92,93] Hydrogels and hydrogel nanocomposites have attractive properties that allow them to be great candidates for enterosorbents. Reported treatments of hydrogels as enterosorbents include for the sorption of heavy metals, bile acids, and toxins, and treatment of gastric disorders. (Table 2)
Table 2.
Hydrogel and hydrogel nanocomposites used as prophylactic treatments in the prevention and development of chronic disease.
| Nanocomposite | Material | Contaminant Treated | Key Result | Reference | |
|---|---|---|---|---|---|
| Heavy metals remediation | Enterosgel | Polymethylsiloxane polyhydrate | Cu2+, Ni2+, Zn2+, Pb2+ | Metal complexation: Cu < Ni < Zn < Pb where sorbent efficiency is as follows “White coal Pb2+ 150.28 m g−1” ≥ “POLYSORB Pb2+ 102.11 mg g−1. ” ≥ “Enterosgel” ≥ “Smekta” ≥ “Filtrum” ≥ “Activated carbon 49.18 mg g−1” | [89] |
| Polysorb | Fumed silica | ||||
| Activated carbon | Carbon | ||||
| White coal | Silicon dioxide main component | ||||
| Smekta | Dioctahedralsmectite | ||||
| Filtrum | Lignin-dietary fiber | ||||
| Citrus pectin | Complex of colloid polysaccharides based on galacturonic acid side chains of ramose, arabinose, xylose, and fructose | Cd, Fe, Cu, Zn | In whey milieu the sorption capacity of enterosorbents to metals varies. The received combinations of enterosorbents were of higher adsorption capacity in relation to the investigated metals as compared with monobiopolymer enterosorbents |
[90] | |
| Calcium alginate | Salts of agonic acid molecules | ||||
| Chitosan | Polymer β-(1 → 4)-2-acetamido-2-deoxy-D-glucopyranose (enterosorbent “chitosan”) | ||||
| MCC (microcrystalline cellulose) | Product of hydrolytic cleavage of the polymer of d-glucose | ||||
| Microtone | Polysach-Polymer constricted from the residues of N-acetyl-β-D-glucosamine 1→4 bonds between them(chitin) and β-glucans (β−1,3- and β−1,6) | ||||
| Polisorb | Fumed silica | ||||
| Spectra | Dioctahedralsmectite | ||||
| Poly(AAm-co-AAc)/bentonite | Copolymer of acrylic acid and acrylamide with bentonite | Pb2+ | Sorption capacity is 25% higher than pectin system (max sorption 2250 mg Pb g−1 sorbent) | [91] | |
| Poly(AAm-co-AAc)/pectin | Copolymer of acrylic acid and acrylamide with pectin | Inclusion of pectin increases sorption capacity to that of the hydrogel by 20% (max sorption 1650 mg Pb g−1 sorbent) | |||
| Bile acids removal | Enterosgel | Polymethylsiloxane polyhydrate | Shiga Toxin II subunit B (Stx-2B), Clostridium difficile Toxin A (TcdA), and Toxin B (TcdB), endotoxin taurocholic acid, glycocholic acid, taurochenodeoxycholic acid, and glycochenodeoxycholic acid | Removed 8% taurocholic, taurochenodeoxycholic, 13% glycocholic, and 27% of glycochenodeoxycholic | [88] |
| Charcodote | Charcoal | 100% removal | |||
| Cationic microfibrillated cellulose | Cellulose grafted with N-(2,3-epoxypropyl) trimethylammonium chloride (EPTMAC) | Sodium cholate | 29.74% removal compared to cholestyramine | [92] | |
| Unmodified microfibrillated cellulose | Homogenized pulp | 10.60% removal compared to cholestyramine | |||
| Pulp fibers | Hardwood dissolved | 4.51% removal compared to cholestyramine | |||
| Cholestyramine | Quaternized styrene–divinilbenzene copolymer | 100% removal | |||
| Cationic dextran hydrogels | Dimethylethylamine | Sodium glycocholate, sodium taurocholate, sodium cholate, and sodium deoxycholate | Increased length of the alkyl substituents (R) leads to the increased ionic complex formation rate and stability (both the ionization constant K0 and the stability constant K increase) and a reduction of the aggregation of bile acid molecules (cooperativity parameter u decreases) | [96] | |
| Dimethylbutylamine Dimethyloctylamine Dimethyldodecylamine |
|||||
| Cholestyramine | Benzyltrimethylammonium chloride | ||||
| PAMPMTA-co-PHEA | Poly((3-acrylamidopropyl) trimethylammonium chloride-co-poly(2-hydroxyethyl acrylate) | Sodium cholate | The hydrogels synthesized by SARA ATRP exhibited a considerably higher binding capacity than the one of the hydrogels produced by FRP | [97] | |
| P(AH-co-AHH) | Poly(allylamine hydrochloride with allylhexylamine) | Sodium glycocholate | The maximum adsorption capacity was 859.63 mg g−1, and the adsorption reached equilibrium within only 2 h | [100] | |
| Chitosan–silica composite | Chitosan and fumed silica | Cholic and taurocholic acid | The maximum values of adsorption were found to be up to 97 μmol g−1 for taurocholic acid and 43 μmol g−1 for cholic acid | [100] | |
| Gastric disorders therapy | Enterosgel | Polymethylsiloxane polyhydrate | – | Primary outcome of duration of diarrhea to first nonwatery stool showed a statistically significant decrease (p= 0.03) in the Enterosgel group, which corresponds to a 64% chance of the Enterosgel subject’s diarrhoea resolving first compared with standard therapy alone. | [102] |
| Children with diarrhea syndrome stayed in hospital significantly less time | [98] | ||||
| Patients in main group achieved a statistically significant reduction in the severity of abdominal pain, diarrhea syndrome, dyspepsia scales, and on the total gastrointestinal symptom rating scale | [104] |
3.1. Enterosorbents for Heavy Metal Remediation
Heavy metals are a large class of environmental contaminants that can lead to severe human diseases when accumulated in the body. Enterosorption has been used to remove these substances from the GI tract. Andrusenko et al. evaluated the effectiveness at heavy metal detoxification of some commonly used medical enterosorbents, including Entersogel.[94] Enterosgel is a polymethylsiloxane-based hydrogel with proven binding efficacy toward high molecular weight compounds.[92] All the studied sorbents have a half-sorption period of ≈30 min and appeared to be effective at removing heavy metals ions with the following efficacy: Cu2+< Ni2+< Zn2+< Pb2+. Similarly, Yahiya et al. reported on the sorption isotherms of various heavy metals from a combination of studies and proved that heavy metal sorption is significant and effective, with results varying greatly depending on the sorbent used.[95] These studies show the potential of natural and polymeric intervention for removal of heavy metals and preventing accumulation in the body and potential initiation of disease.
In other research, Uspenskaya et al. developed hydrogel nanocomposites based on a copolymer of acrylic acid and acrylamide loaded with varying concentrations of pectin and bentonite for the sorption of Pb2+ from aqueous solutions. Acrylic acid and acrylamide were crosslinked with N,N′-methylene-bis-acrylamide via chemically initiated free radical polymerization to create a biocompatible, semi-interpenetrating network.[96] Heavy metal complexation occurred rapidly for all hydrogel nanocomposites with those containing higher concentration of pectin having a greater sorption capacity for lead. These results show great promise for the decrease of heavy metal body burden using hydrogels and hydrogel nanocomposites.
3.2. Enterosorbents for Excess Bile Acid Removal due to Organic Contaminants
Bile acids are natural surfactants formed from cholesterol in the liver that, while at low concentrations are harmless, can become harmful as a bile salt if it continues to be released into the body.[93] A large amount of cholesterol in the blood can also lead to obesity, diabetes, and even cardiovascular diseases.[97] Disruption of hepatic bile acid metabolisms has recently been associated with exposure to a slew of organic pollutants, specifically GenX, its analogs, and PFAS.[98–100] The removal of these substances using bile acid sequestrants, or hypocholesterolemic agent-containing polymers, has gained popularity given their high experimental sorption efficiency.[96,101–103] Some of these sequestrants include hydrogels such as Enterosgel and cellulose-based materials. Howell et al. recently, tested the efficacy of Enterosgel as compared to a charcoal sorbent in adsorbing bile acids (i.e., taurocholic acid, glycocholic acid, taurochenodeoxycholic acid, and glycochenodeoxycholic acid), bacterial entero- and endotoxins, and pharmaceutical drugs.[93] Here, Enterosgel showed a strong potential for cholic acid treatment through its continuous sorption, as well as high binding of the bacterial toxins Shiga Toxin II subunit B (Stx-2B), Clostridium difficile Toxin A (TcdA), and Toxin B (TcdB) (Figure 6). The sorptive properties of the hydrogel keep these substances from being absorbed into the body through enterohepatic circulation and its selective network allow for less diffusion of commonly used pharmaceutical drugs to be adsorbed compared to charcoal.
Figure 6.

Adsorption kinetics for A) TcdB and B) TcdA, showing remaining concentration over time for Enterosgel, charcodote, and positive control (no adsorbent) (mean ± sem). Equilibrium adsorption isotherm (Qe) of C) TcdB and D) TcdA against remaining concentration in solution (Ce) for Enterosgel and Charcodote. Reproduced with permission.[93] Copyright 2019, Nature Publications.
Cellulose can also be functionalized to create a network for bile acid sorption. Zhu et al. used the cationic modifier N-(2,3-epoxypropyl) trimethylammonium chloride (EPTMAC) to create a biocompatible and selective sorbent with increased colloidal stability and large surface area.[92] The resulting cationic microfibrillated cellulose gel along with unmodified microfibrillated cellulose and its derivates, then underwent a series of experiments including the in vitro sorption of sodium cholate, a model bile salt compound. A Langmuir-fitted isotherm showed the best fit of sodium cholate sorption with successful binding of this substance, especially compared to the unmodified controls. Sodium cholate removal was also investigated by Nichifor et al., where they attempted to develop a more selective sorbent with higher sorption capacity compared to the clinically used sorbents cholestyramine and colestipol.[101,104] The cationic dextran hydrogels contained cationic moieties with ethyl, butyl, octyl, and dodecyl alkyl groups to determine optimal sorption of sodium glycocholate, sodium taurocholate, sodium cholate, and sodium deoxycholate.[101] Ultimately, the cationic dextran hydrogels proved to have higher binding efficiency for sodium cholate due to an increased ion-complex formation rate attributed to the presence of alkyl substituents.
Furthermore, for a bile acid sequestrant to be effective, the cationic and hydrophobic regions must remain balanced, and display high swelling ratios. Mendonca et al. inspected the development of poly((3-acrylamidopropyl)trimethylammonium chloride-co-poly(2-hydroxyethyl acrylate) (PAMPMTA-co-PHEA) hydrogels using two different synthesis techniques, and potential hydrogen bonding interactions with acids that are credited to the PHEA addition.[102] The hydrogels demonstrated high swelling capacity, as well as sorption of sodium cholic acid. However, given the decrease in binding of cholic acid with an increase in PHEA concentration, the stated hypothesis attributing hydrogen instead of electrostatic interactions being the most prominent for of attraction can be rejected. Copolymers have also been employed as bile acid sequestrants in China. Wang et al. synthesized an enterosorbent based on P(AH-co-AHH) (allylamine hydrochloride with allylhexylamine hydrogel) with a crosslinker obtained by the oxidation of methyl α-d-glucopyranoside by sodium periodat.[105] PAH and its derivates have shown biocompatibility and chemical reactivity, and therefore are desirable enterosorbents. The gels exhibited large swelling properties alongside high binding of sodium glycocholate with equilibrium sorption reached after 1 h.[105]
Finally, Budnyak et al. also explored the sorption of bile acids, specifically cholic and taurocholic acid, onto a chitosan–pyrogenic silica enterosorbent. Combining the amino groups from chitosan with silica-based hydrogels can impart higher selectivity, with increased resistance to microorganisms and a high surface area.[106] The composites showed strong affinity toward bile acids due to electrostatic interactions determined by their physicochemical properties, with decreasing sorption in high pH environments indicating a pH-dependency. Bile acid therapeutic remediation efforts from enterosorbents have displayed promising results with a range of hydrogels and nanocomposites and have a lot of potential for further exploration.
3.3. Enterosorbents for Gastric Disorders: Acute Diarrhea/Irritable Bowel Syndrome Treatment
Gastric diseases can range in their severity with some resulting in acute pain, requiring medical treatment and hospitalization. Although the cause for gastric disorders is still unknown, it is believed exposure to environmental contaminants must play a role. Acute intestinal infections or disorders are common among children and adults and, if not promptly treated, can cause abdominal pain and discomfort, and even long-term damage to the GI tract. Diarrhea (D), irritable bowel syndrome (IBS), and IBS-D are three gastrointestinal disorders that are still prevalent and account for high morbidity and mortality especially at a younger age.[107,108] Since some of these conditions can become fatal, efforts to prevent and treat the gut are necessary. Howell et al. designed a random, controlled 8-day experiment with a total of 105 adult volunteers with acute diarrhea that were randomly divided between two groups: an Enterosgel-receiving group, and a placebo group for control. Out of the 105 participants, 86 (43 from each group) were analyzed and it was determined that diarrhea resolved more rapidly in patients treated with Enterosgel compared with the placebo.[107] Khavkin et al. also studied the effects of Enterosgel on diarrhea but focused on a population of children with sample size of 64. Once again, the group that received Enterosgel treatment presented faster regression of symptoms, and participants were able to be discharged from constant treatment in an average of four days.[103]
Irritable bowel syndrome remediation has also been studied with daily treatment of Enterosgel.[109,110] Tkachenko et al. performed a small, 30 participant-experiment to determine efficacy and safety of this sorbent through daily ingestion. The 21-day study most significantly demonstrated a decrease of stool sample frequency and abdominal pain.[109] Enterosgel displays auspicious results in the treatment of gastric disorders and should continue to be tested for efficacy as an enterosorbent.
