Abstract
Our knowledge about how low-dose (analgesic) fentanyl affects autonomic cardiovascular regulation is primarily limited to animal experiments. Notably, it is unknown if low-dose fentanyl influences human autonomic cardiovascular responses during painful stimuli in humans. Therefore, we tested the hypothesis that low-dose fentanyl reduces perceived pain and subsequent sympathetic and cardiovascular responses in humans during an experimental noxious stimulus. Twenty-three adults (10 females/13 males; 27 ± 7 yr; 26 ± 3 kg·m−2, means ± SD) completed this randomized, crossover, placebo-controlled trial during two laboratory visits. During each visit, participants completed a cold pressor test (CPT; hand in ∼0.4°C ice bath for 2 min) before and 5 min after drug/placebo administration (75 μg fentanyl or saline). We compared pain perception (100-mm visual analog scale), muscle sympathetic nerve activity (MSNA; microneurography, 11 paired recordings), and beat-to-beat blood pressure (BP; photoplethysmography) between trials (at both pre- and postdrug/placebo timepoints) using paired, two-tailed t tests. Before drug/placebo administration, perceived pain (P = 0.8287), ΔMSNA burst frequency (P = 0.7587), and Δmean BP (P = 0.8649) during the CPT were not different between trials. After the drug/placebo administration, fentanyl attenuated perceived pain (36 vs. 66 mm, P < 0.0001), ΔMSNA burst frequency (9 vs. 17 bursts/min, P = 0.0054), and Δmean BP (7 vs. 13 mmHg, P = 0.0174) during the CPT compared with placebo. Fentanyl-induced reductions in pain perception and Δmean BP were moderately related (r = 0.40, P = 0.0641). These data provide valuable information regarding how low-dose fentanyl reduces autonomic cardiovascular responses during an experimental painful stimulus.
Keywords: algometry, cerebral tissue oxygenation, opioids, respiration, sympathoexcitatory
INTRODUCTION
Pain management is a primary component of medical care (1, 2). For pain medication selection, clinicians consider both the analgesic properties of a drug and the side effects that medication has on vital physiological processes, such as autonomic nervous system control of arterial blood pressure (BP) (3, 4). BP support (i.e., the avoidance of severe hypotension) is critical in the prehospital (i.e., field) setting because advanced cardiovascular support may not be immediately available (1). Currently, various opioid-based medications, fentanyl, morphine, and sufentanil, and ketamine (NMDA antagonist) are used in the prehospital setting to treat pain (2). However, we do not understand how these prehospital analgesics, such as fentanyl (a μ-opioid receptor agonist) (5) affect autonomic cardiovascular regulation during painful stimuli in conscious humans. In other words, the analgesic doses of fentanyl currently employed in the prehospital setting have not been independently studied in awake humans within the context of autonomic cardiovascular regulation at rest and during painful stimuli.
Understanding how low-dose (analgesic) fentanyl affects vital physiological processes, such as autonomic nervous system control of BP (3, 4), would improve clinical risk-benefit analyses for its use in conscious humans. However, our knowledge about the independent effects of low-dose fentanyl lowering sympathetic outflow, BP, and respiration is primarily limited to studies in anesthetized animals (6–16) and anesthetized humans (17–24). Observational studies report how low-dose fentanyl is used in prehospital settings for conscious humans (5, 25, 26), but it is not clear how low-dose fentanyl affects autonomic cardiovascular regulation—particularly in the presence of pain.
Autonomic cardiovascular responses to painful stimuli are dependent on pain perception (2, 27–31). We recently discussed (32), 1) that pain perception is directly related to BP responses during a cold pressor test (CPT) and 2) that BP increases due to higher pain perception are typically mediated via increases in muscle sympathetic nerve activity (MSNA), as reported by us (33) and others (27, 30, 34–39). However, in contrast to point 2, recently we reported that low-dose ketamine—another prehospital analgesic—attenuated BP but not MSNA responses during the CPT (32). Thus, it is unclear to what extent different analgesics alter the relation between pain, MSNA responses, and BP responses normally observed during a painful stimulus. Nonetheless, since fentanyl provides analgesia for moderate pain (40–48), but not severe pain (49), we reasoned that fentanyl-related reductions in pain perception would be accompanied by blunted BP responses during the CPT, an experimental model eliciting mild-to-moderate pain. Notably, the CPT has been demonstrated to be the best experimental pain model in discriminating opioid effects from placebo effects (43). Given that fentanyl-induced alterations in BP are likely dependent on changes in efferent sympathetic outflow (6, 11, 14), we expected that fentanyl would attenuate increases in MSNA, and accompanying increases in BP, during the CPT. To that end, the purpose of this study was to determine to what extent a low dose of fentanyl affects autonomic cardiovascular regulation during a noxious stimulus. Specifically, we tested the hypothesis that low-dose fentanyl attenuates perceived pain, MSNA, and BP responses during the CPT. The results from this work will inform guidelines for the use of fentanyl in both prehospital (2) and hospital-based (49) settings. Moreover, our work will provide fundamental information regarding the effects of low-dose fentanyl on autonomic cardiovascular regulation. Finally, given the absence of human data, we explored whether low-dose fentanyl affected cerebral blood oxygenation during the cold pressor test.
METHODS
Ethical Approval
The United States Army Medical Research & Material Command Human Research Protection Office, Institutional Review Board for Human Subjects Research at the University of Texas Southwestern Medical Center (IRB No. 092017-069), and Texas Health Presbyterian Hospital Dallas approved this protocol. We recruited participants from the Dallas-Fort Worth metroplex. This study conformed with the standards set by the latest revision of the Declaration of Helsinki. Each of the 23 participants provided verbal and written consent before enrollment in the study. The data in this paper are associated with a registered clinical trial (ClinicalTrials.gov Identifier: NCT04136548).
Experimental Overview
This experiment with low-dose fentanyl was a randomized, crossover, placebo-controlled design consisting of two experimental visits separated by at least 48 h. The experimental visits included initial pain assessments (Predrug administration), a drug/placebo administration, a progressive lower-body negative pressure (LBNP) test, a second drug/placebo administration (30 min after the first drug administration), and a second pain assessment (Postdrug administration; see timeline in Fig. 1). The data from this protocol aimed to address two distinct research questions, i.e., the effect of low-dose fentanyl administration on 1) pain perception, sympathetic, and cardiovascular responses during the CPT, from which data are presented herein and 2) tolerance to progressive LBNP along with the integrative sympathetic and cardiovascular responses, which are presented in a companion manuscript (50).
Figure 1.
Experimental protocol timeline. In a randomized crossover fashion, participants completed two experimental trials, on different days, that were identical except during one visit they received two administrations of low-dose fentanyl, and during the other visit, they received two administrations of placebo (saline). Before drug/placebo administration, we collected data during a rest period before participants completed a pressure pain threshold test using algometry and cold pressor test (CPT). Following the second drug/placebo administration, we repeated these assessments. Addressing an unrelated hypothesis, the data from the lower-body negative pressure (LBNP) test are presented in a companion manuscript (50). The primary data of interest for this manuscript are the comparisons between pain assessments made after placebo and fentanyl administration.
Participants
The inclusion criteria for this study included: age between 18 and 45 yr, body mass ≥65 kg, and body mass index <35 kg/m2. We assessed these criteria during the initial screening visit. Study participants were free of any known overt cardiovascular disease and had no evidence of cardiac, neurological, renal, metabolic, or pulmonary disease. Exclusion criteria included current or recent (within the past three years) use of nicotine, prescription-based pain medications, and/or antihypertensive medications. Four participants completed a single placebo trial as a comparative reference for both the present study and a previous investigation using ketamine. Thus, we analyzed the data from these four placebo trials previously (32) and included these data in the present manuscript.
Experimental Protocol
Before the experimental visits, participants were instructed to limit water intake for 2 h; eating for at least 6 h; caffeine for 12 h; strenuous exercise, alcohol, and NSAIDs for 24 h; and over-the-counter cold or allergy medication and aspirin for 36 h before each trial. For female participants, the second experimental visit was completed in the same menstrual cycle phase as the first experimental visit (which was not controlled). Participants arrived at the laboratory and provided a urine sample to confirm 1) a negative urine drug screen, 2) euhydration with a spot urine specific gravity ≤1.025 (51) (Atago Inc., Bellevue, WA), and 3) a negative pregnancy screen for female adults. After an intravenous catheter was placed, participants were instrumented for the measurement of MSNA, brachial BP, beat-to-beat BP, and heart rate. Once these signals were acquired, participants relaxed quietly in the supine position in our temperature-controlled laboratory.
