Abstract
Asthma and its heterogeneity change with age. Increased airspace neutrophil numbers contribute to severe steroid-resistant asthma exacerbation in the elderly, which correlates with the changes seen in adults with asthma. However, whether that resembles the same disease mechanism and pathophysiology in aged and adults is poorly understood. Here, we sought to address the underlying molecular mechanism of steroid-resistant airway inflammation development and response to corticosteroid (Dex) therapy in aged mice. To study the changes in inflammatory mechanism, we used a clinically relevant treatment model of house-dust mite (HDM)-induced allergic asthma and investigated lung adaptive immune response in adult (20–22 wk old) and aged (80–82 wk old) mice. Our result indicates an age-dependent increase in airway hyperresponsiveness (AHR), mixed granulomatous airway inflammation comprising eosinophils and neutrophils, and Th1/Th17 immune response with progressive decrease in frequencies and numbers of HDM-bearing dendritic cells (DC) accumulation in the draining lymph node (DLn) of aged mice as compared with adult mice. RNA-Seq experiments of the aged lung revealed short palate, lung, and nasal epithelial clone 1 (SPLUNC1) as one of the steroid-responsive genes, which progressively declined with age and further by HDM-induced inflammation. Moreover, we found increased glycolytic reprogramming, maturation/activation of DCs, the proliferation of OT-II cells, and Th2 cytokine secretion with recombinant SPLUNC1 (rSPLUNC1) treatment. Our results indicate a novel immunomodulatory role of SPLUNC1 regulating metabolic adaptation/maturation of DC. An age-dependent decline in the SPLUNC1 level may be involved in developing steroid-resistant airway inflammation and asthma heterogeneity.
Keywords: aging, airway inflammation, dendritic cells, SPLUNC1, steroid-resistant asthma
Keywords: INTRODUCTION
Asthma is a chronic disease of the upper airways that influence people differently across the lifespan, affecting 7% of the population older than 65 yr (1–3). The heterogeneity of asthma is known to change with age and complicate responses to therapy, thereby impacting health outcomes (4, 5). Asthma in older adults is superimposed on a background of aging-associated lung and immune changes and classified as long-standing (childhood-onset) or late-onset asthma (6–9). A large body of evidence from human studies and rodent models implicates that airway inflammation associated with asthma and responses to therapy in the elderly differs from that in adult subjects with asthma (9–12). Neutrophilic airway inflammation, similar to that noted in severe noneosinophilic asthma, is present in the airways of older people and responds poorly to steroid therapy (7, 13, 14). Increased airspace neutrophil numbers contribute to asthma exacerbation in the elderly, mirroring the changes seen in patients with severe asthma with neutrophil-predominant and mixed Th1/Th17 cytokine signatures (3, 15–17). However, little is known whether this heterogeneity resembles the same disease in aged individuals as in adults or at least has a different cause and pathophysiology.
The microenvironment within the airways and bronchioles changes during aging and airway inflammation. For example, senescence-associated secretory phenotype (SASP) components wield potent effects on adjacent cells and influence dendritic cell-airway epithelial cell (DC-AEC) cross talk and T cell effector function, each of which regulates adaptive lung immunity ultimately promoting functional airway deterioration (18–23). The airway mucosa is lined by AECs, closely associated with antigen-presenting DCs, and secretes inflammatory mediators, activating the local DC network in the airways (24, 25). Subsequently, the development of an effective T cell-mediated adaptive immune response to inhaled allergens requires activation and efficient allergen recognition by DCs (26, 27). Current geriatric studies suggest that DCs from aged individuals are more proinflammatory and exist in a semiactivated state in the absence of stimuli than DC from adults, which triggers the AECs to secrete chemokines altering the barrier properties (28, 29). Moreover, aged DC are less responsive to retinoic acid (RA), impair mucosal tolerance, and accelerate steroid-resistant airway inflammation (30–32). However, changes in airway signals and molecular mechanisms that impact lung DC function and consequently modify the adaptive immune response to the inhaled allergen, triggering asthma in aging, are poorly understood.
Short palate, lung, and nasal epithelial clone1 (SPLUNC1) are the most abundantly expressed secretory proteins in healthy lungs, which serve as an allosteric modulator of epithelial Na+ channel (ENaC), regulates mucociliary clearance, and maintains epithelial integrity in the upper airways (33–36). Moreover, due to its structural similarity with other bacterial permeability-increasing protein fold-containing (BPIF) family members and LPS-binding capacity, antimicrobial functions against various pathogens have been suggested (37–40). Previous studies have demonstrated that the production of SPLUNC1 by epithelial cells is markedly reduced in Th1-induced or Th2-induced airway inflammation, suggesting a direct role of SPLUNC1 in allergic asthma (38, 41). Interestingly, in an allergic setting, it has been shown that SPLUNC1−/− mice develop severe eosinophilic airway inflammation due to the increased capacity of eotaxin-2 production by alveolar macrophages (42). Furthermore, SPLUNC1 acts as a smooth muscle relaxing factor and suppresses smooth muscle contractility by directly binding and inhibiting Ca2+ influx channel Orai1 (43). Although the ion-channel regulating function and the antimicrobial role of SPLUNC1 are known, the biological significance of an immunomodulatory function remains unclear.
In the present study, we show that the response to common aeroallergen house-dust mite (HDM) and induction of airway inflammation is dysregulated with increasing age, which is manifested by steroid-resistant neutrophilic inflammation and Th1/Th17 signatures in the aged lung as compared with eosinophilic/Th2 high adult lung. We demonstrate that epithelium-derived SPLUNC1 levels in the airways progressively decline with increasing age and further by inflammation. Ex vivo, we show that recombinant (r) SPLUNC1 accelerates DC glycolytic reprogramming, maturation, and promotes proliferation of OTII and Th2 cytokine secretion. Our findings collectively suggest SPLUNC1 as a steroid-responsive gene promoting DC-mediated immune priming function that progressively declines with age. Therefore, we propose that a reduction in SPLUNC1 level may in part accelerate steroid-resistant airway inflammation and asthma heterogeneity in aging.
METHODS
The authors declare that all supporting data are available within the article. All procedures and protocols involving animals were approved by the Institutional Animal Care and Use Committee of Auburn University (Auburn, AL).
Mice Allergen (HDM) Sensitization/Challenge and Dex Treatment Protocol
Young (6–8 wk old), adult (20–22 wk old), and aged (80–82 wk old) wild-type BL/6J mice of both sexes and B6.Cg-Tg(Tcra Tcrb)425Cbn/J, which expresses a transgenic MHCII-restricted TCR that binds the OVA peptide antigen, OVA323–339 mice (44) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were sensitized with 50 μg of HDM in a volume of 40 μL through the intranasal (in) route on days 1, 3, and 5. The challenge phase was divided into three consecutive HDM administration phases (50 μg in administration/mouse) on days 11–13, 18–20, and 25–27, respectively. In the treatment protocol, mice receive dexamethasone injection (Sparhawk Laboratories, Lenexa, KS) (4 mg/kg body wt, ip) or sterile saline every 72 h during the HDM challenge period. After the last HDM challenge, mice were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg) and bronchoalveolar lavage (BAL), mediastinal lymph node (DLn), and lung lobes were harvested and analyzed.
Measurement of Airway Hyperresponsiveness
The trachea was cannulated with a 19-G beveled metal catheter, and airway resistance to increasing concentrations of methacholine (Acetyl β-methyl choline, Sigma Aldrich, St. Louis, MO) was directly measured in mechanically ventilated anesthetized mice using an Elan RC Fine Pointe system (DSI, St. Paul, MN). A nebulizer was used to administer ascending doses of methacholine (0, 2.5, 5.0, and 10 mg/mL), and means ± SE values are presented as cmH2O per mL/s.
Assessment of Airway Inflammation
To quantify the inflammation of the airways, each mice lung was washed three times with 0.5 mL of DPBS. Furthermore, RBCs were lysed with ACK lysis buffer for 2–3 min at 4°C, and cells were resuspended in RPMI medium with 10% FBS. BAL cell counts were performed using a hemocytometer, and differential staining for infiltrating cells was performed by flow cytometry. BAL levels of CCL24 and CXCL1 (KC1) chemokines were determined using quantitative ELISA kits from R& D Systems (Minneapolis, MN). The lung was fixed with 10% formalin for 24 h, dehydrated through gradient ethanol, and embedded in paraffin for histopathology. Lung sagittal sections were cut to a thickness of 5 μm and stained with hematoxylin and eosin.
In Vivo Dendritic Cell Migration
HDM extracts (Dermatophagoides pteronyssinus) were labeled with Alexa Fluor 647 (AF647) Protein Labeling Kit (Molecular Probes, Life Technologies) according to the manufacturer’s instruction. The labeled HDM was administered (50 µL; in administration) to adult (20–22 wk old) and aged (80–82 wk old) mice. Lungs and DLNs were harvested after 72 h, and the number of CD11c+/MHC-IIhi/SSClo/CD11b+/HDM+ DCs were quantified by flow cytometry as reported before (45).
Flow Cytometry
Lungs lobes were harvested and digested by enzymatic digestion using type IV collagenase (1 mg/mL) and DNase I (0.1 mg/mL) (Worthington, Lakewood, NJ) for 30 min at 37°C with agitation. Before performing surface staining, suspensions of single cell (1–2 × 106 cells) were preincubated with Fc Block antibody (BD PharMingen) in staining buffer [1X DPBS, 3% FBS, 2 mM EDTA, and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] at 4°C for 10 min. Furthermore, cells were first stained with Live/Dead Fixable Violet Staining Kit (Invitrogen) in 50 μL of PBS for 15 min at 4°C. After washing cells with staining buffer, cocktails of desired cell surface antigens were incubated in 50 μL of staining buffer for 30 min at 4°C in the dark. BAL eosinophils (Eos), neutrophils (Neu), macrophages (Mac), and lymphocytes (Lym) were identified using antibodies against rat anti-mouse CD45R(B220)-Alexa Fluor 488 (clone, RA3-6B2), CD3-Alexa Fluor 488 (clone, 145-2C11), I-A/I-E (MHC-II)-PE-Cy7 (clone, M5/114.15.2), CD11c-APC-eFluor780 (clone, N418), and F4/80-E-Fluor 450 (clone, BM5) all from eBiosciences except anti-mouse CCR3-PE (clone 83101) (R&D Systems) and Gr1-(Ly6G/Ly6C)-APC (clone, RB6-8C5) from BioLegend. To detect Glut-1 expression, cells were stained with live/dead fixable stain and immediately fixed with Fix/Perm buffer (eBiosciences). After washing, cells were stained with anti-Glut-1-PE antibody (Novus biologicals), and surface and endogenous expressions of Glut-1 were determined by flow cytometry.
