Abstract
Objectives:
Many studies of sudden unexpected infant death (SUID) have focused on individual domains of risk factors (maternal, infant, environmental), resulting in limited capture of this multifactorial outcome. The objective of this study was to examine the geographic distribution of SUID in San Diego County, and assess maternal, infant and environmental risk factors from a large, administrative research platform.
Study design:
Births in California between 2005-2017 were linked to hospital discharge summaries and death files. From this retrospective birth cohort, cases of SUID were identified from infant death files in San Diego County. We estimated adjusted hazard ratios (aHR) for infant, maternal and environmental factors and SUID in multivariable Cox regression analysis. Models were adjusted for maternal sociodemographic characteristics and prenatal nicotine exposure.
Results:
There were 211 (44/100,000 live births; absolute risk 0.04%) infants with a SUID among 484,905 live births. There was heterogeneity in geographic distribution of cases. Multiparity (0.05%; aHR 1.4, 95% CI 1.1, 1.9), maternal depression (0.11%; aHR 1.8, 95% CI 1.0, 3.4), substance-related diagnoses (0.27%; aHR 2.3, 95% CI 1.3, 3.8), cannabis-related diagnosis (0.35%; aHR 2.7, 95% CI 1.5, 5.0), prenatal nicotine use (0.23%; aHR 2.5, 95% CI 1.5, 4.2), preexisting hypertension (0.11%; aHR 2.3, 95% CI 1.2, 4.3), preterm delivery (0.09%; aHR 2.1, 95% CI 1.5, 3.0), infant with a major malformation (0.09%; aHR 2.0, 95% CI 1.1, 3.6), respiratory distress syndrome (0.12%; aHR 2.6, 95% CI 1.5, 4.6) and select environmental factors were all associated with SUID.
Conclusions:
Multiple risk factors were confirmed and expanded upon, and the geographic distribution for SUID in San Diego County was identified. Through this approach, prevention efforts can be targeted to geographies that would benefit the most.
Keywords: epidemiology, sudden unexpected infant death, risk factors
Introduction
Sudden unexpected infant death (SUID) is the sudden, unexpected death of a child younger than one year of age where the cause was not obvious before investigation [1,2]. In 2019, the rate of SUID in the United States was 90 infants for every 100,000 live births [2]. Initially classified as sudden infant death syndrome (SIDS), the category has expanded over the last 20 years to include accidental suffocation in the sleeping environment and other deaths from unknown causes. Although there has been a decline in the rate of SIDS deaths since the 1990’s, the overall SUID rate has decreased less, suggesting a diagnostic shift or changes in reporting [3,4]. In 2019, only 37% of SUID in the National Vital Statistics System was attributed to SIDS [2]. Risk for SUID peaks between one to two months of age, and is the most common cause of death between one month and one year of age in developed countries [1,3]. Several risk factors have previously been noted for SIDS or SUID, including race/ethnicity, prematurity, bed sharing, gestational hypertension, inadequate prenatal care, prenatal alcohol or nicotine exposure, household smoke exposure, and low socioeconomic status [1,5–13]. Recently, it has been noted that select constituents of air pollution may also increase the risk of SIDS [14–16].
Often, risk factors are individually examined, or examined by domain (maternal, infant, pollutants), offering a narrow and potentially incomplete picture of this multifactorial outcome. Further, when different domains of factors are studied in heterogeneous populations, it is not possible to directly compare them to evaluate the magnitude of the potential intervention targets. This is because different populations may not share the same effect modifiers, making direct comparisons problematic. Given the broad range of potential risk factors ranging from the individual to environmental factors, a large dataset that examines these factors collectively in the same population will facilitate comparisons between them. The objective of this study was to examine the geographic distribution of SUID in San Diego County, and assess the maternal, infant and environmental risk factors from a large research platform.
Materials and methods
The Study of Mothers and Infants (SOMI) is birth cohort largely comprised of administrative health records, representing all births in California between 2005-2017. Birth certificates, maintained by California Vital Statistics, were linked to hospital discharge, emergency department, and ambulatory surgery records maintained by the California Office of Statewide Health Planning and Development. Hospital discharge, emergency department, and ambulatory surgery files provided diagnoses codes based on the International Classification of Diseases, 9th Revision (ICD-9) and 10th Revision (ICD-10) as reported to the California Office of Statewide Health Planning and Development by the hospitals [17]. The study dataset consists of discharge records (maternal: one year prior to the birth through one year post-delivery; infant: first year of life) linked to the infant’s birth certificate, resulting in over 6 million linked mother-baby dyads. In addition, maternal and infant death records for any mother or infant who subsequently died in the State in the infant’s first year of life are also linked into the cohort. Linkage is performed using a probabilistic linkage between variables on vital statistics and hospital discharge summaries, including but not limited to maternal date of birth, infant date of birth, zip code, delivery hospital, pregnancy complications and outcomes, payer source. Finally, CalEnviroscreen 3.0 [18] and other publicly available, geographically mapped data sources were linked to the SOMI dataset through mapping zip codes at delivery census tracts, which are the unit of aggregation in CalEnviroScreen. The study was approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California, as well as the University of California San Diego Human Research Protections Program.
