Keywords: adaptation, balance, gait, margin of stability, perturbation
Abstract
Introducing unexpected perturbations to challenge gait stability is an effective approach to investigate balance control strategies. Little is known about the extent to which people can respond to small perturbations during walking. This study aimed to determine how subjects adapted gait stability to multidirectional perturbations with small magnitudes applied on a stride-by-stride basis. Ten healthy young subjects walked on a treadmill that either briefly decelerated belt speed (“stick”), accelerated belt speed (“slip”), or shifted the platform medial-laterally at right leg mid-stance. We quantified gait stability adaptation in both anterior-posterior and medial-lateral directions using margin of stability and its components, base of support, and extrapolated center of mass. Gait stability was disrupted upon initially experiencing the small perturbations as margin of stability decreased in the stick, slip, and medial shift perturbations and increased in the lateral shift perturbation. Gait stability metrics were generally disrupted more for perturbations in the coincident direction. Subjects employed both feedback and feedforward strategies in response to the small perturbations, but mostly used feedback strategies during adaptation. Subjects primarily used base of support (foot placement) control in the lateral shift perturbation and extrapolated center of mass control in the slip and medial shift perturbations. These findings provide new knowledge about the extent of gait stability adaptation to small magnitude perturbations applied on a stride-by-stride basis and reveal potential new approaches for balance training interventions to target foot placement and center of mass control.
NEW & NOTEWORTHY Little is known about if and how humans can adapt to small magnitude perturbations experienced on a stride-by-stride basis during walking. Here, we show that even small perturbations disrupted gait stability and that subjects could still adapt their reactive balance control. Depending on the perturbation direction, subjects might prefer adjusting their foot placement over their center of mass and vice versa. These findings could help potentially tune balance training to target specific aspects of balance.
INTRODUCTION
Perturbations during walking can induce potential losses of balance and elicit corrective locomotor adaptations, which are useful for understanding human balance control (1, 2). There are multiple approaches to perturb gait stability, including split-belt walking (1, 3), visual flow distortions (4, 5), waist pulling (6, 7), platform displacements (8, 9), and rapid changes in treadmill belt speed (10, 11). Studies using discrete perturbations such as waist pulling, platform displacements, and changes in treadmill belt speeds often apply the perturbation once out of every 5–20 strides (6–11). These large magnitude perturbations are less frequent and often require recovery steps to regain balance. Currently, there is no clear optimal frequency and magnitude for perturbation-based balance training during walking (12, 13), but more frequent exposure to perturbations seems to be beneficial for developing long-term fall-resisting skills (14, 15). Small magnitude perturbations are also more easily navigated by older adults (16, 17). As such, applying frequent repeated perturbations with small magnitudes during walking to create small but frequent losses of balance could be an effective approach for balance training during walking. Little is known, however, about the extent to which humans can adapt to small magnitude discrete balance perturbations applied on a stride-by-stride basis during walking. This approach could reveal stride-to-stride adaptation to almost imperceptible perturbations to gait stability, which would provide additional insight about how humans control, maintain, and adapt their stability during walking.
The margin of stability is a well-accepted metric to quantify stability of human walking (18). The margin of stability is the distance between the edge of the base of support and the extrapolated center of mass (19). People tend to maintain a constant margin of stability when walking on different surfaces (20, 21). When external conditions in the environment disrupt the steady state of walking and its margin of stability, increasing the base of support is effective for maintaining stability based on the inverted pendulum model (22) and has been demonstrated in multiple gait studies (6, 23, 24). For steps before and after unexpected treadmill belt acceleration perturbations during walking, one study showed that margin of stability decreased, and subjects took fewer recovery steps, suggesting improved or preserved stability (25). Treadmill belt speed-induced backward loss-of-balance perturbations can lead to larger margin of stability reductions in older adults compared with young adults (26). Interestingly, these margins of stability responses scaled with the perturbation intensity in both the young and older adults (26). The extent to which humans can control their margin of stability in response to small magnitude perturbations applied on a stride-by-stride basis remains unknown.
Analyzing the base of support and the extrapolated center of mass components of margin of stability could provide insights on how the body reacts to perturbations and maintains stability. Controlling foot placement through the swing leg and controlling center of mass through the stance leg are two main balance strategies for perturbed walking (6, 27, 28). In young adults, adaptation to multidirectional waist-pulling perturbations has resulted in increased medial-lateral foot placement at perturbation onset, potentially to proactively regulate balance, and also increased anterior-posterior foot placement and margin of stability at contralateral heel strike, perhaps to reactively regain stability (29). Older adults have demonstrated that they tend to adjust their extrapolated center of mass, potentially to transfer the improved stability of the trained leg to the untrained leg in response to treadmill belt acceleration perturbations (30). Treadmill belt slip perturbations have also resulted in the lengthening of the base of support and shortening the forward excursion of the extrapolated center of mass in poststroke individuals, effectively increasing their anterior-posterior margin of stability and overall stability (31). Thus, separating margin of stability into its components, the base of support and extrapolated center of mass, will provide greater insight on how humans adapt, if at all, to frequent small magnitude perturbations.
The primary purpose of this study was to determine how small treadmill perturbations applied on a stride-by-stride basis in healthy young adults affected margin of stability and its components (base of support and extrapolated center of mass). We rapidly accelerated or decelerated treadmill belt speed to create “slip” or “stick” perturbations, respectively. We also rapidly shifted (i.e., translated) the treadmill medially or laterally to create treadmill shift perturbations in the medial or lateral direction, respectively. We applied the treadmill perturbations at right mid-stance of each stride, except for no-perturbation “catch” strides that occurred randomly in each batch of five strides. The purpose of the “catch” strides was to probe whether subjects were potentially adopting a feedforward strategy in response to the small magnitude perturbations encountered during perturbed walking. Experimental protocols that apply perturbations trial-by-trial with unexpected catch trials have typically been used in goal-directed arm reaching studies (32, 33). We are extending that protocol to walking to gain insights on the adaptability of balance control during walking. Because we applied perturbations on a stride-by-stride basis, the strengths of the perturbations were limited to avoid the need for recovery steps, which are often necessary during larger treadmill-induced trip and slip perturbations that seek to induce a fall.
The first hypothesis was that when subjects first experienced the small perturbations, their gait stability would be disrupted (i.e., become less stable) and then adapt to regain stability as they gained more experience with the perturbations. As such, we expected margin of stability to decrease upon first experiencing the perturbations and then increase over time with more exposure to the perturbations. The second hypothesis was that subjects would adopt more feedforward strategies as they gained more experience with the perturbations. We expected that margin of stability, base of support, and extrapolated center of mass during catch strides would differ from the averaged levels of the unperturbed strides before the perturbed period and deviate more as subjects gained more experience with the perturbations.
To assess the extent of the expected adaptation, we also sought to determine whether perturbation direction and size affected the responses and adaptation to the perturbations. We hypothesized that the anterior-posterior margin of stability and its components would be more sensitive to the treadmill belt perturbations, which were in the coincident (anterior-posterior) direction and likewise, medial-lateral margin of stability and its components would be more sensitive to the treadmill shift perturbations, which were in the coincident (medial-lateral) direction. We also hypothesized that adaptation of margin of stability and its components would scale with perturbation size.
METHODS
Participants, Setup, and Protocol
Ten healthy young subjects (4 males, 6 females; age: 21.7 ± 2.4 yr) participated in this study. The Institutional Review Board of the University of Central Florida approved the experimental protocol (SBE-16-12831). The study met all requirements in accordance with the Declaration of Helsinki, and all subjects provided their written informed consent.
Subjects walked on a dual split-belt instrumented treadmill (M-gait, Motekforce Link, Amsterdam, The Netherlands) operating at 300 Hz as we recorded their lower limb kinematics using the 16-marker Conventional Lower Body Markerset and a passive motion capture system (OptiTrack, NaturalPoint Inc., Corvallis, OR; 13 Prime13W and 9 Prime13 cameras) operating at 240 Hz. The treadmill has independent speed control for each treadmill belt, and the treadmill is mounted on a moveable platform such that the treadmill can rapidly translate side-to-side in real-time. These features were used to produce treadmill perturbations.
We programmed the treadmill (D-Flow, version 3.28.0, Motekforce Link, Amsterdam, The Netherlands) to apply perturbations during mid-stance of the right leg on a stride-by-stride basis. The perturbation onset occurred when the vertical projection of the estimated center of mass position (the average position of the four pelvis markers: left anterior superior iliac spine, right anterior superior iliac spine, left posterior superior iliac spine, and right posterior superior iliac spine) resided between the right toe (2nd metatarsal) and right heel markers in the anterior-posterior direction and when the left heel marker was at least 5 cm higher than the right toe marker (Fig. 1A). The duration of each perturbation from onset to termination was ∼400 ms such that the treadmill could return to the neutral unperturbed position and baseline belt speed (1.0 m/s) before or shortly after left heel strike.
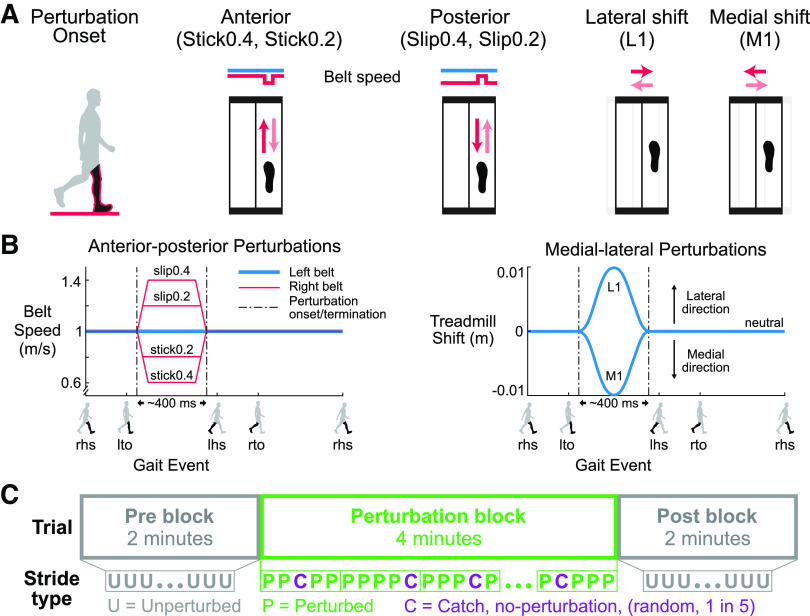
Figure 1.
Schematic of the perturbations and experimental protocol. A: perturbation onset occurred at right leg mid-stance (black leg in red outline). There were 4 perturbation directions, and 2 sizes for the anterior-posterior perturbations, for a total of six perturbation conditions. The red arrows are the initial relative surface displacements, and the faded red arrows are the displacements to return to the unperturbed state. B: the left belt speed was fixed at 1.0 m/s (blue). For the Stick0.2 and Stick0.4 perturbations, the right belt speed (red) decelerated to 0.8 m/s and 0.6 m/s, respectively, and then returned to the tied belt speed. For the Slip0.2 and Slip0.4 perturbations, the right belt speed accelerated to 1.2 m/s and 1.4 m/s, respectively, and then returned to the tied belt speed. For the lateral (L1) and medial (M1) perturbations, the treadmill shifted 1 cm laterally and medially, respectively, and then returned to neutral. The perturbations were ∼400 ms in duration. Gait events: right heel strike (rhs), left toe off (lto), left heel strike (lhs), and right toe off (rto). C: blocks of the experimental protocol. Each trial started with a 2-min unperturbed walking block (pre), followed by a 4-min perturbed walking block (perturbation), and completed with another 2-min unperturbed walking block (post). An unperturbed catch stride occurred randomly one out of every five strides during the perturbation block.
Perturbations were applied in four directions: anterior, posterior, medial, and lateral to examine the effects of perturbation directions (Fig. 1A). We also used two perturbation sizes for the anterior and posterior directions, which could be completed in ∼400 ms, to examine potential scaling effects with perturbation size (Fig. 1B). The baseline walking speed was 1.0 m/s, and the belt acceleration was set to 12.5 m/s2. For anterior “stick” perturbations, the right belt speed decreased from 1.0 m/s to the target speed (0.6 m/s and 0.8 m/s; Stick0.4 and Stick0.2, respectively) and then returned to the baseline speed. For posterior “slip” perturbations, the right belt speed increased to the target speed (1.2 m/s and 1.4 m/s; Slip0.2 and Slip0.4, respectively) and then returned to the baseline speed. For medial perturbations, the treadmill shifted 1 cm toward the medial side of the right leg (i.e., to the left) and then returned to the neutral treadmill location (M1). For lateral perturbations, the treadmill shifted 1 cm toward the lateral side of the right leg (i.e., to the right) and then returned to the neutral treadmill location (L1). The maximum acceleration for the medial-lateral perturbations was 3.6 m/s2.
Each subject completed six trials, one trial for each perturbation condition. We randomized the order of the six trials and did not inform subjects of the perturbation type, timing, direction, and magnitude. Each trial was 8-min long and consisted of three blocks. The trial began with 2 min of unperturbed walking (pre block), followed by 4 min of walking with perturbations (perturbation block) and concluded the trial with another 2 min of unperturbed walking (post block). During the 4-min perturbation block, no-perturbation “catch” strides occurred randomly one out of every five strides to assess whether subjects were anticipating or reacting (Fig. 1C). Subjects wore a safety harness to prevent potential falls during all walking trials.
Data Analysis
We wrote custom MATLAB (MATLAB, RRID:SCR_001622) scripts to process the kinematic data and calculate the gait stability metrics. We low-pass filtered the three-dimensional coordinates of the markers using a 4th-order Butterworth filter with a 12 Hz cut-off frequency, and then extracted the gait events. Heel strike was the instant the vertical position of the heel marker of the foot transitioning from swing to stance, near zero (close to the treadmill), and reached its maximum vertical acceleration (34). Toe off was the instant the toe marker of the foot transitioning from stance to swing, reached its maximum vertical acceleration between two consecutive heel strikes (35).
