Abstract
Purpose: The role of different circulating lymphocyte subsets, as well as their correlation with clinical characteristics of small cell lung cancer patients have not yet been fully understood. This study aims to evaluate the influence of the fluctuating absolute numbers of lymphocyte subpopulations in peripheral blood of patients with small cell lung cancer. Methods: The absolute counts and percentages of lymphocyte subsets in peripheral blood of 329 patients with small cell lung cancer were retrospectively analyzed. The numbers of CD3+, CD3+CD4+, and CD3+CD8+ T lymphocytes, CD3−CD19+ B lymphocytes, and CD3−CD16+CD56+ NK cells were evaluated by flow cytometry. Their relationship with the patients’ clinical characteristics were statistically evaluated. Results: The CD4/CD8 values derived from the absolute number and percentage of CD3+CD4+ cells divided by CD3+CD8+ cells were identical (1.86 ± 0.99). There was no association between any of the lymphocyte subsets levels and age/sex of the 329 patients with small cell lung cancer. The patients with advanced stage had a reduction in CD3+ and CD3+CD4+ T cell counts and a decreased CD4/CD8 ratio. The levels of CD3+CD4+ T cells, CD3−CD19+ B cells, CD3−CD16+CD56+ NK cells, and CD4/CD8 ratio were associated with advanced tumor-node-metastasis stage. Patients who had undergone radiotherapy were characterized by lymphopenia with lower numbers of CD3+, CD3+CD4+, CD3+CD8+ T lymphocyte, B lymphocyte, NK cell, and CD4/CD8 ratio. The evaluation of individual CD4/CD8 ratio should be combined with other clinical parameters. Conclusions: Patients with small cell lung cancer have altered lymphocyte homeostasis. Lymphopenia was a long-lasting feature of the enrolled patients who were treated with radiotherapy. The available lymphocyte subsets levels might be used to manage the clinical treatment scheme.
Keywords: small cell lung cancer, lymphocyte subsets, immune status, radiotherapy, lymphopenia
Introduction
The immunity of cancer patients is closely associated with prognosis. Therefore, it is necessary to monitor their immune status during clinical treatment. 1 In general, evaluating the number of lymphocyte subpopulation in peripheral blood can be used as an effective method for immune surveillance for cancer patients,2,3 such as lung cancer, 4 breast cancer, 5 colon cancer, 6 and others. 7
Lung cancer is one of the malignancies with the highest morbidity and mortality worldwide, which mainly include small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). SCLC accounts for approximately 15% of the whole pulmonary tumor cases.8,9 The remaining cases are almost all NSCLC.
Most of the studies focus on NSCLC. In 2020, Xia et al retrospectively analyzed the absolute number and percentage of peripheral blood lymphocyte subsets in 172 patients with NSCLC after their surgery or postoperative chemotherapy. They found that the absolute count of CD3+, CD3+CD4+, CD3+CD8+, B and NK cells were better indicators of the patient's immune status than the percentage of each lymphocyte subsets. The high level of baseline absolute CD3+CD4+ cells count contributed to longer progression free survival. 10 Up to now, the information concerning the frequency and the function of circulating lymphocyte subsets in SCLC patients are inadequate. A prospective study conducted by Nakamura et al recruited a small number of SCLC cases. Their results suggest that a decreased CD4/CD8 ratio in SCLC (P = .0062) was an independent prognostic factor associated with poor prognosis. 4 Zhou et al examined the circulating lymphocytes and T lymphocyte subsets in 182 lung cancer cases (including 54 SCLC) before, during, and after radiotherapy. The results show that the lymphocyte levels significantly decreased in these patients. 11 These evidences suggest that the potential of lymphocyte subpopulations in patients with SCLC needs to be further explored.
In this study, we evaluate the clinical implication of the number of lymphocyte subsets including CD3+ T cells, CD3+CD4+ helper/inducer T cells, CD3+CD8+ cytotoxic T cells, CD3−CD19+ B cells, and CD3−CD16+CD56+ natural killer (NK) cells in the peripheral blood of 329 SCLC patients.
Materials and Methods
Patients
329 SCLC inpatients in Jilin Cancer Hospital were retrospective enrolled in this study from April 1, 2017 to December 31, 2017 (Table 1) under the approval of the Jilin Province Cancer Hospital Institutional Review Board (202106-01-01). All participants had complete clinical medical records. The TNM stages were verified according to the version 7 staging criteria released by the Union for International Cancer Control and the American Joint Committee on Cancer (UICC/AJCC) in 2009. 12 Tumor grades were categorized by the 2004 World Health Organization system. The exclusive criteria include surgery less than one month before enrollment, infection, chronic inflammatory disease or autoimmune disease, recently used hormones or immunosuppressant, other medical complications that might affect immune function, other malignant tumor, pregnancy, and lactation. Because of the retrospective nature of the study, patient consent for inclusion was waived.