After a 5-min epoch for baseline data collection and blood sample collection, participants completed a pressure pain threshold test and a CPT. Then, over 5 s, we intravenously administered either saline (0.9% NaCl) or 75 μg of fentanyl (fentanyl citrate, Hospira, Inc. Lake Forest, IL). We selected the 75 μg dose of intravenous fentanyl to match the plasma fentanyl concentrations and resultant analgesia attained (42, 44, 52–54) with the 800 μg dose of oral transmucosal fentanyl that is recommended by the United States Army (2), the entity funding this work. A key strength of the intravenous approach was the removal of the confounding effect of between-individual differences in drug absorption, as well as differences in the rate of absorption that occurs depending on the fraction of the oral transmucosal dose that is absorbed through the oral mucosa and the fraction that is swallowed (55, 56). In addition, and importantly, the intravenous route increases participant safety in the research setting given ∼100% bioavailability with intravenous fentanyl administration versus ∼50% with oral fentanyl administration. Finally, providing an absolute dose instead of a dose indexed to body mass increases the ecological validity of our findings, as in the battlefield setting absolute doses are administered (2).
Following this first drug/placebo administration, participants completed a progressive LBNP test, which addressed an unrelated hypothesis with those data presented in a companion paper (50). Because previous work suggests that the plasma concentrations of intravenous fentanyl in the “analgesic range” last less than 30 min (42, 53), we administered a second drug/placebo dose 30 min after the first drug/placebo administration, which is also consistent with the timing of our previous study with low-dose ketamine (32). Thus, we administered either two doses of fentanyl or two doses of saline (placebo) within a given visit. Following this second drug/placebo administration, participants repeated the pain assessments, inclusive of another CPT. For both pain assessments, participants relaxed for 2 min before completing algometer testing (see Pain Assessments) because previous work demonstrated that this would be sufficient to allow plasma fentanyl concentrations to plateau after rising (42). They then relaxed for 2 min before the CPT, at which time another blood sample was collected. Thus, by design, the second CPT started ∼5 min after the drug administration (see timeline in Fig. 1). Participants, but not the researchers or medical personnel, were blind to the experimental conditions.
Pain Assessments
In all 23 participants, the CPT, an experimental noxious stimulus (27, 37–39), was completed by having a laboratory team member submerge the participant’s hand in a stirred ice bath (preplacebo: 0.34 ± 0.05, prefentanyl: 0.34 ± 0.05, postplacebo: 0.35 ± 0.05, postfentanyl: 0.35 ± 0.05°C water temperature; time: P = 0.43, trial: P = 0.95, interaction: P = 0.93; two-way repeated-measures ANOVA) for 2 min. We instructed participants to avoid holding their breath or hyperventilating during the CPT, with compliance monitored through capnography. Immediately after the removal of the hand from the ice bath, we asked participants to rate their pain during the CPT using a 100-mm visual analog scale. The left anchor, at 0 mm, was “no pain or discomfort” and the right anchor, at 100 mm, was “worst imaginable pain or discomfort.” The distance from the left anchor, 0 mm, is reported in results as the pain rating for that CPT.
In 17 participants, we also assessed pressure pain threshold by applying pressure with the tip of a handheld digital algometer (model FPX, Wagner Instruments, Greenwich, CT) just proximal to the webbing of the second and third digit. We instructed participants to “report the first feeling of discomfort, not the most pain that can be tolerated.” We gradually applied force, and the peak force was recorded when the participant first reported a feeling of discomfort. We predetermined that we would stop applying pressure upon reaching 3 kg to limit any potential for injury and would assign that test result a value of 3 kg. We repeated this test three to five times, ∼10 s apart, until we obtained three readings within 10% of one another (57). We report the average of those three values in results.
Muscle Sympathetic Nerve Activity
As previously described (32, 58), we directly recorded MSNA using ultrasound-guided radial microneurography in 11 participants (8 males, 3 females), from which we could obtain adequate MSNA recordings for all assessments in both visits. Briefly, with real-time ultrasound imaging, we inserted a tungsten recording microelectrode into a radial nerve of the upper arm and inserted a reference microelectrode ≤3 cm from the recording electrode. The electrical signal was amplified (80–90,000 times), bandpass filtered (700–2,000 Hz), rectified, and integrated (time constant 0.1 s) using a nerve traffic analyzer (Nerve Traffic Analyzer, model 662c-4; University of Iowa, Bioengineering, Iowa City, IA).
Cardiovascular Measures
We used single-lead ECG to continuously assess heart rate (GE Medical Systems, WI). We used photoplethysmography to assess beat-to-beat BP, as well as Modelflow-derived cardiac output (Finometer; Finapres Medical Systems, The Netherlands) at rest and during the CPT (59). Briefly, Modelflow is a three-element model that uses the arterial input impedance, including continuous correction for estimated variations in the diameter, the compliance of the aorta, and total peripheral resistance, describing the relationship between aortic flow and pressure, and computing valid estimates of stroke volume (60). Thus, Modelflow allows for valid estimates of cardiac output (61). On a beat-to-beat basis, we defined BP waveform peaks as systolic BP, nadirs as diastolic BP, and the average value as mean BP. Total vascular conductance was calculated by dividing cardiac output by finometer-derived mean BP. To assess brachial BP, we used automated auscultatory dimensional K-sound analysis, using a microphone placed over the brachial artery to detect Korotkoff sounds triggered from the ECG signal (Tango M2 Stress Test Monitor, SunTech Medical, SC).
Respiratory and Cerebral Tissue Oxygenation Measures
We measured respiratory rate (n = 15 complete datasets) and the partial pressure of carbon dioxide (n = 12 complete datasets) from expired air using a nasal cannula connected to a capnograph (9004 Capnocheck Plus; Smiths Medical International Ltd, Watford, UK). We also measured cerebral oxygen saturation (n = 16 complete datasets) using near-infrared spectroscopy (Moor Instruments Inc., Wilmington, DE).
Data Analysis
Data were collected at a sampling rate of 625 Hz using Biopac (MP150, Biopac, Santa Barbara, CA). Although blind to condition, an experienced investigator (J.C.W.) (32, 58, 62, 63) visually inspected the sympathetic neurogram on a beat-to-beat basis to determine the presence/absence of MSNA bursts using Ensemble (Elucimed, Wellington, New Zealand). MSNA analysis was conducted per recent guidelines (64) using the following criteria: 1) >3:1 signal-to-noise ratio, 2) burst morphology consistent with MSNA bursts, and 3) a pulse-synchronous signal. MSNA was quantified as burst frequency (bursts·min−1) and burst incidence (bursts·100 heartbeats−1). MSNA burst amplitude and area were not assessed because we could not confirm that the position of the microelectrode was the same for all four CPTs. In other words, MSNA burst amplitude and area varied within participants and between visits given the repeated measures design. For example, we recorded MSNA from opposing arms on separate occasions due to the proximity of experimental trials, coupled with LBNP-related shifts in the neurogram between the repeated CPTs within each visit. Therefore, it would be inappropriate to estimate the MSNA burst amplitude or area for this protocol given that, even with burst amplitude and area normalization, alterations in baseline characteristics of the neurogram have a large influence on the resultant CPT-related changes in burst amplitude and area (64, 65).
We obtained venous blood samples, before drug/placebo administration 1 and approximately 4 min after drug/placebo administration 2, which was just before the second CPT (see Fig. 1). These samples were collected in lithium heparin spray-coated tubes (BD Vacutainer, Oakville, ON, Canada). We stored these tubes on ice until centrifugation (2,000 g for 10 min) less than 60 min later. Finally, we stored plasma samples at −80°C until shipping on dry ice to the laboratory (ARUP Laboratories, Salt Lake City, UT) for high-performance liquid chromatography assessment of plasma epinephrine and norepinephrine concentrations (n = 20 for baseline, n = 18 for postdrug). When plasma epinephrine values were undetectable (laboratory values reported as <10 pg/mL; n = 7 for preplacebo, n = 2 for prefentanyl, n = 2 for postplacebo), we used 9.9 pg/mL for those data points. Removal of these 9.9 pg/mL data points did not meaningfully change the statistical results for either timepoint.