For the purposes of this analysis, we included live-born deliveries in San Diego County (n=484,905) between the years of 2005-2017 (Supplemental Figure 1).
Outcome and exposure variables
SUID cases were identified using diagnostic codes (ICD-10: R95 (sudden infant death syndrome), W75 (accidental suffocation or strangulation in bed), and R99 (unknown cause)) from the cause of death field on infant death records. This group of three diagnostic codes for SUID was recognized by the National Center for Health Statistics in 2015, and for the United States Healthy People 2020 initiative, where SUID is a reported health indicator [3]. In the case of unexpected infant deaths, the cause of death is reported on infant death certificates by a death certifier, who can be a medical examiner, coroner or forensic pathologist. We also identified all other non-SUID deaths in the first year of life from infant death records or hospital discharge summaries.
Maternal sociodemographic factors were identified from hospital discharge records recorded during pregnancy or the delivery episode. These included maternal race/ethnicity (non-Hispanic White, Black, Asian, Hispanic, or other/multiple/unknown), nativity (US born or foreign born), age (<18, 18-34, <34), education (<12th grade or 12th grade or higher), pre-pregnancy body mass index (≥25 kg/m2), prenatal care [19] (inadequate or adequate+), and payer source for delivery (private, public, other).
Maternal and infant characteristics and environmental pollutants were primarily selected from previous literature. However, given the exploratory nature of this project, we chose to also include other variables that signal problems with the pregnancy or developing fetus and could reasonably be on a causal pathway (e.g.- placental abruption or necrotizing enterocolitis). Finally, given the very limited literature on environmental variables, we further selected factors increasingly queried in perinatal epidemiology (e.g. noise, pesticides) or considered particularly important in San Diego (e.g. housing burden). All queried factors are shown in Table 1, and all were pre-specified by the team for inclusion.
Table 1.
Maternal and infant characteristics of live births in San Diego County stratified by cause of death (2005-2017).
| Sudden unexpected infant death | Other deaths | Infant alive at 1 year of age | Chi-square p value (SIDS vs alive at 1 year) | ||||
|---|---|---|---|---|---|---|---|
| n=211 | % | n=1,865 | % | n=482,829 | % | ||
|
|
|||||||
| Maternal sociodemographic factors | |||||||
| Race/ethnicity | 0.00 | ||||||
| Non-Hispanic White | 62 | 29.4 | 509 | 27.3 | 157221 | 32.6 | |
| Hispanic | 91 | 43.1 | 801 | 42.9 | 213114 | 44.1 | |
| Non-Hispanic Black | 20 | 9.5 | 103 | 5.5 | 17394 | 3.6 | |
| Non-Hispanic Asian | 10 | 4.7 | 154 | 8.3 | 49407 | 10.2 | |
| Other, multiple or unknown | 28 | 13.3 | 298 | 16.0 | 45693 | 9.5 | |
| Maternal nativity | 0.00 | ||||||
| Mother born in US | 176 | 83.4 | 1139 | 61.1 | 293070 | 60.7 | |
| Age | 0.00 | ||||||
| <18 | 8 | 3.8 | 55 | 2.9 | 9477 | 2.0 | |
| 18-34 | 182 | 86.3 | 1409 | 75.5 | 375719 | 77.8 | |
| 35+ | 21 | 10.0 | 398 | 21.3 | 97589 | 20.2 | |
| Missing | 0 | 0.0 | 3 | 0.2 | 44 | 0.0 | |
| Education 1 | 0.00 | ||||||
| < 12th grade | 131 | 62.1 | 909 | 48.7 | 198198 | 41.0 | |
| Body mass index 1 | 0.00 | ||||||
| Overweight or obese | 133 | 63.0 | 983 | 52.7 | 230459 | 47.7 | |
| Prenatal care 1 | 0.00 | ||||||
| Inadequate | 51 | 24.2 | 287 | 15.4 | 54070 | 11.2 | |
| Payer source | 0.00 | ||||||
| Private | 67 | 31.8 | 901 | 48.3 | 267489 | 55.4 | |
| Public | 128 | 60.7 | 792 | 42.5 | 185086 | 38.3 | |
| Other | 16 | 7.6 | 172 | 9.2 | 30254 | 6.3 | |
| Maternal characteristics and complications | |||||||
| Nulliparous | 70 | 33.2 | 762 | 40.9 | 197661 | 40.9 | 0.02 |
