Abstract
A child who comes to our attention for the appearance of erythematous, scaly lesions localized to the upper and lower limbs for 2 months. Histological features suggested ichthyosiform disease and concomitant mutations in the SPINK5 and FLG2 genes confirmed Netherton syndrome with severe atopic manifestations.
Keywords: atopic dermatitis, filaggrin 2, ichthyosis, Netherton syndrome, SPINK5, trichorrhexis invaginata
Our case is particularly interesting because it clearly highlights the clinical characteristics of a rare disease whose atopic manifestations have been aggravated by a concurrence of FLG2 and SPINK5 mutations. Furthermore, trichoscopy showing invaginated trichoresis could suggest an early diagnosis of Netherton syndrome.

1. INTRODUCTION
In 1964, Wilkinson et al. 1 defined as Netherton syndrome the triad consisting of congenital ichthyosis, trichorrhexis invaginata, and atopy. Netherton syndrome is an autosomal recessive disease due to a biallelic mutation of serine protease inhibitor Kazal type 5 gene (SPINK5), characterized by widespread disorder of skin keratinization, atopic diathesis, and alteration of the hair shaft, especially of hair and eyebrows. The SPINK5 mutations previously described may be of different types such as frameshift, nonsense, missense, or altered splicing determining the complete absence of the enzyme or its aberrant function. 2 Multiple cutaneous manifestations may be observed in Netherton's syndrome such as an ichthyosiform erythrodermia, continuous skin peeling, and localized ichthyosis circumflexa.
2. CASE REPORT
A 2‐year‐old child with a previous diagnosis of atopic dermatitis presented because of the appearance since 2 months of itchy erythematous, scaly lesions localized at the hands, feet, and knees. At the time of the visit, there were erosions with peripheral fine scale, symmetrically located at the hands, forearms, groins, and heels. In addition, at face and neck there were well‐defined erythematous, scaly patches (Figure 1A,B). There were also thin, fragile hairs, some broken, especially in the temporal and occipital areas. Trichoscopy and light microscopy examination showed typical trichorrhexis invaginata of hairs, eyelashes, and eyebrows (Figure 1C,D). No family history of atopic dermatitis was reported; the parents were consanguineous and in good health. Abnormal laboratory investigations included elevated IgE levels (306 kU/L). Histological examination of lesional skin showed epidermal hyperplasia with moderate hypergranulosis and marked hyperortho‐parakeratosis arranged in parallel laminae. Furthermore, at the edge of the sample, a very thickened orthokeratotic stratum corneum was present forming a collarette scale. In the papillary dermis, a mild perivascular lympho‐histiocytic infiltrate with some intravascular neutrophils was observed (Figure 1E, F). Genetic revealed the presence of a variant c877A> C in heterozygosity in the filaggrin 2 gene (FLG2) and of a variant c882+1G> C in homozygosity in the serine protease inhibitor Kazal type 5 gene (SPINK5). At the follow‐up visit, the patient presented diffuse eczematous lesions, excoriations, and post inflammatory hyperpigmentation. In addition, at the lower limbs, there were serpiginous and polycyclic erythemato‐desquamative lesions. Dermatoscopy showed double‐edged scale suggestive for ichthyosis linearis circumflexa (Figure 2A). Topical tacrolimus 0.03% on the face and topical corticosteroid on eczematous patches was prescribed, plus urea‐based emollients. During the follow‐up, the patient reported the onset of fever (37.7°C) and a painful rash, with serous‐hemorrhagic vesicles and honey crusts localized mainly on the right hemiface and in the right retroauricular region (Figure 2B). Microbiological culture and molecular testing revealed the presence of Staphylococcus aureus and human herpes simplex virus type 1 (HSV‐1) DNA confirming the diagnosis of bacterial infection associated with eczema herpeticum (Kaposi varicelliform eruption). In addition, a nasopharyngeal swab revealed the presence of Sars‐CoV‐2 RNA. The patient was treated with ceftriaxone 50–100 mg/kg intravenously, once daily for 7 days and acyclovir 20 mg/kg every 8 h intravenously for 7 days and topical rifamicin once daily for 14 days. At follow‐up visit, the skin manifestations showed a marked improvement (Figure 2C,D).
FIGURE 1.
