Abstract
Radioembolization is a well-established treatment for primary and metastatic liver cancer. There is increasing interest in personalized treatment planning supported by dosimetry, as it provides an opportunity to optimize dose delivery to tumor and minimize nontarget deposition, which demonstrably increases the efficacy and safety of this therapy. However, the optimal dosimetry procedure in the radioembolization setting is still evolving; existing data are limited as few trials have prospectively tailored dose based on personalized planning and predominantly semi-empirical methods are used for dose calculation. Since the pretreatment or “scout” procedure forms the basis of dosimetry calculations, an accurate and reliable technique is essential. 99m Tc-MAA SPECT constitutes the current accepted standard for pretreatment imaging; however, inconsistent patterns in published data raise the question whether this is the optimal agent. Alternative particles are now being introduced to the market, and early indications suggest use of an identical scout and treatment particle may be superior to the current standard. This review will undertake an evaluation of the increasingly refined dosimetric methods driving radioembolization practices, and a horizon scanning exercise identifying alternative scout particle solutions. Together these constitute a compelling vision for future treatment planning methods that prioritize individualized care.
Keywords: radioembolization, Y90, novel techniques, positron emission tomography, technetium-MAA, single-photon emission computed tomography
Radioembolization or selective internal radiation therapy (SIRT) is a treatment option for primary and metastatic liver cancer which was first introduced into clinical practice two decades ago. 1 Since then, more than 70,000 patients have been treated globally and it is now acknowledged as integral to the treatment algorithm for liver cancer. 2 3 The procedure involves delivery of β-emitting microspheres of either yttrium-90 ( 90 Y) or holmium-166 ( 166 Ho) to tumors via catheterization of the hepatic artery. The uniquely organized dual blood supply to the liver, which feeds the tumor and normal tissue compartments via different vessels (the hepatic artery and portal vein, respectively), means a high tumor-absorbed dose may be achieved while largely sparing the normal liver parenchyma.
Conventionally, the radioembolization treatment pathway comprises of the following steps: first, baseline diagnostic imaging is conducted, followed by angiographic mapping of the liver vasculature and catheter-directed injection of diagnostic technetium-99m macroaggregated albumin [( 99m Tc) MAA] particles in the feeding artery of choice (which mimic the distribution of the therapeutic microspheres). A 99m Tc MAA single-photon emission computed tomography (SPECT) acquisition is performed for the assessment of extrahepatic shunting and lung shunt fraction (LSF) calculation. Increasingly, this procedure also serves as the “scout procedure” which is used to calculate the activity to be injected to achieve a certain tumor and healthy liver dose. The therapeutic procedure then takes place, typically a few days later, and finally the posttreatment assessment is conducted to assess the dose distribution using either a bremsstrahlung SPECT or positron emission tomography (PET) imaging.
Clinical experience gained over the past 20 years of treating hepatocellular carcinoma (HCC) with radioembolization has demonstrated the complex interplay between the presentation of disease and achieving a tumoricidal radiation dose to target volume, together with minimizing dose to healthy normal parenchyma. Understanding this interplay, specifically in regard to the relationship between dose to tumor and normal tissue and the resultant efficacy and safety of the therapy, has driven advancements in radioembolization dosimetry, and led to the concept of personalized treatment via dose optimization.
“Dose optimization” is regarded as the tailoring of a treatment to ensure delivery of an absorbed dose exceeding a tumoricidal threshold while simultaneously not exceeding a critical exposure of normal liver and lung tissue. The requirement to “individually plan exposure of target volumes and verify delivery, taking into account that doses to nontarget volumes shall be as low as reasonably achievable and consistent with the intended radiotherapeutic purpose of the exposure,” is specified by the European Council Directive 59/2013, 4 and in the context of radioembolization treatments, there is a growing evidence base that demonstrates the clear correlation of dosimetry, tumor response, and survival benefit.
However, the optimal clinical dosimetry model for radioembolization treatment planning is still emerging. Various methods exist, ranging from simplistic semi-empirical models which base activity calculation on estimates of the body surface area (BSA), to models which separately consider tumor and normal tissue uptake using information about prognosed dose derived from the scout procedure.
Since more sophisticated, personalized dosimetry methods rely on the scout procedure as a reference for treatment, the accuracy of this procedure is paramount. One of the barriers to routine implementation of personalized dosimetry is the persistent uncertainty regarding the validity of 99m Tc-MAA as a surrogate for therapeutic microspheres. A scout agent which improves predictive assessment of both intrahepatic dose distribution and LSF is likely to drive a concomitant increase in clinician confidence to implement personalized dosimetry techniques.
In this review, we aim to provide an overview of practices in personalized treatment planning, the evolution of increasingly sophisticated dosimetry approaches, and the scout agents that facilitate these dosimetry calculations.
Dosimetry Models
Four different dosimetry approaches with increasing complexity are employed in current practice. The commonly used BSA method of calculation employed for resin microspheres is a semi-empirical approach geared toward limiting toxicity. The fundamental assumption of BSA dosimetry is a correlation between BSA and liver volume. During activity calculation, the planned activity is adjusted to account for the patient's BSA and fractional tumor burden (where tumor and liver volumes are derived from 3D imaging). The activity can be reduced proportionally in case of lobar or superselective treatment, and also in case of lung shunting. However, a close examination of the BSA formula reveals that across a wide variety of input data, a generalized response results, with a dose of 1.0 to 3.0 GBq being output in the majority of cases. 5 For patients requiring an activity beyond this range, the BSA approach will result in suboptimal dosing. Since the estimated liver volume is derived from a cohort of healthy patients, the BSA will not necessarily result in an accurate estimation of individual liver volume for patients in a disease state. The activity is calculated as shown in Fig. 1 .
Fig. 1.
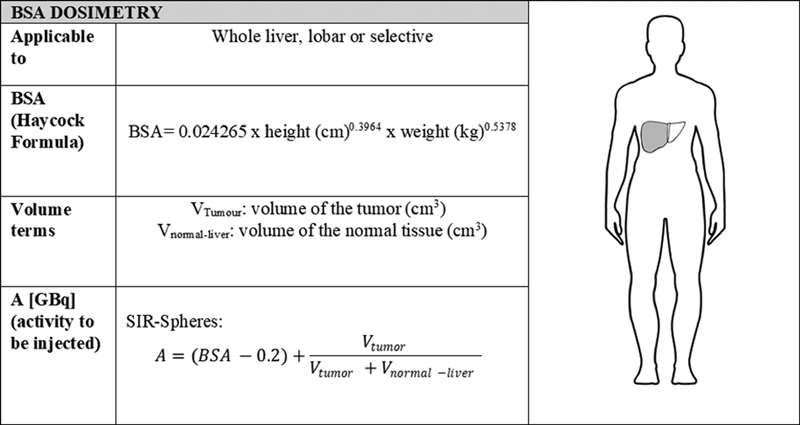
BSA dosimetry calculation method. The equation for activity calculation is equivalent to that presented in Giammarile et al. 6
Due to the shortcomings of the BSA method and lack of personalization, this method has largely been retired and radioembolization dosimetry can now be considered as evolving along two distinct paths, single compartment and multi-compartment MIRD (Medical Internal Radiation Dose). The MIRD formalism expresses the absorbed dose in a volume of interest (VOI) as the ratio of energy deposited in that volume over time and its mass. 7 Typically, in radionuclide therapy (where the radionuclide distribution varies over time) the distribution of activity is estimated at numerous time points and the cumulated activity extrapolated via mathematical modeling. In radioembolization, microspheres are permanently implanted into the arterial tree and the activity distribution is fixed. In this unique situation, a single time point is sufficient to estimate the energy in a VOI and the MIRD formalism is simplified. 8 9 The fundamental assumption of the MIRD formalism is that activity is uniformly distributed throughout the VOI, and provided that a satisfactory calculation method is used to model the energy deposition through the material (local deposition model, kernel, Monte Carlo) which takes account of the material density heterogeneity and particle range, MIRD may be applied at various scales. 10
The most simplistic interpretation of the MIRD formalism is the single-compartment model; this model assumes homogenous distribution of microspheres at a whole liver or lobar level, and therefore the absorbed dose is distributed uniformly within the treated liver volume. In the single-compartment model, the difference in distribution pattern of microspheres (especially between tumors and normal liver) is not considered; in reality, this represents a simplification of the actual distribution, as dose will be escalated within the hypervascular tumors relative to the surrounding normal hepatic parenchyma. Single-compartment dosimetry is most often utilized in cases of selective, ablative radiation dosing, that is, radiation segmentectomy; in this clinical scenario despite the simplicity of the standard model, clear clinical benefit has been demonstrated ( Fig. 2 ).