| Mental health | |||||||
| Anxiety | 9 | 4.3 | 54 | 2.9 | 10900 | 2.3 | 0.05 |
| Depression | 11 | 5.2 | 49 | 2.6 | 9603 | 2.0 | 0.00 |
| Substance related diagnosis | |||||||
| Alcohol related diagnosis | 4 | 1.9 | 8 | 0.4 | 1502 | 0.3 | 0.00 |
| Other substance related diagnosis | 19 | 9.0 | 57 | 3.1 | 7055 | 1.5 | 0.00 |
| Cannabis related diagnosis | 14 | 6.6 | 29 | 1.6 | 3993 | 0.8 | 0.00 |
| Prenatal nicotine use | 19 | 9.0 | 56 | 3.0 | 8315 | 1.7 | 0.00 |
| Comorbidities | |||||||
| Antepartum migraine | 3 | 1.4 | 23 | 1.2 | 5211 | 1.1 | 0.63 |
| Preexisting diabetes | 1 | 0.5 | 31 | 1.7 | 3736 | 0.8 | 0.61 |
| Preexisting hypertension | 10 | 4.7 | 88 | 4.7 | 8720 | 1.8 | 0.00 |
| Pregnancy complications | |||||||
| Gestational diabetes | 20 | 9.5 | 181 | 9.7 | 45722 | 9.5 | 0.99 |
| Preeclampsia/eclampsia | 9 | 4.3 | 125 | 6.7 | 19055 | 3.9 | 0.81 |
| Gestational hypertension | 9 | 4.3 | 30 | 1.6 | 12377 | 2.6 | 0.12 |
| Any infection in pregnancy | 25 | 11.8 | 232 | 12.4 | 40278 | 8.3 | 0.07 |
| Placental abruption | 3 | 1.4 | 181 | 9.7 | 4670 | 1.0 | 0.50 |
| Cesarean delivery | 63 | 29.9 | 851 | 45.6 | 166436 | 34.5 | 0.15 |
| Polyhydramnios | 1 | 0.5 | 133 | 7.1 | 2676 | 0.6 | 0.88 |
| Infant characteristics and complications | |||||||
| Infant sex (male) | 119 | 56.4 | 1001 | 53.7 | 246058 | 51.0 | 0.11 |
| Preterm delivery | 36 | 17.1 | 1080 | 57.9 | 39987 | 8.3 | 0.00 |
| missing | 3 | 1.4 | 122 | 6.5 | 3579 | 0.7 | |
| Multiple gestation | 6 | 2.8 | 225 | 12.1 | 13055 | 2.7 | 0.90 |
| Major structural defect | 12 | 5.7 | 527 | 28.3 | 13334 | 2.8 | 0.01 |
| Respiratory distress syndrome | 13 | 6.2 | 452 | 24.2 | 10724 | 2.2 | 0.01 |
| Intraventricular hemorrhage | 2 | 0.9 | 254 | 13.6 | 1374 | 0.3 | 0.08 |
| Necrotizing enterocolitis | 0 | 0.0 | 47 | 2.5 | 371 | 0.1 | 0.68 |
| Periventricular leukomalacia | 1 | 0.5 | 19 | 1.0 | 106 | 0.0 | 0.00 |
| Patent ductus arteriosus | 2 | 0.9 | 403 | 21.6 | 6119 | 1.3 | 0.67 |
| Bronchopulmonary dysplasia | 0 | 0.0 | 55 | 2.9 | 1256 | 0.3 | 0.46 |
| Environmental exposures | |||||||
| Cal Enviroscreen 3.0 (mean, SD) | 32.3 | 19.8 | 30.1 | 20.8 | 28.8 | 20.7 | 0.02 |
| Ozone percentile (mean, SD) | 36.6 | 12.6 | 37.0 | 13.2 | 37 | 12.8 | 0.63 |
| Particulate matter 2.5 percentile (mean, SD) | 59.0 | 16.5 | 56.6 | 18.1 | 56.6 | 17.5 | 0.04 |
| Diesel particulate matter emissions percentile (mean, SD) | 45.6 | 17.6 | 44.9 | 18.1 | 44.9 | 17.7 | 0.42 |
| Drinking water contaminant index percentile (mean, SD) | 32.3 | 11.8 | 31.6 | 13.2 | 31.3 | 12.6 | 0.29 |
| Total pounds of selected pesticide percentile (mean, SD) | 10.8 | 14.7 | 12.9 | 15.9 | 12.7 | 15.6 | 0.08 |
| Chemical releases percentile (mean, SD) | 32.4 | 13.6 | 31.7 | 14.7 | 31.1 | 14.6 | 0.19 |
| Traffic density percentile (mean, SD) | 49.7 | 17.2 | 48.5 | 18.3 | 48.8 | 19.2 | 0.47 |
| Solid waste sites percentile (mean, SD) | 25.3 | 10.4 | 25.2 | 12.3 | 24.7 | 11.8 | 0.38 |
| Poverty percentile (mean, SD) | 46.9 | 19.3 | 43.6 | 20.4 | 41.7 | 20.5 | 0.00 |
| Housing burden percentile (mean, SD) | 49.2 | 23.5 | 43.7 | 24.2 | 41.9 | 23.9 | 0.00 |
| Noise mean (mean, SD) | 37.7 | 8.0 | 36.4 | 8.9 | 36.4 | 8.2 | 0.02 |
Imputed frequencies
SD=standard deviation
Maternal characteristics and complications included parity (nulliparous vs multiparous), and the following diagnoses during pregnancy (all binary as present/not present): anxiety, depression, alcohol-related diagnosis, cannabis-related diagnosis, other substance-related diagnosis, prenatal nicotine use, antepartum migraine diagnosis, preexisting diabetes, preexisting hypertension, gestational diabetes, preeclampsia/eclampsia, gestational hypertension, any infection in pregnancy, placental abruption, cesarean delivery, and polyhydramnios.