We calculated the margin of stability at every left heel strike (lhs) to assess reactive control and left toe off (lto) to assess anticipatory control as
| (1) |
where MOS is the margin of stability, BOS is the boundary of the base of support, and XCOM is the extrapolated center of mass (19). We calculated the extrapolated center of mass using Eq. 2, which also accounts for the movement velocity of the treadmill surface (36, 37)
| (2) |
COM is the center of mass position, and VCOM is the center of mass velocity, which was derived as the first derivative of the center of mass position. Vtreadmill is the belt velocity for the anterior-posterior perturbations and the platform velocity for the medial-lateral perturbations, respectively. The remaining variables are g, the gravitational constant (9.81 m/s2) and l, the equivalent pendulum length, which was the distance from the ankle marker (lateral malleolus) of the leading leg to the center of mass at left heel strike and left toe off.
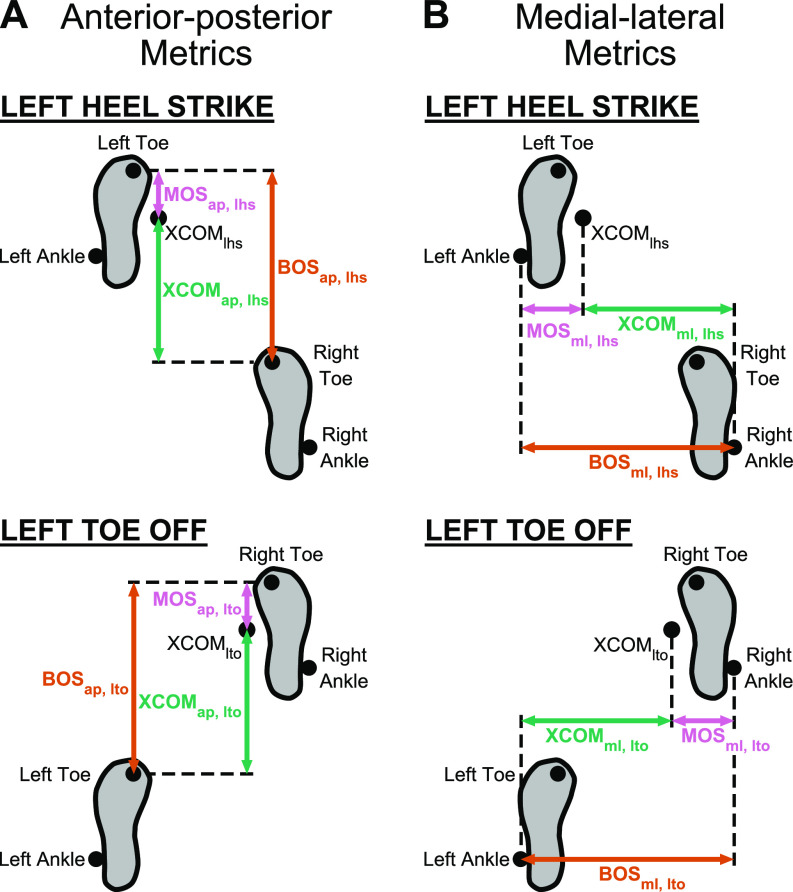
We calculated the anterior-posterior margin of stability (MOSap) as the anterior-posterior distance between the extrapolated center of mass and the toe marker of the leading leg in the sagittal plane, which defined the anterior-posterior base of support (BOSap) (Fig. 2A). The anterior-posterior extrapolated center of mass (XCOMap) was the anterior-posterior distance between the extrapolated center of mass and the toe marker of the trailing leg. We calculated the medial-lateral margin of stability (MOSml) as the medial-lateral distance between the extrapolated center of mass and the ankle marker of the leading leg in the frontal plane, which defined the medial-lateral base of support (BOSml) (Fig. 2B). The medial-lateral extrapolated center of mass (XCOMml) was the medial-lateral distance between the extrapolated center of mass and the ankle marker of the trailing leg.
Figure 2.
Anterior-posterior and medial-lateral metrics of gait stability at left heel strike (lhs) and left toe off (lto). A: the anterior-posterior base of support (BOSap, orange arrow) was the anterior-posterior distance of the toe marker of the leading leg relative to the toe marker of the trailing leg. The anterior-posterior extrapolated center of mass (XCOMap, green arrow) was the anterior-posterior distance of XCOM relative to the toe marker of the trailing leg. The anterior-posterior margin of stability (MOSap, pink arrow) was the BOSap − XCOMap. B: the medial-lateral base of support (BOSml) was the medial-lateral distance of the ankle marker of the leading leg relative to the ankle marker of the trailing leg. The medial-lateral extrapolated center of mass (XCOMml) was the medial-lateral distance of XCOM relative to the ankle marker of the trailing leg. The medial-lateral margin of stability (MOSml) was the BOSml − XCOMml.
We quantified margin of stability and its components at different phases throughout the protocol: pre, early perturbed, late perturbed, early catch, late catch, early post, and late post. Pre referred to all strides during the first 2-min prewalking block except for the first 11 strides during which the treadmill belts were getting up to speed (38). Early perturbed was the first three perturbed strides during the 4-min perturbation block. We used the first three perturbed strides as people tend to adapt quickly in arm reaching (39) and split-belt walking studies (40). For left toe off metrics, early perturbed used perturbed strides 2–4, which excluded the first perturbed stride since left toe off occurred before the perturbation onset. Late perturbed was the last three perturbed strides during the perturbation block, regardless of when the metrics were calculated. Early catch and late catch referred to the first two and last two unperturbed strides during the 4-min perturbation block, respectively. Early post was the first three strides while late post was the last three strides during the last 2-min post-walking block.
Statistics
We performed statistical procedures using JMP Pro (JMP, RRID:SCR_014242) and set the significance level to 0.05. First, we checked and excluded outliers as being beyond 3 standard deviations from the mean for each data set. We also used a Shapiro–Wilk W test, Levene’s test, and Mauchly’s test to check for the distribution normality, homogeneity of variance, and sphericity, respectively. For the first three hypotheses related to adaptation, using a feedforward strategy, and effects of perturbation directions, we analyzed the anterior (Stick0.4), posterior (Slip0.4), medial (M1), and lateral (L1) perturbation conditions. For the fourth hypothesis related to perturbation size effect, we analyzed the data of the two sizes of the anterior (Stick0.4, Stick0.2) and posterior (Slip0.4, Slip0.2) perturbations.
For the first hypothesis regarding adaptation, we performed one-way repeated-measures analysis of variance (rANOVA) for the perturbed strides on each metric (MOSap, lhs, MOSml, lhs, MOSap, lto, MOSml, lto, BOSap, lhs, BOSml, lhs, BOSap, lto, BOSml, lto, XCOMap, lhs, XCOMml, lhs, XCOMap, lto, and XCOMml, lto) with phase (pre, early perturbed, late perturbed, early post, and late post) as a factor by perturbation condition. When phase was a main effect, we performed post hoc pairwise comparisons using Tukey’s honest significant difference (HSD) tests to account for multiple comparisons for the following a priori comparisons: 1) pre to early perturbed to assess the disruption of gait stability, 2) early perturbed to late perturbed to assess adaptation, 3) early post to late post to assess de-adaptation. To determine which component of margin of stability contributed more to changes in margin of stability (|ΔMOS|), we compared the magnitudes of the changes of the base of support (|ΔBOS|) and extrapolated center of mass position (|ΔXCOM|) within a phase comparison (pre and early perturbed; early and late perturbed) using paired t tests (i.e., for pre and early perturbed, |ΔBOS| = |BOSpre – BOSearly_perturbed| vs. |ΔXCOM| = |XCOMpre – XCOMearly_perturbed|; for early to late perturbed, |ΔBOS| = |BOSearly_perturbed – BOSlate_perturbed| vs. |ΔXCOM| = |XCOMearly_perturbed – XCOMlate_perturbed|).
For the second hypothesis regarding whether subjects used a feedforward strategy for adaptation, we performed one-way rANOVA for the catch strides on each metric with phase (pre, early catch, late catch) as a factor by perturbation condition. If phase was a main effect, we performed Tukey’s HSD post hoc tests for the following comparisons: 1) pre to early catch to assess anticipatory responses at early catch, 2) pre to late catch to assess anticipatory responses at late catch, and 3) early catch to late catch to assess if subjects used more feedforward strategies during adaptation.
For the third hypothesis regarding perturbation direction, we also used a one-way rANOVA for each metric (ΔMOS, ΔBOS, and ΔXCOM for early perturbed minus pre; ΔMOS, ΔBOS, and ΔXCOM for late perturbed minus early perturbed) with perturbation condition as a factor. Here, the direction of the change, not just the magnitude, from pre to early and from early to late was important. We performed post hoc pairwise comparisons using Tukey’s HSD if perturbation condition was a main effect.
For the fourth hypothesis regarding a scaling effect, we used a two-way rANOVA on each metric (|ΔMOS|, |ΔBOS|, and |ΔXCOM| for pre to early perturbed; |ΔMOS|, |ΔBOS|, and |ΔXCOM| for early to late perturbed) with perturbation condition (stick, slip) and size (regular, 0.4; half-size, 0.2) as factors. If a significant interaction effect was detected, we performed paired t tests with Bonferroni correction (α = 0.025) to identify differences between sizes for each perturbation condition.
We calculated effect size using partial eta squared () for the rANOVA results and Cohen’s d for post hoc pairwise comparisons and paired t tests (41). Complete statistical results are provided in Supplemental Tables S1–S6 (all Supplemental tables are available at https://doi.org/10.6084/m9.figshare.16910683).
RESULTS
Representative Data
Changes of MOS and its components during perturbed walking were evident throughout the gait cycle (Fig. 3). When perturbations first occurred at early perturbed, MOSlhs, BOSlhs, and XCOMlhs all deviated from the pre unperturbed strides, indicating that the perturbations disrupted the subjects. As subjects gained more experience with the perturbations, MOSlhs and BOSlhs of late perturbed generally trended back to pre levels, suggesting adaptation occurred. When the perturbations were removed at the post block, MOSlhs and its components were almost the same as pre, suggesting a return to pre levels. The MOSap, lto of the Stick0.4 perturbation, BOSap, lto and XCOMap, lto of the Slip0.4 perturbation, and BOSml, lto and XCOMml, lto of the L1 perturbation deviated from pre at early perturbed, suggesting an anticipatory response. The BOSlto and XCOMlto of the Slip0.4 and L1 perturbations then trended to their pre levels by late perturbed, an indication of adaptation, and returned to the pre levels by the end of post.
Figure 3.
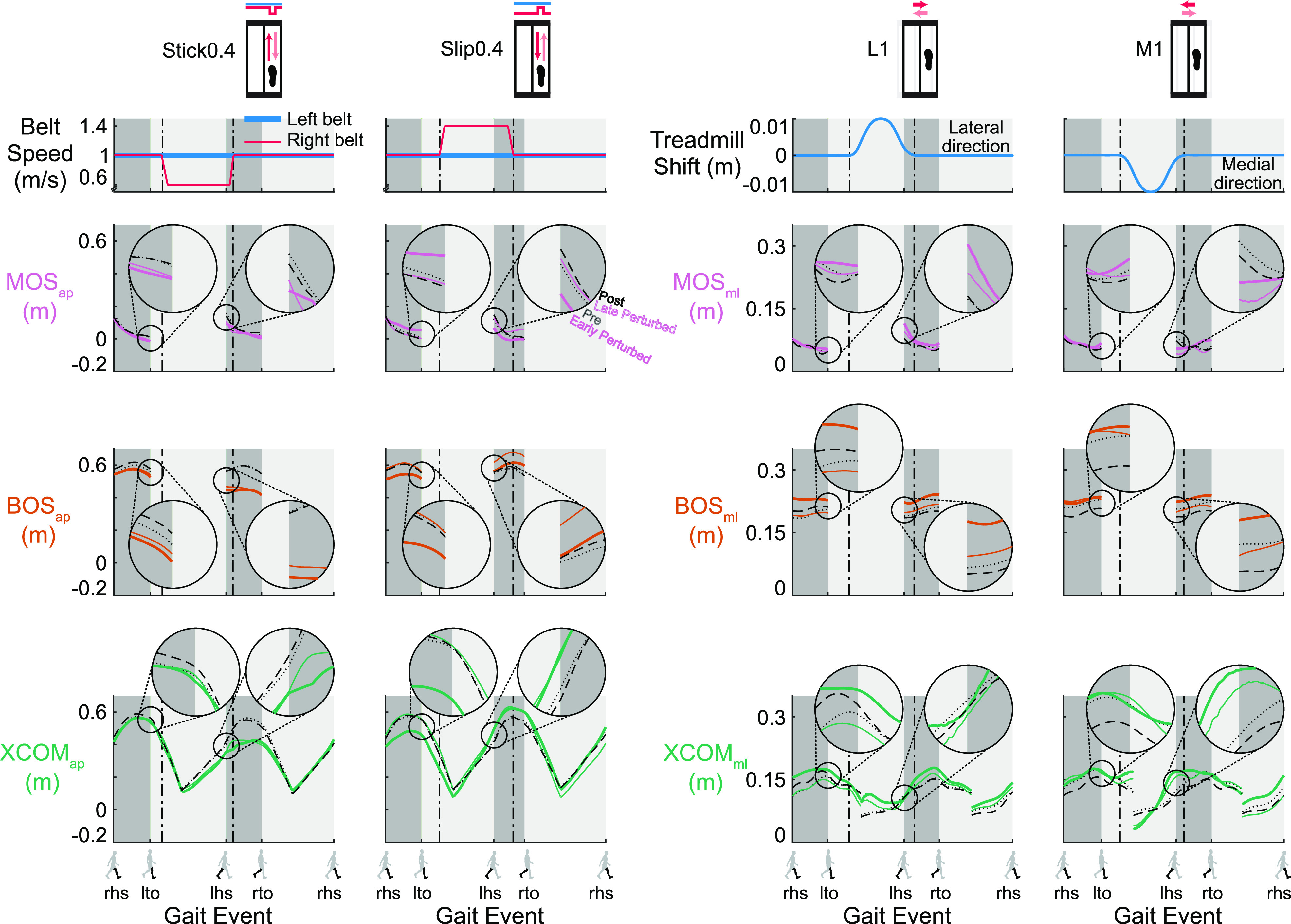
Trajectories of the gait stability metrics [margin of stability (MOS), pink; base of support (BOS), orange, and extrapolated center of mass (XCOM), green] for specific strides of a representative subject for the Stick0.4, Slip0.4, L1, and M1 perturbations (black dotted line: the 2nd to last stride of pre; colored thick line: the 2nd stride of early perturbed; colored thin line: the 2nd to last stride of late perturbed; and black dashed line: the 2nd to last stride of post). The MOSap, lhs, BOSap, lhs, and XCOMap, lhs of the Stick0.4 and Slip0.4 perturbations, and MOSml, lhs, BOSml, lhs, and XCOMml, lhs of the lateral (L1) and medial (M1) perturbations deviated from pre at early perturbed, then MOSlhs and BOSlhs generally trended to the pre trajectory by late perturbed and returned to the pre trajectory by post, shown in the exploded view in the inset circles. The MOSap, lto of the Stick0.4 perturbation, BOSap, lto and XCOMap, lto of the Slip0.4 perturbation, and BOSml, lto and XCOMml, lto of the L1 perturbation deviated from pre at early perturbed, then BOSlto and XCOMlto trended to the pre trajectory by late perturbed and returned to the pre trajectory by post, shown in the exploded view in the inset circles. Gait events: right heel strike (rhs), left toe off (lto), left heel strike (lhs), and right toe off (rto). Dark gray area: double support phase. Light gray area: single support phase. The plots show the MOS and BOS during double support and XCOM for the entire gait cycle. ap, anterior-posterior; ml, medial-lateral.