Fig. 2.
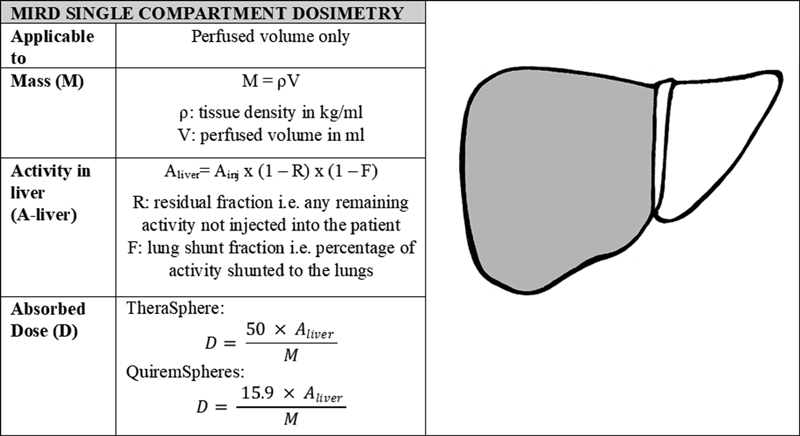
MIRD single-compartment dosimetry calculation method. The factors of 50 (J/GBq) and 15.9 (J/GBq) account for the energy deposited in the region over time per activity injected for 90 Y and 166 Ho, respectively. The equations for absorbed dose have been adapted from those presented by Gulec et al 11 and are the formalism used in Simplicit 90 Y (Mirada medical Ltd, Oxford, UK). 11
Multi-compartment dosimetry is an alternative approach in which the mean absorbed dose is calculated in more than one VOI (tumor, normal liver tissue, lung tissue, etc.). As such, a separate evaluation of tumor and normal liver dose can be performed, which in turn facilitates an activity prescription that maximizes dose to tumor and minimizes dose to normal tissue. The seminal work by Lau et al 12 demonstrated the differential uptake of 99m Tc-MAA to tumor versus normal liver tissue. The objective demonstration that a preoperative tumor to normal tissue (T:N) ratio determined via 99m Tc MAA correlated well with the T:N ratio of the therapeutic 90 Y microspheres was key in establishing 99m Tc MAA as the accepted agent for the pretreatment safety evaluation prior to 90 Y radioembolization. In addition, the essential principle that the therapeutic T:N ratio can be predicted using a 99m Tc MAA SPECT informed many subsequent studies which demonstrate superior clinical outcomes by tailoring treatments to maximize tumor-absorbed dose, resulting in higher response rates (RRs) and improved overall survival (OS) ( Fig. 3 ).
Fig. 3.
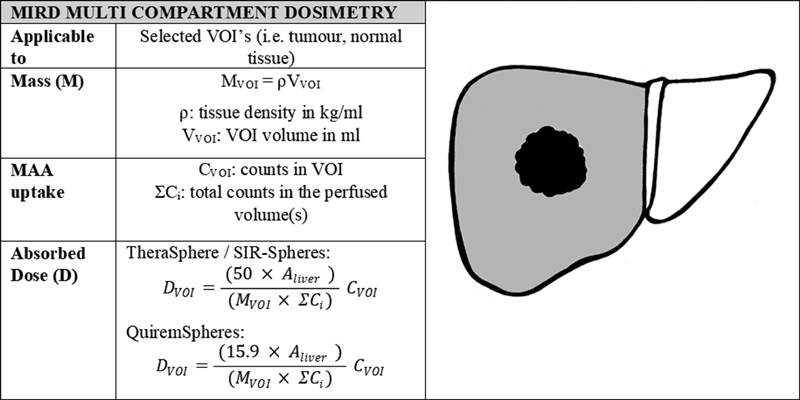
MIRD multi-compartment dosimetry calculation method. The factors of 50 (J/GBq) and 15.9 (J/GBq) account for the energy deposited in the region over time per activity injected for 90 Y and 166 Ho, respectively. The equations for absorbed dose have been adapted from those presented by Gulec et al 11 and are the formalism used in Simplicit 90 Y (Mirada medical Ltd). 11 (Printed with permission from Hermann A, Dieudonné A, Ronot M, et al. Relationship of tumor radiation–absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial Radioembolization with 90 Y in the SARAH Study. Radiology 2020;296:673–684). 21
While multi-compartment dosimetry is considered the most appropriate method in practice today, the precision of dosimetry may be further improved by evaluating intratumoral dose distribution via voxel dosimetry. Unlike radiation oncology where the dosimetric approach has been standardized and at the voxel level for the past 50 years, 13 voxel-level dosimetry is still a nascent technique in radioembolization. Voxel dosimetry applies the MIRD schema at the voxel level to generate a 3D voxel-wise dose map. Importantly, in this model the spatial distribution of activity is preserved. While in single- and multi-compartment methods the assumption is made that all radiation from a specific compartment is deposited in the same compartment (local deposition model), this does not necessarily hold true when looking at a small spatial scale. For a SPECT reconstruction with a small matrix size of 64 × 64, local deposition methods are not optimal and dose kernels or direct Monte Carlo calculations may be applied to estimate the local influence of radiation originating from a neighboring location or voxel. 14 For larger SPECT reconstruction, matrix 256 × 256 local deposition is adequate. 15 The dose distribution and dose volume histogram (DVH) determined via voxel dosimetry can be considered the most clinically representative of those generated by all the models discussed thus far, as it avoids many of their associated limitations; for example, wherein the multi-compartment model–absorbed dose is averaged over tumor volume, voxel-wise dosimetry provides absorbed dose gradients across tumor volumes (which reflect the true intratumoral heterogeneity). However, due to the limitations of nuclear medicine imaging resolution at the voxel level, quantitative assessments of DVHs should be made with caution. Ideally, high-resolution image data (i.e., computed tomography [CT] or magnetic resonance imaging [MRI]) would be used as the basis of voxel dosimetry calculations to accurately express absorbed dose gradients on a small spatial scale ( Fig. 4 ).
Fig. 4.
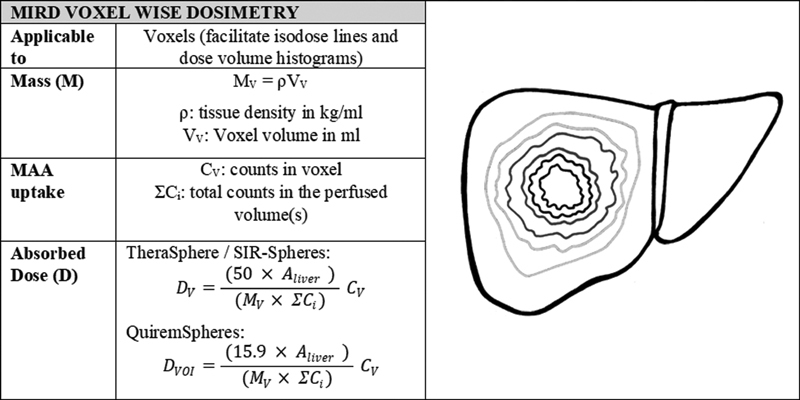
MIRD voxel-wise dosimetry calculation method. The factors of 50 (J/GBq) and 15.9 (J/GBq) account for the energy deposited in the region over time per activity injected for 90 Y and 166 Ho, respectively. The equations for absorbed dose have been adapted from those presented by Gulec et al 11 and are the formalism used in Simplicit 90 Y (Mirada medical Ltd). 11 This formalism assumes application of local deposition model.
