Abstract
Introduction/Aims
Progressive axonal loss in multifocal motor neuropathy (MMN) is often assessed with nerve conduction studies (NCS), by recording maximum compound muscle action potentials (CMAPs). However, reinnervation maintains the CMAP amplitude until a significant portion of the motor unit (MU) pool is lost. Therefore, we performed more informative CMAP scans to study MU characteristics in a large cohort of patients with MMN.
Methods
We derived the maximum CMAP amplitude (CMAPmax), an MU number estimate (MUNE), and the largest MU amplitude stimulus current required to elicit 5%, 50%, and 95% of CMAPmax (S5, S50, S95) and relative ranges ([S95 − S5] × 100 / S50) from the scans. These metrics were compared with clinical, laboratory, and NCS results.
Results
Forty MMN patients and 24 healthy controls were included in the study. CMAPmax and MUNE were reduced in MMN patients (both P < .001). Largest MU amplitude as a percentage of CMAPmax was increased in MMN patients (P < .001). Disease duration and treatment duration were not associated with MUNE. Relative range was larger in patients with anti‐GM1 antibodies than in those without anti‐GM1 antibodies (P = .016) and controls (P < .001). The largest MU amplitudes were larger in patients without anti‐GM1 antibodies than in patients with anti‐GM1 antibodies (P = .037) and controls (P = .044).
Discussion
We found that MU loss is common in MMN and accompanied by enlarged MUs. Presence of anti‐GM1 antibodies was associated with increased relative range of MU thresholds and reduction in largest MU amplitude. Our findings indicate that CMAP scans complement routine NCS, and may have potential for practical monitoring of treatment efficacy and disease progression.
Keywords: anti‐ganglioside antibodies, CMAP scan, motor unit integrity, motor unit number estimation, multifocal motor neuropathy
Abbreviations
- APB
abductor pollicis brevis
- CB
conduction block
- CMAP
compound muscle action potential
- D50
indicator of largest discontinuities in a CMAP scan
- DML
distal motor latency
- IVIg
intravenous immunoglobulins
- MCV
motor conduction velocity
- MMN
multifocal motor neuropathy
- MPS
multiple point stimulation
- MRC
medical research council
- MU
motor unit
- MUNE
motor unit number estimation
- MUNIX
motor unit number index
- NCS
nerve conduction studies
- S5
stimulus current to reach 5% of maximum CMAP amplitude
- S50
stimulus current to reach 50% of maximum CMAP amplitude
- S95
stimulus current to reach 95% of maximum CMAP amplitude
1. INTRODUCTION
Multifocal motor neuropathy (MMN) is a chronic dysimmune neuropathy characterized by exclusive involvement of motor axons, resulting in asymmetric distal limb weakness and a slowly progressive course. 1 The electrophysiological finding of motor conduction block (CB) with normal sensory conduction, is considered its diagnostic hallmark. 1 When nerve conduction studies (NCS) fail to show these features in patients with suspected MMN, the diagnosis may be supported by the presence of GM1 immunoglobulin M (IgM) antibodies and nerve imaging. 1
Despite treatment, mainly intravenous immunoglobulin (IVIg), gradual progression of muscle weakness over time is common. 2 , 3 Axonal degeneration, time without treatment, low Medical Research Council (MRC) sum‐score and absence of reflexes at presentation are associated with development of more severe muscle weakness over time. 4 , 5 Loss of motor axons is often assessed with routine NCS, based on recording of maximal distal compound muscle action potential (CMAP); however, this method has a relatively low utility as CMAP amplitudes are preserved mainly by ongoing reinnervation and they only drop after the motor axon population has been reduced considerably. 6 , 7 , 8
The CMAP scan is a reliable practical bedside test, that produces a stimulus‐response curve from which the size and number of motor units (MUs) can be estimated quickly 9 , 10 and with a high sensitivity to detect early MU loss in various (lower) motor neuron syndromes. 7 , 9 , 11 The CMAP scan can also be used to derive the activation thresholds of all MUs that innervate the muscle. Several studies indicated that demyelinating neuropathies harbor MUs with increased thresholds to external stimuli, but this has not yet been studied systematically in MMN. 12 , 13 , 14 Consequently, CMAP scan provides detailed information on MU integrity in terms of denervation, reinnervation, and demyelination, all of which are common in MMN. At present, only one study has explored its ability to capture MU loss in a smaller sample of MMN patients. 8 Therefore, we aimed to systematically study CMAP scan metrics pertaining to MU integrity in a large sample of MMN patients and compare these with clinical characteristics.
2. METHODS
2.1. Study design and subjects
We performed an observational cohort study in consecutive MMN patients visiting our neuromuscular outpatient clinic at the University Medical Centre Utrecht, between 2016 and 2018. All patients underwent the relevant routine ancillary investigations, including an extensive NCS protocol and testing for GM1 IgM antibodies. Patients with definite and probable MMN, according to diagnostic consensus criteria, 15 were eligible for inclusion. Exclusion criteria for participation in this study were carpal tunnel syndrome or other disorders commonly associated with polyneuropathy. The patients underwent standardized NCS, CMAP scans, and clinical and laboratory testing. In addition, we recruited age‐matched healthy controls who underwent CMAP scans only. All patients and healthy controls gave informed consent. The study was performed in accordance with the Declaration of Helsinki and was approved by the medical ethics committee of the University Medical Centre Utrecht.
