Abstract
Background: Minocycline has multiple neuroprotective roles in abundant brain diseases, including the prevention and treatment of Alzheimer’s disease (AD). Cdk5/p25 signaling plays an important role in the onset and development of Alzheimer’s-like pathology. The aim of the present work was to further explore the underlying mechanism which minocycline effects on Cdk5/p25 signaling related to Alzheimer’s-like pathology.
Methods: The cognitive function of animals was measured by the Morris water maze test. The levels of Aβ were determined by an enzyme-linked immunosorbent assay. The levels of APP, β- and γ-secretases, and the biomarkers of tau (total tau and hyperphosphorylated tau), inflammatory cytokine and matrix metalloproteinases (MMP-2 and MMP-9), and biomarkers of synapse and Cdk5/p25 signaling, were detected by the Western blotting. The biomarkers of the synapse, inflammatory cytokine, and matrix metalloproteinases (MMP-2 and MMP-9) were also determined by immunofluorescence.
Results: Minocycline improved learning and memory in APP/PS1 mice. It limited the production of Aβ and hyperphosphorylation of tau in the hippocampus and ameliorated synaptic deficit. Moreover, it also inhibited the activation of Cdk5/p25 signaling, inflammation, and matrix metalloproteinases.
Conclusion: Minocycline mitigates Alzheimer’s-like pathology via limiting the activation of Cdk5/p25 signaling pathway and improves cognitive deficits.
Keywords: Minocycline, Alzheimer’s disease, pathology, synapse, cyclin-dependent kinase 5, anti-inflammatory drugs
1. INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disease of the brain featured by cognitive impairment and dementia in its later stage. The prevalence of AD increases substantially after the age of 70 and may affect around 50% of people over the age of 85. With the rapid growth of the world's aging With the rapid growth of aging population,, a global focus on reducing mortality and morbidity from AD is more urgent than ever. To date, a variety of medications are used to prevent the progress of AD, such as melatonin [1], donepezil [2, 3], galantamine [4], and memantine [1]. Some of them may help in maintaining thinking skills, slowing memory loss, improving communication skills, and helping with certain behavioral problems. However, all these drugs have either limited side effects or adverse side effects [5]. Until now, none of the drugs is likely to successfully cure AD [6, 7]. It is well accepted that there is an association between the inflammatory system and Alzheimer's disease, such as the release of inflammatory cytokines, matrix metalloproteinases (MMPs), oxidative stress, and apoptotic molecule [8-11]. Therefore, it is of great significance to seek and develop anti-inflammatory drugs for AD [11].
Minocycline, a semisynthetic tetracycline, has originally been applied to treat a variety of infectious diseases with relatively few adverse effects. Minocycline can easily penetrate through the blood-brain barrier since it has high lipophilicity [12], indicating that minocycline is an ideal candidate for treating the diseases of the central nervous system [13]. An effective neuroprotective property of minocycline has been well reported, such as anti-inflammatory, antioxidant, and anti-apoptotic properties. It is also known as a powerful inhibitor of matrix metalloproteinases (MMPs) [14-17]. Furthermore, the pharmacological effects of minocycline on several neurological disorders have been evidenced, including stroke [18, 19], multiple sclerosis [13, 20], Alzheimer’s disease [21], and other neurodegenerative disorders [1].
Cyclin-dependent kinase 5 (Cdk5), a unique member of the cyclin-dependent kinases (Cdks), has been found to associate with the pathogenesis of AD as Cdk5/p25 is of vital importance in the development of synaptic functions and cognitive impairment [22, 23]. Over-activation of Cdk5/p25 signaling induces aberrant hyperphosphorylation of various substrates, like amyloid precursor protein (APP), tau, and neurofilament, resulting in Alzheimer’s-like pathology [24]. Increasing studies have demonstrated the roles of Cdk5/p25 signaling in the pathophysiological mechanisms of AD [25-27]. In this study, we analyzed the changes in the hippocampus of APP/PS1 transgenic mice regarding Alzheimer’s-like pathology after minocycline administration. These parameters indicated that that minocycline alleviates the pathology of AD via inhibiting the activation of Cdk5/p25 signaling, inhibiting the production of Aβ and hyperphosphorylation of tau, and improving synaptic deficit.
2. MATERIALS AND METHODS
2.1. Animals and Drugs
In this study, the 120-day male APP/PS1 transgenic mice were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences & Peking Union Medical College. They were housed in individual cages under controlled light and environmental conditions (12h light/12h dark cycle at 23±2°C and 50±10% humidity) with access to standard laboratory food and water. All rats were habituated to the cage for 7 days before the experiments. The experimental process and schematic schedule are demonstrated in Fig. (1). The transgenic mice were randomly divided into two groups: AD model control group (n=6) and minocycline-treated group (50mg/kg/d, n=6).
Fig. (1).
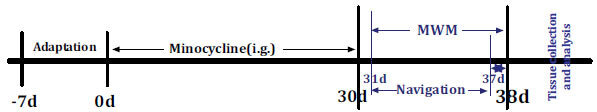
The experimental process and schematic schedule. i.g.: intragastric administration; MWM, Morris water maze.
Minocycline (100 mg/capsule, Huishi Pharmaceutical Limited Company, China) was diluted to 0.5 mg/mL concentration by normal saline. The animals in the minocycline intervention group were administered a dose of 50 mg/kg body weight once per day by intragastric administration (i.g.) for 30 consecutive days, while each mouse with vehicle treatment received an equal volume of normal saline as control. The minocycline dosage was determined according to the previous studies [28, 29]. Each test was performed with 12 samples (6/Control, 6/Minocycline) and three times.
All the experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were in accordance with the ethical standards approved by the Research Ethics Committee of the Hubei University of Medicine.
