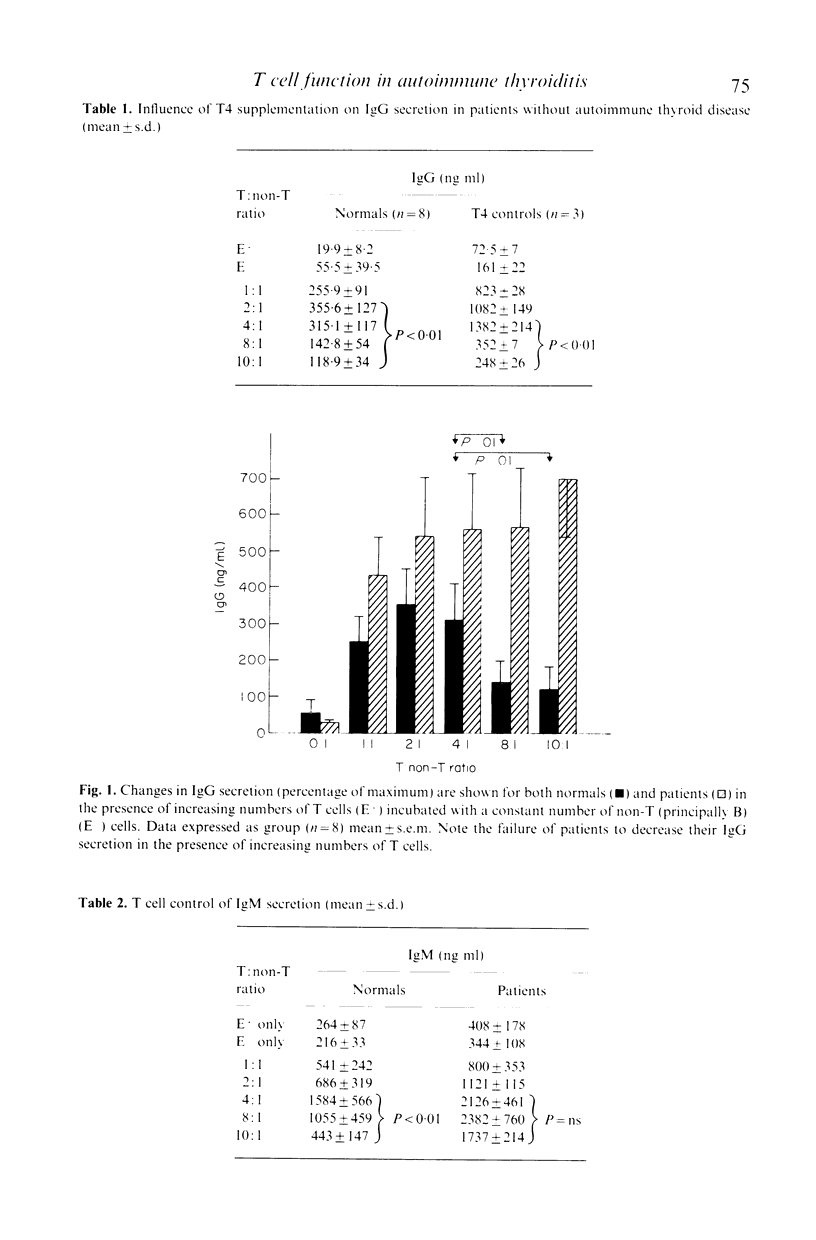
Abstract
We examined T cell function in patients with autoimmune thyroiditis in relation to mitogen-stimulated thyroglobulin autoantibody secretion and used total IgG and IgM secretion as a measure of non-specific polyclonal activation. Control subjects (n = 8) showed enhanced immunoglobulin secretion (both IgG and IgM) in the presence of increasing autologous T cells up to a ratio of 4:1 T:non-T followed by suppression of such secretion when the number of T cells was increased still further (the T cell suppressor effect). Patients (n = 8) demonstrated similar enhancement of immunoglobulin secretion when compared to normals but the T cell suppressor effect was markedly reduced. Autoantibodies to human thyroglobulin antigen, measured by specific ELISA techniques, were detected in cultures from five patients and demonstrated a similar pattern of enhancement but reduced suppression as observed with the immunoglobulin assays. However, normal suppression of immunoglobulin and thyroglobulin autoantibody secretion in such patients was obtained with the use of allogeneic normal T cells. These data confirm the presence of a T cell suppressor defect in autoimmune thyroiditis but indicate that the abnormality is not restricted to the control of thyroid autoantibodies. Such T cell dysfunction may allow the abnormal amplification of a distinct and separate primary event and may be viewed as an abnormal set point for a hypothetical 'autoimmunostat'.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. D., Knight J. G. H gene theory of inherited autoimmune disease. Lancet. 1980 Feb 23;1(8165):396–398. doi: 10.1016/s0140-6736(80)90946-0. [DOI] [PubMed] [Google Scholar]
- Beall G. N., Kruger S. R. Production of human antithyroglobulin in vitro. II. Regulation by T cells. Clin Immunol Immunopathol. 1980 Aug;16(4):498–503. doi: 10.1016/0090-1229(80)90191-9. [DOI] [PubMed] [Google Scholar]
- De Bernardo E., Davies T. F. Antigen-specific B-cell function in human autoimmune thyroiditis. J Clin Immunol. 1983 Oct;3(4):392–398. doi: 10.1007/BF00915801. [DOI] [PubMed] [Google Scholar]
- Fawcett J., Hutton C., Mclachlan S. M., Clark F., Rees Smith B. Defective regulation of the immune response to tetanus toxoid in Hashimoto's disease. Immunology. 1984 Jul;52(3):525–528. [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Deak B. D., Shreffler D. C., Klein J., Stimpfling J. H., Snell G. D. Genetic control of the immune response. Mapping of the Ir-1 locus. J Exp Med. 1972 Jun 1;135(6):1259–1278. doi: 10.1084/jem.135.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Dickinson A. M., Malcolm A., Farndon J. R., Young E., Proctor S. J., Smith B. R. Thyroid autoantibody synthesis by cultures of thyroid and peripheral blood lymphocytes. I. Lymphocyte markers and response to pokeweed mitogen. Clin Exp Immunol. 1983 Apr;52(1):45–53. [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Wee S. L., McGregor A. M., Smith B. R., Hall R. In vitro studies on the control of thyroid autoantibody synthesis. J Clin Lab Immunol. 1980 Jan;3(1):15–21. [PubMed] [Google Scholar]
