Abstract
This review summarizes high-quality evidence supporting delayed umbilical cord clamping to promote placental transfusion to preterm and term neonates. In preterm neonates, delayed cord clamping may decrease mortality and the need for blood transfusions. Although robust data are lacking to guide cord management strategies in many clinical scenarios, emerging literature is reviewed on numerous topics including delivery mode, twin gestations, maternal comorbidities (eg, gestational diabetes, red blood cell alloimmunization, human immunodeficiency virus [HIV] infection, and severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection) and neonatal complications (eg, fetal growth restriction, congenital heart disease, and the depressed neonate). Umbilical cord milking is an alternate method of rapid placental transfusion, but has been associated with severe intraventricular hemorrhage in extremely preterm neonates. Data on long-term outcomes are discussed, as well as potential contraindications to delayed cord clamping. Overall, delayed cord clamping offers potential benefits to the estimated 140 million neonates born globally every year, emphasizing the importance of this simple and no-cost strategy.
Obstetric practitioners determine when to clamp and cut the umbilical cord after the neonate’s birth. The risks and benefits of various umbilical cord management strategies affect the estimated 140 million neonates born worldwide every year.1 Preterm neonates (less than 37 weeks of gestation) are at increased risk for morbidity and mortality.2 In preterm neonates, delayed cord clamping may decrease mortality and the need for blood transfusions. Globally, an estimated 14.84 million neonates were born prematurely in 20143; performing delayed cord clamping could potentially reduce hospital mortality, resulting in 148,400–742,000 additional annual survivors.4 Importantly, delayed cord clamping is a simple and no-cost intervention.
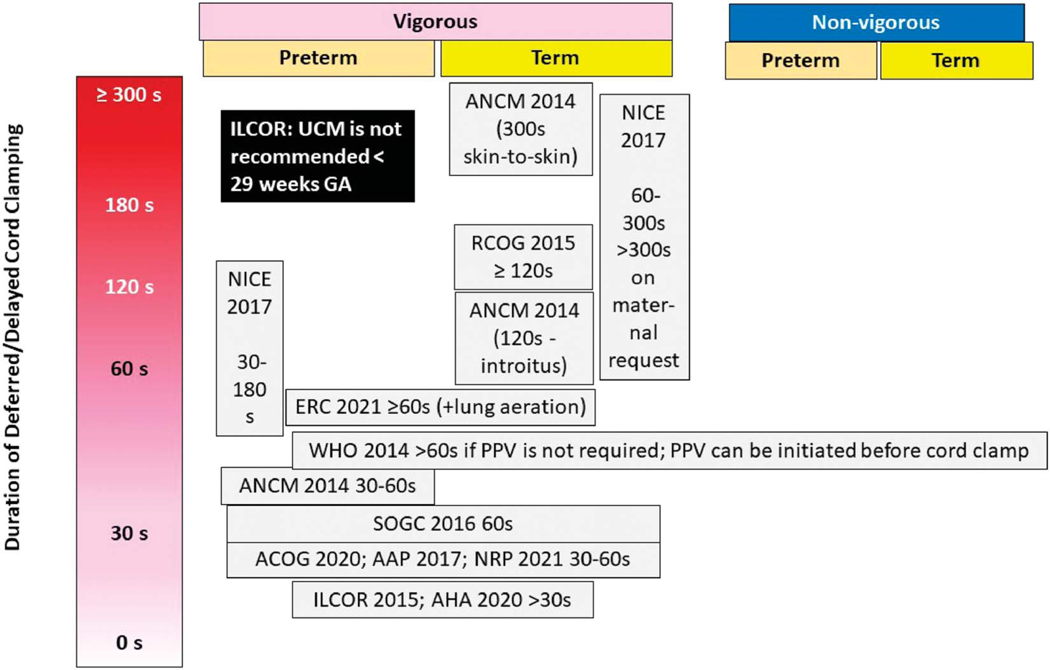
Worldwide, numerous governing bodies recommend delayed cord clamping in preterm and term newborns (Fig. 1).5–10 Since publication of the American College of Obstetricians and Gynecologists’ Committee Opinion in 201211 and the updated Committee Opinion in 2020,6 delayed cord clamping is recommended for at least 30–60 seconds in preterm and term neonates, except when early cord clamping is necessary because of neonatal or maternal indications.6 The following sections address methods of umbilical cord management, physiologic factors influencing placental transfusion volume, short-term and long-term outcomes in term and preterm neonates.
Fig. 1.
Delayed umbilical cord clamping recommendations by various organizations. The vertical axis shows duration of delay between birth and umbilical cord clamping. The recommendations for vigorous and nonvigorous neonates (preterm and term) are aligned under the respective legends. There is significant variability in these recommendations. ILCOR, International Liaison Committee on Resuscitation; UCM, umbilical cord milking; GA, gestational age; ACNM, American College of Nurse Midwives; NICE, National Institute for Health and Care Excellence; RCOG, Royal College of Obstetricians and Gynaecologists; ERC, European Resuscitation Council Guidelines; WHO, World Health Organization; PPV, positive pressure ventilation; SOGC, Society of Obstetricians and Gynaecologists of Canada; ACOG, American College of Obstetricians and Gynecologists; AAP, American Academy of Pediatrics; NRP, neonatal resuscitation program; AHA, American Heart Association.
UMBILICAL CORD MANAGEMENT STRATEGIES
Three umbilical cord management strategies are commonly used—early cord clamping (or immediate cord clamping), delayed cord clamping (or deferred cord clamping) and umbilical cord milking.12 Although synonymous, this review will use “early cord clamping” in place of “immediate cord clamping” because “immediate” implies that the cord was clamped the instant after delivery, instead of before a specific duration of time has passed after delivery (eg, 30 seconds), which is often the case. With early cord clamping, umbilical arterial blood flow stays in the neonate, and oxygenated umbilical venous blood stays in the placenta.
The practice of early cord clamping, which is not based on evidence or physiology, is no longer a part of the active management of the third stage of labor unless there is a perceived need for urgent resuscitation.13 In preterm neonates not requiring resuscitation, early cord clamping is associated with an increased risk for adverse outcomes and resources, as demonstrated in the Canadian Neonatal Network retrospective review of preterm neonates delivered at less than 33 weeks of gestation, which included 4,916 neonates who received early cord clamping and 4,419 who received delayed cord clamping.14 Compared with delayed cord clamping, early cord clamping was associated with an increased risk for death (adjusted odds ratio [aOR] 1.6; 95% CI 1.22–2.1), intraventricular hemorrhage (aOR 1.29; 95% CI 1.19–1.41), blood transfusion (aOR 1.68; 95% CI 1.35–2.09), surfactant use (aOR 1.40; 95% CI 1.26–1.57), inotropic support in the first 48 hours (aOR 2.07; 95% CI 1.58–2.70), late-onset sepsis (aOR 1.33; 95% CI 1.20–1.48), and patent ductus arteriosus (aOR 1.14; 95% CI 1.01–1.27). Similarly, a Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network study demonstrated lower mortality by 36 weeks of postmenstrual age and decreased treatment for hypotension in the first 24 hours after birth in 3,116 preterm neonates delivered at less than 29 weeks of gestation after exposure to delayed cord clamping or umbilical cord milking compared with early cord clamping.15 The preterm neonates in this study undergoing umbilical cord milking were sicker with higher need for positive pressure ventilation, intubation, surfactant, and chest compressions. However, even after adjustment for some of these factors, umbilical cord milking was associated with a higher risk of severe intraventricular hemorrhage (19.8 vs 11.8%) compared with delayed cord clamping.16
Umbilical cord milking involves manual expression of umbilical cord blood by milking blood 3–4 times down a 20–30 cm umbilical cord segment at a rate of ~10 cm/second. Umbilical cord milking can be performed through a cut or intact umbilical cord.17 Katheria et al18 compared umbilical cord milking with delayed cord clamping in a randomized trial of preterm neonates delivered at less than 32 weeks of gestation. The primary composite outcome of the trial was death or severe intraventricular hemorrhage. The trial was stopped early by the data safety monitoring board due to concerns about severe intraventricular hemorrhage in the milking group. The incidence of the primary outcome was similar overall (12% in the umbilical cord milking group and 8% in the delayed cord clamping group, P=.16). However, umbilical cord milking was associated with severe intraventricular hemorrhage in extremely preterm neonates (23–27 weeks of gestation). In addition, death or severe intraventricular hemorrhage was more common (21%) with umbilical cord milking compared with delayed cord clamping (8%) among preterm neonates delivered by the vaginal route. An exploratory post hoc analysis showed that the presence of maternal chorioamnionitis significantly modified the association between umbilical cord milking and severe intraventricular hemorrhage.
