Abstract
The environment and personnel are both exposed to powdered pharmaceuticals inside pharmacies. This makes developing new methods for rapidly determining such contaminants an important objective. In this study, we developed a liquid-chromatography tandem-mass-spectrometry (LC–MS/MS) method for the simultaneous qualitative and quantitative determination of powdered medicinal drugs, such as famotidine, risperidone, lansoprazole, olanzapine, haloperidol, clarithromycin, promethazine, levomepromazine, and chlorpromazine. The method involves the use of acetaminophen as the internal standard, an LC–MS/MS method with a core–shell column, and a 10 mM ammonium formate/acetonitrile gradient mobile phase. The analytes were separated within 14 min, and MS with an electrospray ionization source in positive-ion mode was used. The limits of detection for the 9 drugs were .1-8.4 ng/mL. Linear calibration curves in the 10-50 000 ng/mL range were constructed, and inter-day accuracies of 92.6-113.8% were determined for the 9 drugs. The coefficients of variation were less than 14.6%. These data suggest that the proposed method is applicable for the routine assaying of powdered-medicine contamination in pharmacies.
Keywords: liquid-chromatography tandem-mass-spectrometry, powdered medicinal drugs, pharmacy, environmental contamination, core–shell column
Introduction
Environmental contamination by and the exposure of pharmacy personnel to biologically active compounds must be avoided. Several medicinal drugs are dispensed as powders in Japan. 1 It is important that powdered medicines do not contaminate other drugs, and that pharmacists avoid exposure by inhalation or adsorption, 2 especially since allergic symptoms and irritation resulting from environmental exposure have been reported.3-5 The degree of danger that pharmacists are subject to depends on whether they work in a community- or hospital-based pharmacy. Each of these workplace settings presents different hazards that need to be addressed to prevent harm, and sensitive, specific, high-throughput analytical methods are required to quantify medicinal drugs in various pharmacy settings.
Various analytical methods are available for identifying and quantifying drugs. Established methods include high performance liquid-chromatography (HPLC) with ultraviolet detection using conventional reverse-phase C18 (octadecyl-silica: ODS) columns. Recently, mass spectrometry (MS) has been recognized as a powerful tool for determining several drugs because of its high selectivity and sensitivity, and liquid-chromatography tandem-mass-spectrometry (LC–MS/MS) is ideally suited to the analysis of medicinal drugs. 6 We previously reported the use of a conventional ODS column and LC–MS/MS methods for determining environmental contamination by powdered medicines. 7 Core–shell ODS columns have been reported to have shorter retention times, leading to lower solvent consumption.8,9 Therefore, we explored the possibility of using a high-throughput core–shell ODS column for the determination of powdered medicinal drug samples.
Materials and Methods
Chemicals and Materials
We measured the concentration of suspended drug ingredients. Nine drugs, capable of being quantitatively determined, were selected from 25 drugs reported to be compounded frequently in 15 hospitals in Gifu prefecture. 7 The drugs targeted in this study were the psychotropic drugs (chlorpromazine, haloperidol, levomepromazine, olanzapine, and risperidone), anti-Parkinson drug (promethazine), anti-peptic ulcer drugs (famotidine and lansoprazole), antipyretic and analgesic drug (acetaminophen), and antibiotic drug (clarithromycin). The drugs that could not be detected or quantified were albumin tannate, amoxicillin, bethanechol chloride, bromhexine hydrochloride, carbocysteine, codeine phosphate, domperidone, furosemide, loperamide hydrochloride, pantethine, pranlukast, sodium valproate, teprenone, ursodeoxycholic acid, and zopiclone. Acetaminophen (paracetamol, internal standard (IS)) and haloperidol were purchased from the Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Famotidine, risperidone, lansoprazole, olanzapine, haloperidol, clarithromycin, promethazine, levomepromazine, and chlorpromazine were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Ammonium formate was obtained from Nakarai Tesuque (Kyoto, Japan), and HPLC grade methanol was obtained from Kishida Chemical (Osaka, Japan). Purified water from a Milli-Q system (Millipore, Bedford, MA, USA) was used throughout this study. Other analytical reagent grade chemicals were also used.