To assess the intracellular cytokines, single-cell lung suspensions were first stained with surface markers against rat anti-mouse CD3-Alexa Fluor 647 (clone 17-A2), CD4-APC-eFluor 780 (clone GK1.5) from eBiosciences. After a single washing with staining buffer, cells were further fixed for 30 min with IC Fixation Buffer (eBiosciences). Followed by a wash with 1X Perm/Wash buffer (BD Biosciences), cytokines were detected after incubation with monoclonal antibody cocktails of rat anti-mouse IL-13-Alexa Fluor 488 (eBio13A), IL-17-eFluor 450 (eBio17B7), and IFN-γ-PerCP-Cy5.5 (cone, XMG1.2), (all from eBioscience) in 50 μL 1X Perm/Wash buffer (BD Biosciences) for 30 min at room temperature. Cells were washed and resuspended in staining buffer and analyzed by flow cytometry. Absolute cell counts were determined using hemocytometer, and flow cytometry data were acquired on a cytometer (LSR-II; BD Biosciences). Data were further analyzed using FlowJo software (TreeStar).
Magnetic Sorting of Lung Dendritic Cells and Airway Epithelial Cells
Single-cell suspension was prepared using freshly isolated mice lungs, as stated earlier. Airway epithelial cells (AECs) were isolated with EasySep APC Positive Selection Kit (StemCell Technologies, UK) according to the manufacturer’s instructions in 5-mL polystyrene round-bottom tubes on the EasySep magnet. Cell suspensions (1 × 108 cells/mL) were centrifuged at 300 g for 10 min at 4°C and resuspended in 0.5 mL of recommended medium (PBS containing 2% FBS and 1 mM EDTA) in a 5-mL polystyrene tube. Furthermore, mouse-specific FcR block (1 µg/mL) was added and stained with epithelial cell-specific CD326 (EpCAM) monoclonal antibody (1 µg/mL; clone: G8.8, APC, eBioscience). Using selection cocktail and Rapid Spheres, EpCAM+ cells were selected and were processed for further DC isolation (EasySep Mouse Pan-DC Enrichment Kit; StemCell Technologies) according to the manufacturer’s instructions. Positively selected lung AECs and negatively sorted lung DCs were processed for RNA isolation.
Quantitative Real-Time Polymerase Chain Reaction
RNA from lung tissue and isolated DCs, AECs, or cultured BMDCs were isolated using TRIzol reagent (Life Technologies, Grand Island, NY), and cDNA was prepared with a High-Capacity RNA to cDNA kit (Applied Biosystems). Quantitative real-time polymerase chain reaction (qRTPCR) was performed using the QuantStudio 6 sand 7 Flex Real-Time PCR Systems (Applied Biosystems). All the genes were quantified using SYBR green primers (Table 1). After amplification, Ct values were obtained and analyzed according to the 2−ΔΔCt method considering quantitative PCR efficiency. The Ct value of each target gene was normalized to housekeeping gene acidic ribosomal phosphoprotein P0 (36B4).
Table 1.
List of primer sequences used in the study
| Forward Primer | Reverse Primer | |
|---|---|---|
| BPIFA1 | 5′- GTCCACCCTTGCCACTGAACCA-3′ | 5′- CACCGCTGAGAGCATCTGTGAA-3′ |
| TGF-β | 5′- GGATGCATTCATGAGTATTGC-3′ | 5′- GCTTCCTGAGGCTGGATTC-3′ |
| Col1 | 5′- TGGACGCCATCAAGGTCTACTGC-3′ | 5′- GGAGGTCTTGGTGGTTTTGTATTCG-3′ |
| MMP10 | 5′- CCTGTGTTGTCTGTCTCTCCAAGA-3′ | 5′- CGTGCTGACTGAATCAAAGGAC-3′ |
| MMP12 | 5′- AATTACACTCCGGACATGAAGCGT-3′ | 5′- GGCTAGTGTACCACCTTTGCCATC-3′ |
| GR1-α | 5′- AAAGAGCTAGGAAAAGCCATTGTC-3′ | 5′- TCAGCTAACATCTCTGGGAATTCA-3′ |
| GR1-β | 5′- AAAGAGCTAGGAAAAGCCATTGTC-3′ | 5′- CTGTCTTTGGGCTTTTGAGATAGG-3′ |
| PKM2 | 5′- TTAGGCCAGCAACGCTTGTAGTGC-3′ | 5′- AGATGCTGCCGCCCTTCTGTGATA-3′ |
| Hif-1α | 5′- GGTTCCAGCAGACCCAGTTA-3′ | 5′- AGGCTCCTTGGATGAGCTTT-3′ |
| HK2 | 5′- TGATCGCCTGCTTATTCACGG-3′ | 5′- AACCGCCTAGAAATCTCCAGA-3′ |
| IRF4 | 5′- ACAGCACCTTATGGCTCTCTG-3′ | 5′- ATGGGGTGGCATCAT GTAGT-3’ |
| ICOSL | 5′- AGCTTGAACTTACAGACCACGC-3′ | 5′- CTCTGAAGTTGTGTCTGACATC-3′ |
| Zbtb46 | 5′- AGAGAGCACATGAAGCGACA-3′ | 5′- CTGGCTGCAGACATGAACAC-3′ |
| OX40L | 5′- ATGGAAGGGGAAGGGGTTCAACC-3′ | 5′- TCACAGTGGTACTTGGTTCACAG-3′ |
| Glut1 | 5′- CATCCTTATTGCCCAGGTGTTT-3′ | 5′- GAAGACGACACTGAGCAGCAGA-3′ |
| LDHA | 5′- CACTGACTCCTGAGGAAGAGGCCC-3′ | 5′- AGCTCAGACGAGAAGGGTGTGGTC-3′ |
| Zbtb46 | 5′- AGAGAGCACATGAAGCGACA-3′ | 5′- CTGGCTGCAGACATGAACAC-3′ |
| 36B4 | 5′- GGACCCGAGAAGACCTCCTT-3′ | 5′- GCACATCACTCAGAATTTCAATGG-3′ |
p16 (INK4a) (Cdkn2a): TaqMan primer; assay ID: Mm00494449_m1 (Invitrogen: Cat No: 4453320).
Flow Cytometric Detection of SA-β-Galactosidase
SA-β-galactosidase was detected by flow cytometry in the lung CD45−/EpCAM+ epithelial cells (AECs), CD45−/CD31+ endothelial cells (Endo), and CD45+/CD11c+/CD11b+/MHCII+ dendritic cells (DC). In brief, single-cell lung suspensions from adult and aged mice were obtained by enzymatic digestion as described above. Lung cell suspensions were incubated for 2 h with C12FDG (33 µM, Thermo Fischer) followed by washing with ice-cold PBS before fluorescence measurement using a flow cytometer (LSR-II, BD Bioscience). Data were analyzed using the FlowJo software.
Bone Marrow-Derived Dendritic Cell Cultures and Stimulation
Bone marrow-derived dendritic cells (BMDCs) were generated as previously described (46, 47). Briefly, cells were isolated from leg bones of euthanized mice and cultured at a density of 1 × 106 cells/mL in differentiation media [Iscove modified Dulbecco medium (Gibco/Life Technologies, CA) containing 10% heat-inactivated FCS, penicillin-streptomycin (100 U/mL), L-glutamine (2 mM), 2-mercaptoethanol (50 µM), recombinant mouse granulocytes macrophage colony-stimulating factor (20 ng/mL), and recombinant mouse IL-4 (10 ng/mL, PeproTech]. Cultures were supplemented with an equal volume of medium (day 3 and day 5), and nonadherent cells were collected on day 6 and plated in six-well tissue culture plates at a density of 2 ×106 cells/well in differentiation media. Nonadherent cells were collected on day 7 for analysis. BMDCs (2−5 × 105) were cultured on 96-well plates and pulsed with HDM (100 µg/mL) in the presence or absence of rSPLUNC1 (5 µg/mL). BMDCs were further processed for RNA isolation and Western blot analysis. In separate experiments, BMDCs were used for XFp assays and extracellular acidification rates (ECARs) measurements.
BMDC and OT-II Coculture
The ability of BMDCs to induce ex vivo antigen-specific T cell proliferation was assessed using CFSE-labeled splenic CD4+ T cells obtained from naïve [B6.Cg-Tg(Tcra Tcrb)425Cbn/J] transgenic mice (Jackson Laboratories, Bar Harbor, ME) that express a transgenic MHCII-restricted TCR that recognizes the OVA peptide antigen (44). Splenic naive CD4+ T cells were purified using EasySep Mouse CD4+ T Cell Isolation Kit (Stem cells, Vancouver, CA) and were labeled with 5 µM CFDA-SE (carboxyfluorescein diacetate succinimidyl ester; Cayman Chemical, MI) in DPBS for 20 min at 37°C. BMDCs were pulsed overnight with 5 µg·mL−1 of OVA323–339 peptide (AnaSpec, Fremont, CA) or PBS in the presence or absence of rSPLUNC1 (5 µg/mL. Furthermore, 1 × 105 OVA peptide-specific CD4+ OT-II cells were cocultured with 2 × 104 CD11c+ BMDCs in 96-well plates for 4 days, and T cell proliferation was quantified by flow cytometry using CFSE dye dilution. Gated CD4+ T cells were analyzed using proliferation profile and percent divided as calculated by the FlowJo Proliferation Platform. The quantity of IL4 and IL13 released into the culture supernatant were measured using ELISA. In addition, cells were stained with CD3-Alexa Fluor 647 (clone 17-A2), CD4-APC-Cy7 780 (clone GK1.5) before incubation with GATA3-BV405 (clone TWAJ) for flow cytometry.