We compared pressure pain threshold via the algometer, and pain perception during the CPT, between trial days before saline/drug administration (i.e., during the “Predrug” assessment in Fig. 1). We also compared absolute cardiovascular and sympathetic measures at rest and during the CPT between trial days before drug/placebo administration. We calculated cardiovascular and sympathetic responses (Δ values) during each CPT by subtracting the values collected during the second minute of the CPT from the values collected during the entire rest period before that CPT. The sample size for preplacebo/fentanyl analyses includes 22 of the 23 total participants because one participant requested an early termination from their prefentanyl CPT (60 s in, pain rating of 80 on the 100-mm visual analog scale). We used ECG-derived heart rate data and finometer-derived hemodynamic data for all comparisons, aside from one prefentanyl trial where we report brachial BP instead of finometer due to an aberrant finometer signal during the CPT. Therefore, our prefentanyl versus preplacebo analyses for Modelflow-derived data (e.g., cardiac output, etc.) will include 21 participants. Separately, and pertinent to testing the hypotheses of this manuscript, we compared the same CPT data between trial days as described earlier within the “Postdrug/Placebo” timepoint in all 23 participants.
Statistical Analysis
The hypotheses in this manuscript address a secondary aim from a larger registered clinical trial (ClinicalTrials.gov Identifier: NCT04136548). Thus, we did not conduct an a priori power analysis for this dataset. We compared spot urine specific gravity values between trials using paired, two-tailed t tests. We compared pain measurements between drug and placebo trials separately within each timepoint (i.e., Predrug/Placebo and Postdrug/Placebo) using paired, two-tailed t tests or the Wilcoxon matched-pairs signed-rank test when data failed (P < 0.05) the Shapiro–Wilk normality test. For all such nonparametric analyses, we report median [interquartile range] instead of means ± SD. We compared plasma catecholamine concentrations between trials separately within each timepoint (i.e., Predrug and Postdrug) using paired, two-tailed t tests. We compared absolute sympathetic and cardiovascular measures at rest and during the CPT between trials separately within each timepoint (i.e., Predrug and Postdrug) using repeated-measures two-way ANOVAs [main effects of perturbation (i.e., CPT) and trial]. We employed Tukey’s multiple comparison testing for post hoc analyses when appropriate. We compared the magnitude of the change in sympathetic and cardiovascular measures during the CPT between trials within each timepoint (i.e., Predrug and Postdrug) using paired, two-tailed t tests. We examined the relation between reductions in pain and mean BP responses after low-dose fentanyl administration using a Pearson correlation. Finally, we used Pearson correlations to examine the relations between changes in perceived pain, changes in MSNA responses, and changes in BP responses during the CPTs conducted within placebo trials (postplacebo CPT minus preplacebo CPT) and within fentanyl trials (postfentanyl CPT minus prefentanyl CPT). We analyzed these data using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA). We report exact P values for all comparisons. We did not create a dichotomous line of significance/nonsignificance (66, 67). However, when P values were below 0.10, we considered that value along with the physiological relevance for each variable to draw conclusions about the result of a given comparison. Lastly, we report effect sizes for the primary data of interest—perceived CPT pain, BP responses during the CPT, and MSNA burst frequency responses during the CPT between postfentanyl and postplacebo trials—to aid with interpretation.
RESULTS
A schematic of the experimental visit timeline is included in Fig. 1. Participant screening characteristics are provided in Table 1. Upon arrival to the laboratory, urine-specific gravity was not different between trials (preplacebo 1.014 ± 0.010 vs. prefentanyl 1.013 ± 0.010; P = 0.6535; paired, two-tailed t test).
Table 1.
Participant screening information
| Characteristic | Means ± SD (Range) |
|---|---|
| Number of participants | 23 (10 F/13 M) |
| Age, yr | 27 ± 7 (20–45) |
| Body mass, kg | 79 ± 11 (65–110) |
| Body mass index, kg·m−2 | 26 ± 3 (22–34) |
| Systolic BP, mmHg | 121 ± 10 (102–136) |
| Diastolic BP, mmHg | 76 ± 10 (60–94) |
We present data as means ± SD with ranges. BP, blood pressure; F, female; M, male.
Predrug Administration Measures
Plasma epinephrine concentrations (preplacebo 17 [10–27] vs. prefentanyl 16 [10–28] pg/mL, P = 0.9294; Wilcoxon matched-pairs signed-rank test) and norepinephrine concentrations (preplacebo 220 ± 91 vs. prefentanyl 210 ± 82 pg/mL, P = 0.4826; paired, two-tailed t test) were not different between trials. Pressure pain threshold was not different between trials (preplacebo 1.1 [1.0–1.5] vs. prefentanyl 1.2 [0.9–1.4] kg, P = 0.3983; Wilcoxon matched-pairs signed-rank test).
Perceived pain during the CPT was not different between trials (preplacebo 70 [47–80] vs. prefentanyl 66 [56–80] mm, P = 0.8287; Wilcoxon matched-pairs signed-rank test). MSNA burst frequency increased from rest to CPT and was not different between trials (Rest: preplacebo 12 ± 8 vs. prefentanyl 13 ± 6; CPT: preplacebo 29 ± 12 vs. prefentanyl 28 ± 10 bursts·min−1; perturbation: P < 0.0001, trial: P = 0.9803, interaction: P = 0.7587; two-way repeated-measures ANOVA). Mean BP increased from rest to CPT and was not different between trials (Rest: preplacebo 93 ± 9 vs. prefentanyl 93 ± 11; CPT: preplacebo 107 ± 11 vs. prefentanyl 107 ± 12 mmHg; perturbation: P < 0.0001, trial: P = 0.8956, interaction: P = 0.9344; two-way repeated-measures ANOVA). Increases in MSNA burst frequency (preplacebo Δ: 16 ± 10 vs. prefentanyl 15 ± 8 bursts·min−1, P = 0.7587; paired, two-tailed t test) and mean BP (preplacebo Δ: 14 [9–23] vs. prefentanyl 14 [7–20] mmHg, P = 0.8649; Wilcoxon matched-pairs signed-rank test) during the CPT were not different between trials. Similarly, changes in MSNA burst incidence, systolic BP, diastolic BP, heart rate, cardiac output, and total vascular conductance during the CPT did not differ between trials (P ≥ 0.2348 for all Predrug data, data not shown).
Respiratory rate was not different between rest and CPT or between trials (Rest: preplacebo 14 ± 4 vs. prefentanyl 15 ± 4; CPT: preplacebo 14 ± 5 vs. prefentanyl 12 ± 3 breaths·min−1; perturbation: P = 0.3165, trial: P = 0.3094, interaction: P = 0.0137; P ≥ 0.1219 for all post hoc comparisons; two-way repeated measures ANOVA). End-tidal carbon dioxide was lower during the CPT and was not different between trials (Rest: preplacebo 46 ± 6 vs. prefentanyl 46 ± 4; CPT: preplacebo 43 ± 6 vs. prefentanyl 41 ± 6 mmHg; perturbation: P = 0.0015, trial: P = 0.8199, interaction: P = 0.1759; two-way repeated-measures ANOVA). Cerebral tissue oxygenation (Rest: preplacebo 58 ± 13 vs. prefentanyl 59 ± 14; CPT: preplacebo 59 ± 12 vs. prefentanyl 59 ± 16%; perturbation: P = 0.5598, trial: P = 0.9806, interaction: P = 0.3254; two-way repeated-measures ANOVA) was not different between rest and CPT or between trials.
Postdrug Administration Pain Assessments
Pressure pain threshold was higher after fentanyl compared with placebo administration (postfentanyl 1.6 [0.9–2.2] vs. postplacebo 1.1 [0.8–1.3] kg; P = 0.0110; Wilcoxon matched-pairs signed-rank test). None of these participants reached our predetermined upper limit of 3 kg before, or after, fentanyl/placebo administration. Pain perception during the CPT was lower (Fig. 2; Cohen’s d = 1.48) after fentanyl compared with placebo administration. None of the participants reported “0” as their CPT pain rating before, or after, fentanyl/placebo administration.
Figure 2.
Pain perception during the postdrug/placebo administration cold pressor test (CPT). Perceived pain during the CPT was lower after low-dose fentanyl compared with placebo administration. We present data as means ± SD with individual responses (n = 23). We compared data using a paired, two-tailed t test.
Postdrug Administration Sympathetic Measures
We present representative BP and MSNA tracings before and during the CPT from one participant after placebo and fentanyl administrations in Fig. 3. MSNA burst frequency and burst incidence values increased from rest to the CPT in both trials (Fig. 4, A and C). The magnitude of the increases in MSNA burst frequency (Cohen’s d = 1.50) and burst incidence during the CPT were attenuated following fentanyl compared with placebo trials (Fig. 4, B and D). Plasma epinephrine concentrations (postplacebo 27 [16–36] vs. postfentanyl 68 [57–110] pg/mL, P < 0.0001; Wilcoxon matched-pairs signed-rank test), but not norepinephrine concentrations (postplacebo 229 ± 98 vs. postfentanyl 223 ± 79 pg/mL, P = 0.8313; paired, two-tailed t test), were higher after fentanyl compared with placebo administration.
