Abstract
Anastomotic leaks after colorectal surgery is associated with increased morbidity and mortality. Understanding the impact of anastomotic leaks and their risk factors can help the surgeon avoid any modifiable pitfalls. The diagnosis of an anastomotic leak can be elusive but can be discerned by the patient's global clinical assessment, adjunctive laboratory data and radiological assessment. The use of inflammatory markers such as C-Reactive Protein and Procalcitonin have recently gained traction as harbingers for a leak. A CT scan and/or a water soluble contrast study can further elucidate the location and severity of a leak. Further intervention is then individualized on the spectrum of simple observation with resolution or surgical intervention.
Keywords: anastomosis, leak, complications, CT scan
Since the first modern description of the gastrointestinal anastomosis by Ramdohr in 1727, surgeons have continuously improved upon and refined anastomotic technique. Lembert's discovery of the importance of serosal apposition in 1826, Halstead's recognition in 1887 of the significance of submucosal collagen in anastomotic strength, improvements in suture technology, and the widespread use of surgical stapling devices have helped transform a morbid and risky surgical venture into an indispensable technique performed routinely in modern surgical therapy. 1 2 Yet, in spite of the advancements in surgical technique and perioperative care over the past 300 years, anastomotic leakage (AL) remains a dreaded complication and significant source of harm for patients undergoing abdominal surgery. For colorectal surgery patients, specifically, AL dramatically increases postoperative mortality, increases the risk for a permanent stoma, and leads to worse oncologic outcomes following resection for colorectal cancer. 3 4 5 6
Given the potential morbidity associated with anastomotic leaks in colon and rectal surgery, early detection of AL followed by timely intervention is of the utmost importance. This review aims to provide colorectal surgeons with the knowledge necessary to facilitate the early diagnosis of AL including the clinical and operative risk factors for AL, the typical clinical manifestations of AL, the diagnostic tools that are available to confirm the presence and severity of AL.
Patient Risk Factors for Anastomotic Leak
Prior to diagnosing an anastomotic leak, the patient risk factors for AL (as discussed in detail elsewhere in this edition) have to be reiterated to guide the surgeon's level of suspicion for AL given the individual at risk.
Location of Anastomosis
One of the most significant risk factor for AL is the location of the anastomosis. In general, the more distal the anastomosis, the higher the risk of AL with enteroenteric anastomoses having the lowest leak rates (1–2%), followed by ileocolic (1–4%), colocolic (2–3%), and ileorectal (3–7%) anastomoses. Extraperitoneal anastomoses including ileoanal pouch anastomoses (4–7%) and low colorectal and coloanal anastomoses (5–19%) have the highest leak rate. 7 Among high-risk low anastomoses, the odds of leak can be further stratified by distance from the anal verge. 8 9 10 11 12 13 For example, in their series of 296 patients undergoing low anterior resection (LAR), Hamabe and colleagues reported that anastomoses ≤7 cm from the anal verge had a significantly higher risk for AL compared with higher colorectal anastomoses (odds ratio = 3.8; p = 0.0039). 12
Male Sex
Several investigators have demonstrated increased rates of AL among male patients, especially among patients undergoing LAR. 12 14 15 16 17 18 In a prospective study of 395 patients undergoing laparoscopic LAR, Tanaka and colleagues demonstrated that male sex was associated with as high as a four-fold increase in the rate of AL. 19 While this sex-based difference in AL is hypothesized to result from the technical difficulties encountered when operating in the narrow, male pelvis, attempts to correlate objective measures of pelvic geometry with AL have generated mixed data. 20 21
Chronic Immunosuppression Medications
The use of chronic immunosuppression medications is associated with increased risk of AL, and this association has been most strongly established for patients being treated with corticosteroids in the perioperative period. 18 22 23 24 In a pooled analysis of 12 studies with a total of 9,564 patients undergoing colorectal surgery for a variety of diagnoses, Eriksen and colleagues demonstrated a doubling in the rate of AL from 3.26% in the noncorticosteroid group, to 6.77% among patients treated with corticosteroids in the perioperative period. 23 There is also a smaller body of evidence suggesting increased rates of AL among patients treated with medications frequently used to prevent rejection after solid organ transplantation, including tacrolimus, cyclosporine A, mycophenolate mofetil, and evrolimus. 25 26 27 28
There is significantly less data in the literature examining the risk of AL among patients treated with newer anticytokine biologic therapy commonly used in the treatment of inflammatory bowel disease. 29 30 Though this research is ongoing, a small, retrospective study has shown no significant increase in rates of AL among patients with Crohn's disease being treated with either infliximab or adalimumab and undergoing ileocolic resection. 31 32
Obesity
The association between obesity and AL in colorectal surgery is well established. 17 33 34 35 An early study by Benoist and colleges examined 584 consecutive patients undergoing colorectal surgery. In their cohort, obesity, defined as body mass index (BMI) >27 kg/m 2 , was an independent risk factor for AL among patients undergoing LAR with AL occurring in 16% in the obese group as compared with 7% of the nonobese group ( p < 0.05). 33 Kiran et al similarly demonstrated that obesity is also a risk factor for AL among patients undergoing ileopouch anal anastomosis (IPAA). Among their cohort of over 2,000 patients, 10.4% of patients with BMI >30 kg/m 2 suffered AL compared with 5.4% of patients with BMI <30 kg/m 2 ( p < 0.001). 34 Biondo and colleagues investigated a cohort of 208 patients who underwent left colonic resection and primary anastomosis for distal colonic emergencies. In this cohort, the authors also demonstrated that obese patients were significantly more likely to experience AL (odds ratio [OR] = 9, p = 0.016). 35 Though the precise mechanism for the increased rate of AL in obese patients is unclear, the increase risk might be explained by technical failure of the anastomosis due to tension and ischemia caused by a short, thick mesentery and increase visceral adiposity.