Infant factors were identified from birth through the first year of life and modeled as binary variables. These include prematurity, sex, being a multiple, major structural birth defect, respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, periventricular leukomalacia, patent ductus arteriosus, and bronchopulmonary dysplasia.
Finally, we considered environmental and neighborhood level factors based on maternal residential census tract at delivery. Environmental exposures were primarily identified from CalEnviroScreen 3.0., which identifies California communities by census tract that are disproportionately burdened by, and vulnerable to, multiple sources of pollution [18]. CalEnviroscreen produces measure of pollution’s impacts and vulnerabilities in California communities. A more detailed description of CalEnviroscreen, full list of data sources, ICD codes and variable definitions, and environmental variables are available in Supplemental Table 1. Variables were all modeled as continuous variables, and included the Cal Enviorscreen score, ozone percentile, particulate matter (PM) 2.5 percentile, diesel PM emissions percentile, drinking water contaminant index percentile, total pounds of pesticide percentile, chemical releases percentile, traffic density percentile, solid waste sites percentile, poverty percentile, housing burden percentile, and noise mean.
Three covariates (maternal education, pre-pregnancy body mass index [BMI], and prenatal care) were missing >5% among the SUID strata (Supplemental Table 5). Due to the small number of SUID cases, we performed imputation using multivariable linear regression. Maternal age, race/ethnicity and payer source (which were all predictive of each of the missing covariates) were used to predict the unobserved values.
Statistical analysis
Data were first summarized by the disposition of the infant at one year of life: death attributed to SUID, death from cause other than SUID, and alive. We assessed the univariate association between each risk factor and SUID vs. alive at one year with chi-square and Fisher’s exact tests where necessary, or with t-tests. Subsequently, variables with univariate probability ≤ 0.10 and a cell count of at least five were included in multivariable regression. The significance was set to 0.10 due to the small strata for SUID (n=211). Cox multivariable regression was used to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI). Age at death (in days) was the unit of time in models. Non-SUID deaths were censored at the day of death, and infants alive at one year of follow up were censored at one year. We first focused on maternal sociodemographic models, regressing SUID on maternal race/ethnicity, nativity, age, education, pre-pregnancy BMI, prenatal care, and payer source in a single model. We performed models with the imputed variables, and then repeated all models with the unimputed variables. Observing no marked differences between imputed and non-imputed models (Supplemental Table 6), we used imputed variables in all subsequent analyses.
We then constructed individual models for each maternal characteristic or complication, infant characteristic or complication, and environmental factor that was significant in univariate analyses, each adjusted for the aforementioned maternal sociodemographic characteristics. We considered that maternal smoking, a well-documented risk factor for SUID, may confound the variables in the prenatal and infant models. Therefore, we also adjusted each model for maternal nicotine use (except the model estimating the risk for maternal nicotine). In a sensitivity analysis, we limited the outcome to SIDS (censoring for the other two death diagnoses (R99 and W75) along with other non-SIDS deaths) to assess the impact on results. All analyses were conducted in SAS, version 9.4 (SAS Institute, Cary, NC).
Results
There were 484,905 live births in San Diego County between 2005-2017; 2,076 (428 per 100,000 live births) died within the first year of life. From the full live-born cohort, 211 (44/100,000 live births) had a classification of SUID on the infant death record. There has been a negative secular trend over the study period in the incidence of SUID (−0.10, p<0.001) and non-SUID deaths (−0.02, p=0.006), with steeper declines in the rates of SUID deaths. Deaths attributable to SUID peaked in 2008 at 69 per 100,000 live births, and had the lowest incidence in 2017 with 25 deaths per 100,000 live births (Supplemental Figure 2). From the three ICD codes constituting SUID, 86% were attributed to SIDS, with declining proportion over time (94% SIDS in 2007, 66% SIDS in 2017). The mean age at death in infants with SUID was 105 days (standard deviation 77 days).