ELISA and Immunoblot Assay for SPLUNC1 Detection
ELISA plates (Nunc MaxiSorp) were coated with rSPLUNC1 and BAL samples and further incubated with polyclonal sheep anti-mouse SPLUNC1 antibody (R&D, Minneapolis, MN), Sheep IgG horseradish peroxidase (HRP) (R&D, Minneapolis, MN), and tetramethylbenzidine substrate (Thermo Fisher Scientific). Data were measured at an optical density of 450 and 560 nm after background correction. To quantify SPLUNC1 level in BAL samples, an equal amount of BAL were resolved by SDS-PAGE using 4%–12% Bis-Tris gel (Thermo Fisher Scientific) and transferred on nitrocellulose membranes (Thermo Fisher Scientific) and probed with anti-mouse SPLUNC1 (1:500; R&D Systems) and anti-rabbit IgG, HRP-linked antibody (1:2,000; Cell Signaling Technologies).
RNA Sequencing Experiments and Analysis
Total RNA from lung samples of the adult (20–22 wk old) and aged (80–82 wk old) naïve, HDM-treated, and HDM + Dex-treated (n = 5) were extracted using RNeasy plus Universal Kits (QIAGEN, MD). The quality of RNA was assessed before being processed for library preparation using Bioanalyzer (Agilent Technologies). The whole transcriptome was amplified, and the library was constructed using TruSeq Stranded mRNA (Illumina; San Diego, CA). Quantitative assessment of library was done using Qubit 2.0 fluorometer (Invitrogen) and evaluated on the high-sensitivity DNA chip (Agilent Technologies). Libraries were sequenced on a NovaSeq 6000 platform (Illumina) using a pair-end 50 bp sequencing strategy. RNA sequencing data were preprocessed and analyzed using the Picard-STAR-limma pipeline. Raw read counts and fragments per kilobase of transcript per million (FPKM) mapped reads abundance were estimated at the transcript and gene levels. Principal component analysis (PCA) was used to identify outliers. Both upregulated and downregulated gene sets are reported on three GO subcategories: biological process (BP), cellular component (CC), and molecular function (MF). FDR q-values were estimated to correct the P-values for the multiple testing issue. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE178770 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE178770).
Seahorse Analysis
The Seahorse XFp Extracellular Flux Analyzer (Agilent, Santa Clara, CA) was used to analyze real-time changes of extracellular acidification rates (ECARs) as described earlier (48). BMDCs were plated (120,000 cells/well) in 200 µL in XFp mini-culture plates overnight in an IMDM medium containing 5% FBS at 37°C in the CO2 incubator. The culture medium was replaced the next day with a fresh medium containing 5% FCS, stimulated with HDM (100 µg/mL), and treated with and without rSPLUNC1 protein for 16 h. Furthermore, the medium was replaced with a warm XF DMEM base medium (Agilent) containing 2 mM L-glutamine (Agilent, 103579) and 1% FBS. The assay plate was incubated in a non-CO2 incubator for 1 h at 37°C. Glycolysis stress tests were performed according to the manufacturer’s protocol with sequential injections of glucose, oligomycin (Agilent), and 2-DG (Sigma) in ports A, B, and C, respectively. The effects on ECAR were recorded three times every 5 min interval, and data were analyzed using Wave software 2.6.1 (Seahorse Bioscience) after normalization with total protein.
Statistics
Data were analyzed using GraphPad Prism version 7.0 b using either an unpaired t test for normally distributed data or a Mann–Whitney test for non-normally distributed data. Multiple comparisons were analyzed using a one-way ANOVA with Sidak’s multiple comparison test or a two-way ANOVA. Data are presented as means + SE. A P value < 0.05 was considered significant.
RESULTS
Age-Dependent Increase in Steroid-Resistant Neutrophilic Airway Inflammation
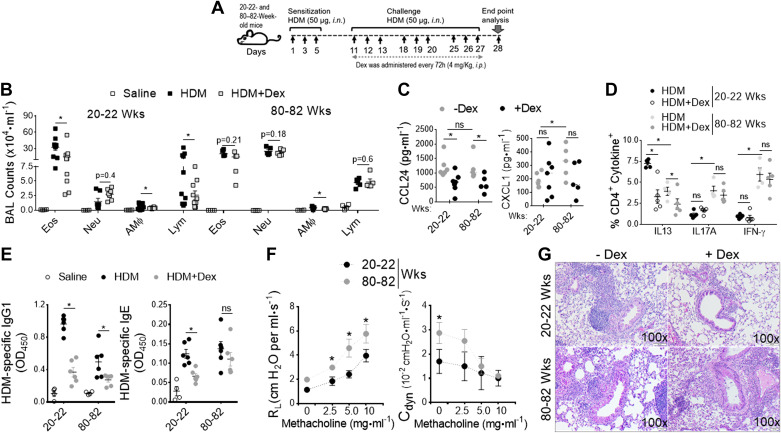
Using a clinically relevant murine model of asthma induced by repeated exposure to HDM (D. pteronyssinus), a common aeroallergen that is an environmental trigger and risk factor for the development of persistent asthma in the elderly (49), we sought to identify the underlying molecular mechanism of disease severity in the context of aging. We employed a treatment model of HDM-induced asthma in adult (20–22 wk old) and aged (80–82 wk old) B6 mice (Fig. 1A). Mice were sensitized and challenged with HDM thrice for three consecutive days with 4 days intervals and received intraperitoneal injections of Dex (4 mg/kg) every 72 h during the challenge period. Differential counts in the bronchoalveolar lavage (BAL) fluid recovered from mice 24 h after the last HDM challenge showed greater neutrophil recruitment in aged mice but more eosinophil predominant airway inflammation in adults (Fig. 1B). Dex treatment substantially reduced airway inflammation in adult mice but was ineffective in reducing airway inflammation in the aged. BAL levels of C-C chemokines (CCL24) that recruit eosinophils were significantly decreased with Dex treatment in both ages. However, C-X-C chemokines (KC) that recruit neutrophils were unresponsive to Dex therapy and increased in aged mice compared with adult mice (Fig. 1C). In addition, the increase in lung IL13+ CD4+ T cell numbers was greater in adults than in aged mice, and the cells were reduced by 50% upon Dex treatment (Fig. 1D). We observed a significantly higher frequency of IL17A+ CD4+ and IFN-γ+ CD4+ T cells in aging lungs compared with adults, which was not suppressed by Dex treatment. Although serum levels of HDM-specific IgG1 were significantly decreased with Dex treatment compared with HDM-challenged mice of both ages, HDM-specific IgE levels were not altered in aged mice after Dex treatment (Fig. 1E), demonstrating an age-dependent increase in steroid-resistant airway inflammation. Moreover, the aged mice had significantly higher lung resistance and dynamic compliance than HDM-challenged adult mice with increasing doses of methacholine (Fig. 1F). In support, histological examinations revealed marked peribronchial inflammatory cell infiltrates in HDM-challenged aged mice, which was not reduced by Dex treatment (Fig. 1G). These results demonstrate that the HDM challenge causes predominantly eosinophilic airway inflammation that is fully responsive to Dex treatment in adults, whereas aged lung augments mixed granulomatous neutrophilic inflammation and refractory AHR to Dex treatment.
Figure 1.
Aging promotes Dex-resistant mixed granulomatous airway inflammation. A: adult (20–22 wk old) and aged (80-82 wk old) B6 mice were intranasally sensitized and challenged with HDM (50 μg) and PBS. Mice received Dex (4 mg/kg body wt, ip) or sterile saline every 72 h during the HDM-challenged period as indicated. Endpoint analysis was performed 24 h after the last administration of HDM. B: numbers of BAL inflammatory cell types (Eos; eosinophils), (Neu; neutrophils), (AM; alveolar macrophages), and (Lym; lymphocytes) from PBS−, HDM−, and HDM+ Dex-challenged were compared; (n = 8–10 mice, significance denoted by *P< 0.01, HDM vs. HDM+ Dex, one-way ANOVA with Sidak’s multiple comparison test). Bar graph shows (C) BAL levels of CCL24 and CXCL1 chemokines; (D) frequency of CD4+ cytokines+ T cells in lung; and (E) serum levels of HDM specific IgG1 and HDM-specific IgE (n = 8–10 mice, significance denoted by *P < 0.05, HDM vs. HDM+ Dex, one-way ANOVA with Sidak’s multiple comparison test). F: graph plot show AHR (left, airway resistance and right, lung dynamic compliance to increasing dose of inhaled methacholine in HDM-treated mice) (n = 8–10 mice, significance denoted by *P < 0.05, 20–22 wk old vs. 80–82 wk old, one-way ANOVA with Sidak’s multiple-comparison test), and G: representative lung histology sections stained with H&E and PAS. Scale bars = 100 μm for the ×100 images. Data are shown as means ± SE of 5–10 mice per group and representative from three independent experiments. AHR, airway hyperresponsiveness; BAL, bronchoalveolar lavage; HDM, house-dust mite.