Standard Solutions
Stock solutions of compounds and the IS (1 mg/mL) were prepared in methanol and stored at −60°C until use. Working solutions for calibration and control purposes were prepared via appropriate dilution in a 1:1 mixture of acetonitrile and 10 mM ammonium formate. Calibration curves were constructed from data acquired at different concentrations (10, 50, 100, 500, 1000, 5000, 10 000, and 50 000 ng/mL). Quality control (QC) samples at concentrations of 10, 50, 100, 500, and 1000 ng/mL were also prepared. The prepared standard and QC solutions were pipetted into 1.5-mL polypropylene tubes and stored at −60°C until required.
LC–MS/MS Methods
LC–MS/MS analyses were carried out on an Agilent 1260 infinity system coupled to an Agilent Technologies 6460 Triple Quad LC/MS (Santa Clara, CA, USA). The mass spectrometer was operated using an atmospheric-pressure electrospray ionization (ESI) source in positive-ion mode (ESI+) with multiple reaction monitoring (MRM). Chromatography was performed under gradient conditions using a Kinetex C18 column (core–shell ODS with 2.6-μm particles, 2.1 × 100 mm, Phenomenex, Torrance, CA, USA). The mobile phase comprised solvent A (10 mM aqueous ammonium formate) and solvent B (acetonitrile). The following gradient program was used: 0-1 min, isocratic conditions with 80:20 A/B; 1-3 min, linear gradient from 80:20 A/B to 10:90 A/B; 3-14 min, isocratic conditions with 10:90 A/B. The program included a washing cycle with solvent B to eliminate any carryover from the previous sample. The flow rate was set to .12 mL/min. A conventional XBridge C18 column (particle size: 3.5 μm, 2.1 × 100 mm, Waters, Milford, MA, USA) was used to compare the chromatographic data. The column and autosampler tray temperatures were stabilized at 40 and 5°C, respectively. The injection volume was 10 μL, and the LC effluent was directed to the ESI source in the absence of splitting; the analytical run time was 14 min. The MS/MS instrument was operated with a capillary voltage of 4 kV; the desolvation gas (nitrogen) was heated to 350°C and delivered at 10 L/min, and the nebulizer was maintained at 50 psi. The optimized MRM, cone voltage, and collision energy of each analyte are listed in Table 1. The peak areas of all components were automatically integrated using the Agilent MassHunter software.
Table 1.
Parameters for the LC–MS/MS method.
| Compounds | Multiple reaction monitoring transition (m/z) | Fragmentor voltage (V) | Collision Energy (eV) |
|---|---|---|---|
| Famotidine | 338.0 > 189.0 | 90 | 15 |
| Acetaminophen (internal standard) | 152.1 > 110.0 | 90 | 15 |
| Risperidone | 411.2 > 191.0 | 140 | 25 |
| Lansoprazole | 370.1 > 252.0 | 50 | 5 |
| Olanzapine | 313.1 > 256.0 | 140 | 20 |
| Haloperidol | 377.1 > 123.0 | 140 | 45 |
| Clarithromycin | 748.5 > 158.0 | 140 | 25 |
| Promethazine | 285.1 > 86.0 | 90 | 15 |
| Levomepromazine | 329.2 > 100.0 | 140 | 20 |
| Chlorpromazine | 320.0 > 86.0 | 90 | 20 |
Validation samples were prepared and analyzed to evaluate inter-day accuracies and precisions of the analytical method for the authentic standard solutions (10, 50, 100, 500, and 1000 ng/mL). Six replicates of each validation-concentration solution were analyzed along with one set of standard samples on each of the six days using the same instrument. Each limit of detection (LOD) was determined from a signal-to-noise ratio (S/N) of 3, and each limit of quantification (LOQ) was determined from an S/N of 10. Both of these S/Ns are in accordance with the November 2005 ICH Q2(R1) guideline. 10
We determined the concentrations of the suspended particles in the dispensary of a hospital in accordance with the working environment measurement standards 11 provided by the Ministry of Health, Labour and Welfare, Japan. Dust concentrations were determined at locations and times at which exposure levels to pharmacists were considered to be average (A-measurement) and maximum (B-measurement) (Figure 1). 11 Six A-measurement points were set at intervals of about 6 m. Meanwhile, B-measurement points were taken to be those points where hospital pharmacists considered the drug dust to be scattered the most. The measurements were taken at heights of between 1.2 and 1.5 m from the floor for 10 min to simulate the pharmacist breathing zone. The suspended particles in air were captured on polytetrafluoroethylene (PTFE) filters (PF-4) via vacuum filtration at a total flow volume of 300 L for 10 min using an air sampler (model ESH-501H; Okano Works Ltd., Osaka, Japan) at the air sampling point (Figure 1). The absorbed filter was immersed in 1:1 water: methanol (10 mL) and ultrasonicated for 30 min. The extracted samples were then filtered through a .45-μm membrane filter. Each sample was then evaporated under nitrogen at 40°C. The residue was resolved in 200 μL of a 1:1 mixture of acetonitrile and 10 mM ammonium formate, and a 10-μL aliquot of the sample was injected into the LC–MS/MS system.