Table 1.
Demographics of the 329 SCLC Patients.
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| Male | 223 (67.78) |
| Female | 106 (32.22) |
| Age, years | |
| <65 | 222 (67.48) |
| ≥65 | 107 (32.52) |
| Smoking history | |
| Never smokers | 89 (27.05) |
| Current or former smokers | 240 (72.95) |
| TNM Stage | |
| I | 23 (6.99) |
| II | 14 (4.26) |
| III | 105 (31.91) |
| IV | 187 (56.84) |
| Tumor extent | |
| Limited stage | 144 (43.47) |
| Extensive stage | 185 (56.23) |
| Degree of myelosuppression | |
| 0 | 190 (57.75) |
| I | 72 (21.88) |
| II | 34 (10.33) |
| III | 24 (7.29) |
| IV | 9 (2.74) |
| Inflammation | |
| No | 71 (21.58) |
| Pneumonia | 97 (29.48) |
| Obstructive inflammation | 95 (28.88) |
| Streaky inflammation | 33 (10.03) |
| Other inflammation | 33 (10.03) |
| ECOG PS | |
| 0 to 1 | 302 (91.79) |
| 2 to 4 | 27 (8.21) |
| Radiotherapy | |
| Yes | 130 (39.51) |
| No | 199 (60.49) |
ECOG-PS, Eastern Cooperative Oncology Group Performance Status; TNM, tumour node metastasis.
Flow Cytometry
Peripheral blood samples (2 ml/patient) were collected into EDTA-K2 anticoagulative tubes and analyzed within 6 h. Briefly, 100 μl blood was incubated respectively with 10 μl fluorochrome conjugated antibody (anti-CD3, anti-CD4, anti-CD8, anti-CD19, anti-CD16/CD56) and the absolute count microspheres (BD Biosciences, NJ, USA) according to the manufacturer's instruction. The corresponding lymphocytes were discriminated by the intensity of positive CD45 and lower side scatter signals after the erythrocytes were fragmented by BD FACS Lysing Solution (BD Biosciences, NJ, USA). The relative and absolute amount of lymphocyte subsets (CD3+, CD3+CD4+, CD3+CD8+, CD3−CD19+, and CD3−CD16+CD56+) were detected on the FACSCalibur Flow Cytometer (BD Biosciences, NJ, USA) and analyzed with the FlowJo software (Tree Star, Ashland, OR, USA). The formula for calculating the absolute number of the certain cells (cells/ml) = the number of cells counted × the concentration of beads/the number of beads counted.
Statistical Analysis
Data were presented as mean ± standard deviation. Comparison between different groups was executed using one-way analysis of variance (ANOVA), Student t-test or Mann-Whitney U test. The Pearson chi-squared test was adopted to calculate the association of lymphocyte subsets with the patients’ clinicopathological characteristics. The ability of lymphocyte subsets to distinguish subgroups of SCLC patients was assessed with receiver operating characteristic (ROC) curve. Statistical significance was set at P < .05 (2-tailed).
Results
Patients’ Demographics
Patients’ demographics are presented in Table 1. There are a total of 329 SCLC cases including 223 men and 106 women. Their median age is 61 years old (range, 35-81 years old).
Alteration of the Relative and Absolute Numbers of Lymphocyte Subsets in SCLC Patients
We evaluated both the percentage and absolute amount of CD3+, CD3+CD4+, CD3+CD8+, CD3−CD19+, and CD3−CD16+CD56+ cells in all enrolled SCLC patients. The results are presented in Table 2 with the customary reference range in our department (t-test or Mann-Whitney U test). 13 The CD4/CD8 value determined from the absolute number and percentage of CD3+CD4+ T cells divided by CD3+CD8+ T cells were identical (1.86 ± 0.99), which demonstrated that the results obtained by the two methods were consistent.
Table 2.
Absolute Count and Percentage of Lymphocyte Subsets in the Peripheral Blood of SCLC Patients (n = 329, means ± SD).