A Decline in DC Migration to Draining Mediastinal Lymph Node Is Impacted by Senescence
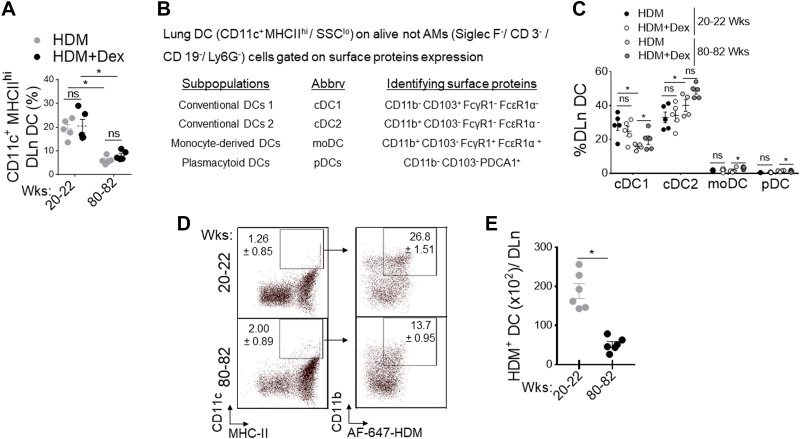
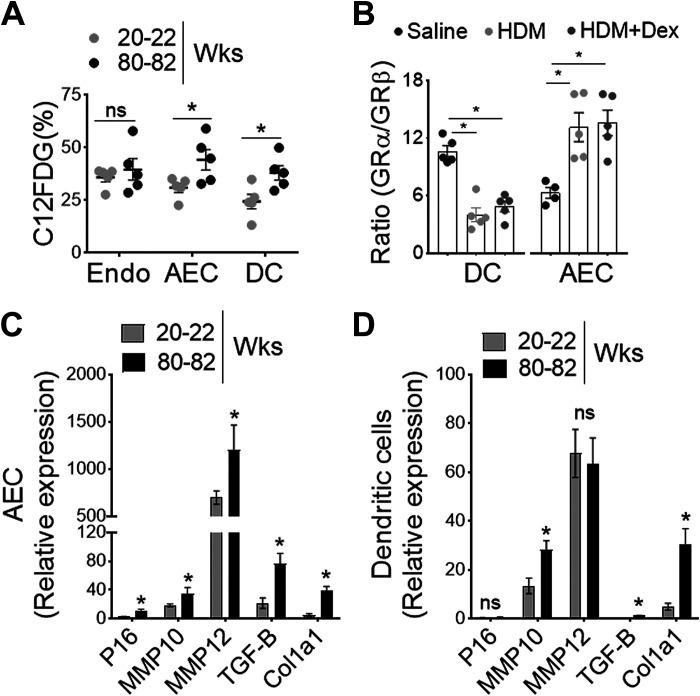
Migration of activated tissue DC from the site of inflammation to DLn is an essential step in the induction of lung adaptive immune response to inhaled allergens (27, 46). To investigate whether increasing age influences the lung DC repertoire, we evaluated the numbers of different DC subsets in the HDM-challenged lungs of adult and aged mice with and without Dex treatment. Frequencies of total DC in DLn were markedly reduced in aged mice compared with adult mice and are unresponsive to Dex treatment (Fig. 2A). Using a previously described approach (Fig. 2B) (45), we found a significant reduction in the cDC1 subset in aged mice. However, the cDC2, moDC, and pDC were comparable in aging and adult mice with HDM challenge and after Dex treatment (Fig. 2C). Next, we analyzed the capacity of lung DCs to capture and transport HDM-derived antigens to DLn in response to inhaled HDM extracts after 72 h. We found a progressive decline in frequencies (Fig. 2D) and numbers (Fig. 2E) of HDM bearing Alexa-647+ DC in the DLn as mice aged, suggesting that the intrinsic ability of DC to migrate to DLn is compromised in aged mice. In addition, using C12FDG (5-dodecanoyl amino fluorescein di- β-D-galactopyranoside), a fluorogenic substrate for β-galactosidase activity (50, 51) demonstrated that aged lungs (80–82 wk old) had increased frequency of senescent EpCAM+ AEC and CD11c+ MHCIIhi DC. In contrast, most CD31+ endothelial cells were nonsenescent compared with naïve adult lungs (Fig. 3A).
Figure 2.
Defective DC migration in aging. Adult (20–22 wk old) and aged (80–82 wk old) wild-type mice were sensitized and challenged by administration of PBS, HDM (50 μg), and or treated with Dex (4 mg/kg, ip). A: flowcytometry assessed the frequency of CD11c+ MHC-IIhi CD11b+ DC in DLn (n = 4–6 mice per group, significance denoted by *P< 0.01, HDM vs. HDM+ Dex, adult vs. aged, one-way ANOVA with Sidak’s multiple comparison test). B: the panel of surface markers used to identify lung DC subsets and (C) frequency in adult and aged mice after HDM challenge and Dex treatment (each circle indicates individual mice, *P< 0.01, HDM vs. HDM+ Dex, adult vs. aged, one-way ANOVA with Sidak’s multiple comparison test). Activated DC migration to DLn during the sensitization phase of allergic airway inflammation: Naïve B6 mice intranasally inoculated with AF647-labeled HDM (100 µg/mouse in 50 µL of PBS) and enumerated at 72 h in DLn and analyzed for CD11c+/MHC-IIhi/CD11b+/HDM+ DC by flow cytometry. D: representative dot plot and (E) enumeration of migrated AF647+HDM+ DC per DLn. Data are represented as means ± SE of six mice per group and representative of two independent experiments (*P< 0.05, adult vs. aged, unpaired t test). DC, dendritic cell; DLn, draining lymph node; HDM, house-dust mite.
Figure 3.
Impact of lung senescence in airway epithelial cells (AECs) and DC. A: frequency of C12FDG+ lung endothelial cells (Endo), AECs, and DC from adult and aged mice as measured by SA-β-gal activity-based flow cytometric staining (n = 4 or 5 mice per group, *P< 0.01, adult vs. aged, unpaired t test), B: bar chart shows the ratio of GRα/GRβ expression in isolated lung DCs and AECs from HDM or PBS-challenged aged mice treated with Dex (n = 4 or 5 mice per group, *P< 0.01, saline vs. HDM, saline vs. HDM + Dex, one-way ANOVA with Sidak’s multiple comparison test). Bar chart showing relative expression of senescence-associated secretory phenotype (SASP) in isolated (C) AECs and (D) lung DCs. Data are represented as means ± SE from two independent experiments (n = 4 or 5 mice per group, *P< 0.01, adult vs. aged, unpaired t test). DC, dendritic cell; DLn, draining lymph node; HDM, house-dust mite; SA-β-gal, SA-β-galactosidase.
Glucocorticoids (GC) such as Dex exerts its immunosuppressive functions via the cytosolic receptor, glucocorticoid receptors (GRs encoded by the Nr3c1 gene), a member of the nuclear hormone receptor superfamily (52). GR is primarily located in the cytosol and remains inactive in the absence of ligand binding (53); however, it diffuses across the cell membrane and undergoes conformational change following activation (54). Based on the alternative use of exons 9a and 9b, a single GR mRNA can generate functionally distinct two isoforms GRα and GRβ (55). The predominant GRα isoform of the receptor operates as a classical agonist receptor (56), whereas the GRβ lacks the ligand-binding domain and functions as a dominant-negative regulator (57, 58). The elevated level of GRβ suppresses GRα-mediated anti-inflammatory gene activation and is potentially linked to steroid resistance (58, 59). Our results show that GR isoforms expression was reciprocally regulated in lung DCs and airway epithelial cells (AECs) with HDM challenge and Dex treatment in aged mice (Fig. 3B). Since the ratio of GRα/GRβ expression was significantly lower in lung DCs than in steroid-responsive AECs with HDM and Dex treatment, these findings strongly suggest an indispensable contribution of aged DC in steroid insensitivity. Senescence-associated secretory phenotype (SASP) such as P16, MMP10, MMP12, and Col1a1 that wield potent effects on adjacent DC, were markedly elevated with aging both in AECs and lung DCs (Fig. 3, C and D). Collectively, these findings suggest that senescence-associated changes in aged mice may influence DC activity such as antigen uptake and migration to the DLn, thereby influencing airway inflammation and T cell-mediated allergic immune response to the inhaled aeroallergen HDM.
An Increase in Age Progressively Decreases SPLUNC1 Levels
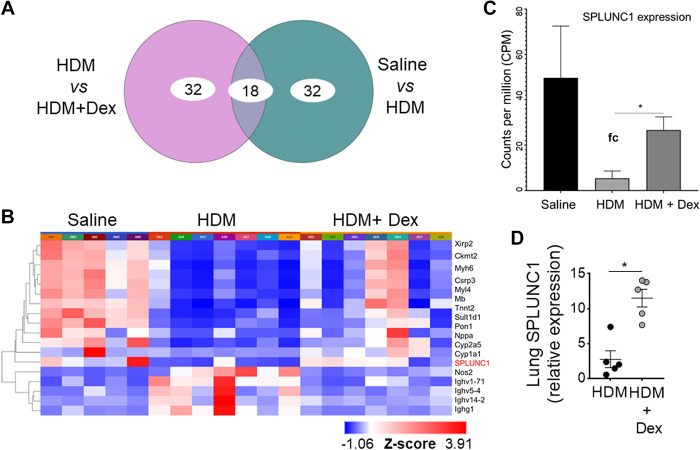
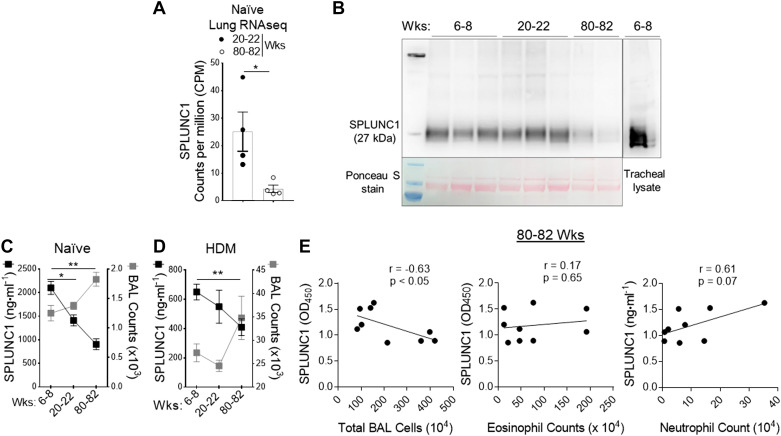
We used genome-wide expression profiling of the asthmatic lung transcriptome by RNA-Seq, using the treatment model of HDM-induced asthma in aged mice (Fig. 4) to directly identify lung genes that demonstrate persistently upregulated expression despite Dex therapy during aging. All samples passed stringent quality control criteria. RNA-Seq-based gene expression analysis of aged lungs showed significantly differentially expressed genes with or without Dex treatment (Fig. 4, A and B). We observed that mRNA expression of SPLUNC1 was significantly modulated with HDM and further by Dex treatment (Fig. 4, C and D). In addition, we extracted RNA-Seq data of counts per million (CPM)-normalized SPLUNC1 gene expression from naïve mice. Comparison of adult (20–22 wk old) and aged (80–82 wk old) naïve mice revealed a significant decrease in lung SPLUNC1 transcript level in aged as compared with adult mice (Fig. 5A).