Dosimetry Models in Practice
Over the past 20 years, various dosimetry approaches have been used and documented in trials. The continual refinement of dosimetric techniques over this time period, together with research into the effect of tumor and nontumoral doses on outcomes, has culminated in the development of dose–response relationships and the definition of tumor and normal tissue metrics that correlate with objective response and acceptable toxicity, respectively.
An overview of the evolution of how dosimetry is applied is now presented, as well as the lessons learned from practices and trials as published in literature, including the establishment of associated tumor and normal tissue dose thresholds.
Body Surface Area Dosimetry
Outcomes from trials utilizing the BSA dosimetry approach highlight the potential negative clinical implications that may arise from utilization of an inadequate dosimetry model. This semi-empirical method is known to forgo accuracy as it fails to incorporate the differential uptake between tumor and normal liver tissue and will invariably result in the planned activity failing within a low, constrained range. The BSA method was intended as a simple and fast way to calculate 90 Y-microspheres activity, which proponents of this method cite as a distinct advantage, together with the fact that it has a strong foundation of historical data demonstrating it limits unacceptable toxicity. However, a technique that offers a more generic evaluation and focuses on margins of safety is liable to deliver subtherapeutic radiation doses, impairing clinical outcomes. Historically, resin microspheres recommended use of BSA dosimetry; however, the recent resin microspheres dosimetry update specifies that if personalized therapeutic activity prescription is feasible, it is preferred to the BSA method. 16 This has been heralded as a “welcome advancement that gleans lessons from negative trials” 17 in the user community.
Three phase 3 trials with radioembolization failed to demonstrate survival benefit when compared with the tyrosine kinase inhibitor (sorafenib) in advanced HCC. SARAH and SIRveNIB compared radioembolization using resin 90 Y-microspheres with sorafenib in a European population and Asia Pacific population, respectively, and found similar efficacy between radioembolization and sorafenib. SORAMIC compared radioembolization followed by sorafenib with sorafenib as a monotherapy and found no difference in OS between the groups. These trials were not designed with a specific dosimetric endpoint and all utilized a simplistic dosimetry approach, primarily using the BSA algorithm for dose calculation. Some works have suggested “the lack of dosimetry-based personalized treatment planning may have diluted the clinical outcomes for radioembolization” 18 and challenge the relevance of these negative results, especially in light of recent publications. Sposito and Mazzaferro 19 highlighted the lack of dosimetric consideration in both SIRveNIB and SARAH which use the BSA dosimetry method and contrast with several other studies utilizing the multi-compartment MIRD method, in which a clear tumor-dose–response relationship was demonstrated. 20 While SARAH did not evaluate dose-related efficacy, a post hoc analysis of SARAH examined tumor dose and outcome, and a distinct dose–response relationship was found. Patients receiving a tumor dose of greater than 100 Gy with an optimal correlation between pretreatment MAA and posttreatment dose distribution had a significant survival improvement (24.9 vs. 6.7 months; Fig. 5 ).
Fig. 5.
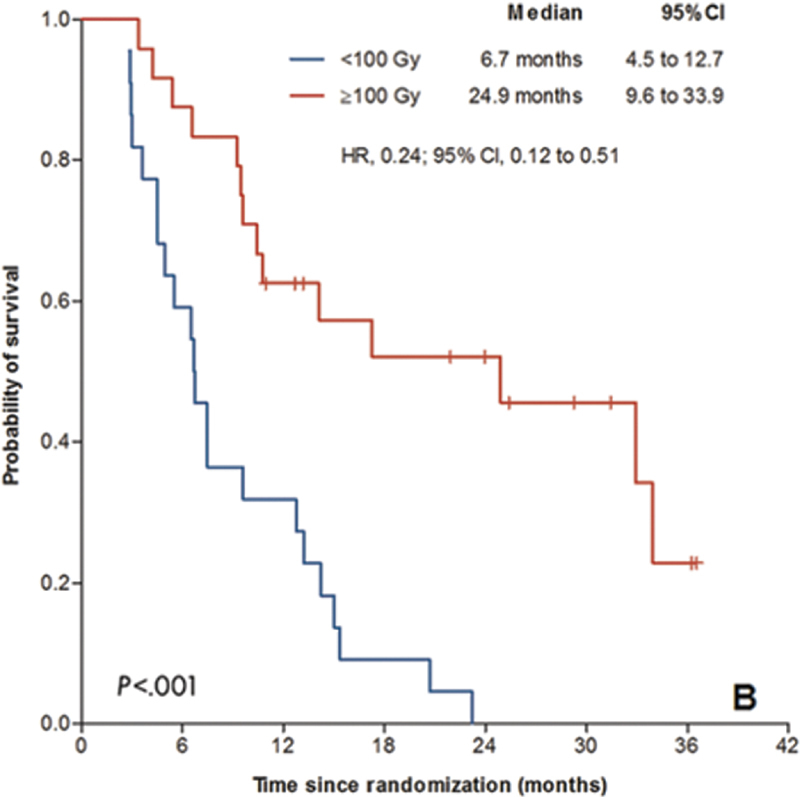
Kaplan–Meier curves for overall survival for participants with optimal agreement. This research was initially published in Radiology by Hermann et al. 21 (Copyright by CC BY 4.0.)
The same study also demonstrated that the correlation between 99m Tc-MAA distribution and posttreatment 90 Y distribution was medium to poor in 47% of patients—in almost half of patients, the scout procedure was not predictive of the final outcome. This has been identified as a shortcoming of 99m Tc-MAA and a potential inroad for new scout agents that better reflect the posttreatment distribution and therefore lead to better control over both the lung-shunt calculation and intrahepatic distribution. 22 Since more sophisticated dosimetry models rely on a scout dose as a reference for treatment, an agent that accurately reflects the posttreatment intrahepatic distribution is essential.
A treatment workup that accurately identifies which patients stand to benefit from radioembolization (i.e., where administering a tumoricidal dose is feasible and safe), and a dosimetry model that facilitates maximal dose to tumor and minimal nontarget deposition, is essential, and the BSA approach falls short on both counts. The results of the negative trials utilizing BSA activity calculation prompted the move away from this simplistic dosimetry technique, but also demonstrated that it is only possible to leverage the benefits of more sophisticated dosimetry approaches when a scout procedure that is highly predictive of the final outcome is utilized.
