Abstract
Leaks from anastomoses can be a serious complication of any gastrointestinal resection. Leaks lead to increased morbidity, delayed postoperative recovery, and potential delays in adjuvant treatment in cancer cases. Prevention of anastomotic leak has been an area of ongoing research for decades. Methods of assessing bowel perfusion have been developed that may provide forewarning of anastomotic compromise. Physical reinforcement of the anastomosis with buttressing material is an available method employed with the goal of preventing leaks. Liquid-based sealants have also been explored. Lastly, interactions between the gut microbiome and anastomotic healing have been investigated as a mean of manipulating the microenvironment to reduce leak rates. Though no single technology has been successful in eliminating leaks, an understanding of these developing fields will be important for all surgeons who operate on the gastrointestinal tract.
Keywords: anastomosis, colorectal surgery, anastomotic leak, bowel perfusion, endoscopy
Anastomotic leaks are a dreaded complication in colorectal surgery. The morbidity incurred by the patient is substantial, and the sequelae can be challenging to manage for both the patient and surgeon. Several techniques have been developed in an attempt to reduce leaks after colorectal anastomoses. Sealants applied to the exterior of anastomoses, reinforcement of staple lines with bioabsorbable material, placement of omental flaps, and perfusion assessment with fluoroscopy have been explored over the last two decades. Though no one technique is clearly superior to the others, perfusion assessment is the most extensively studied in colorectal patients and has the strongest data supporting its use in selected cases.
Fibrin and Other Sealants
The use of fibrin-based sealants to reduce anastomotic leak has been investigated for over 30 years. Fibrin is a biologic polymer protein that is the end product of a complex chemical pathway activated after thrombin lyses plasma fibrinogen. Fibrin clots are resistant to tension and compression but remain porous to cytokines and other proteins. In addition, the polymer scaffold facilitates passage of immune cells. 1 Fibrin can be obtained from donated plasma, and it does not induce an immune response when purified. These properties make it a potential substrate for wound healing and aggregation of procoagulant cells which are the primary purposes of endogenous fibrin.
Fibrin solutions have been used for hemostasis in renal surgery in Europe for nearly half a century. 2 Fibrin was approved by the Food and Drug Administration in 1998 as a hemostatic agent, and its use has expanded to nearly all surgical subspecialties. 3 Initial canine studies were performed with rectal devascularization to augment the risk of leak in high colorectal anastomoses and demonstrated no statistical difference in rates of barium-enema detected anastomotic leak (65% without fibrin vs. 47% with fibrin). 4 The majority of leaks in both groups developed into contained abscesses. As in all subsequent studies, fibrin was applied to coat the exterior of the anastomosis after the anastomosis was created. The first published report in rat models involved four arms to compare control, steroids, fibrin plus steroids, and fibrin alone. They found no improvement in the bursting pressure of colonic anastomoses treated with a fibrin sealant but did note an increase in perianastomotic abscesses. 5 Further studies in rat and rabbit models found that fibrin sealants may actually inhibit collagen maturation and induce a marked inflammatory response. 6 7 8 Subsequent studies in large animal models have been more promising, with two studies demonstrating reduced leaks in a porcine model. 9 10 These studies demonstrated a reduction in the leak rate from 20 to 0 to 5%. 10 Interestingly, in the setting of an anastomotic leak, both studies identified that the fibrin gel physically occluded the site of anastomotic dehiscence, suggesting that the fibrin acted as both a scaffold for healing and as a physical barrier.
Alternate substances, such as platelet-rich plasma (PRP), cross-linking gelatins, and intestine submucosal wraps have been found to yield higher bursting pressures in animal models. 11 12 13 PRP is an autologous solution derived from patients' serum. Plasma is concentrated from whole blood and processed into an injectable liquid or instilled into a collagen matrix. It has been used in clinical trials for arthroscopic rotator cuff repair, and a recent meta-analysis suggests the PRP-infused collagen matrices reduce retearing rates in selected circumstances. 14 Rat models have shown mixed benefits in anastomotic bursting pressure, but did not investigate anastomotic leak rates. 11 15 A pig model of small bowel anastomoses did not find any difference in leaks. 16 No human trials of PRP for colorectal anastomoses have been published to date, and the utility of PRP for anastomotic healing thus remains unclear.
Cross-linking gelatin is a sealant derived from pigs and exposed to high-intensity light to induce polymerization after application to the anastomosis. Preliminary animal studies indicate that it is well tolerated with a minimal inflammatory response compared with fibrin-based sealants. 12 Similarly, the utilization of small intestine submucosa as a sealant has been shown to increase collagen deposition and bursting pressures in rats. 13 However, studies of in vivo leak rates after animal or human colorectal surgery have yet to be reported with these sealants.