Adaptation to Small Anterior-Posterior Perturbations Applied on a Stride-by-Stride Basis (Hypothesis 1)
Subjects adapted to the Stick0.4 perturbation after an initial disruption to gait stability (Fig. 4A and Supplemental Fig. S1A; all Supplemental figures are available at https://doi.org/10.6084/m9.figshare.16910713). Phase had a significant effect on all metrics except for BOSap, lto for the Stick0.4 perturbation (see Supplemental Table S1 for metric-specific F, P, and ). Upon initial exposure to the perturbations, MOSap, lhs was disrupted and decreased significantly by 41.3% (P < 0.001, Cohen’s d = 2.49). After the initial perturbation, subjects immediately took shorter and wider steps and shifted their XCOM backward at left heel strike (pre to early perturbed: BOSap, lhs decreased, P < 0.001, Cohen’s d = 4.95; BOSml, lhs increased, P = 0.03, Cohen’s d = 0.99; XCOMap, lhs decreased, P < 0.001, Cohen’s d = 2.32). Starting with the second Stick0.4 perturbation, subjects also took wider steps (determined by the previous right heel strike) and shifted their XCOM forward at left toe off in anticipation of an impending perturbation at right leg mid-stance (pre to early perturbed: BOSml, lto increased, P < 0.001, Cohen’s d = 2.21; XCOMap, lto increased, P < 0.001, Cohen’s d = 1.83). MOSap, lhs increased significantly by 44.4% (P < 0.001, Cohen’s d = 1.57) after the initial disruption and trended toward pre level by late perturbed, indicating adaptation occurred. Subjects also took longer steps at left heel strike and took narrower steps and shifted their XCOM backward at left toe off (early to late perturbed: BOSap, lhs increased, P = 0.01, Cohen’s d = 1.09; BOSml, lto decreased, P = 0.001, Cohen’s d = 1.37; XCOMap, lto decreased, P < 0.001, Cohen’s d = 1.77) as they adapted. MOSap, lhs continued trending to pre level during the post block (early post to late post, P = 0.02, Cohen’s d = 1.02).
Figure 4.
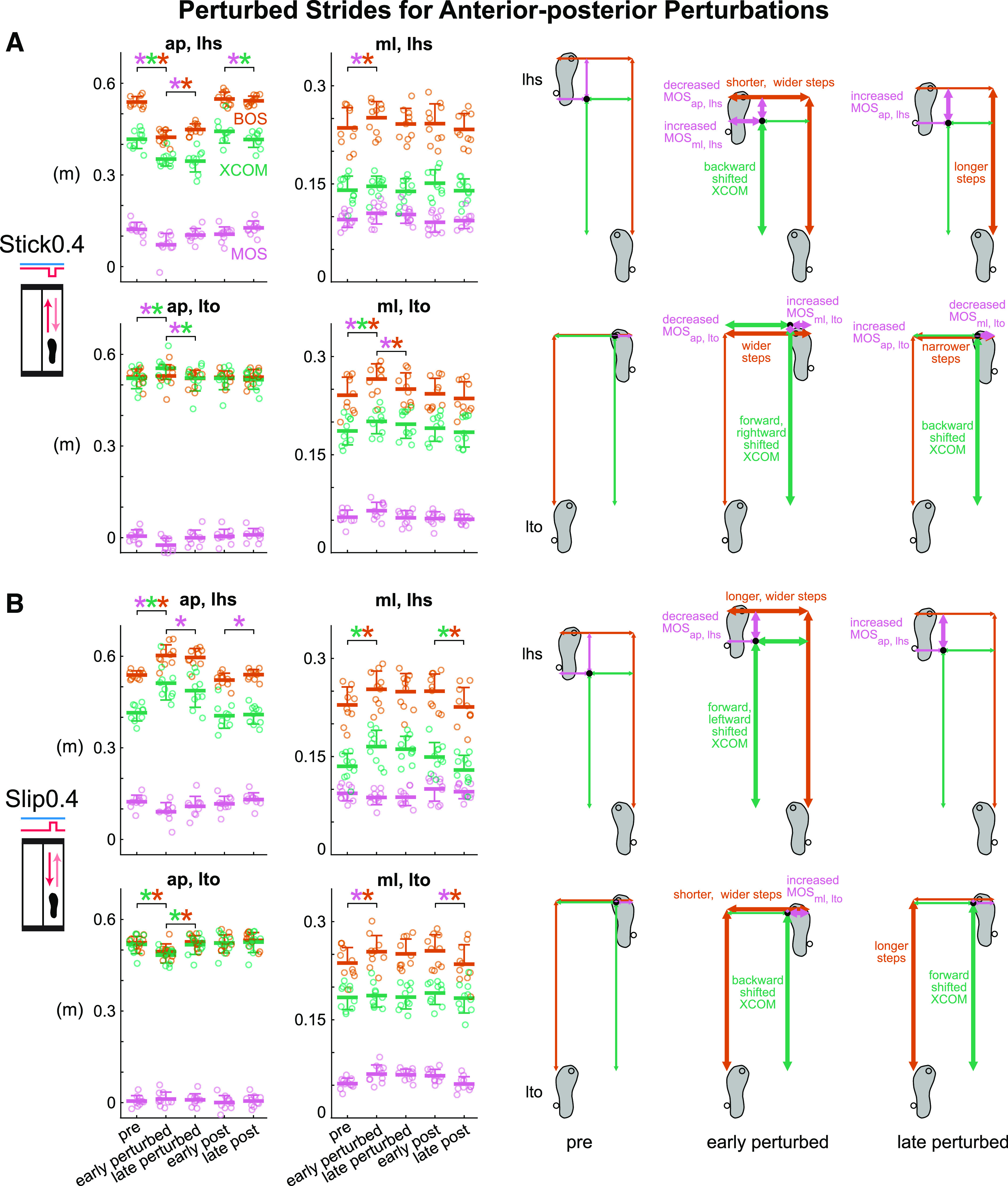
Group-averaged (mean and standard deviation indicated by thick horizontal lines and one-sided error bar, n = 10) anterior-posterior and medial-lateral margin of stability (MOS, pink), base of support (BOS, orange), and extrapolated center of mass (XCOM, green) at left heel strike and left toe off for pre, early perturbed, late perturbed, early post, and late post during the anterior-posterior perturbations and the corresponding footprints with metrics at pre, early perturbed, and late perturbed. Thick arrows indicate significant changes from the previous phase. In the group-averaged plots, circles are individual subjects. A: subjects were destabilized at early perturbed then adapted by late perturbed during the Stick0.4 perturbation. B: subjects were destabilized and adapted during the Slip0.4 perturbation. *P < 0.05 and color-coded for the specific metrics with the significant differences between phases indicated by the brackets. ap, anterior-posterior; lhs, left heel strike; lto, left toe off; ml, medial-lateral.
Like the Stick0.4 perturbation, subjects adapted to the Slip0.4 perturbation at both left heel strike and left toe off after initially being disrupted (Fig. 4B and Supplemental Fig. S1B). Phase had a significant effect on all metrics except for MOSap, lto and XCOMml, lto for the Slip0.4 perturbation (see Supplemental Table S1 for metric-specific F, P, and ). Upon initially experiencing the perturbations, anterior-posterior gait stability was disrupted as MOSap, lhs decreased significantly by 26.6% (P < 0.001, Cohen’s d = 2.34). Subjects also immediately took longer and wider steps and shifted their XCOM forward and leftward at left heel strike (pre to early perturbed: BOSap, lhs increased, P < 0.001, Cohen’s d = 2.92; BOSml, lhs increased, P = 0.02, Cohen’s d = 1.05; XCOMap, lhs increased, P < 0.001, Cohen’s d = 3.39; XCOMml, lhs increased, P < 0.001, Cohen’s d = 1.53). At left toe off, subjects also immediately increased their step width and shifted their XCOM backward in anticipation of the impending perturbation at mid-stance (pre to early perturbed: BOSml, lto increased, P = 0.02, Cohen’s d = 1.02; XCOMap, lto decreased, P < 0.001, Cohen’s d = 1.98). The MOSap, lhs increased significantly by 19.6% (P = 0.003, Cohen’s d = 1.27) and trended toward pre level from early to late perturbed during adaptation. Subjects also took longer steps and shifted their XCOM forward at left toe off (early to late perturbed: BOSap, lto increased, P < 0.001, Cohen’s d = 1.49; XCOMap, lto increased, P < 0.001, Cohen’s d = 1.92). From early post to late post, MOSap, lhs continued trending to pre level (P = 0.02, Cohen’s d = 1.01).
Adaptation to Small Medial-Lateral Perturbations Applied on a Stride-by-Stride Basis (Hypothesis 1)
The L1 perturbation was initially disruptive, but subjects adapted with more experience with the perturbation (Fig. 5A and Supplemental Fig. S1C). Phase had a significant effect on all metrics except for XCOMap, lto and MOSml, lto for the L1 perturbation (see Supplemental Table S1 for metric-specific F, P, and ). After initial exposure to the perturbations, MOSml, lhs was disrupted and increased significantly by 47.3% (P < 0.001, Cohen’s d = 4.27). Subjects also immediately took shorter and wider steps and shifted their XCOM backward and rightward at left heel strike (pre to early perturbed: BOSap, lhs decreased, P < 0.001, Cohen’s d = 1.46; BOSml, lhs increased, P = 0.008, Cohen’s d = 1.14; XCOMap, lhs decreased, P = 0.004, Cohen’s d = 1.22; XCOMml, lhs decreased, P < 0.001, Cohen’s d = 1.84). Starting with the second L1 perturbation, subjects showed anticipatory responses based on their wider steps at left toe off (pre to early perturbed: BOSml, lto increased, P = 0.005, Cohen’s d = 1.20). From early to late perturbed, MOSml, lhs decreased significantly by 8.0% (P = 0.02, Cohen’s d = 1.06) and trended toward pre level, indicating adaptation to the L1 perturbation. Subjects also took longer and narrower steps at both left heel strike and left toe off (early to late perturbed: BOSap, lhs increased, P = 0.01, Cohen’s d = 1.09; BOSml, lhs decreased, P = 0.05, Cohen’s d = 0.91; BOSap, lto increased, P = 0.02, Cohen’s d = 1.02; BOSml, lto decreased, P = 0.004, Cohen’s d = 1.22) as they adapted. From early post to late post, MOSml, lhs did not change significantly (P = 0.87, Cohen’s d = 0.30), suggesting that subjects rapidly restored pre level of MOSlhs preference in the medial-lateral direction during the first few strides of the post block.
Figure 5.
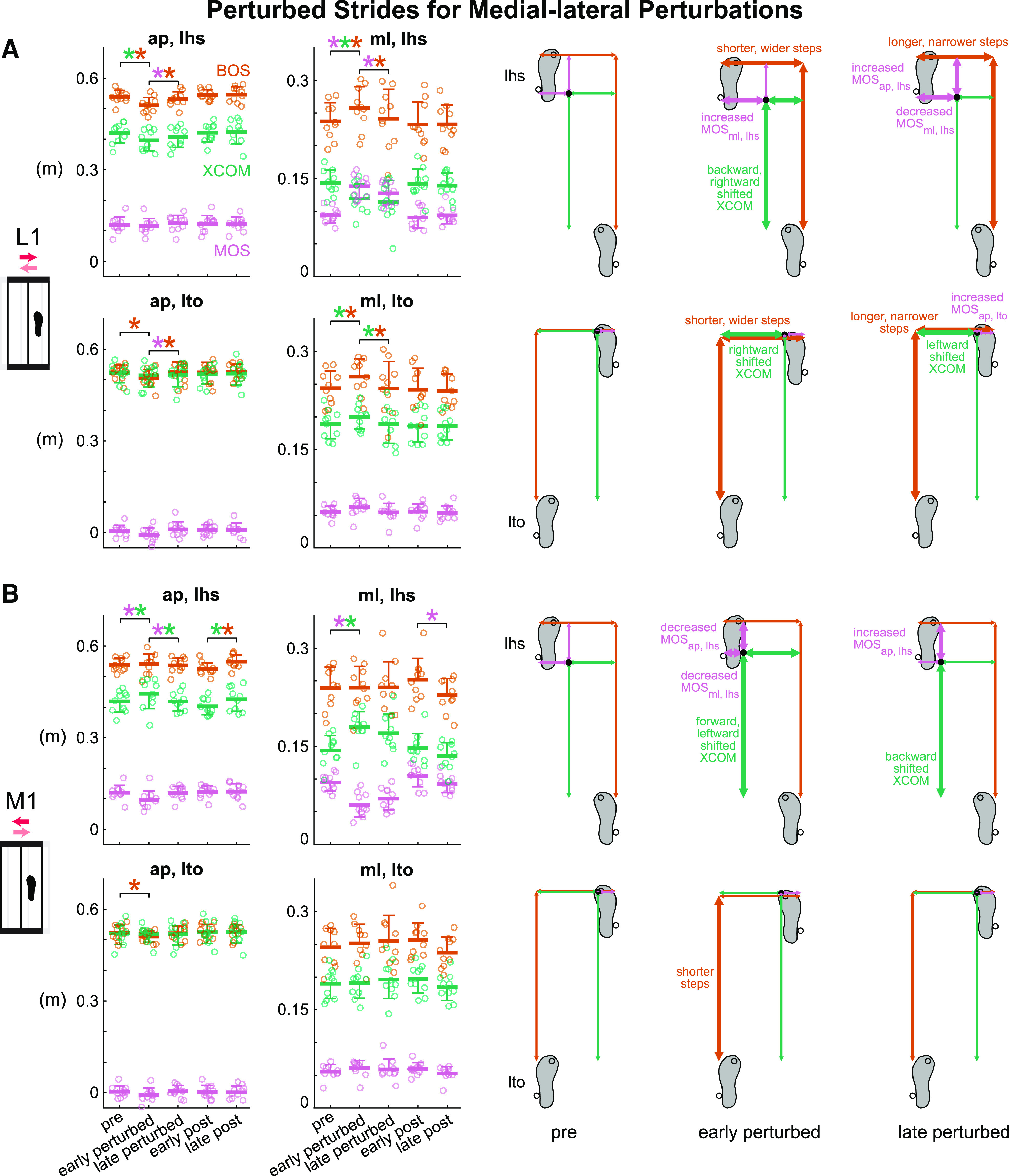
Group-averaged (mean and standard deviation indicated by thick horizontal lines and one-sided error bar, n = 10) anterior-posterior and medial-lateral margin of stability (MOS, pink), base of support (BOS, orange), and extrapolated center of mass (XCOM, green) at left heel strike and left toe off for pre, early perturbed, late perturbed, early post, and late post during the medial-lateral perturbations and the corresponding footprints with metrics at pre, early perturbed, and late perturbed. Thick arrows indicate significant changes from the previous phase. In the group-averaged plots, circles are individual subjects. A: subjects adapted to the lateral (L1) perturbation after initially being disrupted. B: subjects adapted to the medial (M1) perturbation after initially being disrupted. *P < 0.05 and color-coded for the specific metrics with the significant differences between phases indicated by the brackets. ap, anterior-posterior; lhs, left heel strike; lto, left toe off; ml, medial-lateral.