2.2. Morris Water Maze (MWM) Test
The implemented MWM test includes place navigation and spatial probe. Firstly, the mice were adapted to the pool environment for 3 days at a steady temperature (20°C-24°C) and humidity (60-80%) before the MWM test with 4 trials per day. All mice were placed in a circular pool of water and allowed to escape and reach a hidden platform. The mice were given a maximum of 60s to find the hidden platform. Then, the acquisition trial was considered the training trial to find the hidden platform. The time spent to reach the hidden platform was recorded as the escape latency for the spatial learning performance. On day 8, the mice were subjected to the probe trial to assess spatial memory. The time in the target quadrant and the times crossing the former platform area were recorded for testing spatial memory performance. The experimental procedures of the MWM test were monitored using Morris Image System (Shanghai DOiT Industrial Co., Ltd.).
2.3. Enzyme-linked Immunosorbent Assay (ELISA)
After behavioral testing, all the mice were anesthetized and perfused transcardially with ice-cold normal saline. Brains were quickly removed, dissected at the midsagittal plane, and placed on ice. Tissues were homogenized in tissue protein extraction reagent buffer (Biosource International, Inc, Camarillo, CA, USA). 4-benzenesulfonyl ñuoride hydrochloride (Sigma Chemical Co,) was added to tissue lysates to prevent the degradation of Aβ. The concentrations of Aβ1-40 and Aβ1-42 were measured using a sandwich ELISA kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The optical densities in each well were measured using a plate reader at 450 nm. A standard curve for the ELISA test was measured to determine the concentration of antigen in a sample, while a standard curve using a solution of known concentration was prepared in advance.
2.4. Immunofluorescence Staining
One hemisphere was fixed in 4% paraformaldehyde, followed by embedding in paraffin for immunohistochemical analyses. Briefly, sections (50 μm) were cut with a freezing microtome. Permeabilization and blocking were obtained by incubating the sections for 1h at room temperature in PBS containing 0.5% Triton X-100 (vol/vol) and 3% bovine serum albumin. Paraffin-embedded tissue sections were deparaffinized and hydrated through a series of graded ethanol solutions, followed by 0.1 M Tris, pH 7.6. Sample sections were blocked in 5% goat serum with 0.3% Triton X-100 for 1 h at RT and then incubated overnight at 4 °C with the following primary antibodies: mouse monoclonal anti-MAP1 (Abcam, Cambridge, MA, USA), mouse monoclonal anti-SYP (Abcam, Cambridge, MA, USA), mouse monoclonal anti-PSD95 (Abcam, Cambridge, MA, USA), mouse monoclonal anti-IL-1β (Santa Cruz, CA, USA), mouse monoclonal anti-MMP-2 (Santa Cruz, CA, USA), and mouse monoclonal anti-MMP-9 (Santa Cruz, CA, USA). The sections were then incubated with fluorescence-conjugated secondary antibodies (Alexa Fluor Cy3 and Alexa Fluor 488, Beyotime, China). Nuclei were stained with 4’,6-diamidino-2-phenylin- dole (DAPI) (Beyotime, China) at RT for 10 min. The coverslips were installed onto glass slides using Antifade Mounting Medium (Beyotime), and the sections were visualized and photographed with a laser scanning confocal microscope (Leica TCS-SP2, Germany) or NEX-247 COPE microscope (NE900, USA). Quantification of the fluorescent signal intensity was done by image analysis software (Image-Pro Plus 6.2, Media Cybernetics).
2.5. Western Blotting
Western blotting was carried out according to the manufacturer’s instructions and previous studies [30, 31]. Cerebral cortex and hippocampus extracts were loaded onto 10-16% Tris/tricine SDS gels and transferred to nitrocellulose membranes prior to overnight incubation with one of the following primary antibodies: BACE, BACE1, sAPPβ, PS1, NCT, Aph-1α, Pen-2, p-Ser199, p-Ser202, p-Thr205, p-Thr231, p-Ser396, p-Ser404, HT7, p25, p35, and Cdk5 (purchased from Abcam, Cambridge, MA, USA). MAP1, SYP, and PSD95 were obtained from Cell Signaling Technology, Inc. USA. Mouse monoclonal anti-IL-1β, mouse monoclonal anti-MMP-2, mouse monoclonal anti-MMP-9, and goat anti-mouse IgG labeled with biotin were procured from Santa Cruz Biotechnology, Inc. USA. Rabbit anti-mouse β-actin was also obtained from Santa Cruz Biotechnology, Inc. USA. The optical densities of the specific bands were achieved by image analysis software (HPIAS 2000, Tongji Qianping Company, Wuhan, China).
2.6. Statistical Analysis
All statistical work was performed using the GraphPad Prism 6.0 software for Windows (GraphPad Software, La Jolla, CA, USA). Quantitative data were expressed as the mean ± standard error of the mean (SEM). For statistical evaluation of intergroup differences, paired t-test was used. A significant difference was considered at P<0.05.
3. RESULTS
3.1. Minocycline Ameliorates Cognitive Impairment
The spatial learning of all mice was evaluated by the acquisition trials using Morris water maze (MWM). As shown in Fig. (2A), minocycline treatment significantly lowered the escape latencies of the 10-month APP/PS1 mice for spatial learning performance (P<0.01). After the last training session, the spatial memory of all mice was assessed by the probe trial. The minocycline-treated APP/PS1 mice spent more time in the target quadrant (platform located) (P<0.01), (Fig. 2B) and had more crossing times in the target quadrant (P<0.01), (Fig. 2C) than the controls, indicating that impaired spatial memory in the APP/PS1 mice was markedly ameliorated after minocycline treatment. No significant difference was observed in the swimming speeds between the minocycline-treated APP/PS1 and the controls, suggesting that minocycline itself did not directly influence the spatial learning and memory of the mice. Considering this data, it can be concluded that minocycline administration remarkably relieves cognitive deficits in AD mice.
Fig. (2).