Neoadjuvant Therapy in Rectal Cancer
Current treatment recommendations for patients with locally advanced rectal cancer typically involves neoadjuvant radiotherapy with or without chemotherapy followed by total mesorectal excision (TME). 36 37 While neoadjuvant therapy has been shown to decrease rates of local recurrence, the detrimental effect of chemotherapy and radiation on wound healing has led to concerns that this treatment strategy is associated with increased rates of AL. The data implicating neoadjuvant radiotherapy, with or without chemotherapy, as a risk factor for AL is mixed, however. Several large retrospective studies have demonstrated increased risk for AL after LAR among patients treated with neoadjuvant chemoradiotherapy or radiotherapy alone after multivariate analysis. 38 39 40 Other authors have shown no increased risk for anastomotic leak, including a meta-analysis randomized controlled trials (RCTs) performed by Qin and colleagues. 41 42 43 In their study of seven RCTs which included 3,375 patients treated with either neoadjuvant radiotherapy or neoadjuvant chemoradiotherapy prior to LAR, the authors showed no increased risk of AL on subgroup analysis though their results may be confounded by variations in tumor location, definition of AL, and the use of diverting ostomy among the studies. 43
Given the heterogeneity in the data surrounding neoadjuvant (chemo)radiotherapy, many authors argue that proximal diversion should be considered in these patients.
Medical Comorbidity
Several retrospective and prospective studies have demonstrated a correlation between increased rates of AL and a patient's overall burden of medical comorbidities as defined by the American Society of Anesthesiologists physical status classification system (ASA). 17 44 45 46 47 As an example, a meta-analysis performed by Qu and colleagues included 14 prospective and retrospective studies totaling 4,580 patients undergoing LAR and demonstrated significant increased risk of AL among patients with ASA > 2 (OR = 1.75, p = 0.04). 17 Similar results were demonstrated in a review of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) performed by Rencuzogullari et al that included 10,392 patients over the age of 65 years undergoing colectomy at different levels for a variety of diagnoses. The authors similarly demonstrated a significant association between ASA score > 2 and AL in this cohort. 46
In terms of specific comorbid diseases that confer increased risk of AL, diabetes mellitus and cardiovascular disease have been identified to be independent risk factors for AL by several studies. 45 48 49 50 51 Given the effect of diabetes mellitus and cardiovascular disease on the vasculature, it follows that these comorbidities negatively impact the microcirculation required for a healthy anastomosis.
Smoking
Smoking is not only an independent risk factor for the development of colorectal cancer and inflammatory bowel disease, two of the common indications for colorectal resection, but it has also been demonstrated to be an independent risk factor for AL in several studies. 18 22 45 46 52 53 54 55 In a series of 246 patients undergoing left hemicolectomy for various indications, Baucom et al demonstrated a 17% rate of AL among smokers compared with 5% in nonsmoker, and smoking remained strongly associated with AL after multivariate analysis (OR = 4.2, p = 0.02). 53 A similar association with smoking and increased risk for AL was demonstrated by Kwak and colleagues in a series of 423 patients undergoing right hemicolectomy for cancer (OR = 6.592, p = 0.007). 55
The relationship between smoking an AL is likely explained by the reduction in tissue perfusion and oxygenation, the impairment of inflammatory cell functions, and the decreased synthesis and deposition of collagen by fibroblasts that have all been demonstrated to be effects of smoking. 56
Malnutrition
Malnutrition is a well-established risk factor for complications after major abdominal surgery. 57 58 59 60 Among colorectal surgery patients, hypoalbuminemia, used as a surrogate marker for malnutrition, has been demonstrated to be an independent risk factor for AL. 45 61 62 In a review of the 2013 NSQIP database by Parthasarathy et al, which included 17,518 patients undergoing colorectal resection with anastomosis, demonstrated serum albumin >4 g/dL to be independently correlated to anastomotic leak on multivariate analysis (OR = 1.32 p = 0.03). 45 Similar results were demonstrated by Suding and colleagues in their series of 672 patients undergoing open colorectal resection. The authors demonstrated that a baseline albumin level of less than 3.5 g/dL was associated with anastomotic leaks in both univariate and multivariate analyses (OR = 2.56 p = 0.4). 61
Emergency Surgery
Large retrospective studies have demonstrated emergency surgery to be an independent predictor of AL. 17 63 64 This is likely explained by common confounders that occur in the setting of emergency colorectal surgery that are not always controlled for in multivariate analysis, including fecal contamination of the abdomen, pre- or postoperative hypotension, and overwhelming systemic inflammatory response. 46 65 66
Diagnosis of Anastomotic Leakage
Clinical Diagnosis
Clinical signs and symptoms of anastomotic leak include common, nonspecific markers of intra-abdominal inflammation and infection such as increasing abdominal pain, tachycardia, fever, tachypnea, and ileus. If an intraperitoneal drain has been placed, enteric, feculent, or purulent drain output is frequently noted. Severe cases can also involve evidence of end-organ dysfunction including low urine output, and mental status changes. Laboratory markers of systemic inflammation are also frequently elevated, including increased white blood cell count with left shift. Severe AL may also result in severe sepsis and end-organ dysfunction which can manifest as altered mental status, elevated liver function test, and low urine output accompanied by markers of decreased renal clearance including elevated blood urea nitrogen and serum creatinine.
C-Reactive Protein and Serum Procalcitonin
There has been significant interest in other biomarkers of inflammation as screening tests for AL, namely, C-reactive protein (CRP) and serum procalcitonin (PCT). As acute phase reactants, the circulating concentrations of CRP and PCT rise rapidly and extensively in a cytokine-mediated response to tissue injury, infection, and inflammation.