A recent study of six different sealants used for hemostasis or air leaks commonly used after lung resection (one fibrin, two albumin, two polyethylene, and one cyanoacrylate based) was performed in a mouse colorectal anastomosis model. 17 These sealants have been identified in clinical trials to decrease air leaks after lung surgery. 18 A common mouse colorectal anastomosis model with a baseline of 40% leak rate by 1 week was used. One albumin-based sealant had no leaks but caused severe hepatotoxicity. The remaining five sealants had no observable effect on leak rates but increased ileus and mortality. The authors concluded that none of the sealants could be recommended for clinical trials. In fact, a systemic review of 40 studies using fibrin or hyaluronic acid substrates for colon or colorectal anastomoses in multiple animal models found no benefit with either substrate, and potential harm from hyaluronic acid substrates. 19 Though animal studies have not consistently demonstrated benefits, fibrin has been used in clinical trial settings for a variety of anastomoses in patients.
As anticipated from the experimental models, clinical studies of fibrin and other sealants have provided mixed results in other gastrointestinal anastomoses. A randomized trial of 340 bariatric patients undergoing laparoscopic Roux-en-Y gastric bypass demonstrated a lower reoperation rate but no statistical difference in clinically detected leaks. 20 . Randomized trials of patients undergoing the Whipple procedures at a high-volume center also demonstrated no decrease in pancreatic leak rates. 21 22 A series of 48 patients undergoing an emergency laparotomy with bowel resection for sepsis had an 8% leak rate but no controls were included. 23
Few human trials of fibrin glue for protection of colorectal anastomoses have been reported. A nonrandomized prospective study of fibrin in 223 rectal cancer patients undergoing laparoscopic low anterior resection was reported in 2010. 24 Fibrin glue application was not associated with anastomotic leak on multivariate analysis (5.8% in fibrin group and 10.9% in nonfibrin group, p = 0.169). The same authors published an updated series of 1,148 rectal cancer patients and, after propensity-score matching, there was no difference in leak rates (5.1% with fibrin vs. 7.0% without fibrin, p = 0.33). 25 To date, no robust clinical trials have demonstrated a benefit from liquid or gelatinous sealants around any gastrointestinal anastomosis. Further research into these field is needed before any exogenous product should be considered outside of a trial setting.
Staple Line Reinforcements
An alternative means to reduce anastomotic leaks is reinforcement of stapled anastomoses with bioabsorbable materials placed along the length of the staple line ( Fig. 1 ). The buttressing material is placed between opposing tissues to be stapled together. When the stapler is fired, the material is secured in place between these tissues. This technique was initially used in the early 1990s to reduce leaks after lung-reduction surgery which is plagued by high air leak rates. 26 A multicenter randomized trial conducted in the late 1990s examined staple-line reinforcement with bovine pericardium and found a trend toward lower air leak duration. 27 A larger single-center trial demonstrated a lower air leak rate (2 vs. 20%, p = 0.016), but the study was closed early due to a manufacturer recall. 28 This setback has not dampened interest in using buttresses for bowel anastomoses.
Fig. 1.
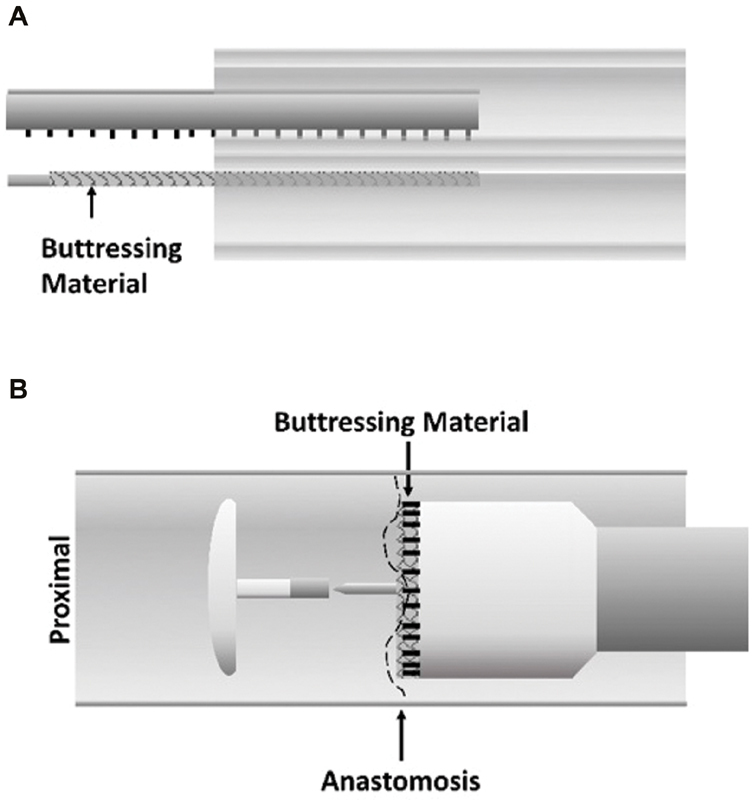
Staple line reinforcement. Biodegradable material is placed on the stapler along its length, and is secured by staple fires. ( A ) Configuration for linear stapler. ( B ) Configuration for circular stapler.