Examining the deaths geospatially, there was heterogeneity in incidence rates of SUID by sub-regional area (SRA) in San Diego County (Supplemental Figure 3). The highest risk was in the north-east and south-east regions of the county, with lower incidence in the coastal and central regions of the county. The geospatial patterns observed were not explained by race/ethnicity or socioeconomic status, as residents in the south east SRAs tend to identify as Latina or non-Hispanic Black and have lower socioeconomic status, while residents in the north east are more likely to identify as non-Hispanic White and have a higher socioeconomic status [20].
In univariate analyses, women with infants with a SUID were more likely to identify as non-Hispanic Black or other/multiple races, and less likely to identify as non-Hispanic Asian than women who had an infant who survived the first year of life (Table 1). Further, mothers of infants with a SUID were more likely to be born in the US, to be young, to have less than a high-school education, to be overweight/obese, to have inadequate prenatal care, and use public insurance. Multiple maternal characteristics and complications, infant characteristics, and environmental variables in Table 1 had a univariate p-value <0.10 for inclusion in multivariable models. Based on low frequency, we were not able to include alcohol-related diagnosis, intraventricular hemorrhage, or periventricular leukomalacia in multivariable models.
In the multiply adjusted model for maternal sociodemographic characteristics, non-Hispanic Black women were more likely than non-Hispanic White women to have an infant with SUID (graphed in Figure 1, point estimates and confidence intervals in Supplemental Table 3). Additionally, women born outside of the US were less likely to have an infant with SUID. Finally, having less than a high school education, being overweight or obese pre-pregnancy, having inadequate prenatal care, and public or other non-private insurance all were associated with an increase likelihood of having an infant with SUID.
Figure 1.
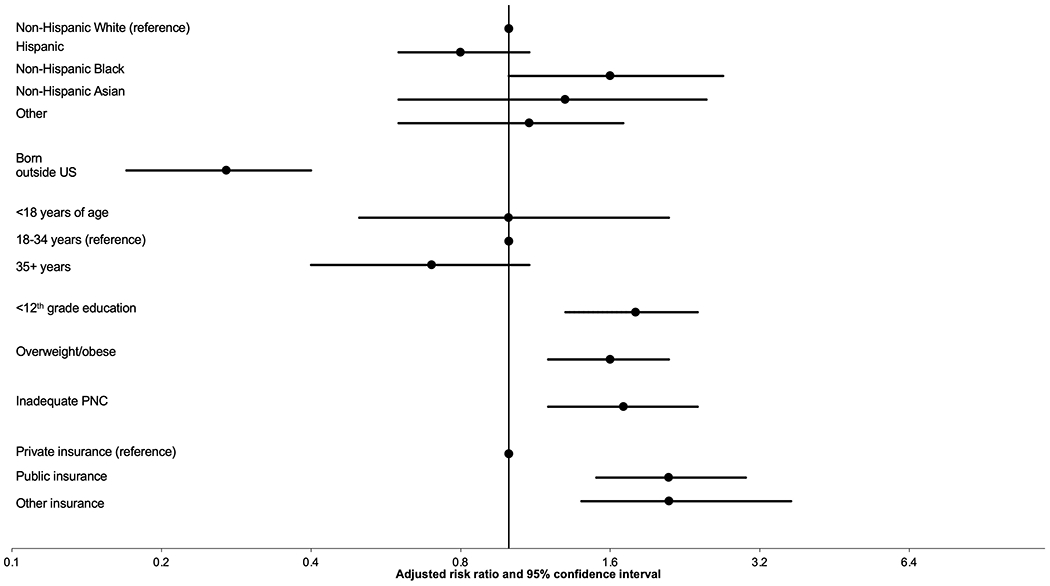
Adjusted hazard ratios for maternal sociodemographic factors derived from a single model. Reference not plotted for binary factors. Reference group is infants who did not die in the first year of life. PNC=prenatal care.
When maternal characteristics or complications, infant characteristics or complications, and environmental characteristics were queried in individual models, after adjusting for maternal sociodemographic characteristics and nicotine, several factors were associated with SUID (Figure 2, Supplemental Table 4). These included multiparity, maternal depression, cannabis, nicotine and other substance-related diagnoses, preexisting hypertension, preterm delivery, the infant having a major structural malformation, or having respiratory distress syndrome. Neighborhood level environmental exposures, including poverty, housing burden and noise pollution also were associated with SUID, although with very modest hazard estimates. The strongest risk factors were cannabis-related diagnosis and respiratory distress syndrome, although confidence intervals were wide.
Figure 2.