Figure 3.
Representative blood pressure and sympathetic tracings during the postdrug/placebo administration cold pressor test. We present representative mean blood pressure (BP) and muscle sympathetic nerve activity (MSNA) tracings during the cold pressor test from one participant after a placebo (left) and low-dose fentanyl (right) administration. The x-axis line break indicates the onset of the 2-min cold pressor test.
Figure 4.
Muscle sympathetic nerve activity (MSNA) responses during the postdrug/placebo administration cold pressor test (CPT). MSNA burst frequency and burst incidence values increased from rest to the CPT in both trials (A and C). The increases in MSNA burst frequency (Cohen’s d = 1.50) and burst incidence during the CPT were attenuated for fentanyl compared with placebo trials (B and D). We present data as means ± SD with individual responses (n = 11). For A and C, we compared data using repeated-measures two-way ANOVAs (main effects of rest/CPT and trial). We employed Tukey’s multiple comparison testing for post hoc analyses when appropriate. For B and D, we compared data using paired, two-tailed t tests.
Postdrug Administration Cardiovascular Measures
Systolic, but not mean or diastolic, BP values were lower during rest after fentanyl compared with placebo administration. In addition, systolic, mean, and diastolic BP increased from rest to CPT in both trials (Fig. 5, A, C, and E). Increases in systolic BP during the CPT were 6 mmHg lower on average after fentanyl compared with placebo administration (Cohen’s d = 0.60, Fig. 5B). Increases in mean (Cohen’s d = 0.68) and diastolic (Cohen’s d = 0.66) BP during the CPT were also attenuated after fentanyl compared with placebo administration (Fig. 5, D and F).
Figure 5.
Blood pressure (BP) responses during the postdrug/placebo administration cold pressor test (CPT). Systolic, but not mean or diastolic, BP values were lower during rest after fentanyl compared with placebo administration and increased from rest to CPT in both trials (A, C, and E). Increases in systolic BP during the CPT were 6 mmHg lower on average for fentanyl compared with placebo trials (Cohen’s d = 0.60, B). Increases in mean (Cohen’s d = 0.68) and diastolic (Cohen’s d = 0.66) BP during the CPT were attenuated for fentanyl compared with placebo trials (D and F). We present data as means ± SD with individual responses (n = 23). For A, C, and E, we compared data using repeated-measures two-way ANOVAs (main effects of rest/CPT and trial). We employed Tukey’s multiple comparison testing for post hoc analyses when appropriate. For B, D, and F, we compared data using paired, two-tailed t tests.
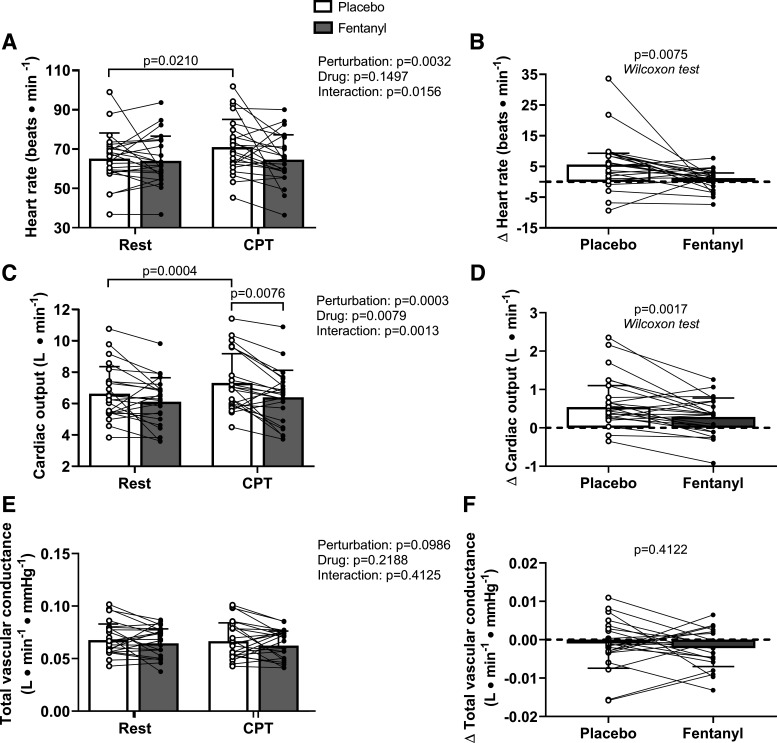
Heart rate and cardiac output, but not total vascular conductance, were higher during the CPT compared with rest (Fig. 6, A, C, and E). In addition, cardiac output was lower postfentanyl compared with postplacebo during the CPT (Fig. 6C). The increases in heart rate and cardiac output, but not total vascular conductance, during the CPT were attenuated after fentanyl compared with placebo administration (Fig. 6, B, D, and F).
Figure 6.
Hemodynamic responses during the postdrug/placebo administration cold pressor test (CPT). Heart rate and cardiac output, but not total vascular conductance, were higher during CPT compared with rest (A, C, and E). In addition, cardiac output was lower for fentanyl compared with placebo during the CPT (C). The increases in heart rate and cardiac output, but not total vascular conductance, during the CPT were attenuated for fentanyl compared with placebo (B, D, and F). For A, C, and E, we compared data using repeated-measures two-way ANOVAs (main effects of rest/CPT and trial). We employed Tukey’s multiple comparison testing for post hoc analyses when appropriate. We present these data as means ± SD with individual responses. For B and D, we compared data using Wilcoxon match-pairs signed-rank tests and present data as medians with 75% interquartile range bars and individual responses. For F, we compared data using a paired, two-tailed t test and are presented as means ± SD with individual responses. n = 23 for all comparisons.
Postdrug Administration Respiratory and Cerebral Tissue Oxygen Saturation Measures
Respiratory rate was lower during the CPT compared with rest in both trials (Rest: postplacebo 18 ± 3 vs. postfentanyl 17 ± 4; CPT: postplacebo 14 ± 4 vs. postfentanyl 12 ± 4 breaths·min−1; perturbation: P = 0.0001, trial: P = 0.1132, interaction: P = 0.4901; two-way repeated-measures ANOVA). End-tidal carbon dioxide was 5 mmHg higher on average during rest (post hoc P = 0.0754) and 8 mmHg higher during the CPT (post hoc P = 0.0115) during the postfentanyl compared with postplacebo condition (Rest: postplacebo 42 ± 7 vs. postfentanyl 47 ± 6; CPT: postplacebo 39 ± 8 vs. postfentanyl 47 ± 8 mmHg; perturbation: P = 0.0607, trial: P = 0.0047, interaction: P = 0.0969; two-way repeated measures ANOVA). Cerebral tissue oxygenation was not different from rest to CPT and was not different between trials (Rest: postplacebo 59 ± 14 vs. postfentanyl 64 ± 10; CPT: postplacebo 58 ± 12 vs. postfentanyl 63 ± 11%; perturbation: P = 0.3049, trial: P = 0.0946, interaction: P = 0.9979; two-way repeated-measures ANOVA).
Interplay between Pain Perception, MSNA Responses, and BP Responses during the CPT
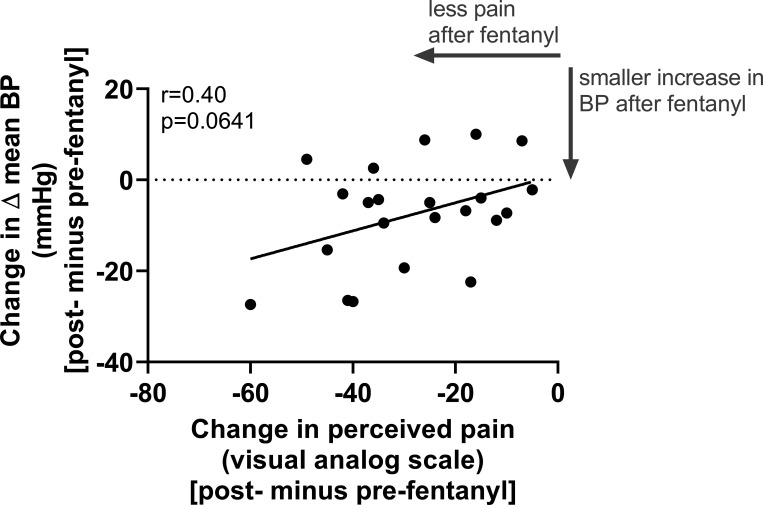
Between the preplacebo and postplacebo CPTs, the changes in pain perception and changes in ΔMSNA were not meaningfully related (r < 0.01, P = 0.9896), the changes in ΔMSNA burst frequency and changes in Δmean BP were moderately inversely related (r = −0.65, P = 0.0316), and the changes in pain perception and changes in Δmean BP were not meaningfully related (r = −0.10, P = 0.6557). Between the prefentanyl and postfentanyl CPTs, the changes in pain perception and changes in ΔMSNA were weakly related (r = 0.25, P = 0.4602) and the changes in ΔMSNA burst frequency and changes in Δmean BP were weakly related (r = 0.33, P = 0.3155). Finally, we identified a moderate relation between reductions in pain perception and attenuations in Δmean BP after fentanyl administration (postfentanyl CPT minus prefentanyl CPT; Fig. 7).