3.4. Other Prophylactic Treatment Examples
Environmental contaminants continue to pose significant health risks and, despite the insurmountable efforts to remove them from the environment, their pervasiveness has proven complete remediation to be incredibly difficult and cost-prohibitive.[2,111] There is a pressing need for biomedical solutions to reduce susceptibility to environmental pollutant exposure, stop progression of chronic diseases and improve prognosis. Studies over the last decade have shown the impotence of nutrition in the modulation of inflammation and antioxidant pathways, decreasing toxicity of proinflammatory pollutants ultimately decreasing their body burden.[112–122] Hydrogels and hydrogel nanocomposites can act as successful delivery platforms to target the area of interest, increase bioavailability, maintain antioxidant/nutrient activity and can respond to external stimuli for trigger release. Poly(beta-amino ester) (PBAE) polymers are a class of hydrogels with demonstrated improved drug delivery by enhancing stability and allowing controlled release by degradation through hydrolysis.[74,123–125] Jordan et al. developed curcumin conjugates PBAE crosslinked polymers capable of releasing curcumin in physiological relevant concentrations of H2O2 showcasing an accelerated release in response to higher incorporation of curcumin in the hydrogel.[126] Curcumin, a hydrophobic plant-derived polyphenol with high antioxidant capacity, can be acrylated and integrated into the polymer chains for its targeted delivery upon PBAE degradation.[127–129] Recently, Babaei et al. have explored the use of curcumin as a therapeutic for prolonged and localized treatment of glioma by incorporation into a biodegradable thermoresponsive hydrogel applied as an injection.[130]
In another application, Chen et al. developed a recombinant protein crosslinked hydrogel loaded with magnesium and zinc ions for bone regeneration via their synergistic and sustained release.[131] Glycol chitosan, gelatin, silk fibroin, alginate, and cell-laden based hydrogels have also been studied for bone tissue engineering.[132–138] The development of pH responsive injectable hydrogels have been shown to be good candidates for localized and sustained delivery of RNAi.[139–145]
4. Treating Diseases Resulting from Chronic Exposure to Environmental Contaminants
As mentioned above, environmental contaminants are ubiquitous, and exposures are simply unavoidable by humans. The significant body burden of anthropogenic contaminants being detected in humans worldwide continues to put forth the need to address the adverse health effects associated with their exposure and body accumulation. The need for cutting edge treatments to tackle all the gene-environment interactions and increasing number of noncommunicable diseases continues to grow. Hydrogels and hydrogel nanocomposites have been widely studied in the field of medicine and biological research. Their highly hydrated and tunable structures allow hydrogels to mimic the mechanical properties of extracellular matrices of many living tissues, resulting in excellent biocompatibility in many cases. In addition, their physical, morphological, mechanical, electrical, chemical, toxicological, and compositional properties can be modified to suit a variety of applications like drug delivery, biosensing, cancer therapies, regenerative medicine, pathogen detection and treatment, and treatment of other chronic diseases. This section highlights some of the most unique and recent advances in chronic disease treatment/management with hydrogels and hydrogel nanocomposites, specifically relating to cancer, diabetes, and cardiovascular diseases (Table 3).
Table 3.
Hydrogel and hydrogel nanocomposites for treating diseases resulting from chronic exposure to environmental contaminants.
| Hydrogel/hydrogel nanocomposite | Drug | Application | Key results | Reference | |
|---|---|---|---|---|---|
| Cancer | ERT MS-embedded HP9/HD cross-linked hydrogel (Erlotnib microsphere-embedded hyaluronic acid-phenylboronic acid/dopamine hydrogel) | Erlotinib (ERT) | Peritumoral injection of an anticancer agent for local cancer therapy | Sustained locoregional delivery of ERT, enhanced cancer curing efficiencies, sustained local delivery, short gelation time, single syringe injection, self-healing potential, high retention time | [180] |
| Visible light-cured glycol chitosan (GC) hydrogel containing paclitaxel (PTX)-complexed beta-cyclodextrin(-CD) (GC/CD/PTX) | Paclitaxel (PTX) | Injectable drug delivery depot system for ovarian cancer | Sustained and controlled release of PTX, single local administration showed antitumor efffect for 7 days in mice | [181] | |
| Self-assembled dextran sulfate (DS)-DOX complexes encapsulated in agarose hydrogel | Doxorubicin (DOX) | Local injected into the cavity after lumpectomy for sustained delivery of low dose DOX | Completely elimination of MDA-MB-231 breast cancer cells with low cytotoxicity to NIH 3T3 fibroblasts | [182] | |
| CREKA-conjugated dextran-coated iron oxide nanoparticles | Cisplatin | Systemic tumor targeting to treat lung cancer | Enhanced permeation and stability retention in cell culture, effective at targeting fibrinogen overexpressed in tumor tissues, nontoxic over long period of time, synergistic effect of cisplatin and hyperthermia | [194] | |
| Hyaluronic acid (HA) hydrogel covalently embedded with doxorubicin loaded and triphenylphosphine (TPP) core–shell gold mesoporous silica nanoparticles | Doxorubicin (DOX) and triphenylphosphine (TPP) | Local drug-delivery system for sustained stomach cancer treatment | Development of as an implantable drug-delivery system for local synergistic chemophotothermal cancer therapy | [195] | |
| Diabetes | 4-Carboxy-3-fluorophenylboronic acid (FPBA) modified with biodegradable poly(l-lysine) (PLL) polymers for constructing polymer−insulin complexes | Insulin | Glucose-stimulated insulin delivery | Construction of polymer–insulin complexes, blood glucose regulation ability, glucose-triggered insulin release in type 1 diabetic mice | [212] |
| Silk fibroin hydrogel (iSFH) | Insulin | Subcutanoues injectable hydrogel for sustained insulin delivery | Insulin–iSFH in diabetic rats forms active depot under skin for slow sustained release of insulin and restoration glucose homeostasis for 4 days, insulin–iSFH did not cause hypoglycemia | [213] | |
| Oligomer serine-b-poly(lactide)-b-poly(ethyleneglycol)-bpoly(lactide)-b-oligomer serine (OS-PLA-PEG-PLA-OS) pentablock copolymer, as matrix and chitosan–insulin electrosprayed nanospheres (CIN) as constituent materials | Insulin | In situ injectable pH-temperature sensive hydrogel system—biodegradable after 5 weeks | Steady state insulin delivery into induced diabetic mice with no changes in plasma concentrations, blodd glucose level reduction effects for over 60 h | [214] | |
| Polyacrylamide bidentate ß-cyclodextrin-based hydrogel with preloaded insulin | Insulin | Injectable biomimetic glucose trigger-insulin release system | Dual self-regulated system shows a specific d-glucose response to realize accurate monitoring and simultaneous on-demand trigger insulin release, enables long lasting blood glucose control | [215] | |
| pH-sensitive semi-interpenetrating polymer network (IPN) hydrogel | Insulin | Oral insulin delivery | In vitro insulin release is pH dependent, blood glucose level reduction with oral administration of insulin-loaded hydrogel in mice studies | [226] | |
| Cardiovascular disease | Controllable NO-releasing redox injectable hydrogel (NO-RIG) composed of dual bifunctional triblock copolymers | NO | Injectable hydrogel system | Scavenges overproduced ROS and regulates local NO expression level simultaneously, exhibited therapeutic efficiency without any conventional drugs or biomolecules | [244] |
| Mixed component hydrogel capable of releasing both bioactive curcumin and NO | Curcumin and NO | Injectable hydrogel for myocardial infraction (MI) | Combinational treatment of curcumin and NO reduces collagen deposition, improves cardiac function, ameliorates adverse myocardium remodeling, suppresses apoptosis, and hypertrophy (synergistic effect) | [247] | |
| GST-TIMP-bFGF/collagen-GSH hydrogels | Recombinant protein GST-TIMP-bFGF (basic fibroblast growth factor) | Dual function, MI-responsive on-demand growth factory delivery system | Promotes recovery of MI rats by enhancing vascularization and ameliorating myocardium remodeling in in vitro and in vivo studies | [252] | |
| Tissue-derived extracellular matrix (ECM)/silk fibroin (SF) composite scaffolds with Au nanoparticles and mesenchymal stem cells (MSCs) | – | Cardiac patch for infarcted myocardium regeneration | Formation of well-interconnected porous surface ECM cardiac composite patches with Au and SF proteins improves cell proliferation and migration with suitable physiological properties. Au–ECM/SF patches made suitable atmosphere for cell growth at higher number and adhered cardiomyocytes with homogeneous spreading on the patches | [256] | |
| Polyvinylalcohol/dextran (PVA/Dex) elastic hydrogel patches | Astaxanthin | Cardiac patch to assist responses against myofibril stress | Elastic hydrogel materials can load astaxanthin without affecting antioxidant properties, sustained antioxidant release, patches can be implanted in rats without damage to surrounding tissue | [257] | |
| Alzheimer’s disease | liposomal donepezil HCl (LDH) dispersed into thiolated chitosan hydrogel (TCH) | Donepezil HCl (DH) | Intranasal delivery | In vivo rabbits’ studies showed LDH incorporated into TCH significantly increasing the blood and brain of DH compared to the oral tablets | [267] |
| Mixture of two hydrophilic polymers (Poloxamer 407 and Poloxamer 188) | Active pharmaceutical ingredient (API) | Controlled intranasal delivery of an active pharmaceutical ingredient (API) via liposomes | Good natural mucoadhesive characteristics of in situ gel formulations which increased when liposomes were added, controlled API release, decreased systemic exposure with increased bioavialbility in the CNS | [268] | |
| Dual temperature/ion-sensitive in situ hydrogel (ISG) | Timosaponin BII | Intranasal delivery | Brain-targeted drug delivery signaling good Alzheimer’s prevention | [269] | |
| Chronic respiratory disease | Poly(lactic-co-glycolic acid) (PLGA) microspheres embedded in a poly(N-isopropylacrylamide) (p-NIPAAm)-based hydrogel | Mometasone furoate | Thermogel, Extended-release Microsphere-based-delivery to the Paranasal Sinuses (TEMPS) for reduction of sinonasal inflammation | System undergoes reversible sol–gel transition at 34–35 °C applied as a liquid at ambient temperature and conforming to the sinonasal epithelium as it gels, TEMPS was maintained in rabbit sinuses and effectively reduced sinonasal inflammation, thermogel matrix is non-biodegradable but the system is temporary and can be readily removed and reapplied as needed | [281] |
| Hyaluronan and heparin-based hydrogel system with encapsulated IL-10 | IL-10 | Intranasal delivery for idiopathic pulmonary fibrosis (IPF) in the lung | Hhydrogel delivery system for IL-10 attenuates lung fibrosis due to inhibition of TGFβ−1 activation of fibroblasts in an animal model of IPF, intranasal administration effectively delivers sufficient hydrogel deposition to small airways and lung parenchyma | [282] | |
| Hydrogel scaffold based on natural polymers gelatin and alginate | – | Catheter-injectable gelatin–alginate hydrogel | Hydrogel scaffold can be injected through long catheters, exhibiting physical and mechanical properties necessary for dual treatment of remodeling the lung architecture as a lung volume reduction material and developing a platform for tissue regeneration to allow for cell or organoid implant | [283] |
4.1. Treating Cancer with Hydrogels and Hydrogel Nanocomposites
Cancer is currently one of the most life-threatening diseases, causing an average of 1 in every 6 deaths worldwide, which translates into 606 520 deaths in 2020 in the US alone.[146,147] It is estimated that 19% of annual worldwide cancer death are attributed to environmental contaminant exposures, including PCBs, pesticides (i.e., heavy metals and their compounds (i.e., cadmium, chromium, nickel), benzidine-based dyes, asbestos and aflatoxins.[6,148–157] Additionally, a variety of environmental contaminants have been identified as “known human carcinogens” by the international agency for research on cancer, the American Cancer Society, and the National Cancer Institute.[158–160] The common anticancer treatments today (i.e., surgical removal, chemotherapy, radiotherapy, etc.), all cause intense severe side-effects, risk unmanageable recurrence due to cancer cell proliferation and infiltrative growth, and cause other physical and psychological health issues. The use of hydrogels and hydrogel nanocomposites based cancer therapies has been demonstrated to be superior in many cases for their unique properties, including 1) their biocompatibility avoids severe immune rejection and systemic organ toxicity, 2) controlled drug release compared to traditional methods, 3) high drug loading capacity of one or more drugs or essential components addressing issues of low solubility of most anticancer therapeutics (i.e., doxorubicin, cisplatin, paclitaxel, magnetic particles, etc.), and/or 4) less invasiveness thanks to ease of injectability.[161–169] Our group has extensively studied magnetic hydrogel nanocomposite for cancer therapy applications such as controlled drug delivery, controlled thermal delivery, and combinations of these therapies [170–176] There have also been a few insightful reviews highlighting the breadth of activities in this area.[177–179]
Injectable hydrogels have gained interest for tumor treatment because of their ability to be locally administered, high swelling capacity, which can increase contact area between the therapeutic gel and irregularly shaped/distributed carcinoma, in situ polymerization capability as a response to external stimulus (i.e., like pH or temperature), ability to mimic the biological environment, prevent degradation of the drug during transport, and enhanced localized therapeutic effects. Lee et al. developed pH-modulated hydrogels with optimized viscoelastic properties for short gelation time based on boronate ester and polydopamine linkages for its application in cancer treatment as a localized, single peritumoral injection (Figure 7A).[180] This single syringe injection operates near physiological pH, alleviating pain caused by pH variation between the injection site and injected therapy. The anticancer drug erlotinib (ERT), an inhibitor of the epidermal growth factor tyrosine-kinase, was encapsulated into hydrogel microspheres and gelation pH was adjusted for fast polymerization upon injection based on boronate bond formation. Anticancer efficacy of the designed hydrogels was tested by antiproliferation using A549 cells (a lung adenocarcinoma cell line) showing negligible cytotoxicity in a 50/20–1000/1000 μg mL−1 concentration range, demonstrating a safe tumor-localized erlotinib delivery.[180] Furthermore, in vivo degradation kinetics of the hydrogel systems were observed using real-time optical imaging. The gels were injected subcutaneously with Cy5.5, as a near infrared spectroscopy dye, to trace in vivo fate and estimate the fraction of residual hydrogel. The Cy5.5 group disappeared faster in its free form, while increased retention was observed for the three different hydrogel formulations (Figure 7B). The composite with encapsulated erlotinib exhibited a higher integrated density value from 144 to 672 h as compared to the other systems.[180] Overall, Lee at al. were able to successfully design a hydrogel system with enhanced cancer treatment efficiency by providing sustained release and localized delivery of erlotinib, and thus preventing systemic toxicity while maintaining sufficient tumor suppression capacity, potentially making it a safe and applicable lung cancer treatment option.