CRP, which is predominantly produced and secreted by hepatocytes, is thought to promote the recognition and elimination of pathogens and enhance the clearance of necrotic and apoptotic cells. 67 68 Baseline serum CRP levels typically range from 3 to 10 mg/L in healthy patients. 69 CRP has a half-life of approximately 19 hours, begins to rise 12 to 24 hours after an inciting stimulus, and peaks within 2 to 3 days. 70
PCT is normally produced by the parafollicular cells of the thyroid gland as a precursor to the calcium homeostasis hormone calcitonin. As PCT is converted to calcitonin prior to secretion from the thyroid in healthy individuals, serum PCT levels are typically below the level of detection for most laboratories (<0.15 ng/mL) in the absence of infection. 71 In the setting of systemic inflammation, and in particular bacterial infection, animal models have demonstrated a dramatic expansion of PCT expression with dramatically elevated levels of PCT mRNA detected in spleen, kidney, adipocytes, pancreas, colon, and brain. Compared with CRP, serum PCT levels rise more rapidly, becoming elevated between 2 and 6 hours, and peak within 6 to 24 hours following an inciting stimulus. 71
C-Reactive Protein in the Diagnosis of Anastomotic Leakage
An early prospective study by Garcia-Granero and colleagues demonstrated the utility of CRP in ruling out AL among their cohort of 205 undergoing elective colorectal surgery. Similar to previous studies, the authors demonstrated that serum CRP concentrations significantly increased in all patients immediately after surgery but normalized by postoperative day (POD) 3 in patients without complications. Among their cohort, 17 (8.3%) patients were diagnosed with AL, 11 (5.4%) of which were classified as “major,” requiring drainage or surgical intervention. Using area under the cure analysis, the authors were able to establish CRP cut-offs of 147 mg/L on POD 3 and 135 mg/L on POD 5 (99 and 98% negative-predictive values, respectively) among those patients with major AL. Most importantly, the majority of major AL in the cohort was diagnosed by clinical examination or imaging after POD 5 (mean time to diagnosis = POD 7.5), suggesting that the detection of AL using CRP may precede the clinical or radiologic manifestations of major leak, potentially shortening the time to intervention. 72
Similar results were demonstrated in a 2014 meta-analysis of seven studies investigating the role of CRP in the exclusion of AL following colorectal surgery. In their pooled analysis of 2,483 patients, the authors established CRP cut-off values of 172 mg/L on POD 3, 124 mg/L on POD 4 and 144 mg/L on POD 5 each with corresponding negative predictive values of 97%. Unsurprisingly, given CRP is a nonspecific marker of inflammation, the positive predictive values of the cut-offs that they established were low, ranging between 21 and 23%. 73
Procalcitonin in the Diagnosis of Anastomotic Leakage
One of the largest studies evaluating the use of PCT in the exclusion of AL comes from Giaccaglia and the authors of PREDICS study. In their cohort of 504 patients undergoing elective colorectal surgery for cancer of which 75% of cases were laparoscopic, 28 (5.6%) patients were diagnosed with AL. The authors established PCT cut-offs of 2.7 ng/mL on POD 3 and 2.3 ng/mL on POD 5 with negative predictive values of 96.9 and 98.3%, respectively. 74 A 2018 meta-analysis of eight studies by Tan and colleagues further demonstrates the utility of PCT in excluding AL in colorectal surgery. In their pooled analysis of eight studies including 1,629 patients, the authors were able to further refine PCT cut-off values obtaining 1.45 ng/mL on POD 3, 1.28 ng/mL on POD 4, and 1.26 ng/mL on POD 5 using area under the curve analysis. The author demonstrated that PCT had the highest diagnostic capability on POD 5 with diagnostic OR of 32.9 (95% confidence interval [CI]: 15.01–69.88), sensitivity of 0.78 (95% CI: 0.65–0.89), and specificity of 0.88 (95% CI: 0.85–0.90). 75
Most of the literature to date examining the use of CRP and PCT in the diagnosis of AL has demonstrated that the value of these tests lies in their ability to rule out AL with a high degree of certainty within the first 5 PODs, and many authors maintain that the diagnostic accuracy of these tests is further improved when they are used in conjunction rather than individually. 72 74 76 77 78 79 80 81 82 83 The ability to rule out AL at an early stage, even before they become clinically relevant, is becoming increasingly important in the era of enhanced recovery after surgery (ERAS) protocols that are designed to speed hospital discharge (ERAS citation). Screening patients prior to discharge with both CRP and PCT can help facilitate application of ERAS protocols by identifying those patients who may require extended observation or imaging studies prior to discharge.
Though there has been no official consensus or society guidelines recommending specific CRP or PCT thresholds to help rule out AL after colorectal surgery, the values obtained by the meta-analyses detailed above can be used with reasonable confidence to help guide the safe discharge of patients postoperatively.
Radiologic Diagnosis of Anastomotic Leakage
Imaging technique for identification of an AL depends on the location of the anastomosis in the gastrointestinal (GI) tract. The most common initial imaging after bowel surgery for evaluation of postsurgical complications is computed tomography (CT) with or without intravenous (IV) contrast medium. 84 Oral or rectal contrast (preferably water soluble) may be administered depending on the type and location of surgery. It is extremely important for the reading radiologist to be knowledgeable of the type of surgical technique and postoperative altered surgical anatomy. This is extremely vital to avoid over call of complications and also not to miss complications. CT imaging findings may be definitive of anastomotic leak, but additional imaging with water soluble contrast enema or small bowel follow through may be necessary to confirm the leak. 85
Lower Anterior Resection
There is a wide spectrum of imaging findings on CT imaging in the immediate postoperative follow-up. Presacral soft tissue thickening with or without small foci air is common in the immediate postoperative period and are not indicative of a clinically significant anastomotic leak. 86 87 Presence of perianastomotic free air, fluid collections, or both should raise concern for anastomotic leak. A water-soluble enema under fluoroscopy or CT of pelvis with water soluble contrast enema can confirm the presence of leak. It is important not to mistake intraluminal air and fluid in the blind loop of the colon at the anastomosis tethered to the presacral soft tissues as anastomotic leak. 88 Careful imaging with appropriate fluoroscopic spot imaging or multiplanar reconstruction of CT images would be useful in differentiating extra luminal contrast from contrast within a blind limb of the colon ( Fig. 1 ). Presacral air and fluid collections from anastomotic leak can extend extraperitoneally along the iliac vessels. Fluid collections can resolve, but soft-tissue inflammatory changes in the presacral space can persist for months and even years after surgery. 87 These may present as presacral mass, difficult to differentiate from local recurrence of malignancy, and may be needed additional imaging with magnetic resonance imaging (MRI) and fluorodeoxyglucose–positron emission tomography (FDG-PET) imaging ( Fig. 2 ).