Subjects also adapted to the M1 perturbation that initially disrupted gait stability (Fig. 5B and Supplemental Fig. S1D). Phase had a significant effect on MOSap, lhs, BOSap, lhs, XCOMap, lhs, MOSml, lhs, XCOMml, lhs, and BOSap, lto for the M1 perturbation (see Supplemental Table S1 for metric-specific F, P, and ). Upon initially responding to the perturbations, gait stability was disrupted in both directions as MOSap, lhs decreased significantly by 20.3% (P = 0.002, Cohen’s d = 1.28) and MOSml, lhs decreased significantly by 36.3% (P < 0.001, Cohen’s d = 2.80). After the initial perturbation, subjects immediately shifted their XCOM forward and leftward at left heel strike (pre to early perturbed: XCOMap, lhs increased, P = 0.01, Cohen’s d = 1.09; XCOMml, lhs increased, P < 0.001, Cohen’s d = 1.88). At left toe off, subjects also immediately took shorter steps (pre to early perturbed: BOSap, lto decreased, P = 0.04, Cohen’s d = 0.94). Subjects adapted after the initial disruption as MOSap, lhs increased significantly by 23.9% (P = 0.004, Cohen’s d = 1.21) and trended toward pre level by late perturbed. Subjects also shifted their XCOM backward at left heel strike (early to late perturbed: XCOMap, lhs decreased, P = 0.01, Cohen’s d = 1.11).
Which Component of Margin of Stability Was Affected More by the Small Perturbations?
During disruption, perturbations had different effects on the magnitudes of the changes in the BOS and XCOM at left heel strike (Fig. 6A) and left toe off (Supplemental Fig. S2A). For the Stick0.4 perturbation, the BOSlhs was disrupted more than XCOMlhs based on the larger changes in the BOSlhs compared with the changes in XCOMlhs that were significantly different in the anterior-posterior direction and trending toward significance in the medial-lateral direction (|ΔBOSap, lhs| > |ΔXCOMap, lhs| P = 0.001, Cohen’s d = 1.60); |ΔBOSml, lhs| > |ΔXCOMml, lhs| P = 0.08, Cohen’s d = 0.62). For the Slip0.4 and M1 perturbations, the XCOMlhs was disrupted more than BOSlhs as the change in XCOMlhs was greater than the change in BOSlhs in both directions (Slip0.4: |ΔXCOMap, lhs| > |ΔBOSap, lhs| P < 0.001, Cohen’s d = 2.00; |ΔXCOMml, lhs| > |ΔBOSml, lhs| P = 0.04, Cohen’s d = 0.73; M1: |ΔXCOMap, lhs| > |ΔBOSap, lhs| P = 0.06, Cohen’s d = 0.68; |ΔXCOMml, lhs| > |ΔBOSml, lhs| P < 0.001, Cohen’s d = 1.93). For the L1 perturbation, there was no difference between the changes in BOSlhs and XCOMlhs in either direction (P values > 0.21, Cohen’s d values < 0.43).
Figure 6.
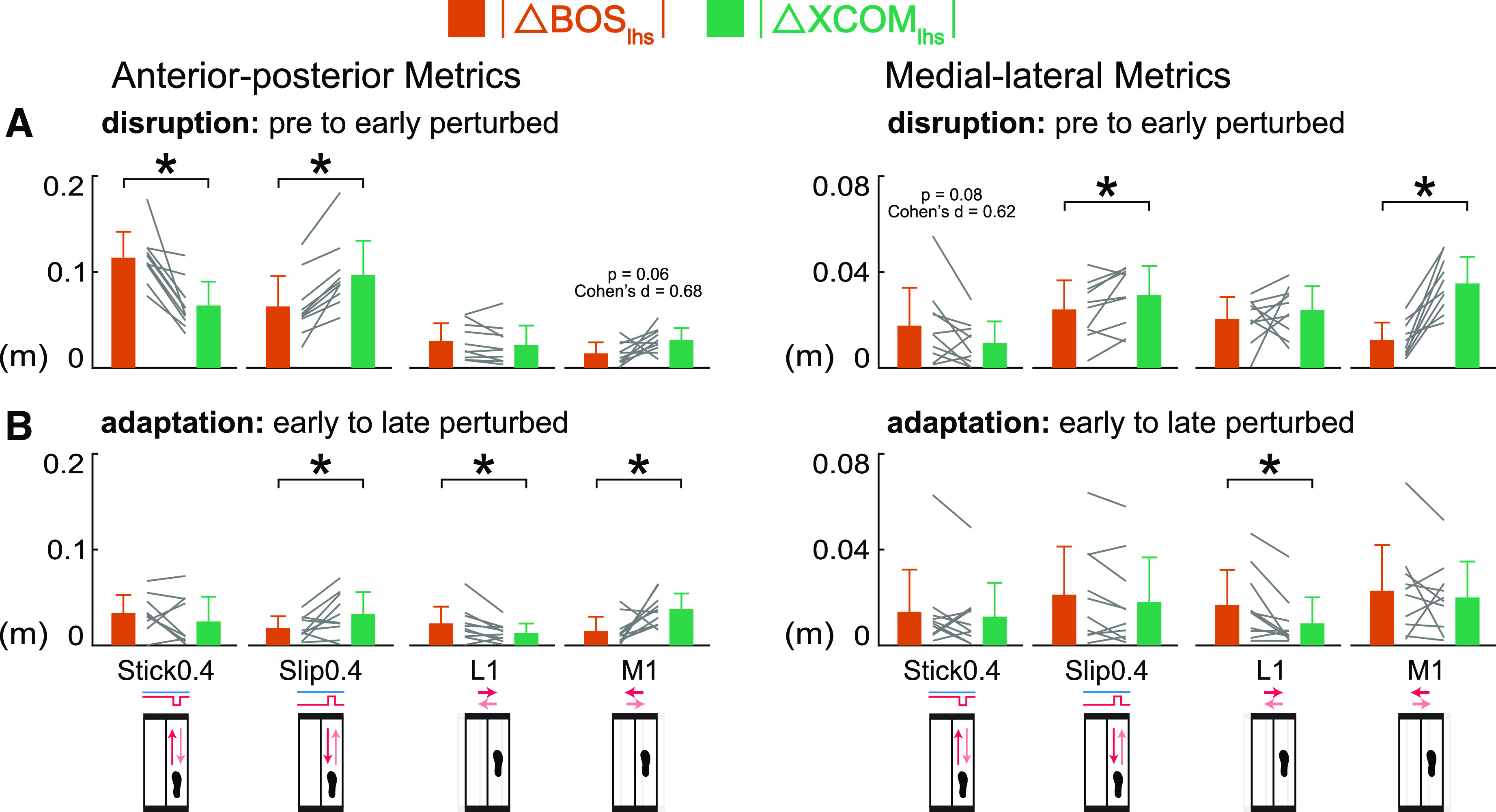
Group-averaged (mean and standard deviation, n = 10) |Δ left heel strike base of support (BOSlhs)| (orange) compared with |Δ left heel strike extrapolated center of mass (XCOMlhs)| (green) during disruption (pre to early perturbed) and adaptation (early to late perturbed). Gray lines between bars are individual subjects. A: during disruption, BOSlhs was disrupted more by the Stick0.4 perturbation, XCOMlhs was disrupted more by the Slip0.4 and M1 perturbations. B: during adaptation, BOSap, lhs adapted more in the L1 perturbation, XCOMap, lhs adapted more in the Slip0.4 and M1 perturbations, BOSml, lhs adapted more in the L1 perturbation. *P < 0.05 and significant differences between |ΔBOSlhs| and |ΔXCOMlhs|. ap, anterior-posterior; ml, medial-lateral.
Which Component Contributed More to the Adaptation of Margin of Stability?
During adaptation, the component that was the primary contributor to the adaptation of MOS at left heel strike (Fig. 6B) and left toe off (Supplemental Fig. S2B) depended on the perturbation. The |ΔBOSap, lhs| was larger than the |ΔXCOMap, lhs| for the L1 perturbation (P = 0.03, Cohen’s d = 0.85). The |ΔXCOMap, lhs| was greater than the |ΔBOSap, lhs| for the Slip0.4 and M1 perturbations (P values < 0.05, Cohen’s d values > 0.72), suggesting that XCOMap, lhs adapted more than BOSap, lhs during those perturbations. There was no significant difference between |ΔBOSap, lhs| and |ΔXCOMap, lhs| in the Stick0.4 perturbation (P = 0.27, Cohen’s d = 0.37). For the medial-lateral metrics, the only significant difference was the larger |ΔBOSml, lhs| compared with the |ΔXCOMml, lhs| for the L1 perturbation (P = 0.02, Cohen’s d = 0.90), suggesting that BOSml, lhs adapted more in the lateral shift perturbation. There were no significant differences between the |ΔBOSml, lhs| and |ΔXCOMml, lhs| in the other perturbations (P values > 0.12, Cohen’s d values < 0.53).
Did Subjects Use More Feedforward Strategies When Adapting to Small Perturbations Applied on a Stride-by-Stride Basis? (Hypothesis 2)
Catch strides revealed that subjects used feedforward strategies upon initially experiencing the anterior-posterior perturbations but did not use more anticipatory control as adaptation progressed (Fig. 7, A and B and Supplemental Fig. S1, A and B). For the Stick0.4 perturbation, phase had a significant effect on all metrics except for BOSap, lto, BOSap, lhs, MOSml, lhs whereas for the Slip0.4 perturbation phase had a significant effect on MOSml, lto, BOSml, lto, MOSap, lhs, XCOMap, lhs, MOSml, lhs, BOSml, lhs, and XCOMml, lhs (see Supplemental Table S4 for metric-specific F, P, and ). At early catch, MOSap, lto and MOSml, lto deviated significantly from their pre levels for the Stick0.4 (MOSap, lto decreased, P = 0.001, Cohen’s d = 1.44; MOSml, lto increased, P = 0.02, Cohen’s d = 0.96) and the Slip0.4 (MOSml, lto increased, P = 0.004, Cohen’s d = 1.20) perturbations. Subjects also took wider steps for both perturbations (pre to early catch: BOSml, lto increased, P values < 0.003, Cohen’s d values > 1.25) and shifted their XCOM forward and rightward at left toe off for the Stick0.4 perturbation (pre to early catch: XCOMap, lto increased, P = 0.02, Cohen’s d = 0.99; XCOMml, lto increased, P = 0.009, Cohen’s d = 1.07). At left heel strike for the Stick0.4 perturbation, subjects marginally shifted their XCOM forward to 0.44 ± 0.05 m (pre to early catch: XCOMap, lhs, P = 0.130, Cohen’s d = 0.65), which was in the opposite direction of the perturbed response of 0.35 ± 0.02 m, revealing evidence of anticipating an impending perturbation. MOSml, lto and MOSap, lhs trended to their pre levels by late catch for the Stick0.4 perturbation (early to late catch: MOSml. lto decreased, P = 0.02, Cohen’s d = 0.96; MOSap, lhs increased, P = 0.02, Cohen’s d = 0.99). Subjects also took narrower steps for the Stick0.4 perturbation (early to late catch: BOSml, lto decreased, P = 0.003, Cohen’s d = 1.22; BOSml, lhs decreased, P = 0.007, Cohen’s d = 1.10) and shifted their XCOM rightward at left heel strike for both perturbations (early to late catch: XCOMml, lhs decreased, P values <0.007, Cohen’s d values >1.11). Although MOSml, lto and MOSml, lhs increased significantly from pre to late catch for the Slip0.4 perturbation (MOSml, lto, P = 0.03, Cohen’s d = 0.91; MOSml, lhs, P = 0.03, Cohen’s d = 0.88), there were no significant differences between early catch and late catch (MOSml, lto, P = 0.64, Cohen’s d = 0.29; MOSml, lhs, P = 0.11, Cohen’s d = 0.67), suggesting that subjects did not use more feedforward strategies as they experienced more anterior-posterior perturbations.
Figure 7.