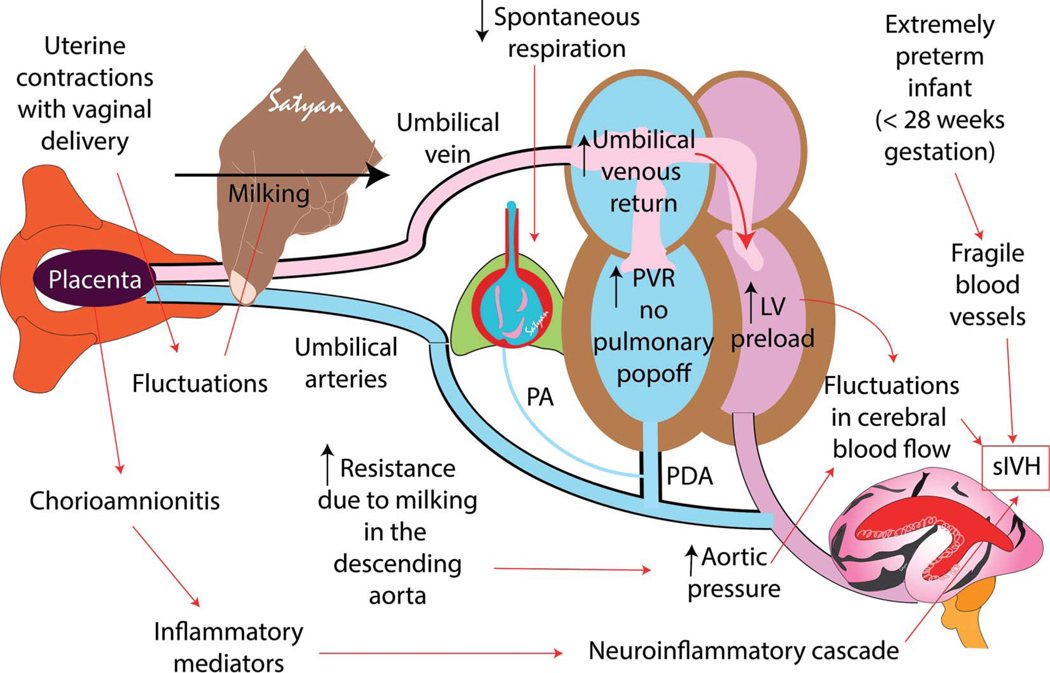
We speculate that in the presence of uterine contractions, vaginal delivery and cord milking significantly increase aortic pressure. In the absence of spontaneous breathing effort, pulmonary vascular resistance remains high with a bidirectional or right-to-left ductal shunt preventing a surge in pulmonary blood flow with milking. Hence, the increase in aortic pressure, without a pulmonary pop-off can potentially cause fluctuations in cerebral blood flow. Similar fluctuations in cerebral blood flow have been described in preterm lamb models.19 Associated chorioamnionitis could also contribute to fragility of germinal matrix vessels increasing the risk of intraventricular hemorrhage with fluctuating cerebral blood flow (Fig. 2). After publication of this article by Katheria et al, some organizations have recommended against umbilical cord milking in preterm neonates delivered at less than 28–29 weeks of gestation (Fig. 1).
Fig. 2.
Speculative pathogenesis of umbilical cord milking associated severe intraventricular hemorrhage (sIVH) in extremely preterm neonates. After vaginal delivery, uterine contractions propel blood toward the neonate. Umbilical cord milking will enhance umbilical venous return to the right atrium. Because most of the extremely preterm neonates do not have established lung ventilation soon after birth, pulmonary vascular resistance (PVR) and right ventricular pressure remain high, diverting most of umbilical venous return to the left ventricle (LV), thereby increasing LV preload and increasing LV output to the ascending aorta. Milking and uterine contractions increase resistance in the descending aorta. High PVR leads to right-to-left shunt at the patent ductus arteriosus (PDA) level. Fluctuant increase in ascending aorta flow is mostly directed to the cerebral circulation due to high PVR and resistance in descending aorta. Chorioamnionitis and inflammatory mediators along with extreme prematurity contribute to fragility of germinal matrix vasculature precipitating sIVH. PA, pulmonary artery. Courtesy of Satyan Lakshminrusimha. Used with permission.
Most recommendations regarding delayed cord clamping are time-based and range from 30 seconds to a few minutes. Compared with umbilical cord milking, a physiologic delayed cord clamping approach highlights the importance of establishing lung ventilation when placental circulation is still intact to promote left ventricular preload, which should optimize postnatal left ventricle function and promote an optimal hemodynamic transition at birth.20 Based on established recommendations and available data, this article will focus on delayed cord clamping because most organizations endorse this placental transfusion method.6–8,21–25
PLACENTAL TRANSFUSION VOLUME
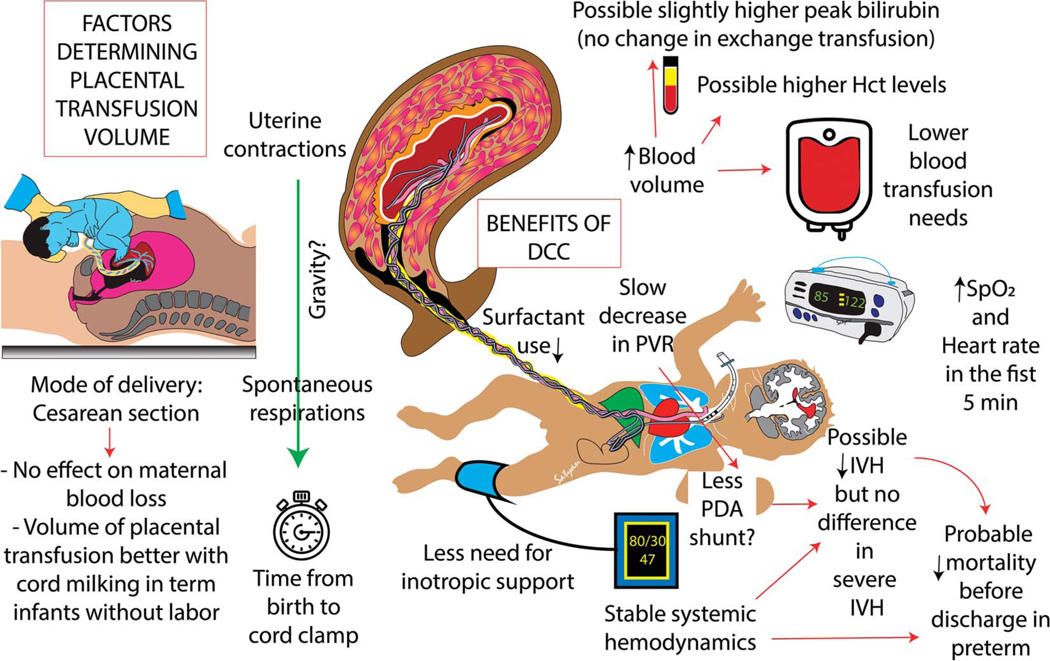
After birth, umbilical cord blood flow typically persists for a few minutes, with net-flow from the placenta to the neonate, resulting in a time-dependent decrease in placental blood volume. The net blood volume transferred to the neonate after birth before clamping the umbilical cord is referred to as the placental transfusion volume; this volume contributes to neonatal hemodynamic stability and red cell volume. The net placental blood volume transferred to the newborn during delayed cord clamping may be influenced by numerous factors beyond timing of umbilical cord clamping, including delivery mode, the presence and strength of uterine contractions, the position of the newborn, and the presence and timing of sustained spontaneous breathing (Fig. 3).
Fig. 3.
Factors determining placental transfusion and benefits and risks of delayed cord clamping (DCC). Uterine contractions, mode of delivery, time from birth to cord clamp, and possibly gravity and the neonate’s spontaneous respirations may influence the volume of placental transfusion. Benefits of DCC are shown on the right side of the diagram. Hct, hematocrit; SpO2, oxygen saturation by pulse oximetry; PVR, pulmonary vascular resistance; PDA, patent ductus arteriosus; IVH, intraventricular hemorrhage. Courtesy of Satyan Lakshminrusimha. Used with permission.
In term newborns, postnatal placental transfusion provides an additional 80–100 mL of blood, representing between one third and one quarter of the neonatal blood volume.26,27 In a study by Yao et al28 of 301 healthy term neonates delivered vaginally, delayed cord clamping transferred ~55% of the neonate’s total blood volume; this transfer was in a stepwise manner with up to 30% of the blood volume transferred within 10–15 seconds, followed by a short period of no gain in neonatal blood volume, and then transfer of the remaining blood volume by 3 minutes.
In preterm newborns, postnatal placental transfusion also increases neonatal blood volumes. In a small study of vaginally delivered preterm newborns, delayed cord clamping for 60–90 seconds resulted in a significantly increased mean blood volume (80.5±11.2 mL/kg; n=9) compared with early cord clamping (61.3±5.4 mL/kg; n=11).29
Duration of Delayed Umbilical Cord Clamping
Most studies in preterm neonates include duration of delayed cord clamping ranging from 30 to 60 seconds and 60 seconds or more in term neonates. Although current delayed cord clamping recommendations are time-based, insufficient evidence exists in preterm and term neonates to determine the optimal time to clamp the umbilical cord. Future trials comparing different lengths of delay before umbilical cord clamping in different gestational age groups may inform more precise recommendations. The concept of “physiological” cord clamping refers to determination of the duration of placental transfusion based on establishment of lung ventilation.30
Position of the Neonate During Delayed Umbilical Cord Clamping After Vaginal Delivery
In preterm vaginal deliveries, data are lacking on the feasibility and safety of placement of the neonate on the maternal abdomen or chest during delayed cord clamping, especially with extremely preterm neonates who have shorter umbilical cord lengths and often require resuscitation. In term vaginal deliveries, to promote bonding by skin-to-skin contact after birth, neonates can be placed at the mother’s anterior thighs, abdomen, or chest while the cord is intact because this appears safe and does not hinder placental transfusion based on weight gain or hematocrit at 24 hours after birth.31,32 In general, after spontaneous vaginal delivery, uterine contractions generate an adequate pressure gradient (Fig. 3) to facilitate placental transfusion irrespective of the neonate’s position.
Umbilical Cord Management After Cesarean Delivery
Compared with research on neonates delivered vaginally, fewer studies have investigated outcomes after delayed cord clamping performed at the time of cesarean delivery. Debate exists on whether placental-to-neonatal transfusion results in net increases in neonatal blood volumes with delayed cord clamping at the time of cesarean delivery. In elective cesarean deliveries, the absence of uterine contractions preceding delivery may influence the effectiveness of placental-to-neonatal transfusion. With preterm, twin (or higher-order multifetal gestations), or emergency cesarean deliveries, the effects of delayed cord clamping on maternal and neonatal outcomes remains understudied. Table 1 illustrates a summary of neonatal outcomes associated with delayed cord clamping performed at the time of cesarean delivery.29,33–37
Table 1.