In gastrointestinal surgery, the proposed benefit of buttressing the anastomosis is a reduction in leaks through better apposition of the stapled walls and additional tensile strength to resist intraluminal pressures. This is supported by mouse models of buttressed colo-colonic anastomoses that demonstrated a higher bursting pressure, such that the point of failure was adjacent normal colonic wall. 29 30 However, studies of colorectal anastomoses in a porcine model have been conflicting, with one study showing no differences in burst pressures, and another finding improved burst pressures with reinforcement. 31 32 As porcine bowels more closely resemble human organs in caliber, this model is thought to be more accurately represented the physiologic forces acting on human anastomoses.
The increased use of stapled anastomoses in clinical practice has allowed for the easier incorporation of bioabsorbable buttressing materials. This has been studied extensively in bariatric operations. Collagen-based materials have been found to reduce rates of bleeding, both in sleeve gastrectomy staple lines apposing cut edges of gastric wall and gastrojejunostomy anastomoses in gastric bypass operations. 33 34 Leak rates decreased from 2.6 to 1.1% for sleeve gastrectomy ( p = 0.001), but increased from 0.7 to 4.9% with gastrojejunostomy anastomoses ( p = 0.02). Interestingly, reductions in strictures (0.7 vs. 9.3%, p < 0.01) were found in a large series of gastrojejunostomies fashioned with buttressed circular staples. 35
Three large clinical trials of staple-line reinforcement in colorectal surgery have been performed. A single-arm trial of 117 patients undergoing a left-sided anastomosis evaluated the use of a commercially available bioabsorbable polyglycolic acid-based (PGA) material. 36 The overall leak rate was 3.4% (4/117), and reoperation rate was 1.7%. There were no apparent complications related to the material itself. Subsequently, a single-center randomized trial of 302 left-sided circular stapled colorectal anastomoses was performed. Patients were randomized at the time of surgery to buttressing with the above-tested bioabsorbable material. There was no significant difference in overall complication rate, leak rates, bleeding, or stenosis. 37 A multicenter randomized trial with 258 patients was performed in patients requiring a low rectal anastomosis (mean distance to anal verge of 4.3 cm), primarily for rectal cancer. 38 All anastomoses were performed with a circular stapler, and a PGA-based buttressing material was used. The trial was terminated at the interim efficacy analysis due to futility on account of nearly equal leak rates (12 vs. 11.4%, p = 0.85). In addition, there were no differences in bleeding rates (1.6 vs. 4.4%, p = 0.29). Interestingly, patients with buttressing had a lower stricture rate (0.8 vs. 8.1%, p = 0.006). Based on the findings of these trials, the utility of buttressing stapled colorectal anastomoses remains unclear.
Omental Flaps
The use of omental flaps is common in clinical practice. This technique involves wrapping or approximating a portion of well-vascularized omentum against an anastomosis. Though generally considered a relatively low-risk technique, clinical studies of omentoplasty in preventing colorectal anastomotic leaks are limited. A 1998 randomized study of 705 patients who underwent elective colorectal operations for benign and malignant conditions was performed in France. 39 The trial resulted in similar leak rates in patients who underwent omentoplasty compared with those who did not (4.7 vs. 5.2%) and the rates of reoperation for leaks were identical (1.7%). A subsequent study performed on 112 patients undergoing low anterior resection randomized patients to omentoplasty or not, and found a lower clinically detected leak rate in the omentoplasty arm for this higher risk anastomosis (3.1 vs. 10.9%, p < 0.05). 40 A third randomized trial also demonstrated a lower leak rate in patients randomized to omentoplasty (6.4 vs. 21.9%, p < 0.05). 41 However, this trial had an unusually high leak rate in the nonomentoplasty arm, likely due to the inclusion of emergency operations. A meta-analysis combining these trials demonstrated no differences in radiologic leak rates (risk ratio [RR] = 0.76, 95% confidence interval [CI]: 0.41–1.40), but did identify a decrease in clinical leaks (RR = 0.36, 95% CI: 0.16–0.78). 42 The authors noted heterogeneity between trials in defining a clinical leak which may account for this difference. Interestingly, a meta-analysis of esophagogastric anastomoses, which have similar leak rates to colorectal anastomoses, demonstrated a substantial benefit to omentoplasty in preventing radiologically evident leaks. 43 The reasons for these differences are unclear but may be related to devascularization that can occur when fashioning omentum to have sufficient length to reach the pelvis. The data on use of omentoplasty for protecting colorectal anastomoses is conflicting but appears to benefit high-risk anastomoses. Larger randomized trials evaluating this technique are needed to confirm this potential benefit.