2.2. Nerve conduction study
NCS was performed by M.K. after warming limbs in water at 37°C for 45 minutes with an electromyography (EMG) machine (Nicolet Viking IV; Nicolet Biomedical, Madison, Wisconsin). We investigated median nerve motor conduction by supramaximal stimulation at the wrist and elbow and recording from the abductor pollicis brevis muscle; the distance between wrist and the active recording electrode was 7 cm. We determined distal CMAP amplitude (baseline to negative peak), distal motor latency (DML), motor conduction velocity (MCV), percent CMAP area reduction between wrist and elbow, and percent CMAP duration prolongation between wrist and elbow. Conduction slowing consistent with demyelination was defined according to the following values established in our own laboratory 16 : DML ≥5.3 milliseconds, MCV ≤38 m/s, or CMAP duration prolongation ≥30%. CB was defined as a CMAP area reduction between wrist and elbow fulfilling the criteria established by Van Asseldonk et al, 3 which also take into account the amount of temporal dispersion. Axon loss on the basis of this median nerve motor conduction study was considered if distal CMAP amplitude was <3.5 mV.
2.3. CMAP scan recordings
All CMAP scan recordings were performed on a Nicolet Viking IV machine and a DS5 isolated bipolar constant current stimulator (Model D185‐HB4; Digitimer, Welwyn Garden City, UK), both coupled to a computer via a data acquisition system (PCI‐6221; National Instruments, Austin, Texas) that ran the MScanFit application (QtracS; Institute of Neurology, Queen Square, London, UK). To optimize temperature conditions, we applied a warming protocol with a water‐heated infant hyper/hypothermia blanket for single patient use (Norm‐O‐Temp & Maxi‐Therm Lite; Cincinnati Sub‐Zero LLC, Cincinatti, Ohio) at 37°C during the 30 minutes before each recording. 17 , 18 , 19 CMAP scans of patients and controls were recorded by M.K. and D.S., respectively, who were not blinded to the participants' clinical or treatment status. To minimize a possible influence of IVIg treatment, the CMAP scan timing was standardized to 1 to 2 weeks after IVIg administration.
Recordings were made from the thenar muscles using 10‐millimeter disk surface electrodes with the active recording electrode on the muscle belly and the reference electrode on the proximal metacarpophalangeal joint. Stimuli were delivered to the median nerve via a set of nonpolarizable surface electrodes (Red Dot; 3M Health Care, Germany), with the cathode at the wrist (7 cm proximal to the active recording electrode) and the anode 10 cm proximal over the radial side of the arm. CMAP scan recordings started by determining the minimal stimulus current that elicited the maximum CMAP manually. The stimulus current was reduced in steps of 0.2% at a frequency of 2 Hz (stimulus duration: 1 millisecond) until no more MU potentials were elicited. 7 Next, the CMAP scan amplitudes and corresponding stimulus currents were processed in MATLAB R2019b (The MathWorks, Natick, Massachusetts). We derived maximum CMAP amplitude (CMAPmax); S5, S50, and S95 (stimulus current to reach 5%, 50% and 95% of CMAPmax); absolute range, defined as S95 − S5; relative range defined as [(S95 − S5) × 100] / S50; and indicator of largest discontinuities in a CMAP scan (D50), defined as the number of largest consecutive differences required to build up to 50% of the CMAPmax. 6 D50 was considered reduced if it was below 25, which denotes loss of MUs and/or enlarged MUs due to reinnervation. 6 , 7 An overview of the study setup and the CMAP scan metrics are shown in Figure S1.
A motor unit number estimate (MUNE) and the amplitude of the largest MU potential (absolute and as percentage of CMAPmax) were obtained with the MScanFit tool, part of the QtracP software (Institute of Neurology, Queen Square, London, UK). MScanFit is based on a mathematical model that simulates the CMAP scan by including properties such as the number of MUs, their sizes, thresholds, and the threshold variability of individual MUs. 20 First, an initial estimate of the number of MUs is derived based on the slope (mV/mA) and the response variance (mV2) of the measured CMAP scan (also in case of a continuous change of the CMAP amplitude). In a second and final step, the model is further refined by adjusting the variables (including the number of MUs) to improve the fit between the measured CMAP scan and simulated scans, resulting in an optimized estimate of the number of MUs. 10 This technique was shown to have better reproducibility and sensitivity to axon loss than common MUNE methods, such as multiple point stimulation (MPS) and the motor unit number index (MUNIX). 9 , 11 , 21
We defined the threshold for significant MU loss as a MUNE lower than half of the 5th percentile of MUNE values in our control population. We omitted MU thresholds of patients with MUNE <5 for analysis, as their relative and absolute ranges were not considered reliable.