Fig. 1.

CT scan and fluoroscopy with extra-luminal contrast via anastomotic leak (arrows).
Fig 2.

CT scan ( A ) magnetic resonance imaging ( B & C ) and fluorodeoxyglucose-positron emission tomography (FDG-PET) imaging ( D ) used to distinguish persistent leak from tumor recurrence or inflammatory changes.
Entero-Enteric, Ileo-Colic, and Colo-Colic Anastomotic Leaks
CT of the abdomen and pelvis has become the imaging modality of choice for suspected leaks in the immediate postoperative period. CT with IV contrast and without positive oral contrast is most often performed. Free intraperitoneal fluid and air are common in the immediate postoperative period which have high sensitivity but low specificity for AL, as there is overlap between patients with and without clinically important leak. Perianastomotic fat stranding and gas and fluid collections have high sensitivity for leak ( Fig. 3 ). 85 89 Mural edema of the bowel, decreased mural enhancement, and obstruction at the anastomosis are more specific but less commonly seen imaging signs for an AL ( Fig. 3 ). 85 Documentation of extravasation of contrast form the bowel anastomosis on a CT with intraluminal positive contrast is the most specific sign of a clinically significant AL ( Figs. 4 and 5 ). 85 In the CT protocol with positive intraluminal contrast, it is important to see that contrast reaches the bowel anastomosis site. The main disadvantage with positive intraluminal contrast is that the bowel wall enhancement and possible ischemia cannot be assessed. When CT findings are inconclusive in the context of a strong clinical suspicion, fluoroscopic water soluble enema are confirmatory for colo-colic and ileo-colic AL ( Fig. 5 ). A single-center retrospective case control study for bowel AL has sensitivity of 64.9% for CT without intraluminal contrast and increased to a sensitivity of 74.3% for CT with positive intraluminal contrast. 85 CT is also useful to assess more distant peritoneal collections and guidance for percutaneous abscess drainage. Patients with inflammatory bowel disease, on immunosuppressive therapy or anti–vascular endothelial growth factor (VEGF) agents, such as bevacizumab (Avastin), are at high risk for postoperative anastomotic leaks with complex collections and fistulas, sometimes involving multiple bowel segments due to poor wound healing ( Fig. 6 ). 90 91 92
Fig. 3.
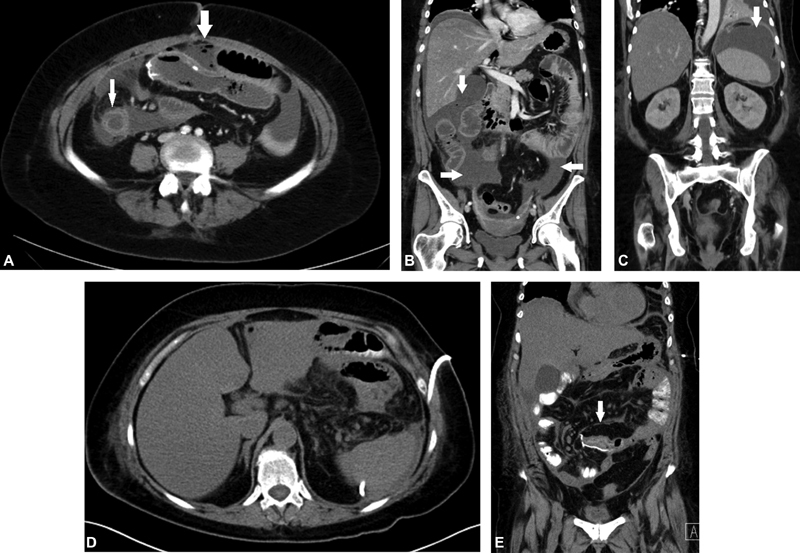
CT scans with perianastomotic fat stranding ( A ) and gas and fluid collections ( B & C ) have high sensitivity for leak; mural edema of the bowel ( D & E ).
Fig. 4.

CT scans with contrast extravasation outside the bowel lumen (arrows).
Fig. 5.
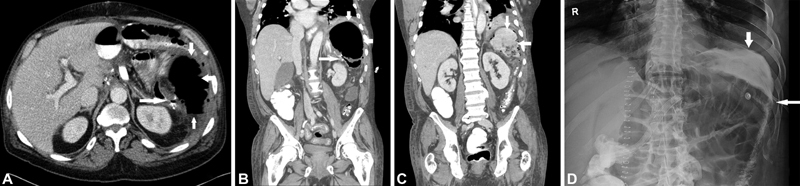
CT scan and fluoroscopy indicating contrast extravasation and AL.
Fig. 6.

CT scan and fluoroscopy indicating complex collections and fistulas (arrows) in delayed AL.