Minocycline alleviates spatial learning and memory of AD mice. After treatment with minocycline (50 mg/kg/d, i.g.) for 30 consecutive days, mice were examined using the Morris water maze (MWM) task. The latency to find the platform during 7 days in the acquisition trials was recorded in the navigation training. The minocycline-treated mice exhibited lower escape latency on days 3, 4, 5, 6, and 7 during training trials compared to vehicle-treated APP/PS1 mice (**P<0.01) (A). The number of times that mice cross the target quadrant (removed platform) (B) and the time that mice stay in the target quadrant (C) were calculated. A significant increase in the number of times mice cross the target quadrant and the time of mice staying in the target quadrant (**P<0.01) was observed in the minocycline-treated mice compared to APP/PS1 transgenic mice. No difference was observed between the two groups (D) regarding swimming speed. Values were represented as mean ± SD (n=10). AD: Alzheimer’s disease, MINO: minocycline.
3.2. Down-regulation of Aβ in the Brain of AD Mice by Minocycline
The alterations of Aβ levels in the cortex and hippocampal tissues of the brain of transgenic mice after minocycline treatment were measured using the colorimetric ELISA. As shown in Fig. (3), both Aβ40 and Aβ42 levels in the cortex and hippocampal tissues were found to be significantly decreased (P<0.01). These results indicated that minocycline is strongly effective in inhibiting the expression of Aβ in the brain of AD transgenic mice.
Fig. (3).
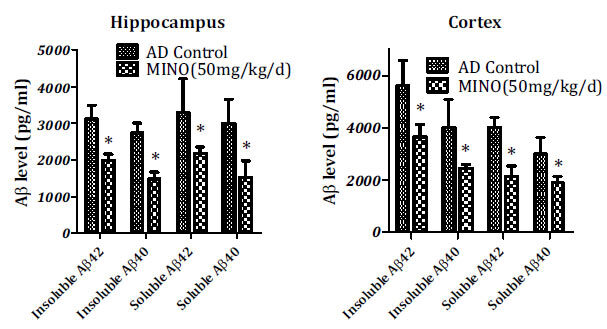
Down-regulation of Aβ in the brain of AD mice by minocycline. Both Aβ42 and Aβ40 levels significantly decreased in the hippocampal and cortex tissues after minocycline treatment (*P<0.01). Values were represented as mean ± SD (n=10). Aβ: beta-amyloid peptides, AD: Alzheimer’s disease, MINO: minocycline.
3.3. Minocycline Lowers the Activity of β- and γ-secretases
The generation of Aβ peptides results from the sequential cleavage of beta-amyloid precursor protein (APP) by β-secretase (BACE) and γ-secretase. This amyloidogenic pathway generates N-terminal ectodomain (sAPPβ) and Aβ peptides. The levels of BACE, BACE1, and sAPPβ in the hippocampus of minocycline-treated mice were significantly lower than the model control ones (P<0.05), as shown in Fig. (4A).
Fig. (4).
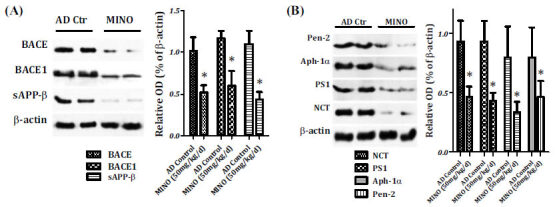
The effects of minocycline on β- and γ-secretases. Relative amounts of sAPP-β, BACE, and BACE1 were expressed as the densitometry OD ratio to β-actin (mean ± SED) for Western blotting. The sAPP-β, BACE, and BACE1 levels in the hippocampus of minocycline-treated mice were found to be lower than the model control ones (P<0.05) (A). Minocycline treatment also markedly decreased the expression of PS1, NCT, Aph-1α, and Pen-2 in the hippocampus of AD mice (B). AD: Alzheimer’s disease, Aph-1α: anterior pharynx-defective 1alpha, BACE1: the beta-amyloid precursor protein cleavage enzyme 1, MINO: minocycline, NCT: nicastrin, OD: optical density, Pen-2: presenilin enhancer 2, PS1: presenilin 1.
γ-secretase is a protease complex that has at least four components: the amino- and carboxy-terminal fragments of presenilin 1 (PS1), a highly glycosylated form of nicastrin (NCT), anterior pharynx-defective 1alpha (Aph-1α), and presenilin enhancer 2 (Pen-2). Therefore, the four components of the γ-secretase complex were investigated to examine the role of minocycline on the Aβ production. As shown in Fig. (4B), minocycline treatment markedly decreased the expression of PS1, NCT, Aph-1α, and Pen-2 in the hippocampus of AD mice. Hence, minocycline was found to inhibit the activity of β-secretase and γ-secretase complex in the hippocampus and down-regulate the Aβ levels in the brain of AD mice.
3.4. Anti-hyperphosphorylation of Tau by Minocycline
Hyperphosphorylation of tau plays an important role in the formation of NFTs in AD. In the present study, the total tau levels and phosphorylation of tau were investigated by western blot. Hippocampal samples were obtained from AD mice to measure hyperphosphorylation of tau. We selected 6 phosphorylated tau monoclonal antibodies (p-Ser199, p-Ser202, p-Thr205, p-Thr231, p-Ser396 and p-Ser404) and total tau (HT7) to evaluate changes in tau protein. Western blot revealed a substantial decrease in the levels of both total tau (HT7) and phosphorylated tau proteins, including pre-tangle marker phospho-tau protein TG3(pThr205/pThr231), intraneuronal tangle marker phospho-tau protein (pSer199/pSer202), and extracellular tangle marker PHD finger gerprotein-1 ([PHF-1] pSer396/pSer404), after minocycline treatment, compared to model control animals (Fig. 5).
Fig. (5).