- Noma T., Yata J., Shishiba Y., Inatsuki B. In vitro detection of anti-thyroglobulin antibody forming cells from the lymphocytes of chronic thyroiditis patients and analysis of their regulation. Clin Exp Immunol. 1982 Sep;49(3):565–571. [PMC free article] [PubMed] [Google Scholar]
- Okita N., Row V. V., Volpe R. Suppressor T-lymphocyte deficiency in Graves' disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1981 Mar;52(3):528–533. doi: 10.1210/jcem-52-3-528. [DOI] [PubMed] [Google Scholar]
- Pacini F., DeGroot L. J. Studies of immunoglobulin synthesis in cultures of peripheral T and B lymphocytes: reduced T-suppressor cell activity in Graves' disease. Clin Endocrinol (Oxf) 1983 Mar;18(3):219–232. doi: 10.1111/j.1365-2265.1983.tb03206.x. [DOI] [PubMed] [Google Scholar]
- Roman S. H., Korn F., Davies T. F. Enzyme-linked immunosorbent microassay and hemagglutination compared for detection of thyroglobulin and thyroid microsomal autoantibodies. Clin Chem. 1984 Feb;30(2):246–251. [PubMed] [Google Scholar]
- Thompson P. M., McLachlan S. M., Parkes A., Clark F., Howel D., Rees Smith B. The IgG subclass distribution of thyroglobulin antibody synthesized in culture. Scand J Immunol. 1983 Aug;18(2):123–129. doi: 10.1111/j.1365-3083.1983.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Topliss D., How J., Lewis M., Row V., Volpé R. Evidence for cell-mediated immunity and specific suppressor T lymphocyte dysfunction in Graves' disease and diabetes mellitus. J Clin Endocrinol Metab. 1983 Oct;57(4):700–705. doi: 10.1210/jcem-57-4-700. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M. Autoimmune thyroid disease: developments in our understanding. Endocr Rev. 1984 Spring;5(2):309–355. doi: 10.1210/edrv-5-2-309. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M., Hall R. Methimazole inhibits thyroid autoantibody production by an action on accessory cells. Clin Immunol Immunopathol. 1983 Jul;28(1):39–45. doi: 10.1016/0090-1229(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Weiss I., De Bernardo E., Davies T. F. Plaque-forming cells in autoimmune thyroid disease. Clin Immunol Immunopathol. 1982 Apr;23(1):50–57. doi: 10.1016/0090-1229(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Weksler M. E., Moody C. E., Jr, Kozak R. W. The autologous mixed-lymphocyte reaction. Adv Immunol. 1981;31:271–312. doi: 10.1016/s0065-2776(08)60923-2. [DOI] [PubMed] [Google Scholar]
- Zakarija M. The thyroid-stimulating antibody of Graves' disease: evidence for restricted heterogeneity. Horm Res. 1980;13(1):1–15. doi: 10.1159/000179266. [DOI] [PubMed] [Google Scholar]