Figure 7.
Relation between fentanyl-induced reductions in perceived pain and attenuated increases in mean blood pressure (BP) during the cold pressor test. We identified a moderate relation between reductions in pain perception and attenuations in the increases in mean BP after low-dose fentanyl administration (postfentanyl minus prefentanyl). We conducted Pearson’s correlations and present individual data for 22 paired datasets within fentanyl trials.
DISCUSSION
Our findings provide new information for the independent effects of low-dose fentanyl administration on autonomic cardiovascular regulation during the CPT in conscious humans. The primary findings of this study are that during the CPT, low-dose fentanyl 1) reduced pain perception, 2) attenuated increases in MSNA burst frequency, and 3) attenuated increases in systolic, mean, and diastolic BP. In addition, we identified a moderate relation between reductions in pain perception and attenuations in the increases in mean BP after low-dose fentanyl administration. Separately, we found that the administration of low-dose fentanyl, compared with placebo, increased pressure pain threshold. Together, these data suggest that low-dose fentanyl effectively reduces pain perception during two different acute experimental pain stimuli and that low-dose fentanyl attenuates MSNA and BP responses during the CPT in conscious humans.
Resting Cardiovascular Effects
In the resting state, previous studies in humans have reported that fentanyl reduces resting heart rate, cardiac output, and BP (17, 22, 68, 69). Such effects may be due to a downward resetting of the carotid baroreflex mean BP and heart rate operating points (70). Others have reported that low-dose fentanyl (1.00 vs. ∼0.95 μg of fentanyl per kg of body mass used in this study) reduces the low-frequency power and low-frequency power-to-high-frequency power ratio of heart rate variability during both spontaneous and paced breathing in nonanesthetized adults (71). Such findings suggest that fentanyl may elicit vagal activation of the autonomic nervous system at low doses. Related to the autonomic nervous system, we found that low-dose fentanyl did not affect resting MSNA burst frequency, though fentanyl attenuated increases in MSNA burst frequency during an acute sympathoexcitatory stimulus (i.e., the CPT). We speculate that this attenuation in sympathetic responsiveness was more likely due to reductions in pain perception during the CPT rather than a direct sympatholytic effect of the drug. Nonetheless, additional studies are necessary to determine to what extent low-dose fentanyl elicits sympatholytic effects, which such investigations should include longer epochs without perturbations.
Respiratory Effects
We found that fentanyl administration raised end-tidal carbon dioxide by 5 mmHg during rest, and by 8 mmHg during the CPT, both compared with placebo administration. Our finding of low-dose fentanyl not affecting respiratory rate is consistent with another previous study reporting no changes in respiratory rate after ∼0.71 μg of fentanyl per kg of body mass among healthy young male adults (72). Previous work has demonstrated that opioid medications, such as fentanyl, elicit respiratory depression—i.e., reduce minute ventilation or respiratory rate × tidal volume (53, 73, 74). The observed increases in end-tidal carbon dioxide could be due to fentanyl-induced hypoventilation (i.e., reductions in minute ventilation), which is likely given the observed reductions in respiratory rate. However, we did not assess tidal volume in the present study, therefore, we could not determine if fentanyl affected minute ventilation. Future work is necessary to fill this gap in knowledge with clinical importance. Finally, low-dose fentanyl did not affect cerebral tissue oxygen saturation at rest or during the CPT. This information may be useful to a practitioner’s risks/benefit analysis for using low-dose fentanyl.
Analgesic Effects
Our finding that a 75 μg intravenous fentanyl administration (∼1.0 μg per kg of body mass) reduces pain perception during the CPT is consistent with other studies using lower (∼0.4–0.7 μg of intranasal fentanyl per kg of body mass; Ref. 40) and similar (1.0–1.4 μg of intravenous fentanyl per kg of body mass; Refs. 45, 46, 48) doses of fentanyl. Our finding of fentanyl increasing pressure pain threshold is consistent with another study targeting fentanyl administration to achieve plasma fentanyl concentrations of 0.80 and 1.20 ng/mL (44); i.e., fentanyl concentrations that would be expected after the recommended 800 μg dose of oral transmucosal fentanyl (2) and after the 75 μg dose administered in the present work (53–56). Taken together, our findings confirm previous results regarding the analgesic effects of fentanyl when studied using absolute dosing that is provided in the field setting (5). These findings complement observational studies supporting fentanyl’s use in real-world settings.
Cardiovascular and Sympathetic Nervous System Responses during Sympathoexcitatory Stimuli
Perceived pain during noxious stimuli is associated with sympathetically mediated (30, 36–38) increases in BP (27, 39). The response to the CPT, a somatosensory reflex, stimulates pain- and temperature-sensitive free nerve endings in the skin and activates the spinal anterolateral system of ascending tracts (36). In animals, fentanyl reduces BP responses during certain acute sympathoexcitatory stimuli (75, 76). Furthermore, previous reports in anesthetized animals demonstrated that actions of fentanyl on the cardiovascular system are dependent on sympathetic outflow (6, 11, 14). In humans, it is unknown how synthetic opioid agonists (e.g., fentanyl) affect autonomic cardiovascular responses during a painful stimulus such as the CPT. Nonetheless, we summarize previous work with opioid receptor antagonists below to discuss what is known regarding the interplay between endogenous opioids and cardiovascular systems during the CPT. For example, low-dose (0.075 mg/kg body mass) and high-dose (0.15 mg/kg body mass) naloxone (an opioid receptor antagonist) does not affect heart rate, MSNA, or mean BP responses during the CPT compared with predrug administration (77). In addition, there may be an interplay between endogenous opioid activity and BP responses during the CPT. However, this notion is limited to interpreting the effects of naltrexone administration on such responses (78, 79). Together, these data only can inform us on how opioid receptor antagonists affect CPT responses and not how opioid agonists (e.g., fentanyl) affect CPT responses. Another pair of studies found low doses of fentanyl (range 25–50 μg) to attenuate mean BP responses during cycling, but not handgrip, exercise (68, 69), which is relevant because exercise also evokes sympathetically mediated increases in BP. Previous work also demonstrated that CPT pain intensity is reduced 24 h after transdermal fentanyl administration (41) in a dose-dependent manner (43). These studies helped to inform our hypothesis that low-dose fentanyl will attenuate perceived pain, MSNA burst frequency, and BP responses during the CPT in conscious humans.
In support of our hypothesis, we found that low-dose fentanyl attenuated perceived pain, MSNA burst frequency responses, and BP responses during the CPT. Thus, our data extend the knowledge base about how low-dose fentanyl affects the integrated responses during painful stimuli to include directly recorded MSNA responses. Importantly, these findings have high relevance to doses administered in real-world settings, such as prehospital (e.g., battlefield) settings (2). Understanding how low-dose fentanyl affects cardiovascular variables in the presence of pain (e.g., during the CPT) may help to guide its use, particularly in field settings where advanced cardiorespiratory support may not be immediately available for use (1).
Interestingly, with an identical experimental design, we recently demonstrated that perceived pain and BP responses, but not MSNA responses, were attenuated during the CPT following administration of low-dose ketamine compared with placebo trials (32). Moreover, the reductions in pain perception were weakly and inversely related (r = −0.27) to the attenuations in mean BP increases during the CPT, which was in opposition to our prior hypothesis, as we expected that reductions in perceived pain would be directly related to reductions in mean BP increases. Thus, while it is tempting to assume that all analgesics currently employed in prehospital settings (i.e., ketamine, fentanyl, and morphine) similarly reduce the pain-associated BP and MSNA increases, it is critical to independently study each drug to understand what mechanisms may be at play. Here, we identified a moderate relation between reductions in pain perception and attenuations in the increases in mean BP after low-dose fentanyl administration (postfentanyl CPT minus prefentanyl CPT, r = 0.40, P = 0.0641; see Fig. 7). Given the strong relation between perceived pain and mean BP responses during the CPT in the absence of analgesic agents (33), future studies are warranted to better understand the interplay between pain and cardiovascular responses following administration of low-dose fentanyl with the potential inclusion of a wider array of noxious stimuli.