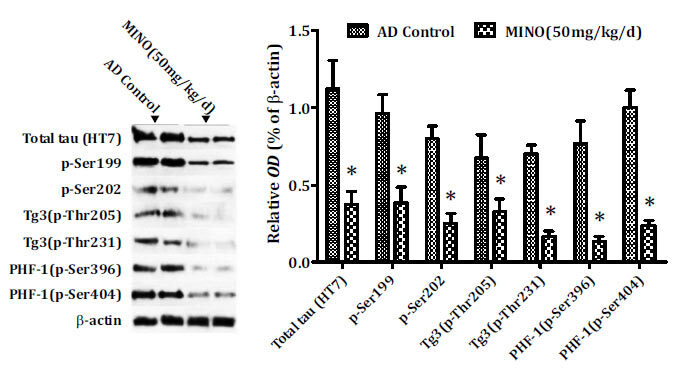
Minocycline inhibits phosphorylation of tau. Minocycline down-regulated the levels of both total tau (HT7) and phosphorylated tau proteins, including pre-tangle marker phospho-tau antibody TG3 (pThr205/pThr231), intraneuronal tangle marker phospho-tau protein (pSer199/pSer202), and extracellular tangle marker PHD finger gerprotein-1 ([PHF-1] pSer396/pSer404) in the hippocampus of AD mice. AD: Alzheimer’s disease, MINO: minocycline; OD: optical density.
3.5. Minocycline Attenuates Synaptic Deficit
The synaptic deficit of neurons directly results in a deficit in learning and memory. The synaptic deficit is another primary pathologic characteristic of AD, including the reduction of synaptic proteins and abnormal synaptic plasticity processes [32]. Thus, the three synaptic proteins (microtubule-associated protein 1, synaptophysin and post-synaptic density protein 95) were measured to determine whether minocycline treatment could rescue the synaptic deficit in AD. The results showed that minocycline increases the levels of the three synaptic proteins in the hippocampus compared to the model control mice (Fig. 6A and B).
Fig. (6).
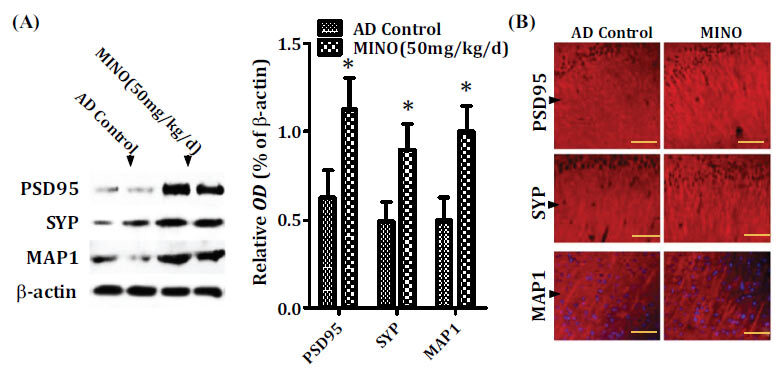
Minocycline treatment reduces the synaptic deficit. Western blot (A) and immunofluorescence staining (B) analysis showed that minocycline increased the levels of the three synaptic proteins (microtubule-associated protein 1, synaptophysin, and post-synaptic density protein 95) in the hippocampus compared to the model control mice (A and B). Quantitative results were found to be normalized against the levels of β-actin. Values were presented as the group mean ± SEM (n = 6). *p< 0.001 vs. the AD control group. The scale bar was 50 µm. AD: Alzheimer’s disease, MAP1: microtubule-associated protein 1, MINO: minocycline; OD: optical density, PSD95: post-synaptic density protein 95, SYP: synaptophysin.
3.6. Minocycline Inhibits the Activation of Cdk5/p25 Signaling
Cyclin-dependent kinase 5 (Cdk5), a unique member of the cyclin-dependent kinases (Cdks), is well recognized to be associated with the pathogenesis of AD, ranging from the formation of senile plaques and neurofibrillary tangles, synaptic damage, mitochondrial dysfunction to cell cycle reactivation as well as neuronal apoptosis. To investigate further the molecular mechanism responsible for the effect of minocycline pharmacologic blockade on tau phosphorylation, we observed that the levels of p25 and Cdk5 were significantly decreased in the minocycline-treated mice while the levels of p35 were markedly increased compared to the control ones (Fig. 7). Therefore, anti-hyperphosphorylation of tau by minocycline may be via inhibiting the activation of Cdk5/p25 signaling in the brain of AD.
Fig. (7).
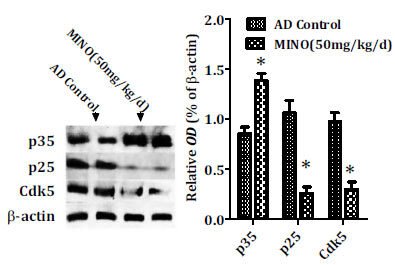
Minocycline inhibits the activity of Cdk5/p25 signaling. Minocycline significantly decreased the levels of p25 and Cdk5 while the levels of p35 were markedly increased after minocycline administration compared to the control ones (*P<0.001 vs. the control group). AD: Alzheimer’s disease, Cdk5: cyclin-dependent kinase 5, MINO: minocycline; OD: optical density.
3.7. Minocycline Limits Inflammation and Activation of Matrix Metalloproteinases
Increasing studies have demonstrated that the inflammatory system contributes to the pathogenesis of Alzheimer's disease, including the release of inflammatory cytokines, matrix metalloproteinases (MMPs), oxidative stress, and apoptotic molecule [8-11]. Thus, the inflammatory cytokine (Interleukin-1beta, IL-1β) and matrix metalloproteinases (MMP-2 and MMP-9) were measured to determine whether minocycline treatment could limit the inflammation and activation of matrix metalloproteinases in the pathogenesis of Alzheimer's disease. The results showed that minocycline lowed the levels of IL-1β and matrix metalloproteinases (MMP-2 and MMP-9) proteins in the cerebral cortex compared to the model control mice (Fig. 8A and B).