2.4. Clinical and laboratory testing
We assessed muscle strength of the abductor pollicis brevis (APB) by MRC grading and grip strength recording with the Martin Vigorimeter (Martin Medizintechnik, Tuttlingen, Germany). 22 We only included grip strength data obtained on routine outpatient clinic visits that occurred around the time of the CMAP scan recording. Presence of anti‐GM1 antibodies was assessed as described in previous studies. 23 , 24 , 25
2.5. Statistical analysis
For continuous variables we determined median, interquartile range, and absolute range. The association between MUNE and muscle strength scores was examined by restricted cubic splines with three knots. Linear regression analysis with the Spearman rank correlation coefficient was used to associate the remaining clinical and laboratory characteristics and NCS variables with the CMAP scan characteristics. Correlations were described as poor, moderate, or strong (R < 0.4, 0.4 ≤ R < 0.6, and R ≥ 0.6, respectively 26 ). The effect of the presence of anti‐GM1 antibodies and CB on the CMAP scan characteristics was examined with the Mann‐Whitney U test. P < .05 was considered significant. All statistical analyses were performed using R (R Core Team [2020], R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
A total of 40 consecutive MMN patients were included (Table 1), as well as 24 age‐matched, healthy controls (10 men; median age, 52 years; range, 30‐71 years). Only 1 of 40 (3%) of the MMN patients was treatment‐naive and 5 of 40 (13%) received IVIg induction treatment with a cumulative dose of 2 g/kg of IVIg over 5 days. All other patients were IVIg responders on regular maintenance IVIg treatment (0.4‐1.0 g/kg of IVIg every 2‐6 weeks). 22 Vigorimeter scores were obtained in 26 of 40 (65%) of the enrolled MMN patients within an appropriate interval of the study procedures.
TABLE 1.
Summary characteristics MMN patients
| Demographic characteristics | MMN (n = 40) |
|---|---|
| Age in years, median (range) | 53 (22‐78) |
| Male/female | 31/9 |
| MRC score APB (number of patients) | 0 (1), 1 (1), 2 (2), 3 (22), 4 (8), 5 (6) |
| Vigorimeter scores a in kPa, median (range) | 77.5 (18‐160) |
| Time to diagnosis in months, median (range) | 24 (0‐293) |
| Disease duration in months, median (range) | 74 (3‐557) |
| Treatment duration in months, median (range) | 20 (1‐97) |
| IVIg responders | 34 (85%) |
| Presence of anti‐GM1 antibodies | 14 (35%) |
Abbreviations: APB, abductor pollicis brevis muscle; MMN, multifocal motor neuropathy; MRC, Medical Research Council.
n = 26.
3.1. MU characteristics
In total, 40 CMAP scans were recorded from our patient population, of which 1 had to be excluded due to inability to relax the muscles, thus leaving 39 scans for further analysis. A summary of the CMAP scan data can be found in Table 2. Figure 1 shows nine CMAP scans obtained in consecutive patients with comparable muscle strength (MRC = 3). We found considerable variability in MUNE values and the presence of large gaps or discontinuities in their CMAP scans. Figure 2 shows the decreased MUNE values in MMN patients (P < .001) and the amplitude of the largest MU as a percentage of CMAPmax in comparison to controls (P < .001). The threshold for significant MU loss was defined as MUNE lower than half of the 5th percentile of healthy controls (MUNE <27). We found significant MU loss in 13 of 39 (33%) patients, with 1 patient who appeared to have only a single MU left. Of the 7 of 39 (18%) patients with reduced CMAPmax (≤3.5 mV), all showed significant MU loss. In contrast, of the 32 of 39 (82%) without reduced CMAPmax, we found that the CMAP scan still showed significant MU loss in 6 of 32 (19%). Interestingly, in 8 of 39 (21%) patients, CMAPmax was normal (>3.5 mV), whereas D50 was reduced (<25). Five of 39 (13%) patients had MUs exceeding an amplitude of 1 mV, the largest being 1.82 mV. The association between CMAPmax and MUNE was more apparent in patients than in controls (r = 0.79, P < .001; r = 0.58, P = .003). In contrast to controls, MUNE of patients was inversely related to largest MU amplitude as a percentage of CMAPmax (r = −0.91, P < .001). We also found that a reduction in D50 correlated with MUNE (r = 0.71, P < .001).
TABLE 2.
Summary CMAP scan results in MMN patients and healthy controls
| Median (range) | P value | ||
|---|---|---|---|
| Measures | MMN (n = 39) | Controls (n = 24) | |
| CMAPmax, mV | 7.2 (0.5‐16.9) | 11.3 (4.0‐16.5) | <.001 |
| MUNE | 48 (1‐117) | 86 (41‐176) | <.001 |
| Amplitude of largest MU, mV | 0.6 (0.2‐1.8) | 0.5 (0.2‐1.1) | .24 |
| Amplitude of largest MU, % | 8.6 (2.3‐100) | 4.2 (1.5‐10.4) | <.001 |
| D50 | 39 (1‐55) | 42 (30‐63) | .16 |
| S5 a , mA | 3.2 (1.2‐8.1) | 3.8 (2.0‐8.6) | .054 |
| S50 a , mA | 4.5 (1.9‐18.8) | 5.1 (2.9‐10.8) | .28 |
| S95 a , mA | 6.0 (2.0‐24.7) | 6.4 (3.5‐13.7) | .75 |
| Absolute range a , mA | 3.1 (0.8‐16.6) | 2.8 (1.4‐5.1) | .38 |
| Relative range a , % | 63 (36‐182) | 53 (36‐78) | .056 |
Abbreviations: CMAPmax, maximum compound muscle action potential amplitude; D50, number of largest consecutive differences to reach 50% of CMAPmax; MMN, multifocal motor neuropathy; MUNE, motor unit number estimate; S5, S50, and S95, stimulus current at 5%, 50% and 95% of CMAPmax.
n = 38 MMN patients.