Conclusion
Anastomotic leak is a dreaded but unfortunate reality in the specialty of colorectal surgery. There are many clinical and operative risk factors for anastomotic leak and the surgeon must exercise astute clinical judgement to mitigate those who are modifiable and optimize the patient for surgery when possible. In the event of a suspected leak, a surgeon must maintain a high clinical suspicion in the setting of an acute change in the clinical picture as demonstrated by instability of vital signs, change in physical examination, and possibly the change in the character of intraoperative drains. An increased lactic acidosis or leukocytosis along with other laboratory derangements may be seen in the acute phases of AL. An elevated CRP and PCT levels may be used as an adjunctive to diagnosis AL but neither should be relied on solely apart from the overall clinical picture. A CT scan has become the standard bearer for diagnosis along with patient's global clinical assessment. Based on the location of the anastomosis, water-soluble contrast via oral/nasogastric tube or with an enema is a preferred adjunct to aid in the diagnosis. Finally, the treatment for an anastomotic leak is individualized to the location of the anastomosis, the level of containment of the contamination and stability of the patient. Interventions may be as minimal as observation with antibiotics or percutaneous drainage or to the extreme of immediate surgical intervention with source control and likely fecal diversion with or without addressing the anastomotic defect.
Footnotes
Conflict of Interest None declared.
References
- 1.Dietz U A, Debus E-S. Intestinal anastomoses prior to 1882; a legacy of ingenuity, persistence, and research form a foundation for modern gastrointestinal surgery. World J Surg. 2005;29(03):396–401. doi: 10.1007/s00268-004-7720-x. [DOI] [PubMed] [Google Scholar]
- 2.Hardy K J. Bowel surgery: some 18th and 19th century experience. Aust N Z J Surg. 1988;58(04):335–338. doi: 10.1111/j.1445-2197.1988.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 3.Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum. 2011;54(01):41–47. doi: 10.1007/DCR.0b013e3181fd2948. [DOI] [PubMed] [Google Scholar]
- 4.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(05):890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 5.Nordholm-Carstensen A, Rolff H C, Krarup P-M. Differential impact of anastomotic leak in patients with stage IV colonic or rectal cancer: a nationwide cohort study. Dis Colon Rectum. 2017;60(05):497–507. doi: 10.1097/DCR.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 6.Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2017;32(04):549–556. doi: 10.1007/s00384-016-2744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott F D, Heeney A, Kelly M E, Steele R J, Carlson G L, Winter D C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102(05):462–479. doi: 10.1002/bjs.9697. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Wu Z, Wu Y. Laparoscopic surgery may decrease the risk of clinical anastomotic leakage and a nomogram to predict anastomotic leakage after anterior resection for rectal cancer. Int J Colorectal Dis. 2019;34(02):319–328. doi: 10.1007/s00384-018-3199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Lou Z, Liu Q. Multicenter analysis of risk factors for anastomotic leakage after middle and low rectal cancer resection without diverting stoma: a retrospective study of 319 consecutive patients. Int J Colorectal Dis. 2017;32(10):1431–1437. doi: 10.1007/s00384-017-2875-8. [DOI] [PubMed] [Google Scholar]
- 10.Vignali A, Fazio V W, Lavery I C. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg. 1997;185(02):105–113. doi: 10.1016/s1072-7515(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 11.Pakkastie T E, Luukkonen P E, Järvinen H J.Anastomotic leakage after anterior resection of the rectum Eur J Surg 199416005293–297., discussion 299–300 [PubMed] [Google Scholar]
- 12.Hamabe A, Ito M, Nishigori H, Nishizawa Y, Sasaki T. Preventive effect of diverting stoma on anastomotic leakage after laparoscopic low anterior resection with double stapling technique reconstruction applied based on risk stratification. Asian J Endosc Surg. 2018;11(03):220–226. doi: 10.1111/ases.12439. [DOI] [PubMed] [Google Scholar]
- 13.Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007;9(01):71–79. doi: 10.1111/j.1463-1318.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 14.Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc. 2011;25(09):2972–2979. doi: 10.1007/s00464-011-1655-8. [DOI] [PubMed] [Google Scholar]
- 15.Park J S, Choi G S, Kim S H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257(04):665–671. doi: 10.1097/SLA.0b013e31827b8ed9. [DOI] [PubMed] [Google Scholar]
- 16.Kim S H, Park I J, Joh Y G, Hahn K Y. Laparoscopic resection of rectal cancer: a comparison of surgical and oncologic outcomes between extraperitoneal and intraperitoneal disease locations. Dis Colon Rectum. 2008;51(06):844–851. doi: 10.1007/s10350-008-9256-x. [DOI] [PubMed] [Google Scholar]
- 17.Qu H, Liu Y, Bi D S. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015;29(12):3608–3617. doi: 10.1007/s00464-015-4117-x. [DOI] [PubMed] [Google Scholar]
- 18.Midura E F, Hanseman D, Davis B R. Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum. 2015;58(03):333–338. doi: 10.1097/DCR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Okuda J, Yamamoto S. Risk factors for anastomotic leakage after laparoscopic surgery with the double stapling technique for stage 0/I rectal carcinoma: a subgroup analysis of a multicenter, single-arm phase II trial. Surg Today. 2017;47(10):1215–1222. doi: 10.1007/s00595-017-1496-8. [DOI] [PubMed] [Google Scholar]