Figure 7.

A) Scheme of the pH-controlled hydrogel loaded with erlotinib-encapsulated microspheres for peritumoral injection. B) In vivo degradation kinetics of scanned NIRF images of the control, Cy5.5, HP/HD/Cy5.5, HP9/HD/Cy5.5, and HP9/HD/ERTMS/Cy5.5 groups in mice from 0 to 672 h postperitumoral injection. Reproduced with permission.[180] Copyright 2021, American Chemical Society.
Additional chemotherapy drugs have shown great potential for localized delivery with the use of hydrogels, given their ability to provide a stable environment for the encapsulated drug and prevent degradation. Hyun et al. designed a light-cured glycol chitosan (GC) beta-cyclodextrin (β-CD)-paclitaxel (PTX) loaded composite hydrogel for ovarian cancer drug delivery.[181] As is the case with most anticancer drugs, PTX is hydrophilic and has low water solubility that can be improved by β-CD addition. A single administration of GC/β-CD/PTX demonstrated controlled release for up to 7 days, with the added benefit of hydrogel formation upon 10 s of visible light exposure to riboflavin, thereby eliminating the use of harsher UV-light.[181] Another form of dextran, dextran sulfate (DS), has been explored as a drug carrier for doxorubicin (DOX), a hydrophilic nonspecific anticancer drug typically employed for breast cancer treatment. Niu et al. encapsulated DS-DOX complexes into a biocompatible, biodegradable, and injectable agarose hydrogel and demonstrated high efficacy against MDA-MB-231 breast cancer cells with low toxicity to NIH 3T3 fibroblast.[182]
Recent in vivo animal studies have shown that a more favorable therapeutic outcome can be obtained with combination chemotherapy as compared to single drug therapy.[183–192] Among the most desirable outcomes is a synergistic effect, where the efficacy of multiple chemotherapeutics administered together is greater than the sum of the individual therapeutics administered independently.[193,194] Because of their intrinsic properties, hydrogels can be introduced by direct injection into the localized tumor area and have become the go-to drug delivery system for synergistic cancer treatment. Additionally, release kinetics can be easily tuned by adjusting polymer/water ratio. Schneible et al. developed a homogeneous composite hydrogel made of DOX-loaded modified-graphene oxide (GO) nanoparticles suspended in gemcitabine (GEM)-loaded Max8 hydrogel for scheduled and synergistic release of DOX and, being lower than that reported by for the DOX-GEM combination as free drugs, represented a step forward in the development of successful, translatable cancer treatment solutions. Another type of synergistic effect on cancer therapy and tumor reduction can be achieved by combining chemo- and photothermal therapy with targeted drug delivery. Zhou et al. develop a hyaluronic acid (HA) hydrogel covalently embedded with DOX-loaded and triphenyl phosphate (TPP)-modified core–shell gold mesoporous silica nanoparticles for chemo–photothermal multistage targeting and sustained tumor treatment for stomach cancer.[195] The hydrogel nanocomposites demonstrated tumor specific hyaluronidase degradation of the HA hydrogel to obtain in situ controlled nanoparticle release and sustained drug delivery as DOX was transported into cancer cells and mitochondria, thus resulting in high toxicity for cancer cells without any detectable side effects on healthy cells. Additionally, the gold nanoparticles successfully converted near infrared light into heat and effectively suppressed tumor growth, suggesting a promising application for synergistic chemo–photothermal cancer treatment.
4.2. Treating Diabetes with Hydrogels and Hydrogel Nanocomposites
Diabetes is a chronic disease that alters the way the body converts insulin into energy and occurs when either the pancreas does not produce enough insulin or the body is incapable of properly using the insulin it does produce.[196,197] Diabetes is one of the world’s fastest growing chronic diseases and was directly responsible for the death of 1.5 million people worldwide in 2019.[197] In the U.S. alone there are 1.5 million new cases of diabetes diagnosed each year, with a striking 11% of the population already having been diagnosed.[196,198] Over time, diabetes can cause significant damage to the heart, blood vessels, eyes, kidneys, and nerves, and eventually lead to stroke and/or lower limb amputations. Exposure to environmental contaminants like toxic metals, persistent organic pollutants, per and polyfluoroalkyl substances (PFAS), pesticides, and plasticizers have been linked to the pathogenesis of diabetes.[199–205] These contaminants tend to interact with the kidneys, liver, or pancreas where detrimental/toxic interactions with major enzymes can occur and potentially interfere with the metabolic pathways of glucose (i.e., glycolysis, glycogenesis, glucogenesis).[201,206,207] High levels of glucose are a common trait for Type 2 diabetes, and are usually encountered when the kidney and liver have been impaired through metabolic interactions with environmental contaminants, the pancreas has shown a decrease in muscle function, and specific endocrine functions, such as insulin secretion and maintenance of glucose homeostasis, are inhibited.[201,203,204,208–210] To date, diabetic therapy consists of constant monitoring of glucose levels, subcutaneous injectable delivery of insulin, and restoring euglycemia.[211]
The ideal closed-loop diabetes treatment requires an advanced material that is able to rapidly detect shifts in glucose levels and can respond to them by providing appropriate insulin or other therapeutic delivery, which is one of the innate properties of responsive hydrogels. As an example, Wang et al. recently developed biodegradable polymer–insulin complexes for glucose-stimulated insulin delivery based on a poly(L-lysine)-derived (PLL) glucose-responsive cationic polymer.[212] The complex is formed by first modifying biodegradable PLL with 4-carboxy-3-fluoro-phenylboronoc acid (FPBA: a glucose-sensing component), followed by a complexation process with negative insulin via electrostatic interactions at physiological pH.[212] This complex is capable of releasing insulin upon interaction with glucose by decreasing the pKa of FPBA as it binds with glucose, introducing a negative charge which in turn weakens the insulin-polymer interaction and results in its release (Figure 8A). In vivo studies were conducted in C57BL/6 mice with Type 1 diabetes induced by streptozotocin to evaluate the ability of the complexes to regulate blood glucose. IVIS images of the mice after subcutaneous injection shows the ability of all complexes to extend the antihyperglycemic effect of native insulin and achieve normoglycemia for over 20 h, with some even remaining effective after 72 h of treatment (Figure 8B). Furthermore, all systems were biocompatible and completely biodegradable as confirmed by their absence in the subcutaneous tissue 3 months after treatment.
Figure 8.

A) Schematic illustration of the formation of the biodegradable polymer-insulin complexes and mechanisms of glucose binding and insulin release. B) Representative IVIS images of diabetic mice treated with insulin and four different biodegradable polymer–insulin complexes from injection time (0 h) to 48 h post-treatment. Reproduced with permission. [212] Copyright 2021, American Chemical Society.
Maity et al. developed an injectable silk fibroin-based hydrogel (iSFH) for sustained insulin release over a prolonged period in diabetic conditions.[213] By effectively modulating the SF protein gelation using ethylene glycol (EG) and triethylamine glycol additives, they were able to obtain gelation in 50 min as compared to 4 days for SF alone, and successfully maintained a mesoporous structure ideal for human recombinant insulin encapsulation. In vivo delivery studies using T1DM Wistar rats demonstrated the formation of an active depot of insulin-iSHF under the skin upon subcutaneous injection from which the insulin doles out over a period of 4 days and maintains glucose homeostasis, proving iSFH is a viable insulin delivery therapeutic. Additionally, by providing continuous release for a period of several days, iSFH can increase patient compliance by circumventing the need for multiple daily injections and local amyloidosis.
In another application, Trinh et al. prepared a novel injectable pH-temperature sensitive hydrogel nanocomposite made up of an oligomer serine-b-poly(lactide)-b-poly(ethylene glycol)-b-poly(lactide)-b oligomer serine (OS-PLA-PEG-PLA-OS) pentablock copolymer with electrosprayed chitosan-insulin nanospheres for treatment of diabetes Type 1.[214] The hydrogel nanocomposites solution is in an expanded state at high pH and temperature. Gel transition occurs under body-like conditions, allowing for its application as an in situ injectable system. The hydrogel nanocomposites maintained steady-state blood insulin concentration, showed a prolonged reduction of blood glucose levels for over 60 h, and biodegraded 5 weeks after injection to the back of a mouse.
Taking a different approach, Wang et al. developed a biomimetic glucose trigger-insulin release hydrogel system based on a bidentate-β-cyclodextrin preloaded with insulin.[215] The dual self-regulated hydrogel showed specific binding between d-glucose and bidentate-β-cyclodextrin and exhibited macroscopic swelling due to proton release. While hydrogel expansion triggered release of preloaded insulin, isomers of D-glucose caused shrinkage and therefore delayed the release of insulin, enabling steady and prolonged blood glucose control (12+h). These results demonstrate the feasibility of β-cyclodextrin to respond in specific host–guest interactions for diabetes treatment and other targeted biomolecules.
Hydrogels and hydrogel nanocomposites allow for the design and development of “smart” responsive platforms that react to variations in pH or glucose levels and can be administered orally. Oral delivery of insulin increases patience compliance by eliminating the need for several subcutaneous injections. There are several challenges with oral delivery, however, and most center around the low stability and poor membrane permeability of the delivery system for insulin, specifically in the GI. The Peppas research group has made great progress in optimizing hydrogels for oral delivery of insulin.[216–221] They have developed a complexation hydrogel based in poly(methacrylic acid-g-ethylene glycol) [P(MMA-g-EG)] that can rapidly release insulin in the intestine due to its pH-dependent complexation, and also exhibits mucoadhesive characteristics, high-insulin loading efficiency, and enzyme inhibiting properties.[216,222–224] Further research demonstrated that cell-penetrating peptides (CPPs), like oligoarginines which are composed of six arginine resides (hexa-arginine or R6), can enhance penetration for oral delivery.[225] Recently, the combination of both strategies, the P(MMA-g-EG) hydrogel and the CPP R6, was examined and successfully demonstrated immediate release of insulin and R6 at pH of 7.4, resulting in a strong hypoglycemic response (reduction of blood glucose level by ≈18%) complemented by improvement in insulin absorption.[219] These results suggest the combination hydrogel carriers are a promising strategy for oral delivery of insulin for diabetes treatment. Another approach for oral delivery of insulin composed of semi-interpenetrating polymer networks of sodium carboxymethyl cellulose and poly(methacrylic acid) developed by Li et al. takes advantage of the intrinsic swelling properties of hydrogels and their pH responsiveness. In the collapsed stage at very acidic pH of 1.2, the hydrogels protected insulin from being released, and then at a higher pH of 6.8, swell and allowed for insulin to diffuse out of the polymeric network.[226] This hydrogel network showed a sustained release of insulin and lowered blood glucose levels in diabetic rats, showing potential as an oral insulin formulation.
4.3. Treating Cardiovascular Diseases with Hydrogels and Hydrogel Nanocomposites
Cardiovascular diseases (CVDs) are a group of pathological disorders of the heart and blood vessels that include but are not limited to coronary heart disease, atherosclerosis, myocardial infarction, cerebrovascular disease, stroke, and heart failure, and are the number one leading cause of death worldwide.[227,228] In2017, it was estimated 17.9 million people died from CVDs, accounting for 32% of all global deaths.[227,229] In the U.S., costs associated with CVDs each year are approximately $219 billion USD including health care services (46%), medications and loss of labor.[229] Environmental exposures have an important role in the development, severity and mortality of CVDs, with air pollution being the world’s largest environmental health risk according to the WHO.[230] Although the specific pathological mechanisms are unknown, evidence suggests the impact environmental contaminants have on the development and progression of CVDs is largely due to effected signaling pathways resulting in endothelial dysfunction, increased thrombus formation, inflammatory processes, oxidative stress, and an imbalance of the autonomic nervous system (contributing to arterial hypertension).[230–237]
Myocardial infarction(MI), or a heart attack, is the number one cause of cardiovascular mortality and disability worldwide and is responsible for roughly 805 000 heart attack cases per year in the U.S. alone. [229] MI occurs when blood flow to the heart muscle is blocked, hindering oxygen flow and resulting in ischemic myocardial tissue, progressive negative ventricular remodeling due to the obstructed coronary artery and, in some cases, irreversible necrosis of the heart muscle. The use of injectable hydrogels for cell therapy, resaturation of blood flow, regulation of cardiovascular tissues, and delivery of other beneficial molecules have shown promising results in cardiac repair following MI, all of which is attributed to the hydrogel’s increased biocompatibility, tunable biodegradability, properly tunable mechanical strength, and porous structure.[238–240]
Endogenously produced nitric oxide (NO) performs an important role in protecting and regulating cardiac function though vascular-dependent and independent functions.[241–243] Vong et al. have recently developed a NO-releasing redox injectable hydrogel (NO-RIG) showing therapeutic potential in cardiovascular disorder by controlling the NO levels and simultaneously the redox equilibrium at the injection site.[244] NO-RIG was prepared from polyion complex flower-type micelles composed of 1) a NO releasing polymer: PArg-PEG-PArg (Parg: Poly-Arginine) and 2) a redox scavenging polymer: PMNT-PEG-PMNT (PMNT: poly(methylnitroxidestyrene)), two bifunctional polycationic polymers capable of gelation at 37 °C (physiological conditions).[244] The therapeutic effect of NO-RIG was studied using the ligation of the left anterior descending artery, demonstrating improvement in cardiac functions in MI mice as well as decreased reduction in the infraction size.[244] The mechanism of action by which NO-RIG protects the heart functions after MI consists of two complementary effects: 1) PArg-PEG-PArg containing hydrogel induced the generation of NO to the regulated required level stimulating angiogenesis at the site of the diseased tissue, and 2) PMNT-PEG-PMNT containing hydrogel’s capacity to catalytically scavenge ROS, improving the bioavailability of NO production from PArg-PEG-PArg, regardless of endogenous NO production (Figure 9A). Vong et al. successfully assessed the ability of NO-RIG to act as a therapeutic by itself upon injection to the affected MI site, without delivery of active drugs or biomolecules, to regulate angiogenesis and other cardiac functions. Additionally, NO-RIG’s therapeutic effect can be enhanced by entrapping drugs, biomolecules, or cells within the hydrogel to treat MI and other cardiovascular diseases.[238,245]
Figure 9.