FIGURE 1.
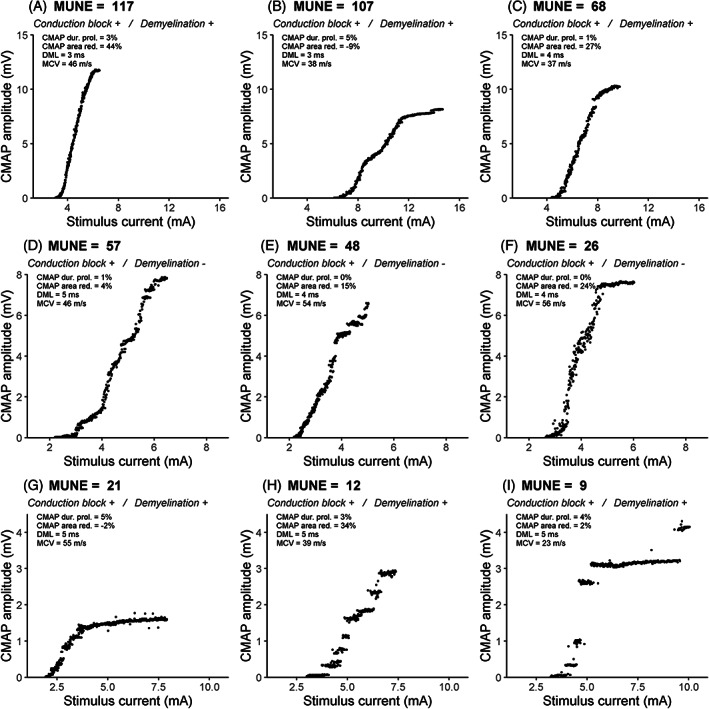
CMAP scans of patients with comparable strength scores (MRC = 3), showing wide variability in motor unit number estimates (MUNEs), illustrated by decreasing MUNE values from A‐G. Features of demyelinative slowing were present in A‐C and G‐I. Conduction block (CB) was not observed. NCS variables are depicted at the top left of each scan. Note the intersubject differences in maximum CMAP amplitude (y axis) and stimulus intensities (x axis). Abbreviations: DML, distal motor latency; MCV, motor conduction velocity; red., reduction; dur. prol., duration prolongation
FIGURE 2.
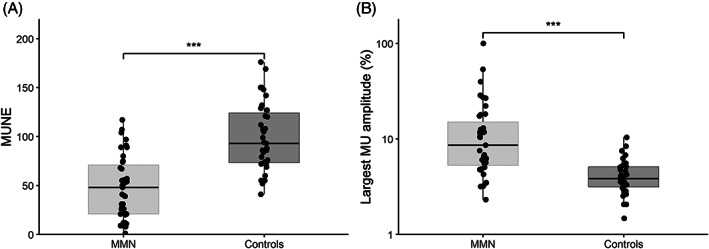
A, Boxplot showing motor unit number estimates (MUNEs). B, Boxplot showing largest motor unit amplitude as percentage of maximum CMAP of 39 MMN patients and 24 controls. Solid line: median value; box edges: 25th and 75th percentiles; whiskers: entire range except for outliers. ***Significant difference (P < .001)
The distribution of absolute thresholds to electrically recruit the entire MU pool reflected by S5, S50, and S95 showed wide ranges (Table 2). The thresholds of one patient with only a single MU left were omitted for analysis. We found no differences between S5, S50, S95 absolute and relative ranges of MMN patients and controls. Relative range only had a moderate inverse correlation with increasing CMAPmax (r = −0.40, P = .012). Neither MU thresholds (S5, S50, S95) nor absolute and relative range correlated with MUNE.
3.2. Relation of MU characteristics with clinical and laboratory characteristics
We found a poor inverse correlation between age and MUNE (r = −0.32, P = .046). MRC and vigorimeter scores were both associated with MUNE (R2 = 0.26, P = .004; R2 = 0.28, P = .027; Figure 3A and B). We found no association between MUNE values and disease duration (time from onset of symptoms), treatment duration (P = .51, P = .85), time to diagnosis (P = .99), and presence of anti‐GM1 antibodies (P = .80; Figure 4A). The relation between CMAPmax and MUNE for patients (with and without anti‐GM1 antibodies) and controls is shown in Figure 4B. Patients with anti‐GM1 antibodies also had elevated relative ranges of MU thresholds in comparison to patients without antibodies (P = .016) and controls (P < .001; Figure 4C). In contrast, patients without anti‐GM1 antibodies had elevated absolute largest MU amplitudes compared to patients with anti‐GM1 antibodies (P = .037) and controls (P = .044; Figure 4D).
FIGURE 3.