Figure 4.
RNA-Seq of HDM-challenged aged mice treated with or without Dex. RNA sequencing data were preprocessed and analyzed using the Picard-STAR-limma pipeline. A: Venn diagram shows the top 50 genes that were significantly differentially expressed between no treatment vs. Dex treatment. B: using unsupervised hierarchical clustering (HC) analysis based on the differentially expressed genes (DEGs), heatmaps for the top genes among the three groups were generated. Bar graph shows (C) three-group comparison of SPLUNC1/BPIFA1 gene expression using counts per million (CPM) data and (D) qRT-PCR of lung SPLUNC1 expression. Data are represented as means ± SE (n = 4 or 5 mice per group, *P< 0.01, HDM vs. HDM +Dex, unpaired t test). HDM, house-dust mite; SPLUNC1, short palate, lung, and nasal epithelial clone 1.
Figure 5.
Age-dependent progressive decline of epithelium-derived SPLUNC1 levels. A: the bar chart shows counts per million (CPM)-normalized to SPLUNC1 gene expression extracted from naïve adult (20–22 wk old) and aged (80–82 wk old) mice lung RNA-Seq data. B: immunoblot showing SPLUNC1 level in BAL from the young, adult, and aged naïve mice; [upper lane, SPLUNC1 protein in BAL samples (50 µL/lane), and tracheal lysate; lower lane, corresponding Ponceau S staining of the nitrocellulose membrane developed from the same blot]. SPLUNC1 level compared with corresponding total cell counts in BAL from (C) naïve and (D) HDM-challenged allergic mice as measured by ELISA. E: correlations between BAL levels of SPLUNC1 with absolute numbers of total and inflammatory cell types from HDM-inflamed aged lung. Pearson correlation coefficients and associated P values are shown for significant relationships only. Results are represented as means ± SE of two independent experiments, *P< 0.05, **P< 0.01. BAL, bronchoalveolar lavage; HDM, house-dust mite; SPLUNC1, short palate, lung, and nasal epithelial clone 1.
Furthermore, we detect SPLUNC1 levels in BAL from the young, adult, and aged naïve mice (Fig. 5B). As shown in Fig. 5, C and D, SPLUNC1 levels in BAL were markedly decreased with increasing age and further by HDM-induced airway inflammation. A negative correlation was found between SPLUNC1 protein level and total inflammatory cell numbers in HDM-induced aged mice; however, differential cell analysis reveals a modest association between inflammatory cell types (Fig. 5E). Congruently, these results showed that an age-dependent decline in SPLUNC1 level significantly correlated with airway inflammation, suggesting an anti-inflammatory role of SPLINC1 in the aging lung.
SPLUNC1 Induces DC Activation and Maturation
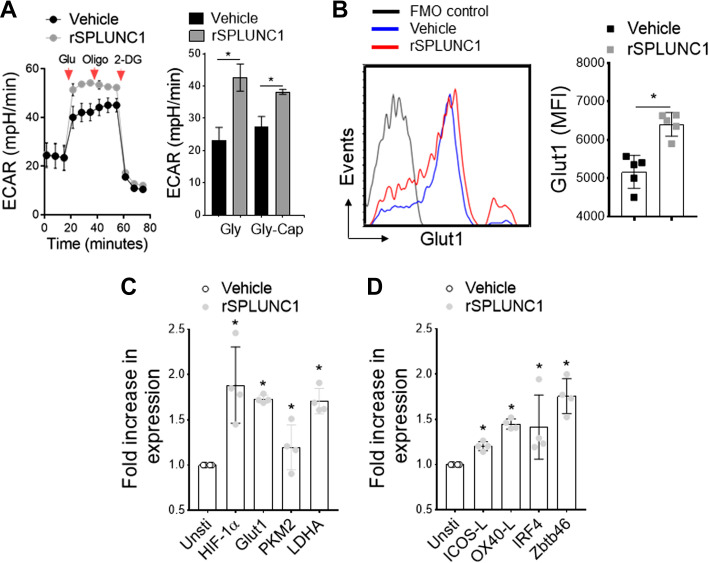
We and others have shown that allergen-mediated DC activation results in a striking Warburg-like metabolic shift to glycolysis, which is required for enhanced lysosomal acidification in mature DCs for activation and antigen presentation to T cells (48, 60, 61). To determine the biological function of SPLUNC1 on DC activation and maturation, we used real-time measurement of glycolysis in BMDCs isolated from young mice (6–8 wk old) and stimulated with and without recombinant SPLUNC1 (rSPLUNC1). In the presence of HDM, BMDCs showed typical ECAR changes in response to sequential addition of glucose (Glu), inhibition of mitochondrial ATP synthase by oligomycin (Oligo), and glycolysis inhibition by 2-DG, whereas significantly increased by rSPLUNC1 (5 μg/mL) in glycolysis (Gly) and glycolysis-capacity (Gly-Cap) as measured by XFp seahorse analyzer (Fig. 6A). Next, we considered whether rSPLUNC1 stimulation could induce glycolytic markers in HDM-pulsed BMDCs. As shown in Fig. 6B, incubation with rSPLUNC1 mediate a significant increase in Glut1 surface expression compared with HDM-pulsed control BMDCs. Furthermore, rSPLUNC1 markedly induces expression of glycolytic-associated genes, including HIF-1α, Glut1, PKM2, and LDHA (Fig. 6C), as well as activation/maturation markers (ICOS-L, OX40-L) and DC-specific transcription factors (IRF4, Zbtb46) (Fig. 6D). These data demonstrate a novel immunomodulatory function of SPLUNC1, inducing the DC activation/maturation process, thereby influencing allergic airway inflammation.
Figure 6.
SPLUNC1 induces DC glycolytic metabolism. HDM-pulsed BMDCs (6- to 8-wk-old mice) were stimulated with and without recombinant (r) SPLUNC1 (rSPLUNC1; 5 µg/mL) for 16 h before metabolic measurements. A: kinetic extracellular acidification rate (ECAR) in response to glucose (10 mM), oligomycin (2 µM), and 2-DG (100 mM) measuring glycolytic flux/capacity were graphed over time (arrowhead indicates sequential drug injection). Bar graph (right) represents glycolysis (Gly) and glycolytic capacity (Gly-Cap) of HDM-pulsed BMDCs in response to rSPLUNC1. Data are represented as means ± SE of two independent experiments. B: overlay flow histogram (left) and bar graph (right) showing mean fluorescence intensity of Glut1+ CD11c+MHCII+CD11b+ HDM-pulsed BMDCs treated with rSPLUNC1 and/or vehicle control. C and D: qRT-PCR of glycolytic genes, DC activation markers, and DC-specific transcription factors. Data are represented as means ± SE (n = 4–6, *P < 0.05, unpaired t test) of at least two independent experiment. BMDCs, bone marrow-derived dendritic cells; DC, dendritic cell; HDM, house-dust mite; SPLUNC1, short palate, lung, and nasal epithelial clone 1.
SPLUNC1 Modulates the Antigen Presentation Capacity of DC
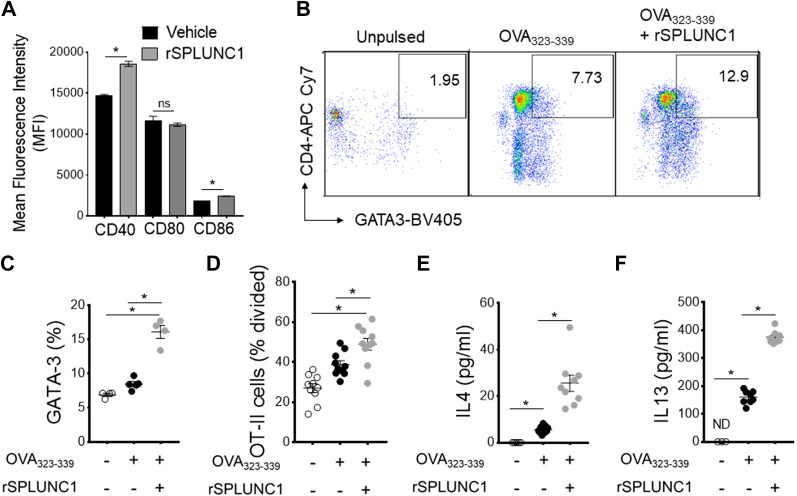
Since SPLUNC1 induces allergen-induced DC glycolytic reprogramming and maturation, we next hypothesized that SPLUNC1 might interfere with the immune priming capacity of DC to T cells. As shown in Fig. 7A, rSPLUNC1 significantly induces the surface expression of maturation markers CD40 and CD86 in HDM-pulsed BMDCs. Coculture experiments of splenic CD4+ T cells from mice expressing transgenic MHCII-restricted TCR that recognizes the OVA323–339 peptide showed that BMDCs incubated with rSPLUNC1 mediate significant increases in both Th2-specific transcription factor GATA3 expression and T cell proliferation (Fig. 7, B–D) compared with BMDCs cultured without rSPLUNC1 after ex vivo stimulation with the OVA323–339 peptide. Similarly, BMDCs stimulated with rSPLUNC1 showed augmented ability to induce Th2 cytokines, IL13, and IL4 productions in the supernatant following ex vivo stimulation with OVA323–339 peptide when cocultured with splenic T cells isolated from OT-II mice as compared with BMDCs culture in the absence of rSPLUNC1 (Fig. 7, E and F). These experiments collectively show the immune priming capacity of DC by SPLUNC1 to present antigen to CD4+ T cells, thereby promoting the Th2 immune response.