- 20.Tsuruta A, Tashiro J, Ishii T. Prediction of anastomotic leakage after laparoscopic low anterior resection in male rectal cancer by pelvic measurement in magnetic resonance imaging. Surg Laparosc Endosc Percutan Tech. 2017;27(01):54–59. doi: 10.1097/SLE.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zur Hausen G, Gröne J, Kaufmann D. Influence of pelvic volume on surgical outcome after low anterior resection for rectal cancer. Int J Colorectal Dis. 2017;32(08):1125–1135. doi: 10.1007/s00384-017-2793-9. [DOI] [PubMed] [Google Scholar]
- 22.Nikolian V C, Kamdar N S, Regenbogen S E. Anastomotic leak after colorectal resection: a population-based study of risk factors and hospital variation. Surgery. 2017;161(06):1619–1627. doi: 10.1016/j.surg.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksen T F, Lassen C B, Gögenur I. Treatment with corticosteroids and the risk of anastomotic leakage following lower gastrointestinal surgery: a literature survey. Colorectal Dis. 2014;16(05):O154–O160. doi: 10.1111/codi.12490. [DOI] [PubMed] [Google Scholar]
- 24.Slieker J C, Komen N, Mannaerts G H. Long-term and perioperative corticosteroids in anastomotic leakage: a prospective study of 259 left-sided colorectal anastomoses. Arch Surg. 2012;147(05):447–452. doi: 10.1001/archsurg.2011.1690. [DOI] [PubMed] [Google Scholar]
- 25.Zeeh J, Inglin R, Baumann G. Mycophenolate mofetil impairs healing of left-sided colon anastomoses. Transplantation. 2001;71(10):1429–1435. doi: 10.1097/00007890-200105270-00013. [DOI] [PubMed] [Google Scholar]
- 26.Petri J B, Schurk S, Gebauer S, Haustein U F. Cyclosporine A delays wound healing and apoptosis and suppresses activin beta-A expression in rats. Eur J Dermatol. 1998;8(02):104–113. [PubMed] [Google Scholar]
- 27.Schäffer M R, Fuchs N, Proksch B, Bongartz M, Beiter T, Becker H D. Tacrolimus impairs wound healing: a possible role of decreased nitric oxide synthesis. Transplantation. 1998;65(06):813–818. doi: 10.1097/00007890-199803270-00008. [DOI] [PubMed] [Google Scholar]
- 28.van der Vliet J A, Willems M CM, de Man B M, Lomme R MLM, Hendriks T. Everolimus interferes with healing of experimental intestinal anastomoses. Transplantation. 2006;82(11):1477–1483. doi: 10.1097/01.tp.0000246078.09845.9c. [DOI] [PubMed] [Google Scholar]
- 29.Belgian Inflammatory Bowel Disease Research Group North-Holland Gut Club D'Haens G, Baert F, van Assche G.Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial Lancet 2008371(9613):660–667. [DOI] [PubMed] [Google Scholar]
- 30.REACT Study Investigators Khanna R, Bressler B, Levesque B G.Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial Lancet 2015386(10006):1825–1834. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Spinelli A, Suzuki Y. Risk factors for complications after ileocolonic resection for Crohn's disease with a major focus on the impact of preoperative immunosuppressive and biologic therapy: a retrospective international multicentre study. United European Gastroenterol J. 2016;4(06):784–793. doi: 10.1177/2050640615600116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W, Tang Y, Nong L, Sun Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn's disease: A meta-analysis of observational studies. J Crohn's Colitis. 2015;9(03):293–301. doi: 10.1093/ecco-jcc/jju028. [DOI] [PubMed] [Google Scholar]
- 33.Benoist S, Panis Y, Alves A, Valleur P. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179(04):275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 34.Kiran R P, Remzi F H, Fazio V W. Complications and functional results after ileoanal pouch formation in obese patients. J Gastrointest Surg. 2008;12(04):668–674. doi: 10.1007/s11605-008-0465-3. [DOI] [PubMed] [Google Scholar]
- 35.Biondo S, Parés D, Kreisler E. Anastomotic dehiscence after resection and primary anastomosis in left-sided colonic emergencies. Dis Colon Rectum. 2005;48(12):2272–2280. doi: 10.1007/s10350-005-0159-9. [DOI] [PubMed] [Google Scholar]
- 36.EORTC Radiotherapy Group Trial 22921 . Bosset J-F, Collette L, Calais G. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 37.Sebag-Montefiore D, Stephens R J, Steele R.Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial Lancet 2009373(9666):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Q, Ma T, Deng Y. Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum. 2016;59(10):934–942. doi: 10.1097/DCR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 39.Park J S, Huh J W, Park Y A. Risk factors of anastomotic leakage and long-term survival after colorectal surgery. Medicine (Baltimore) 2016;95(08):e2890. doi: 10.1097/MD.0000000000002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthiessen P, Hallböök O, Andersson M, Rutegård J, Sjödahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004;6(06):462–469. doi: 10.1111/j.1463-1318.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 41.Hu M-H, Huang R-K, Zhao R-S, Yang K-L, Wang H. Does neoadjuvant therapy increase the incidence of anastomotic leakage after anterior resection for mid and low rectal cancer? A systematic review and meta-analysis. Colorectal Dis. 2017;19(01):16–26. doi: 10.1111/codi.13424. [DOI] [PubMed] [Google Scholar]
- 42.Chang J S, Keum K C, Kim N K. Preoperative chemoradiotherapy effects on anastomotic leakage after rectal cancer resection: a propensity score matching analysis. Ann Surg. 2014;259(03):516–521. doi: 10.1097/SLA.0b013e31829068c5. [DOI] [PubMed] [Google Scholar]
- 43.Qin C, Ren X, Xu K, Chen Z, He Y, Song X. Does preoperative radio(chemo)therapy increase anastomotic leakage in rectal cancer surgery? A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2014;2014:910956. doi: 10.1155/2014/910956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park J-H, Kim D-H, Kim B-R, Kim Y-W. The American Society of Anesthesiologists score influences on postoperative complications and total hospital charges after laparoscopic colorectal cancer surgery. Medicine (Baltimore) 2018;97(18):e0653. doi: 10.1097/MD.0000000000010653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis. 2017;19(03):288–298. doi: 10.1111/codi.13476. [DOI] [PubMed] [Google Scholar]