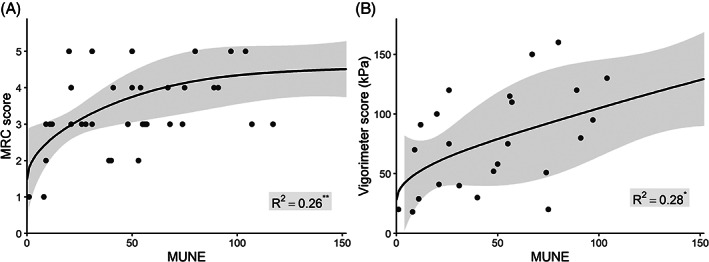
Association of strength scores with motor unit number estimates (MUNEs). Restricted cubic splines (solid lines) and 95% confidence intervals (gray areas) depicting (A) the relation between MRC scores and MUNE and (B) the relation between vigorimeter scores and MUNE
FIGURE 4.
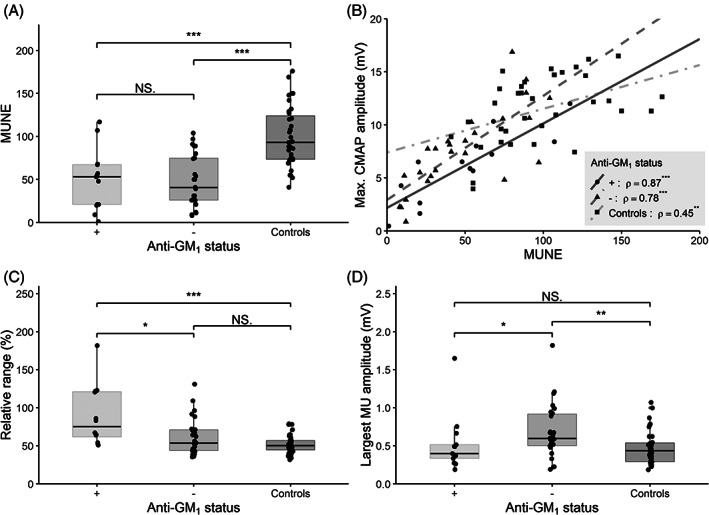
Motor unit characteristics in patients stratified by presence of anti‐GM1 antibodies and compared with controls. A, Boxplot showing motor unit number estimates (MUNEs). B, Boxplot showing relation between maximum CMAP amplitude and MUNE of patients with (circles and solid line) and without (triangles and dashed line) anti‐GM1 antibodies, and controls (squares and dashed dotted line), with rho indicating Spearman rank coefficient. C. Boxplot showing relative range. D, Boxplot showing largest motor unit amplitude. Solid line: median value; box edges: 25th and 75th percentiles; whiskers: entire range except outliers. Significant differences: *P < .05, **P < .01, and ***P < .001
3.3. Relation between CMAP scan and NCS
Table 3 summarizes the NCS findings in the MMN patients. In 4 of 39 patients, the presence of a CB in the forearm led to the inability to derive the MCV, CMAP area reduction, and duration prolongation. On NCS, 15 of 39 (38%) patients had motor conduction slowing compatible with demyelination, and 9 of 39 had CB in the forearm. Patients with either motor conduction slowing or CB showed no association with any of the CMAP scan variables. We found that only DML was inversely correlated with MUNE (r = −0.70, P < .001).
TABLE 3.
Summary nerve conduction study results in MMN
| Measures | Median (range) |
|---|---|
| Distal CMAP (mV) | 10 (0.5‐18.9) |
| DML (ms) | 3.9 (3.0‐5.7) |
| MCV (m/s) | 51 (23‐67) |
| CMAP area reduction (%) | 17.5 (−8.9 to 93.3) |
| CMAP duration prolongation (%) | 17.2 (−26.7 to 54.7) |
| Features compatible with demyelination (number of patients) | |
| (a) DML ≥5.3 ms | 2 |
| (b) MCV ≤38 m/s | 2 |
| (c) CMAP duration prolongation ≥30% | 5 |
| (d) CMAP duration prolongation ≥30%, DML ≥5.3 ms | 1 |
| (e) CMAP duration prolongation ≥30%, MCV ≤38 m/s | 4 |
| (f) CMAP duration prolongation ≥30%, MCV ≤38 m/s, DML ≥5.3 ms | 1 |
Note: The nerve conduction studies were obtained in median nerve unilaterally.
Abbreviations: CMAP, compound muscle action potential; DML, distal motor latency; MCV, motor conduction velocity; MMN, multifocal motor neuropathy.
4. DISCUSSION
Our study has shown that the variability of MU loss derived from CMAP scans in MMN patients is high, irrespective of disease duration and treatment duration. We also found that MU loss in MMN is compensated by successful reinnervation, as indicated by an increase in the largest MU amplitude and preserved distal CMAPmax. The relative range of MU thresholds of patients with anti‐GM1 antibodies was wider than in controls. The identification of MU loss and changes in MU thresholds not captured with routine NCS indicates that the CMAP scan provides important complementary information to standard NCS.