Limitations
Although our study contributes new knowledge related to how low-dose fentanyl alters pain perception, sympathetic, and cardiovascular responses during an acute experimental pain stimulus, there are several limitations to mention. First, we present sympathetic and cardiovascular responses during an experimental pain stimulus following intravenous, and not oral, fentanyl administration, the latter of which is recommended for prehospital use (2). As discussed in methods, we chose the intravenous administration route to remove confoundment from variations in the bioavailability of fentanyl that occurs via the oral administration route. Thus, the present dataset does not allow us to conclude how oral (or intranasal, intramuscular, etc.) fentanyl might affect autonomic cardiovascular function with pain, especially severe forms of pain (and concomitant fear and/or anxiety) associated with real-world trauma. Of note, there are well-founded ethical concerns rightfully preventing the use of extremely painful stimuli for investigative purposes, and recent data suggest this dose of fentanyl is inadequate for the treatment of such severe acute pain (49). Thus, despite the aforementioned dosing challenges, future studies using the oral administration route may be warranted to confirm the observations in our present study. Second, using a nearly identical protocol to our prior work with ketamine (32), the experimental design of the present investigation included a progressive LBNP test between the pre- and postdrug administration pain assessments (Fig. 1), and relies on the repeatability of responses during the CPT. For brevity, we direct the reader to our previous work that includes an extended discussion of these limitations and their potential implications (32). Taken together, future studies are warranted to confirm our findings and to fill the aforementioned knowledge gaps.
Perspectives and Significance
Consistent with previous literature, we observed reductions in resting systolic BP following low-dose (analgesic) fentanyl administration. Our current data support that fentanyl, in similar doses similar as those used in the prehospital setting, reduces pain perception. Importantly, these data are the first to demonstrate that low-dose fentanyl administration attenuates increases in BP and MSNA burst frequency during a painful stimulus (e.g., the CPT). Finally, we identified a moderate relation between reductions in pain perception and attenuations in the increases in mean BP after low-dose fentanyl administration. The results from this work will inform guidelines for the use of fentanyl in both prehospital and hospital settings, as well as inform future investigations for this drug.
DATA AVAILABILITY
Data are available upon reasonable request to the principal investigator after institutional data transfer agreement approvals.
GRANTS
This research was supported by the Department of Defense—United States Army Grant W81XWH1820012 (to C. G. Crandall), National Institutes of Health (NIH) Grant F32HL154559 (to J. C. Watso), NIH Grant F32HL154565 (to L. N. Belval), and by the American Physiological Society Postdoctoral Fellowship (to J. C. Watso).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.W., M.H., J.M.H., C.H.-L., and C.G.C. conceived and designed research; J.C.W., M.H., L.N.B., F.A.C., C.P.J., J.M.H., and C.G.C. performed experiments; J.C.W., M.H., F.A.C., C.P.J., and C.G.C. analyzed data; J.C.W., M.H., L.N.B., F.A.C., C.P.J., J.M.H., C.H.-L., and C.G.C. interpreted results of experiments; J.C.W. prepared figures; J.C.W. drafted manuscript; J.C.W., M.H., L.N.B., F.A.C., C.P.J., J.M.H., C.H.-L., and C.G.C. edited and revised manuscript; J.C.W., M.H., L.N.B., F.A.C., C.P.J., J.M.H., C.H.-L., and C.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all study volunteers for participation. We thank graduate student Mads Fischer and research nurses Ileana Hill, Margot Morris, and Naomi Kennedy for contributions to this project. We performed experiments in the Thermal and Vascular Physiology Research Laboratory within the Institute of Exercise and Environmental Medicine of Texas Health Presbyterian Hospital Dallas.
REFERENCES
- 1.Gausche-Hill M, Brown KM, Oliver ZJ, Sasson C, Dayan PS, Eschmann NM, Weik TS, Lawner BJ, Sahni R, Falck-Ytter Y, Wright JL, Todd K, Lang ES. An evidence-based guideline for prehospital analgesia in trauma. Prehosp Emerg Care 18: 25–34, 2014. doi: 10.3109/10903127.2013.844873. [DOI] [PubMed] [Google Scholar]
- 2.Butler FK, Kotwal RS, Buckenmaier CC, Edgar EP, O'Connor KC, Montgomery HR, Shackelford SA, Gandy JV, Wedmore IS, Timby JW, Gross KR, Bailey JA. A triple-option analgesia plan for tactical combat casualty care: TCCC guidelines change 13-04. J Spec Oper Med 14: 13–25, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst 6: 293–302, 1982. doi: 10.1016/0165-1838(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 4.Joyner MJ, Charkoudian N, Wallin GB. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56: 10–16, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauer SG, Naylor JF, Maddry JK, Hinojosa-Laborde C, April MD. Trends in prehospital analgesia administration by US forces from 2007 through 2016. Prehosp Emerg Care 23: 271–276, 2019. doi: 10.1080/10903127.2018.1489022. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Whitwam JG. Effects of intravenous fentanyl on spontaneous renal sympathetic nerve activity in normal and vagotomized rabbits. Chin Med Sci J 19: 282–285, 2004. [PubMed] [Google Scholar]
- 7.Caffrey JL. Enkephalin inhibits vagal control of heart rate, contractile force and coronary blood flow in the canine heart in vivo. J Auton Nerv Syst 76: 75–82, 1999. doi: 10.1016/s0165-1838(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 8.White DA, Reitan JA, Kien ND, Thorup SJ. Decrease in vascular resistance in the isolated canine hindlimb after graded doses of alfentanil, fentanyl, and sufentanil. Anesth Analg 71: 29–34, 1990. doi: 10.1213/00000539-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Thämer V, Deussen A, Schipke JD, Tölle T, Heusch G. Pain and myocardial ischemia: the role of sympathetic activation. Basic Res Cardiol 85, Suppl 1: 253–266, 1990. doi: 10.1007/978-3-662-11038-6_21. [DOI] [PubMed] [Google Scholar]
- 10.Ohsumi H, Sakamoto M, Yamazaki T, Okumura F. Effects of fentanyl on carotid sinus baroreflex control of circulation in rabbits. Am J Physiol Regul Integr Comp Physiol 256: R625–R631, 1989. doi: 10.1152/ajpregu.1989.256.3.R625. [DOI] [PubMed] [Google Scholar]
- 11.Gautret B, Schmitt H. Multiple sites for the cardiovascular actions of fentanyl in rats. J Cardiovasc Pharmacol 7: 649–652, 1985. doi: 10.1097/00005344-198507000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Flacke JW, Flacke WE, Bloor BC, Olewine S. Effects of fentanyl, naloxone, and clonidine on hemodynamics and plasma catecholamine levels in dogs. Anesth Analg 62: 305–313, 1983. [PubMed] [Google Scholar]
- 13.McPherson RW, Traystman RJ. Fentanyl and cerebral vascular responsivity in dogs. Anesthesiology 60: 180–186, 1984. doi: 10.1097/00000542-198403000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Flacke JW, Davis LJ, Flacke WE, Bloor BC, Van Etten AP. Effects of fentanyl and diazepam in dogs deprived of autonomic tone. Anesth Analg 64: 1053–1059, 1985. [PubMed] [Google Scholar]
- 15.Xiang L, Calderon AS, Klemcke HG, Scott LL, Hinojosa-Laborde C, Ryan KL. Fentanyl impairs but ketamine preserves the microcirculatory response to hemorrhage. J Trauma Acute Care Surg 89: S93–S99, 2020. doi: 10.1097/TA.0000000000002604. [DOI] [PubMed] [Google Scholar]