Neonatal Outcomes Associated With Delayed Umbilical Cord Clamping at the Time of Cesarean Delivery
| Author, Study Type, Setting | Gestational Age (wk) | Management or Intervention | Outcome Measures | Results |
|---|---|---|---|---|
|
| ||||
| Aladangady et al29 | 24 0/7–32 6/ | ECC (n=12) | Blood volume | Blood volume greater with DCC (70.4 |
| RCT | 7 | DCC 30–90 | RBC volume (biotin | ±10.2) than ECC group (64.0 69.1 |
| The Queen Mother’s Hospital, | s (n=14) | labeling method | mL/kg)* | |
| Glasgow, United Kingdom | and with fetal | P<.1; 95% CI 21.5 to 14.2 | ||
| hemoglobin | No difference in Hct levels | |||
| dilution) | measured 1 h after birth | |||
| Cavallin et al33 | 39 or more | ECC within 10 | Hct (%) at day 2 | Hct greater with DCC (54±6) than |
| RCT | s (n=40) | with ECC (48%±5%), | ||
| Padua Teaching Hospital, Italy | DCC more | MD 6, 95% CI 3–8; P<.001 | ||
| than 60 s | ||||
| (n=40) | ||||
| De Bernardo et al34 RCT |
37 or more | ECC (n=66) DCC 60 s |
Preductal SpO2 after delivery |
No differences in oxygen saturation, heart rate, or temperature |
| Department of Mother and | (n=66) | Hct at 72 h | Hct greater with DCC | |
| Child’s Health Poliambulanza | P=.001 | |||
| Foundation, Brescia, Italy | ||||
| Purisch et al35 | 37 or more | ECC (n=56) | Neonatal hemoglobin | Hemoglobin level higher with DCC |
| RCT | within 15 s | level at 24–72 h | (mean 18.1 g/dL; 95% CI 17.4– | |
| 1 academic hospital and 1 | after birth | after birth | 18.8) vs immediate (16.4 g/dL; 95% | |
| community hospital within a | DCC (n=57) | (secondary | CI 15.9–17.0) cord clamping (MD | |
| single university-based medical | 60 s after | outcome) | 1.67 g/dL; 95% CI 0.75–2.59; | |
| center in New York City | birth | P<.001) | ||
| Jenusaitus et al37 | 37 or more | ECC (n=416) | Apgar scores at 1 and | Apgar scores slightly lower in the |
| Retrospective cohort study | DCC more | 5 min | DCC group at 1 min (median [IQR] | |
| Tertiary care hospital, | than 60 s | 8.0 [8.0–9.0] vs 9.0 [8.0–9.0], | ||
| Connecticut | (n=317) | P=.035), but were similar at 5 min | ||
| Consonni et al36 | 37 or more | ECC (median | Primary outcome was | Elective CD: UCM led to higher |
| Retrospective cohort study | 20 s, IQR | neonatal Hct at 48 h | neonatal Hct (mean±SD; 61.5% | |
| 2 hospitals, Italy | 20–40; | ±5.5) vs ECC (55.1% ±5.5) and | ||
| n=53) | DCC (56.4% ±5.7, P=.001) | |||
| UCM | CD with labor: no significant | |||
| (median 40 | differences among the placental | |||
| s, IQR 30– | transfusion strategies | |||
| 60; n=33) | ||||
| DCC (60 s; | ||||
| n=137) | ||||
RCT, randomized controlled trial; DCC, delayed cord clamping; ECC, early cord clamping; RBC, red blood cell; Hct; hematocrit; MD, mean difference; IQR, interquartile range; UCM, umbilical cord milking; CD, cesarean delivery.
Data are mean±SD.
Reanalysis excluding three neonates assigned randomly to delayed umbilical cord clamping but who received immediate clamping demonstrated that neonates who had delayed umbilical cord clamping had significantly increased blood volumes (72.8 vs 63.6 mL/kg 95% CI 2.0–16.4, P=.01).
NEONATAL OUTCOMES
Many studies have evaluated the effects of placental transfusion strategies, with most focused on short-term outcomes in preterm and term neonates.6 The main findings from recent robust systematic reviews and meta-analyses will be discussed to provide further insight into potential risks and benefits of delayed cord clamping compared with early cord clamping in preterm and term neonates. Because strategies may affect preterm neonates differently based on their level of prematurity, consideration of gestational age at delivery is recommended.
Six systematic reviews and meta-analyses published between 2018 and 2021 that evaluated delayed cord clamping in neonates are reviewed in Table 2.4,38–42
Table 2.
A Review of Recent Systematic Reviews and Meta-analyses Evaluating Delayed Cord Clamping Compared With Early Cord Clamping in Neonates
| Study—1st Author |
||||||
|---|---|---|---|---|---|---|
| Fogarty4 | Rabe39 (Cochrane) | Jasani40 | Persad41 | Seidler42 (ILCOR) | Gomersall38 | |
|
| ||||||
| No. of included studies | 18 | 25 | 31 | 19 | 23 | 33 |
| Gestational age (wk) | Less than 37 | Less than 37 | Less than 37 | Less than 32 | Less than 34 | 34 or more |
| Total N | 2,834 | 3,100 | 3,823 | 3,010 | 3,514 | 5,263 |
| Mortality—hospital | ↓ | ↓ | ↓ | Uncertain | Uncertain | Uncertain |
| or neonatal death | n=2,834, RR | n=2,680, | n=3,083, | n=595, RR | n=2,988, RR 1.02 | n=537, RR |
| before discharge | 0.68 (95% CI | aRR 0.73 | OR 0.64 | 0.51 (95% | (95% CI 0.993– | 2.54 |
| 0.52–0.90) | (95% CI | (95% CI | CI 0.26– | 1.04) | (95% CI 0.50– | |
| 28 wk or less | 0.54–0.98) | 0.39–0.99) | 1.00) | number needed to | 12.74) | |
| n=996, RR | benefit: 50, 95% | |||||
| 0.70 (95% CI | CI 25 to no | |||||
| 0.51–0.95; P | benefit | |||||
| 5.02) | ||||||
| Hb (g/dL) or Hct | ↑Peak Hct | Hb at 24 h – no | ↑ Hb from 1 | ↑ Hct within 24 h | Hb at 24 h—no | |
| (%) | n=1,587, MD | change | d to 7 d after | n=1,100, MD | change | |
| 2.73 (95% CI | n=42, MD | birth | 2.63 (95% CI | n=1,352, MD | ||
| 1.94–3.52; | 0.8 (95% CI | n=921, MD | 1.85–3.42) | 1.17 (95% CI | ||
| P<.001) | −0.02 to | 2.39 (95% | Within 7 d after | 0.48–1.86) | ||
| 1.62) | CI 2.17– | birth n=1,550, | 7 d after birth | |||
| 2.61) | MD 2.7 (95% CI | n=695, MD | ||||
| 1.88–3.52) | 1.11 (95% CI | |||||
| 0.40–1.82) | ||||||
| No difference | ||||||
| in anemia 4–6 | ||||||
| mo of life | ||||||
| n=937, RR | ||||||
| 1.01 (95% CI | ||||||
| 0.75–1.37) | ||||||
| PRBC transfusions | ↓ | ↓ | ↓ | ↓ | ↓ | |
| n=2,595, RR | n=2,280, RR | n=2,904, RR | n=769, RR | n=2,910, RR 0.83 | ||
| 0.81 (95% CI | 0.66 (95% CI | 0.48 (95% CI | 0.82 (95% | (95% CI 0.77– | ||
| 0.74–0.87) | 0.5–0.86) | 0.32–0.66) | CI 0.69– | 0.90) | ||
| 0.98) | Total # blood | |||||
| transfusions | ||||||
| during hospital | ||||||
| course n=242, | ||||||
| MD −0.63 (95% | ||||||
| CI −1.08 to | ||||||
| −0.17) | ||||||
| Polycythemia (Hct | ↑ | |||||
| greater than | n=2,529, RR | |||||
| 65%) | 2.65 (95% CI | |||||
| 1.61–4.37)* | ||||||
| Hemodynamics, BP | ↑ Mean BP | ↑Mean BP during | ||||
| (mm Hg) | n=208, MD | first 12 h MD 1.79 | ||||
| 2.87 (95% CI | (95% CI 0.53– | |||||
| 1.09–4.64) | 3.05) | |||||
| ↓ Inotropic | ||||||
| support first 24 h RR 0.36 (95% CI 0.17–0.75) | ||||||
| IVH | No difference | ↓ any grade | ↓ any grade | Uncertain-any | No difference- | Not applicable |
| any IVH | IVH | IVH | grade IVH | grade 3 and 4 IVH | due to | |
| n=2,871, RR | n=2,333, | n=3,316, | n=1,310, | n=2,972, RR 0.98 | gestational | |
| 0.87 (95% CI | aRR 0.83 | OR 0.73 | RR,0.86 | (95% CI 0.67– | age | |
| 0.75–1.00) | (95% CI 0.7– | (95% CI | (95% CI | 1.42) | ||
| No difference | 0.99) | 0.54–0.97) | 0.7–1.05) | |||
| grade 3 and 4 | No | No | Uncertain- | |||
| IVH n=2,300, | difference | difference | grade 3 and | |||
| RR 0.87 (95% | grade 3 and 4 | grade 3 and 4 | 4 IVH | |||
| CI 0.59–.027) | IVH | IVH | n=2,324, | |||
| n=2058, | n=2,469, | RR 0.98 | ||||
| aRR 0.94 | OR 0.83 | (95% CI | ||||
| (95% CI | (95% CI | 0.62–1.54) | ||||
| 0.63–1.39) | 0.47–1.34) | |||||
| Hyperbilirubinemia | ↑Peak bilirubin | No difference | No difference in | Possibly higher | ||
| n=2,358, MD | in | phototherapy | use of | |||
| 0.26 mg/dL | phototherapy | n=908, RR, 0.99 | phototherapy | |||
| (95% CI 0.07– | n=495, aRR | (95% CI 0.95– | n=2,814, RR | |||
| 0.45, P=.008) | 1.05 (95% CI | 1.03) | 1.28 (95% CI | |||
| No difference | 0.95–1.16) | 0.90–1.82) | ||||
| with exchange transfusions n=2,139, RR 0.29 (95% CI 0.05–1.73) | ||||||
ILCOR, International Liaison Committee on Resuscitation; RR, risk ratio; aRR, average risk ratio; OR, odds ratio; Hb, hemoglobin; Hct, hematocrit; MD, mean difference; PRBC, packed red blood cell; BP, blood pressure; IVH, intraventricular hemorrhage.