- 46.Rencuzogullari A, Benlice C, Valente M, Abbas M A, Remzi F H, Gorgun E. Predictors of anastomotic leak in elderly patients after colectomy: nomogram-based assessment from the American College of Surgeons National Surgical Quality Program procedure-targeted cohort. Dis Colon Rectum. 2017;60(05):527–536. doi: 10.1097/DCR.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 47.Tan W P, Talbott V A, Leong Q Q, Isenberg G A, Goldstein S D. American Society of Anesthesiologists class and Charlson's comorbidity index as predictors of postoperative colorectal anastomotic leak: a single-institution experience. J Surg Res. 2013;184(01):115–119. doi: 10.1016/j.jss.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 48.Shinji S, Ueda Y, Yamada T. Male sex and history of ischemic heart disease are major risk factors for anastomotic leakage after laparoscopic anterior resection in patients with rectal cancer. BMC Gastroenterol. 2018;18(01):117. doi: 10.1186/s12876-018-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cong Z J, Fu C G, Wang H T, Liu L J, Zhang W, Wang H. Influencing factors of symptomatic anastomotic leakage after anterior resection of the rectum for cancer. World J Surg. 2009;33(06):1292–1297. doi: 10.1007/s00268-009-0008-4. [DOI] [PubMed] [Google Scholar]
- 50.Volk A, Kersting S, Held H C, Saeger H D. Risk factors for morbidity and mortality after single-layer continuous suture for ileocolonic anastomosis. Int J Colorectal Dis. 2011;26(03):321–327. doi: 10.1007/s00384-010-1040-4. [DOI] [PubMed] [Google Scholar]
- 51.Warschkow R, Steffen T, Thierbach J, Bruckner T, Lange J, Tarantino I. Risk factors for anastomotic leakage after rectal cancer resection and reconstruction with colorectostomy. A retrospective study with bootstrap analysis. Ann Surg Oncol. 2011;18(10):2772–2782. doi: 10.1245/s10434-011-1696-1. [DOI] [PubMed] [Google Scholar]
- 52.Alberg A J, Shopland D R, Cummings K M. The 2014 Surgeon General's report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179(04):403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baucom R B, Poulose B K, Herline A J, Muldoon R L, Cone M M, Geiger T M. Smoking as dominant risk factor for anastomotic leak after left colon resection. Am J Surg. 2015;210(01):1–5. doi: 10.1016/j.amjsurg.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 54.Sørensen L T, Jørgensen T, Kirkeby L T, Skovdal J, Vennits B, Wille-Jørgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86(07):927–931. doi: 10.1046/j.1365-2168.1999.01165.x. [DOI] [PubMed] [Google Scholar]
- 55.Kwak H D, Kim S H, Kang D W, Baek S J, Kwak J M, Kim J. Risk factors and oncologic outcomes of anastomosis leakage after laparoscopic right colectomy. Surg Laparosc Endosc Percutan Tech. 2017;27(06):440–444. doi: 10.1097/SLE.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 56.Sørensen L T. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255(06):1069–1079. doi: 10.1097/SLA.0b013e31824f632d. [DOI] [PubMed] [Google Scholar]
- 57.Sungurtekin H, Sungurtekin U, Balci C, Zencir M, Erdem E. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr. 2004;23(03):227–232. doi: 10.1080/07315724.2004.10719365. [DOI] [PubMed] [Google Scholar]
- 58.Schiesser M, Müller S, Kirchhoff P, Breitenstein S, Schäfer M, Clavien P A. Assessment of a novel screening score for nutritional risk in predicting complications in gastro-intestinal surgery. Clin Nutr. 2008;27(04):565–570. doi: 10.1016/j.clnu.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Mullen J L, Gertner M H, Buzby G P, Goodhart G L, Rosato E F. Implications of malnutrition in the surgical patient. Arch Surg. 1979;114(02):121–125. doi: 10.1001/archsurg.1979.01370260011001. [DOI] [PubMed] [Google Scholar]
- 60.Montomoli J, Erichsen R, Antonsen S, Nilsson T, Sørensen H T. Impact of preoperative serum albumin on 30-day mortality following surgery for colorectal cancer: a population-based cohort study. BMJ Open Gastroenterol. 2015;2(01):e000047. doi: 10.1136/bmjgast-2015-000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suding P, Jensen E, Abramson M A, Itani K, Wilson S E.Definitive risk factors for anastomotic leaks in elective open colorectal resection Arch Surg 200814309907–911., discussion 911–912 [DOI] [PubMed] [Google Scholar]
- 62.MD S, Chase S, Roopavathana S B, Nadarajan A R, Nayak S. Serum prealbumin, a novel indicator of risk of anastomotic leak in bowel anastomosis. International Surgery Journal. 2018;5:1724. [Google Scholar]
- 63.Calin M D, Bălălău C, Popa F, Voiculescu S, Scăunaşu R V. Colic anastomotic leakage risk factors. J Med Life. 2013;6(04):420–423. [PMC free article] [PubMed] [Google Scholar]
- 64.Bakker I S, Grossmann I, Henneman D, Havenga K, Wiggers T.Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit Br J Surg 201410104424–432., discussion 432 [DOI] [PubMed] [Google Scholar]
- 65.Choudhuri A H, Uppal R, Kumar M. Influence of non-surgical risk factors on anastomotic leakage after major gastrointestinal surgery: Audit from a tertiary care teaching institute. Int J Crit Illn Inj Sci. 2013;3(04):246–249. doi: 10.4103/2229-5151.124117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakr A, Emile S H, Abdallah E, Thabet W, Khafagy W. Predictive factors for small intestinal and colonic anastomotic leak: a multivariate analysis. Indian J Surg. 2017;79(06):555–562. doi: 10.1007/s12262-016-1556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volanakis J E.Human C-reactive protein: expression, structure, and function Mol Immunol 200138(2,3(:189–197. [DOI] [PubMed] [Google Scholar]