Figure 1.
Setting the measurement points for suspended-particle concentrations in a hospital dispensary (more than 450 hospital beds, dispensary floor space of about 72 m2, and an average of 50 powdered-drug prescriptions dispensed per day).
Results and Discussion
Optimal desolvation temperature, collision energy, and argon gas-flow rate were determined by observing the maximum responses of product ions. Furthermore, 10 mM aqueous ammonium acetate/methanol and 10 mM aqueous ammonium acetate/acetonitrile were examined as mobile phases; optimal conditions in terms of peak intensity and peak shape were obtained with a mobile phase comprising 10 mM aqueous ammonium formate and acetonitrile.
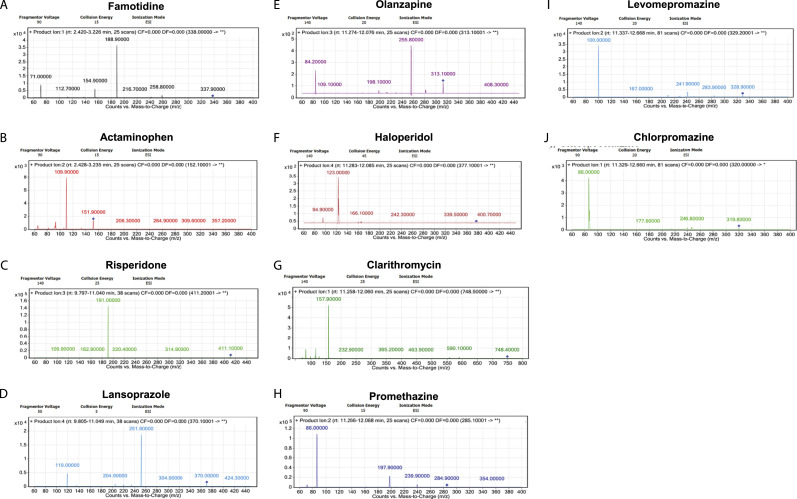
The samples were quantitatively analyzed in MRM mode because highly selective and sensitive data are acquired in this mode (Table 1). The MS spectrum for each compound is shown in Figure 2. Figure 3 shows the representative total ion chromatograms for a Kinetex C18 column and an XBridge C18 column, as well as the MRM chromatograms of authentic standard solutions for the Kinetex C18 column. A core–shell column is typically used because of its advantages including analysis speed and resolution power; however, a common HPLC instrument with minimal adjustments can be used, owing to the low back pressure guaranteed by this type of column. We used 2 columns, namely, Kinetex C18 and XBridge C18, to examine the chromatographic resolution of the 9 compounds; the Kinetex C18 column provided short retention times that led to the use of less solvent. The typical retention times for famotidine, acetaminophen, risperidone, lansoprazole, olanzapine, haloperidol, clarithromycin, promethazine, levomepromazine, and chlorpromazine were 2.2, 2.3, 10.2, 10.3, 10.4, 10.7 11.2, 11.4, 11.5, and 11.9 min, respectively, (Table 2). All compounds were separated under the specified conditions, and all peaks were detected within 14 min. A sample carryover effect was not observed (data not shown). The XBridge C18 column provided improved retention times for famotidine, acetaminophen, risperidone, lansoprazole, olanzapine, haloperidol, clarithromycin, promethazine, levomepromazine, and chlorpromazine in aqueous solutions of 2.6, 2.7, 10.9, 11.0, 11.3, 11.7, 12.0, 12.1, 12.4, and 12.7 min, respectively, (data not shown) using the high-resolution ODS column (XBridge C18). These retention times were longer than those obtained with the core–shell column.