A) Schematic showing the proposed mechanism of NO-RIG in inhibiting MI in mice. NO-RIG is composed of two biofuntional polymers: PArg-PEG-PArg, which generates NO by the activated macrophages in inflamed tissues, and PMNT-PEG-PMNT, which scavenges ROS to enhance NO activity. NO-RIG can sustainably control the NO delivery and redox equilibrium balance in the infarcted tissues. As the results, NO-RIG treatment significantly prevents the progression of MI and improve cardiac functions. SMC: smooth muscle cell; EC: endothelial cell. Reproduced with permission.[244] Copyright 2018, Elsevier. B) Schematic representations of MMP-responsive hydrogel preparation and the process of drug release in the wound bed of MI model rats. (A) Preparation of GST-TIMP-bFGF via a recombinant protein expression method. (B) GSH was loaded into the hydrogel by a chemical crosslinking process to obtain Gel-GSH. C) GST-TIMP-bFGF was mixed with Gel-GSH, and the two components were linked with a bond between GST and GSH. D) Intramyocardial injection of the mixed hydrogel to the wound of a rat after MI. E) In the wound microenvironment, substrate peptides were degraded by MMP-2/9, and specific targeting peptides were released, achieving the dual functions of angiogenesis and MMP inhibition. Reproduced with permission.[252] Copyright 2019, Wiley-VCH GmbH.
The use of angiogenic growth factors for MI treatment has been extensively studied, but their application in CVD treatment is limited given their inefficient release from drug delivery carriers which can result in low drug accumulation on the infarcted region and severe induced side effects.[246–251] Fan et al. have developed a dual metalloproteinase (MMP) and pH responsive hydrogel for on-demand drug delivery of basic fibroblast growth factor (bFGF) to promote angiogenesis and inhibit cardiac remodeling after MI.[252] MMPs are part of the zinc endopeptidases family and have the ability to cleave extracellular matrix components, which are known to be overexpressed in heart disease and other pathologies.[253–255] The strategy of Von et al. employed to develop their hydrogel is shown in Figure 9B, where 1) a glutathione (GSH)-modified collagen hydrogel is prepared via conjugation of collagen amine groups with GSH sulfide groups to obtain gel-GSH and 2) bFGF is fused with glutathione-S-transferase (GST) and MMP-2/9 cleavable peptide PLGLAG (TIMP) to obtain the recombinant protein GST–TIMP–bFGF.[252] The MMP cleavable peptide TIMP, enclosed between GST and bFGF, responds to overexpressed MMPs upon intramyocardial injection to the affected area, where released TIMP inhibits excessive degradation of the cardiac matrix, hence achieving both angiogenesis and MMP inhibition. The dual-responsive injectable hydrogel platform presented here is a promising approach for the treatment of ischemic heart disease.
The use of cell therapy to aid in myocardium tissue regeneration has been an area of interest to treat CVDs. One approach is the development of cardiac patches to aid in repair of the infarcted area and ameliorate toxic side effects associated with heart transplants. Dong et al. developed a hydrogel nanocomposite composed of morphology-modified gold nanoparticles embedded in extracellular matrix (ECM)/SF proteins for cell proliferation and expansion of cardiomyocyte.[256] The Au-ECM/SF patches had significant influence on cardiomyocyte differentiation, suggesting the Au nanoparticles help maintain contractability of the engineered cardiac tissue and aided in electrical coupling between cells and the patch. Additionally, the patches confirm antioxidant properties of the ECM/SF matrix that interact with ROS at the infarcted area and allowed the embedded mesenchymal cells to proliferate and grow.[256] Another application for cardiac patches was explored by Zuluaga et al. to develop a PVA/dextran (D) elastic hydrogel for the delivery of the antioxidant astaxanthin to assist against myofibril stress generated after MI.[257] The developed patches were able to encapsulate and maintain antioxidant properties of astaxanthin for 24 h post-injection, and demonstrated high in vivo compatibility, stability, and mechanical strength able to support heart movement.[258] This hydrogel patch has potential to be implanted during heart transplant surgery or in conjunction with other CVD treatment to help decrease myofibril stress generated after MI and reduce the oxidative stress in the ischemic heart.
4.4. Treating Other Chronic Diseases with Hydrogels and Hydrogel Nanocomposites
Over the last few decades, chronic diseases have become a major focus of public health concern. The World Health Organization reported the top 10 causes of death in 2019 accounted for 55% of the 55.4 million deaths worldwide, with noncommunicable or chronic diseases being responsible for 74% of all global deaths.[259] Among those top 10 causes, the world’s major killers are cardiovascular diseases corresponding to 27% of all deaths, followed by chronic respiratory disease (9.3%), Alzheimer’s disease and other dementias (3%), diabetes (2.8%), and kidney diseases (2.6%).[259] All of these diseases have delineable associations with environmental contaminant exposures that influence their mortality, incidence, and severity. Environmental exposures can come from air and water pollution, endocrine-disrupting chemicals, persistent organic pollutants, metals, ultraviolet radiation, personal care products, pesticides, and food packaging amongst others. Hydrogel and hydrogel nanocomposites can mimic the tissue microenvironments because of their hydrophilic and porous network structures, and some also have ability to change their material properties dynamically to mimic naturally occurring scenarios where there are progressive changes in the chemical and/or physical microenvironments.
Neurodegenerative diseases, like Alzheimer’s disease (AD) and dementia, stem from alterations in the structure and function of the central nervous system primarily caused by increases in oxidative stress and neuroinflammation.[260,261] Environmental contaminants related to neurotoxic manifestation are industrial waste, pesticides, automobile exhaust, laboratory waste, and burning of terrestrial waste.[261–264] The lack of effective treatments for neurodegenerative disease represents a major public health issue. Available treatments and diagnostics for AD and other dementias are limited, given the challenges drug carriers must address crossing the blood-brain barrier while also maintaining therapeutic properties.[265] Hydrogels and hydrogel nanocomposites have been shown to effectively target drug delivery to the brain and have potential to be used as therapeutic treatments and diagnostic AD marker detection tools. In recent years, the nasal passage has been the most prominently studied approach for administration of brain drug delivery for treatment and diagnosis of AD.[266] Harthi et al. developed a thiolated chitosan hydrogel with dispersed liposomal donepezil HCl (DH), a drug used to treat dementia related to AD, capable of increasing the mean drug content in the brain by 107% as compared to the oral DH tablets when delivered intranasally.[267] Similarly, Adnet et al. designed and characterized thermoresponsive hydrogels based on poloxamer 407 and/or Poloxamer 188, embedded with a promising active pharmaceutical ingredient (API) for outpatient treatment of AD.[268] This hydrogel nanocomposite presented excellent mucoadhesive properties and its ability to intranasally deliver an API was successfully demonstrated. Taking advantage of the multi stimuli-responsiveness characteristic of some hydrogels, Chen et al. fabricated a timosaponin BII loaded temperature/ion-sensitive in situ hydrogel capable of improving spatial memory and spontaneous behavior on AD model mice upon intranasal administration, thus demonstrating a promising medication for local prevention of AD.[269]
Chronic respiratory diseases (CRDs) are diseases of the airways and other structures of the lung, such as chronic obstructive pulmonary disease, asthma, occupational lung diseases, and pulmonary hypertension.[270] The development and exacerbation of CRDs are greatly influenced by environmental contaminant exposures, specifically air pollution.[271–273] The pathogenesis of CRDs exhibits a diverse spectrum of manifestation including oxidative stress, autophagy, mitochondrial dysfunction, formation of fibrosis and/or emphysema, and significant alterations in the ECM, affecting mechanical support and biochemical cell signals.[274–276] Treatments currently available can only help temporarily alleviate discomfort, while the only long term therapeutic solution currently consists of highly invasive organ transplants. Unfortunately, there is no current treatment option to slow down disease progression, hence the pressing need to develop new and innovative therapies for CRDs. A variety of approaches have emerged in the last decade to tackle this challenging issue and employ hydrogels and hydrogel nanocomposites.[277–280] Schilling et al. have made progress in paranasal delivery of a long-acting corticosteroid drug (mometasone) using a polymer microsphere with reversible sol–gel transition at 34–35 °C, capable of reducing sinonasal inflammation associated with CRDs.[281] Scamskhou et al. demonstrated targeted pulmonary delivery via intranasal application of a hydrogel loaded with interleukin-10 (IL-10), a potent anti-inflammatory and antifibrotic cytokine, to prevent and reverse pulmonary fibrosis.[282] Going beyond the nasal pathway, Shilimzan et al. developed a hydrogel scaffold from gelatin and alginate to be applied via catheter to the lung that serves the dual purpose of remodeling the lung and acting as a platform for tissue regeneration.[283] A different approach to treat CRDs is to harness the ECM microenvironment to develop native human ECM hydrogels as a novel model that mimics the body ECM (resemble shape and electrochemical properties), acting as an ex vivo matrix and allowing for the further development of other technologies.[274,284]
The kidney is particularly vulnerable to water contaminants because of its excretory function and associated high blood flow. Association between environmental contaminants and chronic kidney disease (CKD) is well known for heavy metals like arsenic, cadmium, lead, and mercury, which tend to bioaccumulate and are known to cause CKD.[285–289] Research continues to elucidate synergistic effects between genetics and other environmentalcontaminants(i.e.,industrialchemicals)thatcanincrease the risk for CKD or accelerate progression.[290–294] CKD is characterized by a gradual loss of kidney function over time, and can increase risk of high blood pressure, heart disease, stroke, and premature death.[230,294,295] CKD can also be caused by diabetes and high blood pressure. Effective therapies to reverse or stabilize renal function in individuals with CKD are very limited or nonexistent. Over the last few years, research efforts have been geared toward the therapeutic potential of stem-cell therapy hydrogel delivery systems to optimize targeting efficacy and minimize loss of cell function.[296–299] The most widely studied human cell regenerative therapy focuses on the use of mesenchymal stem cells (MSCs) or endothelial progenitor cells because of their ability to mediate kidney repair through distinct paracrine effects.[296,300–303] As an example, Huling et al. fabricated a scaffold with vascularized mesenchymal cell 1 (MS1) endothelial cell coating and incorporated 3D renal constructs that demonstrated improved vascularization effects, and showed significant renal cell infiltration.[304] Furthermore, the MS-1-coated scaffold was able to enhance tubular structure regeneration after 14 days of incubation, demonstrating the feasibility of cell therapies for regeneration of renal tissues. Ki et al. developed 3D-printed polydopamine (DOPA)-modified bioceramic (BC) scaffolds with a mussel-inspired surface coating in order to evaluate the therapeutic effect of adipose-derived MSCs (Ad-MSCs) and their ability to regulate paracrine function.[305] The DOPA-BC scaffolds cultured with Ad-MSC enhanced vascularization in skin-healing-model rats, thus successfully illustrating the ability of these materials to accelerate tissue regeneration.
The Peppas research group has recently developed a nanoscale pH-responsive cationic hydrogel and evaluated the synthesized materials for cytocompatibility, membrane disruption (hemolysis), therapeutic loading and transfection of siRNA and their physicochemical dependent biological effects.[168] The most influential nanogel property identified to directly correlate to toxicity, hemolytic activity is nanogel pKa. From in vitro studies, it was determined that nanogels with pKa values in the range of 6.5–7.05 are the most promising formulations, producing over 60% cell viability and over 40% GFP expression (used to assess transfection). Finally, the most successful hydrogels for delivery of siRNA, or other therapeutics, exhibited the lowest degrees of cytotoxicity and pH-dependent cell membrane disruption potential, highest siRNA loading, and highest transfection efficacies (Figure 10).[168] This nanogel platform shows great promise as a drug delivery platform for a variety of chronic diseases.
Figure 10.

Optimal range for delivery of siRNA using nanohydrogels. Reproduced with permission.[168] Copyright 2021, Elsevier.
5. Conclusion and Future Perspectives
Lifetime exposure to environmental contaminants is an unavoidable consequence of an industrialized society. Human beings can be exposed to environmental contaminants through four major pathways. 1) Inhalation – major route of entry for vapor, gases, and particulates, where they can be exhaled or further deposited in the respiratory tract and potentially diffuse into the blood, giving rise to systemic effects. 2) Skin (or eye) absorption – dermal contact with exogenous chemicals may directly harm the skin or cross the dermal barrier into the blood, again, generating systemic effects. Exposures through the eye follow this same principle. 3) Ingestion – food/water contaminated with environmental pollutants can damage the GI tract itself or be adsorbed by the GI tract lining and enter the blood, bioaccumulating in specific organs. 4) Injection – refers to chemical exposure via penetration of the skin by contaminated objects that can circulate in the body and bioaccumulate.[306–308] Once in the human body, the chemical can be metabolized, stored (bioaccumulate), or excreted. Given the wide range of environmental contaminants, all with distinct chemical, physical, and biological properties, the specific pathological interactions, inflammation, and contributions to body burden of disease for each species and especially mixtures of these species for the vast majority remain unknown. Pathway elucidation is further challenging when taking into consideration chronic exposures where there are years/decade-long delays between exposure to an environmental contaminant and onset of disease. Although a spectrum of organic and inorganic contaminants have been associated with the development, severity, and mortality of chronic diseases, in many cases indisputable evidence for these effects in human studies is elusive and will remain so as development of additional man-made chemicals continues. Therefore, it is imperative that current development of hydrogel and hydrogel nanocomposite materials for environmental, prophylactic, and disease treatment continue to incorporate more complex scenarios that involve interactions between several chemical pollutants, beneficial species in the human body/environment, and contribute to a better understanding of the human exposome.
Due to the ubiquity of environmental contaminants worldwide, to reduce and limit human exposure, complete environmental remediation remains an ultimate goal.[111] Achieving this goal, however, is cost prohibitive and extremely difficult, given shortcomings of available remediation technologies, their contaminant detection limits, and the multitude of emerging contaminants being detected and associated with chronic diseases worldwide. Consequently, there is a growing need to bolster environmental remediation efforts with risk recognition and chemical assessment from toxicological studies. Together these tools can help inform exposure reduction strategies for disease prevention, amelioration, and treatment. The complex web of disease and environmental contributors requires a multilevel response to design and implement other biologically relevant means of buffering against toxicant exposure before, during, and after remediation activities.