Fig. (8).
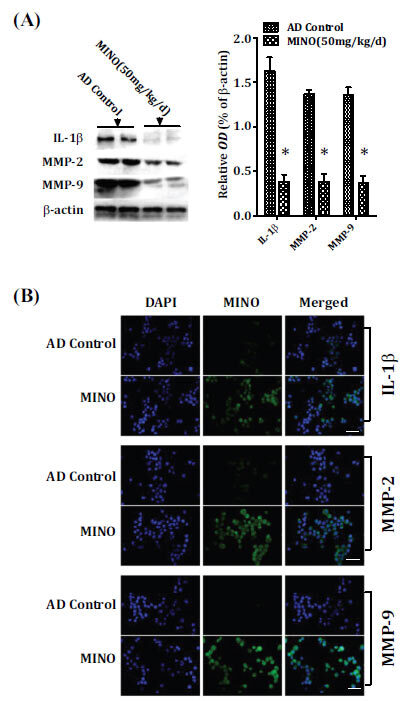
Minocycline limits inflammation and activation of matrix metalloproteinases. Western blot (A) and immunofluorescence staining (B) analysis showed that minocycline reduced the levels of IL-1β and matrix metalloproteinases (MMP-2 and MMP-9) proteins in the cerebral cortex compared to the model control mice (A and B). Quantitative results were found to be normalized against the levels of β-actin. Values were presented as the group mean ± SEM (n = 6). *p< 0.001 vs. the AD control group. The scale bar was 20µm. AD: Alzheimer’s disease, IL-1β: Interleukin-1beta, MINO: minocycline; MMP-2:matrix metalloproteinase 2, MMP-9:matrix metalloproteinase 9, OD: optical density.
4. DISCUSSION
The present study unveils a new facet of the neurobiology of minocycline which strongly retards the pathogenic development of AD. We showed that minocycline decreases the production of Aβ and hyperphosphorylation of tau in the hippocampus, ameliorates synaptic deficit, and improves cognitive impairment in APP/PS1 transgenic mice. Studies have also shown that minocycline markedly inhibits the activation of Cdk5/p25 signaling, which is closely associated with Alzheimer’s-like pathology. Hence, all of these parameters have indicated that minocycline attenuates the pathology of AD via inhibiting the activation of Cdk5/p25 signaling, including limiting the production of Aβ and hyperphosphorylation of tau and improving the synaptic deficit. This study has demonstrated the therapeutic potential of minocycline in AD.
The production and accumulation of Aβ have been widely accepted as the initial causative events in AD. The Aβ is generated from amyloid precursor protein (APP) by the sequential cleavage of β-secretase and γ-secretase. APP is sequentially cleaved first by β-secretase to form soluble amyloid precursor protein β (sAPPβ). The beta-amyloid precursor protein cleavage enzyme 1 (BACE1) has been identified as β-secretase, which is the leading function in Aβ production. Minocycline, an antibiotic that effectively crosses the blood-brain barrier, has been found to have significant neuroprotective effects on AD [1, 33, 34]. Minocycline might represent a promising drug for preventing or delaying the development of Alzheimer's-like neuropathology at its initial, pre-clinical stages by ameliorating early neuroinflammation and inhibiting BACE1 activity [33].
To further clarify the role of minocycline in the Aβ pathology, we investigated the change in the levels of BACE and BACE1 in the brain of AD mice after minocycline treatment. It has been found that minocycline significantly lowers BACE, BACE1, and sAPPβ levels in the hippocampus of AD mice. These findings strongly support the role of minocycline in inhibiting Aβ generation, acting as a specific inhibitor of β-secretase.
It is now recognized that a key role is played by γ-secretase that cleaves sAPPβ to produce the Aβ fragment, ultimately resulting in the formation of amyloid plaques. γ-secretase, as a protease complex, has at least four components: the amino- and carboxy-terminal fragments of presenilin 1 (PS1), a highly glycosylated form of nicastrin (NCT), anterior pharynx-defective 1alpha (Aph-1α), and presenilin enhancer 2 (Pen-2) [35]. Thus, the four components of the γ-secretase complex were investigated to determine the role of minocycline on the Aβ production. It has been demonstrated that minocycline treatment markedly suppressed the activity of PS1, NCT, Aph-1α, and Pen-2 in the hippocampus of AD mice. Taken together, minocycline made a remarkable contribution to inhibiting the activity of β-secretase and γ-secretase complex and then decreasing the Aβ levels in the brain of AD mice.
Intracellular deposits of hyperphosphorylated tau protein are one of the classical pathological hallmarks in AD [36]. This study investigated the levels of phosphorylated tau proteins, including pre-tangle marker phospho-tau protein (TG3: pThr205/pThr231), intraneuronal tangle marker phospho-tau protein (pSer199/pSer202), and extracellular tangle marker PHD finger gerprotein-1 ([PHF-1] pSer396/pSer404), and total tau (HT7). These parameters showed that minocycline strongly limits the phosphorylation of tau protein in the different stages of tau hyperphosphorylation, including pre-tangle phospho-tau protein, intraneuronal tangle phospho-tau protein, extracellular tangle PHD finger gerprotein-1, and total tau. These data indicated that one of the neuroprotective roles of minocycline is to suppress the tau hyperphosphorylation.
Synaptic dysfunction and the loss of synapses are pathological features of AD [37]. It is well reported that synaptic dysfunction results in cognitive deficit in AD [38, 39]. The reversibility of early synaptic dysfunction at the preclinical stage of AD highlights the importance of delaying or preventing the disease processes [40]. It has been reported that minocycline benefits synaptic plasticity and improves cognitive impairment in AD models [21]. This study has also shown that minocycline increases the levels of the three synaptic proteins (microtubule-associated protein 1, synaptophysin, and post-synaptic density protein 95) in the hippocampus, implicating that minocycline attenuates synaptic deficit and then improves cognitive impairment in AD.