Perfusion Assessment
Adequate perfusion has been long recognized as an essential aspect of successful anastomosis creation. The most straight-forward assessment of perfusion is evaluating bleeding after transection of the colon along with examination of the mucosa and serosa prior to performing the anastomosis. Despite ensuring adequate perfusion, anastomotic leaks persist. Fluorescent imaging has been developed to further improve on this visual assessment with the goal of further reducing leak rates.
The most commonly used fluorophore for vascular assessment is indocyanine green (ICG). This compound has been used for several decades in retinal angiography with only rare reports of allergic reactions and no toxicity. 44 The compound is ultimately excreted in bile, and has the advantage of not staining tissues if extravasated. Visualization does require specialized light sources and a camera system with near-infrared capabilities for optimal contrast against nonperfused tissues. Laparoscopic and robotic platforms often have these options available. After intravenous injection, ICG circulates through the vascular system in 2 to 3 minutes and washes out after 3 to 5 minutes. Repeat injections can be performed if needed. Once the bowel is perfused with ICG, both the colon and rectal stump can be assessed. Well-perfused bowel will fluoresce green on infrared imaging. A sharp demarcation between vascularized and devascularized bowel can be readily identified ( Fig. 2 ). Bowel mucosa can also be assessed with direct visualization prior to anastomosis creation, or more commonly afterward through rigid proctoscopy using specialized lighting.
Fig. 2.
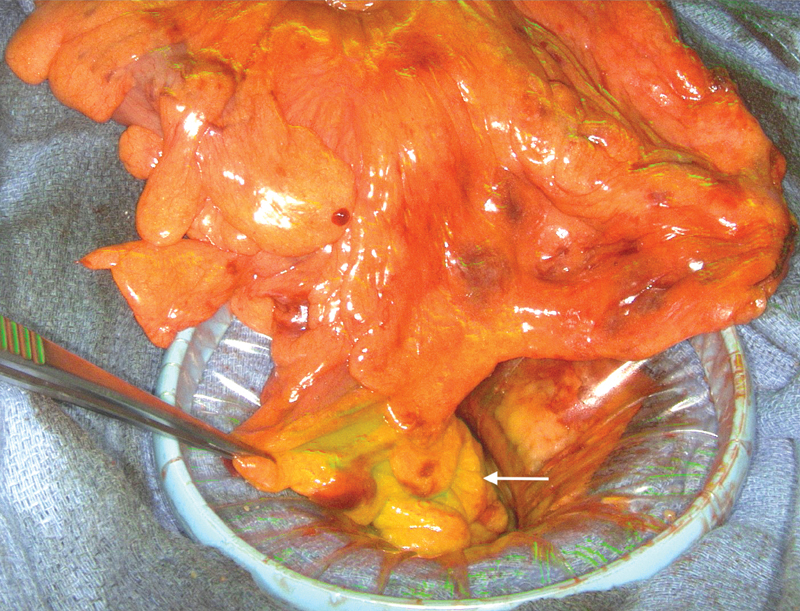
Intraoperative perfusion assessment. After intravenous injection of indocyanine green (ICG), perfused bowel will fluoresce under an appropriate light source (arrow).