- 68.Thompson D, Pepys M B, Wood S P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7(02):169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 69.Kushner I, Antonelli M J. What should we regard as an “elevated” C-reactive protein level? Ann Intern Med. 2015;163(04):326. doi: 10.7326/L15-5126. [DOI] [PubMed] [Google Scholar]
- 70.Pepys M B, Hirschfield G M. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vijayan A L, Ravindran S. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51. doi: 10.1186/s40560-017-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Granero A, Frasson M, Flor-Lorente B. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum. 2013;56(04):475–483. doi: 10.1097/DCR.0b013e31826ce825. [DOI] [PubMed] [Google Scholar]
- 73.Singh P P, Zeng I S, Srinivasa S, Lemanu D P, Connolly A B, Hill A G. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101(04):339–346. doi: 10.1002/bjs.9354. [DOI] [PubMed] [Google Scholar]
- 74.Giaccaglia V, Salvi P F, Antonelli M S. Procalcitonin reveals early dehiscence in colorectal surgery: the PREDICS study. Ann Surg. 2016;263(05):967–972. doi: 10.1097/SLA.0000000000001365. [DOI] [PubMed] [Google Scholar]
- 75.Tan W J, Ng W Q, Sultana R. Systematic review and meta-analysis of the use of serum procalcitonin levels to predict intra-abdominal infections after colorectal surgery. Int J Colorectal Dis. 2018;33(02):171–180. doi: 10.1007/s00384-017-2956-8. [DOI] [PubMed] [Google Scholar]
- 76.Wacker C, Prkno A, Brunkhorst F M, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(05):426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 77.IMACORS Study . Facy O, Paquette B, Orry D. Diagnostic accuracy of inflammatory markers as early predictors of infection after elective colorectal surgery: results from the IMACORS study. Ann Surg. 2016;263(05):961–966. doi: 10.1097/SLA.0000000000001303. [DOI] [PubMed] [Google Scholar]
- 78.Zawadzki M, Czarnecki R, Rzaca M, Obuszko Z, Velchuru V R, Witkiewicz W. C-reactive protein and procalcitonin predict anastomotic leaks following colorectal cancer resections - a prospective study. Wideochir Inne Tech Malo Inwazyjne. 2016;10(04):567–573. doi: 10.5114/wiitm.2015.56999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adamina M, Warschkow R, Näf F. Monitoring c-reactive protein after laparoscopic colorectal surgery excludes infectious complications and allows for safe and early discharge. Surg Endosc. 2014;28(10):2939–2948. doi: 10.1007/s00464-014-3556-0. [DOI] [PubMed] [Google Scholar]
- 80.Giaccaglia V, Salvi P F, Cunsolo G V. Procalcitonin, as an early biomarker of colorectal anastomotic leak, facilitates enhanced recovery after surgery. J Crit Care. 2014;29(04):528–532. doi: 10.1016/j.jcrc.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 81.Kørner H, Nielsen H J, Søreide J A, Nedrebø B S, Søreide K, Knapp J C. Diagnostic accuracy of C-reactive protein for intraabdominal infections after colorectal resections. J Gastrointest Surg. 2009;13(09):1599–1606. doi: 10.1007/s11605-009-0928-1. [DOI] [PubMed] [Google Scholar]
- 82.MacKay G J, Molloy R G, O'Dwyer P J. C-reactive protein as a predictor of postoperative infective complications following elective colorectal resection. Colorectal Dis. 2011;13(05):583–587. doi: 10.1111/j.1463-1318.2010.02236.x. [DOI] [PubMed] [Google Scholar]
- 83.Platt J J, Ramanathan M L, Crosbie R A. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012;19(13):4168–4177. doi: 10.1245/s10434-012-2498-9. [DOI] [PubMed] [Google Scholar]
- 84.Nicksa G A, Dring R V, Johnson K H, Sardella W V, Vignati P V, Cohen J L. Anastomotic leaks: what is the best diagnostic imaging study? Dis Colon Rectum. 2007;50(02):197–203. doi: 10.1007/s10350-006-0708-x. [DOI] [PubMed] [Google Scholar]
- 85.Samji K B, Kielar A Z, Connolly M. Anastomotic leaks after small- and large-bowel surgery: diagnostic performance of ct and the importance of intraluminal contrast administration. AJR Am J Roentgenol. 2018;210(06):1259–1265. doi: 10.2214/AJR.17.18642. [DOI] [PubMed] [Google Scholar]
- 86.Lim M, Akhtar S, Sasapu K. Clinical and subclinical leaks after low colorectal anastomosis: a clinical and radiologic study. Dis Colon Rectum. 2006;49(10):1611–1619. doi: 10.1007/s10350-006-0663-6. [DOI] [PubMed] [Google Scholar]
- 87.DuBrow R A, David C L, Curley S A. Anastomotic leaks after low anterior resection for rectal carcinoma: evaluation with CT and barium enema. AJR Am J Roentgenol. 1995;165(03):567–571. doi: 10.2214/ajr.165.3.7645472. [DOI] [PubMed] [Google Scholar]
- 88.Katory M, McLean R, Osman K. The novel appearance of low rectal anastomosis on contrast enema following laparoscopic anterior resection: discriminating anastomotic leaks from “dog-ears” on water-soluble contrast enema and flexible sigmoidoscopy. Abdom Radiol (NY) 2017;42(02):435–441. doi: 10.1007/s00261-016-0885-6. [DOI] [PubMed] [Google Scholar]
- 89.Power N, Atri M, Ryan S, Haddad R, Smith A. CT assessment of anastomotic bowel leak. Clin Radiol. 2007;62(01):37–42. doi: 10.1016/j.crad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Kaur P, Karandikar S S, Roy-Choudhury S. Accuracy of multidetector CT in detecting anastomotic leaks following stapled left-sided colonic anastomosis. Clin Radiol. 2014;69(01):59–62. doi: 10.1016/j.crad.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Saif M W, Elfiky A, Salem R R. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14(06):1860–1869. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 92.Heinzerling J H, Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006;63(05):334–337. doi: 10.1016/j.cursur.2006.06.002. [DOI] [PubMed] [Google Scholar]