No increase in partial exchange transfusions.
Mortality
Overall, compared with early cord clamping, delayed cord clamping appears to be associated with reduced in-hospital mortality in preterm neonates (Table 2). In late preterm or term neonates, compared with early cord clamping, delayed umbilical cord clamping (30 seconds or more) is not associated with a reduction in mortality.4,38–40 Stating that the relative risk depends on an event’s incidence and the incidence of survival is higher than mortality, the ILCOR (International Liaison Committee on Resuscitation) review by Seidler et al42 assessed survival, the inverse of mortality. The benefit of delayed cord clamping regarding enhanced survival was suggested, but not compelling (RR 1.02, 95% CI 1.00–1.04).
How delayed cord clamping helps decrease mortality in premature neonates is yet to be determined. Mercer et al43 proposed that the enhanced blood volume provided by placental transfusion strategies, such as delayed cord clamping, may promote increased vascular perfusion of the neonate, which triggers mechanical stimuli-induced electrochemical activity, facilitating organ-specific endothelial cell signaling, essential for normal growth, maintenance, and repair; this enhanced blood volume may also increase progesterone levels and pulmonary artery pressure, which supports neonatal adaptation after birth. Early cord clamping prevents these potential benefits of enhanced vascular perfusion.
Hematologic Parameters and Red Blood Cell Transfusions
Hematologic parameters (hemoglobin or hematocrit—peak values at 24 hours or 7 days) are higher with delayed cord clamping in most studies and delayed cord clamping is associated with a decreased need for packed red cell transfusions (Table 2).4,39–42
Most extremely low birth weight (less than 1,000 g) preterm neonates become anemic and receive at least one blood transfusion during their neonatal intensive care unit (NICU) hospitalization,44 with frequent phlebotomies done for testing and monitoring purposes playing a major role in overall blood losses. Extremely preterm neonates have been reported to lose 58% of their endogenous blood volume (median 40.4 mL/kg, interquartile range 23.9–53.3 mL/kg) related to blood losses in the first 2 weeks of life.45 Transfusion exposure has been associated with increased risk for morbidity and mortality in premature neonates.44,46,47 Because delayed cord clamping reduces the need for blood transfusions, this is a safe approach to increase starting blood volume and avoid transfusion-associated risks in vulnerable premature neonates.
Polycythemia (Hematocrit Greater Than 65%)
Fogarty et al4 concluded delayed cord clamping increased the incidence of polycythemia (13 trials; 2,529 neonates; RR 2.65; 95% CI 1.61–4.37) without an increased need for partial exchange transfusion (four trials; 1,743 neonates; RR 0.14; 95% CI 0.01–2.74).
Hemodynamic Parameters
In general, there is a tendency toward higher blood pressure and reduced need for inotropic support after delayed cord clamping.39,42 Because blood pressure data are usually not collected within the first hour after delivery, the immediate cardiovascular effects of delayed cord clamping are unclear, as are correlations of early blood pressure values and neurodevelopmental outcomes.
Intraventricular Hemorrhage
Data are mixed regarding delayed cord clamping and intraventricular hemorrhage (any grade or severe, grade 3 or 4) with some studies showing protective effect against any intraventricular hemorrhage and no studies showing any difference in severe intraventricular hemorrhage (Table 2).4,39–42 The mechanism of reduction in intraventricular hemorrhage with delayed cord clamping in some studies is not clear. We speculate that hemodynamic stability with higher systemic blood pressure and a slow decrease in pulmonary vascular resistance associated with delayed cord clamping48 limits ductal shunting and leads to less fluctuations in cerebral blood flow.
Hyperbilirubinemia
Although peak bilirubin was slightly elevated in neonates with delayed cord clamping, there is no evidence of increased need for phototherapy or exchange transfusion in existing systematic reviews (Table 2).4,39,42
UMBILICAL CORD BLOOD GASES
Routine umbilical cord blood gas analysis, which helps to retrospectively evaluate labor and delivery events, is typically performed to diagnose and treat potentially acidemic neonates after birth.49 Current cord blood gas reference ranges are based on measurements established when early cord clamping was standard practice. The effect of delayed cord clamping on umbilical cord blood gas analysis in preterm neonates is unclear. In vaginally delivered, healthy, term singletons, a 2020 systematic review concluded that delayed cord clamping (n=218), compared with early cord clamping (n=234), does not affect or has a minimal, clinically insignificant effect on umbilical cord blood acid–base balance.50 Blood gas values associated with delayed cord clamping were slightly more acidemic, but still within normal reference ranges.
Limited information is available regarding the effects of delayed cord clamping on umbilical cord blood gas values in cesarean deliveries. In a cohort of term neonates delivered vaginally (n=97) or by elective cesarean (n=124), arterial cord blood pH, bicarbonate, and base excess decreased significantly after 3-minutes of delayed cord clamping compared with early cord clamping values, both in vaginal and cesarean deliveries. Delayed cord clamping was associated with an increase in pCO2 and lactate in cesarean compared with vaginal deliveries. The neonates in this cohort were vigorous after delivery based on their median 5-minute Apgar score of 10 in both groups.51
IMMEDIATE POSTNATAL HEART RATE AND OXYGEN SATURATIONS
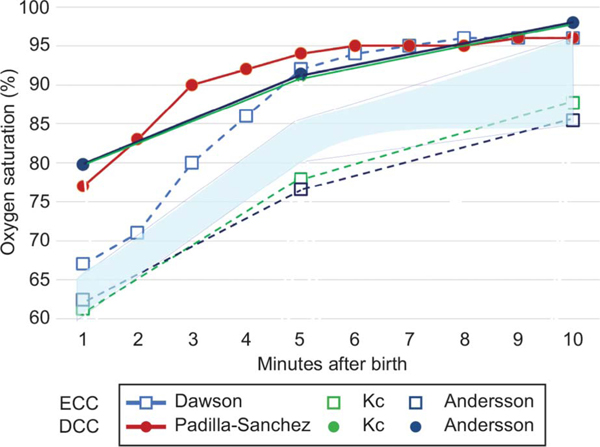
Delayed cord clamping may affect postnatal heart rate and pulse oximetry oxygen saturation levels after delivery (Fig. 4). Studies demonstrate that delayed cord clamping may promote earlier postnatal heart rate and oxygenation stabilization in the first minutes after birth in healthy term newborns.52 As demonstrated in Figure 4, in vaginal deliveries, delayed cord clamping is associated with higher oxygen saturations at five and 10 minutes after birth compared with early cord clamping.52–57 It is possible that oxygenated blood (~SpO2 85%) in the umbilical venous return improves preductal saturations during the first 5 minutes after birth. Because delayed cord clamping is the standard for vigorous preterm and term neonates, as more normative data accumulate, initial target ranges for heart rate and pulse oximetry oxygen saturation may need to be reconsidered to guide resuscitation efforts.
Fig. 4.
Oxygen saturations (preductal: median or mean) after early cord clamping (ECC) or delayed cord clamping (DCC) in various studies with vaginal deliveries. Closed circles depict DCC, and open squares depict ECC. The blue area represents the preductal oxygen saturation (SpO2) values recommended by American Academy of Pediatrics (AAP)/American Heart Association (AHA) Neonatal Resuscitation Program.
MATERNAL BLOOD LOSS
Delayed cord clamping does not appear to affect maternal blood loss. A retrospective cohort study compared two time periods related to cesarean deliveries, one in which early cord clamping was routine (n=416) and one after delayed cord clamping (60 seconds or more; n=317) became standard of care. Delivering in the time period with delayed cord clamping was not associated with negative effects on maternal hematologic characteristics and was, in fact, associated with reduced estimated blood loss.37 In another study of patients undergoing scheduled cesarean delivery of term singleton pregnancies, delayed cord clamping (60 seconds), compared with early cord clamping (15 seconds), resulted in no significant difference in the change in maternal hemoglobin level (an objective measure of blood loss) on postoperative day 1.35
Compared with early cord clamping, delayed cord clamping in preterm deliveries (delivered at less than 37 weeks of gestation) does not appear to affect the risk of women with postpartum hemorrhage (greater than 500 mL).4 In the two comprehensive ILCOR reviews (neonates grouped as 34 weeks of gestation or less or 34 weeks or more), the risk of postpartum hemorrhage (greater than 500 mL) or severe postpartum hemorrhage (greater than 1,000 mL) was not affected by delayed cord clamping.38,42
MATERNAL COMORBIDITIES
Gestational and Pre-existing Diabetes Mellitus
Gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy. Globally, the prevalence of GDM range from 8.4% to 24.5% (median estimate of 12.9%), with an estimated prevalence of 8.9% in North America.58 Neonates born to mothers with GDM have higher perinatal morbidity and mortality compared with those born to mothers without diabetes.59,60 In a randomized trial of 80 term neonates, compared with early cord clamping (median 4 seconds), delayed cord clamping (median 60 seconds) did not affect the incidence of hypoglycemia or jaundice requiring phototherapy, was associated with higher postnatal hematocrit levels at 6 and 24 hours of age, led to increased polycythemia rates, but without the need for partial exchange transfusion treatment, and was associated with decreased NICU admissions of neonates with respiratory distress.61
Red Blood Cell Alloimmunization
Hemolytic disease of the newborn is an alloimmune disorder due to transplacentally transmitted maternal immunoglobulin G antibodies that bind to paternally inherited antigens present on fetal red blood cells leading to hemolysis and elevated bilirubin levels.62 Hyperbilirubinemia refractory to intensive phototherapy treatment may require exchange transfusions; this intervention may help prevent central nervous system damage, such as kernicterus. Given the risks associated with hemolytic disease of the newborn, decisions surrounding placental transfusion practices warrant consideration; however, limited evidence-based guidance is available on this topic.