In this review, we have presented three main junctures where the use of hydrogel and hydrogel nanocomposite materials can intervene to positively impact human health: 1) preventing exposures to environmental contaminants; 2) prophylactic treatments to prevent chronic disease initiation; 3) treating diseases after they have developed. The intrinsic properties of hydrogels and hydrogel nanocomposites have made them powerful platforms for removal of environmental contaminants, detoxification and reduction of body burden of disease associated with exogenous chemical exposures, and treatment of exposure associated chronic diseases like cancer, diabetes, and cardiovascular disease. It is important to highlight the versatility and wide-ranging use of hydrogels and hydrogel nanocomposites to improve human health and the broader implications that existing and developing technologies/therapeutic platforms have on overall human health and the potential public health benefits associated from improved health and productivity.
Environmental exposures to individual toxicants or complex mixtures of organic and inorganic environmental contaminants will unfortunately continue due to the large spectrum of harmful chemicals in commercial use and the essential services some of these chemicals provide to our industrialized society. Hence, efforts to limit the human exposure to environmental contaminants, and to prevent, inhibit progression, and treat associated diseases will continue to be an essential focus for public health improvement while also reducing, mental, physical, and economical costs related to chronic disease.
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42ES007380].
Biography

Angela M. Gutierrez received her Ph.D. in chemical and materials engineering from the University of Kentucky in 2019. Her research focused on the development on nanomaterials for the removal of organic pollutants form water systems. She works as postdoctoral scholar at the University of Kentucky. Her research interests now include the development of polymeric flocculants for a variety of environmental applications which led to an NIEHS SBIR phase 1 project where she is the principal investigator. She has been recognized in multiple occasions by NIEHS and was the 2017 recipient of the Karen Wetterhahn Memorial Award.

Erin Molly Frazar received her B.S. degree in chemistry from the University of Texas in 2010 and B.S. degree in chemical engineering from the University of Kentucky in 2017. She is currently a Ph.D. student in the Chemical and Materials Engineering Department at the University of Kentucky. Her current research interests are in the development and assessment of polymeric materials used for PFAS remediation in aqueous environments.

Maria Victoria X. Klaus is a Ph.D. student in the Department of Chemical and Materials Engineering at the University of Kentucky. Her current research focuses on the development of polymeric enterosorbents for removal of environmental contaminants. She earned her B.Sc. in chemical engineering from the University of Arkansas in May 2019. As an undergraduate student she participated in the Research Experience for Undergraduates at the University of Kentucky focusing on graphene-based membranes for environmental treatment.

Pranto Paul is a Ph.D. student in the Department of Chemical and Materials Engineering at the University of Kentucky. His current research focuses on the synthesis and functionalization of magnetic nanoparticles and magnetic nanocomposite hydrogels, as well as their application in virus inactivation and contaminants removal from the environment. He earned his B.Sc. in chemical engineering from Bangladesh University of Engineering and Technology (BUET) in 2019 prior to entering graduate school.

J. Zach Hilt is the Gill Eminent Professor of Chemical Engineering in the Department of Chemical and Materials Engineering at the University of Kentucky. He received his bachelor degrees in chemistry and physics from Miami University (Ohio). He completed his Masters degree in Chemical Engineering from Purdue University and his Doctor of Philosophy in Chemical Engineering from the University of Texas at Austin. His research laboratory focuses on the rational design, synthesis, and application of nanoparticle, polymeric, and nanocomposite materials, and it is particularly interested in designing and applying advanced materials based on magnetic nanoparticles and magnetic hydrogel nanocomposites.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Angela M. Gutierrez, Department of Chemical and Materials Engineering, University of Kentucky, 177 F Paul Anderson Tower, Lexington, KY 40506, USA; Superfund Research Center, University of Kentucky, Lexington, KY 40506, USA
Erin Molly Frazar, Department of Chemical and Materials Engineering, University of Kentucky, 177 F Paul Anderson Tower, Lexington, KY 40506, USA; Superfund Research Center, University of Kentucky, Lexington, KY 40506, USA.
Maria Victoria X. Klaus, Department of Chemical and Materials Engineering, University of Kentucky, 177 F Paul Anderson Tower, Lexington, KY 40506, USA; Superfund Research Center, University of Kentucky, Lexington, KY 40506, USA
Pranto Paul, Department of Chemical and Materials Engineering, University of Kentucky, 177 F Paul Anderson Tower, Lexington, KY 40506, USA; Superfund Research Center, University of Kentucky, Lexington, KY 40506, USA.
J. Zach Hilt, Department of Chemical and Materials Engineering, University of Kentucky, 177 F Paul Anderson Tower, Lexington, KY 40506, USA; Superfund Research Center, University of Kentucky, Lexington, KY 40506, USA.
References
- [1].Brinbaum LS, Toxicol. Lett 2016, 259, S1. [Google Scholar]
- [2].Suk WA, Heacock ML, Trottier BA, Amolegbe SM, Avakian MD, Carlin DJ, Henry HF, Lopez AR, Skalla LA, Rev. Environ. Health 2020, 35, 85. [DOI] [PubMed] [Google Scholar]
- [3].Heacock ML, Amolegbe SM, Skalla LA, Trottier BA, Carlin DJ, Henry HF, Lopez AR, Duncan CG, Lawler CP, Balshaw DM, Suk WA, Rev. Environ. Health 2020, 35, 111. [DOI] [PubMed] [Google Scholar]
- [4].Baccarelli AA, J. Am. Coll. Cardiol 2019, 74, 1330. [DOI] [PubMed] [Google Scholar]
- [5].WHO Global Strategy on Health, Environment and Climate Change, WHO, Geneva: 2020, pp. 1–36. [Google Scholar]
- [6].Prüss-Ustün A, Wolf J, Corvalán C, Neville T, Bos R, Neira M, J. Public Health 2017, 30, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henry HF, Suk WA, Rev. Environ. Health 2017, 32, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].National Environmental Health Partnership Council 2016. The Value of Environmental Health Services: Exploring the Evidence https://apha.org/-/media/files/pdf/topics/environment/eh_values.ashx (accessed: August 2021)
- [9].Grandjean P, Bellanger M, Environ. Health 2017, 10.1186/s12940-017-0340-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Henry H, Carlin D, Heacock H, Trottier B, Suk W Economic impact of environmental health research: A case study of the NIH/NIEHS superfund research program. Abstracts of Papers of the American Chemical Society 2017. Vol. 254 Accession Number: WOS:000429525604818 [Google Scholar]
- [11].D’Surney SJ, Smith MD, Encyclopedia of Toxicology, (Ed: Wexler P), 2nd ed., Elsevier, Amsterdam: 2005, pp. 526–530. [Google Scholar]
- [12].Henry HF, Suk WA, in Karst Groundwater Contamination and Public Health: Beyond Case Studies (Ed: White WB, Herman JS, Herman EK, Rutigliano M), Springer, Berlin: 2018, pp. 7–14. [Google Scholar]
- [13].Norman RE, Carpenter DO, Scott J, Brune MN, Sly PD, Rev. Environ. Health 2013, 28, 59. [DOI] [PubMed] [Google Scholar]
- [14].De Long NE, Holloway AC, Diabetes, Metab. Syndr. Obes.: Targets Ther 2017, 10, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zarean M, Poursafa P, in Primordial Prevention of Non Communicable Disease. Advances in Experimental Medicine and Biology (Ed: R.), Springer, Advances in Experimental Medicine and Biology, 1121 Berlin: 2019, pp. 21–31. [DOI] [PubMed] [Google Scholar]
- [16].Tonne C, Basagaña X, Chaix B, Huynen M, Hystad P, Nawrot TS, Slama R, Vermeulen R, Weuve J, Nieuwenhuijsen M, Environ. Int 2017, 104, 155. [DOI] [PubMed] [Google Scholar]
- [17].Adeola FO, Handbook of Global Health, (Eds: Haring RK, Kickbusch I, Ganten D, Moeti M), Springer, Berlin: 2021, pp. 1–30. [Google Scholar]
- [18].Singh RP, Tripathi RD, Sinha SK, Maheshwari R, Srivastava HS, Chemosphere 1997, 34, 2467. [DOI] [PubMed] [Google Scholar]
- [19].Pourrut B, Shahid M, Dumat C, Winerton P, Pinelli E, Rev. Environ. Contam. Toxicol 2011, 213, 113. [DOI] [PubMed] [Google Scholar]
- [20].Environmental Protection Agency Lead - What are the health effects of lead? https://www.epa.gov/lead/learn-about-lead (accessed: August 2021).
- [21].Altmann L, Weinsberg F, Sveinsson K, Lilienthal H, Wiegand H, Winneke G, Toxicol. Lett 1993, 66, 105. [DOI] [PubMed] [Google Scholar]
- [22].Lin J-L, Lin-Tan D-T, Hsu K-H, Yu C-C, N. Engl. J. Med 2003, 348, 277. [DOI] [PubMed] [Google Scholar]
- [23].Mohammad MT, Cauli O, Environ. Toxicol. Pharmacol 2009, 27, 307. [DOI] [PubMed] [Google Scholar]
- [24].Sears ME, Genuis SJ, J. Environ. Public Health 2012, 2012, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Adv. Mater 2006, 18, 1345. [Google Scholar]
- [26].Ye J, Fu S, Zhou S, Li M, Li K, Sun W, Zhai Y, Eur. Polym. J 2020, 139, 110024. [Google Scholar]
- [27].Gaharwar AK, Peppas NA, Khademhosseini A, Biotechnol. Bioeng 2013, 111, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Richbourg NR, Peppas NA, Prog. Polym. Sci 2020, 105, 101243. [Google Scholar]
- [29].Michalik R, Wandzik I, Polymers 2020, 12, 2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mhinroosta M, Farsangi ZJ, Allahverdi A, Shakoori Z, Mater. Today Chem 2018, 8, 45. [Google Scholar]
- [31].Richbourg NR, Wancura M, Gilchrist AE, Toubbeh S, Harley BAC, Cosgriff-Hernandez E, Peppas NA, Sci. Adv 2021, 7, eabe324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jayakumar A, Jose VK, Lee J-M, Small Methods 2020, 4, 1900735. [Google Scholar]
- [33].Daly AC, Riley L, Segura T, Burdick JA, Nat. Rev. Mater 2020, 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qu B, Luo Y, Int. J. Biol. Macromol 2020, 152, 437. [DOI] [PubMed] [Google Scholar]
- [35].Li J, Jia X, Yin L, Food Rev. Int 2021, 37, 313. [Google Scholar]
- [36].Koetting MC, Peters JT, Steichen SD, Peppas NA, Mater. Sci. Eng.: R: Reports 2015, 93, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Richbourg NR, Ravikumar A, Peppas NA, Macromol. Chem. Phys 2021, 222, 2100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sulaiman NS, Hamzah N, Zakaria SF, Che Othman SF, Mohamed Suffian IF, Nanomedicine 2021, 16, 81. [DOI] [PubMed] [Google Scholar]
- [39].Kaniewska K, Karbarz M, Katz E, Appl. Mater. Today 2020, 20, 100776. [Google Scholar]
- [40].Merino S, Martín C, Kostarelos K, Prato M, Vázquez E, ACS Nano 2015, 9, 4686. [DOI] [PubMed] [Google Scholar]
- [41].Du H, Shi S, Liu W, Teng H, Piao M, Environ. Sci. Pollut. Res 2020, 27, 12967. [DOI] [PubMed] [Google Scholar]
- [42].Hauser AK, Wydra RJ, Stocke NA, Anderson KW, Hilt JZ, J. Controlled Release 2015, 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J, Annu. Rev. Biomed. Eng 2000, 2, 9. [DOI] [PubMed] [Google Scholar]
- [44].Peppas N, Bures P, Leobandung W, Ichikawa H, Eur. J. Pharm. Biopharm 2000, 50, 27. [DOI] [PubMed] [Google Scholar]
- [45].Ahmed EM, J. Adv. Res 2015, 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Caló E, Khutoryanskiy VV, Eur. Polym. J 2015, 65, 252. [Google Scholar]
- [47].Thoniyot P, Tan MJ, Karim AA, James D, Loh XJ, Adv. Sci 2015, 4, 1400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Frazar EM, Shah RA, Dziubla TD, Hilt JZ, J. Appl. Polym. Sci 2020, 137, 48770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Janarthanan G, Noh I, J. Mater. Sci. Technol 2021, 63, 35. [Google Scholar]
- [50].Shah RA, Frazar EM, Hilt JZ, Curr. Opin. Chem. Eng 2020, 30, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tang L, Wang L, Yang X, Feng Y, u Li Y, Feng W, Prog. Mater. Sci 2021, 115, 100702. [Google Scholar]
- [52].Downs FG, Lunn DJ, Booth MJ, Sauer JB, Ramsay WJ, Klemperer RG, Hawker CJ, Bayley H, Nat. Chem 2020, 12, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zarrintaj P, Jouyandeh M, Ganjali MR, Hadavand BS, Mozafari M, Sheiko SS, Vatankhah-Varnoosfaderani M, Gutiérrez TJ, Saeb MR, Eur. Polym. J 2019, 117, 402. [Google Scholar]
- [54].Tang S, Floy M, Bhandari R, Sunkara M, Morris AJ, Dziubla TD, Hilt JZ, ACS Omega 2017, 2, 8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tang S, Floy M, Bhandari R, Dziubla T, Hilt J, Gels 2017, 3, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tang S, Bhandari R, Delaney SP, Munson EJ, Dziubla TD, Hilt JZ, Mater. Today Commun 2017, 10, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Interstate Technology Regulatory Council, Regulations, Guidance, and Advisories for Per- and Polyfluoroalkyl Substances (PFAS) 2018 pp. 1. https://pfas-1.itrcweb.org/fact-sheets/ (accessed: August 2021).