Figure 2.
Mass spectra of authentic standards.
Figure 3.
Representative total ion chromatograms for a Kinetex C18 (a) and an XBridge C18 (m) column, and multiple reaction monitoring chromatograms of: (b-k) authentic standards (100 ng/mL) and (l) air at the sampling point, using a core–shell ODS column as described in the Methods section.
Table 2.
Retention time and validation data in the authentic standard solution.
| Compounds | Retention time (min) | Limit of detection (ng/mL) | Limit of quantification (ng/mL) | Linearity range (ng/mL) | Correlation coefficient (r2) |
|---|---|---|---|---|---|
| Famotidine | 2.2 | 5.7 | 17.1 | 10-50 000 | .9992 |
| Acetaminophen (internal standard) | 2.3 | 50.0 | 150.0 | 50-50 000 | .9998 |
| Risperidone | 10.2 | .1 | 0.3 | 10-50 000 | .9883 |
| Lansoprazole | 10.3 | 8.4 | 25.2 | 10-50 000 | .9989 |
| Olanzapine | 10.4 | 5.5 | 16.5 | 10-50 000 | .9945 |
| Haloperidol | 10.7 | .5 | 1.5 | 10-50 000 | .9694 |
| Clarithromycin | 11.2 | 1.5 | 4.5 | 10-50 000 | .9787 |
| Promethazine | 11.4 | .5 | 1.5 | 10-50 000 | .9906 |
| Levomepromazine | 11.5 | .8 | 2.4 | 10-50 000 | .9925 |
| Chlorpromazine | 11.9 | 7.1 | 21.3 | 10-50 000 | .9899 |
Our previous study was conducted using a GL Sciences LC800 HPLC (GL Sciences Inc, Tokyo, Japan) coupled with an AB SCIEX QTRAP 5500 mass spectrometer (AB SCIEX, Foster City, CA, USA). 7 The separation was performed with a Shiseido Capcell Pak C18 column (particle size: 3 μm, 1.0 × 75 mm) (Shiseido Co. Ltd., Tokyo, Japan) at 40°C and an injection volume of 5 μL. The mobile phase comprised solvent A (10-mM ammonium acetate in .1% aqueous formic acid) and solvent B (methanol). The flow rate was .15 mL/min. The following gradient program was used: 0-6 min, linear gradient from 95:5 A/B to 0:100 A/B; 6-8 min, isocratic conditions with 0:100 A/B; 8-14 min, linear gradient back to the initial conditions (from 0:100 A/B to 95:5 A/B). A detailed comparison between the Kinetex C18 and Shiseido Capcell Pak C18 methods was not possible. This was because of the differences in column sizes, ODS packing-material properties, particle sizes, and mobile phase compositions. However, the total amount of the mobile phase that was required per column volume for the Kinetex C18 column was smaller than that for the Shiseido Capcell Pak C18 column (data not shown).
The LODs, LOQs, and linearity ranges are listed in Table 2. The peak-area ratios of authentic standard solutions as functions of concentration were subjected to linear regression over the 10-50 000 ng/mL concentration range. The LODs and LOQs for the 9 drugs ranged from .1 to 8.4 ng/mL and .3 to 25.2 ng/mL, respectively. Satisfactory LODs, LOQs, and linear ranges were observed for all compounds. The LOQs in our previous research were over 10 ng/mL (data not shown), 7 indicating that the sensitivity of the present method compares favorably with that of our previous method.
Inter-day precisions were calculated from the data obtained from the 6 replicated analyses at 5 concentrations (Table 3). The inter-day precision of the 9 drugs ranged from 2.2% to 14.6% at each concentration, with the mean accuracy ranging between 92.6% and 113.8%. Generally, the accuracy for each compound was within the quantitative bioanalytical method-validation guidelines set by the ICH, that is, 85-115%, and the precision was below 15%. 10 The accuracies and precisions during the determinations of these compounds were satisfactory.