A single-center, retrospective cohort study by Garabedian et al63 assessed the risk and benefits of delayed cord clamping (more than 30 seconds) in cases of red blood cell alloimmunization involving 72 neonates who received in utero transfusions for fetal anemia primarily for anti-D. Compared with a historic cohort of neonates who had early cord clamping (median 34.1, range 27.6– 37.0 weeks of gestation, n=36), neonates who received delayed cord clamping (median 34.9, range 28.6–37.9 weeks of gestation, n=36) had higher hemoglobin levels measured within 1 hour after birth and required less postnatal exchange transfusions (19.4% vs 47.2%, P<.013) without significant differences in maximum bilirubin levels, intensive phototherapy rates, or total phototherapy treatment duration between the two groups.
A single-center randomized trial by Sahoo et al evaluated the effect of delayed cord clamping (60 seconds or more; n=36) compared with early cord clamping (10 seconds or less; n=34) in Rh-alloimmunized neonates; the median (interquartile range) gestation of enrolled neonates was 3532–36 weeks of gestation. Compared with early cord clamping, delayed cord clamping was associated with a higher hematocrit 2 hours after delivery (mean difference 4.9%; 95% CI 0.6–9.1, P=.03), without significant differences in the duration of phototherapy, requirement for partial exchange and double volume exchange transfusion, hemodynamic parameters based on functional echocardiography in initial 12 hours after delivery, and blood transfusion requirements until 14 weeks of age.64
Although these preliminary studies suggest that delayed cord clamping may be beneficial in the setting of alloimmunization, additional large multicenter studies including neonates with severe anemia and hydrops with long-term neurodevelopmental follow-up are needed.
MATERNAL HUMAN IMMUNODEFICIENCY VIRUS (HIV) INFECTION
Antiretroviral drugs for mothers living with HIV and postnatal prophylaxis in newborns can result in neonatal anemia. WHO guidelines recommend delayed cord clamping for at least 1–3 minutes among women living with HIV because, “the benefits of delaying umbilical cord clamping outweigh the risks” of HIV transmission.24 Women living with HIV and their neonates should receive antiretroviral therapy to prevent mother-to-child transmission of HIV.
In a randomized study of 64 mother–newborn dyads, Pogliani et al65 compared the effect of delayed cord clamping (120 seconds) with that of early cord clamping (less than 30 seconds) in term neonates delivered by planned cesarean to mothers on stable antiretroviral therapy. Neonates who received delayed cord clamping had significantly higher mean hemoglobin concentrations at 24 hours and 1 month of age. After 12 months of age, no between-group hemoglobin concentration differences were detected. None of the neonates had positive HIV–polymerase chain reaction from birth through 18 months of age. The risk of vertical transmission in mothers not treated with antiretroviral therapies during pregnancy or with high viral loads remains unclear, but would be anticipated to be no higher than in utero acquisition of the virus.
MATERNAL SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-CoV-2) INFECTION
Given the potential newborn benefits, delayed cord clamping is recommended with maternal SARS-CoV-2 infection by major scientific societies.66–68 A prospective observational study of pregnant women with SARS-CoV-2 demonstrated that compared with early cord clamping (less than 30 seconds; n=231), delayed cord clamping (more than 30 seconds; n=172) was not associated with significant differences in positive neonatal test results for SARS-CoV-2 infection; no confirmed vertical transmission cases were detected.69
Anti–SARS CoV-2 immunoglobulin G antibodies are detected in umbilical cord blood of neonates born to mothers who received coronavirus disease 2019 (COVID-19) mRNA vaccines during pregnancy.70–72 Placental transfusion may enhance passive immunity in the neonate, but there are not studies to date to confirm this possibility. Although rare, maternal SARS-CoV-2 infection and passive transfer of transplacental antibodies may result in multisystem inflammation, atrioventricular conduction abnormalities, and thrombosis in the early neonatal period, consistent with neonatal multisystem inflammatory syndrome.73 The effect of delayed cord clamping on neonatal multisystem inflammatory syndrome is unclear. With the emergence of new SARS-CoV-2 viral genetic variants, with mutations that may affect transmissibility and spread, antigenicity, or virulence, close viral surveillance and ongoing risk assessment for maternal-to-neonatal transmission will be needed to guide clinical practice.
NEONATAL SPECIAL CIRCUMSTANCES
Multifetal Gestations
Published trials demonstrating actual risks or benefits of delayed cord clamping specific to dichorionic twins in vaginal or cesarean deliveries are lacking; however, given each twin has his or her own placenta, this placental transfusion strategy should be feasible. In monochorionic twins with known or suspected twin-to-twin transfusion syndrome, delayed cord clamping is not recommended due to the risk of inter-twin transfusion at birth. Although most twin gestations with monochorionic diamniotic placentation are not complicated by twin-to-twin transfusion syndrome, there are no available data to guide placental transfusion practice in these circumstances.
Fetal Growth Restriction
Fetal growth restriction in both preterm and term neonates is associated with an increased risk for morbidity, mortality, and significantly worse cognitive outcomes compared with neonates born at a size appropriate for gestational age.74,75 Limited information is available on the effect of delayed cord clamping in preterm neonates with fetal growth restriction. Due to concerns that delayed cord clamping may exacerbate the risk for symptomatic polycythemia, neonates with fetal growth restriction have typically been excluded from the clinical practice of delayed cord clamping. In a randomized controlled trial of preterm neonates with fetal growth restriction born at less than 34 weeks of gestation, compared with early cord clamping (10 seconds or less), delayed cord clamping (60 seconds) was associated with significantly increased peripheral blood percentage of CD34, a marker of stem cell levels. Delayed cord clamping was also associated with significantly higher hemoglobin levels at 1 hour after birth and 2 months of age, higher peak bilirubin level without increased phototherapy needs, a decreased duration of supplemental oxygen during hospitalization, and no increased risk of polycythemia.76 Notably, in preterm neonates without fetal growth restriction born at less than 32 weeks of gestation, delayed cord clamping (30–45 seconds) has been associated with lower peripheral blood hematopoietic stem cell counts compared with early cord clamping (less than 10 seconds),77 highlighting the need for more studies in this population.
A secondary analysis of a subset of neonates with fetal growth restriction who were enrolled in a randomized controlled trial by Mercer et al,78 demonstrated delayed cord clamping (30–45 seconds plus umbilical cord milking × 1; n=25) was safe and associated with normal, but higher, initial temperatures on NICU admission compared with neonates who received early cord clamping (n=32).79
In a trial of neonates with fetal growth restriction delivered at more than 28 weeks of gestation, compared with early cord clamping (within 30 seconds; n=55, mean gestational age 35.9±3.0 weeks), delayed cord clamping (more than 60 seconds; n=55, mean gestational age 36.5±2.9 weeks) was associated with improved systemic blood flow at 24 hours based on superior vena cava blood flow measurements.80 Delayed cord clamping was also associated with increased hematocrit at 24 hours of age and serum ferritin levels at 3 months of age without higher incidences of polycythemia or phototherapy requirements for significant hyperbilirubinemia in the newborn period. No differences in major morbidities or mortality were noted between the groups.
In a randomized trial of term small-for-gestational-age neonates, the majority who were delivered by cesarean, delayed cord clamping (mean±SD 62.6±6.5 seconds; n=55) resulted in similar outcomes as early cord clamping (12.1±3.7 seconds; n=58) with no differences in symptomatic polycythemia or the need for partial exchange transfusions.81 At 3 months of age, the serum ferritin levels were higher in the delayed cord clamping group.
Congenital Heart Disease
A systematic review and meta-analysis of 260 studies, encompassing 130,758,851 live births from 2010 to 2017, demonstrated a birth prevalence of congenital heart diseases of 9.41 per 1,000 (95% CI 8.602–10.253).82 Although not all neonates with congenital heart disease will be critically ill at birth, decisions on umbilical cord management may influence outcomes. In theory, by increasing blood volume and hematocrit through delayed cord clamping, neonates with cyanotic heart disease could benefit from improved oxygen carrying capacity and hemodynamics.
A randomized pilot study of term neonates with critical congenital heart disease who were anticipated to require surgery or catheterization within the first month of life, demonstrated that delayed cord clamping (up to 120 seconds; n=15) compared with early cord clamping (less than 30 seconds; n=15) resulted in increased hematocrit in the first 72 hours after birth and a higher probability of not having received a blood transfusion throughout the hospitalization (7% vs 43%; P=.02).83
Cardiac defect-specific hematocrit and blood volume goals associated with the best short- and long-term outcomes have yet to be determined. Given the high prevalence of congenital heart disease, further research is needed to determine ideal umbilical cord management strategies in preterm and term neonates based on anatomic and pathophysiology classifications of cardiac malformations.
NONVIGOROUS NEONATES
Most guidelines include only neonates who are vigorous and “not requiring resuscitation.” In addition, the underlying etiologies contributing to why a preterm or term neonate is deemed not vigorous may be different. Many extremely preterm neonates may not appear vigorous after delivery, yet these neonates may benefit most from delayed cord clamping. A small randomized study that included neonates born at 22–27 weeks of gestation who had a median 1-minute Apgar score of 2, consistent with the need for resuscitation, demonstrated that neonates who received delayed cord clamping (37.4±5.7 seconds, n=20), compared with early cord clamping (3.8±1 seconds; n=20), had similar safety outcomes, but higher normal NICU admission temperatures, higher blood pressures in the first 24 hours, increased hematocrit at 72 hours, and decreased number of red blood cell transfusions in the first 28 days of life.84
Although limited in number, studies support that most preterm newborns, including extremely preterm neonates, will initiate breathing within the first 60 seconds after delivery, with both delayed cord clamping and early cord clamping.85,86 In a randomized trial that included 150 preterm neonates who received 60 seconds of delayed cord clamping, spontaneous breathing occurred, on average, within 25 seconds of delivery with both vaginal and cesarean deliveries.87 Neonates that received early stimulation had spontaneous breathing without evidence of bradycardia or hypoxemia, and did not benefit from face mask positive pressure ventilation.