| Variables | Absolute nos. | P | Normal values (cells/l) | Percentages | P | Normal values (%) | ||
|---|---|---|---|---|---|---|---|---|
| control | SCLC | control | SCLC | |||||
| CD3+ | 1441.05 ± 498.80 | 1386.16 ± 938.61 | .5264 | 955.0 to −2860.0 | 70.39 ± 7.39 | 70.47 ± 10.18 | .9414 | 50.0 to −84.0 |
| CD3+CD4+ | 855.71 ± 316.26 | 844.30 ± 602.59 | .8361 | 550.0 to −1440.0 | 41.83 ± 7.17 | 42.06 ± 10.27 | .8392 | 27.0 to −51.0 |
| CD3+CD8+ | 520.92 ± 219.91 | 516.45 ± 384.61 | .9043 | 320.0 to −1250.0 | 25.56 ± 6.84 | 26.36 ± 9.33 | .4555 | 15.0 to −44.0 |
| CD3−CD19+ | 235.70 ± 114.08 | 209.84 ± 635.95 | .5018 | 90.0 to −560.0 | 11.46 ± 3.42 | 7.24 ± 4.77 | .0000 | 5.0 to −18.0 |
| CD3−CD16+CD56+ | 249.29 ± 124.71 | 344.88 ± 502.57 | .0037 | 150.0 to −1100.0 | 12.44 ± 4.63 | 15.22 ± 8.26 | .0006 | 7.0 to −40.0 |
| CD4/CD8 | 1.80 ± 0.73 | 1.86 ± 0.99 | .5863 | 1.4 to −2.0 | 1.80 ± 0.73 | 1.86 ± 0.99 | .5642 | 1.4 to −2.0 |
The data of the control group were collected from 52 healthy people who voluntarily tested lymphocyte subsets among the population undergoing physical examination at Jilin Cancer Hospital in 2017. CD3+, T lymphocytes; CD3+CD4+, T helper/inducer cells; CD3+CD8+, cytotoxic T cells, CD3−CD19+, B lymphocytes; CD3−CD16+CD56+, natural killer cells.
Clinical Significance of the Number of Lymphocyte Subsets in SCLC Patients
Grouping by age, sex, smoking history, tumor stage, and tumor extent, we used t-test or Mann-Whitney U test to identify the relationship between the clinicopathological features of SCLC patients and their circulating lymphocyte subsets number (Table 3). The results showed that the absolute counts of each lymphocyte subcategory were not correlated with sex and age. The reduction of total T cells was only statistically related to tumor extent. The decreased quantities of CD3+CD4+ cells also notable associated with tumor extent and smoking history. Furthermore, CD4/CD8 ratio was considerably related with tumor stage (I-II vs III-IV), smoking history, and tumor extent. It is noteworthy that with regard to patients’ smoking history, the absolute counts of B lymphocytes and NK cells fluctuated within a very large range.
Table 3.
Correlation between the Absolute Number of Lymphocyte Subsets in SCLC Patients and their Clinicopathological Features.
| Variables | Age (years) | Sex | Stage | Smoking history | Extent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥65 | <65 | P | Male | Female | P | I-II | III- IV | P | Never smokers | smokers | P | Limited | Extensive | P | |
| CD3+ | 1276.50 ± 712.16 | 1439.01 ± 1027.44 | .0965 | 1347.75 ± 996.37 | 1466.97 ± 802.07 | .2462 | 1457.30 ± 657.10 | 1377.14 ± 968.95 | .5138 | 1257.91 ± 753.39 | 1433.72 ± 995.83 | .0879 | 1539.01 ± 1090.58 | 1267.18 ± 783.32 | .0121 |
| CD3+CD4+ | 804.81 ± 471.08 | 863.34 ± 656.78 | .3566 | 804.75 ± 622.67 | 927.50 ± 551.57 | .0842 | 944.07 ± 494.70 | 831.66 ± 614.47 | .2857 | 723.90 ± 501.77 | 888.95 ± 631.05 | .0146 | 980.40 ± 695.88 | 738.37 ± 495.13 | .0005 |
| CD3+CD8+ | 465.85 ± 295.08 | 540.83 ± 419.46 | .0624 | 523.08 ± 421.54 | 502.48 ± 293.43 | .6080 | 461.77 ± 195.43 | 523.37 ± 401.97 | .1257 | 487.85 ± 304.53 | 527.05 ± 410.43 | .3490 | 532.30 ± 398.38 | 504.11 ± 374.17 | .5104 |
| CD3−CD19+ | 211.04 ± 714.93 | 209.26 ± 595.90 | .9822 | 199.65 ± 650.11 | 231.28 ± 607.54 | .6740 | 259.34 ± 303.01 | 203.57 ± 666.45 | .3804 | 145.40 ± 160.60 | 233.74 ± 737.17 | .0815 | 279.98 ± 741.99 | 155.25 ± 535.01 | .0900 |
| CD3−CD16+CD56+ | 316.73 ± 706.59 | 358.45 ± 367.19 | .5666 | 363.42 ± 576.21 | 305.87 ± 291.13 | .2298 | 357.35 ± 211.86 | 343.30 ± 528.32 | .7635 | 328.19 ± 349.77 | 351.07 ± 549.04 | .6559 | 396.99 ± 375.17 | 304.32 ± 580.59 | .0809 |
| CD4/CD8 | 1.97 ± 0.93 | 1.81 ± 1.01 | .1624 | 1.79 ± 1.00 | 2.01 ± 0.95 | .0533 | 2.10 ± 0.69 | 1.83 ± 1.01 | .0385 | 1.67 ± 0.94 | 1.93 ± 1.00 | .0375 | 2.08 ± 1.03 | 1.69 ± 0.92 | .0004 |
CD3+, T lymphocytes; CD3+CD4+, T helper/inducer cells; CD3+CD8+, cytotoxic T cells, CD3−CD19+, B lymphocytes; CD3−CD16+CD56+, natural killer cells.