Single-Compartment Dosimetry
The dosimetry approach advised for both glass 90 Y microspheres and 166 Ho microspheres is single-compartment dosimetry. The instructions for use for the respective devices specify the desired absorbed dose to the perfused volume, and that a single-compartment dosimetry calculation be used to determine the activity required to deliver this dose according to the MIRD schema. For glass 90 Y microspheres, an absorbed dose range of 80 to 150 Gy is advised, and a limit of 30 Gy 8 to the lungs. For 166 Ho microspheres, the maximum tolerated absorbed dose is 60 Gy. 23
In current practice, the single-compartment dosimetry approach is directed toward more selective treatments, with radiation segmentectomy being the primary example. A radiation segmentectomy technique was utilized in LEGACY, a retrospective, multicenter study reporting on RR following 90 Y TheraSphere treatment of patients with solitary HCC. Delivery of an ablative radiation dose via selective infusion of the tumor-loaded hepatic segment was used to achieve a high median dose to the perfused volume of 410 Gy. 24 Ablative radiation dosing is possible in the context of this locoregional therapy, as the high dose is typically limited to ≤2 hepatic segments, uninvolved healthy tissue is spared, and it is accepted that the normal tissue within the perfused volume will be sacrificed. Since there is no goal to spare the perfused volume normal tissue, a single-compartment dosimetry approach that considers solely the average dose across the perfused volume is adequate. The compelling results of LEGACY (objective RR of 72.2% via mRECIST, and OS rate in patients with transplant or resection following radioembolization of 93% at 3 years) demonstrate the efficacy of radiation segmentectomy guided by single-compartment dosing as a curative intent option for a specific cohort of patients. Validation of this approach via a prospective investigation would be a valuable addition to the evidence base.
Multi-Compartment Dosimetry
Multi-compartment dosimetry differs from both the BSA method and single-compartment MIRD by basing activity calculation on more than one VOI. Typically, three separate compartments are considered: tumor, normal tissue, and lung tissue, such that an activity prescription can be tailored to boost dose to tumor and minimize dose to the latter compartments. However, additional volumes of interest including viable tumor and whole liver, which can be important determinants of outcome and complications, can also be considered.
A study evaluating the effectiveness of glass 90 Y microspheres in intermediate- to late-stage HCC using a personalized multi-compartment dosimetry approach versus standard dosimetry was published in 2020. DOSISPHERE-01 25 was a prospective, multicentric, randomized controlled phase 2 study in which patients were randomized to either a personalized dosimetry arm (using a multi-compartment dosimetry model which targeted >205 Gy to the target lesion) or a standard dosimetry arm (using a single-compartment dosimetry model which targeted 120 Gy ± 20 Gy to the perfused liver). Patients were eligible if they had unresectable locally advanced HCC with at least one lesion ≥7 cm, hepatic reserve ≥30% post-SIRT, unilateral or minimal bilateral involvement, and no extrahepatic spread. The primary endpoint was radiologic response at 3 months post-radioembolization via the European Association for the Study of Liver disease (EASL) criteria.
Results revealed that personalized dosimetry based on 99m Tc MAA imaging significantly increased RR of the index lesion in comparison with a standard dosimetry approach, 71.4 versus 35.7%, respectively. A retrospective central review confirmed the superiority of personalized dosimetry (RR = 78.6%) versus standard dosimetry (RR = 43%). Per investigator assessment, index lesion RR for tumor dose ≥205 Gy was 76.6 versus 22.2% for tumor dose less than 205 Gy ( p = 0.0002). The mean tumor doses for the personalized and standard arms were 331.1 ± 131.5 Gy and 221.3 ± 139.4 Gy, respectively. The dose escalation and corresponding RR improvement in the personalized arm was achieved with no increase in the toxicity profile; in fact, the personalized dosimetry arm demonstrated a lower number and severity of adverse events per the Common Terminology Criteria for Adverse Events (CTCAE). A meaningful improvement in OS was also observed 25 ; it was demonstrated that patients treated using personalized dosimetry had a median OS of 26.7 months compared with 10.7 months in the standard dosimetry group. Finally, bridging to curative intent was achievable to a greater degree in the personalized arm where 36% of patients were downstaged to resection versus 4% in the standard arm.
The notable OS improvement associated with treatment planning via 99m Tc MAA SPECT multi-compartment dosimetry (which characterized the personalized arm of the DOSISPHERE-01 study) highlights the efficacy of this technique. Implementation of multi-compartment dosimetry is advantageous for all clinical presentations, as it leads to a more accurate understanding of dose distribution in target and normal tissue. However, the most significant clinical benefit will be added for advanced stage, whole liver treatments where achieving an appropriate balance between efficacy and safety is paramount.
A limitation associated with multi-compartment MIRD is the reproducibility of the technique. The level of complexity associated with this method is significantly greater than the currently promoted single-compartment method, and it is a matter of debate as to whether centers can successfully replicate the approach, and therefore whether it can be implemented on a large scale. There is a need for a standardization of the workflow to ensure consistency in VOI definition, as currently practice varies from site to site, with some users opting for an anatomical image-guided VOI contouring, some using functional image-guided VOI contouring, and others a hybrid approach. A study evaluating errors propagated throughout the multi-compartment dosimetry process would be very valuable and no doubt help instigate uptake of this personalized dosimetry method.
Scout Procedure
Limitations of MAA
The multi-compartment MIRD method offers many potential advantages; however, the success of this approach is dictated by the predictive capability of the scout procedure used to simulate the posttreatment dose distribution. 99m Tc MAA is the current accepted scout agent; however, it has been demonstrated in several studies that there is a suboptimal coincidence of 99m Tc MAA and 90 Y in certain settings. Several factors may influence the difference in biodistribution between 99m Tc MAA and 90 Y; the random, irregular morphology of 99m Tc MAA contrasts sharply with the spherical 90 Y microspheres ( Fig. 6 ), and the density also differs significantly, where 99m Tc MAA particles have a density of 1.1 g/mL and 90 Y microspheres have a higher density of 1.6 g/mL and 3.3 g/mL for resin and glass, respectively. 26 The particle sizes of 99m Tc MAA (∼10–40 µm) and 90 Y microspheres (20–30 µm for glass microspheres and 20–60 µm for resin microspheres) are comparable but not identical. 27 Considering all these factors, it may be expected that 99m Tc MAA and 90 Y would distribute in a nonequivalent manner in vivo.
Fig. 6.
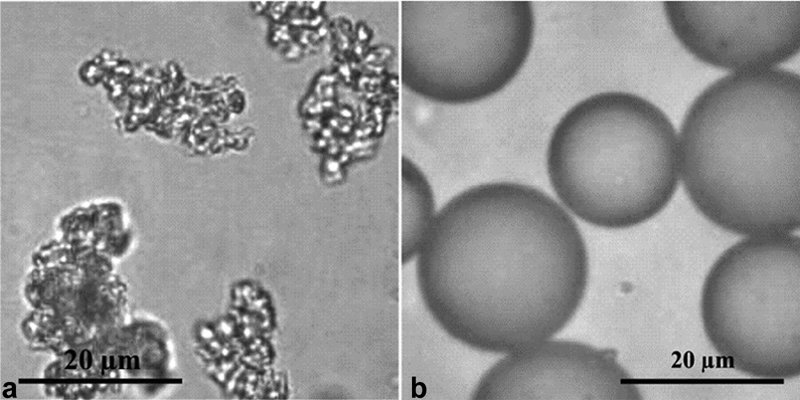
MAA particles ( a ) and TheraSphere (Boston Scientific, Marlborough, MA) microspheres ( b ) as viewed under a microscope. It is evident that the morphology differences between the two particles are significant. (Images provided by Boston Scientific.)
The number of injected particles in the scout and treatment procedures may also impact the respective distributions. It follows that a low number of particles may distribute in a different manner than a high particle load where embolic effects come into play. 26 The number of particles typically administered in a 99m Tc MAA scout procedure 26 is 1 to 2 × 10 6 ; in a treatment procedure using resin microspheres, the number of 90 Y microspheres 26 can be as high as 80 × 10 6 .
Another criticism of 99m Tc MAA SPECT dosimetry methodology is the fact that it is highly influenced by catheter position. Wondergem et al noted in their study that a suboptimal agreement in catheter tip position between the 99m Tc MAA SPECT and subsequent 90 Y procedure resulted in a significantly decreased agreement between the respective distributions, and this was particularly marked for patients with a mismatch in catheter position close to bifurcations. 26 This limitation was also recognized in DOSISPHERE where it was recommended that the same catheter position (equivalent artery, distance from arterial bifurcations, and intraluminal angulation) be used for both scout and treatment procedures.