- [58].Ateia M, Arifuzzaman M, Pellizzeri S, Attia MF, Tharayil N, Anker JN, Karanfil T, Water Res 2019, 163, 114874. [DOI] [PubMed] [Google Scholar]
- [59].Huang P-J, Hwangbo M, Chen Z, Liu Y, Kameoka J, Chu K−H, ACS Omega 2018, 3, 17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang Q, Liu N.a, Wei Y, Feng L, Soft Matter 2018, 14, 2649. [DOI] [PubMed] [Google Scholar]
- [61].Xiao S, Davis JT, Chem. Commun 2018, 54, 11300. [DOI] [PubMed] [Google Scholar]
- [62].Su T, Wu L, Zuo G, Pan X, Shi M, Zhang C, Qi X, Dong W, Environ. Res 2020, 182, 109010. [DOI] [PubMed] [Google Scholar]
- [63].Carvalho IC, Medeiros Borsagli FGL, Mansur AAP, Caldeira CL, Haas DJ, Lage AP, Ciminelli VST, Mansur HS, Environ. Technol 2021, 42, 2046. [DOI] [PubMed] [Google Scholar]
- [64].Mei JF, Zhang H, Li Z, Ou H, Carbohydr. Polym 2019, 224, 115154. [DOI] [PubMed] [Google Scholar]
- [65].Midya L, Das R, Bhaumik M, Sarkar T, Maity A, Pal S, J. Colloid Interface Sci 2019, 542, 187. [DOI] [PubMed] [Google Scholar]
- [66].Chen CH, Guo Y, Long L, Chen K, Hu X, Xue Y, Environ. Sci. Pollut. Res 2020, 27, 32762. [DOI] [PubMed] [Google Scholar]
- [67].Godiya CB, Chen X, Deng G, Li D, Lu X, J. Environ. Chem. Eng 2019, 7, 102806. [Google Scholar]
- [68].Hou X, Li Y, Pan Y, Jin Y, Xiao H, Chem. Commun 2018, 54, 13714. [DOI] [PubMed] [Google Scholar]
- [69].Song X, An J, He C, Zhou J, Xu Y, Ji H, Yang L.i, Yin J, Zhao W, Zhao C, J. Mater. Chem. A 2019, 7, 21386. [Google Scholar]
- [70].Sharma G, Thakur B, Naushad Mu., Kumar A, Stadler FJ, Alfadul SM, Mola GT, Environ. Chem. Lett 2018, 16, 113. [Google Scholar]
- [71].Gutierrez AM, Bhandari R, Weng J, Stromberg A, Dziubla TD, Hilt JZ, J. Appl. Polym. Sci 2020, 137, 49109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gutierrez AM, Bhandari R, Weng J, Stromberg A, Dziubla TD, Hilt JZ, Mater. Chem. Phys 2019, 223, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gutierrez AM, Dziubla TD, Hilt JZ, Rev. Environ. Health 2017, 32, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lakes A, Puleo D, Hilt J, Dziubla T, Gels 2018, 4, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhou A, Chen W, Liao L, Xie P, Zhang TC, Wu X, Feng X, Sci. Total Environ 2019, 671, 377. [DOI] [PubMed] [Google Scholar]
- [76].Ohemeng-Boahen G, Sewu DD, Tran HN, Woo SH, Colloids Surf., A 2021, 625, 126911. [Google Scholar]
- [77].Chen MY, Shen Y, Xu L, Xiang G, Ni Z, Colloids Surf., A 2021, 613, 126050. [Google Scholar]
- [78].Umbreen N, Sohni S, Ahmad I, Khattak NU, Gul K, J. Colloid Interface Sci 2018, 527, 356. [DOI] [PubMed] [Google Scholar]
- [79].Cheng SY, Zheng C, Li J, Pan X, Zhai X, Jiao Y, Dong W, Qi X, Carbohydr. Polym 2021, 262, 117951. [DOI] [PubMed] [Google Scholar]
- [80].Yue Y, Wang X, Wu Q, Han J, Jiang J, J. Colloid Interface Sci 2020, 564, 99. [DOI] [PubMed] [Google Scholar]
- [81].Sharma G, Kumar A, Naushad Mu., Thakur B, Vo D-VN, Gao B, Al-Kahtani AA, Stadler FJ, J. Hazard. Mater 2021, 416, 125714. [DOI] [PubMed] [Google Scholar]
- [82].Pandey N, Shukla SK, Singh NB, Nanocomposites 2017, 3, 47. [Google Scholar]
- [83].Badsha MAH, Lo IMC, J. Hazard. Mater 2020, 381, 121000. [DOI] [PubMed] [Google Scholar]
- [84].Moreno-Sader K, García-Padilla A, Realpe A, Acevedo-Morantes M, Soares JBP, ACS Omega 2019, 4, 10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yu K, Wang D.i, Wang Q, Polymers 2018, 10, 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].>E. P. Agency Indicators: Phosphorus, National Aquatic Resource Surveys, https://www.epa.gov/national-aquatic-resource-surveys/indicators-phosphorus (accessed: August 2021).
- [87].Wan J, Zhu C, Hu J, Zhang TC, Richter-Egger D, Feng X, Zhou A, Tao T, Appl. Surf. Sci 2017, 423, 484. [Google Scholar]
- [88].Sanchez LM, Shuttleworth PS, Waiman C, Zanini G, Alvarez VA, Ollier RP, J. Environ. Chem. Eng 2020, 8, 103795. [Google Scholar]
- [89].Yu YR, Zhang G, Ye L, J. Appl. Polym. Sci 2019, 136, 47318. [Google Scholar]
- [90].Kong W, Chang M, Zhang C, Liu X, He B, Ren J, Polymers 2019, 11, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Özkahraman B, Acar I, Emik S, Clean: Soil, Air, Water 2011, 39, 658. [Google Scholar]
- [92].Nikolaev VG, in Biodefence: Advanced Materials and Methods for Health Protection (Eds: Mikhalovsky S, Khajibaev A), Springer, NATO Science for Peace and Security Series A: Chemistry and Biology, Berlin: 2011. pp. 199. [Google Scholar]
- [93].Howell CA, Mikhalovsky SV, Markaryan EN, Khovanov AV, Sci. Rep 2019, 9, 5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Andrusenko SF, Anfinogenova OI, Denisova EV, Entomol. Appl. Sci. Lett 2020, 7, 10. [Google Scholar]
- [95].Yahiya Tunakova A, Fayzullin RI, Valiev VS, Res. J. Pharm. Biol. Chem. Sci 2015, 6, 202. [Google Scholar]
- [96].Uspenskaya MV, Sitnikova VE, Dovbeta MA, Olekhnovich RO, Yu I, Çankaya N, Recent Research in Polmerization, IntechOpen, Rijeka, Croatia: 2018, pp. 95. [Google Scholar]
- [97].Zhu XH, Weng Y, Wang L, Li C, Cheng D, Zhang H, Zhang H, Ind. Eng. Chem. Res 2014, 53, 18508. [Google Scholar]
- [98].Guo H, Chen J, Zhang H, Yao Z, Sheng J, Li Q, Guo Y, Wu C, Xie W, Dai J, Environ. Sci. Technol 2021, 10.1021/acs.est.1c02471. [DOI] [PubMed] [Google Scholar]
- [99].Dale K, Yadetie F, Müller MB, Pampanin DM, Gilabert A, Zhang X, Tairova Z, Haarr A, Lille-Langøy R, Lyche JL, Porte C, Karlsen OA, Goksøyr A, Aquat. Toxicol 2020, 227, 105590. [DOI] [PubMed] [Google Scholar]
- [100].Meyer SK, Probert PME, Lakey AF, Axon AR, Leitch AC, Williams FM, Jowsey PA, Blain PG, Kass GEN, Wright MC, Toxicol. Lett 2017, 273, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Nichifor M, Zhu XX, Baille W, Cristea D, Carpov A, J. Pharm. Sci 2001, 90, 681. [DOI] [PubMed] [Google Scholar]
- [102].Mendonça PV, Matos A, Sousa AF, Serra AC, Simões S, Coelho JFJ, Pharm. Res 2017, 34, 1934. [DOI] [PubMed] [Google Scholar]
- [103].Ai K, Gv P, Vn P, EC Paediatr 2020, 9, 17. [Google Scholar]
- [104].Nichifor M, Cristea D, Carpov A, Int. J. Biol. Macromol 2000, 28, 15. [DOI] [PubMed] [Google Scholar]
- [105].Wang X, Jing S, Qiu X, Zhao S, Liu Y, Tan Y, Chem. Eng. J 2016, 304, 493. [Google Scholar]
- [106].Budnyak TM, Vlasova NN, Golovkova P, Slabon A, Tertykh VA, Colloid Interface Sci. Commun 2019, 32, 100194. [Google Scholar]
- [107].Howell CA, Markaryan E, Allgar V, Kemppinen A, Khovanov A, Pandya P, Arasaradnam A, BMJ Open Gastroenterol 2019, 6, https://bmjopengastro.bmj.com/content/6/1/e000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Howell CA, Markaryan EN, Mikhalovsky SV, Ann. Pediatr. Child Health 2020, 8, 12202. [Google Scholar]
- [109].Tkachenko EI, Avalueva EB, Skazyvaeva EV, Ivanov SV, Pushkina AV, Lapinskii IV, Minerva Gastroenterol. Dietol 2015, 61, 1.25288202 [Google Scholar]
- [110].Kemppinen A, Howell C, Allgar V, Dodd M, Gregson J, Knowles C, Mclaughlin J, Pandya P, Whorwell P, Markaryan E, Yiannakou Y, Trials 2020, 21, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Petriello MC, Newsome BJ, Dziubla TD, Hilt JZ, Bhattacharyya D, Hennig B, Sci. Total Environ 2014, 491, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hennig B, Petriello MC, Gamble MV, Surh Y-J, Kresty LA, Frank N, Rangkadilok N, Ruchirawat M, Suk WA, Rev. Environ. Health 2018, 33, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hoffman JB, Hennig B, Ann. N. Y. Acad. Sci 2017, 1398, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hoffman JB, Petriello MC, Hennig B, Rev. Environ. Health 2017, 32, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Cano-Sancho G, Casas M, Epidemiol J Community Health 2021, 75, 108. [DOI] [PubMed] [Google Scholar]
- [116].Choi BSY, Varin TV, St-Piere P, Pilon G, Tremblay A, MArette A, Food Chem. Toxicol 2020, 146, 111832. [DOI] [PubMed] [Google Scholar]
- [117].Huang T, Zhang Y, Zhang W, Lin T, Chen L, Yang B, Wu L, Yang J, Zhang D, BioMed Res. Int 2020, 2020, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yun KL, Wang ZY, Biomed. Pharmacother 2017, 91, 1122. [DOI] [PubMed] [Google Scholar]
- [119].Chung RTM, Environ. Sci. Pollut. Res 2017, 24, 8946. [DOI] [PubMed] [Google Scholar]
- [120].Chen L, Mo H, Zhao L, Gao W, Wang S, Cromie MM, Lu C, Wang J-S, Shen C-L, J. Nutr. Biochem 2017, 40, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang H, Tsao R, Curr. Opin. Food Sci 2016, 8, 33. [Google Scholar]
- [122].Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, De Cabo R, Sinclair DA, Nature 2006, 444, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lakes AL, Jordan CT, Gupta P, Puleo DA, Hilt JZ, Dziubla TD, Acta Biomater 2018, 68, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gupta P, Lacerda C, Patil VS, Biswal D, Wattamwar P, Zach Hilt J, Dziubla TD, J. Polym. Sci., Part A: Polym. Chem 2017, 55, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Gupta P, Jordan CT, Mitov MI, Butterfield DA, Hilt JZ, Dziubla TD, Int. J. Pharm 2016, 511, 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Jordan Carolyn T., Hilt JZ, Dziubla TD, J. Appl. Polym. Sci 2020, 137, 48647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Patil VS, Gutierrez AM, Sunkara M, Morris AJ, Hilt JZ, Kalika DS, Dziubla TD, J. Nat. Prod 2017, 80, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gupta P, Thompson BL, Wahlang B, Jordan CT, Zach Hilt J, Hennig B, Dziubla T, Drug Delivery Transl. Res 2018, 8, 740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hussain Z, Thu HE, Amjad MW, Hussain F, Ahmed TA, Khan S, Mater. Sci. Eng.: C 2017, 77, 1316. [DOI] [PubMed] [Google Scholar]
- [130].Babaei M, Davoodi J, Dehghan R, Zahiri M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M, J. Drug Delivery Sci. Technol 2020, 59, 101885. [Google Scholar]
- [131].Chen X, Tan B, Wang S, Tang R, Bao Z, Chen G, Chen S, Tang W, Wang Z, Long C, Lu WW, Yang D, Bian L, Peng S, Biomaterials 2021, 274, 120895. [DOI] [PubMed] [Google Scholar]
- [132].Cui Z-K, Kim S, Baljon JJ, Wu BM, Aghaloo T, Lee M, Nat. Commun 2019, 10, 3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Douglas TEL, Pilarz M, Lopez-Heredia M, Brackman G, Schaubroeck D, Balcaen L, Bliznuk V, Dubruel P, Knabe-Ducheyne C, Vanhaecke F, Coenye T, Pamula E, J. Tissue Eng. Regener. Med 2017, 11, 1610. [DOI] [PubMed] [Google Scholar]
- [134].Tan H, Jin D, Qu X, Liu H, Chen X, Yin M, Liu C, Biomaterials 2019, 192, 392. [DOI] [PubMed] [Google Scholar]
- [135].Zhang K, Lin S, Feng Q, Dong C, Yang Y, Li G, Bian L, Acta Biomater 2017, 64, 389. [DOI] [PubMed] [Google Scholar]
- [136].Suttenun N, Punyamoonwongsa P, Macromol. Symp 2015, 354, 273. [Google Scholar]
- [137].Ito F, Usui K, Kawahara D, Suenaga A, Maki T, Kidoaki S, Suzuki H, Taiji M, Itoh M, Hayashizaki Y, Matsuda T, Biomaterials 2010, 31, 58. [DOI] [PubMed] [Google Scholar]
- [138].Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E, J. Biomed. Mater. Res. Part A 2009, 10.1002/jbm.a.32441 [DOI] [PubMed] [Google Scholar]
- [139].Nakamura H, Lee AA, Afshar AS, Watanabe S, Rho E, Razavi S, Suarez A, Lin Y.u-C., Tanigawa M, Huang B, Derose R, Bobb D, Hong W, Gabelli SB, Goutsias J, Inoue T, Nat. Mater 2018, 17, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Huynh CT, Nguyen MK, Tonga GY, Longé L, Rotello VM, Alsberg E, Adv. Healthcare Mater 2016, 5, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bakker MH, Van Rooij E, Dankers PYW, Chem. Asian J 2018, 13, 3501. [DOI] [PubMed] [Google Scholar]
- [142].Conde J, Oliva N, Atilano M, Song HS, Artzi N, Nat. Mater 2016, 15, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hill MC, Nguyen MK, Jeon O, Alsberg E, Adv. Healthcare Mater 2015, 4, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ma Z, Yang C, Song W, Wang Q, Kjems J, Gao S, J. Nanobiotechnol 2014, 12, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Li J, Kooger R, He M, Xiao X, Zheng L.i, Zhang Y, Chem. Commun 2014, 50, 3722. [DOI] [PubMed] [Google Scholar]
- [146].A. C. Society Cancer Facts & Figures 2020, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed: August 2020).