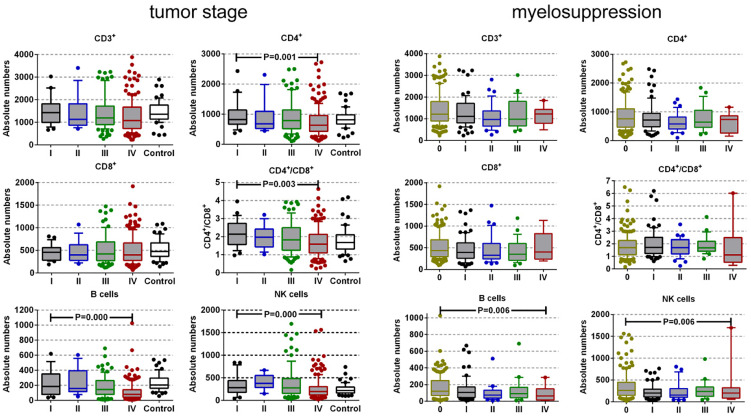
Next, we utilized the Kruskal-Wallis test to further interpret the association between the absolute number of lymphocyte subsets and tumor stage, myelosuppression, and inflammation of the 329 patients (Figure 1). The levels of CD3+CD4+ T cells, CD3−CD19+ B cells, NK cells, and CD4/CD8 ratio were associated with advanced TNM stage (P = .011, P = .000, P = .000, P = .003, respectively). The changes in the number of CD3+ and CD3+CD8+ cells had no substantial difference across I to IV tumor stage (P = .120 and P = .978). The levels of B and NK cells were associated with the degree of myelosuppression in SCLC patients (P = .006 and P = .008). The alteration in the number of CD3+, CD3+CD4+, and CD3+CD8+ T cells, and CD4/CD8 ratio had no significant difference across 0 to IV degree of myelosuppression (P = .072, P = .198, P = .170, and P = .741, respectively). There was no correlation between the variation of lymphocyte subsets and the patients’ infection.
Figure 1.
Absolute numbers of lymphocyte subpopulations and CD4/CD8 values across tumor stage and myelosuppression in SCLC patients. The bar indicates median value; the box represents the (10-90) % range. CD3+, T lymphocytes; CD3+CD4+, T helper/inducer cells; CD3+CD8+, cytotoxic T cells, CD3−CD19+, B lymphocytes; CD3−CD16+CD56+, natural killer cells.
The Effect of Radiotherapy on the Absolute Lymphocyte Subset Counts in SCLC Patients
At the time of blood samples collected, 130/329 SCLC patients had received radiotherapy. We tried to use radiotherapy as a grouping parameter to draw ROC curve to evaluate the stratified capability of lymphocyte subsets in SCLC patients (Figure 2A). The area under the ROC curve (AUC) was 0.692 for CD3+ cells, 0.762 for CD3+CD4+ cells, 0.569 for CD3+CD8+ cells, 0.689 for B cells, 0.599 for NK cells, and 0.734 for CD4/CD8 ratio, respectively. Then we performed a combination set of ROC analysis with all detected lymphocyte parameters by logistic regression. The result showed that the combined detection exhibits a good ability to distinguish SCLC patients with or without radiotherapy (AUC = 0.814). We also plot the ROC curve to calculate the stratified capability of lymphocyte subsets between SCLC and control group (Figure 2B). The results suggested that these parameters could not distinguish SCLC patients and control group. Nevertheless, this may be due to the relative small numbers of the control group. Therefore, we need to expand the sample size for further verification.
Figure 2.
A: ROC curve for the absolute number of lymphocyte subpopulations in SCLC with or without radiotherapy. B: ROC curve for the absolute number of lymphocyte subpopulations in SCLC and control group. CD3+, T lymphocytes; CD3+CD4+, T helper/inducer cells; CD3+CD8+, cytotoxic T cells, CD3−CD19+, B lymphocytes; CD3−CD16+CD56+, natural killer cells; ROC, receiver operating characteristic.
The patients who had undergone radiotherapy had considerably inferior absolute quantities of CD3+ cells, CD3+CD4+ cells, NK cells, and CD4/CD8 value (P = .0000, P = .0000, P = .0324, P = .0000, respectively) compared with those patients unexperienced radiation treatment (Figure 3), which suggested that radiotherapy might contribute to lymphopenia in the SCLC cases in our study. Nevertheless, the counts of CD3+CD8+ cells and B lymphocytes slightly declined after irradiation (P = .0864 and P = .8039) implying that these cells were not sensitive to radiation probably.