Figure 7.
SPLUNC1 modulates the antigen presentation capacity of DC. A: mean fluorescence intensity (MFI) of activation markers in CD11c+ MHCII+CD11b+ OVA323–339 peptide-pulsed BMDCs stimulated with or without rSPLUNC1 (5 µg/mL). OVA323–339 peptide-pulsed BMDCs were incubated (1: 5 ratio) with CFSE-labeled splenic OT-II cells for 4 days, and the effect of rSPLUNC1 on Th2 proliferation and differentiation were enumerated. B: pseudocolor plots show illustrative flow cytometry data and (C) frequencies of CD4+GATA3+ OT-II cells in coculture. D: OVA323–339 peptide-specific proliferation was represented as percentage divided OT-II cells and E and F: Th2 cytokines in coculture supernatant after 4 days were measured by ELISA (n = 4–9 per group; *P < 0.01, unpaired t test). Data are represented as means ± SE of at least two independent experiments. BMDCs, bone marrow-derived dendritic cells; DC, dendritic cell; SPLUNC1, short palate, lung, and nasal epithelial clone 1.
DISCUSSION
Among the many hallmarks of the aging process (in both mice and humans), is a progressive decline in immune function (immunosenescence) (62, 63). Asthma and its heterogeneity change according to age (4, 64, 65). Asthma is superimposed on a background of aging-associated immune changes in the lung, resulting from complex interactions with various factors such as environmental exposure, microbial triggers, or multiple comorbidities. Asthma in adults is highest among those in middle age; however, mortality is more significant in the older age group (2, 3). According to the World Allergy Organization (WAO), asthma in older adults is phenotypically different from that in young patients, which convolute the diagnosis, assessment, and management in this population (4, 7, 8, 66). Current pharmaceutical interventions to treat asthma in older adults are almost exclusively designed to alleviate symptoms. However, inflammation plays a seminal role in asthma development and progression. A recent clinical trial revealed that mediator-specific asthma controllers such as leukotriene-modifying agents (LTMs) significantly decreased the rate of recurring airway inflammation and are more effective in older adults than inhaled corticosteroids (67–69). The initiation and propagation of airway inflammation arise from several factors, including mediators generated by resident airway cells and recruited leukocytes. Developing a robust T cell response in the lungs requires efficient allergen recognition and activation and migration of lung DCs to the draining DLn, in which the T cell response is primed. Defects in DC function have been identified in some, but not all, studies of older populations (70–72).
Recent studies have detected a very high level of secreted SPLUNC1 (short palate, lung, and nasal epithelial clone1) proteins at the airway (nasal, tracheal, and bronchial) epithelium under the physiological conditions, which act as an airway sensor of innate immunity. SPLUNC1 exerts antimicrobial properties due to structural similarity with the bactericidal/permeability-increasing (BPI) protein fold-containing heterogeneous group of proteins (39, 40). Using SPLUNC1−/− mice, previous studies have demonstrated that lack of SPLUNC1 expression in the lung enhances eosinophilic airway inflammation and Th2 allergic asthma in an eotaxin-2-dependent mechanism by alveolar macrophages. However, it is unknown if SPLUNC1 has an immunomodulatory role in regulating the maturation of DC and responses to allergic sensitization impacting airway inflammation. Here, we investigated whether increased age modifies epithelium-derived SPLUNC1 levels and controls DC effector function, thereby bridging lung adaptive T cell-mediated immune response to inhaled allergen. HDM-inflamed lungs of aged mice manifested 1) decline in pulmonary function; 2) mixed granulomatous airway inflammation (predominantly of neutrophils and eosinophils), 3) Th1/Th17-high and Th2-low immune response (which is poorly controlled by Dex treatment) as compared with adult mice. This is consistent with previous findings from people with asthma and mouse models showing allergen-induced eosinophilic inflammation and Th2 immune response that are fully responsive to Dex treatment in adults, whereas allergen-induced neutrophilic inflammation and Th1/Th17 immune response that are refractory to Dex treatment in aging lungs (7, 11, 13, 65).
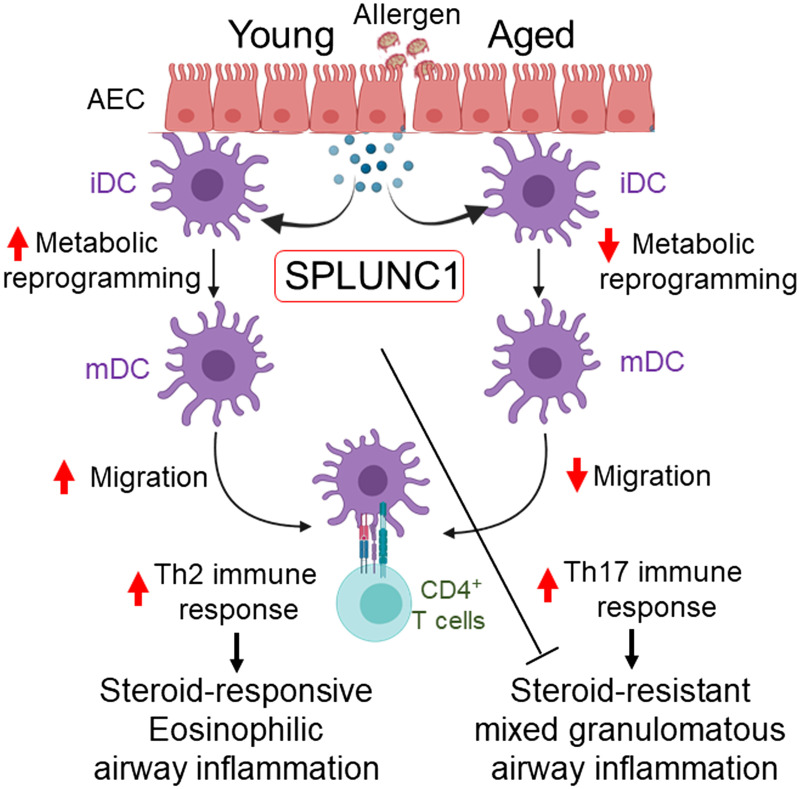
Interestingly, nasal AECs isolated from subjects with asthma (Severe Asthma Research Program 3 cohort) carrying CC allele expressed less SPLUNC1, which negatively correlated with the severity of the disease (asthma endpoints such as FEV%, fractional exhaled nitric oxide, and serum IgE) as compared with CT and TT genotypes (73). Moreover, IL13-induced increased eotaxin-3 production in AECs by CC genotype is reversed by rSPLUNC1 treatment. In accord with that, our results showed an age-dependent decline in SPLUNC1 level that impacts DC immune priming function and subsequent T cell polarization, thereby resulting in Th17/neutrophilic polarized allergic response and accelerating steroid-resistant severe asthma (Fig. 8).
Figure 8.
Schematic representation and proposed mechanism of SPLUNC1 and asthma heterogeneity in aging. Increasing age progressively decreases epithelium-derived SPLUNC1 levels, modulating DC effector function and developing dysregulated T cell-mediated immune response to the inhaled allergen, which results in severe airway inflammation and asthma heterogeneity in aging. DC, dendritic cell; iDC, immature DC; mDC, mature DC; SPLUNC1, short palate, lung, and nasal epithelial clone 1.
The lung microenvironment changes with age that compromise DC migration, distribution, and T cell priming ability (74, 75). Within the lung, in the absence of inflammation, the respiratory mucosa has an integrated network of two conventional DC (cDC) subsets (CD103+ and CD11b+) (45, 76). It has been posited that aging does not change lung DC numbers; however, it alters the effector functions and subset frequencies (28). DC from aged subjects was reported in the semiactivated state with an elevated basal level of NF-κB and proinflammatory cytokines (29). Consistent with these, we found a progressive decrease in frequencies and numbers of HDM-bearing Alexa-647+ DC in the DLn as mice aged, suggesting that the intrinsic ability of DC to migrate to DLn is compromised in aged mice. Candidate molecules that might vary in expression and affect DC maturation and migration include epithelium-derived chemokines, cytokines, pleiotropic bioactive lipid, and protein mediators. Congruently, our findings revealed that HDM-induced eosinophilic inflammation and Th2 immunity in adults are fully responsive to Dex treatment and correlated with the SPLUNC1 level. In contrast, we found HDM promotes mixed granulomatous airway inflammation comprising neutrophils in the aged lung, which are unresponsive to Dex treatment and induce SPLUNC1 expression, suggesting an anti-inflammatory function of SPLUNC1 in the aging lung.
Activation of the primary antigen-presenting DC is coupled to rapid glycolytic shift and enhanced lysosomal acidification, which is required for an effective immune-priming effector function (48, 60). We further examined if SPLUNC1 could exert an immunomodulatory effect on DC metabolic reprogramming and immune priming function. Glycolytic reprogramming was assessed in vitro in HDM-pulsed cultured BMDCs treated with recombinant (r) SPLUNC1. Our results reveal that rSPLUNC1 accelerates HDM-induced glycolytic shift and upregulates Glut1 expressions in DC, accompanied by an increase in expression of glycolytic pathway associated genes including HIF1-α, PKM2, and LDHA. Furthermore, in response to rSPLUNC1 stimulation, we observed increased expression of costimulatory molecules, Zbtb46 (cDC-specific zinc finger transcription factor) (77) and IRF4 (78), which are crucial for maturation and Th2 immune priming capacity of DC. In a potential mechanistic link, our results showed enhanced antigen presentation capabilities of OVA323–339 peptide-pulsed BMDCs in the presence of rSPLUNC1, as evidenced by increased proliferation of naïve OT-II cells and Th2 effector cytokines IL4 and IL13 production. Collectively, these data indicate that SPLUNC1 modulates DC glycolytic reprogramming and accompanies DC maturation and Th2 differentiation.