Cdk5, a multi-function kinase, has both physiological and pathological functions. In the central nervous system, Cdk5 is implicated in neuronal survival and death [41]. The combination of monomeric Cdk5 and p35 (inhibiting Cdk5 activity) implements its diverse functions, such as neuronal migration and differentiation, synaptic growth and functions, memory consolidation as well as normal cerebellar development [42]. In another condition, p35 will be cleaved into p10 and p25, which can activate Cdk5. A combination of p25 with Cdk5 could form a stable complex, p25/Cdk5, which leads to various pathological events, including Aβ production, hyperphosphorylation of tau, synaptic dysfunction, and neuronal cell apoptosis [41, 43] (Fig. 9). This study demonstrated a significant decrease in the levels of p25 and Cdk5d in the minocycline-treated mice, while the levels of p35 were markedly increased by minocycline treatment compared to the control ones. It can be concluded that minocycline plays an important role in Alzheimer’s-like pathology, including the production of anti-Aβ, anti-hyperphosphorylation of tau, and improvement of synaptic dysfunction, via inhibiting the activation of Cdk5/p25 signaling.
Fig. (9).
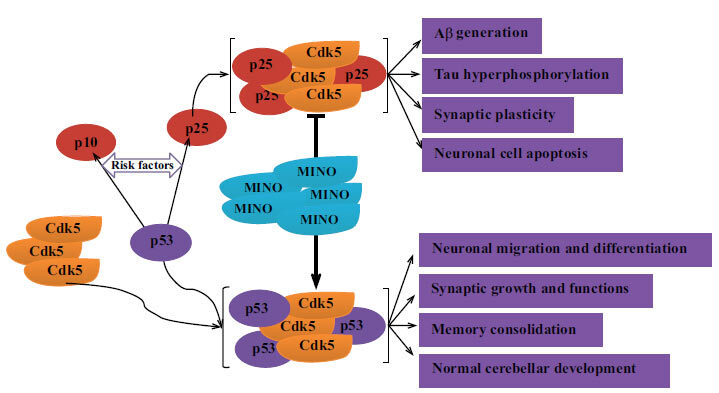
The possible mechanisms of anti-pathology in AD by minocycline. Minocycline inhibits the activation of Cdk5/p25 signaling and limits the development of Alzheimer’s-like pathology, including production of anti-Aβ, anti-hyperphosphorylation of tau, and improvement of synaptic dysfunction. Aβ: beta-amyloid peptides; AD: Alzheimer’s disease; Cdk5: cyclin-dependent kinase 5; MINO: minocycline.
CONCLUSION
This study has evaluated the inñuence of minocycline on the Alzheimer’s-like pathology, including Aβ production, hyperphosphorylation of tau, and improvement of synaptic dysfunction in an APP/PS1 transgenic model. Our research showed the neuroprotective property of minocycline against AD with respect to inhibiting Aβ production and hyperphosphorylation of tau and improving synaptic dysfunction and cognitive deficits. Furthermore, a significant decrease in the p25 and Cdk5d levels in the minocycline-treated mice was observed while the levels of p35 were markedly increased by minocycline treatment. Therefore, the ability of minocycline concerning Alzheimer’s-like pathology may be of great importance in selecting neuroprotective agents in the treatment and prevention of AD.
AUTHORS’ CONTRIBUTIONS
Zhiyou Cai and Yu Zhao participated in designing the experiments, analyzing the data, and writing the manuscript; Wenbo He and Chuanling Wang performed the experiments and analyzed the data; Wenbo He and Chuanling Wang helped with designing experiments and writing the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animal experiments the ethical standards approved by the Research Ethics Committee of the Hubei University of Medicine, China.
HUMAN AND ANIMAL RIGHTS
All the experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was supported by grants from the Chongqing Natural Science Foundation.
ACKNOWLEDGEMENTS
This work was supported by grants from the Chongqing Natural Science Foundation (cstc2018jcyjA4051), Chongqing Health and Natural Science Foundation (2018ZDXM004), the Natural Science Foundation of Hubei Province (2015 CFB260), and the Hubei Province Health and Family Planning Scientific Research Project (WJ2015MB219) and the Shiyan Natural Science Foundation (15K70), and Chongqing General Hospital provided to Dr. Zhiyou Cai.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Daulatzai M.A. Pharmacotherpy and Alzheimer’s disease: The M-Drugs (Melatonin, Minocycline, Modafinil, and Memantine) approach. Curr. Pharm. Des. 2016;22(16):2411–2430. doi: 10.2174/1381612822666160203142111. [DOI] [PubMed] [Google Scholar]
- 2.Chase T.N., Farlow M.R., Clarence-Smith K. Donepezil plus solifenacin (CPC-201) treatment for Alzheimer’s disease. Neurotherapeutics. 2017;14(2):405–416. doi: 10.1007/s13311-016-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han S.H., Lee J.H., Kim S.Y., Park K.W., Chen C., Tripathi M., Dash A., Kubota N. Donepezil 23 mg in Asian patients with moderate-to-severe Alzheimer’s disease. Acta Neurol. Scand. 2017;135(2):252–256. doi: 10.1111/ane.12571. [DOI] [PubMed] [Google Scholar]
- 4.Blautzik J., Keeser D., Paolini M., Kirsch V., Berman A., Coates U., Reiser M., Teipel S.J., Meindl T. Functional connectivity increase in the default-mode network of patients with Alzheimer’s disease after long-term treatment with Galantamine. Eur. Neuropsychopharmacol. 2016;26(3):602–613. doi: 10.1016/j.euroneuro.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Mancuso C., Siciliano R., Barone E., Butterfield D.A., Preziosi P. Pharmacologists and Alzheimer disease therapy: To boldly go where no scientist has gone before. Expert Opin. Investig. Drugs. 2011;20(9):1243–1261. doi: 10.1517/13543784.2011.601740. [DOI] [PubMed] [Google Scholar]
- 6.Ehret M.J., Chamberlin K.W. Current practices in the treatment of Alzheimer disease: Where is the evidence after the phase iii trials? Clin. Ther. 2015;37(8):1604–1616. doi: 10.1016/j.clinthera.2015.05.510. [DOI] [PubMed] [Google Scholar]