Fluorescent imaging with ICG has been studied extensively in the clinical setting. A phase-II multicenter trial demonstrated the technique is logistically feasible and widely applicable for left-sided anastomoses. During this trial, 8% of operative plans were modified based on ICG perfusion assessment. None of these patients developed a leak, and an overall clinically detected leak rate of 1.4% was reported in the total population of 139 patients. 45 Smaller single-institution series noted similar rates of operative modifications based on ICG imaging and similarly low leak rates. A small series of 42 patients undergoing low anterior resections noted a 0% clinically detected leak rate, while the level of two patients' anastomoses was modified based on ICG findings. 46 This same group reported a 3.7% operative change rate and only one leak after 107 right- and left-sided anastomoses. 47 A recent meta-analysis of approximately 1,000 patients in five nonrandomized series, including the three mentioned above, identified a significantly lower leak rate for low anterior resections performed for rectal cancer when ICG imaging was utilized (1.1 vs. 6.1%, p = 0.02). 48 However, inclusion of colorectal operations for benign disease diluted this effect, and no statistically significant difference in leak rates were noted in the overall cohort (hazard ratio [HR] = 0.51, 95% CI: 0.23–1.13). Most recently, a multicenter randomized trial of 252 patients undergoing left-sided colon or rectal resections was performed in Italy. Additional colon resection due to inadequate perfusion occurred in 11% of the ICG arm, but the anastomotic leak rate was not statistically different (9% in control group and 5% in ICG group). 49 However, the study was significantly underpowered as the leak rate in the ICG group was higher than anticipated.
Perfusion assessment with ICG is an inexpensive, easily performed technique that may alter surgical plans to the benefit of patients. Further study with larger, well-powered randomized trials are needed to confirm its benefit in reducing leak rates. However, ICG imaging provides a clear benefit in situations where there is clinical concern for hypoperfusion and an additional confirmatory test is desired.
Intraluminal Sheaths
Though the above technologies focus on reinforcing or optimizing the anastomosis during its creation, an alternate family of devices that seek to protect the anastomosis and mitigate leaks has been explored. These devices center around an endoscopically deployed intraluminal sheath that is secured to the proximal bowel, extends across the anastomosis, and terminates in the distal bowel ( Fig. 3 ). Small studies in the 1980s used a nonbiodegradable sheath attached with Vicryl sutures demonstrated the feasibility of this technique, but larger trials were not conducted. 50 51 A biodegradable sheath was developed 30 years later, and small pilot studies demonstrated the safety of deploying an endoluminal sheath as part of a circular stapled anastomosis. 52 53 However, a subsequent randomized controlled trial involving 402 patients undergoing a colorectal anastomosis, primarily for cancer, showed no difference in leak rates on univariate analysis (10.4% with sheath vs. 5.0% without, p = 0.06). 54 The trial was thus terminated due to futility. Concerningly, a higher leak rate was noted in the experimental group after adjustment for other factors (odds ratio [OR] = 2.21, 95% CI: 1.01–4.82). In addition, 15.8% of sheath deployments encountered technical difficulties. At this time, no device is approved for use as an endoluminal sheath for protecting an anastomosis, although a large trial of a new device is currently underway.
Fig. 3.
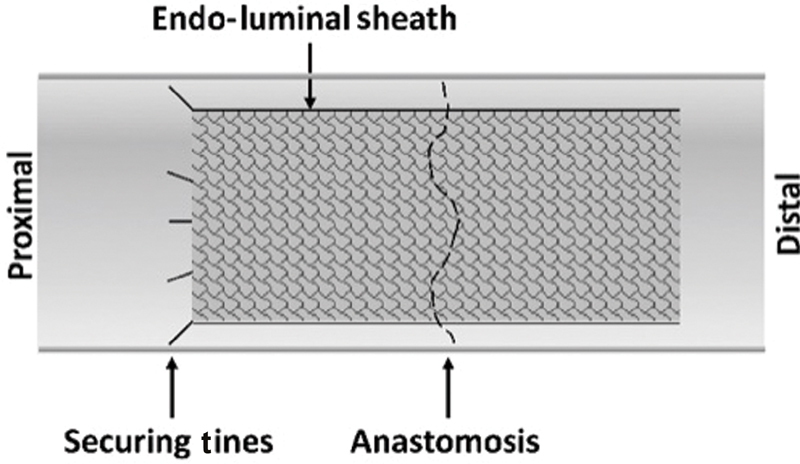
Intraluminal sheath covering an anastomosis. The sheath is secured with biodegradable tines or hooks that are expelled several weeks after surgery.
Conclusion
Multiple techniques to reduce the rate of anastomotic leaks after colorectal operations have been developed and researched over decades. Though preclinical experiments have produced promising results for several methods, and the materials involved are generally safely utilized in other settings, results have been mixed in large randomized clinical trials. Despite this, research is continuing into novel materials and methods that may reduce the morbidity of colorectal surgery.
Footnotes
Conflict of Interest None declared.