Differences in physical characteristic between 99m Tc MAA and 90 Y inherently hinder the success of 99m Tc MAA as a scout agent; however, limitations associated with SPECT imaging also detract from 99m Tc MAA. The limited resolution of SPECT imaging dictates the minimum tumor volume that can be accurately contoured and registered, and for lesions of a comparable size or smaller than the resolution of the SPECT camera, partial volume effects will severely impact quantification. Due to the lack of intrinsic landmarks on SPECT data, registration to other image data is challenging; therefore, it is generally not recommended that SPECT is used in isolation when registering datasets. SPECT/CT is preferable as the CT component may be used for registration with the diagnostic anatomical scan.
Despite these limitations, DOSISPHERE demonstrated that for a certain cohort of patients, Tc 99m MAA SPECT can be used effectively for pretreatment dosimetry planning. A noteworthy feature of the DOSISPHERE-01 patient cohort was the large median lesion size (11 cm). Wondergem et al 26 highlighted in their study that 99m Tc-MAA and 90 Y-microspheres distribution differences are relatively less for segments with greater tumor involvement and attributed this to reduced random distribution of activity. Anecdotally, this is consistent with observations at our center that large, hypervascular tumors are associated with greater levels of 99m Tc-MAA and 90 Y-microspheres agreement.
The combined effects of reduced random activity distribution for large, hypervascular lesions (as all injected material is avidly taken up by tumor), and reduced co-registration artifacts due to minimal impact of SPECT imaging limitations for large lesions, could have contributed to the success of DOSISPHERE-01 which predominantly focused on tumors of a larger size ( Fig. 7 ).
Fig. 7.
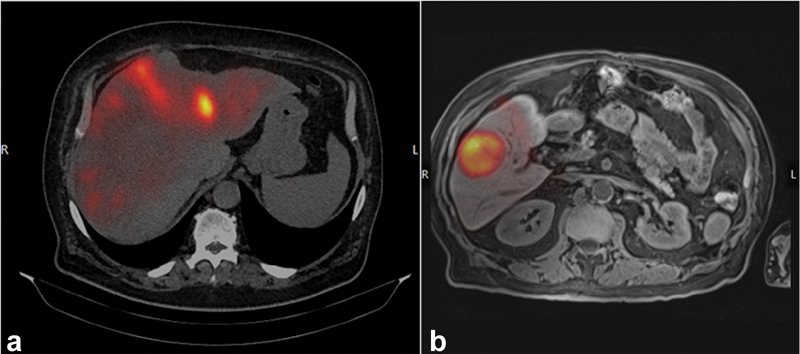
( a ) A patient with diffuse uptake, and multiple small lesions. Registration of the baseline diagnostic dataset and the 99m Tc MAA is difficult and therefore the alignment is suboptimal. ( b ) A patient with a large, hypervascular lesion. There is less random activity distribution and it is inherently easier to register the datasets; therefore, the alignment is optimal.
It seems that while there will be a place for 99m Tc MAA SPECT in certain settings (mimicking the hyperselective conditions of DOSISPHERE), a departure from 99m Tc MAA SPECT is foreseeable for patients with diffuse, small metastases, or when the embolic effect dominates the distribution mechanism such as high-dose resin microspheres. 26
166 Ho Microspheres as Alternative Scout Particles
A microsphere that can be visualized on multiple imaging modalities and is optimized for both treatment and imaging is beneficial from a dosimetry perspective. 166 Ho microspheres satisfy both these requirements and therefore represent an optimized theranostic agent that is potentially advantageous compared with the accepted 99m Tc MAA/ 90 Y procedure. 166 Ho emits a somewhat less energetic β radiation spectrum than 90 Y (Eβ-max = 1.85 MeV [48.8%], 1.77 MeV [49.9%]), 1 but has the significant benefit that it may be imaged on both SPECT and MRI using the gamma emissions and paramagnetic properties of holmium, respectively. 28 SPECT imaging is the current standard for dosimetry but is limited by poor spatial resolution (typically 1 cm) and can be problematic when targeting small volumes; conversely, MRI has excellent spatial resolution (typically 1–2 mm for most sequences) 29 and hence is less susceptible to partial volume effects. MRI relies on variations in susceptibility, this attribute permits assessment of the biodistribution of microspheres for prolonged periods posttherapy; however, measurements can be impacted by susceptibility artifacts (particularly extrahepatic distribution around air-containing organs where this effect is most pronounced). SPECT, therefore, remains crucial for this assessment. 28 In short, the respective benefits each of these modalities offer allow for a more accurate assessment of tumor-absorbed dose and nontarget embolization after treatment.
Use of an identical scout and treatment particle is theoretically beneficial as particles should distribute in an equivalent manner, improving predictive capability of the scout procedure. A recent study by Smits et al compared discrepancies arising between the therapeutic intrahepatic distribution of 166 Ho microspheres and distributions of 166 Ho scout dose and 99m Tc MAA, respectively. The study found that greater agreement was reached when using the 166 Ho scout compared with 99m Tc MAA across both qualitative and quantitative measures. 30 This outcome would tend to suggest there is merit in investigating future scout doses that utilize identical particles to the therapeutic counterpart ( Fig. 8 ).
Fig. 8.

166 Ho microspheres as viewed under a scanning electron microscope. This research was initially published in JECCR by Lin and Alessio. 29 (Copyright by CC BY 2.0.)
90 Y Microspheres as Alternative Scout Particles
The concept of imaging 90 Y microspheres at diagnostic activity levels for pretreatment planning has been investigated in several publications. 31 32 As with 166 Ho microspheres, the benefit of utilizing 90 Y microspheres for the pretreatment procedure as opposed to 99m Tc MAA is that the predictive capability may be maximized by using the same particle for pretreatment and treatment. However, in the case of 90 Y, this is technically difficult due to the high specific activity per microsphere, which effectively limits the activity that can be safely injected, and consequently the signal that can be detected during image acquisition. Since 90 Y is a pure β-emitter, consideration should be given to the safety of using it as a scout agent. Kunnen et al reported that a safety threshold of 100 MBq is required to avoid radiation damage. 32 Image acquisition at activities that fall under this critical threshold is challenging. 90 Y imaging may be conducted on two modalities (PET/CT and SPECT/CT) and each presents their own challenges.
PET/CT is generally regarded as superior in terms of image quality due to higher spatial resolution; however, reconstructed PET images have a high associated noise and the low positron branching ratio of 90 Y (30,000 times lower than 18 F) can hinder the sensitivity of PET images, especially at low activities. SPECT/CT has a lower noise level than PET and is more sensitive, and therefore might be more suitable when working with low activities 32 ; however, model-based scatter correction (i.e., modeling the physics of photon transport) is necessary for 90 Y SPECT because energy window–based methods are not feasible with the continuous bremsstrahlung energy spectrum. Therefore, the routine clinical reconstruction protocol may not be utilized.