Compared with preterm neonates, most term neonates appear to spontaneously breathe sooner after birth. In a randomized clinical trial by Kc et al52 involving 1,510 term neonates with low-risk vaginal delivery, neonates who received early cord clamping 60 seconds or less had their first breath at 14.5±7.6 seconds with regular breathing established by 19±13.1 seconds compared with neonates with delayed cord clamping for 180 seconds or more who had their first breath at 10.4±7.2 seconds with regular breathing established by 14.9±13.0 seconds. A small (n=15) prospective observational study of healthy term neonates assessed by echocardiographic ultrasonogram after delivery (positioned on their mother’s chest) demonstrated that inspiration appears to direct ductus venosus blood flow into the right atrium, which may promote placental-to-neonatal blood flow and increase neonatal blood volume.88 In addition, a large multicenter trial (MINVI, NCT03631940) has recently completed enrollment of 1,200 nonvigorous term neonates (35–42 weeks of gestation) who received early cord clamping or umbilical cord milking (four times along the entire length of the umbilical cord within 15–20 seconds) using a cluster randomized crossover design to evaluate incidence of NICU admission (primary outcome) and mortality, need for therapeutic hypothermia, hemoglobin levels, hyperbilirubinemia and need for volume expanders (secondary outcomes).
LONG-TERM NEURODEVELOPMENTAL OUTCOMES
Overall, robust data on the effects of delayed cord clamping on long-term neurodevelopmental outcomes are lacking. A follow-up study at 18–22 months corrected age of premature singleton neonates born at less than 32 weeks of gestation demonstrated that delayed cord clamping (30–45 seconds; n=82), compared with early cord clamping (less than 10 seconds; n=79), was associated with fewer motor scores lower than 85 on the Bayley Scales of Infant Development, Third Edition (odds ratio 0.32, 95% CI 0.10–0.90, P=.03).89 A follow-up study at 2 years corrected age of premature neonates born at less than 32 weeks of gestation demonstrated that fewer children allocated to delayed cord clamping (2 minutes or more; 24/155, 21%) died or had an adverse neurodevelopmental outcome compared with early cord clamping (20 seconds or less; 35/103, 34%); RR 0.61 (95% CI 0.39–0.96); risk difference −13% (95% CI −25% to −21%).90
Analysis of secondary outcomes from a follow-up study of a randomized trial that included low-risk term pregnancies demonstrated that compared with early cord clamping (less than 10 seconds), delayed cord clamping (180 seconds or more) in 4-year-old boys (but not girls) was associated with the higher scores in tests that assessed fine motor and personal–social domains, the processing speed quotient and the bicycle trail task.91 A 12-month follow-up study of term singleton neonates from healthy pregnancies demonstrated that compared with early cord clamping (20 seconds or less), delayed cord clamping (5 minutes or more) was associated with increased regional white matter brain growth within the right and left internal capsules, the right parietal, occipital, and prefrontal cortex, without gender or developmental testing differences between the two groups.92 Although these follow-up studies are reassuring for potential benefits of delayed cord clamping, robust long-term follow-up studies in high-risk preterm and term neonates are needed.
CONTRAINDICATIONS TO DELAYED UMBILICAL CORD CLAMPING
Early cord clamping is recommended in clinical situations of maternal hemorrhage, hemodynamic instability, or both, and when placental circulation is interrupted (eg, abruption, bleeding previa, known true umbilical cord knot, cord avulsion, fetal growth restriction with abnormal cord Doppler evaluation).6 In many scenarios (eg, nonvigorous newborns requiring resuscitation) evidence on the risks and benefits of delayed cord clamping is still lacking; therefore, it is reasonable to perform early cord clamping in these situations until more data are available.
CONCLUSION
Interpreting the literature on the potential benefits and risks of delayed cord clamping compared with early cord clamping can be challenging, even when considering systematic reviews and meta-analyses. Meta-analyses are hindered by heterogeneity in the definition of delayed cord clamping, and differences in gestational age at recruitment, comparison methodologies, assessment of bias risk, and lack of studies with sufficient sample size to evaluate specific outcomes and neonatal subgroups.
Despite the heterogeneity in studies, substantial, high-quality evidence from systematic reviews and meta-analyses supports the safety and potential benefits of delayed cord clamping, supporting this placental transfusion strategy in preterm (more than 30 seconds) and term (more than 60 seconds) neonates. In clinical situations where placental circulation is interrupted, early cord clamping is indicated. In preterm neonates, delayed cord clamping may decrease mortality and the need for blood transfusions. In neonates delivered at 34 weeks of gestation to term, delayed cord clamping increases hemoglobin and hematocrit immediately after birth and may increase the risk for hyperbilirubinemia requiring phototherapy, so these neonates require monitoring for hyperbilirubinemia. Delayed cord clamping appears to be safe for mothers and does not increase the risk of postpartum hemorrhage.
CME FOR THE CLINICAL EXPERT SERIES.
Learning Objectives for “Management of Placental Transfusion to Neonates After Delivery”
After completing this continuing education activity, you will be able to:
Describe what is meant by placental transfusion;
Discuss existing evidence for benefits of delayed umbilical cord clamping for premature and other neonates;
Compare gravity-assisted transfusion with umbilical cord milking, and;
Implement an evidence-based approach to using delayed umbilical cords clamping in your practice.
Instructions for Obtaining AMA PRA Category 1 Credits™
Continuing Medical Education credit is provided through joint providership with The American College of Obstetricians and Gynecologists.
Obstetrics & Gynecology includes CME-certified content that is designed to meet the educational needs of its readers. This article is certified for 2 AMA PRA Category 1 Credits.™ This activity is available for credit through January 31, 2025.
Accreditation Statement
ACCME Accreditation
The American College of Obstetricians and Gynecologists is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
AMA PRA Category 1 Credit(s)™
The American College of Obstetricians and Gynecologists designates this journal-based CME activity for a maximum of 2 AMA PRA Category 1 Credits.™ Physicians should claim only the credit commensurate with the extent of their participation in the activity.
College Cognate Credit(s)
The American College of Obstetricians and Gynecologists designates this journal-based CME activity for a maximum of 2 Category 1 College Cognate Credits. The College has a reciprocity agreement with the AMA that allows AMA PRA Category 1 Credits™ to be equivalent to College Cognate Credits.
Disclosure of Faculty and Planning Committee
Industry Relationships
In accordance with the College policy, all faculty and planning committee members have signed a conflict of interest statement in which they have disclosed any financial interests or other relationships with industry relative to article topics. Such disclosures allow the participant to evaluate better the objectivity of the information presented in the articles.
How to Earn CME Credit
To earn CME credit, you must read the article in Obstetrics & Gynecology and complete the quiz, answering at least 70 percent of the questions correctly. For more information on this CME educational offering, visit the Lippincott CMEConnection portal at https://cme.lww.com/browse/sources/196 to register and to complete the CME activity online. ACOG Fellows will receive 50% off by using coupon code, ONG50.
Hardware/software requirements are a desktop or laptop computer (Mac or PC) and an Internet browser. This activity is available for credit through January 31, 2025. To receive proper credits for this activity, each participant will need to make sure that the information on their profile for the CME platform (where this activity is located) is updated with 1) their date of birth (month and day only) and 2) their ACOG ID. In addition, participants should select that they are board-certified in obstetrics and gynecology.
The privacy policies for the Obstetrics & Gynecology website and the Lippincott CMEConnection portal are available at http://www.greenjournal.org and https://cme.lww.com/browse/sources/196, respectively.
Contact Information
Questions related to transcripts may be directed to educationcme@acog.org. For other queries, please contact the Obstetrics & Gynecology Editorial Office, 202–314-2317 or obgyn@greenjournal.org. For queries related to the CME test online, please contact ceconnection@wolterskluwer.com or 1-800-787-8985.