- [147].Morgan KK How Many People Die of Cancer a Year? https://www.webmd.com/cancer/how-many-cancer-deaths-per-year (accessed: August 2020).
- [148].Shankar A, Dubey A, Saini D, Singh M, Prasad CP, Roy S, Bharati SJ, Rinki M, Singh N, Seth T, Khanna M, Sethi N, Kumar S, Sirohi B, Mohan A, Guleria R, Rath GK, Transl. Lung Cancer Res 2019, 8, S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Simonds NI, Ghazarian AA, Pimentel CB, Schully SD, Ellison GL, Gillanders EM, Mechanic LE, Genet. Epidemiol 2016, 40, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhao Y, Yang L, Wu D.i, He H, Wang M, Ge T, Liu Y, Tian H, Cui J, Jia L, Wan Z, Han F, Oncotarget 2016, 7, 76867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Izumi M, Oyanagi J, Sawa K, Fukui M, Ogawa K, Matsumoto Y, Tani Y, Suzumura T, Watanabe T, Kaneda H, Mitsuoka S, Asai K, Ohsawa M, Yamamoto N, Koh Y, Kawaguchi T, Sci. Rep 2021, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ghosh R, Rojanasakul Y, Nanotechnology Characterization Tools for Environment, Health, and Safety (Ed: Kumar C), Springer, Berlin, Heidelberg: 2019. pp. 103. [Google Scholar]
- [153].Melendez Gelvez I, Vargas MJQ, Quijano Parra A, Rev. Int. Contam. Ambiental 2016, 32, 435. [Google Scholar]
- [154].Luanpitpong S, Chen M, Knuckles T, Wen S, Luo J, Ellis E, Hendryx M, Rojanasakul Y, Environ. Sci. Technol 2014, 48, 12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Luanpitpong S, Wang L, Castranova V, Rojanasakul Y, Part. Fibre Toxicol 2014, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Fucic A, Gamulin M, Ferencic Z, Katic J, Krayer Von Krauss M, Bartonova A, Merlo DF, Environ. Health 2012, 11, S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].J. LEWTAS, Mutat. Res./Rev. Mutat. Res 2007, 636, 95. [DOI] [PubMed] [Google Scholar]
- [158].W.H.O. IARC Monographs on the Identification of Carcinogenic Hazards to Humans, https://monographs.iarc.who.int/cards_page/publications-monographs/ (accessed: August 2021).
- [159].American Chemical Society, Known and Probable Human Carcinogens, https://www.cancer.org/cancer/cancer-causes/general-info/known-and-probable-human-carcinogens.html (accessed: August 2021).
- [160].National Cancer Institute, Cancer-Causing Substances in the Environment, https://www.cancer.gov/about-cancer/causes-prevention/risk/substances (accessed: August 2021).
- [161].Fan D-Y, Tian Y, Liu Z-J, Front. Chem 2019, 7, 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Naahidi S, Jafari M, Logan M, Wang Y, Yuan Y, Bae H, Dixon B, Chen P, Biotechnol. Adv 2017, 35, 530. [DOI] [PubMed] [Google Scholar]
- [163].Sun Z, Song C, Wang C, Hu Y, Wu J, Mol. Pharmaceutics 2020, 10.1021/acs.molpharmaceut.9b01020 [DOI] [PubMed] [Google Scholar]
- [164].Sepantafar M, Maheronnaghsh R, Mohammadi H, Radmanesh F, Hasani-Sadrabadi MM, Ebrahimi M, Baharvand H, Trends Biotechnol 2017, 35, 1074. [DOI] [PubMed] [Google Scholar]
- [165].Cirillo C, Nicoletta I, Pharmaceutics 2019, 11, 486. [Google Scholar]
- [166].Tan B, Huang L, Wu Y, Liao J, J. Biomed. Mater. Res., Part A 2021, 109, 404. [DOI] [PubMed] [Google Scholar]
- [167].Ward Deidra M., Shodeinde Aaliyah B., Peppas Nicholas A., Adv. Healthcare Mater 2021, 10, 2100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Spencer DS, Shodeinde AB, Beckman DW, Luu BC, Hodges HR, Peppas NA, J. Controlled Release 2021, 332, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Schoener CA, Hutson HN, Peppas NA, Journal of Biomedical Materials Research Part A 2013, 101A, 2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Stocke NA, Meenach SA, Arnold SM, Mansour HM, Hilt JZ, Int. J. Pharm 2015, 479, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wydra RJ, Kruse AM, Bae Y, Anderson KW, Hilt JZ, Mater. Sci. Eng.: C 2013, 33, 4660. [DOI] [PubMed] [Google Scholar]
- [172].Meenach SA, Shapiro JM, Hilt JZ, Anderson KW, J. Biomater. Sci., Polym. Ed 2013, 24, 1112. [DOI] [PubMed] [Google Scholar]
- [173].Hawkins AM, Bottom CE, Liang Z, Puleo DA, Hilt JZ, Adv. Healthcare Mater 2012, 1, 96. [DOI] [PubMed] [Google Scholar]
- [174].Meenach SA, Otu CG, Anderson KW, Hilt JZ, Int. J. Pharm 2012, 427, 177. [DOI] [PubMed] [Google Scholar]
- [175].Meenach SA, Hilt JZ, Anderson KW, Acta Biomaterialia 2010, 6, 1039. [DOI] [PubMed] [Google Scholar]
- [176].Meenach SA, Hilt JZ, Anderson KW, Acta Biomater 2010, 6, 1039. [DOI] [PubMed] [Google Scholar]
- [177].Hauser AK, Wydra RJ, Stocke NA, Anderson KW, Hilt JZ, J. Controlled Release 2015, 219, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Frimpong RA, Hilt JZ, Nanomedicine 2010, 5, 1401. [DOI] [PubMed] [Google Scholar]
- [179].Satarkar NS, Biswal D, Hilt JZ, Soft Matter 2010, 6, 2364. [Google Scholar]
- [180].Lee SY, Yang M, Seo J-H, Jeong DI, Hwang C, Kim H-J, Lee J, Lee K, Park J, Cho H-J, ACS Appl. Mater. Interfaces 2021, 13, 2189. [DOI] [PubMed] [Google Scholar]
- [181].Hyun H, Park M, Jo G, Kim S, Chun H, Yang D, Marine Drugs 2019, 17, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Niu XY, Zhang ZL, Zhong YH, Mater. Sci. Eng., C 2017, 77, 888. [DOI] [PubMed] [Google Scholar]
- [183].Cardoso F, Bedard PL, Winer EP, Pagani O, Senkus-Konefka E, Fallowfield LJ, Kyriakides S, Costa A, Cufer T, Albain KS, JNCI: J. National Cancer Institute 2009, 101, 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Balakrishnan B, Joshi N, Thorat K, Kaur S, Chandan R, Banerjee R, J. Mater. Chem. B 2019, 7, 2920. [Google Scholar]
- [185].Camacho KM, Kumar S, Menegatti S, Vogus DR, Anselmo AC, Mitragotri S, J. Controlled Release 2015, 210, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Camacho KM, Menegatti S, Mitragotri S, Nanomedicine 2016, 11, 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].de Melo-Diogo D, Costa EC, Alves CG, Lima-Sousa R, Ferreira P, Louro RO, Correia IJ, Eur. J. Pharm. Biopharm 2018, 131, 162. [DOI] [PubMed] [Google Scholar]
- [188].Deb A, Andrews NG, Raghavan V, Int. J. Biol. Macromol 2018, 113, 515. [DOI] [PubMed] [Google Scholar]
- [189].Tang R-Z, Liu Z-Z, Gu S-S, Liu X-Q, J. Mater. Chem. B 2021, 9, 1521. [DOI] [PubMed] [Google Scholar]
- [190].Thoniyot P, Tan MJ, Karim AA, Young DJ, Loh XJ, Adv. Sci 2015, 2, 1400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wu X, Wu Y, Ye H, Yu S, He C, Chen X, J. Controlled Release 2017, 255, 81. [DOI] [PubMed] [Google Scholar]
- [192].Wagner AM, Spencer DS, Peppas NA, J. Appl. Poly. Sci 2018, 135, 46154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Roell KR, Havener TM, Reif DM, Jack J, McLeod HL, Wiltshire T, Motsinger-Reif AA, Frontiers in Genetics 2019, 10, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Kruse AM, Meenach SA, Anderson KW, Hilt JZ, Acta Biomater 2014, 10, 2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Zhou J, Wang M, Han Y, Lai J, Chen J, ACS Appl. Bio Mater 2019, 3, 421. [DOI] [PubMed] [Google Scholar]
- [196].Diabetes, https://www.cdc.gov/diabetes/basics/diabetes.html (accessed: August2021).
- [197].W.H.O. on Diabetes, https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed: August 2021).
- [198].National Diabetes Statistics Report 2020, https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html (accessed: December 2020).
- [199].Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK, Diabetes 2005, 54, S125. [DOI] [PubMed] [Google Scholar]
- [200].Rewers M, Hyöty H, Lernmark Å, Hagopian W, She JX, Schatz D, Ziegler A-G, Toppari J, Akolkar B, Krischer J, Curr. Diabetes Rep 2018, 18, 136. [Google Scholar]
- [201].Sant KE, Annunziato K, Conlin S, Teicher G, Chen P, Venezia O, Downes GB, Park Y, Timme-Laragy AR, Environ. Pollut 2021, 275, 116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Dendup T, Feng X, Clingan S, Astell-Burt T, Int. J. Environ. Res. Public Health 2018, 15, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Javaid A, Akbar I, Javed H, Khan U, Iftikhar H, Zahra D, Rashid F, Ashfaq UA, Crit. Rev. Eukaryotic Gene Expression 2021, 31, 65. [DOI] [PubMed] [Google Scholar]
- [204].Ding Y, Xu T, Mao G, Chen Y, Qiu X, Yang L, Zhao T, Xu X, Feng W, Wu X, Food Chem. Toxicol 2021, 149, 112003. [DOI] [PubMed] [Google Scholar]
- [205].Kolb H, Martin S, BMC Med 2017, 15, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Lee DH, Lind PM, Jacobs DR, Salihovic S, Van Bavel B, Lind L, Diabetes Care 2011, 34, 1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K, Occup. Environ. Med 2018, 75, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA, J. Hazard. Mater 2017, 340, 360. [DOI] [PubMed] [Google Scholar]
- [209].Qi W, Clark JM, Timme-Laragy AR, Park Y, Toxicol. Environ. Chem 2020, 102, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Misra BB, Misra A, Diabetes Metab. Syndr 2020, 14, 23. [DOI] [PubMed] [Google Scholar]
- [211].Reyes-Martínez JE, Ruiz-Pacheco JA, Flores-Valdéz MA, Elsawy MA, Vallejo-Cardona AA, Castillo-Díaz LA, J. Tissue Eng. Regener. Med 2019, 13, 1375. [DOI] [PubMed] [Google Scholar]
- [212].Wang J, Wang Z, Chen G, Wang Y, Ci T, Li H, Liu X, Zhou D, Kahkoska AR, Zhou Z, Meng H, Buse JB, Gu Z, ACS Nano 2021, 15, 4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Maity B, Samanta S, Sarkar S, Alam S, Govindaraju T, ACS Appl. Bio Mater 2020, 3, 3544. [DOI] [PubMed] [Google Scholar]
- [214].Trinh TA, Duy Le TM, Ho HGV, To TCT, Nguyen VVL, Huynh DP, Lee DS, Biomater. Sci 2020, 8, 3830. [DOI] [PubMed] [Google Scholar]
- [215].Wang R, Tian Y, Wang J, Song W, Cong Y, Wei X, Mei Y, Miyatake H, Ito Y, Chen YM, Adv. Funct. Mater 2021, 31, 2104488. [Google Scholar]
- [216].Marek SR, Peppas NA, AIChE J 2013, 59, 3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Koetting MC, Guido JF, Gupta M, Zhang A, Peppas NA, J. Controlled Release 2016, 221, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Caldorera-Moore M, Maass K, Hegab R, Fletcher G, Peppas N, J. Drug Delivery Sci. Technol 2015, 30, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Fukuoka Y, Khafagy ES, Goto T, Kamei N, Takayama K, Peppas NA, Takeda-Morishita M, Biological and Pharmaceutical Bulletin 2018, 41, 811. [DOI] [PubMed] [Google Scholar]
- [220].Steichen S, O’Connor C, Peppas NA, Macromolecular Bioscience 2017, 17, 1600266. [DOI] [PubMed] [Google Scholar]
- [221].Sharpe LA, Daily AM, Horava SD, Peppas NA, Expert Opin. Drug Delivery 2014, 11, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Morishita M, Goto T, Peppas NA, Joseph JI, Torjman MC, Munsick C, Nakamura K, Yamagata T, Takayama K, Lowman AM, J. Controlled Release 2004, 97, 115. [DOI] [PubMed] [Google Scholar]
- [223].Yamagata T, Morishita M, Kavimandan NJ, Nakamura K, Fukuoka Y.u, Takayama K, Peppas NA, J. Controlled Release 2006, 112, 343. [DOI] [PubMed] [Google Scholar]
- [224].Goto T, Morishita M, Kavimandan NJ, Takayama K, Peppas NA, J. Pharm. Sci 2006, 95, 462. [DOI] [PubMed] [Google Scholar]
- [225].Morishita M, Kamei N, Ehara J, Isowa K, Takayama K, J. Controlled Release 2007, 118, 177. [DOI] [PubMed] [Google Scholar]
- [226].Li S, Chen Z, Wang J, Yan L, Chen T, Zeng Q, J. Biomater. Appl 2020, 35, 3. [DOI] [PubMed] [Google Scholar]
- [227].W.H.O. Cardiovascular diseases, https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed: June 2021).
- [228].A. H. Association What is Cardiovascular Disease? https://www.heart.org/en/health-topics/consumer-healthcare/whatis-cardiovascular-disease (accessed: September 2017).
- [229].Heart Disease Facts, https://www.cdc.gov/heartdisease/facts.htm (accessed: August 2021).