Group-averaged (mean and standard deviation indicated by thick horizontal lines and one-sided error bar, n = 10) anterior-posterior and medial-lateral margin of stability (MOS, pink), base of support (BOS, orange), and extrapolated center of mass (XCOM, green) at left heel strike and left toe off for pre, early catch, late catch for the Stick0.4, Slip0.4, lateral (L1), and medial (M1) perturbations. Circles are individual subjects. Stick0.4 perturbation (A), Slip0.4 perturbation (B), L1 perturbation (C), and M1 perturbation (D) induced anticipatory responses at early catch, but subjects did not use more feedforward strategies by late catch. *P < 0.05 and color-coded for the specific metrics with the significant differences between phases indicated by the brackets. ap, anterior-posterior; lhs, left heel strike; lto, left toe off; ml, medial-lateral.
Based on the responses to the catches, subjects employed feedforward strategies after the initial medial-lateral perturbations, however, more feedforward strategies were not used during adaptation (Fig. 7, C and D and Supplemental Fig. S1, C and D). Phase had a significant effect on MOSml, lto, BOSml, lto, MOSap, lhs, XCOMap, lhs, BOSml, lhs, and XCOMml, lhs for the L1 perturbation and MOSap, lto, BOSap, lto, MOSml, lto, BOSap, lhs, XCOMap, lhs, and MOSml, lhs for the M1 perturbation (see Supplemental Table S4 for metric-specific F, P, and ). From pre to early catch, MOSml, lto increased significantly for both perturbations (P values < 0.02, Cohen’s d values > 1.04). Subjects also took wider steps at left toe off for the L1 perturbation (pre to early catch: BOSml, lto increased, P = 0.004, Cohen’s d = 1.20). At left heel strike, subjects shifted their XCOM forward and leftward for the L1 perturbation (pre to early catch: XCOMap, lhs increased, P = 0.04, Cohen’s d = 0.85; XCOMml, lhs increased, P = 0.002, Cohen’s d = 1.28) and increased their MOSml, lhs for the M1 perturbation (pre to early catch, P = 0.002, Cohen’s d = 1.27), these trends were in the opposite direction of the responses for pre to early perturbed. These results suggest that subjects had anticipatory behavior after the initial medial-lateral perturbations. From early to late catch, MOSml, lto decreased significantly for both perturbations (P values < 0.009, Cohen’s d values > 1.08). Subjects also took narrower steps at left toe off and shifted their XCOM rightward at left heel strike for the L1 perturbation (early to late catch: BOSml, lto decreased, P = 0.006, Cohen’s d = 1.13; XCOMml, lhs decreased, P = 0.01, Cohen’s d = 1.02). For the M1 perturbation, MOSml, lhs increased and subjects took shorter steps and shifted their XCOM rightward at left heel strike (pre to late catch: MOSml, lhs increased, P < 0.0001, Cohen’s d = 1.88; BOSap, lhs decreased, P = 0.01, Cohen’s d = 1.06; XCOMap, lhs decreased, P = 0.004, Cohen’s d = 1.20), whereas no significant differences were found between their early catch and late catch except for XCOMap, lhs (early to late catch: MOSml, lhs, P = 0.16, Cohen’s d = 0.61; BOSap, lhs, P = 0.29, Cohen’s d = 0.49; XCOMap, lhs decreased, P = 0.02, Cohen’s d = 0.96). These results suggest that subjects generally did not use more feedforward strategies as they adapted to the medial-lateral perturbations.
Are Margin of Stability and Its Components Sensitive to Directions of Small Perturbations? (Hypothesis 3)
Anterior-posterior metrics at left heel strike were more sensitive to the anterior-posterior perturbations when subjects were disrupted at early perturbed but not during adaptation from early to late perturbed (Fig. 8A). During disruption, perturbation direction had a significant effect on each anterior-posterior metric at left heel strike [F values (3, 27) > 7.92, P values < 0.002, values > 0.46]. All perturbations produced a decrease in MOSap, lhs, during disruption of which, the decreases for the Stick0.4 and Slip0.4 perturbations were observed in all subjects and significantly larger than the decrease for the L1 perturbation (P values < 0.04, Cohen’s d values > 0.94). For early perturbed, the BOSap, lhs and XCOMap, lhs had the largest decrease with the Stick0.4 perturbation compared with the decrease with the L1 perturbation (P values < 0.02, Cohen’s d values > 1.03). The BOSap, lhs and XCOMap, lhs had the largest increase with the Slip0.4 perturbation compared with the increase with the M1 perturbation for early perturbed (P values < 0.001, Cohen’s d values > 1.72). During adaptation, all perturbations resulted in an increased MOSap, lhs of similar magnitude [F (3, 27) = 1.36, P = 0.28, = 0.13]. The Stick0.4 and L1 perturbations resulted in increases of BOSap, lhs during adaptation, whereas the only significant difference was between Stick0.4 and Slip0.4 perturbations mainly due to differences in sign (P = 0.04, Cohen’s d = 0.92). Conversely, although perturbation direction did not have a significant main effect on XCOMap, lhs [F (3, 27) = 2.83, P = 0.06, = 0.24], the Slip0.4 and M1 perturbations resulted in decreases of XCOMap, lhs during adaptation.
Figure 8.
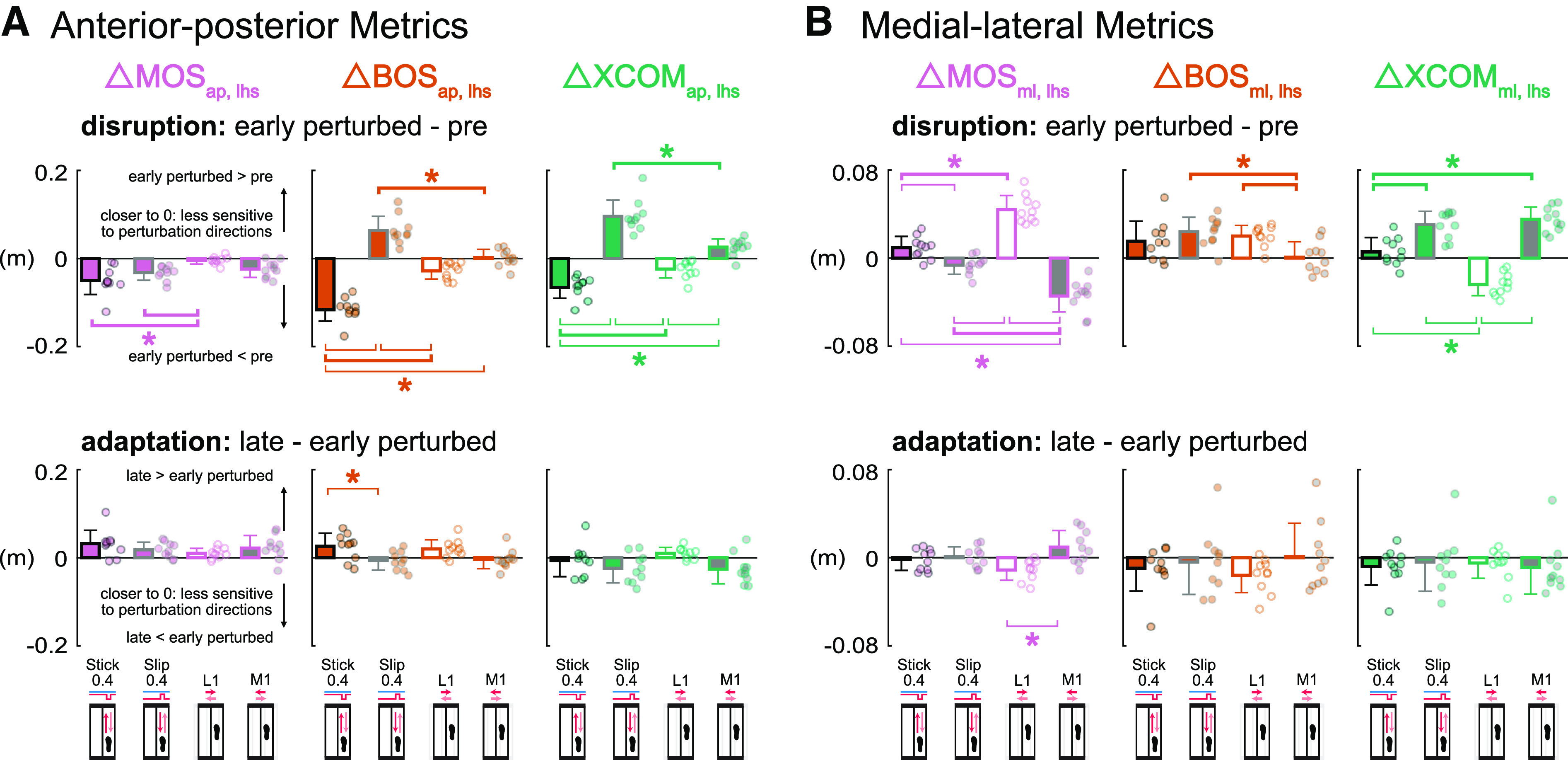
Group-averaged (mean and standard deviation, n = 10) Δ left heel strike margin of stability (MOSlhs, pink), Δ left heel strike base of support (BOSlhs, orange), Δ left heel strike extrapolated center of mass (XCOMlhs, green) during disruption (early perturbed minus pre) and adaptation (late minus early perturbed) for the Stick0.4 (black outline), Slip0.4 (gray outline), L1 (white fill), and M1 (gray fill) perturbations. Circles are individual subjects. A: anterior-posterior metrics at left heel strike were more sensitive to the anterior-posterior perturbations during disruption but not adaptation. B: MOSml, lhs and XCOMml, lhs were more sensitive to the medial-lateral perturbations during disruption but not adaptation. *With thick brackets: P < 0.05 and significant same signed differences between two perturbation conditions; *with thin brackets: P < 0.05 and significant opposite signed differences between two perturbation conditions. ap, anterior-posterior; ml, medial-lateral.
The medial-lateral metrics at left heel strike, MOSml, lhs and XCOMml, lhs, were more sensitive to the medial-lateral perturbations during disruption but not during adaptation (Fig. 8B). During disruption, perturbation direction had a significant effect on each medial-lateral metric at left heel strike [F values (3, 27) > 5.32, P values < 0.006, values > 0.36]. For early perturbed, all subjects increased and decreased MOSml, lhs in the L1 and M1 perturbations, respectively. MOSml, lhs had the largest increase with the L1 perturbation compared with the increase with the Stick0.4 perturbation (P < 0.001, Cohen’s d = 2.07), whereas the MOSml, lhs had the largest decrease with the M1 perturbation compared with the decrease with the Slip0.4 perturbation (P < 0.001, Cohen’s d = 1.67). All perturbations produced an increase in BOSml, lhs, during disruption of which, the increase for the M1 perturbation was significantly smaller than the increases for the Slip0.4 and L1 perturbations (P values < 0.03, Cohen’s d values > 0.98). For the XCOMml, lhs for early perturbed, the increase of XCOMml, lhs for all subjects in the M1 perturbation was significantly larger than the increase in the Stick0.4 perturbation (P < 0.001, Cohen’s d = 1.79), whereas the decrease of XCOMml, lhs for all subjects in the L1 perturbation was significantly different than the increases in the Stick0.4 and Slip0.4 perturbations (P values < 0.001, Cohen’s d values > 1.83). During adaptation, perturbation direction had a significant effect on ΔMOSml, lhs [F (3, 27) = 5.89, P = 0.003, = 0.40]. The increase of MOSml, lhs in the M1 perturbation was significantly different than the decrease in the L1 perturbation (P = 0.002, Cohen’s d = 1.32), largely due to differences in sign. For BOSml, lhs and XCOMml, lhs, nearly all perturbations resulted in a decrease of similar magnitudes during adaptation [F values (3, 27) < 0.93, P values > 0.44, values < 0.10].
Do Responses in Margin of Stability and Its Components Scale with Perturbation Size? (Hypothesis 4)
Anterior-posterior metrics at left heel strike scaled with perturbation size when subjects were disrupted at early perturbed but not during adaptation from early to late perturbed (Fig. 9A). Perturbation size had a main effect on the changes during disruption for each anterior-posterior metric at left heel strike [F values (1, 27) > 9.51, P values < 0.006, values > 0.25]. There was a significant interaction effect between perturbation size and perturbation condition for the changes in BOSap, lhs during disruption [F (1, 27) = 5.53, P = 0.03, = 0.17]. Paired t tests revealed that the half-size perturbations produced significantly smaller magnitude changes in BOSap, lhs than the regular perturbations within each perturbation condition (Stick0.2 vs. Stick0.4 P = 0.001, Cohen’s d = 1.61; Slip0.2 vs. Slip0.4 P = 0.01, Cohen’s d = 1.01). These results suggest that subjects scaled BOSap, lhs more in the stick perturbations than slip perturbations at early perturbed. During adaptation, perturbation size had a significant effect on |ΔBOSap, lhs| [F (1, 27) = 4.75, P = 0.04, = 0.15] but not on |ΔMOSap, lhs| and |ΔXCOMap, lhs| [F values (1, 27) < 1.70, P values > 0.20, values < 0.07], indicating most anterior-posterior metrics at left heel strike did not scale with perturbation size during adaptation.
Figure 9.