Acknowledgments
Satyan Lakshminrusimha is supported by NIH grant 5R01HD072929–11.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Our World In Data. Number of births and deaths per year, world. Accessed September 27, 2021. https://ourworldindata.org/grapher/births-and-deaths-projected-to-2100
- 2.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388:3027–35. doi: 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–46. doi: 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogarty M, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol 2018;218:1–18. doi: 10.1016/j.ajog.2017.10.231 [DOI] [PubMed] [Google Scholar]
- 5.Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015;132(16 suppl 1):S204–41. doi: 10.1161/CIR.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 6.Delayed umbilical cord clamping after birth. ACOG Committee Opinion No. 814. American College of Obstetricians and Gynecologists. Obstet Gynecol 2020;136:e100–6. doi: 10.1097/AOG.0000000000004167 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO guidelines approved by the guidelines review committee. Guideline: delayed umbilical cord clamping for improved maternal and infant Health and nutrition outcomes. World Health Organization; 2014. [PubMed] [Google Scholar]
- 8.Royal College of Obstetricians and Gynaecologists. Clamping of the umbilical cord and placental transfusion. Scientific Impact Paper No. 14. RCOG; 2015. [Google Scholar]
- 9.National Institute for Health and Care Excellence. Intrapartum care for healthy women and babies. Clinical guideline: third stage of labour. NICE; 2014. [PubMed] [Google Scholar]
- 10.Royal College of Midwives. Midwifery care in labour guidance for all women in all settings: third stage of labour. RCM Midwifery Blue Top Guidance No. 1, 2018. Accessed October 8, 2021. https://www.rcm.org.uk/media/2539/professionals-blue-top-guidance.pdf [Google Scholar]
- 11.Timing of umbilical cord clamping after birth. Committee Opinion No. 543 [withdrawn]. American College of Obstetricians and Gynecologists. Obstet Gynecol 2012;120:1522–6. doi: 10.1097/01.AOG.0000423817.47165.48 [DOI] [PubMed] [Google Scholar]
- 12.Katheria AC, Lakshminrusimha S, Rabe H, McAdams R, Mercer JS. Placental transfusion: a review. J Perinatol 2017;37:105–11. doi: 10.1038/jp.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weeks AD, Fawcus S. Management of the third stage of labour: (for the Optimal Intrapartum Care series edited by Mercedes Bonet, Femi Oladapo and Metin Gülmezoglu). Best Pract Res Clin Obstet Gynaecol 2020;67:65–79. doi: 10.1016/j.bpobgyn.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 14.El-Naggar W, Afifi J, Dorling J, Bodani J, Cieslak Z, Canning R, et al. A comparison of strategies for managing the umbilical cord at birth in preterm infants. J Pediatr 2020;225:58–e4. doi: 10.1016/j.jpeds.2020.05.018 [DOI] [PubMed] [Google Scholar]
- 15.Kumbhat N, Eggleston B, Davis AS, Van Meurs KP, DeMauro SB, Foglia EE, et al. Placental transfusion and short-term outcomes among extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2021;106:62–8. doi: 10.1136/archdischild-2019-318710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumbhat N, Eggleston B, Davis AS, DeMauro SB, Van Meurs KP, Foglia EE, et al. Umbilical cord milking vs delayed cord clamping and associations with in-hospital outcomes among extremely premature infants. J Pediatr 2021;232:87–e4. doi: 10.1016/j.jpeds.2020.12.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basile S, Pinelli S, Micelli E, Caretto M, Benedetti Panici P. Milking of the umbilical cord in term and late preterm infants. Biomed Res Int 2019;2019:9185059. doi: 10.1155/2019/9185059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA 2019;322: 1877–86. doi: 10.1001/jama.2019.16004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blank DA, Polglase GR, Kluckow M, Gill AW, Crossley KJ, Moxham A, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed 2018;103:F539–46. doi: 10.1136/archdischild-2017-314005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooper SB, Crossley KJ, Zahra VA, van Vonderen J, Moxham A, Gill AW, et al. Effect of body position and ventilation on umbilical artery and venous blood flows during delayed umbilical cord clamping in preterm lambs. Arch Dis Child Fetal Neonatal Ed 2017;102:F312–9. doi: 10.1136/archdischild-2016-311159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madar J, Roehr CC, Ainsworth S, Ersdal H, Morley C, Rüdiger M, et al. European Resuscitation Council Guidelines 2021: newborn resuscitation and support of transition of infants at birth. Resuscitation 2021;161:291–326. doi: 10.1016/j.resuscitation.2021.02.014 [DOI] [PubMed] [Google Scholar]
- 22.Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, Kattwinkel J, Perlman JM, et al. Part 13: neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132(18 suppl 2):S543–60. doi: 10.1161/CIR.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 23.Lee L, Dy J, Azzam H. Management of spontaneous labour at term in healthy women. J Obstet Gynaecol Can 2016;38:843–65. doi: 10.1016/j.jogc.2016.04.093 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Guideline: delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. 2014. Accessed October 8, 2021. https://www.who.int/elena/titles/full_recommendations/cord_clamping/en/ [PubMed]
- 25.American College of Nurse Midwives. Position statement: delayed umbilical cord clamping. Cited September 28, 2021. Accessed October 8, 2021. https://www.midwife.org/ACNM/files/ACNMLibraryData/UPLOADFILENAME/000000000290/Delayed-Umbilical-Cord-Clamping-May-2014.pdf
- 26.Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG 2011;118:70–5. doi: 10.1111/j.1471-0528.2010.02781.x [DOI] [PubMed] [Google Scholar]
- 27.Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet 1969;2:871–3. doi: 10.1016/s0140-6736(69)92328-9 [DOI] [PubMed] [Google Scholar]
- 28.Yao AC, Hirvensalo M, Lind J. Placental transfusion-rate and uterine contraction. Lancet 1968;1:380–3. doi: 10.1016/s0140-6736(68)91352-4 [DOI] [PubMed] [Google Scholar]
- 29.Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics 2006;117:93–8. doi: 10.1542/peds.2004-1773 [DOI] [PubMed] [Google Scholar]
- 30.Hooper SB, Polglase GR, te Pas AB. A physiological approach to the timing of umbilical cord clamping at birth. Arch Dis Child Fetal Neonatal Ed 2015;100:F355–60. doi: 10.1136/archdischild-2013-305703 [DOI] [PubMed] [Google Scholar]
- 31.Vain NE, Satragno DS, Gorenstein AN, Gordillo JE, Berazategui JP, Alda MG, et al. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non-inferiority trial. Lancet 2014;384:235–40. doi: 10.1016/S0140-6736(14)60197-5 [DOI] [PubMed] [Google Scholar]
- 32.Mansaray A, Yetman R, Berens P. Effect of delayed cord clamping above versus below the perineum on neonatal hematocrit: a randomized controlled trial. Breastfeed Med 2015;10: 464–7. doi: 10.1089/bfm.2015.0109 [DOI] [PubMed] [Google Scholar]
- 33.Cavallin F, Galeazzo B, Loretelli V, Madella S, Pizzolato M, Visentin S, et al. Delayed cord clamping versus early cord clamping in elective cesarean section: a randomized controlled trial. Neonatology 2019;116:252–9. doi: 10.1159/000500325 [DOI] [PubMed] [Google Scholar]
- 34.De Bernardo G, Giordano M, De Santis R, Castelli P, Sordino D, Trevisanuto D, et al. A randomized controlled study of immediate versus delayed umbilical cord clamping in infants born by elective caesarean section. Ital J Pediatr 2020;46:71. doi: 10.1186/s13052-020-00835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purisch SE, Ananth CV, Arditi B, Mauney L, Ajemian B, Heiderich A, et al. Effect of delayed vs immediate umbilical cord clamping on maternal blood loss in term cesarean delivery: a randomized clinical trial. JAMA 2019;322:1869–76. doi: 10.1001/jama.2019.15995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Consonni S, Vaglio Tessitore I, Conti C, Plevani C, Condo M, Torcasio F, et al. Umbilical cord management strategies at cesarean section. J Obstet Gynaecol Res 2020. Sep 22 [Epub ahead of print]. doi: 10.1111/jog.14501 [DOI] [PubMed] [Google Scholar]
- 37.Jenusaitis L, Keplinger KB, Dean K, Madan I, Jonathan PP, Shepherd. Impact of a delayed cord clamping protocol on maternal and neonatal outcomes in patients undergoing term cesarean section. J Matern Fetal Neonatal Med 2020. Dec 7 [Epub ahead of print]. doi: 10.1080/14767058.2020.1857357 [DOI] [PubMed] [Google Scholar]
- 38.Gomersall J, Berber S, Middleton P, McDonald SJ, Niermeyer S, El-Naggar W, et al. Umbilical cord management at term and late preterm birth: a meta-analysis. Pediatrics 2021;147: e2020015404. doi: 10.1542/peds.2020-015404 [DOI] [PubMed] [Google Scholar]
- 39.Rabe H, Gyte GM, Díaz-Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. The Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No.: CD003248. doi: 10.1002/14651858.CD003248.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jasani B, Torgalkar R, Ye XY, Syed S, Shah PS. Association of umbilical cord management strategies with outcomes of preterm infants: a systematic review and Network meta-analysis. JAMA Pediatr 2021;175:e210102. doi: 10.1001/jamapediatrics.2021.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persad E, Sibrecht G, Ringsten M, Karlelid S, Romantsik O, Ulinder T, et al. Interventions to minimize blood loss in very preterm infants—a systematic review and meta-analysis. PLoS One 2021;16:e0246353. doi: 10.1371/journal.pone.0246353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seidler AL, Gyte GML, Rabe H, Díaz-Rossello JL, Duley L, Aziz K, et al. Umbilical cord management for newborns, <34 weeks’ gestation: a meta-analysis. Pediatrics 2021;147: e20200576. doi: 10.1542/peds.2020-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mercer JS, Erickson-Owens DA, Rabe H. Placental transfusion: may the “force” be with the baby. J Perinatol 2021;41:1495–504. doi: 10.1038/s41372-021-01055-0 [DOI] [PubMed] [Google Scholar]
- 44.Wang YC, Chan OW, Chiang MC, Yang PH, Chu SM, Hsu JF, et al. Red blood cell transfusion and clinical outcomes in extremely low birth weight preterm infants. Pediatr Neonatol 2017;58:216–22. doi: 10.1016/j.pedneo.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 45.Hellström W, Forssell L, Morsing E, Sävman K, Ley D. Neonatal clinical blood sampling led to major blood loss and was associated with bronchopulmonary dysplasia. Acta Paediatr 2020;109:679–87. doi: 10.1111/apa.15003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawford TM, Andersen CC, Hodyl NA, Robertson SA, Stark MJ. The contribution of red blood cell transfusion to neonatal morbidity and mortality. J Paediatr Child Health 2019;55:387–92. doi: 10.1111/jpc.14402 [DOI] [PubMed] [Google Scholar]