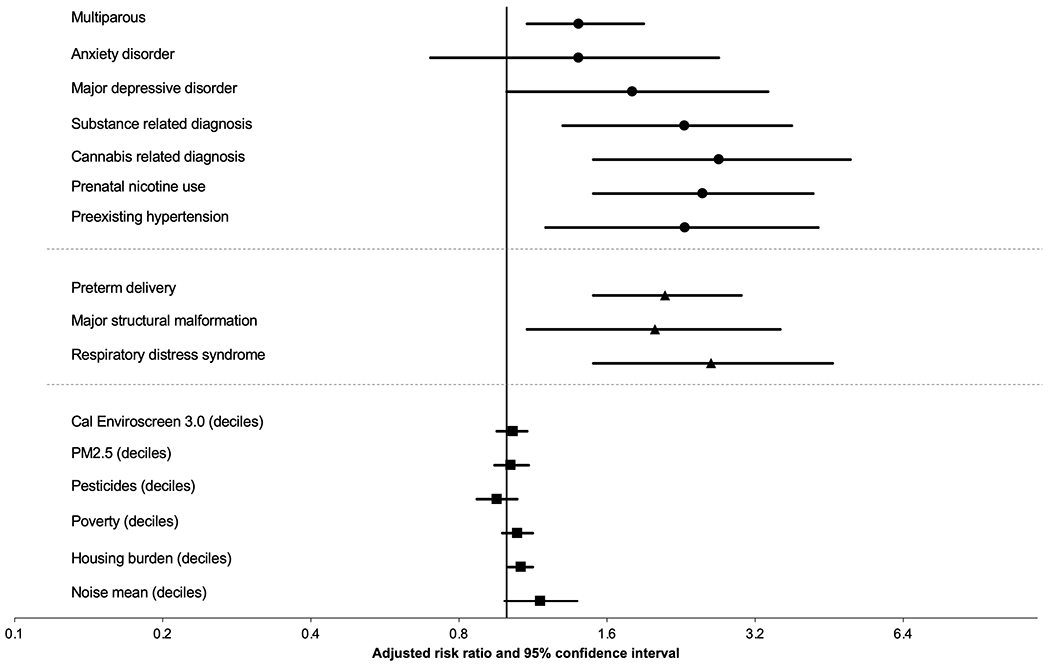
Adjusted hazard ratios for maternal characteristics and complications (circle markers), infant characteristic and complications (triangle markers), and environmental (square markers) factors. Reference not plotted for binary factors. Adjusted hazard ratios derived from individual models adjusted for sociodemographic factors and prenatal nicotine use (nicotine model only adjusted for sociodemographic factors). Reference group is infants who did not die in the first year of life.
In a sensitivity analysis, we limited our outcome to SIDS (n=181) and repeated all models (data not shown). The only difference was that the estimate for parity now included the null; all other results and interpretations were unchanged. Finally, unimputed frequencies (Supplemental Table 5) and multivariable models for maternal sociodemographic characteristics without imputation (Supplemental Table 6) are available in the supplemental material.
Discussion
SUID is a devastating outcome that is known to be multifactorial [21]. Between 2005-2017, death rates from SUID have declined in San Diego County, although from these data one cannot establish a true decline in prevalence from other factors (e.g. scientific advances in classifying cause of death). To fully appreciate the collective risk factors, it is necessary to analyze multiple domains of risk factors from a given population. Upon examination of maternal characteristics, identifying as non-Hispanic Black, having low educational achievement, being overweight or obese, having inadequate prenatal care, and having public insurance all increased the risk of having an infant with SUID. Maternal nativity (born outside of the US) was associated with a reduced risk. In adjusted models, there were positive associations between SUID and parity, maternal depression, substance-related diagnoses, cannabis-related diagnosis, prenatal nicotine exposure, preexisting hypertension, preterm delivery, the infant having a major structural malformation, or having respiratory distress syndrome, and neighborhood level economic and noise pollution factors.
Some of these factors have been noted in previous studies, with the literature primarily limited to SIDS. In a large administrative data set from the Centers for Disease Control and Prevention of 37 million births (1995-2004), women who identified as Black, who smoked or use alcohol during pregnancy, had inadequate prenatal care, had increasing parity and who delivered prematurely had a higher risk for having an infant with SIDS [7]. When an updated analysis from the same source was carried out using data through 2010, similar risk factors were again identified [10]. It is important to note that, particularly within the US, factors such as race, education, payer source and prenatal care often proxy for structural factors that negatively impact birth and infant outcomes, including racism, red-lining, chronic stress and environmental adversity, and should not be viewed as causal [22,23].
Others have examined the association between maternal mental health and the risk of SIDS. From population-based data from Denmark between 1973-1998, psychiatric admissions for either parent conferred a two-fold increased risk of SIDS for the infant. However, when admission causes were examined, affective disorders and alcohol and drug-related disorders conferred the increased risk, while schizophrenia and related disorders did not increase the risk of having an infant with SIDS [6]. A second study from national data in Sweden (1978-2004) also noted the increased risk of SIDS with maternal admission for a psychiatric illness, and found that 50% of the excess risk was explained by maternal smoking and social adversity measures (low education, poverty) and less so by obstetric complications [9]. In our models, depression was a risk factor for SUID, while the confidence intervals for anxiety disorders crossed the null. Notably, these associations were independent of prenatal smoking. Finally, like many others [5–7,10,13,24], we noted an increased risk of SUID/SIDS with prenatal alcohol and nicotine use. Further, we found that a cannabis-related diagnosis in pregnancy conferred a 2.7-fold increased risk of SUID, which included adjustment for maternal nicotine use. To our knowledge, few have examined cannabis as a risk factor. In a study of 393 SIDS cases in New Zealand, authors noted that frequent maternal cannabis (> weekly use) was a weak risk factor for SIDS after adjusting for tobacco smoke. Given the increased legality and potency of cannabis in the United States [25], this finding warrants additional follow up.