SPLUNC1 protein comprises an S18-ENaC-regulatory domain, a central lipid-binding region (tubular lipid-binding proteins), and a C-terminal domain that binds to Ca2+ influx channel Orai1 (43, 79). Interestingly, lung DC activation and migration are driven by calcium-activated potassium channel KCa3.1 (80), which could act as a binding partner for epithelial-derived SPLUNC1 proteins regulating DC effector function, and needs to be addressed in the future. Moreover, it is unlikely that a single factor is driving asthma heterogeneity and airway inflammation in aging. Thus, a deeper understanding of the DC-AEC cross talk and how senescence impacts airway inflammation and influences asthma progression are further warranted through state-of-the-art single-cell RNA-Seq (scRNA-Seq) signatures of aging (12, 64).
In conclusion, the present study demonstrates an explanation for asthma heterogeneity and airway inflammation in the aging lung that correlates with SPLUNC1 expression. Moreover, RNA-Seq of aged lungs reveals that SPLUNC1 expression is induced by steroid (Dex) treatment, suggesting an anti-inflammatory function of SPLUNC1, which further influences T cell-mediated adaptive immune responses by modulating effector DC activation/maturation. Nevertheless, the results of our study highlight the causal role of SPLUNC1 expression that may be in part coupled with exaggerated airway inflammation and asthma heterogeneity in aging.
GRANTS
This study was supported by the Department of Pathobiology, College of Veterinary Medicine, Auburn University and by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under Award No. R03AI153794-01A1 (to A. Mishra).
DISCLAIMERS
This article was prepared by A.M. The opinions expressed in this article are the authors’ own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.J., J.Y., S.M., M.S., and A.M. performed experiments; A.K.J., J.Y., M.S., A.S., A.K.M., and A.M. analyzed data; A.K.J., J.Y., A.S., and A.M. interpreted results of experiments; A.K.J., J.Y., M.S., A.S., A.K.M., and A.M. prepared figures; A.K.J., J.Y., and A.M. drafted manuscript; A.K.J., J.Y., S.M., and A.M. edited and revised manuscript; A.K.J., J.Y., and A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the University of Minnesota Informatics Institute (UMII) for the support and analysis of RNA Sequence experiments.
REFERENCES
- 1.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest 116: 603–613, 1999. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 2.Moorman JE, Mannino DM. Increasing U.S. asthma mortality rates: who is really dying? J Asthma 38: 65–71, 2001. doi: 10.1081/jas-100000023. [DOI] [PubMed] [Google Scholar]
- 3.Baptist AP, Busse PJ. Asthma over the age of 65: all's well that ends well. J Allergy Clin Immunol Pract 6: 764–773, 2018. doi: 10.1016/j.jaip.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr TF, Bleecker E. Asthma heterogeneity and severity. World Allergy Organ J 9: 41, 2016. doi: 10.1186/s40413-016-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore WC, Bleecker ER. Asthma heterogeneity and severity-why is comprehensive phenotyping important? Lancet Respir Med 2: 10–11, 2014. doi: 10.1016/S2213-2600(13)70288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellia V, Pedone C, Catalano F, Zito A, Davi E, Palange S, Forastiere F, Incalzi RA. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest 132: 1175–1182, 2007. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 7.Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general-population. Chest 100: 935–942, 1991. doi: 10.1378/chest.100.4.935. [DOI] [PubMed] [Google Scholar]
- 8.Meyer KC, Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax 54: 697–700, 1999. doi: 10.1136/thx.54.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zureik M, Orehek J. Diagnosis and severity of asthma in the elderly: results of a large survey in 1,485 asthmatics recruited by lung specialists. Respiration 69: 223–228, 2002. doi: 10.1159/000063624. [DOI] [PubMed] [Google Scholar]
- 10.Mathur SK, Schwantes EA, Jarjour NN, Busse WW. Age-related changes in eosinophil function in human subjects. Chest 133: 412–419, 2008. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yagi T, Sato A, Hayakawa H, Ide K. Failure of aged rats to accumulate eosinophils in allergic inflammation of the airway. J Allergy Clin Immunol 99: 38–47, 1997. doi: 10.1016/s0091-6749(97)70298-7. [DOI] [PubMed] [Google Scholar]
- 12.Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol 126: 690–699, 2010. doi: 10.1016/j.jaci.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyenhuis SM, Schwantes EA, Evans MD, Mathur SK. Airway neutrophil inflammatory phenotype in older subjects with asthma. J Allergy Clin Immunol 125: 1163–1165, 2010. doi: 10.1016/j.jaci.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho YJ, Ke. L. Decreased glucocorticoid binding affinity to glucocorticoid receptor is important in the poor response to steroid therapy of older-aged patients with severe bronchial asthma. Allergy Asthma Proc 24: 353–358, 2003. [PubMed] [Google Scholar]
- 15.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, Ray P, Ray A. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest 125: 3037–3050, 2015. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 181: 4089–4097, 2008. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high) immunophenotypes: potential benefits of calcitriol. J Allergy Clin Immunol 136: 628–637.e4, 2015. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh P, Wicher S, Khandalavala K, Pabelick CM, Britt RD Jr, Prakash YS. Cellular senescence in the lung across the age spectrum. Am J Physiol Lung Cell Mol Physiol 316: L826–L842, 2019. doi: 10.1152/ajplung.00424.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat Immunol 3: 673–680, 2002. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 20.Yanagi S, Tsubouchi H, Miura A, Matsuo A, Matsumoto N, Nakazato M. The impacts of cellular senescence in elderly pneumonia and in age-related lung diseases that increase the risk of respiratory infections. Int J Mol Sci 18: 503, 2017. doi: 10.3390/ijms18030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan L, Du XZ, Tang S, Wu SY, Wang LY, Xiang Y, Qu XP, Liu HJ, Qin XQ, Liu C. ITGB4 deficiency induces senescence of airway epithelial cells through p53 activation. FEBS J 286: 1191–1203, 2019. doi: 10.1111/febs.14749. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZN, Su RN, Yang BY, Yang KX, Yang LF, Yan Y, Chen ZG. Potential role of cellular senescence in asthma. Front Cell Dev Biol 8: 59, 2020. doi: 10.3389/fcell.2020.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal A. Dendritic cell-airway epithelial cell cross-talk changes with age and contributes to chronic lung inflammatory diseases in the elderly. Int J Mol Sci 18: 1206, 2017. doi: 10.3390/ijms18061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein K, Schilling J, Zissler U, Schmidt-Weber C, Heinbockel L, Goldmann T, Heine H. Studies of the influence of culture conditions and asthma-associated cytokines on airway epithelial cell activation and interaction with dendritic cells (Abstract). Allergy 73, Suppl 105: 828–828, 2018. https://onlinelibrary.wiley.com/doi/epdf/10.1111/all.13540. [Google Scholar]
- 25.Paplinska-Goryca M, Misiukiewicz-Stepien P, Proboszcz M, Gorska K, Krenke R. The impact of the interactions between airway epithelium, dendritic cells and macrophages on TSLP and IL-33 epithelial expression in asthma and healthy controls (Abstract). Allergy 74, Suppl 106: 136–136, 2019. https://onlinelibrary.wiley.com/doi/full/10.1111/all.13959. [Google Scholar]
- 26.Kool M, Willart MAM, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis e Sousa C, Hammad H, Lambrecht BN. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 34: 527–540, 2011. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Nobs SP, Pohlmeier L, Li F, Kayhan M, Becher B, Kopf M. GM-CSF instigates a dendritic cell-T-cell inflammatory circuit that drives chronic asthma development. J Allergy Clin Immunol 147: 2118–2133.e3, 2021. doi: 10.1016/j.jaci.2020.12.638. [DOI] [PubMed] [Google Scholar]
- 28.Prakash S, Agrawal S, Vahed H, Ngyuen M, BenMohamed L, BenMohamad L, Gupta S, Agrawal A. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol 7: 1386–1394, 2014. [Erratum in Mucosal Immunol 7:1280, 2014]. doi: 10.1038/mi.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev 10: 336–345, 2011. doi: 10.1016/j.arr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal S, Ganguly S, Tran A, Sundaram P, Agrawal A. Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging (Albany NY) 8: 1223–1235, 2016. doi: 10.18632/aging.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wansleeben C, Bowie E, Hotten DF, Yu YRA, Hogan BLM. Age-related changes in the cellular composition and epithelial organization of the mouse trachea. PLoS One 9: e93496, 2014. doi: 10.1371/journal.pone.0093496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol 6: 335–346, 2013. doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Caballero A, Rasmussen JE, Gaillard E, Watson MJ, Olsen JC, Donaldson SH, Stutts MJ, Tarran R. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc Natl Acad Sci USA 106: 11412–11417, 2009. [Erratum in Proc Natl Acad Sci USA 106: 15091, 2009]. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hobbs CA, Blanchard MG, Alijevic O, Tan CD, Kellenberger S, Bencharit S, Cao R, Kesimer M, Walton WG, Henderson AG, Redinbo MR, Stutts MJ, Tarran R. Identification of the SPLUNC1 ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol 305: L990–L1001, 2013. [Erratum in Am J Physiol Lung Cell Mol Physiol 306: L708, 2014]. doi: 10.1152/ajplung.00103.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bingle L, Bingle CD. Distribution of human PLUNC/BPI fold-containing (BPIF) proteins. Biochem Soc Trans 39: 1023–1027, 2011. doi: 10.1042/BST0391023. [DOI] [PubMed] [Google Scholar]
- 36.Campos MA, Abreu AR, Nlend MC, Cobas MA, Conner GE, Whitney PL. Purification and characterization of PLUNC from human tracheobronchial secretions. Am J Respir Cell Mol Biol 30: 184–192, 2004. doi: 10.1165/rcmb.2003-0142OC. [DOI] [PubMed] [Google Scholar]