References
- 1.Litvinov R I, Weisel J W. What is the biological and clinical relevance of fibrin? Semin Thromb Hemost. 2016;42(04):333–343. doi: 10.1055/s-0036-1571342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urlesberger H, Rauchenwald K, Henning K. Fibrin adhesives in surgery of the renal parenchyma. Eur Urol. 1979;5(04):260–261. doi: 10.1159/000473125. [DOI] [PubMed] [Google Scholar]
- 3.Spotnitz W D. Fibrin sealant: the only approved hemostat, sealant, and adhesive-a laboratory and clinical perspective. ISRN Surg. 2014;2014:203943. doi: 10.1155/2014/203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oka H, Harrison R C, Burhenne H J. Effect of a biologic glue on the leakage rate of experimental rectal anastomoses. Am J Surg. 1982;143(05):561–564. doi: 10.1016/0002-9610(82)90162-3. [DOI] [PubMed] [Google Scholar]
- 5.Houston K A, Rotstein O D. Fibrin sealant in high-risk colonic anastomoses. Arch Surg. 1988;123(02):230–234. doi: 10.1001/archsurg.1988.01400260118015. [DOI] [PubMed] [Google Scholar]
- 6.van der Ham A C, Kort W J, Weijma I M, van den Ingh H F, Jeekel J. Effect of fibrin sealant on the healing colonic anastomosis in the rat. Br J Surg. 1991;78(01):49–53. doi: 10.1002/bjs.1800780117. [DOI] [PubMed] [Google Scholar]
- 7.van der Ham A C, Kort W J, Weijma I M, van den Ingh H F, Jeekel H. Healing of ischemic colonic anastomosis: fibrin sealant does not improve wound healing. Dis Colon Rectum. 1992;35(09):884–891. doi: 10.1007/BF02047878. [DOI] [PubMed] [Google Scholar]
- 8.Giuratrabocchetta S, Rinaldi M, Cuccia F. Protection of intestinal anastomosis with biological glues: an experimental randomized controlled trial. Tech Coloproctol. 2011;15(02):153–158. doi: 10.1007/s10151-010-0674-0. [DOI] [PubMed] [Google Scholar]
- 9.Wenger F A, Szucsik E, Hoinoiu B F, Cimpean A M, Ionac M, Raica M. Circular anastomotic experimental fibrin sealant protection in deep colorectal anastomosis in pigs in a randomized 9-day survival study. Int J Colorectal Dis. 2015;30(08):1029–1039. doi: 10.1007/s00384-015-2260-4. [DOI] [PubMed] [Google Scholar]
- 10.Wenger F A, Szucsik E, Hoinoiu B F. Is circular fibrin sealing of low rectal anastomosis able to prevent leakage in 21-day follow-up? randomized experimental trial in pigs. Surg Innov. 2019;26(04):408–419. doi: 10.1177/1553350619834786. [DOI] [PubMed] [Google Scholar]
- 11.Yol S, Tekin A, Yilmaz H. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008;146(02):190–194. doi: 10.1016/j.jss.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Vuocolo T, Haddad R, Edwards G A. A highly elastic and adhesive gelatin tissue sealant for gastrointestinal surgery and colon anastomosis. J Gastrointest Surg. 2012;16(04):744–752. doi: 10.1007/s11605-011-1771-8. [DOI] [PubMed] [Google Scholar]
- 13.Hoeppner J, Wassmuth B, Marjanovic G, Timme S, Hopt U T, Keck T. Anastomotic sealing by extracellular matrices (ECM) improves healing of colonic anastomoses in the critical early phase. J Gastrointest Surg. 2010;14(06):977–986. doi: 10.1007/s11605-010-1191-1. [DOI] [PubMed] [Google Scholar]
- 14.Saltzman B M, Jain A, Campbell K A. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(05):906–918. doi: 10.1016/j.arthro.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173(02):258–266. doi: 10.1016/j.jss.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Giusto G, Vercelli C, Iussich S, Tursi M, Perona G, Gandini M. Comparison of the effects of platelet-rich or growth factor-rich plasma on intestinal anastomosis healing in pigs. BMC Vet Res. 2017;13(01):188. doi: 10.1186/s12917-017-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slieker J C, Vakalopoulos K A, Komen N A, Jeekel J, Lange J F. Prevention of leakage by sealing colon anastomosis: experimental study in a mouse model. J Surg Res. 2013;184(02):819–824. doi: 10.1016/j.jss.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 18.McGuire A L, Yee J. Clinical outcomes of polymeric sealant use in pulmonary resection: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis. 2018;10 32:S3728–S3739. doi: 10.21037/jtd.2018.10.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pommergaard H C, Achiam M P, Rosenberg J. External coating of colonic anastomoses: a systematic review. Int J Colorectal Dis. 2012;27(10):1247–1258. doi: 10.1007/s00384-012-1547-y. [DOI] [PubMed] [Google Scholar]