- 16.Taneyama C, Goto H, Kohno N, Benson KT, Sasao J, Arakawa K. Effects of fentanyl, diazepam, and the combination of both on arterial baroreflex and sympathetic nerve activity in intact and baro-denervated dogs. Anesth Analg 77: 44–48, 1993. doi: 10.1213/00000539-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Schmittner MD, Vajkoczy SL, Horn P, Bertsch T, Quintel M, Vajkoczy P, Muench E. Effects of fentanyl and S (+)-ketamine on cerebral hemodynamics, gastrointestinal motility, and need of vasopressors in patients with intracranial pathologies: a pilot study. J Neurosurg Anesthesiol 19: 257–262, 2007. doi: 10.1097/ANA.0b013e31811f3feb. [DOI] [PubMed] [Google Scholar]
- 18.Pacentine GG, Muzi M, Ebert TJ. Effects of fentanyl on sympathetic activation associated with the administration of desflurane. Anesthesiology 82: 823–831, 1995. doi: 10.1097/00000542-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Mayer M, Ochmann O, Doenicke A, Angster R, Suttmann H. The effect of propofol-ketamine anesthesia on hemodynamics and analgesia in comparison with propofol-fentanyl. Anaesthesist 39: 609–616, 1990. [PubMed] [Google Scholar]
- 20.Burgos LG, Ebert TJ, Asiddao C, Turner LA, Pattison CZ, Wang-Cheng R, Kampine JP. Increased intraoperative cardiovascular morbidity in diabetics with autonomic neuropathy. Anesthesiology 70: 591–597, 1989. doi: 10.1097/00000542-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Stanley TH, Webster LR. Anesthetic requirements and cardiovascular effects of fentanyl-oxygen and fentanyl-diazepam-oxygen anesthesia in man. Anesth Analg 57: 411–416, 1978. doi: 10.1213/00000539-197807000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Patschke D, Brückner JB, Eberlein HJ, Hess W, Tarnow J, Weymar A. Effects of althesin, etomidate and fentanyl on haemodynamics and myocardial oxygen consumption in man. Can Anaesth Soc J 24: 57–69, 1977. doi: 10.1007/BF03006813. [DOI] [PubMed] [Google Scholar]
- 23.Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth 18: 24–28, 2006. doi: 10.1016/j.jclinane.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Ebert TJ, Kotrly KJ, Madsen KE, Bernstein JS, Kampine JP. Fentanyl-diazepam anesthesia with or without N2O does not attenuate cardiopulmonary baroreflex-mediated vasoconstrictor responses to controlled hypovolemia in humans. Anesth Analg 67: 548–554, 1988. [PubMed] [Google Scholar]
- 25.Shackelford SA, Fowler M, Schultz K, Summers A, Galvagno SM, Gross KR, Mabry RL, Bailey JA, Kotwal RS, Butler FK. Prehospital pain medication use by US Forces in Afghanistan. Mil Med 180: 304–309, 2015. doi: 10.7205/MILMED-D-14-00257. [DOI] [PubMed] [Google Scholar]
- 26.Petz LN, Tyner S, Barnard E, Ervin A, Mora A, Clifford J, Fowler M, Vs. B. Prehospital and en route analgesic use in the combat setting: a prospectively designed, multicenter, observational study. Mil Med 180: 14–18, 2015. doi: 10.7205/MILMED-D-14-00383. [DOI] [PubMed] [Google Scholar]
- 27.Peckerman A, Hurwitz BE, Saab PG, Llabre MM, McCabe PM, Schneiderman N. Stimulus dimensions of the cold pressor test and the associated patterns of cardiovascular response. Psychophysiology 31: 282–290, 1994. doi: 10.1111/j.1469-8986.1994.tb02217.x. [DOI] [PubMed] [Google Scholar]
- 28.Metzler-Wilson K, Vrable A, Schaub A, Schmale TK, Rodimel BV, Krause BA, Wilson TE. Effect of suboccipital release on pain perception and autonomic reflex responses to ischemic and cold pain. Pain Med 21: 3024–3033, 2020. doi: 10.1093/pm/pnaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stancák A, Yamamotová A, Kulls IP, Sekyra IV. Cardiovascular adjustments and pain during repeated cold pressor test. Clin Auton Res 6: 83–89, 1996. doi: 10.1007/BF02291228. [DOI] [PubMed] [Google Scholar]
- 30.Fagius J, Karhuvaara S, Sundlöf G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand 137: 325–334, 1989. doi: 10.1111/j.1748-1716.1989.tb08760.x. [DOI] [PubMed] [Google Scholar]
- 31.Fazalbhoy A, Birznieks I, Macefield V. Consistent interindividual increases or decreases in muscle sympathetic nerve activity during experimental muscle pain. Exp Brain Res 232: 1309–1315, 2014. doi: 10.1007/s00221-014-3847-7. [DOI] [PubMed] [Google Scholar]
- 32.Watso JC, Huang M, Moralez G, Cramer MN, Hendrix JM, Cimino FA, Belval LN, Hinojosa‐Laborde C, Crandall CG. Low dose ketamine reduces pain perception and blood pressure, but not muscle sympathetic nerve activity, responses during a cold pressor test. J Physiol 599: 67–81, 2021. doi: 10.1113/JP280706. [DOI] [PubMed] [Google Scholar]
- 33.Huang M, Yoo JK, Stickford ASL, Moore JP, Hendrix JM, Crandall CG, Fu Q. Early sympathetic neural responses during a cold pressor test linked to pain perception. Clin Auton Res 31: 215–224, 2021. doi: 10.1007/s10286-019-00635-7. [DOI] [PubMed] [Google Scholar]
- 34.Fazalbhoy A, Birznieks I, Macefield V. Individual differences in the cardiovascular responses to tonic muscle pain: parallel increases or decreases in muscle sympathetic nerve activity, blood pressure and heart rate. Exp Physiol 97: 1084–1092, 2012. doi: 10.1113/expphysiol.2012.066191. [DOI] [PubMed] [Google Scholar]
- 35.Schobel HP, Ringkamp M, Behrmann A, Forster C, Schmieder RE, Handwerker HO. Hemodynamic and sympathetic nerve responses to painful stimuli in normotensive and borderline hypertensive subjects. Pain 66: 117–124, 1996. doi: 10.1016/0304-3959(96)03079-5. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Kawabe K, Sapru HN. Cold pressor test in the rat: medullary and spinal pathways and neurotransmitters. Am J Physiol Heart Circ Physiol 295: H1780–H1787, 2008. doi: 10.1152/ajpheart.646.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- 38.Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 454: 359–371, 1992. doi: 10.1113/jphysiol.1992.sp019268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peckerman A, Saab PG, McCabe PM, Skyler JS, Winters RW, Llabre MM, Schneiderman N. Blood pressure reactivity and perception of pain during the forehead cold pressor test. Psychophysiology 28: 485–495, 1991. doi: 10.1111/j.1469-8986.1991.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 40.Lenz H, Høiseth L, Comelon M, Draegni T, Rosseland LA. Determination of equi-analgesic doses of inhaled methoxyflurane versus intravenous fentanyl using the cold pressor test in volunteers: a randomised, double-blinded, placebo-controlled crossover study. Br J Anaesth 126: 1038–1045, 2021. doi: 10.1016/j.bja.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andresen T, Upton RN, Foster DJ, Christrup LL, Arendt-Nielsen L, Drewes AM. Pharmacokinetic/pharmacodynamic relationships of transdermal buprenorphine and fentanyl in experimental human pain models. Basic Clin Pharmacol Toxicol 108: 274–284, 2011. doi: 10.1111/j.1742-7843.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- 42.Christrup LL, Foster D, Popper LD, Troen T, Upton R. Pharmacokinetics, efficacy, and tolerability of fentanyl following intranasal versus intravenous administration in adults undergoing third-molar extraction: a randomized, double-blind, double-dummy, two-way, crossover study. Clin Ther 30: 469–481, 2008. doi: 10.1016/j.clinthera.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Koltzenburg M, Pokorny R, Gasser UE, Richarz U. Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain 126: 165–174, 2006. doi: 10.1016/j.pain.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Tucker AP, Kim YI, Nadeson R, Goodchild CS. Investigation of the potentiation of the analgesic effects of fentanyl by ketamine in humans: a double-blinded, randomised, placebo controlled, crossover study of experimental pain [ISRCTN83088383]. BMC Anesthesiol 5: 2, 2005. doi: 10.1186/1471-2253-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zacny JP, Coalson DW, Klafta JM, Klock PA, Alessi R, Rupani G, Young CJ, Patil PG, Apfelbaum JL. Midazolam does not influence intravenous fentanyl-induced analgesia in healthy volunteers. Pharmacol Biochem Behav 55: 275–280, 1996. doi: 10.1016/S0091-3057(96)00082-2. [DOI] [PubMed] [Google Scholar]