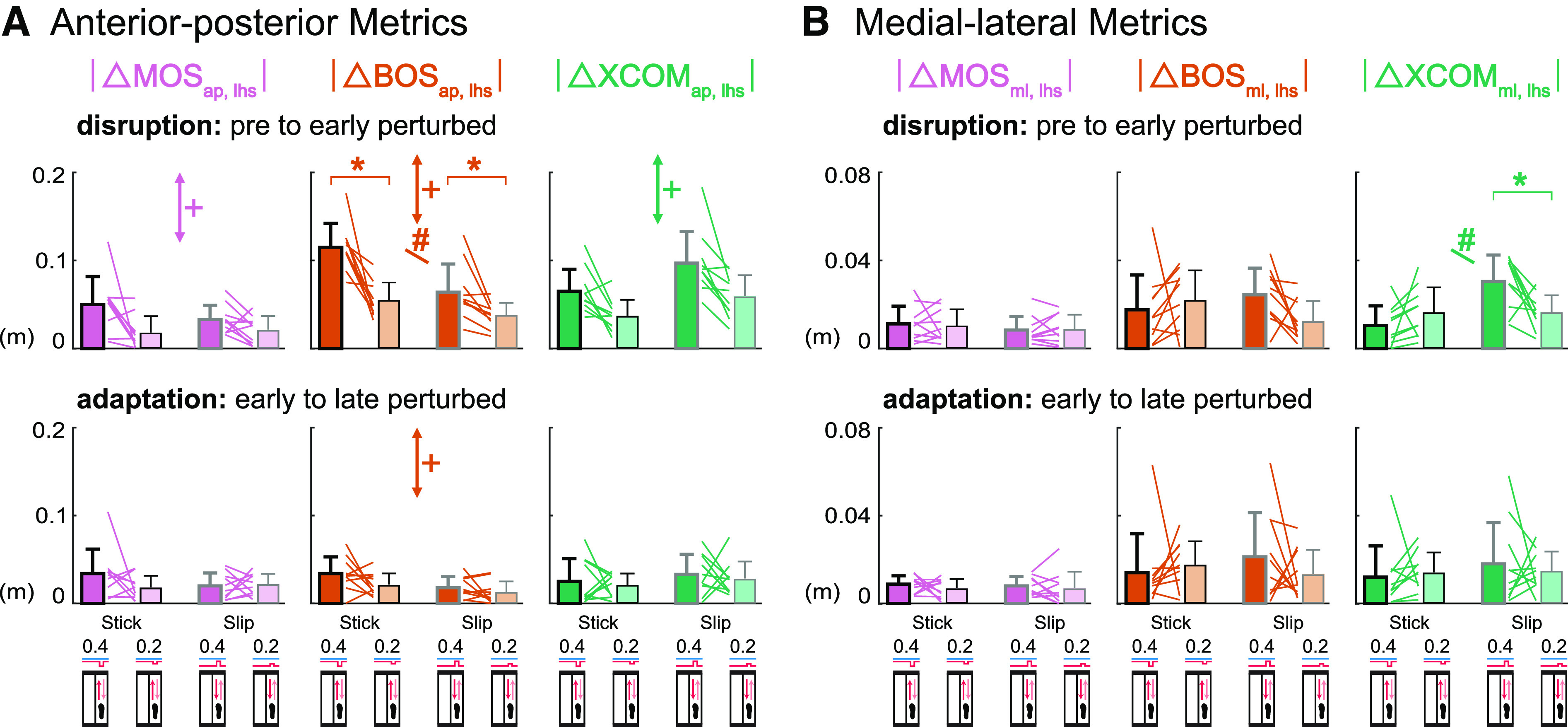
Group-averaged (mean and standard deviation, n = 10) |Δ left heel strike margin of stability (MOSlhs)| (pink), |Δ left heel strike base of support (BOSlhs)| (orange), |Δ left heel strike extrapolated center of mass (XCOMlhs)| (green) during disruption (pre to early perturbed) and adaptation (early to late perturbed) for the anterior-posterior perturbations. Dark bars indicate the regular size perturbations (Stick0.4 and Slip0.4) and light bars indicate the half-size perturbations (Stick0.2 and Slip0.2). Colored lines between bars are individual subjects. A: anterior-posterior metrics at left heel strike scaled with perturbation size during disruption but not adaptation. B: medial-lateral metrics at left heel strike did not scale with perturbation size during disruption and adaptation. +P < 0.05, perturbation size effect (perturbation condition effect was not reported). #P < 0.05, interaction effect between perturbation size (regular, 0.4; half-size, 0.2) and perturbation condition (stick, slip). *P < 0.025, significant differences between the regular and half-size perturbations of same condition. ap, anterior-posterior; ml, medial-lateral.
Medial-lateral metrics at left heel strike did not scale with perturbation size when subjects were disrupted or during adaptation (Fig. 9B). Perturbation size did not have a main effect on the changes during disruption for any medial-lateral metric at left heel strike [F values (1, 27) < 1.19, P values > 0.28, values < 0.05]. Despite the significant interaction effect on |ΔXCOMml, lhs| during disruption [F (1, 27) = 7.96, P = 0.009, = 0.23], paired t tests only detected significant differences between Slip perturbations (P = 0.02, Cohen’s d = 0.93) but not between Stick perturbations (P = 0.21, Cohen’s d = 0.43). For the changes of each medial-lateral metric at left heel strike during adaptation, there was neither a main effect of perturbation size nor a significant interaction effect [F values (1, 27) < 1.23, P values > 0.27, values < 0.05]. These results suggest that medial-lateral metrics at left heel strike did not scale with perturbation size during disruption or adaptation.
DISCUSSION
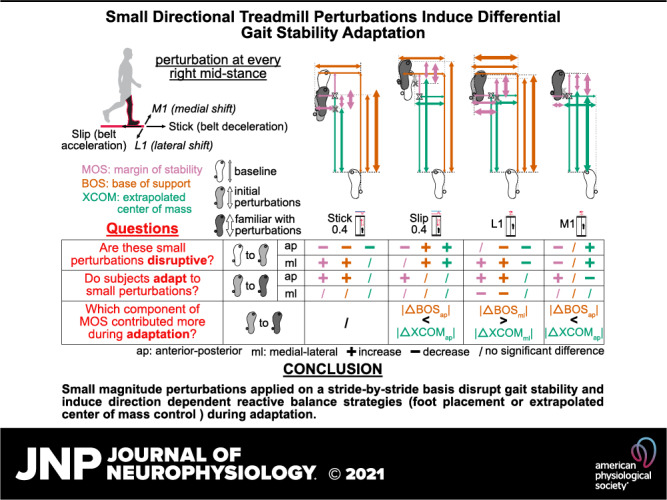
We sought to determine how small magnitude multidirectional perturbations affect modulation of margin of stability, base of support, and extrapolated center of mass on a stride-by-stride basis. In the anterior-posterior direction, subjects mainly modulated XCOMap, lhs when adapting to the belt acceleration (Slip0.4) and medial shift (M1) perturbations. In the medial-lateral direction, subjects primarily modulated BOSml, lhs when adapting to the lateral shift (L1) perturbation. Metrics at left toe off and catch strides revealed that subjects immediately took wider steps for the Stick0.4, Slip0.4, and L1 perturbations and shifted their XCOMap, lto forward for the Stick0.4 perturbation before perturbation onset, providing evidence of feedforward strategies. Despite the combination of feedback and feedforward strategies upon initial exposure to the perturbations, subjects did not use more feedforward strategies during adaptation. When first experiencing the perturbations, anterior-posterior metrics at left heel strike for perturbed strides were more sensitive to perturbations in the coincident anterior-posterior direction, and likewise, MOSml, lhs and XCOMml, lhs were more sensitive to perturbations in the coincident medial-lateral direction. In addition, anterior-posterior metrics at left heel strike scaled with perturbation size upon initially responding to the perturbations (at disruption). These findings suggest that applying small perturbations on a stride-by-stride basis led to the adaptation of the margin of stability, providing new insights regarding the extent of locomotor adaptation to small perturbations. Furthermore, perturbation directions could be used to target specific aspects of gait stability control (e.g., primarily modulating base of support or center of mass).
One of the main findings was that small magnitude perturbations altered gait stability, i.e., the margin of stability at left heel strike, in specific directions. For most perturbations, the MOSap, lhs and MOSml, lhs initially decreased before adapting back to pre levels (Figs. 4 and 5), suggesting that subjects were destabilized initially before gradually regaining stability during the Stick0.4, Slip0.4, and M1 perturbations. These results are consistent with previous studies that showed that treadmill belt and shift perturbations decreased MOSap (11, 25, 42). Adjusting foot placement and BOS is a common approach to modulate MOS (6, 23), which was evident in our Stick0.4 perturbation. Other studies demonstrated the importance of center of mass control to modulate MOS (11, 30), which was evident in the Slip0.4 and M1 perturbations. MOSlhs did not always decrease in response to perturbations. For the Stick0.4 and L1 perturbation, the MOSml, lhs initially increased, however, instead of decreasing as seen in the other perturbations. This increased MOSml, lhs likely resulted from a combination of a more lateral (leftward) foot placement (i.e., increased BOSml, lhs) and redirected XCOMlhs rightward during the lateral shift perturbation. In another study that applied lateral shift perturbations at early single leg support, people used the gluteus medius of their swing leg to place their subsequent foot more outward (9) in the direction of the potential fall (27, 43), which would help increase the MOSml to maintain a more stable body position (19). Larger MOSml often occurs in response to medial-lateral treadmill shift perturbations (42, 44, 45), similar to the increased MOSml, lhs in our L1 perturbation. Interestingly, subjects maintained this increased MOSml, lhs for the Stick0.4 perturbation but gradually reduced the MOSml, lhs for the L1 perturbation, suggesting that subjects either could not or did not have a reason to decrease MOSml, lhs while gaining more experience with the Stick0.4 perturbation.
At left heel strike, subjects primarily modulated foot placement compared with the extrapolated center of mass in the belt deceleration (during disruption) and lateral shift (during adaptation) perturbations. Researchers are increasingly finding that BOS control is an active neuromechanical balance strategy that is adjusted based on the state of the center of mass during walking (4, 43, 46, 47). In addition, the central nervous system is actively involved in this foot placement strategy (48, 49). In our study, both the BOSap, lhs and XCOMap, lhs decreased in response to the Stick0.4 perturbation, but subjects primarily adjusted BOSap, lhs by taking shorter steps during disruption. The reduction and smaller adjustment of XCOMap, lhs compared with the BOSap, lhs was likely a consequence of the rotation and acceleration of the upper body backward relative to the lower body created by the stick perturbations. The significant BOSap, lhs adjustment was likely needed to rapidly interrupt the forward progression of the swing leg to compensate for the interrupted progression of the stance leg caused by the Stick0.4 perturbation (26). These reactions conform to the theory of stepping in the direction of the potential fall and to the maintenance of forward progression on the treadmill (27, 43, 50). During adaptation to the Stick0.4 perturbation, there were similar magnitude changes in the BOSap, lhs and XCOMap, lhs, suggesting a less dominant stepping strategy during adaptation compared with the dominant foot placement strategy during disruption. For the lateral shift perturbation, subjects appeared to employ combination of a foot placement strategy through swing leg control that increased the BOSml, lhs (9), while also using an ankle strategy with the stance leg that decreased XCOMml, lhs by a similar magnitude (51). Changes in the BOSml, lhs then dominated during adaptation to the L1 perturbation. This aligns with the predominant stepping strategy people often employ, taking shorter and wider steps during medial waist-pull perturbations that produced relative movements of the BOS and XCOM similar to our L1 perturbation (52).
Our results also showed that subjects primarily modulated the extrapolated center of mass at left heel strike in the belt acceleration and medial shift perturbations. Both the BOSap, lhs and XCOMap, lhs increased in response to our Slip0.4 perturbation, but subjects mainly modulated their XCOMap, lhs while adapting to the Slip0.4 perturbation. The XCOMap, lhs responses were likely related to the decreased hip flexor moment of the right stance leg to reduce the forward rotation and acceleration of the upper body (11). Even though the BOSap, lhs also changed during adaptation, these changes were smaller than the XCOMap, lhs and were probably a consequence of the facilitated forward swing phase induced by the Slip0.4 perturbation. For the medial shift perturbation, subjects primarily adjusted XCOMlhs, interestingly, in the orthogonal anterior-posterior direction, likely by employing an ankle strategy (27) that incorporated increased tibialis anterior activity of the stance leg (51). Subjects barely adjusted the BOSap, lhs, likely due to the small magnitude of our M1 perturbation. In other medial-lateral perturbations, subjects often take crossover steps to adjust BOS in the anterior-posterior direction (53, 54).
Our results suggest that subjects can flexibly employ balance strategies in response to small magnitude perturbations applied on a stride-by-stride basis, depending on the perturbation direction. When the central nervous system detects a perceived fall, the foot placement strategy shifts the swing foot to the direction of the potential fall, such that at heel strike the undesired movement induced by perturbations could be reversed or at least mitigated (27, 28). Another strategy is adjusting the extrapolated center of mass to stop the potential fall through the stance leg, which is similar to the ankle strategy in other studies (27, 52, 55). The foot placement strategy has the advantage of a large adjustment range, however, it acts slowly due to the time needed for active integration of sensory information from the proprioceptive, visual, and vestibular systems (27, 28). Conversely, the extrapolated center of mass strategy allows for quick responses during single stance, whereas its modulation range is constrained to the relatively small contact area under the stance foot (27, 55, 56). A recent visual perturbation study shows that foot placement and ankle strategies are interdependent and negatively correlated, suggesting that these two strategies complement each other (57). Both strategies may increase the energetic cost as a consequence of the cost of redirecting foot placement and extrapolated center of mass (58–61), but adaptation over time usually leads to a more economical gait (62, 63). Thus, depending on the perturbation direction, people appear to recruit more readily available and efficient balance strategies for gait stability when experiencing small magnitude perturbations applied on a stride-by-stride basis.
In our study, subjects employed a feedforward strategy that involved direction-independent foot placement but direction-dependent extrapolated center of mass control after being initially perturbed. Subjects immediately took wider steps at left toe off for the early catch strides after experiencing the Stick0.4, Slip0.4, and L1 perturbations, indicating a direction-independent anticipatory strategy (Fig. 7, A–C). These changes reflect anticipatory responses because left toe off occurs immediately before the expected perturbation at right mid-stance. For pre to early perturbed, like the catches, subjects also took wider steps at left toe off (Fig. 4 and Fig. 5A), suggesting a general feedforward strategy of increasing step width. Taking wider steps can increase the dynamic stability in the medial-lateral direction (27, 64) and can help prepare for an upcoming perturbation in any direction. For the Stick0.4 perturbation, XCOMap, lto shifted forward for early catch (Fig. 7A) and early perturbed (Fig. 4A), which was opposite to the potential fall direction of the Stick0.4 perturbation, revealing direction-dependent anticipatory XCOM adjustments. Forward shifts of the COM or XCOM position to prepare for impending backward fall inducing perturbations (65–67) and a reduction of forward COM velocity to prepare for impending forward fall inducing perturbations (68, 69) are common anticipatory balance strategies in response to anterior-posterior perturbations. The lack of anterior-posterior XCOM adjustments for the Slip0.4 perturbation was likely due to the less risk of forward fall compared with backward fall (10, 70).