We observed both geospatial variation and a higher overall environmental burden among SUID cases in univariate analyses. Respective to the geospatial variation, some identified factors had very similar geospatial variation to SUIDs, e.g. rates of preterm birth. Other factors, like cannabis diagnoses, had similar geospatial patterns in some parts of the county, but differed in others. These disparate patterns by risk factor highlight the importance of community-level dissemination and prevention strategies. Additionally, infants with SUID were born in areas with higher PM2.5, pesticides, poverty, housing burden and noise pollution. In multivariable analyses, these factors were either not significant (overall burden, PM2.5, pesticide) or very marginally significant (poverty, housing burden and noise pollution), although environmental factors often have very small risk estimates [26]. Previous studies of SIDS and constituents of air pollution have either not adjusted for individual level maternal factors [14,16] or adjusted for some but did not adjust for smoking [15]. Given the correlation between areas of high environmental burden, low socioeconomic status, and behaviors such as prenatal smoking, it is unclear whether those reported associations were confounded by maternal smoking. It is important to highlight that our estimates of environmental burden were static averages (i.e. not temporally estimated for each individual) and were relative measures within California communities. Thus, these estimates cannot be directly compared to absolute values from other work. Further, the averaged measures may lead to misclassification for pollutants with strong temporal variation or pollutants in areas with less regional representation (e.g. density of air monitoring stations). Nonetheless, the literature is increasingly supportive of the role of the environment in child health outcomes, including SUID, and future research should model pollutants with greater precision than the estimates available to this analysis.
While our findings from this study are novel in the breadth of factors analyzed, they should be viewed in light of the limitations. Administrative data, which benefits from large numbers to study rare outcomes, relies on ICD codes and birth records for demographic factors and comorbidities. Reliance on these data can lead to misclassification, although given that these data were recorded prior to the known outcome would likely be non-differential and bias results towards the null. Further, some variables such as substance-related diagnoses are known to be under-reported in hospital discharge summaries and likely represent disordered use. It is unclear how these biases would ultimately influence results. Also, we are only capturing infants who died in California in the first year of life. Families who leave the state would be assumed to be alive at one year, which may not be the case. Within the cohort, we do not have a good variable to identify siblings, and were unable to adjust standard errors for non-independence of siblings. Finally, given the source of the data, we did not have information on sleep position, which is a well-established risk factor for SUID. Some of our identified factors, particularly the socioeconomic factors used for model adjustment, likely operate through this pathway, but we were unable to query this further. Despite these limitations, the platform represents virtually the entire population of infants in San Diego County, an ethnically and economically diverse sample. Therefore, results should be reasonably generalizable to other similar populations.
In summary this analysis confirmed and expanded maternal, infant and environmental factors and the risk of SUID. Further, the geospatial distribution noted in the findings allows for targeted public outreach campaigns for SUID prevention, and shared learnings from areas with low incidence of SUID.
Supplementary Material
Funding
This study was funded by the San Diego Study of Mothers and Infants at the University of California San Diego and the Rady Children’s Institute for Genomic Medicine, as well as an NIH award (R01 HD101540). Gretchen Bandoli is funded by a NIH award (K01 AA027811).
Role of the funder/sponsor:
No funders/sponsors participated in this work.