- 7.Mehta D., Jackson R., Paul G., Shi J., Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin. Investig. Drugs. 2017;26(6):735–739. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azizi G., Khannazer N., Mirshafiey A. The potential role of chemokines in Alzheimer’s disease pathogenesis. Am. J. Alzheimers Dis. Other Demen. 2014;29(5):415–425. doi: 10.1177/1533317513518651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padurariu M., Ciobica A., Hritcu L., Stoica B., Bild W., Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2010;469(1):6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Azizi G., Navabi S.S., Al-Shukaili A., Seyedzadeh M.H., Yazdani R., Mirshafiey A. The role of inflammatory mediators in the pathogenesis of Alzheimer’s disease. Sultan Qaboos Univ. Med. J. 2015;15(3):e305–e316. doi: 10.18295/squmj.2015.15.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan A., Park T.J., Ikram M., Ahmad S., Ahmad R., Jo M.G., Kim M.O. Antioxidative and Anti-inflammatory effects of kojic acid in Aβ-induced mouse model of Alzheimer’s disease. Mol. Neurobiol. 2021;58(10):5127–5140. doi: 10.1007/s12035-021-02460-4. [DOI] [PubMed] [Google Scholar]
- 12.Di Stefano A., Sozio P., Iannitelli A., Cerasa L.S., Fontana A., Di Biase G., D’Amico G., Di Giulio M., Carpentiero C., Grumetto L., Barbato F. Characterization of alkanoyl-10-O-minocyclines in micellar dispersions as potential agents for treatment of human neurodegenerative disorders. Eur. J. Pharm. Sci. 2008;34(2-3):118–128. doi: 10.1016/j.ejps.2008.02.123. [DOI] [PubMed] [Google Scholar]
- 13.Hahn J.N., Kaushik D.K., Mishra M.K., Wang J., Silva C., Yong V.W. Impact of minocycline on extracellular matrix metalloproteinase inducer, a factor implicated in multiple sclerosis immunopathogenesis. J. Immunol. 2016;197(10):3850–3860. doi: 10.4049/jimmunol.1600436. [DOI] [PubMed] [Google Scholar]
- 14.Soliman S., Ishrat T., Fouda A.Y., Patel A., Pillai B., Fagan S.C. Sequential therapy with minocycline and candesartan improves long-term recovery after experimental stroke. Transl. Stroke Res. 2015;6(4):309–322. doi: 10.1007/s12975-015-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Z.Y., Yan Y., Chen R. Minocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat model. Neurosci. Bull. 2010;26(1):28–36. doi: 10.1007/s12264-010-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry C.J., Huang Y., Wynne A., Hanke M., Himler J., Bailey M.T., Sheridan J.F., Godbout J.P. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y., Salayandia V.M., Thompson J.F., Yang L.Y., Estrada E.Y., Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J. Neuroinflammation. 2015;12:26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klaas J.P., Matzke T., Makol A., Fulgham J.R. Minocycline-induced polyarteritis nodosa-like vasculitis presenting as brainstem stroke. J. Clin. Neurosci. 2015;22(5):904–907. doi: 10.1016/j.jocn.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Kohler E., Prentice D.A., Bates T.R., Hankey G.J., Claxton A., van Heerden J., Blacker D. Intravenous minocycline in acute stroke: A randomized, controlled pilot study and meta-analysis. Stroke. 2013;44(9):2493–2499. doi: 10.1161/STROKEAHA.113.000780. [DOI] [PubMed] [Google Scholar]
- 20.Sørensen P.S., Sellebjerg F., Lycke J., Färkkilä M., Créange A., Lund C.G., Schluep M., Frederiksen J.L., Stenager E., Pfleger C., Garde E., Kinnunen E., Marhardt K. Minocycline added to subcutaneous interferon β-1a in multiple sclerosis: Randomized RECYCLINE study. Eur. J. Neurol. 2016;23(5):861–870. doi: 10.1111/ene.12953. [DOI] [PubMed] [Google Scholar]
- 21.Choi Y., Kim H.S., Shin K.Y., Kim E.M., Kim M., Kim H.S., Park C.H., Jeong Y.H., Yoo J., Lee J.P., Chang K.A., Kim S., Suh Y.H. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology. 2007;32(11):2393–2404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- 22.Mushtaq G., Greig N.H., Anwar F., Al-Abbasi F.A., Zamzami M.A., Al-Talhi H.A., Kamal M.A. Neuroprotective Mechanisms Mediated by CDK5 Inhibition. Curr. Pharm. Des. 2016;22(5):527–534. doi: 10.2174/1381612822666151124235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon K.J., Park J.H., Jo I., Song K.H., Han J.S., Park S.H., Han S.H., Cho D.H. Disruption of neuronal nitric oxide synthase dimerization contributes to the development of Alzheimer’s disease: Involvement of cyclin-dependent kinase 5-mediated phosphorylation of neuronal nitric oxide synthase at Ser(293). Neurochem. Int. 2016;99:52–61. doi: 10.1016/j.neuint.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra N., Gupta R., Kumar P. Pharmacological relevance of CDK inhibitors in Alzheimer’s disease. Neurochem. Int. 2021;148:105115. doi: 10.1016/j.neuint.2021.105115. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y., Huang W., Huang Y., Song P., Zhang M., Zhang H.T., Pan S., Hu Y. Cdk5 inhibitory peptide prevents loss of neurons and alleviates behavioral changes in p25 transgenic mice. J. Alzheimers Dis. 2020;74(4):1231–1242. doi: 10.3233/JAD-191098. [DOI] [PubMed] [Google Scholar]