Early studies on axon loss in MMN mainly relied on decrease of distal CMAPmax, 3 , 25 , 27 and a few included needle EMG. However, distal CMAPmax may also decrease, due to distal CB, and only arrives at thresholds below clinically used cutoffs after failure of reinnervation processes to compensate for considerable axon loss. 6 , 7 , 8 Moreover, although needle EMG is sensitive, it is also invasive, often painful for patients, and subject to variability in rater interpretation. 28 The CMAP scan avoids most of these drawbacks as a sensitive, reproducible, and noninvasive tool for quantitative assessment of motor unit integrity. 9 , 10 Corroborating a previous exploratory study, 8 we found a substantial reduction in number of MUs combined with increase in MU sizes, compatible with axon loss and reinnervation in our MMN patients. However, we found that these features were highly variable between MMN patients. This difference may be explained by the fact that we evaluated a larger sample (39 vs 8) and also included MMN patients without CB in the median nerve forearm segment. In contrast to the previous study, 8 we detected a moderate association between MUNE and our focused evaluation of muscle strength (MRC score and handgrip strength, in median nerve territory). It is possible that the expanded MRC score that was used may have masked potential associations with muscle strength. Furthermore, we did not find a relation between MUNE, disease duration, treatment duration, time to diagnosis, or presence of anti‐GM1 antibodies. We also determined MU activation thresholds and found wide variability in our MMN patients, as reflected by S95 and the absolute and relative range, to electrically recruit the entire MU pool. Similar results have been noted in nerve excitability studies in MMN, which reported a small but inconsistent increase in stimulus current to elicit 50% of the maximum CMAP (S50). 24 , 29 , 30 Taken together, our findings confirm that the CMAP scan is a powerful tool to evaluate MU loss in MMN, and can also detect compensatory reinnervation and more subtle changes in both axonal excitability and demyelination.
Unfortunately, IVIg is not sufficient to arrest ongoing axonal degeneration, ultimately leading to progressive disability in MMN. 5 The prominent MU loss detected by CMAP scan in our study may help to explain this finding, as successful reinnervation can initially compensate for MU loss and help to preserve function. However, over time, as axonal degeneration progresses, these mechanisms will ultimately fail and inevitably lead to functional decline. Therefore, CMAP scan could also serve as potential prognostic marker in development of future treatment strategies, and aid in their evaluation on efficacy regarding limiting the axonal burden in MMN.
We also found increased relative ranges reflecting increased spread of the MU thresholds in our MMN patients with anti‐GM1 antibodies vs those without. Furthermore, the absolute largest MU amplitude was lower in patients with anti‐GM1 antibodies than those without. The presence of anti‐GM1 in MMN is believed to inhibit remyelination and reorganization of Na+ channels, 31 which are typically overexpressed in sprouting axons during reinnervation. 32 In doing so, anti‐GM1 may both reduce successful reinnervation of MUs and increase the relative range of activation thresholds. Other factors (eg, age and disease duration) may further affect these properties, although we did not observe such a relation in our data. These alterations in MU integrity could explain why patients with anti‐GM1 antibodies have more pronounced weakness, disability, and reduction of their maximum CMAP than those without. 33 , 34
Our study has several limitations. The number of patients was relatively small due to the rarity of MMN, but the variability of MU loss in our MMN patients corroborates the clinical heterogeneity and indicates that we have included a sufficiently representative sample. No scores reflecting fine motor function were obtained during this study, which could provide more details on the relation between clinical and CMAP scan metrics. Although not specifically evaluated, timing of the CMAP scan with respect to IVIg treatment may have some effect on MU thresholds. 35 Therefore, we aimed to standardized the time between the CMAP scan and IVIg treatment in order to minimize the introduction of timing effects. The CMAP scans were restricted to examining a single nerve innervating the distal thenar muscles only. However, distal muscles in the upper limb, particularly those innervated by the median nerve, are a hotspot for clinical and electrophysiological involvement in MMN. Therefore, the finding of MU loss and changes in MU sizes and thresholds in our cohort are considered representative for MMN, likely with comparable yield in other affected nerves.
In conclusion, our study has shown that CMAP scan can detect important changes in MU integrity in MMN, demonstrating a wide spectrum of MU loss, enlarged MUs due to reinnervation, and focal increases in MU activation thresholds. These characteristic alterations occur irrespective of treatment duration, indicating that the CMAP scan can be used across the whole spectrum of MMN to evaluate efficacy of potentially new interventions aimed at limiting ongoing axonal degeneration and thereby decreasing progressive disability. Studying multiple muscle groups with CMAP scan may provide further insight on the distribution of MU integrity changes in MMN that may be masked in routine NCS.
CONFLICT OF INTEREST
None of the authors has any conflicts of interest to disclose. L.H.v.d.B. reports grants from ALS Foundation Netherlands; grants from the Netherlands Organization for Health Research and Development (Vici Scheme); grants from the Netherlands Organization for Health Research and Development (SOPHIA, STRENGTH, ALS‐CarE Project), funded through the EU Joint Programme—Neurodegenerative Disease Research; and personal fees from Shire, Biogen, Cytokinetics, and Treeway; unrelated to this study.
AUTHOR CONTRIBUTIONS
D.J.L.S.G. conceived and designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, and drafted the manuscript. M.O.K. conceived and designed research and performed experiments. H.S.G. and B.T.H.M.S. conceived and designed research, interpreted results of the experiments, and drafted the manuscript. L.J.v.S. developed and maintained study setup and provided technical expertise. H.F. and L.H.v.d.B. conceived and designed research. All authors edited and revised the manuscript and approved the final version.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Overview of study setup, recordings, and compound muscle action potential (CMAP) scan metrics. A, Placement of the recording and stimulation electrodes for recording CMAPs on the abductor pollicis brevis. B, Depiction of all CMAP amplitudes recorded during a CMAP scan in a healthy control. C, CMAP scan corresponding to (B) together with the maximum CMAP amplitude (CMAPmax) and the stimuli required to reach 5%, 50%, and 95% of CMAPmax (S5, S50, and S95).