The efficacy of PET/CT and SPECT/CT in accurately depicting a pretreatment 90 Y distribution intrahepatically and extrahepatically was investigated in a 2020 publication by Kunnen et al. They reported that a pretreatment SPECT/CT scan of an anthropomorphic phantom containing 100 MBq of 90 Y was successfully used to determine LSF, tumor dose and normal tissue dose with 1 percentage point accuracy, and was able to detect all extrahepatic depositions. PET/CT performed less well, as LSF was overestimated, normal tissue dose was underestimated, and extrahepatic depositions could not be detected. They cited the high noise component at low activities in PET images as a likely contributing factor. A simulation of a pretreatment 90 Y SPECT/CT was also conducted in a patient by acquiring a scan over a shortened time period and compared with a standard 99m Tc MAA SPECT. Quantitative measures (T:N, LSF, tumor dose, etc.) were comparable between 99m Tc and 90 Y pretreatment scans; however, 99m Tc MAA SPECT image quality was superior. 32 In this study, the effect of scattered radiation within the SPECT images was reduced through the adoption of a novel Monte Carlo–based reconstruction method; in a clinical setting, it may not be possible to take this approach due to both lack of availability and time constraints. Yue et al presented a comparative study, which suggested that with appropriate reconstruction methods and measured calibration correction factors a 90 Y SPECT/CT may be used for quantitative measurements; however, this was using posttherapy images at a higher activity level and so may not be relevant in a discussion of scout dose imaging. 33 Due to the necessity for specialist reconstruction techniques in 90 Y SPECT as well as the superior image quality reported for 99m Tc SPECT, the potential use of a 99m Tc-labeled microsphere within a pretreatment setting may be advantageous.
Novel Scout Particles
Development of an optimized scout particle as a precursor to radioembolization treatment is the subject of ongoing research efforts. An avenue currently being explored is the development of a microsphere that may be visualized in situ using imaging modalities in the interventional radiology (IR) suite, which would facilitate real-time dosimetry, that is, allow for dose to be estimated intraoperatively. Currently, either c-arm fluoroscopy or cone beam CT (CBCT) is used to guide procedures and so a radiopaque microsphere would be required to facilitate intraprocedural imaging. The development of an investigational 90 Y product “Eye90” (ABK Biomedical Inc., Halifax, Canada) is underway of which preclinical testing has confirmed strong X-ray fluoroscopic and CBCT radiopacity. 34 It is foreseeable that this product may facilitate real-time intraprocedural confirmation of tumor targeting. 166 Ho microspheres may also be utilized in this setting due to its paramagnetic qualities, but only in selected centers that have access to MR scanning in the IR suite. Testing of 166 Ho microspheres was also conducted under CT imaging, but quantification of normal liver dose via this method was challenging, as it was possible only in high uptake areas with maximal microsphere deposition.
PET imaging has advantages over SPECT imaging in terms of spatial resolution and accuracy of quantification, and consequently is regarded as a superior option for conducting in vivo measurements for radionuclide dosimetry. As such, potential scout agents that are PET imageable are of great interest. Selwyn et al reported on their development of a novel positron-emitting resin microsphere to be used as a PET imaging surrogate for resin 90 Y microspheres. 35 In this study, a new treatment procedure whereby the 99m Tc MAA SPECT was replaced with a PET scan of 18 F-loaded resin microspheres was conducted to capitalize on the benefits of PET. They reported that the radiolabeling process was equivalent to the standard MAA radiolabeling procedure in terms of difficulty and time, and PET-derived uptake ratios were shown to be accurate to histologic ratio to 3%, which leads to the conclusion that this method could be used to determine uptake ratio and tumor dose using the MIRD formalism. 35 A downside of using 18 F as a surrogate agent is the high dose to (radiopharmacy) personnel and relatively short half-life, which limits the window for distribution to clinical sites. 64 Cu was proposed as an alternative nuclide, which may warrant further investigation due to the longer half-life; however, literature suggested that 64 Cu has a high binding affinity for albumin and so is likely to leach into the blood in vivo. This finding effectively rules out 64 Cu as an imaging surrogate. 35
Avila-Rodriguez et al evaluated a 89 Zr microsphere in their 2007 publication. This was considered optimal due to the 78-hour half-life 89 Zr, which facilitates worldwide distribution. 36 They determined that a resin microsphere labeled with 89 Zr had adequate in vivo stability for clinical application, but they did not explicitly report on agreement between 89 Zr PET biodistribution and 90 Y distribution. Therefore, a further study focusing specifically on the dosimetry application of 89 Zr would be of interest. Perk et al discussed the use of a positron-emitting analog of the 90 Y therapeutic nuclide 86 Y, and identified that a drawback of utilizing 86 Y as a surrogate was that 86 Y emits prompt gamma photons. These photons partnered with 511-keV annihilation photons, which resulted in spurious true coincidences and consequently introduced quantification artifacts. 37 Lubberink and Herzog highlighted that an isotope with similar chemical properties to 90 Y, such as 89 Zr, 38 may in fact be preferable, as the characteristics of 89 Zr (favorable decay scheme, longer half-life) are desirable for imaging and transport, respectively.
As discussed, PET offers excellent spatial resolution, and thus is an accurate and desirable technique to facilitate dosimetry. However, as demonstrated in Kunnen et al's 2020 publication, 32 PET can be of limited value when imaging low activities. SPECT is the most widely used modality for the evaluation of pre- and post- 90 Y imaging. Due to the prevalence of SPECT in clinical centers globally, and the longstanding protocol of 99m Tc MAA SPECT usage for workup, it is reasonable to assume that SPECT scanning will remain a mainstay of radioembolization treatment for the foreseeable future. It would therefore be of interest to evaluate a surrogate agent that utilizes an identical particle as the therapeutic analog (to maximize predictability of the scout), but is labeled with 99m Tc, which is readily available and benefits from being integrated into current practice.
Table 1 describes the relative benefits and drawbacks of each of the scout particles described earlier.
Table 1. Relative pros and cons of the various scout particles available.
| Scout particles and isotope | Similarity to therapeutic counterpart | Optimal imaging properties | Logistics of distribution | Easy to integrate into current practice | Stable in vivo |
|---|---|---|---|---|---|
| 99m Tc MAA | + | ++ | +++ | +++ | ++ |
| 166 Ho scout dose | ++ + a | ++ | ++ | ++ | +++ |
| 89 Zr-labeled microsphere | ++ + a | ++ | + | + | ++ |
| 18 F-labeled microsphere | ++ + a | +++ | + | + | ++ |
| Radiopaque “Eye 90” microsphere | ++ + a | ? | +++ | ++ | ? |
| 64 Cu-labeled microsphere | ++ + a | ++ | + | + | + |
Abbreviation: MAA, macroaggregated albumin.
Note: Rating has been conducted on a 3-point scale where +++ is optimal, ++ is moderate, and + is suboptimal.
Identical particles used for scout and treatment. ?As yet unknown as there is no published data
Future Perspectives
The treatment planning evolution that has taken place over the past 20 years of treating with radioembolization has seen a paradigm shift away from “off the shelf,” generic techniques such as BSA, toward bespoke treatment planning that enables an understanding of dose to tumor and normal tissue, and thus the expected efficacy and safety prior to treatment. The concept of personalized dosimetry that accounts for these parameters is still in its infancy; currently, single-compartment MIRD dosimetry still represents the most commonly used methodology, but slowly more instances of multi-compartment MIRD are being reported and implemented clinically. The demonstration in DOSISPHERE-01, that for a specific patient cohort an experienced user can implement 99m Tc MAA SPECT multi-compartment dosimetry with good effect, marked a turning point in the acceptance of multi-compartment MIRD techniques. It is expected that the number of users opting for multi-compartment methods will continue to grow, and the publication of key studies (TARGET) may see the manufacturers recognizing multi-compartment methods in their instructions for use.