- 37.Levy O, Ooi CE, Elsbach P, Doerfler ME, Lehrer RI, Weiss J. Antibacterial proteins of granulocytes differ in interaction with endotoxin. Comparison of bactericidal/permeability-increasing protein, p15s, and defensins. J Immunol 154: 5403–5410, 1995. [PubMed] [Google Scholar]
- 38.Chu HW, Thaikoottathil J, Rino JG, Zhang G, Wu Q, Moss T, Refaeli Y, Bowler R, Wenzel SE, Chen Z, Zdunek J, Breed R, Young R, Allaire E, Martin RJ. Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J Immunol 179: 3995–4002, 2007. doi: 10.4049/jimmunol.179.6.3995. [DOI] [PubMed] [Google Scholar]
- 39.Gally F, Di YP, Smith SK, Minor MN, Liu Y, Bratton DL, Frasch SC, Michels NM, Case SR, Chu HW. SPLUNC1 promotes lung innate defense against Mycoplasma pneumoniae infection in mice. Am J Pathol 178: 2159–2167, 2011. doi: 10.1016/j.ajpath.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukinskiene L, Liu Y, Reynolds SD, Steele C, Stripp BR, Leikauf GD, Kolls JK, Di YP. Antimicrobial activity of PLUNC protects against Pseudomonas aeruginosa infection. J Immunol 187: 382–390, 2011. doi: 10.4049/jimmunol.1001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Britto CJ, Liu Q, Curran DR, Patham B, Dela Cruz CS, Cohn L. Short palate, lung, and nasal epithelial clone-1 is a tightly regulated airway sensor in innate and adaptive immunity. Am J Respir Cell Mol Biol 48: 717–724, 2013. doi: 10.1165/rcmb.2012-0072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaikoottathil JV, Martin RJ, Di PY, Minor M, Case S, Zhang B, Zhang G, Huang H, Chu HW. SPLUNC1 deficiency enhances airway eosinophilic inflammation in mice. Am J Respir Cell Mol Biol 47: 253–260, 2012. doi: 10.1165/rcmb.2012-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu T, Huang J, Moore PJ, Little MS, Walton WG, Fellner RC, Alexis NE, Peter Di Y, Redinbo MR, Tilley SL, Tarran R. Identification of BPIFA1/SPLUNC1 as an epithelium-derived smooth muscle relaxing factor. Nat Commun 8: 14118, 2017. doi: 10.1038/ncomms14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76: 34–40, 1998. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal AK, Sandey M, Suryawanshi A, Cattley RC, Mishra A. Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function. Immun Inflamm Dis 7: 201–213, 2019. doi: 10.1002/iid3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra A, Brown AL, Yao X, Yang S, Park SJ, Liu C, Dagur PK, McCoy JP, Keeran KJ, Nugent GZ, Jeffries KR, Qu X, Yu ZX, Levine SJ, Chung JH. Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase. Nat Commun 6: 6224, 2015. doi: 10.1038/ncomms7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra A, Gordon EM, Yao X, Saxena A, Dagur PK, McCoy J, Keeran K, Jeffries K, Qua X, Yu ZX, Levine SJ. Ldl-receptor related protein-1 attenuates house dust mite-induced airway inflammation by suppressing dendritic cell-mediated adaptive immune responses. Am J Resp Crit Care 195: A2977, 2017. doi: 10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A2977. [DOI] [Google Scholar]
- 48.Jaiswal AK, Makhija S, Stahr N, Sandey M, Suryawanshi A, Mishra A. Pyruvate kinase M2 in lung APCs regulates alternaria-induced airway inflammation. Immunobiology 225: 151956, 2020. doi: 10.1016/j.imbio.2020.151956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King MJ, Bukantz SC, Phillips S, Mohapatra SS, Tamulis T, Lockey RF. Serum total IgE and specific IgE to Dermatophagoides pteronyssinus, but not eosinophil cationic protein, are more likely to be elevated in elderly asthmatic patients. Allergy Asthma Proc 25: 321–325, 2004. [PubMed] [Google Scholar]
- 50.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, Ota C, Costa R, Schiller HB, Lindner M, Wagner DE, Günther A, Königshoff M. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J 50: 1602367, 2017. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4: 1798–1806, 2009. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 52.Niu N, Manickam V, Kalari KR, Moon I, Pelleymounter LL, Eckloff BW, Wieben ED, Schaid DJ, Wang L. Human glucocorticoid receptor alpha gene (NR3C1) pharmacogenomics: gene resequencing and functional genomics. J Clin Endocrinol Metab 94: 3072–3084, 2009. doi: 10.1210/jc.2008-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krieger S, Sorrells SF, Nickerson M, Pace TWW. Mechanistic insights into corticosteroids in multiple sclerosis: war horse or chameleon? Clin Neurol Neurosurg 119: 6–16, 2014. doi: 10.1016/j.clineuro.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 54.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE 2005: pe48, 2005. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 55.Hinds TD Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, Sanchez ER. Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol Endocrinol 24: 1715–1727, 2010. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 318: 635–641, 1985. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Castro M, Elliot S, Kino T, Bamberger C, Karl M, Webster E, Chrousos GP. The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGR beta): tissue levels, mechanism of action, and potential physiologic role. Mol Med 2: 597–607, 1996. [PMC free article] [PubMed] [Google Scholar]
- 58.Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor beta isoform. Specificity and mechanisms of action. J Biol Chem 274: 27857–27866, 1999. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- 59.Vazquez-Tello A, Halwani R, Hamid Q, Al-Muhsen S. Glucocorticoid receptor-beta up-regulation and steroid resistance induction by IL-17 and IL-23 cytokine stimulation in peripheral mononuclear cells. J Clin Immunol 33: 466–478, 2013. doi: 10.1007/s10875-012-9828-3. [DOI] [PubMed] [Google Scholar]
- 60.Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, Artyomov MN, Jones RG, Pearce EL, Pearce EJ. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol 15: 323–332, 2014. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mishra A. Metabolic plasticity in dendritic cell responses: implications in allergic asthma. J Immunol Res 2017: 5134760, 2017. doi: 10.1155/2017/5134760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, Accardi G. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol 10: 2247, 2019. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 8: 1960, 2017. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, Stempel DA, Lima JJ, Fish JE, Wilson SR, Boyd C, Patel KV, Irvin CG, Yawn BP, Halm EA, Wasserman SI, Sands MF, Ershler WB, Ledford DK; Asthma in Elderly workshop participants. Asthma in the elderly: current understanding and future research needs–a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol 128: S4–S24, 2011. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akuthota P, Busse WW. How sex and age of asthma onset influence difficult asthma heterogeneity. J Allergy Clin Immunol Pract 8: 3407–3408, 2020. doi: 10.1016/j.jaip.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 66.Bellia V, Battaglia S, Catalano F, Scichilone N, Incalzi RA, Imperiale C, Rengo F. Aging and disability affect misdiagnosis of COPD in elderly asthmatics: the SARA study. Chest 123: 1066–1072, 2003. doi: 10.1378/chest.123.4.1066. [DOI] [PubMed] [Google Scholar]
- 67.Barua P, O'Mahony MS. Overcoming gaps in the management of asthma in older patients: new insights. Drugs Aging 22: 1029–1059, 2005. doi: 10.2165/00002512-200522120-00004. [DOI] [PubMed] [Google Scholar]
- 68.Korenblat PE, Kemp JP, Scherger JE, Minkwitz MC, Mezzanotte W. Effect of age on response to zafirlukast in patients with asthma in the Accolate Clinical Experience and Pharmacoepidemiology Trial (ACCEPT). Ann Allergy Asthma Immunol 84: 217–225, 2000. doi: 10.1016/S1081-1206(10)62759-7. [DOI] [PubMed] [Google Scholar]
- 69.Creticos P, Knobil K, Edwards LD, Rickard KA, Dorinsky P. Loss of response to treatment with leukotriene receptor antagonists but not inhaled corticosteroids in patients over 50 years of age. Ann Allerg Asthma Immunol 88: 401–409, 2002. doi: 10.1016/S1081-1206(10)62372-1. [DOI] [PubMed] [Google Scholar]
- 70.Agrawal A, Agrawal S, Cao JN, Su HF, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol 178: 6912–6922, 2007. doi: 10.4049/jimmunol.178.11.6912]. [DOI] [PubMed] [Google Scholar]
- 71.Rahmatpanah F, Agrawal S, Scarfone VM, Kapadia S, Mercola D, Agrawal A. Transcriptional profiling of age-associated gene expression changes in human circulatory CD1c(+) myeloid dendritic cell subset. J Gerontol A Biol Sci Med Sci 74: 9–15, 2019. doi: 10.1093/gerona/gly106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agrawal A, Tay A, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol 182: 1138–1145, 2009. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaefer N, Li X, Seibold MA, Jarjour NN, Denlinger LC, Castro M, Coverstone AM, Teague WG, Boomer J, Bleecker ER, Meyers DA, Moore WC, Hawkins GA, Fahy J, Phillips BR, Mauger DT, Dakhama A, Gellatly S, Pavelka N, Berman R, Di YP, Wenzel SE, Chu HW. The effect of BPIFA1/SPLUNC1 genetic variation on its expression and function in asthmatic airway epithelium. JCI Insight 4: e127237, 2019. doi: 10.1172/jci.insight.127237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res 68: 6341–6349, 2008. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao JC, Zhao JX, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest 121: 4921–4930, 2011. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38: 322–335, 2013. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 77.Satpathy AT, Kc W, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med 209: 1135–1152, 2012. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun 4: 2990, 2013. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ning F, Wang C, Berry KZ, Kandasamy P, Liu H, Murphy RC, Voelker DR, Nho CW, Pan CH, Dai S, Niu L, Chu HW, Zhang G. Structural characterization of the pulmonary innate immune protein SPLUNC1 and identification of lipid ligands. FASEB J 28: 5349–5360, 2014. doi: 10.1096/fj.14-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shao Z, Makinde TO, Agrawal DK. Calcium-activated potassium channel KCa3.1 in lung dendritic cell migration. Am J Respir Cell Mol Biol 45: 962–968, 2011. doi: 10.1165/rcmb.2010-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]