Although subjects initially anticipated, they did not rely more on feedforward strategies when adapting to small magnitude perturbations applied on a stride-by-stride basis. In response to repeated perturbations, people often adapt using feedback (reactive) and feedforward (proactive) control (71, 72). Feedback control relies on sensory detection of unexpected disturbances to dynamic stability, whereas feedforward control relies on knowledge about the expected perturbation generated by prior experience (54, 73). In our study, subjects apparently used feedback control when adapting to the treadmill belt and shift perturbations as MOS and its components did not deviate more from the pre levels with more experience with catch strides. Despite the stride-by-stride exposure to the perturbations, subjects did not appear to develop anticipatory responses. The small perturbations in our study likely contributed to the use of feedback control, as mild medial-lateral perturbations reduce feedforward control and facilitate feedback control in the medial-lateral direction (74). In addition, the repetitive fixed magnitude treadmill belt perturbations also likely contributed to the use of feedback control. Unlike overground walking where subjects can control the displacement of their stance foot and the magnitude of a slip perturbation that allows for more proactive responses (65), subjects are unable to control the displacement of their stance foot during treadmill belt perturbations, resulting in reactive compensatory step lengths and trunk angles responses (75).
Perturbations in the coincident direction more strongly affected gait stability after being initially perturbed. As one might expect, the anterior-posterior and medial-lateral perturbations resulted in a stronger effect in their respective directions (7, 76). In our study, this directional effect was not merely seen in the MOSlhs but was also reflected in its components. Despite the small magnitude, our perturbations also affected metrics in perpendicular directions, though not as strongly as perturbations in coincident directions. Perturbations in one direction often elicit responses perpendicular to the perturbation direction (4, 7, 76, 77) as responses between the sagittal and frontal planes are dynamically coupled (31, 78). Medial-lateral perturbations are often more challenging than the anterior-posterior perturbations (8, 76) because medial-lateral balance requires active control during walking (47, 79). In our study, medial-lateral perturbations were not more challenging than the anterior-posterior perturbations in the anterior-posterior direction, which could be related to the magnitude of the shifts. We used 1 cm medial-lateral shift perturbations as opposed to the 12–15 cm shifts that were used in other studies (8, 76). This suggests that there is a threshold, a medial-lateral shift > 1 cm that is needed to elicit a significant effect on gait stability compared with small anterior-posterior perturbations. We chose relatively small perturbation magnitudes to avoid falls and extra recovery steps as we applied the perturbations on a stride-by-stride basis.
As expected, there was a scaling effect of perturbation magnitude on the anterior-posterior metrics as people tend to magnify their responses proportionally with perturbation size (7, 26, 77). Changes in MOSml, lhs and its components were similar between the two treadmill belt perturbations sizes. In our study, the MOSml, lhs did not respond strongly to the anterior-posterior perturbations (Stick0.4 and Slip0.4) so an even smaller half-sized perturbation (Stick0.2 and Slip0.2) would not be likely to elicit a strong response. Based on the interaction effect for BOSap, lhs observed in our anterior-posterior perturbations (Fig. 9A), we found that the stick perturbations had a stronger scaling effect than slip perturbations for the same increment (from 0.2 to 0.4). Backward instability created by belt decelerations (stick perturbations) tends to be more challenging than forward instability created by the belt accelerations (slip perturbations) (10, 70).
The calculation of MOS is based on an inverted pendulum model and has several assumptions (19). First, the mass of the whole body is modeled as a single mass point of the inverted pendulum, so the possible effects of upper body (arm or trunk) movements in response to perturbations are ignored (18). In this study, we estimated the COM position by averaging the position of the four pelvis markers, which does not account for torso and upper body movements. Although a COM position estimate that includes torso or upper body markers is more accurate, studies have also shown that the average of the four pelvis markers has reasonable COM kinematic accuracy during walking, turning, and medial-lateral platform displacement perturbations (80, 81). Second, the leg is assumed to be rigid and with constant length, which does not consider the large effects of the knee joint motions and moments in response to perturbations (24, 82). Third, the excursions of the COM are small with respect to pendulum length (19), which was likely met in our study due to the small magnitude perturbations. Although all of the assumptions were not likely met in our study, the MOS is a common metric of balance for studies of walking with large perturbations (6, 11, 23–26, 29, 30) and the proposed approaches to fulfill the MOS assumptions vary among studies (83).
The present study had several limitations. One limitation of our study is the small sample size. The sample size was based on previous perturbed walking studies instead of a prespecified effect size to be detected. It is unknown whether all meaningful effect sizes were sufficiently statistically powered for in our analyses. The onsets of the medial-lateral perturbations had a larger delay compared with the anterior-posterior perturbations. The perturbation did not always return to the neutral position or baseline speed before the left heel strike, which likely introduced tiny but extra disturbances. The treadmill system also has a fixed acceleration value for shifting the treadmill platform medial-laterally, which limited the size of the shift perturbation we could use to ≤1 cm for our desired time window such that the treadmill could return to its neutral position at or near left heel strike. To control for the time of the perturbation, we set the duration of the anterior-posterior perturbations to be similar to the medial-lateral perturbations, which again limited the magnitude of the perturbation we could apply. We only applied perturbations to the right leg to limit the experiment to ∼1 h. We would not have expected a difference between perturbing the left or right stepping limbs during walking or in forward falls (84, 85). Finally, we only applied perturbations at one instance of the gait cycle, right leg mid-stance. A recent study applied brief treadmill belt accelerations at push off of each foot on a stride-by-stride basis and that led to sustained increased push-off once the perturbations were removed (86). Applying perturbations at different points of the gait cycle may reveal phase-dependent modulation of margin of stability and its components.
Muscular and cortical mechanisms involved during adaptation of gait stability in response to small magnitude perturbations during walking could provide additional insights about the neural control of walking. We recently found significant differences in electrocortical activity of the anterior cingulate and motor cortices in response to small magnitude mechanical perturbations applied on a stride-by-stride basis during recumbent stepping using electroencephalography (EEG) and source estimation (87). Recording EEG and performing source estimation for subjects experiencing small magnitude perturbations on a stride-by-stride basis during walking may reveal specific cortical networks for modulating foot placement versus center of mass when adapting to perturbations during walking. Currently, EEG gait adaptation studies often use large infrequent perturbations during walking (88, 89).
Conclusions
We investigated how healthy young individuals adjusted their margin of stability, base of support, and extrapolated center of mass in response to small magnitude multidirectional perturbations applied on a stride-by-stride basis. Small magnitude perturbations disrupted gait stability by decreasing the MOSap, lhs in the belt deceleration (Stick0.4), belt acceleration (Slip0.4), and medial treadmill shift (M1) perturbations, while increasing the MOSml, lhs in the lateral treadmill shift (L1) perturbation. During adaptation, BOS (foot placement) control was primarily used in the L1 perturbations, whereas XCOM control was preferred in the Slip0.4 and M1 perturbations. Subjects used both feedback and feedforward strategies after being initially perturbed, but primarily used feedback strategies when adjusting their BOS and XCOM during adaptation. Moreover, MOSlhs and its components were generally more sensitive to perturbations in the coincident direction and scaled with perturbation size upon initial exposure to the perturbations. Overall, our findings provide insights into interstride gait stability and adaptability to small magnitude perturbations experienced on a stride-by-stride basis and have implications for the development of training interventions that could target specific components of gait stability (e.g., base of support or extrapolated center of mass control) for balance-impaired populations.
SUPPLEMENTAL DATA
Supplemental Tables S1–S6: https://doi.org/10.6084/m9.figshare.16910683.
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.16910713.
GRANTS
This study was funded by the National Institute on Aging of the National Institutes of Health (NIH), under Award Number R01AG054621 (to H.J.H.) and a University of Central Florida Office of Research In-House Grant (to H.J.H.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L. and H.J.H. conceived and designed research; J.L. performed experiments; J.L. analyzed data; J.L. and H.J.H. interpreted results of experiments; J.L. prepared figures; J.L. drafted manuscript; J.L. and H.J.H. edited and revised manuscript; J.L. and H.J.H. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at https://doi.org/10.6084/m9.figshare.c.5285258.v3. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
ACKNOWLEDGMENTS
The authors thank Alejandro Hurtado, Kevin Graydon, Amy Lebanoff, and Allen Rahrooh for assistance with data collection.
REFERENCES
- 1.Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ. Locomotor adaptation. Prog Brain Res 191: 65–74, 2011. doi: 10.1016/B978-0-444-53752-2.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansfield A, Wong JS, Bryce J, Knorr S, Patterson KK. Does perturbation-based balance training prevent falls? Systematic review and meta-analysis of preliminary randomized controlled trials. Phys Ther 95: 700–709, 2015. doi: 10.2522/ptj.20140090. [DOI] [PubMed] [Google Scholar]
- 3.Buurke TJW, Lamoth CJC, Vervoort D, van der Woude LHV, den Otter R. Adaptive control of dynamic balance in human gait on a split-belt treadmill. J Exp Biol 221: jeb174896, 2018. doi: 10.1242/jeb.174896. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol 102: 1411–1419, 2009. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franz JR, Francis C, Allen M, Thelen DG. Visuomotor entrainment and the frequency-dependent response of walking balance to perturbations. IEEE Trans Neural Syst Rehabil Eng 25: 1132–1142, 2016. doi: 10.1109/TNSRE.2016.2603340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hof AL, Vermerris SM, Gjaltema WA. Balance responses to lateral perturbations in human treadmill walking. J Exp Biol 213: 2655–2664, 2010. doi: 10.1242/jeb.042572. [DOI] [PubMed] [Google Scholar]
- 7.Vlutters M, van Asseldonk EHF, van der Kooij H. Lower extremity joint-level responses to pelvis perturbation during human walking. Sci Rep 8: 14621, 2018. doi: 10.1038/s41598-018-32839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntosh EI, Zettel JL, Vallis LA. Stepping responses in young and older adults following a perturbation to the support surface during gait. J Mot Behav 49: 288–298, 2017. doi: 10.1080/00222895.2016.1204262. [DOI] [PubMed] [Google Scholar]
- 9.Afschrift M, Pitto L, Aerts W, van Deursen R, Jonkers I, De Groote F. Modulation of gluteus medius activity reflects the potential of the muscle to meet the mechanical demands during perturbed walking. Sci Rep 8: 11675, 2018. doi: 10.1038/s41598-018-30139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel PJ, Bhatt T. Fall risk during opposing stance perturbations among healthy adults and chronic stroke survivors. Exp Brain Res 236: 619–628, 2018. doi: 10.1007/s00221-017-5138-6. [DOI] [PubMed] [Google Scholar]
- 11.Debelle H, Harkness-Armstrong C, Hadwin K, Maganaris CN, O’Brien TD. Recovery from a forward falling slip: measurement of dynamic stability and strength requirements using a split-belt instrumented treadmill. Front Sports Act Living 2: 82, 2020. doi: 10.3389/fspor.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerards MHG, Marcellis RGJ, Poeze M, Lenssen AF, Meijer K, de Bie RA. Perturbation-based balance training to improve balance control and reduce falls in older adults—study protocol for a randomized controlled trial. BMC Geriatr 21: 9, 2021. doi: 10.1186/s12877-020-01944-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper SA, Beethe AZ, Dakin CJ, Bolton DAE. Promoting generalized learning in balance recovery interventions. Brain Sci 11: 402, 2021. doi: 10.3390/brainsci11030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee A, Bhatt T, Liu X, Wang Y, Wang S, Pai Y-CC. Can treadmill slip-perturbation training reduce longer-term fall risk upon overground slip exposure? J Appl Biomech 36: 1–9, 2020. doi: 10.1123/jab.2019-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamanidis K, Epro G, McCrum C, König M. Improving trip- and slip-resisting skills in older people: perturbation dose matters. Exerc Sport Sci Rev 48: 40–47, 2020. doi: 10.1249/JES.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 16.Gerards MHG, McCrum C, Mansfield A, Meijer K. Perturbation-based balance training for falls reduction among older adults: current evidence and implications for clinical practice. Geriatr Gerontol Int 17: 2294–2303, 2017. doi: 10.1111/ggi.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dusane S, Bhatt T. Effect of multisession progressive gait-slip training on fall-resisting skills of people with chronic stroke: examining motor adaptation in reactive stability. Brain Sci 11: 894, 2021. doi: 10.3390/brainsci11070894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruijn SM, Meijer OG, Beek PJ, van Dieën JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface 10: 20120999, 2013. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hof AL, Gazendam MGJ, Sinke WE. The condition for dynamic stability. J Biomech 38: 1–8, 2005. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblatt NJ, Grabiner MD. Measures of frontal plane stability during treadmill and overground walking. Gait Posture 31: 380–384, 2010. doi: 10.1016/j.gaitpost.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Höhne A, Stark C, Brüggemann G-P, Arampatzis A. Effects of reduced plantar cutaneous afferent feedback on locomotor adjustments in dynamic stability during perturbed walking. J Biomech 44: 2194–2200, 2011. doi: 10.1016/j.jbiomech.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Hof AL. The “extrapolated center of mass” concept suggests a simple control of balance in walking. Hum Mov Sci 27: 112–125, 2008. doi: 10.1016/j.humov.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Süptitz F, Moreno Catalá M, Brüggemann G-P, Karamanidis K. Dynamic stability control during perturbed walking can be assessed by a reduced kinematic model across the adult female lifespan. Hum Mov Sci 32: 1404–1414, 2013. doi: 10.1016/j.humov.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Hak L, Houdijk H, van der Wurff P, Prins MR, Mert A, Beek PJ, van Dieën JH. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clin Biomech (Bristol, Avon) 28: 1041–1048, 2013. doi: 10.1016/j.clinbiomech.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 25.McCrum C, Karamanidis K, Willems P, Zijlstra W, Meijer K. Retention, savings and interlimb transfer of reactive gait adaptations in humans following unexpected perturbations. Commun Biol 1: 230, 2018. doi: 10.1038/s42003-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martelli D, Aprigliano F, Tropea P, Pasquini G, Micera S, Monaco V. Stability against backward balance loss: age-related modifications following slip-like perturbations of multiple amplitudes. Gait Posture 53: 207–214, 2017. doi: 10.1016/j.gaitpost.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Reimann H, Fettrow T, Jeka JJ. Strategies for the control of balance during locomotion. Kinesiol Rev 7: 18–25, 2018. doi: 10.1123/kr.2017-0053. [DOI] [Google Scholar]