Figure 3.
The influence of radiotherapy on the absolute number of lymphocyte subpopulations in patients with SCLC. The patients who experienced radiotherapy (n = 130) are compared with those unexperienced cases (n = 199). The bar indicates median value; the box represents the (10-90) % range. CD3+, T lymphocytes; CD3+CD4+, T helper/inducer cells; CD3+CD8+, cytotoxic T cells, CD3−CD19+, B lymphocytes; CD3−CD16+CD56+, natural killer cells.
Radiotherapy and chemotherapy typically cause myelosuppression, which can further affect the level of lymphocytes. In this study, we utilized the Kruskal-Wallis analysis to assess the association between the absolute number of lymphocytes and the degree of myelosuppression based on whether the 329 SCLC patients received radiotherapy or not. The results were as expected (Figure 4), in the group of 130 patients undergone radiation treatment, the decreased absolute amounts of CD3+ cells, CD3+CD4+ cells, CD3+CD8+ cells, B cells, and NK cells were associated with the degree of myelosuppression (P = .030, P = .023, P = .031, P = .001, P = .007, respectively). By contrast, these parameters were not correlated with the degree of myelosuppression in the group of 199 patients without radiotherapy (P = .131, P = .224, P = .787, P = .118, P = .164, respectively). The ratio of CD4/CD8 also had no statistically difference between the both groups (P = .926 and P = .705).
Figure 4.
Absolute number of lymphocyte subpopulations across the degree of myelosuppression in SCLC patients with or without radiotherapy. The bar indicates median value; the box represents the (10-90) % range. CD3+, T lymphocytes; CD3+CD4+, T helper/inducer cells; CD3+CD8+, cytotoxic T cells, CD3−CD19+, B lymphocytes; CD3−CD16+CD56+, natural killer cells.
The Implication of CD4/CD8 Ratio in SCLC Cases
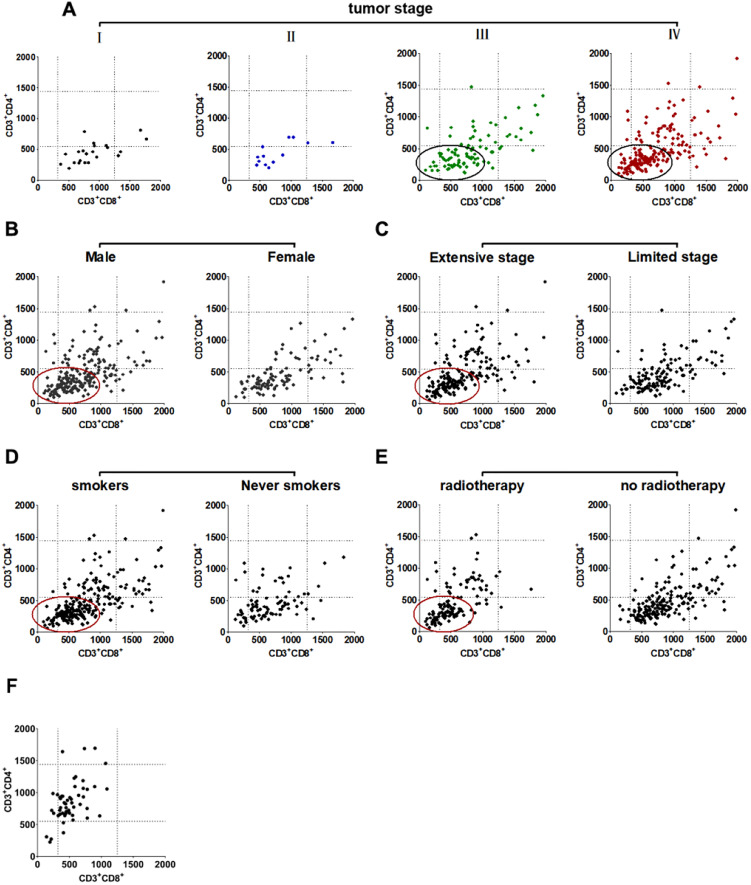
The ratio of CD4/CD8 is commonly used to assess the patient's immune status, as it declines with restrained immune function. However, it should be noted that if the absolute number of CD4+ and CD8+ T cells increases or decreases simultaneously, the identical CD4/CD8 value can be obtained, thus we further dissected the correlation between them in individual patients. The reference parameters included radiotherapy, tumor extent, sex, smoking history, and TNM stage. The results showed that the individual CD4/CD8 ratio of the indicated patients obviously shifts to the lower left quarter in Figure 5.