- 47.Ghirardello S, Dusi E, Cortinovis I, Villa S, Fumagalli M, Agosti M, et al. Effects of red blood cell transfusions on the risk of developing complications or death: an observational study of a cohort of very low birth weight infants. Am J Perinatol 2017;34: 88–95. doi: 10.1055/s-0036-1584300 [DOI] [PubMed] [Google Scholar]
- 48.Arcilla RA, Oh W, Lind J, Gessner IH. Pulmonary arterial pressures of newborn infants born with early and late clamping of the cord. Acta Paediatr Scand 1966;55:305–15. doi: 10.1111/j.1651-2227.1966.tb17659.x [DOI] [PubMed] [Google Scholar]
- 49.Xodo S, Xodo L, Berghella V. Delayed cord clamping and cord gas analysis at birth. Acta Obstet Gynecol Scand 2018;97:7–12. doi: 10.1111/aogs.13233 [DOI] [PubMed] [Google Scholar]
- 50.Nudelman MJR, Belogolovsky E, Jegatheesan P, Govindaswami B, Song D. Effect of delayed cord clamping on umbilical blood gas values in term newborns: a systematic review. Obstet Gynecol 2020;135:576–82. doi: 10.1097/AOG.0000000000003663 [DOI] [PubMed] [Google Scholar]
- 51.Giovannini N, Crippa BL, Denaro E, Raffaeli G, Cortesi V, Consonni D, et al. The effect of delayed umbilical cord clamping on cord blood gas analysis in vaginal and caesarean-delivered term newborns without fetal distress: a prospective observational study. BJOG 2020;127:405–13. doi: 10.1111/1471-0528.16026 [DOI] [PubMed] [Google Scholar]
- 52.Kc A, Singhal N, Gautam J, Rana N, Andersson O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth—randomized clinical trial. Matern Health Neonatol Perinatol 2019;5:7. doi: 10.1186/s40748-019-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersson O, Rana N, Ewald U, Målqvist M, Stripple G, Basnet O, et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (Nepcord III)—a randomized clinical trial. Matern Health Neonatol Perinatol 2019;5:15. doi: 10.1186/s40748-019-0110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bancalari A, Araneda H, Echeverria P, Marinovic A, Manríquez C. Arterial oxygen saturation and heart rate in term new-born in the first hour after birth. Rev Chil Pediatr 2019;90:384–91. doi: 10.32641/rchped.v90i4.964 [DOI] [PubMed] [Google Scholar]
- 55.Padilla-Sánchez C, Baixauli-Alacreu S, Cañada-Martínez AJ, Solaz-García Á, Alemany-Anchel MJ, Vento M. Delayed vs immediate cord clamping changes oxygen saturation and heart rate patterns in the first minutes after birth. J Pediatr 2020;227: 149–56.e1. doi: 10.1016/j.jpeds.2020.07.045 [DOI] [PubMed] [Google Scholar]
- 56.Weiner G, editor. Textbook of neonatal resuscitation. 8th ed. American Academy of Pediatrics/American Heart Association Neonatal Resuscitation Program; 2021. [Google Scholar]
- 57.Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010;125:e1340–7. doi: 10.1542/peds.2009-1510 [DOI] [PubMed] [Google Scholar]
- 58.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16:7. doi: 10.1007/s11892-015-0699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during pregnancy: a maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int J Mol Sci 2021;22:22. doi: 10.3390/ijms22062965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramezani Tehrani F, Naz MSG, Yarandi RB, Behboudi-Gandevani S. The impact of diagnostic criteria for gestational diabetes mellitus on adverse maternal outcomes: a systematic review and meta-analysis. J Clin Med 2021;10:666. doi: 10.3390/jcm10040666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korkut S, Oğuz Y, Bozkaya D, Türkmen GG, Kara Ö, Uygur D, et al. Evaluation of the effects of delayed cord clamping in infants of diabetic mothers. Am J Perinatol 2021;38:242–7. doi: 10.1055/s-0039-1695799 [DOI] [PubMed] [Google Scholar]
- 62.Fasano RM. Hemolytic disease of the fetus and newborn in the molecular era. Semin Fetal Neonatal Med 2016;21:28–34. doi: 10.1016/j.siny.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 63.Garabedian C, Rakza T, Drumez E, Poleszczuk M, Ghesquiere L, Wibaut B, et al. Benefits of delayed cord clamping in red blood cell alloimmunization. Pediatrics 2016;137:e20153236. doi: 10.1542/peds.2015-3236 [DOI] [PubMed] [Google Scholar]
- 64.Sahoo T, Thukral A, Sankar MJ, Gupta SK, Agarwal R, Deorari AK, et al. Delayed cord clamping in Rh-alloimmunised infants: a randomised controlled trial. Eur J Pediatr 2020;179:881–9. doi: 10.1007/s00431-020-03578-8 [DOI] [PubMed] [Google Scholar]
- 65.Pogliani L, Erba P, Nannini P, Giacomet V, Zuccotti GV. Effects and safety of delayed versus early umbilical cord clamping in newborns of HIV-infected mothers. J Matern Fetal Neonatal Med 2019;32:646–9. doi: 10.1080/14767058.2017.1387896 [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization. Clinical management of COVID-19: interim guidance. WHO; 2020. [Google Scholar]
- 67.American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. ACOG; 2020. [Google Scholar]
- 68.Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) infection in pregnancy. Version 13. RCOG; 2021. [Google Scholar]
- 69.Mejía Jiménez I, Salvador López R, García Rosas E, Rodriguez de la Torre I, Montes García J, de la Cruz Conty ML, et al. Umbilical cord clamping and skin-to-skin contact in deliveries from women positive for SARS-CoV-2: a prospective observational study. BJOG 2021;128:908–15. doi: 10.1111/1471-0528.16597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blumberg D, Sridhar A, Lakshminrusimha S, Higgins RD, Saade G. COVID-19 vaccine considerations during pregnancy and lactation. Am J Perinatol 2021;38:523–8. doi: 10.1055/s-0041-1726390 [DOI] [PubMed] [Google Scholar]
- 71.Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol 2021;225:303. e1–17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N Engl J Med 2021;384:2273–82. doi: 10.1056/nejmoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pawar R, Gavade V, Patil N, Mali V, Girwalkar A, Tarkasband V, et al. Neonatal multisystem inflammatory syndrome (mis-N) associated with prenatal maternal SARS-CoV-2: a case series. Children (Basel) 2021;8:572. doi: 10.3390/children8070572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lees C, Marlow N, Arabin B, Bilardo CM, Brezinka C, Derks JB, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol 2013;42:400–8. doi: 10.1002/uog.13190 [DOI] [PubMed] [Google Scholar]
- 75.Sacchi C, Marino C, Nosarti C, Vieno A, Visentin S, Simonelli A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr 2020;174: 772–81. doi: 10.1001/jamapediatrics.2020.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yunis M, Nour I, Gibreel A, Darwish M, Sarhan M, Shouman B, et al. Effect of delayed cord clamping on stem cell transfusion and hematological parameters in preterm infants with placental insufficiency: a pilot randomized trial. Eur J Pediatr 2021;180: 157–66. doi: 10.1007/s00431-020-03730-4 [DOI] [PubMed] [Google Scholar]
- 77.Gokmen Z, Ozkiraz S, Tarcan A, Kozanoglu I, Ozcimen EE, Ozbek N. Effects of delayed umbilical cord clamping on peripheral blood hematopoietic stem cells in premature neonates. J Perinat Med 2011;39:323–9. doi: 10.1515/jpm.2011.021 [DOI] [PubMed] [Google Scholar]
- 78.Mercer JS, Erickson-Owens DA, Collins J, Barcelos MO, Parker AB, Padbury JF. Effects of delayed cord clamping on residual placental blood volume, hemoglobin and bilirubin levels in term infants: a randomized controlled trial. J Perinatol 2017;37:260–4. doi: 10.1038/jp.2016.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang M, Mercer JS, Padbury JF. Delayed cord clamping in infants with suspected intrauterine growth restriction. J Pediatr 2018;201:264–8. doi: 10.1016/j.jpeds.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 80.Digal KC, Singh P, Srivastava Y, Chaturvedi J, Tyagi AK, Basu S. Effects of delayed cord clamping in intrauterine growth-restricted neonates: a randomized controlled trial. Eur J Pediatr 2021;180:1701–10. doi: 10.1007/s00431-021-03959-7 [DOI] [PubMed] [Google Scholar]
- 81.Chopra A, Thakur A, Garg P, Kler N, Gujral K. Early versus delayed cord clamping in small for gestational age infants and iron stores at 3 months of age—a randomized controlled trial. BMC Pediatr 2018;18:234. doi: 10.1186/s12887-018-1214-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455–63. doi: 10.1093/ije/dyz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Backes CH, Rivera BK, Haque U, Bridge JA, Smith CV, Hutchon DJ, et al. Placental transfusion strategies in very preterm neonates: a systematic review and meta-analysis. Obstet Gynecol 2014;124:47–56. doi: 10.1097/AOG.0000000000000324 [DOI] [PubMed] [Google Scholar]
- 84.Backes CH, Huang H, Iams JD, Bauer JA, Giannone PJ. Timing of umbilical cord clamping among infants born at 22 through 27 weeks’ gestation. J Perinatol 2016;36:35–40. doi: 10.1038/jp.2015.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nevill E, Meyer MP. Effect of delayed cord clamping (DCC) on breathing and transition at birth in very preterm infants. Early Hum Dev 2015;91:407–11. doi: 10.1016/j.earlhumdev.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 86.Murphy MC, McCarthy LK, O’Donnell CPF. Crying and breathing by new-born preterm infants after early or delayed cord clamping. Arch Dis Child Fetal Neonatal Ed 2020;105: 331–3. doi: 10.1136/archdischild-2018-316592 [DOI] [PubMed] [Google Scholar]
- 87.Katheria A, Poeltler D, Durham J, Steen J, Rich W, Arnell K, et al. Neonatal resuscitation with an intact cord: a randomized clinical trial. J Pediatr 2016;178:75–80.e3. doi: 10.1016/j.jpeds.2016.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brouwer E, Knol R, Kroushev A, Van Den Akker T, Hooper SB, Roest AA, et al. Effect of breathing on venous return during delayed cord clamping: an observational study. Arch Dis Child Fetal Neonatal Ed 2021. Jun 9 [Epub ahead of print]. doi: 10.1136/archdischild-2020-321431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mercer JS, Erickson-Owens DA, Vohr BR, Tucker RJ, Parker AB, Oh W, et al. Effects of placental transfusion on neonatal and 18 Month outcomes in preterm infants: a randomized controlled trial. J Pediatr 2016;168:50–e1. doi: 10.1016/j.jpeds.2015.09.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Armstrong-Buisseret L, Powers K, Dorling J, Bradshaw L, Johnson S, Mitchell E, et al. Randomised trial of cord clamping at very preterm birth: outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed 2020;105:292–8. doi: 10.1136/archdischild-2019-316912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andersson O, Lindquist B, Lindgren M, Stjernqvist K, Domellöf M, Hellström-Westas L. Effect of delayed cord clamping on neurodevelopment at 4 Years of age: a randomized clinical trial. JAMA Pediatr 2015;169:631–8. doi: 10.1001/jamapediatrics.2015.0358 [DOI] [PubMed] [Google Scholar]
- 92.Mercer JS, Erickson-Owens DA, Deoni SCL, Dean DC III, Tucker R, Parker AB, et al. The effects of delayed cord clamping on 12-month brain myelin content and neurodevelopment: a randomized controlled trial. Am J Perinatol 2020. Jul 21 [Epub ahead of print]. doi: 10.1055/s-0040-1714258 [DOI] [PMC free article] [PubMed] [Google Scholar]