- 26.Rosell-Cardona C., Griñan-Ferré C., Pérez-Bosque A., Polo J., Pallàs M., Amat C., Moretó M., Miró L. Dietary spray-dried porcine plasma reduces neuropathological Alzheimer’s disease Hallmarks in SAMP8 mice. Nutrients. 2021;13(7):2369. doi: 10.3390/nu13072369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S.D., Yang J.L., Lin Y.C., Chao A.C., Yang D.I. Emerging roles of inhibitor of differentiation-1 in Alzheimer’s disease: cell cycle reentry and beyond. Cells. 2020;9(7):E1746. doi: 10.3390/cells9071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohman R.A., Bhattacharya T.K., Kilby C., Bucko P., Rhodes J.S. Effects of minocycline on spatial learning, hippocampal neurogenesis and microglia in aged and adult mice. Behav. Brain Res. 2013;242:17–24. doi: 10.1016/j.bbr.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siopi E., Calabria S., Plotkine M., Marchand-Leroux C., Jafarian-Tehrani M. Minocycline restores olfactory bulb volume and olfactory behavior after traumatic brain injury in mice. J. Neurotrauma. 2012;29(2):354–361. doi: 10.1089/neu.2011.2055. [DOI] [PubMed] [Google Scholar]
- 30.Meng F., Wang J., Ding F., Xie Y., Zhang Y., Zhu J. Neuroprotective effect of matrine on MPTP-induced Parkinson’s disease and on Nrf2 expression. Oncol. Lett. 2017;13(1):296–300. doi: 10.3892/ol.2016.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Z., Yan Y., Wang Y. Minocycline alleviates beta-amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorder. Clin. Interv. Aging. 2013;8:1089–1095. doi: 10.2147/CIA.S46536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarantseva S.V., Bol’shakova O.I., Timoshenko S.I., Rodin D.I., Vitek M.P., Shvartsman A.L. Studying pathogenesis of Alzheimer’s disease in a Drosophila melanogaster model: Human APP overexpression in the brain of transgenic flies leads to deficit of the synaptic protein synaptotagmin. Genetika. 2009;45(1):119–126. [PubMed] [Google Scholar]
- 33.Ferretti M.T., Allard S., Partridge V., Ducatenzeiler A., Cuello A.C. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer’s disease-like amyloid pathology. J. Neuroinflammation. 2012;9:62. doi: 10.1186/1742-2094-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S.D., Yin J.H., Hwang C.S., Tang C.M., Yang D.I. Anti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: Implication in Alzheimer’s disease and cerebral ischemia. Free Radic. Res. 2012;46(8):940–950. doi: 10.3109/10715762.2012.674640. [DOI] [PubMed] [Google Scholar]
- 35.Gwon A.R., Park J.S., Arumugam T.V., Kwon Y.K., Chan S.L., Kim S.H., Baik S.H., Yang S., Yun Y.K., Choi Y., Kim S., Tang S.C., Hyun D.H., Cheng A., Dann C.E., III, Bernier M., Lee J., Markesbery W.R., Mattson M.P., Jo D.G. Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer’s disease. Aging Cell. 2012;11(4):559–568. doi: 10.1111/j.1474-9726.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascoal T.A., Mathotaarachchi S., Mohades S., Benedet A.L., Chung C.O., Shin M., Wang S., Beaudry T., Kang M.S., Soucy J.P., Labbe A., Gauthier S., Rosa-Neto P. Amyloid-β and hyperphosphorylated tau synergy drives metabolic decline in preclinical Alzheimer’s disease. Mol. Psychiatry. 2017;22(2):306–311. doi: 10.1038/mp.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcello E., Di Luca M., Gardoni F. Synapse-to-nucleus communication: From developmental disorders to Alzheimer’s disease. Curr. Opin. Neurobiol. 2018;48:160–166. doi: 10.1016/j.conb.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Schreurs A., Latif-Hernandez A., Uwineza A. Commentary: APP as a Mediator of the Synapse Pathology in Alzheimer’s Disease. Front. Cell. Neurosci. 2018;12:150. doi: 10.3389/fncel.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad F., Das D., Kommaddi R.P., Diwakar L., Gowaikar R., Rupanagudi K.V., Bennett D.A., Ravindranath V. Isoform-specific hyperactivation of calpain-2 occurs presymptomatically at the synapse in Alzheimer’s disease mice and correlates with memory deficits in human subjects. Sci. Rep. 2018;8(1):13119. doi: 10.1038/s41598-018-31073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.H., Kim K.R., Ryu S.Y., Son S., Hong H.S., Mook-Jung I., Lee S.H., Ho W.K. Impaired short-term plasticity in mossy fiber synapses caused by mitochondrial dysfunction of dentate granule cells is the earliest synaptic deficit in a mouse model of Alzheimer’s disease. J. Neurosci. 2012;32(17):5953–5963. doi: 10.1523/JNEUROSCI.0465-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S.L., Wang C., Jiang T., Tan L., Xing A., Yu J.T. The role of Cdk5 in Alzheimer’s disease. Mol. Neurobiol. 2016;53(7):4328–4342. doi: 10.1007/s12035-015-9369-x. [DOI] [PubMed] [Google Scholar]
- 42.Kumazawa A., Mita N., Hirasawa M., Adachi T., Suzuki H., Shafeghat N., Kulkarni A.B., Mikoshiba K., Inoue T., Ohshima T. Cyclin-dependent kinase 5 is required for normal cerebellar development. Mol. Cell. Neurosci. 2013;52:97–105. doi: 10.1016/j.mcn.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopes J.P., Oliveira C.R., Agostinho P. Neurodegeneration in an Abeta-induced model of Alzheimer’s disease: The role of Cdk5. Aging Cell. 2010;9(1):64–77. doi: 10.1111/j.1474-9726.2009.00536.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