- [230].Cosselman KE, Navas-Acien A, Kaufman JD, Nat. Rev. Cardiol 2015, 12, 627. [DOI] [PubMed] [Google Scholar]
- [231].Liao X, Yang X, Deng H, Hao Y, Mao L, Zhang R, Liao W, Yuan M, Front. Bioeng. Biotechnol 2020, 8, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Chaulin A, Duplyakov D, Arch. Euromed 2021, 11, 30. [Google Scholar]
- [233].Cainzos-Achirica M, Fedeli U, Sattar N, Agyemang C, Jenum AK, Mcevoy JW, Murphy JD, Brotons C, Elosua R, Bilal U, Kanaya AM, Kandula NR, Martinez-Amezcua P, Comin-Colet J, Pinto X, Atherosclerosis 2019, 286, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Donat-Vargas C, Bellavia A, Berglund M, Glynn A, Wolk A, Åkesson A, J. Intern. Med 2020, 287, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Donat-Vargas C, Moreno-Franco B, Laclaustra M, Sandoval-Insausti H, Jarauta E, Guallar-Castillon P, Environ. Int 2020, 136, 105433. [DOI] [PubMed] [Google Scholar]
- [236].Mariana M, Cairrao E, J. Cardiovasc. Dev. Dis 2020, 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Perkins JT, Petriello MC, Newsome BJ, Hennig B, Environ. Sci. Pollut. Res 2016, 23, 2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Hoeeg C, Dolatshahi-Pirouz A, Follin B, Gels 2021, 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Trombino S, Curcio F, Cassano R, Curcio M, Cirillo G, Iemma F, Pharmaceutics 2021, 13, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Cattelan G, Guerrero Gerbolés A, Foresti R, Pramstaller PP, Rossini A, Miragoli M, Caffarra Malvezzi C, Front. Bioeng. Biotechnol 2020, 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Massion PB, Feron O, Dessy C, Balligand J-L, Circ. Res 2003, 93, 388. [DOI] [PubMed] [Google Scholar]
- [242].Saraiva RM, Hare JM, Curr. Opin. Cardiol 2006, 21, 221. [DOI] [PubMed] [Google Scholar]
- [243].Krzywonos-Zawadzka A, Franczak A, Olejnik A, Radomski M, Gilmer JF, Sawicki G, Woźniak M, Bil-Lula I, J. Cell. Mol. Med 2019, 23, 2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Vong LB, Bui TQ, Tomita T, Sakamoto H, Hiramatsu Y, Nagasaki Y, Biomaterials 2018, 167, 143. [DOI] [PubMed] [Google Scholar]
- [245].Mancuso A, Barone A, Cristiano MC, Cianflone E, Fresta M, Paolino D, Int. J. Mole. Sci 2020, 21, 7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Brown RD, Ambler SK, Mitchell MD, Long CS, Annu. Rev. Pharmacol. Toxicol 2005, 45, 657. [DOI] [PubMed] [Google Scholar]
- [247].Chen G, Li J, Song M, Wu Z, Zhang W, Wang Z, Gao J, Yang Z, Ou C, Adv. Funct. Mater 2017, 27, 1701798. [Google Scholar]
- [248].Kanazawa T, Kurakami K, Kashima K, Konomi U, Komazawa D, Nakamura K, Matsushima K, Akagi Y, Misawa K, Nishino H, Watanabe Y, Acta Oto-Laryngol 2017, 137, 962. [DOI] [PubMed] [Google Scholar]
- [249].Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, Tanaka S, Yanagi S, Ito-Ihara T, Ikeda T, Murayama T, Teramukai S, Katsura T, Matsubara K, Kawakami K, Yokode M, Shimizu A, Sakata R, Heart Vessels 2016, 31, 713. [DOI] [PubMed] [Google Scholar]
- [250].Z Li, Masumoto H, Jo J-I, Yamazaki K, Ikeda T, Tabata Y, Minatoya K, Gen. Thorac. Cardiovasc. Surg 2018, 66, 641. [DOI] [PubMed] [Google Scholar]
- [251].Si HB, Zeng YI, Lu YR, Cheng JQ, Shen B, Exp. Ther. Med 2017, 13, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Fan C, Shi J, Zhuang Y, Zhang L, Huang L, Yang W, Chen B, Chen Y, Xiao Z, Shen H, Zhao Y, Dai J, Adv. Mater 2019, 31, 1902900. [DOI] [PubMed] [Google Scholar]
- [253].Vartak DG, Gemeinhart RA, J. Drug Targeting 2007, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].van Duijnhoven SMJ, Robillard MS, Nicolay K, Grüll H, J. Nucl Med 2011, 52, 279. [DOI] [PubMed] [Google Scholar]
- [255].Purcell BP, Barlow SC, Perreault PE, Freeburg L, Doviak H, Jacobs J, Hoenes A, Zellars KN, Khakoo AY, Lee T, Burdick JA, Spinale FG, Am. J. Physiol.: Heart Circ. Physiol 2018, 315, H814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Dong Y, Hong M, Dai R, Wu H, Zhu P, Sci. Total Environ 2020, 707, 135976. [DOI] [PubMed] [Google Scholar]
- [257].Zuluaga M, Gregnanin G, Cencetti C, Di Meo C, Gueguen V, Letourneur D, Meddahi-Pellé A, Pavon-Djavid G, Matricardi P, Biomed. Mater 2017, 13, 015020. [DOI] [PubMed] [Google Scholar]
- [258].Shi H, Wang C, Ma Z, APL Bioeng 2021, 5, 011506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].W.H.O., The Top 10 Causes of Death – Fact Sheet,https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed: May 2020).
- [260].Salvi A, Salim S, Front. Neurosci 2019, 13, 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Iqubal A, Ahmed M, Ahmad S, Sahoo CR, Iqubal MK, Haque SE, Environ Sci Pollut Res Int 2020, 27, 41175. [DOI] [PubMed] [Google Scholar]
- [262].Tang BL, Toxics 2020, 8, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Bakulski KM, Seo Y. A.h, Hickman RC, Brandt D, Vadari HS, Hu H, Park SK, J. Alzheimers Dis 2020, 76, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Voorhees JR, Remy MT, Erickson CM, Dutca LM, Brat DJ, Pieper AA, npj Aging Mech. Dis 2019, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Agrawal M, Prathyusha E, Ahmed H, Dubey SK, Kesharwani P, Singhvi G, Naidu VGM, Alexander A, Neurochem. Int 2021, 145, 105008. [DOI] [PubMed] [Google Scholar]
- [266].Agrawal M, Saraf S, Saraf S, Dubey SK, Puri A, Gupta U, Kesharwani P, Ravichandiran V, Kumar P, Naidu VGM, Murty US, Ajazuddin, Alexander A, J. Controlled Release 2020, 327, 235. [DOI] [PubMed] [Google Scholar]
- [267].Al Harthi S, Alavi SE, Radwan MA, El Khatib MM, Alsarra IA, Sci. Rep 2019, 9, 9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Adnet T, Groo AC, Picard C, Davis A, Corvaisier S, Since M, Bounoure F, Rochais C, Le Pluart L, Dallemagne P, Malzert-Fréon A, Pharmaceutics 2020, 12, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Chen W, Li R, Zhu S, Ma J, Pang L, Ma B, Du L, Jin Y, Int. J. Pharm 2020, 578, 119115. [DOI] [PubMed] [Google Scholar]
- [270].W.H.O. Chronic respiratory diseases, https://www.who.int/healthtopics/chronic-respiratory-diseases#tab=tab_1 (accessed: July 2021).
- [271].Kim WJ, Lee CY, Mol. Cell. Toxicol 2017, 13, 251. [Google Scholar]
- [272].Centers for Disease Control and Prevention (CDC) Chronic Obstructive Pulmonary Disease (COPD) https://www.cdc.gov/copd/basics-about.html (accessed: May 2021).
- [273].Ritchie AI, Baker JR, Parekh TM, Allinson JP, Bhatt SP, Donnelly LE, Donaldson GC, Am J. Respir. Crit. Care Med 2021, 204, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].de Hilster RHJ, Sharma PK, Jonker MR, White ES, Gercama EA, Roobeek M, Timens W, Harmsen MC, Hylkema MN, Burgess JK, Am J Physiol Lung Cell Mol Physiol 2020, 318, L698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Elowsson Rendin L, Löfdahl A, Kadefors M, Söderlund Z, Tykesson E, Rolandsson Enes S, Wigén J, Westergren-Thorsson G, Front Pharmacol 2021, 12, 45558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Xu Y, Liu HM, Song L, J. Nanobiotechnol 2020, 18, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Kendre PN, Satav TS, Polym. Bull 2019, 76, 1595. [Google Scholar]
- [278].El-Sherbiny IM, et al. , Functional Hydrogels in Drug Delivery: Key Features and Future Perspectives (Eds: Spizzirri UG, Cirillo G), 1, CRC Press, New York: 2017, pp. 327. [Google Scholar]
- [279].Secret E, Kelly SJ, Crannell KE, Andrew JS, ACS Appl. Mater. Interfaces 2014, 6, 10313. [DOI] [PubMed] [Google Scholar]
- [280].Stocke NA, Arnold SM, Zach Hilt J, J. Drug Delivery Sci. Technol 2015, 29, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Schilling AL, Kulahci Y, Moore J, Wang EW, Lee SE, Little SR, J. Controlled Release 2021, 330, 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Shamskhou EA, Kratochvil MJ, Orcholski ME, Nagy N, Kaber G, Steen E, Balaji S, Yuan K, Keswani S, Danielson B, Gao M, Medina C, Nathan A, Chakraborty A, Bollyky PL, De Jesus Perez VA, Biomaterials 2019, 203, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Shulimzon TR, Giladi S, Zilberman M, Isr. Med. Assoc. J 2020, 22, 736. [PubMed] [Google Scholar]
- [284].Chaudhuri O, Biomater. Sci 2017, 5, 1480. [DOI] [PubMed] [Google Scholar]
- [285].Tsai T-L, Kuo C-C, Pan W-H, Chung Y-T, Chen C-Y, Wu T-N, Wang S-L, Kidney Int 2017, 92, 710. [DOI] [PubMed] [Google Scholar]
- [286].Orr S, Bridges C, Int. J. Mol. Sci 2017, 18, 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Barregard L, Elinder CG, Handbook on the Toxicology of Metals, Vol I and Ii, 4th ed. (Eds: Nordberg GF, Fowler BA, Nordberg M), Elsevier, San Diego, CA: 2015. pp. 333. [Google Scholar]
- [288].Khan H, Singh RD, Tiwari R, Gangopadhyay S, Roy SK, Singh D, Srivastava V, Toxicology 2017, 386, 28. [DOI] [PubMed] [Google Scholar]
- [289].Lentini P, Zanoli L, Granata A, Signorelli SS, Castellino P, Dellaquila R, Mol. Med. Rep 2017, 15, 3413. [DOI] [PubMed] [Google Scholar]
- [290].Tsai H-J, Wu P-Y, Huang J-C, Chen S-C, Int. J. Med. Sci 2021, 18, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Liang Z, Wang Wa., Wang Y, Ma L, Liang C, Li P, Yang C, Wei F, Li S, Zhang L, Environ Int 2021, 156, 106752. [DOI] [PubMed] [Google Scholar]
- [292].Wu CD, Chern YR, Pan WC, Lung SCC, Yao TC, Tsai HJ, Spengler JD, Science of The Total Environment 2020, 723, 137915. [DOI] [PubMed] [Google Scholar]
- [293].Al-Aly Z, Bowe B, Clin. J. Am. Soc. Nephrol 2020, 15, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Kaufman JS, Clin. J. Am. Soc. Nephrol 2020, 15, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Chronic Kidney Disease in the United States 2021 https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html (accessed: June 2021).
- [296].Mcfetridge ML, Del Borgo MP, Aguilar M-I, Ricardo SD, Clin. Sci 2018, 132, 1977. [DOI] [PubMed] [Google Scholar]
- [297].Eirin A, Lerman LO, Hypertension 2021, 78, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Premer C, Schulman IH, Jackson JS, Am. J. Emerg. Med 2021, 46, 572. [DOI] [PubMed] [Google Scholar]
- [299].Tang H, Zhang P, Zeng L, Zhao Y, Xie L, Chen B, Stem Cell Res. Therapy 2021, 12, 409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [300].Zhuang Q, Ma R, Yin Y, Lan T, Yu M, Ming Y, Stem Cells Int 2019, 2019, 8387350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Fan M, Zhang J, Xin H, He X, Zhang X, Frontiers Physiology 2018, 9, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Liu Y, Cui J, Wang H, Hezam K, Zhao X, Huang H, Chen S, Han Z, Han ZC, Guo Z, Li Z, Stem Cell Research & Therapy 2020, 11, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Takayama K, Akita N, Mimura N, Akahira R, Taniguchi Y, Ikeda M, Sakurai F, Ohara O, Morio T, Sekiguchi K, Mizuguchi H, Hepatol Commun 2017, 1, 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Huling J, Min S. i., Kim DS, Ko IK, Atala A, Yoo JJ, Acta Biomaterialia 2019, 95, 328. [DOI] [PubMed] [Google Scholar]
- [305].Li T, Ma H, Ma H, Ma Z, Qiang L, Yang Z, Yang X, Zhou X, Dai K, Wang J, ACS Appl Mater Interfaces 2019, 11, 17134. [DOI] [PubMed] [Google Scholar]
- [306].U.S. Environmental Protection Agency Possible Exposure Pathways During Emergencies, https://www.epa.gov/emergency-response/possible-exposure-pathways-during-emergencies (accessed: August 2021).
- [307].Agency for Toxic Substances and Disease Registry (ATSDR) Exposure Evaluation: Evaluating Exposure Pathways, Public Health Assessment Guidance Manual, 2005, https://www.atsdr.cdc.gov/hac/phamanual/pdfs/phagm_final1-27-05.pdf (accessed: August 2021).
- [308].National Research Council (US) Commission on Engineering and Technical Systems;, National Research Council (US) Commission on Life Sciences, in Strategies to Protect the Health of Deployed U.S. Forces: Detecting, Characterizing, and Documenting Exposures (Ed: McKone TE, Huey BM, Downing E, Duffy LM), National Academic Press, Washington, DC: 2000. [PubMed] [Google Scholar]