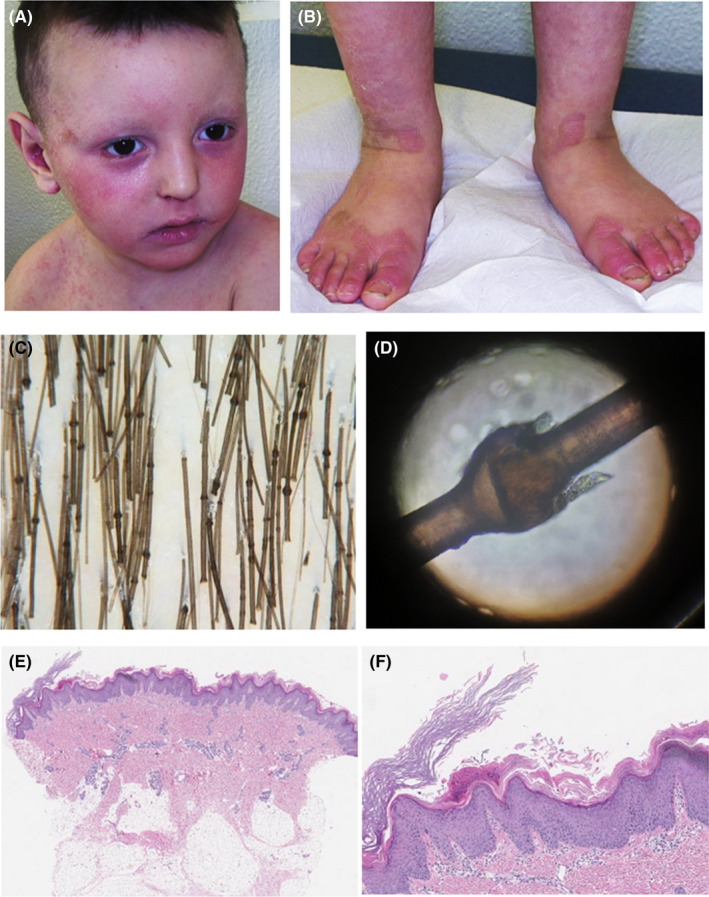
(A, B), erythematous, scaly lesions, and erosions with periferical fine scale; ©, trichoscopy showig trichorrhexis invaginata of hairs; (D), examination of the hair under light microscopy showing the presence of golf‐tee hair; (E, F), marked hyperortoparacheratosis arranged in parallel laminae with an orthokeratotic stratum corneum thicker than the rest of the epidermis, forming the collarette scale, with a mild perivascular lymphohistiocytic infiltrate in the papillary dermis (hematoxylin and eosin, original magnification: (E) ×40; (F) ×100)
FIGURE 2.
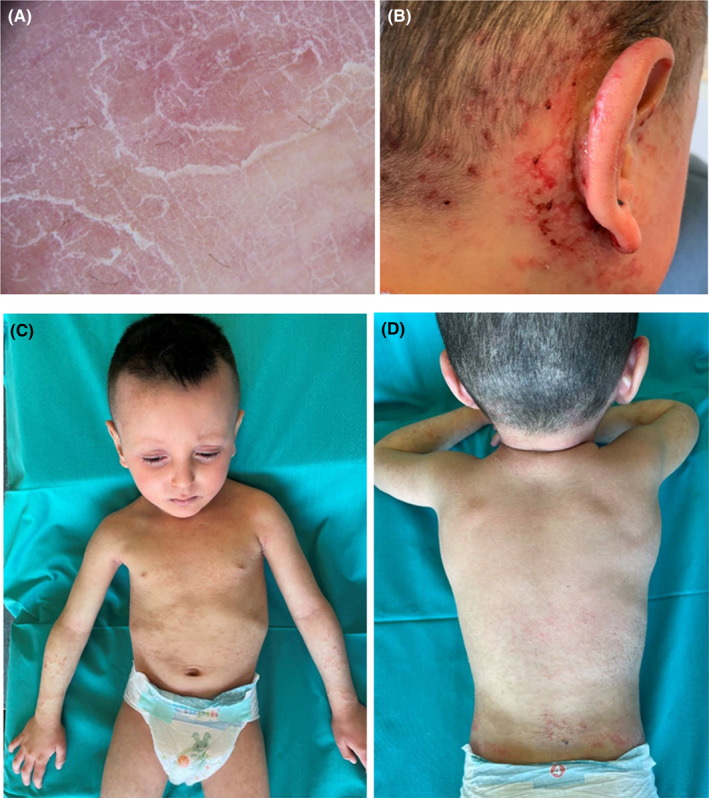
(A) dermatoscopy showing double‐edged scale; (B) vesicles with serous‐hemorrhagic content and honey crusts localized on the right hemiface and in the right retroauricular region were evident; (C, D), marked improvement of skin lesions at the follow‐up visit
3. DISCUSSION
Netherton syndrome is characterized by the biallelic mutation of SPINK5 gene, which encodes for the serine protease inhibitor, LEKTI1. To date, more than 80 different mutations have been identified. 2 , 3 , 4 , 5 , 6 Loss of activity of this inhibitor results in uncontrolled epidermal serine protease activity. In our case, the child had a biallelic mutation in the SPINK5 gene and a mutation in heterozygosis in FLG2 gene, which is typically altered in atopic dermatitis. Sequence analysis revealed the presence of the homozygous variant c.882+1G> C in the SPINK5 gene, which is located in a canonical splicing site that is not present in the general population allele frequency database (gnomAD), and it has not been described previously. Sequence analysis also revealed the heterogeneous variant c.877A> C in the FLG2 gene, which at the protein level determines the amino acid change p. Asn293His (rs1266352893). The missense variant has an allelic frequency of 0.00001062 in the general population (gnomAD) has not been described yet.