Figure 5.
Distribution of the individual absolute CD4+ and CD8+ T cells of SCLC patients. (A) tumor stage, (B) sex, (C) tumor extent, (D) smoking history, (E) radiotherapy, (F) control group. The circle depict the prevalent appearance of the corresponding cells. The dotted line represent the normal reference range used in our laboratory. CD3+CD4+, T helper/inducer cells; CD3+CD8+, cytotoxic T cells.
Discussion
Absolute lymphocyte count (ALC) is an independent prognostic factor in patients with hematological neoplasm and solid tumor, especially in lung cancer14–19 Meanwhile, it is conceivable to individually evaluate the relative and absolute counts of lymphocyte subpopulation in cancer patients by using single-platform flow cytometer. 20 Unfortunately, for SCLC patients, little is known about the impact of the alteration of circulating lymphocytes on tumor pathogenesis, progress, and clinical outcome so far.
We retrospectively evaluated the absolute lymphocyte subsets counts in a cohort of 329 SCLC patients. Our results showed that the number of CD3+ and CD3+CD4+ cells, and CD4+/CD8+ ratio were robustly reduced in the patients with extensive stage (n = 185). The levels of NK and B cells also have decreasing trend in these patients, but not reached statistical significance. Furthermore, we found that the levels of CD3+CD4+ cells, B cells, and NK cells, but also CD4/CD8 ratio were associated with advanced TNM status of SCLC patients (Figure 1). The levels of B and NK cells were associated with the degree of myelosuppression in SCLC patients, But the changes in the number of CD3+, CD3+CD4+, and CD3+CD8+ cells, and CD4/CD8 ratio had no significant difference across 0 to IV degree of myelosuppression. Although we know that inflammation can induce the abnormal alteration of lymphocytes, however, in our study, there was no association between the lymphopenia and the patients’ infection. In addition, the interesting result is that the absolute counts of CD3+CD4+ cells and CD4+/CD8+ value were prominently decreased in those patients who never smoke (Table 3). The reason for this bias might be the limited non-smokers were involved in the study (n = 89).
Lymphocytes are sensitive to radiation which can induce notable and long-term disordered immune homeostasis. 3 Although previous studies propose the existence of radiation-induced anti-tumor immunity,21, 22 a literature investigation yields no indication of the association between circulating lymphocytes and radiation reaction of SCLC patients. In this study, an obvious depression of the absolute value of CD3+ cells, CD3+CD4+ cells, CD3+CD8+ cells, NK cells, and CD4/CD8 ratio appeared in patients who experienced radiotherapy (Figure 3). This appearance suggests that radiation has an important impact on lymphocyte equilibrium in patients with SCLC. In cell-mediated antitumor immune response, CD8+ T cells can recognize and destroy tumor cells. CD4+ T cells also have an essential role in regulating the anti-tumor immune response through their interact with other immune cells. 23 In this study, the value of CD4+ lymphocytes nearly halved in patients experienced radiotherapy. By contrast, the level of CD8+ lymphocytes reduced to only 86.94% of no-radiotherapy values. As an effect, the CD4/CD8 ratio decreased dramatically in parallel with radiotherapy. Furthermore, these effector cells, such as CD4+ and CD8+ T lymphocytes, and B lymphocytes, might not be restored to ordinary levels after radiotherapy. 13 We still need longer observation for these SCLC patients. Higher NK cells level indicates improved prognosis in lung cancer patients with less distant metastasis. 24 Our data failed to display any interaction between NK cells level and clinical outcome. Nevertheless, we could speculate that the significant reduction in NK cells in patients with SCLC after radiotherapy is associated with therapeutic efficacy (Figure 4). Collectively, in the course of radiotherapy, the noticeable drop of circulating lymphocyte counts, which represents as a significant disadvantage, may be used to manage the clinical treatment for SCLC patients.