ACKNOWLEDGMENTS
This project was supported by the European Federation of Neurological Societies scientific Fellowship, grant WOR14‐07 from the Prinses Beatrix Spierfonds, and the Netherlands ALS foundation (Stichting ALS Nederland).
Stikvoort García DJL, Kovalchuk MO, Goedee HS, et al. Motor unit integrity in multifocal motor neuropathy: A systematic evaluation with CMAP scans. Muscle & Nerve. 2022;65(3):317-325. doi: 10.1002/mus.27469
Funding information European Federation of Neurological Societies scientific Fellowship grant; Prinses Beatrix Spierfonds, Grant/Award Number: WOR14‐07; Stichting ALS Nederland
Contributor Information
Diederik J. L. Stikvoort García, Email: d.j.l.stikvoort-2@umcutrecht.nl.
Boudewijn T. H. M. Sleutjes, Email: b.sleutjes@umcutrecht.nl.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Vlam L, van der Pol WL, Cats EA, et al. Multifocal motor neuropathy: diagnosis, pathogenesis and treatment strategies. Nat Rev Neurol. 2012;8:48‐58. [DOI] [PubMed] [Google Scholar]
- 2. Van den Berg‐Vos RM, Franssen H, Wokke JH, Van den Berg LH. Multifocal motor neuropathy: long‐term clinical and electrophysiological assessment of intravenous immunoglobulin maintenance treatment. Brain. 2002;125:1875‐1886. [DOI] [PubMed] [Google Scholar]
- 3. Van Asseldonk JTH, Van den Berg LH, Kalmijn S, et al. Axon loss is an important determinant of weakness in multifocal motor neuropathy. J Neurol Neurosurg Psychiatry. 2006;77:743‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uncini A, Kuwabara S. Nodopathies of the peripheral nerve: an emerging concept. J Neurol Neurosurg Psychiatry. 2015;86:1186‐1195. [DOI] [PubMed] [Google Scholar]
- 5. Herraets I, van Rosmalen M, Bos J, et al. Clinical outcomes in multifocal motor neuropathy: a combined cross‐sectional and follow‐up study. Neurology. 2020;95:e1979‐e1987. [DOI] [PubMed] [Google Scholar]
- 6. Sleutjes B, Montfoort I, Maathuis E, et al. CMAP scan discontinuities: automated detection and relation to motor unit loss. Clin Neurophysiol. 2014;125:388‐395. [DOI] [PubMed] [Google Scholar]
- 7. Sleutjes BT, Wijngaarde CA, Wadman RI, et al. Assessment of motor unit loss in patients with spinal muscular atrophy. Clin Neurophysiol. 2020;131:1280‐1286. [DOI] [PubMed] [Google Scholar]
- 8. Garg N, Howells J, Yiannikas C, et al. Motor unit remodelling in multifocal motor neuropathy: the importance of axonal loss. Clin Neurophysiol. 2017;128:2022‐2028. [DOI] [PubMed] [Google Scholar]
- 9. Jacobsen AB, Bostock H, Fuglsang‐Frederiksen A, et al. Reproducibility, and sensitivity to motor unit loss in amyotrophic lateral sclerosis, of a novel MUNE method: MScanFit MUNE. Clin Neurophysiol. 2017;128:1380‐1388. [DOI] [PubMed] [Google Scholar]
- 10. Bostock H. Estimating motor unit numbers from a CMAP scan. Muscle Nerve. 2016;53:889‐896. [DOI] [PubMed] [Google Scholar]
- 11. Jacobsen AB, Bostock H, Tankisi H. Following disease progression in motor neuron disorders with 3 motor unit number estimation methods. Muscle Nerve. 2019;59:82‐87. [DOI] [PubMed] [Google Scholar]
- 12. Franssen H, Straver DCG. Pathophysiology of immune‐mediated demyelinating neuropathies—part II: Neurology. Muscle Nerve. 2014;49:4‐20. [DOI] [PubMed] [Google Scholar]
- 13. Brismar T. Changes in electrical threshold in human peripheral neuropathy. J Neurol Sci. 1985;68:215‐223. [DOI] [PubMed] [Google Scholar]
- 14. Meulstee J, Darbas A, van Doorn PA, van Briemen L, van der Meche FG. Decreased electrical excitability of peripheral nerves in demyelinating polyneuropathies. J Neurol Neurosurg Psychiatry. 1997;62:398‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Schaik IN, Leger JM, Nobile‐Orazio E, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of multifocal motor neuropathy. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15:295‐301. [DOI] [PubMed] [Google Scholar]
- 16. Van Asseldonk JTH, Franssen H, Van den Berg‐Vos RM, Wokke JH, Van den Berg LH. Multifocal motor neuropathy. Lancet Neurol. 2005;4:309‐319. [DOI] [PubMed] [Google Scholar]
- 17. Franssen H, Wieneke GH. Nerve‐conduction and temperature—necessary warming time. Muscle Nerve. 1994;17:336‐344. [DOI] [PubMed] [Google Scholar]