Children with Netherton syndrome often present with erythroderma in the neonatal period. 7 In most patients, skin lesions evolve into serpiginous, circinate erythematous, and desquamating patches with a characteristic double‐edged scale defined as Ichthyosis linearis circumflexa. The typical atopic diathesis of Netherton syndrome is expressed by eczematous plaques. In addition, hair shaft fragility is determined by a weak cross‐linkage between keratin structures due to a reduced number of disulfide bonds causing an intussusceptions of the distal shaft into the dilated proximal cap, also known as trichorrhexis invaginata (bamboo hair). 8 , 9 , 10 Hair shaft abnormalities generally develop during infancy and early childhood and improve with age. A trichoscopic evaluation is of great help to direct the diagnosis at an early age. 11 , 12 Alterations of skin barrier function as well possible defects in skin innate immune system lead to an increased susceptibility to bacterial or viral infections, including HSV infection. Our patients also resulted positive to Sars‐Cov‐2, but he did not develop respiratory symptoms. In our case, clinical presentation of atopic manifestations was severe possibly because the co‐presence of two mutations acting synergistically. To date, the treatment of Netherton syndrome is to relieve symptoms and improve patients' quality of life. Topical treatments include emollients, keratolytics, tretinoin, brimonidine, calcipotriene, corticosteroids alone or in combination with each other. 13 , 14 In addition, topical calcineurin inhibitors have been shown to be effective in the treatment of skin lesions. 15 The skin barrier dysfunction may lead to a greater absorption of topical drugs (such as topical calcineurin inhibitors), for which it is important to monitor the serum dosage of some drugs. Intravenous immunoglobulins (IVIg) have been used with some efficacy in a limited number of cases. 16 The effect of systemic retinoids remains controversial as it resulted in excellent clinical improvement in some patients, while in others it caused an exacerbation of skin manifestations. 17 Narrow‐band UVB phototherapy, UVA1, PUVA, and balneophototherapy have been reported as effective therapeutic alternatives. 18 , 19 , 20 Recently, cases of Netherton syndrome have been successfully treated with biological drugs such as anti‐TNF alpha, dupilumab, omalizumab, secukinumab, and ustekinumab have been described. 21 , 22 , 23 , 24 , 25
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Agata Moar involved in writing‐original draft preparation. Manfredo Bruni involved in writing‐original draft preparation. Donatella Schena involved in write and review. Erika Rigotti involved in write and review. Chiara Colato involved in write and review. Antonio Novelli involved in write and review. Claudia Cesario involved in write and review. Giampiero Girolomoni involved in writing—review and editing.
ETHICAL APPROVAL
This case was conducted in accordance with the Declaration of Helsinki. All information of the patient's health was collected and evaluated, ensuring his privacy.
CONSENT
Written informed consent was collected for the publication of patient information and images.
ACKNOWLEDGEMENT
None.
Moar A, Bruni M, Schena D, et al. Netherton syndrome plus atopic dermatitis: Two new genetic mutations in the same patient. Clin Case Rep. 2021;9:e05108. doi: 10.1002/ccr3.5108
Funding information
None
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wilkinson RD, George HC, William AH. Netherton’s disease: trichorrhexis invaginata (bamboo hair), congenital ichthyosiform erythroderma and the atopic diathesis. A histopathologic study. Arch Dermatol. 1964;89(1):46‐54. [DOI] [PubMed] [Google Scholar]
- 2. Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25(2):141‐142. [DOI] [PubMed] [Google Scholar]
- 3. Sprecher E, Chavanas S, DiGiovanna JJ, et al. The spectrum of pathogenic mutations in SPINK5 in 19 families with Netherton syndrome: implications for mutation detection and first case of prenatal diagnosis. J Invest Dermatol. 2001;117(2):179‐187. [DOI] [PubMed] [Google Scholar]
- 4. Sarri CA, Roussaki‐Schulze A, Vasilopoulos Y, et al. Netherton syndrome: a genotype‐phenotype review. Mol Diagn Ther. 2017;21(2):137‐152. [DOI] [PubMed] [Google Scholar]