Of equal importance as the widespread adoption of personalized dosimetry is gaining a thorough understanding of the errors inherent in the process. While external beam radiotherapy utilizes a robust framework for quality assuring (QA) the dosimetry process, no such system is currently available for radioembolization. QA in radioembolization currently constitutes checks of vial activity and delivery system functionality pretreatment, and checks of residual activity posttreatment. Plan checking procedures to identify registration quality between diagnostic and functional images is not routine, and no guidance regarding the error that is introduced to the dosimetry process in cases of poor agreement (where there is minimal overlap between structures or large uncertainty in the registration vs. good agreement and where there is global alignment of datasets) currently exists. In some cases, a treatment planning system is used to verify that a plan meets clinical targets with respect to tumor and normal tissue doses by making use of DVH capabilities, but this is not commonplace. Finally, since it has only become possible to evaluate dose intraoperatively in recent years with the advent of scout agents such as 166 Ho, this technique has not been widely implemented. Typically, the only check of treatment quality takes places after treatment via PET or SPECT evaluation, and if a suboptimal treatment has been delivered a repeat procedure is scheduled. An intraoperative check that facilitates real-time dosimetric evaluation and enables the user to adjust accordingly would be more efficient.
Adaptive planning (whereby treatments are reoptimized intraoperatively) is advantageous, as it could lead to both dose escalation and normal tissue sparing. The identification during a procedure that a lesion has a more favorable T:N ratio than was measured during the planning session might encourage the administration of more activity and thus a higher dose. Similarly, if during a procedure it is identified that an embolic effect has been reached and therapeutic beads are distributing into the surrounding healthy parenchyma (an effect which has been reported on for resin microspheres) then the administration may be terminated early to spare normal tissue. To facilitate adaptive planning, real-time imaging is required to guide procedures. As discussed, a novel radiopaque bead is currently in development that would allow for real-time imaging using fluoroscopy or CBCT. 166 Ho may also be used in this regard, exploiting the paramagnetic properties by MRI imaging. A new imaging system comprising a dual-layer detector that is capable of acquiring X-ray and gamma emissions simultaneously 39 40 has recently been integrated into our center and it is hoped this system will improve interventional procedures by facilitating real-time imaging of activity distribution and therefore adaptive planning.
Multicenter harmonization in regard to the treatment planning process is also lacking. European guidelines stipulate the need for dosimetry, but no specific methods are recommended. The recent formation of a Radionuclide Internal Dosimetry Special Interest Group comprising a European network of medical physicists may remedy this by offering guidance as to the clinical implementation of dosimetry in radioembolization. As discussed, the optimal workflow for multi-compartment dosimetry has yet to be defined, with debate as to whether volumes should be delineated on functional imaging, anatomical imaging, or using a hybrid approach, ongoing. Several other procedural refinements are also still being investigated, including the impact of implementing dosimetry software for automatic segmentation and deformable image registration, identical infusion rates of 99m Tc MAA and therapeutic microspheres, and the impact of identical catheter placement. Device manufacturers have published consensus guidelines to summarize current data and recommend best practice 3 16 and there are numerous single-center studies 41 42 43 that evaluate the methods of multi-compartment dosimetry in radioembolization, but ultimately multicenter trials reporting on radioembolization dosimetry are required. DOSISPHERE-01 is the first publication to report on a multicenter, prospective evaluation of 99m Tc-MAA-guided treatment using multi-compartment dosimetry; the TARGET study will make a valuable addition to the evidence base providing guidance on the workflow that should form the basis for volume and absorbed dose assessment, and presenting in an international setting how dosimetry may be performed in a consistent and reproducible way.
Conclusion
An accurate prediction of dose to tumor, healthy liver, and lungs is increasingly considered an essential prerequisite to an optimized radioembolization treatment, as these values can both facilitate optimal patient selection and help realize truly individualized, effective, and safe treatments. Personalized dosimetry is gaining traction in the user community due to its ability to deliver these results, and a growing number of users are incorporating personalized dosimetry methods into treatment planning strategies. Since these methods are reliant on a predictive scout dose distribution, to leverage the benefits of personalized dosimetry, a scout procedure that is highly predictive of the final dose distribution is required. 99m Tc- MAA is an appropriate agent in some settings but is not the ideal solution for all patients. As yet no other agent has demonstrated sufficient efficacy and utility to displace it as the accepted standard. 166 Ho is the only commercially available alternative and has demonstrably excelled in improving the predictive value of pretreatment analysis of distribution—setting a new benchmark which the other radioembolization vendors have yet to address. An agent that is highly predictive of the treatment outcome and thus ensures confidence and control over the intrahepatic distribution and lung shunt calculation is obviously essential, but additionally one which strikes the right balance between desirable imaging properties, a half-life that enables global distribution, and ubiquitous accessibility would be the optimal solution. Ultimately, the “silver bullet” which will fully unlock the potential of SIRT therapy will be the partnership of an optimized scout particle and personalized dosimetry.
Footnotes
Conflict of Interest None declared.
References
- 1.Roosen J, Klaassen N JM, Westlund Gotby L EL.To 1000 Gy and back again: a systematic review on dose-response evaluation in selective internal radiation therapy for primary and secondary liver cancer[published online ahead of print April 10, 2021]Eur J Nucl Med Mol Imaging 2021 10.1007/s00259-021-05340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem R, Gordon A C, Mouli S. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(06):1155–116300. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem R, Padia S A, Lam M. Clinical and dosimetric considerations for Y90: recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imaging. 2019;46(08):1695–1704. doi: 10.1007/s00259-019-04340-5. [DOI] [PubMed] [Google Scholar]
- 4.European Council . European Council Directive 2013/59/Euratom on basic safety standards for protection against the dangers arising from exposure to ionising radiation and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. OJ EU L13. 2014;57:1–73. [Google Scholar]
- 5.Kao Y H, Tan E H, Ng C E, Goh S W. Clinical implications of the body surface area method versus partition model dosimetry for yttrium-90 radioembolization using resin microspheres: a technical review. Ann Nucl Med. 2011;25(07):455–461. doi: 10.1007/s12149-011-0499-6. [DOI] [PubMed] [Google Scholar]
- 6.Therapy, Oncology and Dosimetry Committees . Giammarile F, Bodei L, Chiesa C. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2011;38(07):1393–1406. doi: 10.1007/s00259-011-1812-2. [DOI] [PubMed] [Google Scholar]
- 7.Loevinger R, Budinger T, Watson E. New York: Society of Nuclear Medicine; 1991. MIRD Primer for Absorbed Dose Calculations, revised edition. [Google Scholar]
- 8.Biocompatibles UK Ltd . Farnham UK: Biocompatibles UK Ltd; Therasphere® Yttrium-90 Glass Microspheres: Package Insert. [Google Scholar]