- 28.Bruijn SM, van Dieën JH. Control of human gait stability through foot placement. J R Soc Interface 15: 20170816, 2018. doi: 10.1098/rsif.2017.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martelli D, Kang J, Agrawal SK. A single session of perturbation-based gait training with the A-TPAD improves dynamic stability in healthy young subjects. IEEE Int Conf Rehabil Robot 2017: 479–484, 2017. doi: 10.1109/ICORR.2017.8009294. [DOI] [PubMed] [Google Scholar]
- 30.McCrum C, Karamanidis K, Grevendonk L, Zijlstra W, Meijer K. Older adults demonstrate interlimb transfer of reactive gait adaptations to repeated unpredictable gait perturbations. Geroscience 42: 39–49, 2020. doi: 10.1007/s11357-019-00130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buurke TJW, Liu C, Park S, den Otter R, Finley JM. Maintaining sagittal plane balance compromises frontal plane balance during reactive stepping in people post-stroke. Clin Biomech (Bristol, Avon) 80: 105135, 2020. doi: 10.1016/j.clinbiomech.2020.105135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang HJ, Kram R, Ahmed AA. Reduction of metabolic cost during motor learning of arm reaching dynamics. J Neurosci 32: 2182–2190, 2012. doi: 10.1523/JNEUROSCI.4003-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hreljac A, Marshall RN. Algorithms to determine event timing during normal walking using kinematic data. J Biomech 33: 783–786, 2000. doi: 10.1016/S0021-9290(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 35.De Witt JK. Determination of toe-off event time during treadmill locomotion using kinematic data. J Biomech 43: 3067–3069, 2010. doi: 10.1016/j.jbiomech.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Okubo Y, Brodie MA, Sturnieks DL, Hicks C, Lord SR. A pilot study of reactive balance training using trips and slips with increasing unpredictability in young and older adults: Biomechanical mechanisms, falls and clinical feasibility. Clin Biomech (Bristol, Avon) 67: 171–179, 2019. doi: 10.1016/j.clinbiomech.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Beltran EJ, Dingwell JB, Wilken JM. Margins of stability in young adults with traumatic transtibial amputation walking in destabilizing environments. J Biomech 47: 1138–1143, 2014. doi: 10.1016/j.jbiomech.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J Physiol 591: 1081–1095, 2013. doi: 10.1113/jphysiol.2012.245506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pienciak-Siewert A, Barletta AJ, Ahmed AA. Transfer of postural adaptation depends on context of prior exposure. J Neurophysiol 111: 1466–1478, 2014. doi: 10.1152/jn.00235.2013. [DOI] [PubMed] [Google Scholar]
- 40.Malone LA, Bastian AJ. Age-related forgetting in locomotor adaptation. Neurobiol Learn Mem 128: 1–6, 2016. doi: 10.1016/j.nlm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4: 863, 2013. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAndrew Young PM, Wilken JM, Dingwell JB. Dynamic margins of stability during human walking in destabilizing environments. J Biomech 45: 1053–1059, 2012. doi: 10.1016/j.jbiomech.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Srinivasan M. Stepping in the direction of the fall: the next foot placement can be predicted from current upper body state in steady-state walking. Biol Lett 10: 20140405, 2014. doi: 10.1098/rsbl.2014.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hak L, Houdijk H, Steenbrink F, Mert A, van der Wurff P, Beek PJ, van Dieën JH. Speeding up or slowing down?: Gait adaptations to preserve gait stability in response to balance perturbations. Gait Posture 36: 260–264, 2012. doi: 10.1016/j.gaitpost.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Onushko T, Boerger T, Van Dehy J, Schmit BD. Dynamic stability and stepping strategies of young healthy adults walking on an oscillating treadmill. PLoS One 14: e0212207, 2019. doi: 10.1371/journal.pone.0212207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Leeuwen AM, van Dieën JH, Daffertshofer A, Bruijn SM. Active foot placement control ensures stable gait: effect of constraints on foot placement and ankle moments. PLoS One 15: e0242215, 2020. doi: 10.1371/journal.pone.0242215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reimold NK, Knapp HA, Henderson RE, Wilson L, Chesnutt AN, Dean JC. Altered active control of step width in response to mediolateral leg perturbations while walking. Sci Rep 10: 12197, 2020. doi: 10.1038/s41598-020-69052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruijn SM, Van Dieën JH, Daffertshofer A. Beta activity in the premotor cortex is increased during stabilized as compared to normal walking. Front Hum Neurosci 9: 593, 2015. doi: 10.3389/fnhum.2015.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sipp AR, Gwin JT, Makeig S, Ferris DP. Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J Neurophysiol 110: 2050–2060, 2013. doi: 10.1152/jn.00744.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winter DA. Human balance and posture control during standing and walking. Gait Posture 3: 193–214, 1995. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- 51.Afschrift M, van Deursen R, De Groote F, Jonkers I. Increased use of stepping strategy in response to medio-lateral perturbations in the elderly relates to altered reactive tibialis anterior activity. Gait Posture 68: 575–582, 2019. doi: 10.1016/j.gaitpost.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Matjačić Z, Zadravec M, Olenšek A. Influence of treadmill speed and perturbation intensity on selection of balancing strategies during slow walking perturbed in the frontal plane. Appl Bionics Biomech 2019: 1046459, 2019. doi: 10.1155/2019/1046459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borrelli J, Creath RA, Pizac D, Hsiao H, Sanders OP, Rogers MW. Perturbation-evoked lateral steps in older adults: why take two steps when one will do? Clin Biomech (Bristol, Avon) 63: 41–47, 2019. doi: 10.1016/j.clinbiomech.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Major MJ, Serba CK, Chen X, Reimold N, Ndubuisi-Obi F, Gordon KE. Proactive locomotor adjustments are specific to perturbation uncertainty in below-knee prosthesis users. Sci Rep 8: 1863, 2018. doi: 10.1038/s41598-018-20207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hof AL, Duysens J. Responses of human ankle muscles to mediolateral balance perturbations during walking. Hum Mov Sci 57: 69–82, 2018. doi: 10.1016/j.humov.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Reimann H, Fettrow T, Grenet D, Thompson ED, Jeka JJ. Phase-dependency of medial-lateral balance responses to sensory perturbations during walking. Front Sports Act Living 1: 25, 2019. doi: 10.3389/fspor.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fettrow T, Reimann H, Grenet D, Thompson E, Crenshaw J, Higginson J, Jeka J. Interdependence of balance mechanisms during bipedal locomotion. PLoS One 14: e0225902, 2019. doi: 10.1371/journal.pone.0225902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech 37: 827–835, 2004. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Ijmker T, Houdijk H, Lamoth CJC, Beek PJ, van der Woude LHV. Energy cost of balance control during walking decreases with external stabilizer stiffness independent of walking speed. J Biomech 46: 2109–2114, 2013. doi: 10.1016/j.jbiomech.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 60.IJmker T, Lamoth CJ, Houdijk H, van der Woude LHV, Beek PJ. Postural threat during walking: effects on energy cost and accompanying gait changes. J Neuroeng Rehabil 11: 71, 2014. doi: 10.1186/1743-0003-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu M, Brown GL, Woodward JL, Bruijn SM, Gordon KE. A novel movement amplification environment reveals effects of controlling lateral centre of mass motion on gait stability and metabolic cost. R Soc Open Sci 7: 190889, 2020. doi: 10.1098/rsos.190889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selinger JC, O’Connor SM, Wong JD, Donelan JM. Humans can continuously optimize energetic cost during walking. Curr Biol 25: 2452–2456, 2015. doi: 10.1016/j.cub.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez N, Park S, Finley JM. Evidence of energetic optimization during adaptation differs for metabolic, mechanical, and perceptual estimates of energetic cost. Sci Rep 7: 7682, 2017. doi: 10.1038/s41598-017-08147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu M, Matsubara JH, Gordon KE. General and specific strategies used to facilitate locomotor maneuvers. PLoS One 10: e0132707, 2015. doi: 10.1371/journal.pone.0132707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhatt T, Wening JD, Pai Y-C. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp Brain Res 170: 61–73, 2006. doi: 10.1007/s00221-005-0189-5. [DOI] [PubMed] [Google Scholar]
- 66.Yang F, Pai CY-C. Alteration in community-dwelling older adults’ level walking following perturbation training. J Biomech 46: 2463–2468, 2013. doi: 10.1016/j.jbiomech.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okubo Y, Brodie MA, Sturnieks DL, Hicks C, Carter H, Toson B, Lord SR. Exposure to trips and slips with increasing unpredictability while walking can improve balance recovery responses with minimum predictive gait alterations. PLoS One 13: e0202913, 2018. doi: 10.1371/journal.pone.0202913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Wang S, Bolton R, Kaur T, Bhatt T. Effects of task-specific obstacle-induced trip-perturbation training: proactive and reactive adaptation to reduce fall-risk in community-dwelling older adults. Aging Clin Exp Res 32: 893–905, 2020. doi: 10.1007/s40520-019-01268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang T-Y, Bhatt T, Yang F, Pai Y-C. Adaptive control reduces trip-induced forward gait instability among young adults. J Biomech 45: 1169–1175, 2012. doi: 10.1016/j.jbiomech.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roeles S, Rowe PJ, Bruijn SM, Childs CR, Tarfali GD, Steenbrink F, Pijnappels M. Gait stability in response to platform, belt, and sensory perturbations in young and older adults. Med Biol Eng Comput 56: 2325–2335, 2018. doi: 10.1007/s11517-018-1855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuo AD. The relative roles of feedforward and feedback in the control of rhythmic movements. Motor Control 6: 129–145, 2002. doi: 10.1123/mcj.6.2.129. [DOI] [PubMed] [Google Scholar]
- 72.Bohm S, Mademli L, Mersmann F, Arampatzis A. Predictive and reactive locomotor adaptability in healthy elderly: a systematic review and meta-analysis. Sports Med 45: 1759–1777, 2015. doi: 10.1007/s40279-015-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patla AE. Strategies for dynamic stability during adaptive human locomotion. IEEE Eng Med Biol Mag 22: 48–52, 2003. doi: 10.1109/memb.2003.1195695. [DOI] [PubMed] [Google Scholar]
- 74.Wu M, Brown G, Gordon KE. Control of locomotor stability in stabilizing and destabilizing environments. Gait Posture 55: 191–198, 2017. doi: 10.1016/j.gaitpost.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 75.Lee A, Bhatt T, Pai Y-C. Generalization of treadmill perturbation to overground slip during gait: effect of different perturbation distances on slip recovery. J Biomech 49: 149–154, 2016. doi: 10.1016/j.jbiomech.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Madehkhaksar F, Klenk J, Sczuka K, Gordt K, Melzer I, Schwenk M. The effects of unexpected mechanical perturbations during treadmill walking on spatiotemporal gait parameters, and the dynamic stability measures by which to quantify postural response. PLoS One 13: e0195902, 2018. doi: 10.1371/journal.pone.0195902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martelli D, Vashista V, Micera S, Agrawal SK. Direction-dependent adaptation of dynamic gait stability following waist-pull perturbations. IEEE Trans Neural Syst Rehabil Eng 24: 1304–1313, 2016. doi: 10.1109/TNSRE.2015.2500100. [DOI] [PubMed] [Google Scholar]
- 78.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech 33: 1433–1440, 2000. doi: 10.1016/S0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 79.Rankin BL, Buffo SK, Dean JC. A neuromechanical strategy for mediolateral foot placement in walking humans. J Neurophysiol 112: 374–383, 2014. doi: 10.1152/jn.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Havens KL, Mukherjee T, Finley JM. Analysis of biases in dynamic margins of stability introduced by the use of simplified center of mass estimates during walking and turning. Gait Posture 59: 162–167, 2018. doi: 10.1016/j.gaitpost.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inkol KA, Huntley AH, Vallis LA. Modeling margin of stability with feet in place following a postural perturbation: effect of altered anthropometric models for estimated extrapolated centre of mass. Gait Posture 62: 434–439, 2018. doi: 10.1016/j.gaitpost.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 82.Yoo D, Seo KH, Lee BC. The effect of the most common gait perturbations on the compensatory limb’s ankle, knee, and hip moments during the first stepping response. Gait Posture 71: 98–104, 2019. doi: 10.1016/j.gaitpost.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 83.Watson F, Fino PC, Thornton M, Heracleous C, Loureiro R, Leong JJH. Use of the margin of stability to quantify stability in pathologic gait—a qualitative systematic review. BMC Musculoskelet Disord 22: 597, 2021. doi: 10.1186/s12891-021-04466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Süptitz F, Karamanidis K, Moreno Catalá M, Brüggemann G-P. Symmetry and reproducibility of the components of dynamic stability in young adults at different walking velocities on the treadmill. J Electromyogr Kinesiol 22: 301–307, 2012. doi: 10.1016/j.jelekin.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Madigan ML, Lloyd EM. Age and stepping limb performance differences during a single-step recovery from a forward fall. J Gerontol A Biol Sci Med Sci 60: 481–485, 2005. doi: 10.1093/gerona/60.4.481. [DOI] [PubMed] [Google Scholar]
- 86.Farrens A, Lilley M, Sergi F. Training propulsion via acceleration of the trailing limb. IEEE Trans Neural Syst Rehabil Eng 28: 2816–2825, 2020. doi: 10.1109/TNSRE.2020.3032094. [DOI] [PubMed] [Google Scholar]
- 87.Shirazi SY, Huang HJ. Differential theta-band signatures of the anterior cingulate and motor cortices during seated locomotor perturbations. IEEE Trans Neural Syst Rehabil Eng 29: 468–477, 2021. doi: 10.1109/TNSRE.2021.3057054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagner J, Makeig S, Gola M, Neuper C, Müller-Putz G. Distinct β band oscillatory networks subserving motor and cognitive control during gait adaptation. J Neurosci 36: 2212–2226, 2016. doi: 10.1523/JNEUROSCI.3543-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peterson SM, Ferris DP. Group-level cortical and muscular connectivity during perturbations to walking and standing balance. Neuroimage 198: 93–103, 2019. doi: 10.1016/j.neuroimage.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1–S6: https://doi.org/10.6084/m9.figshare.16910683.
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.16910713.
Data Availability Statement
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at https://doi.org/10.6084/m9.figshare.c.5285258.v3. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.