Table 3.
Inter-day precision and accuracy in the authentic standard solution.
| Compounds | 10 ng/mL | 50 ng/mL | 100 ng/mL | 500 ng/mL | 1000 ng/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean±SD (ng/mL) | CV (%) | Accuracy (%) | Mean±SD (ng/mL) | CV (%) | Accuracy (%) | Mean±SD (ng/mL) | CV (%) | Accuracy (%) | Mean±SD (ng/mL) | CV (%) | Accuracy (%) | Mean±SD (ng/mL) | CV (%) | Accuracy (%) | |
| Famotidine | 9.3±0.4 | 4.6 | 93.3 | 50.4±1.7 | 3.3 | 100.7 | 106.9±8.9 | 8.3 | 106.9 | 487.6±31.6 | 6.5 | 97.5 | 980.0±59.0 | 6.0 | 98.0 |
| Acetaminophen (internal standard) | - | - | - | 46.8±5.7 | 12.2 | 96.2 | 103.5±6.2 | 5.9 | 103.5 | 481.6±36.6 | 7.6 | 96.3 | 990.3±68.6 | 6.9 | 99.0 |
| Risperidone | 9.8±1.2 | 11.9 | 96.2 | 49.8±3.4 | 6.8 | 99.6 | 113.1±7.1 | 6.3 | 113.1 | 478.5±32.4 | 6.8 | 95.7 | 968.7±60.6 | 6.3 | 96.9 |
| Lansoprazole | 10.2±0.4 | 3.6 | 102.5 | 50.4±1.4 | 2.7 | 100.8 | 101.5±5.1 | 5.1 | 101.5 | 482.3±44.6 | 9.2 | 96.5 | 1003.3±50.0 | 5.0 | 100.3 |
| Olanzapine | 9.3±0.6 | 6.7 | 93.5 | 48.3±2.5 | 5.1 | 96.5 | 101.4±4.8 | 4.8 | 101.4 | 498.6±18.3 | 3.7 | 99.7 | 992.9±38.1 | 3.8 | 99.3 |
| Haloperidol | 10.3±1.3 | 12.2 | 103.3 | 47.7±4.7 | 9.9 | 95.3 | 106.8±7.6 | 7.2 | 106.8 | 463.1±38.0 | 8.2 | 92.6 | 965.4±71.8 | 7.4 | 96.5 |
| Clarithromycin | 11.4±1.7 | 14.6 | 113.8 | 48.6±4.6 | 9.5 | 97.2 | 105.3±9.0 | 8.6 | 105.3 | 479.5±46.5 | 9.7 | 95.9 | 987.2±25.4 | 2.6 | 98.7 |
| Promethazine | 9.3±0.8 | 9.0 | 93.1 | 54.2±5.9 | 10.9 | 108.4 | 97.4±14.0 | 14.4 | 97.4 | 508.0±11.3 | 2.2 | 101.6 | 1019.4±52.7 | 5.2 | 101.9 |
| Levomepromazine | 9.5±1.4 | 14.3 | 95.2 | 50.1±2.7 | 5.5 | 100.2 | 93.7±9.5 | 10.2 | 93.7 | 486.4±31.2 | 6.4 | 97.3 | 971.5±59.2 | 6.1 | 97.1 |
| Chlorpromazine | 10.9±1.3 | 11.8 | 109.2 | 50.0±4.2 | 8.3 | 100.0 | 105.1±8.0 | 7.6 | 105.1 | 513.7±35.5 | 6.9 | 102.7 | 959.8±75.1 | 7.8 | 96.0 |
* SD: Standard Deviation.
**CV: Coefficient of Variation.
By observing the working processes in the dispensary, we noted that an average of 50 powdered drugs was prescribed out of 160 prescriptions per day. There were 4 pharmacists on average on the dispensary floor. We measured the suspended particle concentrations in the environmentally contaminated pharmacy in accordance with the standards for working environment measurements. 11 The suspended particle concentrations ranged from .002 to .004 mg/m3 at the A-measurement points. The suspended particle concentration at the B-measurement point was .005 mg/m3. The suspended particle concentration at the air sampling point was .003 mg/m3. None of the drugs in this study was detected at the air sampling point (Figures 3-l). Therefore, it was not possible to compare the identifying MS spectra peaks (Figure 2) with an actual sample.