Immunity surveillance for cancer patients is very important in evaluating clinical response and prognosis. There are many studies report the use of a variety of biomarkers to assess the immune situation of patients during treatment, such as Naïve Th cells, Naïve Tc cells, CM (central memory) Tc cells, and Breg cells. 25 For lung cancer patients, the application of target therapy makes the detection of immune indices increasingly important. 26 In addition to lymphocyte subsets, 10 other parameters such as HLA-DR (%) is reported to be a significant predictor of survival for squamous cell carcinoma and SCLC. 4 Programmed death 1 (PD1) expressed by immune cells is related to survival outcome of lung cancer patients who received target therapy. 27 T cell immunoglobulin and mucin-domain containing-3 (Tim-3) expressed on FOXP3+ Treg cells and innate immune cells 28 also may be potential biomarkers for NSCLC progression following immunotherapy. 29 Additionally, absolute neutrophil count (ANC), absolute monocyte count, and absolute eosinophil count (AEC) were significantly and independently associated with both progression-free survival and overall survival for patients receiving nivolumab treatment. 30
At present, there are no uniform standard for determining which parameters or which combination of parameters can guide clinicians to formulate treatment regimes. Detection of lymphocyte subsets by flow cytometry is a convenient, easily measured method which can obtain highly credible and repeatable results. There are other studies focusing on the ratio of Naïve T cells/Memory T cells, Naïve T cells/EM (effector memory) T cells, Naive Th cells/Memory Th cells, EM Th cells/CM Th cells and EM Tc cells/CM Tc cells, 25 however, none of these ratios has been conventionally used in clinical practice. CD4/CD8 ratio is a commonly used indicator, 31 especially when it is combined with the absolute cell number (Figure 5). Our results are consistent with others.4,32 In short, these data suggest that CD4/CD8 ratio can generally reflect the immune status of tumor patients, and can provide auxiliary reference for doctors.
In fact, we also test other immune cells, such as cytotoxic T cells (CD8+CD28+), Treg (CD4+CD25+), and HLA-DR+ T/B cells in some patients, but we have not obtained informative statistical results due to the less number of SCLC patients who voluntarily choose such testing. Undoubtedly, it is believed that with the rapid progress of technology, more sensitive and accurate biomarkers will play an important role in clinical practice in the future. 33
It has to be stressed that this is a retrospective study. The blood drawing time and frequency are variant obviously among patients, therefore the comparison is obviously flawed from the statistical viewpoint. Nonetheless, other studies also reported that lymphocyte counts at diagnosis were able to discriminate OS of multiple myeloma and non-small cell lung cancer (NSCLC) patients irrespective of their treatment.34,35 There are other limitations in our study. First, the sample size of the control group was too small, but consistent with the recent report by Dovsak, T. et al, 13 the mean level of our tested parameters did not go beyond the reference value range adopted by our laboratory. Second, our total sample size is also insufficient and the fraction of advanced SCLC patients is few in number which may cause inappropriate evaluation of the parameters. Third, hematological biomarkers are dynamically changing with the course of the disease. So our results could not reflect lymphocyte kinetics during treatment process. We also cannot assess all possible internal and external influences. Accordingly, we need to conduct a further prospective study to draw a definite conclusion on these points.
Conclusions
This study demonstrated that circulating lymphocyte subsets were mightily correlated with advanced tumor stage and pathologic grade in patients with SCLC. Radiotherapy caused profound immune depression with a drop in the level of T and B lymphocytes, and NK cells. Peripheral blood lymphocyte subsets are convenient, inexpensive, and reliable biomarkers to stratify SCLC severity.
Acknowledgments
We thank Dr Shuang Li for the statistical guidance for this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This paper was supported by the Scientific Research Project of Jilin Provincial Health and Family Planning Commission (2019J077), the Science and Technology Agency of Jilin Provincial Project (20200201518JC, 202002063JC and 20190201246JC).
Approval of Ethic Committee: Jilin Province Cancer Hospital Institutional Review Board (202106-01-01).
References
- 1.Wang W, Hodkinson P, Mclaren F, et al. Small cell lung cancer tumour cells induce regulatory T lymphocytes, and patient survival correlates negatively with FOXP3+ cells in tumour infiltrate. Int J Cancer. 2012;131(6):E928-E937. [DOI] [PubMed] [Google Scholar]
- 2.Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14(21):6770-6779. [DOI] [PubMed] [Google Scholar]
- 3.Kuss I, Hathaway B, Ferris R L, et al. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10(11):3755-3762. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura H, Saji H, Ogata A, et al. Immunologic parameters as significant prognostic factors in lung cancer. Lung Cancer. 2002;37(2):161-169. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez S V, Macfarlane A WT, Jillab M, et al. Immune phenotype of patients with stage IV metastatic inflammatory breast cancer. Breast Cancer Res. 2020;22(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinkiewicz M, Siemińska I, Małczak P, et al. Perioperative changes in lymphocyte subpopulations in patients undergoing surgery for colorectal cancer. Acta Clin Croat. 2019;58(2):337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Xu X, Ding J, et al. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. J Cancer Res Ther. 2018;14(1):40-45. [DOI] [PubMed] [Google Scholar]