- 9.American Association of Physicists in Medicine . Dezarn W A, Cessna J T, DeWerd L A. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys. 2011;38(08):4824–4845. doi: 10.1118/1.3608909. [DOI] [PubMed] [Google Scholar]
- 10.Medical Internal Radiation Dose Committee . Bolch W E, Bouchet L G, Robertson J S. MIRD pamphlet No. 17: the dosimetry of nonuniform activity distributions – radionuclide S values at the voxel level. J Nucl Med. 1999;40(01):11S–36S. [PubMed] [Google Scholar]
- 11.Gulec S A, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med. 2006;47(07):1209–1211. [PubMed] [Google Scholar]
- 12.Lau W Y, Leung T W, Ho S. Diagnostic pharmaco-scintigraphy with hepatic intra-arterial technetium-99m macroaggregated albumin in the determination of tumour to non-tumour uptake ratio in hepatocellular carcinoma. Br J Radiol. 1994;67(794):136–139. doi: 10.1259/0007-1285-67-794-136. [DOI] [PubMed] [Google Scholar]
- 13.Thariat J, Hannoun-Levi J M, Sun Myint A, Vuong T, Gérard J P. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2013;10(01):52–60. doi: 10.1038/nrclinonc.2012.203. [DOI] [PubMed] [Google Scholar]
- 14.Dieudonné A, Hobbs R F, Lebtahi R. Study of the impact of tissue density heterogeneities on 3-dimensional abdominal dosimetry: comparison between dose kernel convolution and direct Monte Carlo methods. J Nucl Med. 2013;54(02):236–243. doi: 10.2967/jnumed.112.105825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasciak A S, Erwin W D. Effect of voxel size and computation method on Tc-99m MAA SPECT/CT-based dose estimation for Y-90 microsphere therapy. IEEE Trans Med Imaging. 2009;28(11):1754–1758. doi: 10.1109/TMI.2009.2022753. [DOI] [PubMed] [Google Scholar]
- 16.Levillain H, Bagni O, Deroose C M. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imaging. 2021;48(05):1570–1584. doi: 10.1007/s00259-020-05163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toskich B B, Tabori N E, Lewandowski R J. Practical yttrium-90 radioembolization dosimetry for the treatment of hepatocellular carcinoma: current Y-90 dosimetry concepts driving radioembolization practice for HCC treatment. Emb. 2021;20(04):43–48. [Google Scholar]
- 18.Reinders M, Braat A, Lam M. Toxicity and dosimetry in SORAMIC study. J Hepatol. 2020;73(03):734–735. doi: 10.1016/j.jhep.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Sposito C, Mazzaferro V. The SIRveNIB and SARAH trials, radioembolization vs . sorafenib in advanced HCC patients: reasons for a failure, and perspectives for the future . Hepatobiliary Surg Nutr. 2018;7(06):487–489. doi: 10.21037/hbsn.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiesa C, Maccauro M, Romito R. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90)Y microspheres: the experience of the National Tumor Institute of Milan. Q J Nucl Med Mol Imaging. 2011;55(02):168–197. [PubMed] [Google Scholar]
- 21.SARAH Trial Group . Hermann A L, Dieudonné A, Ronot M. Relationship of tumor radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90 Y in the SARAH study . Radiology. 2020;296(03):673–684. doi: 10.1148/radiol.2020191606. [DOI] [PubMed] [Google Scholar]
- 22.Marnix L.Personalised treatment planning “the future” of radioembolization, ECIO hears, in Y-90 vsHo-166 comparison. January 29, 2021. Accessed June 10, 2021 at:https://interventionalnews.com/personalised-treatment-planning-radioembolization
- 23.Bastiaannet R, Kappadath S C, Kunnen B, Braat A JAT, Lam M GEH, de Jong H WAM. The physics of radioembolization. EJNMMI Phys. 2018;5(01):22. doi: 10.1186/s40658-018-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem R, Johnson G, Kim E. Yttrium-90 radioembolization for the treatment of solitary, unresectable hepatocellular carcinoma: the LEGACY study. Hepatology. 2021 doi: 10.1002/hep.31819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DOSISPHERE-01 Study Group . Garin E, Tselikas L, Guiu B. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(01):17–29. doi: 10.1016/S2468-1253(20)30290-9. [DOI] [PubMed] [Google Scholar]
- 26.Wondergem M, Smits M L, Elschot M. 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med. 2013;54(08):1294–1301. doi: 10.2967/jnumed.112.117614. [DOI] [PubMed] [Google Scholar]
- 27.Ilhan H, Goritschan A, Paprottka P. Predictive value of 99mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J Nucl Med. 2015;56(11):1654–1660. doi: 10.2967/jnumed.115.162685. [DOI] [PubMed] [Google Scholar]
- 28.Smits M L, Elschot M, van den Bosch M A. In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J Nucl Med. 2013;54(12):2093–2100. doi: 10.2967/jnumed.113.119768. [DOI] [PubMed] [Google Scholar]
- 29.Lin E, Alessio A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J Cardiovasc Comput Tomogr. 2009;3(06):403–408. doi: 10.1016/j.jcct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smits M LJ, Dassen M G, Prince J F. The superior predictive value of 166 Ho-scout compared with 99m Tc-macroaggregated albumin prior to 166 Ho-microspheres radioembolization in patients with liver metastases . Eur J Nucl Med Mol Imaging. 2020;47(04):798–806. doi: 10.1007/s00259-019-04460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunnen B, Dietze M MA, Braat A JAT, Lam M GEH, Viergever M A, de Jong H WAM. Feasibility of imaging 90 Y microspheres at diagnostic activity levels for hepatic radioembolization treatment planning . Med Phys. 2020;47(03):1105–1114. doi: 10.1002/mp.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunnen B, van der Velden S, Bastiaannet R, Lam M GEH, Viergever M A, de Jong H WAM. Radioembolization lung shunt estimation based on a 90 Y pretreatment procedure: a phantom study . Med Phys. 2018;45(10):4744–4753. doi: 10.1002/mp.13168. [DOI] [PubMed] [Google Scholar]
- 33.Yue J, Mauxion T, Reyes D K. Comparison of quantitative Y-90 SPECT and non-time-of-flight PET imaging in post-therapy radioembolization of liver cancer. Med Phys. 2016;43(10):5779. doi: 10.1118/1.4962472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abraham R, Lewandowski R, Gandhi R. What's New in Y-90? Technical and procedural innovations in Y-90 radioembolization including existing and investigational next-generation technologies. Interv Oncol. 2019;18(10):49–60. [Google Scholar]
- 35.Selwyn R G, Avila-Rodriguez M A, Converse A K. 18F-labeled resin microspheres as surrogates for 90Y resin microspheres used in the treatment of hepatic tumors: a radiolabeling and PET validation study. Phys Med Biol. 2007;52(24):7397–7408. doi: 10.1088/0031-9155/52/24/013. [DOI] [PubMed] [Google Scholar]
- 36.Avila-Rodriguez M A, Selwyn R G, Hampel J A. Positron-emitting resin microspheres as surrogates of 90Y SIR-Spheres: a radiolabeling and stability study. Nucl Med Biol. 2007;34(05):585–590. doi: 10.1016/j.nucmedbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Perk L R, Visser G W, Vosjan M J. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med. 2005;46(11):1898–1906. [PubMed] [Google Scholar]
- 38.Lubberink M, Herzog H. Quantitative imaging of 124I and 86Y with PET. Eur J Nucl Med Mol Imaging. 2011;38 01:S10–S18. doi: 10.1007/s00259-011-1768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Velden S, Kunnen B, Koppert W JC. A dual-layer detector for simultaneous fluoroscopic and nuclear imaging. Radiology. 2019;290(03):833–838. doi: 10.1148/radiol.2018180796. [DOI] [PubMed] [Google Scholar]
- 40.Dietze M MA, Kunnen B, van der Velden S. Performance of a dual-layer scanner for hybrid SPECT/CBCT. Phys Med Biol. 2019;64(10):105020. doi: 10.1088/1361-6560/ab15f6. [DOI] [PubMed] [Google Scholar]
- 41.Ho C L, Chen S, Cheung S K. Radioembolization with 90 Y glass microspheres for hepatocellular carcinoma: significance of pretreatment 11 C-acetate and 18 F-FDG PET/CT and posttreatment 90 Y PET/CT in individualized dose prescription . Eur J Nucl Med Mol Imaging. 2018;45(12):2110–2121. doi: 10.1007/s00259-018-4064-6. [DOI] [PubMed] [Google Scholar]
- 42.Kappadath S C, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumor dose response after 90 Y-radioembolization with glass microspheres using 90 Y-SPECT/CT-based voxel dosimetry . Int J Radiat Oncol Biol Phys. 2018;102(02):451–461. doi: 10.1016/j.ijrobp.2018.05.062. [DOI] [PubMed] [Google Scholar]
- 43.Jadoul A, Bernard C, Lovinfosse P. Comparative dosimetry between 99m Tc-MAA SPECT/CT and 90 Y PET/CT in primary and metastatic liver tumors . Eur J Nucl Med Mol Imaging. 2020;47(04):828–837. doi: 10.1007/s00259-019-04465-7. [DOI] [PubMed] [Google Scholar]