Some analysis limitations should be noted. The IS (acetaminophen) might not be appropriate because acetaminophen may also contaminate the environment. Acetaminophen has been frequently detected in raw waste water in Japan. Acetaminophen powder is commonly prescribed for pediatrics purposes; hence, it possible that the suspended particles in the pharmacy are acetaminophen. It may therefore be better to use a non-medicinal compound as the IS. The use of stable-isotope-labeled compounds as ISs is beneficial for LC–MS. Isotope-labeled analytes as ISs were not available; hence, several compounds were investigated in the initial stages of this study to determine a suitable IS, with acetaminophen determined to be the best. Furthermore, we did not analyze all of the powdered drugs dispensed because many drugs could not be quantified. The survey was conducted for only 1 h. The drugs chosen for testing would not be detected if those drugs are not dispensed; alternatively, if they were dispensed, the amounts dispensed during the survey may be small. Therefore, we may have underestimated the concentrations of the chosen drug ingredients.
The LC–MS/MS with a core–shell ODS column was used to develop an analysis method for dispensed powdered medicinal drugs. The developed method was sensitive, and enabled the simultaneous determination of 9 common powdered drugs dispensed in pharmacies. Therefore, the present method is likely to be applicable to the routine environmental monitoring of drugs.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was partially supported by Japan Society for the Promotion of Science KAKENHI grant number, 24659319 (Grant-in-Aid for Challenging Exploratory Research from Japan Society for the Promotion of Science). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
ORCID iD
Mitsuhiro Nakamura https://orcid.org/0000-0002-5062-5522
References
- 1.Japan Pharmaceutical Shinnin-Yakuzaishi Society. Notameno Chozai-Jiko-Boushi Text. 2nd ed. 2012. Available at: https://www.nichiyaku.or.jp/assets/uploads/pharmacy-info/shinnin_jikoboushi2.pdf (accessed 29 September 2021)
- 2.Mixon B, Nain J. Complying with occupational safety and health administration regulations: a guide for compounding pharmacists. Int J Pharm Compd. 2013;17:182-190. [PubMed] [Google Scholar]
- 3.Lee S-K, Cho H-K, Cho S-H, Kim S-S, Nahm D-H, Park H-S. Occupational asthma and rhinitis caused by multiple herbal agents in a pharmacist. Ann Allergy Asthma Immunol . 2001;86:469-474. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health . NIOSH alert: preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. DHHS (NIOSH) Publication No. 2004-165. 2004.
- 5.Minciullo PL, Imbesi S, Tigano V, Gangemi S. Airborne contact dermatitis to drugs. Allergol Immunopathol. 2013;41:121-126. [DOI] [PubMed] [Google Scholar]
- 6.Boyd RK, Robert B, Bethem RA. Trace Quantitative Analysis by Mass Spectrometry. New Jersey, United States: John Wiley & Sons, Ltd. Hudson County; 2008. [Google Scholar]
- 7.Inaba R, Hioki A, Kondo Y, Nakamura H, Nakamura M. Suspended particle and drug ingredient concentrations in hospital dispensaries and implications for pharmacists’ working environments. Environ Health Prev Med. 2016;21:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preti R. Core-Shell columns in high-performance liquid chromatography: food analysis applications. Int J Anal Chem. 2016;2016:3189724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gritti F, Guiochon G. The current revolution in column technology: how it began, where is it going? J Chromatogr A. 2012;1228:2-19. [DOI] [PubMed] [Google Scholar]
- 10.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use “ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1) (2005)”. Available at: https://www.pmda.go.jp/files/000156867.pdf (accessed 29 September 2021) [Google Scholar]
- 11.Ministry of Health, Labour and Welfare, Working Environment Measurement Standards . Notification No. 46 of the Ministry of Labour, Japan (1976. 4. 22; amended up to Notification No. 377 of the Ministry of Health, Labour and Welfare, 2014. 9. 29)