- 18. Drenthen J, Blok JH, van Heel EBMD, Visser GH. Limb temperature and nerve conduction velocity during warming with hot water blankets. J Clin Neurophysiol. 2008;25:104‐110. [DOI] [PubMed] [Google Scholar]
- 19. Kovalchuk MO, Franssen H, Scheijmans FEV, Van Schelven LJ, Van Den Berg LH, Sleutjes B. Warming nerves for excitability testing. Muscle Nerve. 2019;60:279‐285. [DOI] [PubMed] [Google Scholar]
- 20. Hales JP, Lin CS, Bostock H. Variations in excitability of single human motor axons, related to stochastic properties of nodal sodium channels. J Physiol. 2004;559:953‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobsen AB, Bostock H, Tankisi H. CMAP scan MUNE (MScan)—a novel motor unit number estimation (MUNE) method. J Vis Exp. 2018;136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herraets IJT, Goedee HS, Telleman JA, et al. Nerve ultrasound improves detection of treatment‐responsive chronic inflammatory neuropathies. Neurology. 2020;94:e1470‐e1479. [DOI] [PubMed] [Google Scholar]
- 23. Kovalchuk M, Franssen H, van Schelven LJ, van den Berg L, Sleutjes BTHM. Influence of Ivig on nerve excitability in multifocal motor neuropathy. J Peripher Nerv Syst. 2017;22:319‐320. [Google Scholar]
- 24. Kovalchuk MO, Franssen H, van den Berg LH, van Schelven LJ, Sleutjes BT. Excitability of motor and sensory axons in multifocal motor neuropathy. Clin Neurophysiol. 2020;131:2641‐2650. [DOI] [PubMed] [Google Scholar]
- 25. Van Asseldonk JTH, Van den Berg LH, Van den Berg‐Vos RM, Wieneke GH, Wokke JHJ, Franssen H. Demyelination and axonal loss in multifocal motor neuropathy: distribution and relation to weakness. Brain. 2003;126:186‐198. [DOI] [PubMed] [Google Scholar]
- 26. Dancey CP, Reidy J. Statistics without Maths for Psychology. London: Pearson Education; 2007. [Google Scholar]
- 27. Vucic S, Black K, Chong PS, Cros D. Multifocal motor neuropathy with conduction block: distribution of demyelination and axonal degeneration. Clin Neurophysiol. 2007;118:124‐130. [DOI] [PubMed] [Google Scholar]
- 28. Johnsen B, Pugdahl K, Fuglsang‐Frederiksen A, et al. Diagnostic criteria for amyotrophic lateral sclerosis: a multicentre study of inter‐rater variation and sensitivity. Clin Neurophysiol. 2019;130:307‐314. [DOI] [PubMed] [Google Scholar]
- 29. Kiernan MC, Guglielmi JM, Kaji R, Murray NMF, Bostock H. Evidence for axonal membrane hyperpolarization in multifocal motor neuropathy with conduction block. Brain. 2002;125:664‐675. [DOI] [PubMed] [Google Scholar]
- 30. Garg N, Park SB, Howells J, et al. Conduction block in immune‐mediated neuropathy: paranodopathy versus axonopathy. Eur J Neurol. 2019;26:1121‐1129. [DOI] [PubMed] [Google Scholar]
- 31. Kaji R, Hirota N, Oka N, et al. Anti‐GM(1) antibodies and impaired blood‐nerve barrier may interfere with remyelination in multifocal motor neuropathy. Muscle Nerve. 1994;17:108‐110. [DOI] [PubMed] [Google Scholar]
- 32. Devor M, Keller CH, Deerinck TJ, Levinson SR, Ellisman MH. Na+ channel accumulation on axolemma of afferent endings in nerve end neuromas in Apteronotus. Neurosci Lett. 1989;102:149‐154. [DOI] [PubMed] [Google Scholar]
- 33. van Schaik IN, Bossuyt PM, Brand A, Vermeulen M. Diagnostic value of GM1 antibodies in motor neuron disorders and neuropathies: a meta‐analysis. Neurology. 1995;45:1570‐1577. [DOI] [PubMed] [Google Scholar]
- 34. Cats EA, Jacobs BC, Yuki N, et al. Multifocal motor neuropathy: association of anti‐GM1 IgM antibodies with clinical features. Neurology. 2010;75:1961‐1967. [DOI] [PubMed] [Google Scholar]
- 35. Boerio D, Creange A, Hogrel JY, Gueguen A, Bertrand D, Lefaucheur JP. Nerve excitability changes after intravenous immunoglobulin infusions in multifocal motor neuropathy and chronic inflammatory demyelinating neuropathy. J Neurol Sci. 2010;292:63‐71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overview of study setup, recordings, and compound muscle action potential (CMAP) scan metrics. A, Placement of the recording and stimulation electrodes for recording CMAPs on the abductor pollicis brevis. B, Depiction of all CMAP amplitudes recorded during a CMAP scan in a healthy control. C, CMAP scan corresponding to (B) together with the maximum CMAP amplitude (CMAPmax) and the stimuli required to reach 5%, 50%, and 95% of CMAPmax (S5, S50, and S95).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