- 20.Silecchia G, Boru C E, Mouiel J. The use of fibrin sealant to prevent major complications following laparoscopic gastric bypass: results of a multicenter, randomized trial. Surg Endosc. 2008;22(11):2492–2497. doi: 10.1007/s00464-008-9885-0. [DOI] [PubMed] [Google Scholar]
- 21.Lillemoe K D, Cameron J L, Kim M P.Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial J Gastrointest Surg 2004807766–772., discussion 772–774 [DOI] [PubMed] [Google Scholar]
- 22.Martin I, Au K. Does fibrin glue sealant decrease the rate of anastomotic leak after a pancreaticoduodenectomy? Results of a prospective randomized trial. HPB (Oxford) 2013;15(08):561–566. doi: 10.1111/hpb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Ren J, Zhu W, Li N, Li J. Fibrin sealant prevents gastrointestinal anastomosis dehiscence in intra-abdominal sepsis. Int Surg. 2007;92(01):27–31. [PubMed] [Google Scholar]
- 24.Huh J W, Kim H R, Kim Y J. Anastomotic leakage after laparoscopic resection of rectal cancer: the impact of fibrin glue. Am J Surg. 2010;199(04):435–441. doi: 10.1016/j.amjsurg.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Kim H J, Huh J W, Kim H R, Kim Y J. Oncologic impact of anastomotic leakage in rectal cancer surgery according to the use of fibrin glue: case-control study using propensity score matching method. Am J Surg. 2014;207(06):840–846. doi: 10.1016/j.amjsurg.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 26.Cooper J D. Technique to reduce air leaks after resection of emphysematous lung. Ann Thorac Surg. 1994;57(04):1038–1039. [PubMed] [Google Scholar]
- 27.Miller J I, Jr., Landreneau R J, Wright C E, Santucci T S, Sammons B H.A comparative study of buttressed versus nonbuttressed staple line in pulmonary resections Ann Thorac Surg 20017101319–322., discussion 323 [DOI] [PubMed] [Google Scholar]
- 28.Takamochi K, Oh S, Miyasaka Y. Prospective randomized trial comparing buttressed versus nonbuttressed stapling in patients undergoing pulmonary lobectomy. Thorac Cardiovasc Surg. 2014;62(08):696–704. doi: 10.1055/s-0033-1363295. [DOI] [PubMed] [Google Scholar]
- 29.Hagerman G F, Gaertner W B, Ruth G R, Potter M L, Karulf R E. Bovine pericardium buttress reinforces colorectal anastomoses in a canine model. Dis Colon Rectum. 2007;50(07):1053–1060. doi: 10.1007/s10350-007-0212-y. [DOI] [PubMed] [Google Scholar]
- 30.Gaertner W B, Hagerman G F, Potter M J, Karulf R E. Experimental evaluation of a bovine pericardium-derived collagen matrix buttress in ileocolic and colon anastomoses. J Biomed Mater Res B Appl Biomater. 2010;92(01):48–54. doi: 10.1002/jbm.b.31488. [DOI] [PubMed] [Google Scholar]
- 31.Fajardo A D, Chun J, Stewart D, Safar B, Fleshman J W. 1.5:1 meshed AlloDerm bolsters for stapled rectal anastomoses does not provide any advantage in anastomotic strength in a porcine model. Surg Innov. 2011;18(01):21–28. doi: 10.1177/1553350610370696. [DOI] [PubMed] [Google Scholar]
- 32.Stewart D, Perrone J, Pierce R. Evaluation of unmeshed and 1:1 meshed AlloDerm bolsters for stapled rectal anastomoses in a porcine model. J Laparoendosc Adv Surg Tech A. 2008;18(04):616–625. doi: 10.1089/lap.2007.0141. [DOI] [PubMed] [Google Scholar]
- 33.Ibele A, Garren M, Gould J. Effect of circular staple line buttressing material on gastrojejunostomy failure in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6(01):64–67. doi: 10.1016/j.soard.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Gagner M, Buchwald J N. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014;10(04):713–723. doi: 10.1016/j.soard.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Jones W B, Myers K M, Traxler L B, Bour E S.Clinical results using bioabsorbable staple line reinforcement for circular staplers Am Surg 20087406462–467., discussion 467–468 [PubMed] [Google Scholar]
- 36.Portillo G, Franklin M E., Jr Clinical results using bioabsorbable staple-line reinforcement for circular stapler in colorectal surgery: a multicenter study. J Laparoendosc Adv Surg Tech A. 2010;20(04):323–327. doi: 10.1089/lap.2009.0201. [DOI] [PubMed] [Google Scholar]