Abbreviations
- aHR
Adjusted hazard ratio
- BMI
Body mass index
- CI
Confidence intervals
- ICD
International Classification of Diseases
- PM
Particulate matter
- SOMI
Study of Outcomes in Mothers and Infants
- SIDS
Sudden infant death syndrome
- SUID
Sudden unexpected infant death
Footnotes
Disclosures of interest: The authors have no conflicts of interest relevant to this article to disclose
References
- [1].Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778–783. [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention (CDC). Data and Statistics. Sudd. Unexpected Infant Death Sudd. Infant Death Syndr. 2021. [Google Scholar]
- [3].Shapiro-Mendoza CK, Parks S, Lambert AE, et al. The Epidemiology of Sudden Infant Death Syndrome and Sudden Unexpected Infant Deaths: Diagnostic Shift and other Temporal Changes. In: Duncan JR, Byard RW, editors. Adelaide (AU); 2018. [PubMed] [Google Scholar]
- [4].Shapiro-Mendoza CK, Tomashek KM, Anderson RN, et al. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163:762–769. [DOI] [PubMed] [Google Scholar]
- [5].Elliott AJ, Kinney HC, Haynes RL, et al. Concurrent prenatal drinking and smoking increases risk for SIDS: Safe Passage Study report. EClinicalMedicine. 2020;19:100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].King-Hele SA, Abel KM, Webb RT, et al. Risk of sudden infant death syndrome with parental mental illness. Arch Gen Psychiatry. 2007;64:1323–1330. [DOI] [PubMed] [Google Scholar]
- [7].Hakeem GF, Oddy L, Holcroft CA, et al. Incidence and determinants of sudden infant death syndrome: a population-based study on 37 million births. World J Pediatr. 2015;11:41–47. [DOI] [PubMed] [Google Scholar]
- [8].Moon RY, Horne RSC, Hauck FR. Sudden infant death syndrome. Lancet (London, England). 2007;370:1578–1587. [DOI] [PubMed] [Google Scholar]
- [9].Webb RT, Wicks S, Dalman C, et al. Influence of environmental factors in higher risk of sudden infant death syndrome linked with parental mental illness. Arch Gen Psychiatry. 2010;67:69–77. [DOI] [PubMed] [Google Scholar]
- [10].Friedmann I, Dahdouh EM, Kugler P, et al. Maternal and obstetrical predictors of sudden infant death syndrome (SIDS). J Matern Neonatal Med. 2017;30:2315–2323. [DOI] [PubMed] [Google Scholar]
- [11].Anderson TM, Lavista Ferres JM, Ren SY, et al. Maternal Smoking Before and During Pregnancy and the Risk of Sudden Unexpected Infant Death. Pediatrics. 2019/03/11. 2019;143:e20183325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lavista Ferres JM, Anderson TM, Johnston R, et al. Distinct Populations of Sudden Unexpected Infant Death Based on Age. Pediatrics. 2020;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Allen K, Anderson TM, Chajewska U, et al. Factors associated with age of death in sudden unexpected infant death. Acta Paediatr. 2021;110:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dales R, Burnett RT, Smith-Doiron M, et al. Air Pollution and Sudden Infant Death Syndrome. Pediatrics [Internet]. 2004;113:e628 LP–e631. Available from: http://pediatrics.aappublications.org/content/113/6/e628.abstract. [DOI] [PubMed] [Google Scholar]
- [15].Woodruff TJ, Darrow LA, Parker JD. Air Pollution and Postneonatal Infant Mortality in the United States, 1999–2002. Environ Health Perspect [Internet]. 2008;116:110–115. Available from: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Litchfield IJ, Ayres JG, Jaakkola JJK, et al. Is ambient air pollution associated with onset of sudden infant death syndrome: a case-crossover study in the UK. BMJ Open [Internet]. 2018;8:e018341–e018341. Available from: https://pubmed.ncbi.nlm.nih.gov/29654005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baer RJ, Rogers EE, Partridge JC, et al. Population-based risks of mortality and preterm morbidity by gestational age and birth weight. J Perinatol [Internet]. 2016. [cited 2018 Feb 27];36:1008–1013. Available from: http://www.nature.com/articles/jp2016118. [DOI] [PubMed] [Google Scholar]
- [18].California Office of Environmental Health Hazard Assessment. CalEnviroScreen 3.0 [Internet]. Available from: https://oehha.ca.gov/calenviroscreen/report/calenviroscreen-30.
- [19].Kotelchuck M. The Adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am J Public Health [Internet]. 1994;84:1486–1489. Available from: https://pubmed.ncbi.nlm.nih.gov/8092377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].San Diego Association of Governments: 2010 Census. San Diego Association of Governments (SANDAG) Data Surfer. [Google Scholar]
- [21].Moon RY, Fu L. Sudden Infant Death Syndrome: An Update. Pediatr Rev [Internet]. 2012;33:314–320. Available from: https://pedsinreview.aappublications.org/content/33/7/314. [DOI] [PubMed] [Google Scholar]
- [22].Taylor J, Novoa C, Hamm K, et al. Eliminating Racial Disparities in Maternal and Infant Mortality A Comprehensive Policy Blueprint [Internet]. 2019. Available from: WWW.AMERICANPROGRESS.ORG.
- [23].Chambers BD, Arabia SE, Arega HA, et al. Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health [Internet]. 2020/01/23. 2020;36:213–219. Available from: https://pubmed.ncbi.nlm.nih.gov/31919987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Getahun D, Amre D, Rhoads GG, et al. Maternal and obstetric risk factors for sudden infant death syndrome in the United States. Obstet Gynecol. 2004;103:646–652. [DOI] [PubMed] [Google Scholar]
- [25].Volkow ND, Compton WM, Wargo EM. The Risks ofMarijuana Use During Pregnancy. JAMA - J Am Med Assoc. 2017;317:129–130. [DOI] [PubMed] [Google Scholar]
- [26].Sheppard L, Slaughter JC, Schildcrout J, et al. Exposure and measurement contributions to estimates of acute air pollution effects. J Expo Anal Environ Epidemiol. 2005;15:366–376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