- 46.Zacny JP, Coalson D, Young C, Klafta J, Rupani G, Thapar P, Choi M, Apfelbaum JL. A dose-response study of the effects of intravenous midazolam on cold pressor-induced pain. Anesth Analg 80: 521–525, 1995. doi: 10.1097/00000539-199503000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Zacny JP, Coalson DW, Young CJ, Klafta JM, Lichtor JL, Rupani G, Thapar P, Apfelbaum JL. Propofol at conscious sedation doses produces mild analgesia to cold pressor-induced pain in healthy volunteers. J Clin Anesth 8: 469–474, 1996. doi: 10.1016/0952-8180(96)00126-2. [DOI] [PubMed] [Google Scholar]
- 48.Mauermann E, Filitz J, Dolder P, Rentsch KM, Bandschapp O, Ruppen W. Does fentanyl lead to opioid-induced hyperalgesia in healthy volunteers?: a double-blind, randomized, crossover trial. Anesthesiology 124: 453–463, 2016. doi: 10.1097/ALN.0000000000000976. [DOI] [PubMed] [Google Scholar]
- 49.Patanwala AE, Keim SM, Erstad BL. Intravenous opioids for severe acute pain in the emergency department. Ann Pharmacother 44: 1800–1809, 2010. doi: 10.1345/aph.1P438. [DOI] [PubMed] [Google Scholar]
- 50.Huang M, Watso JC, Belval LN, Cimino FA, Fischer M, Jarrard CP, Hendrix JM, Hinojosa-Laborde C, Crandall CG. Low-dose fentanyl does not alter muscle sympathetic nerve activity, blood pressure, or tolerance during progressive central hypovolemia. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00217.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheuvront SN, Ely BR, Kenefick RW, Sawka MN. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr 92: 565–573, 2010. doi: 10.3945/ajcn.2010.29490. [DOI] [PubMed] [Google Scholar]
- 52.Darwish M, Kirby M, Robertson P Jr, Tracewell W, Jiang JG. Absolute and relative bioavailability of fentanyl buccal tablet and oral transmucosal fentanyl citrate. J Clin Pharmacol 47: 343–350, 2007. doi: 10.1177/0091270006297749. [DOI] [PubMed] [Google Scholar]
- 53.Aronoff GM, Brennan MJ, Pritchard DD, Ginsberg B. Evidence-based oral transmucosal fentanyl citrate (OTFC) dosing guidelines. Pain Med 6: 305–314, 2005. doi: 10.1111/j.1526-4637.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 54.Streisand JB, Varvel JR, Stanski DR, Le Maire L, Ashburn MA, Hague BI, Tarver SD, Stanley TH. Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology 75: 223–229, 1991. doi: 10.1097/00000542-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Parikh N, Goskonda V, Chavan A, Dillaha L. Single-dose pharmacokinetics of fentanyl sublingual spray and oral transmucosal fentanyl citrate in healthy volunteers: a randomized crossover study. Clin Ther 35: 236–243, 2013. doi: 10.1016/j.clinthera.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 56.McIntyre I, Anderson D. Postmortem fentanyl concentrations: a review. J Forensic Res 3: 157, 2012. doi: 10.4172/2157-7145.1000157. [DOI] [Google Scholar]
- 57.Lacourt TE, Houtveen JH, van Doornen LJP. Experimental pressure-pain assessments: test-retest reliability, convergence and dimensionality. Scand J Pain 3: 31–37, 2012. doi: 10.1016/j.sjpain.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Huang M, Watso JC, Moralez G, Cramer MN, Hendrix JM, Yoo JK, Badrov MB, Fu Q, Hinojosa‐Laborde C, Crandall CG. Low‐dose ketamine affects blood pressure, but not muscle sympathetic nerve activity, during progressive central hypovolemia without altering tolerance. J Physiol 598: 5661–5672, 2020. doi: 10.1113/JP280491. [DOI] [PubMed] [Google Scholar]
- 59.Guelen I, Westerhof BE, Van Der Sar GL, Van Montfrans GA, Kiemeneij F, Wesseling KH, Bos WJ. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit 8: 27–30, 2003. doi: 10.1097/00126097-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985) 74: 2566–2573, 1993. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 61.Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212–222, 2001. doi: 10.1093/bja/87.2.212. [DOI] [PubMed] [Google Scholar]
- 62.Watso JC, Robinson AT, Babcock MC, Migdal KU, Wenner MM, Stocker SD, Farquhar WB. Short-term water deprivation does not increase blood pressure variability or impair neurovascular function in healthy young adults. Am J Physiol Regul Integr Comp Physiol 318: R112–R121, 2020. doi: 10.1152/ajpregu.00149.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watso JC, Babcock MC, Robinson AT, Migdal KU, Wenner MM, Stocker SD, Farquhar WB. Water deprivation does not augment sympathetic or pressor responses to sciatic afferent nerve stimulation in rats or to static exercise in humans. J Appl Physiol 127: 235–245, 2019. doi: 10.1152/japplphysiol.00005.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci 193: 12–21, 2015. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macefield VG. Recording and quantifying sympathetic outflow to muscle and skin in humans: methods, caveats and challenges. Clin Auton Res 31: 59–75, 2021. doi: 10.1007/s10286-020-00700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gandevia S. Publications, replication and statistics in physiology plus two neglected curves. J Physiol 599: 1719–1721, 2021. doi: 10.1113/JP281360. [DOI] [PubMed] [Google Scholar]
- 67.Curran-Everett D. Evolution in statistics: P values, statistical significance, kayaks, and walking trees. Adv Physiol Educ 44: 221–224, 2020. doi: 10.1152/advan.00054.2020. [DOI] [PubMed] [Google Scholar]
- 68.Barbosa TC, Vianna LC, Fernandes IA, Prodel E, Rocha HN, Garcia VP, Rocha NG, Secher NH, Nobrega AC. Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. J Physiol 594: 715–725, 2016. doi: 10.1113/JP271335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barbosa TC, Fernandes IA, Magalhães N Jr, Cavalcanti IL, Secher NH, Nóbrega AC, Vianna LC. Oscillatory blood pressure response to the onset of cycling exercise in men: role of group III/IV muscle afferents. Exp Physiol 100: 302–311, 2015. doi: 10.1113/expphysiol.2014.083857. [DOI] [PubMed] [Google Scholar]
- 70.Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Wray DW, Amann M. Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. J Physiol 596: 1373–1384, 2018. doi: 10.1113/JP275465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vettorello M, Colombo R, De Grandis CE, Costantini E, Raimondi F. Effect of fentanyl on heart rate variability during spontaneous and paced breathing in healthy volunteers. Acta Anaesthesiol Scand 52: 1064–1070, 2008. doi: 10.1111/j.1399-6576.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 72.Zacny JP, Lichtor JL, Zaragoza JG, de Wit H. Effects of fasting on responses to intravenous fentanyl in healthy volunteers. J Subst Abuse 4: 197–207, 1992. doi: 10.1016/0899-3289(92)90019-t. [DOI] [PubMed] [Google Scholar]
- 73.Peng PW, Sandler AN. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 90: 576–599, 1999. doi: 10.1097/00000542-199902000-00034. [DOI] [PubMed] [Google Scholar]
- 74.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94: 825–834, 2005. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 75.Niv D, Whitwam JG. Selective effect of fentanyl on group III and IV somatosympathetic reflexes. Neuropharmacology 22: 703–709, 1983. doi: 10.1016/0028-3908(83)90093-X. [DOI] [PubMed] [Google Scholar]
- 76.Daskalopoulos NT, Laubie M, Schmitt H. Localization of the central sympatho-inhibitory effect of a narcotic analgesic agent, fentanyl, in cats. Eur J Pharmacol 33: 91–97, 1975. doi: 10.1016/0014-2999(75)90142-9. [DOI] [PubMed] [Google Scholar]
- 77.Schobel HP, Oren RM, Mark AL, Ferguson DW. Naloxone potentiates cardiopulmonary baroreflex sympathetic control in normal humans. Circ Res 70: 172–183, 1992. doi: 10.1161/01.res.70.1.172. [DOI] [PubMed] [Google Scholar]
- 78.Frew AK, Drummond PD. Opposite effects of opioid blockade on the blood pressure-pain relationship in depressed and non-depressed participants. Pain 142: 68–74, 2009. doi: 10.1016/j.pain.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Lewkowski MD, Young SN, Ghosh S, Ditto B. Effects of opioid blockade on the modulation of pain and mood by sweet taste and blood pressure in young adults. Pain 135: 75–81, 2008. doi: 10.1016/j.pain.2007.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the principal investigator after institutional data transfer agreement approvals.