- 37.Placer C, Enríquez-Navascués J M, Elorza G. Preventing complications in colorectal anastomosis: results of a randomized controlled trial using bioabsorbable staple line reinforcement for circular stapler. Dis Colon Rectum. 2014;57(10):1195–1201. doi: 10.1097/DCR.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 38.Bioabsorbable Staple Line Reinforcement Study Group . Senagore A, Lane F R, Lee E. Bioabsorbable staple line reinforcement in restorative proctectomy and anterior resection: a randomized study. Dis Colon Rectum. 2014;57(03):324–330. doi: 10.1097/DCR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 39.French Associations for Surgical Research . Merad F, Hay J M, Fingerhut A, Flamant Y, Molkhou J M, Laborde Y. Omentoplasty in the prevention of anastomotic leakage after colonic or rectal resection: a prospective randomized study in 712 patients. Ann Surg. 1998;227(02):179–186. doi: 10.1097/00000658-199802000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tocchi A, Mazzoni G, Lepre L. Prospective evaluation of omentoplasty in preventing leakage of colorectal anastomosis. Dis Colon Rectum. 2000;43(07):951–955. doi: 10.1007/BF02237357. [DOI] [PubMed] [Google Scholar]
- 41.Agnifili A, Schietroma M, Carloni A. The value of omentoplasty in protecting colorectal anastomosis from leakage. A prospective randomized study in 126 patients. Hepatogastroenterology. 2004;51(60):1694–1697. [PubMed] [Google Scholar]
- 42.Hao X Y, Yang K H, Guo T K, Ma B, Tian J H, Li H L. Omentoplasty in the prevention of anastomotic leakage after colorectal resection: a meta-analysis. Int J Colorectal Dis. 2008;23(12):1159–1165. doi: 10.1007/s00384-008-0532-y. [DOI] [PubMed] [Google Scholar]
- 43.Wiggins T, Markar S R, Arya S, Hanna G B. Anastomotic reinforcement with omentoplasty following gastrointestinal anastomosis: a systematic review and meta-analysis. Surg Oncol. 2015;24(03):181–186. doi: 10.1016/j.suronc.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Alander J T, Kaartinen I, Laakso A. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jafari M D, Wexner S D, Martz J E. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220(01):82–920. doi: 10.1016/j.jamcollsurg.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc. 2017;31(04):1836–1840. doi: 10.1007/s00464-016-5181-6. [DOI] [PubMed] [Google Scholar]
- 47.Boni L, David G, Dionigi G, Rausei S, Cassinotti E, Fingerhut A. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc. 2016;30(07):2736–2742. doi: 10.1007/s00464-015-4540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanco-Colino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol. 2018;22(01):15–23. doi: 10.1007/s10151-017-1731-8. [DOI] [PubMed] [Google Scholar]
- 49.De Nardi P, Elmore U, Maggi G. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020;34(01):53–60. doi: 10.1007/s00464-019-06730-0. [DOI] [PubMed] [Google Scholar]
- 50.Ravo B. The Coloshield. Dis Colon Rectum. 1988;31(07):579–580. doi: 10.1007/BF02553741. [DOI] [PubMed] [Google Scholar]
- 51.Ravo B. Colorectal anastomotic healing and intracolonic bypass procedure. Surg Clin North Am. 1988;68(06):1267–1294. doi: 10.1016/s0039-6109(16)44686-4. [DOI] [PubMed] [Google Scholar]
- 52.Kolkert J L, Havenga K, ten Cate Hoedemaker H O, Zuidema J, Ploeg R J. Protection of stapled colorectal anastomoses with a biodegradable device: the C-Seal feasibility study. Am J Surg. 2011;201(06):754–758. doi: 10.1016/j.amjsurg.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 53.C-seal Study Group . Morks A N, Havenga K, ten Cate Hoedemaker H O, Leijtens J W, Ploeg R J. Thirty-seven patients treated with the C-seal: protection of stapled colorectal anastomoses with a biodegradable sheath. Int J Colorectal Dis. 2013;28(10):1433–1438. doi: 10.1007/s00384-013-1724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collaborative C-seal Study Group . Bakker I S, Morks A N, Ten Cate Hoedemaker H O. Randomized clinical trial of biodegradeable intraluminal sheath to prevent anastomotic leak after stapled colorectal anastomosis. Br J Surg. 2017;104(08):1010–1019. doi: 10.1002/bjs.10534. [DOI] [PubMed] [Google Scholar]