- 5. Schepis C, Siragusa M, Centofanti A, Vinci M, Calì F. Two siblings affected by Netherton/comèl syndrome. Diagnostic pathology and description of a new SPINK5 variant. Dermatol Online J. 2019;25(7):13030/qt0881q3sk. [PubMed] [Google Scholar]
- 6. Bellon N, Hadj‐Rabia S, Moulin F, et al. The challenging management of a series of 43 infants with Netherton syndrome: unexpected complications and novel mutations. Br J Dermatol. 2021;184(3):532‐537. [DOI] [PubMed] [Google Scholar]
- 7. Pruszkowski A, Bodemer C, Fraitag S, Teillac‐Hamel D, Amoric JC, de Prost Y. Neonatal and infantile erythrodermas: a retrospective study of 51 patients. Arch Dermatol. 2000;136(7):875‐880. [DOI] [PubMed] [Google Scholar]
- 8. Komatsu N, Takata M, Otsuki N, et al. Elevated stratum corneum hydrolytic activity in Netherton syndrome suggests an inhibitory regulation of desquamation by SPINK5‐derived peptides. J Invest Dermatol. 2002;118(3):436‐443. [DOI] [PubMed] [Google Scholar]
- 9. Ishida‐Yamamoto A, Deraison C, Bonnart C, et al. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005;124(2):360‐366. [DOI] [PubMed] [Google Scholar]
- 10. Salodkar AD, Choudhary SV, Jadwani G, Singh A. Bamboo hair in Netherton's syndrome. Int J Trichol. 2009;1(2):143‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schepis C, Failla P, Siragusa M, Vinci M, Calì F. Novel SPINK5 variants in a patient with Netherton syndrome and intellectual disability. The diagnostic value of trichoscopy. G Ital Dermatol Venereol. 2020;155(2):239‐240. [DOI] [PubMed] [Google Scholar]
- 12. Neri I, Balestri R, Starace M, Bardazzi F, Patrizi A. Videodermoscopy of eyelashes in Netherton syndrome. J Eur Acad Dermatol Venereol. 2011;25(11):1360‐1361. [DOI] [PubMed] [Google Scholar]
- 13. Rasheed M, Shahzad S, Zaeem A, Afzal I, Gul A, Khalid S. Updated strategies for the management, pathogenesis and molecular genetics of different forms of ichthyosis syndromes with prominent hair abnormalities. Arch Dermatol Res. 2017;309(10):773‐785. [DOI] [PubMed] [Google Scholar]
- 14. Filoni A, Vestita M, Giudice G, Bonamonte D. Image gallery: brimonidine gel for facial erythema in Netherton syndrome. Br J Dermatol. 2018;178(4):e277. [DOI] [PubMed] [Google Scholar]
- 15. Bin Saif G, Al‐Khenaizan S. Netherton syndrome: successful use of topical tacrolimus and pimecrolimus in four siblings. Int J Dermatol. 2007;46(3):290‐294. [DOI] [PubMed] [Google Scholar]
- 16. Renner ED, Hartl D, Rylaarsdam S, et al. Comèl‐Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol. 2009;124(3):536‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lazaridou E, Apalla Z, Patsatsi A, Trigoni A, Ioannides D. Netherton's syndrome: successful treatment with isotretinoin. J Eur Acad Dermatol Venereol. 2009;23(2):210‐212. [DOI] [PubMed] [Google Scholar]
- 18. Nagata T. Netherton's syndrome which responded to photochemotherapy. Dermatologica. 1980;161(1):51‐56. [DOI] [PubMed] [Google Scholar]
- 19. Capezzera R, Venturini M, Bianchi D, Zane C, Calzavara‐Pinton P. UVA1 phototherapy of Netherton syndrome. Acta Derm Venereol. 2004;84(1):69‐70. [DOI] [PubMed] [Google Scholar]
- 20. Singer R, Çopur M, Yüksel EN, Kocatürk E, Erhan SŞ. Ichthyosis linearis circumflexa in a child. Response to narrowband UVB therapy. J Dermatol Case Rep. 2015;9(4):110‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roda A, Mendonça‐Sanches M, Travassos AR, Soares‐de‐Almeida L, Metze D. Infliximab therapy for Netherton syndrome: a case report. JAAD Case Rep. 2017;3(6):550‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steuer AB, Cohen DE. Treatment of Netherton syndrome with dupilumab. JAMA Dermatol. 2020;156(3):350‐351. [DOI] [PubMed] [Google Scholar]
- 23. Yalcin AD. A case of netherton syndrome: successful treatment with omalizumab and pulse prednisolone and its effects on cytokines and immunoglobulin levels. Immunopharmacol Immunotoxicol. 2016;38(2):162‐166. [DOI] [PubMed] [Google Scholar]
- 24. Luchsinger I, Knöpfel N, Theiler M, et al. Secukinumab therapy for Netherton syndrome. JAMA Dermatol. 2020;156(8):907‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volc S, Maier L, Gritsch A, Aichelburg MC, Volc‐Platzer B. Successful treatment of Netherton syndrome with ustekinumab in a 15‐year‐old girl. Br J Dermatol. 2020;183(1):165‐167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