- 8.Gazdar A F, Bunn P A, Minna J D. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):725-737. [DOI] [PubMed] [Google Scholar]
- 9.Zang R, Shi J F, Lerut T E, et al. Ten-Year trends of clinicopathologic features and surgical treatment of lung cancer in China. Ann Thorac Surg. 2020;109(2):389-395. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, Li W, Li Y, et al. The clinical value of the changes of peripheral lymphocyte subsets absolute counts in patients with non-small cell lung cancer. Transl Oncol. 2020;13(12):100849-100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou P, Chen L, Yan D, et al. Early variations in lymphocytes and T lymphocyte subsets are associated with radiation pneumonitis in lung cancer patients and experimental mice received thoracic irradiation. Cancer Med. 2020;9(10):3437-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-1474. [DOI] [PubMed] [Google Scholar]
- 13.Dovsak T, Ihan A, Didanovic V, et al. Effect of surgery and radiotherapy on complete blood count, lymphocyte subsets and inflammatory response in patients with advanced oral cancer. BMC Cancer. 2018;18(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho O, Oh Y T, Chun M, et al. Minimum absolute lymphocyte count during radiotherapy as a new prognostic factor for nasopharyngeal cancer. Head Neck. 2016;38(Suppl 1):E1061-E1067. [DOI] [PubMed] [Google Scholar]
- 15.Hu P, Shen H, Wang G, et al. Prognostic significance of systemic inflammation-based lymphocyte- monocyte ratio in patients with lung cancer: based on a large cohort study. PLoS One. 2014;9(9):e108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milne K, Alexander C, Webb J R, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanizaki J, Haratani K, Hayashi H, et al. Peripheral blood biomarkers associated with clinical outcome in Non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13(1):97-105. [DOI] [PubMed] [Google Scholar]
- 18.Karantanos T, Karanika S, Seth B, et al. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: a clinical study. Clin Transl Oncol. 2019;21(2):206-212. [DOI] [PubMed] [Google Scholar]
- 19.Song Q, Wu J-Z, Wang S. Perioperative change in lymphocyte count and prognosis in esophageal squamous cell carcinoma. J Thorac Dis. 2019;11(6):2332-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stankovic B, Bjørhovde HaK, Skarshaug R, et al. Immune cell composition in human Non-small cell lung cancer. Front Immunol. 2019;9:3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaue D, Comin-Anduix B, Ribas A, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14(15):4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitayama J, Yasuda K, Kawai K, et al. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caserta S, Kleczkowska J, Mondino A, et al. Reduced functional avidity promotes central and effector memory CD4T cell responses to tumor-associated antigens. J Immunol. 2010;185(11):6545-6554. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura H, Kawasaki N, Hagiwara M, et al. Cellular immunologic parameters related to age, gender, and stage in lung cancer patients. Lung Cancer. 2000;28(2):139-145. [DOI] [PubMed] [Google Scholar]
- 25.Qiu J, Zhou F, Li X, et al. Changes and clinical significance of detailed peripheral lymphocyte subsets in evaluating the immunity for cancer patients. Cancer Manag Res. 2020;12:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X, Gu L, Heng W. T lymphocytes related biomarkers for predicting immunotherapy efficacy in non-small cell lung cancer. Oncol Lett. 2021;21(2):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzaschi G, Facchinetti F, Missale G, et al. The circulating pool of functionally competent NK and CD8+ cells predicts the outcome of anti-PD1 treatment in advanced NSCLC. Lung Cancer. 2019;127:153-163. [DOI] [PubMed] [Google Scholar]
- 28.Kuchroo V K, Umetsu D T, Dekruyff R H, et al. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol. 2003;3(6):454-462. [DOI] [PubMed] [Google Scholar]
- 29.Su H, Xie H, Dai C, et al. Characterization of TIM-3 expression and its prognostic value in patients with surgically resected lung adenocarcinoma. Lung Cancer. 2018;121:18-24. [DOI] [PubMed] [Google Scholar]
- 30.Tanizaki J, Haratani K, Hayashi H, et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non-Small Cell Lung Cancer Patients Treated with Nivolumab. J Thorac Oncol. 2018;13(1):97-105. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Jiang L, Cao H, et al. Predictive value of CD4(+)/CD8(+) ratio in patients with breast cancer receiving recombinant human thrombopoietin. J Interferon Cytokine Res. 2018;38(5):213-220. [DOI] [PubMed] [Google Scholar]
- 32.Lv Y, Song M, Tian X, et al. Impact of radiotherapy on circulating lymphocyte subsets in patients with esophageal cancer. Medicine (Baltimore). 2020;99(36):e20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa R, Brayer J, Restrepo N, et al. High-Dimensional flow cytometry analysis of regulatory receptors on human T cells, NK cells, and NKT cells. Methods Mol Biol. 2021;2194:255-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ege H, Gertz M A, Markovic S N, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol. 2008;141(6):792-798. [DOI] [PubMed] [Google Scholar]
- 35.Kotsakis A, Koinis F, Katsarou A, et al. Prognostic value of circulating regulatory T cell subsets in untreated non-small cell lung cancer patients. Sci Rep. 2016;6:39247. [DOI] [PMC free article] [PubMed] [Google Scholar]