Abstract
Background
Locally advanced esophageal carcinoma is typically treated with neoadjuvant chemoradiation and esophagectomy (trimodality therapy). We compared the long-term oncologic outcomes of minimally invasive Ivor Lewis esophagectomy (M-ILE) cohort with a propensity score weighted cohort of open Ivor Lewis esophagectomy (O-ILE) cases after trimodality therapy.
Methods
This is a retrospective review of 223 patients diagnosed with esophageal carcinoma who underwent neoadjuvant chemoradiation followed by M-ILE or O-ILE from April 2009 to February 2019. Inverse probability of treatment weighting (IPTW) adjustment was used to balance the baseline characteristics between study groups. Kaplan–Meier survival curves were calculated for overall survival and recurrence-free survival comparing the two groups. Multivariate Cox proportional hazards regression models were used to determine predictive variables for overall and recurrence-free survival.
Results
The IPTW cohort included patients with esophageal carcinoma who underwent M-ILE (n = 142) or O-ILE (n = 68). The overall rate of postoperative adverse events was not significantly different after IPTW adjustment between the O-ILE and M-ILE trimodality groups (53.4% vs. 39.2%, p = 0.089). The 3-year overall survival (OS) for the M-ILE group was 59.4% (95% CI: 49.8–67.8) compared to 55.7% (95% CI: 39.2–69.4) for the O-ILE group (p = 0.670). The 3-year recurrence-free survival for the M-ILE group was 59.9% (95% CI: 50.2–68.2) compared to 61.6% (95% CI: 41.9–76.3) for the O-ILE group (p = 0.357). A complete response to neoadjuvant chemoradiation was significantly predictive of improved OS and RFS.
Conclusion
The overall and recurrence-free survival rates for M-ILE were not significantly different from O-ILE for esophageal carcinoma after trimodality therapy. Complete response to neoadjuvant chemoradiation was predictive of improved overall and recurrence- free survival.
Keywords: Esophageal carcinoma, Ivor Lewis esophagectomy, Neoadjuvant therapy, Minimally invasive esophagectomy
Introduction
In the United States, there were an estimated 17,650 newly diagnosed cases of esophageal carcinoma in 2019 [1]. Neoadjuvant chemoradiation (CRT) and esophagectomy combined with comprehensive lymphadenectomy (trimodality therapy) is the current standard treatment for locally advanced esophageal carcinoma [2, 3]. The randomized Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS) Trial demonstrated improved overall survival and disease-free survival for patients who underwent preoperative chemoradiation followed by esophagectomy for locally advanced esophageal carcinoma [2]. Esophagectomy is the mainstay surgical treatment for esophageal carcinoma; however, the complex surgical procedure has been associated with significant morbidity and mortality [4]. Some reports have demonstrated evidence that preoperative chemoradiation may increase the incidence of postoperative complications after open esophagectomy [5, 6].
In recent years, minimally invasive techniques for Ivor Lewis esophagectomy were developed with the hope of minimizing postoperative morbidity and mortality. Luketich and colleagues reported a large single cohort series of over 1000 minimally invasive esophagectomy procedures that demonstrated a low postoperative mortality rate and a relatively low rate of postoperative pulmonary complications [7]. A randomized clinical trial also demonstrated decreased rates of postoperative pulmonary complications for minimally invasive transthoracic esophagectomy compared to the open approach [8]. In addition, a single center cohort study demonstrated fewer postoperative complications and a shorter hospital length of stay for minimally invasive esophagectomy compared to the open approach [9]. The previous comparative studies between minimally invasive Ivor Lewis esophagectomy (M-ILE) and open Ivor Lewis esophagectomy (O-ILE) have mostly focused on short-term clinical outcomes [10, 11]. However, the current gold standard for oncologic outcomes remains overall survival and recurrence-free survival. Only a few studies have evaluated the long-term oncologic results of minimally invasive esophagectomy compared to open esophagectomy [12–14]. In this study, we reported a propensity score adjusted comparison of the long-term oncologic outcomes (overall survival and recurrence-free survival) between M-ILE and O-ILE performed for patients with locally advanced esophageal carcinoma who completed neoadjuvant chemoradiation. We hypothesized that patients undergoing M-ILE after neoadjuvant chemoradiation for esophageal carcinoma will have equivalent long-term oncologic outcomes compared to a similar cohort of patients who underwent O-ILE.
Methods
Patient population
This study is a retrospective observational review of 223 patients who underwent neoadjuvant chemoradiation and Ivor Lewis esophagectomy for esophageal carcinoma on the General Thoracic Surgery Service at the Ohio State Wexner Medical Center between April 2009 and February 2019. The inverse probability of treatment weighting-adjusted (IPTW) cohort included patients with esophageal carcinoma who had undergone M-ILE (n = 142) or O-ILE (n = 68). The CONSORT diagram for the patient selection flowchart is shown in Fig. 1. Patients with adenocarcinoma or squamous cell carcinoma involving the mid-esophagus, distal third, or the gastroesophageal junction were included. The selection of the surgical approach was at the discretion of the treating surgeon. The study was approved by the Institutional Review Board and the requirement for informed consent was waived. The patients were clinically evaluated before the initiation of treatment with endoscopy, Computed Tomography (CT) scans, and clinical history and exam. Endoscopic Ultrasound and Positron Emission Tomography (PET) scans were performed for most patients prior to trimodality therapy (Table 1). The neoadjuvant treatment regimen consisted of weekly carboplatin with paclitaxel with concurrent radiotherapy (41.4, 45, or 50.4 Gray in fractions of 1.8 Gray). A PET CT scan was obtained 3–5 weeks after the completion of neoadjuvant chemoradiation to evaluate patients for clinical response and disease progression.
Fig. 1.
CONSORT diagram for patient selection and allocation
Table 1.
Patient demographics
| Unadjusted | IPTW adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Levels | O-ILE N = 69 |
M-ILE N = 154 |
p-value | O-ILE N = 68 |
M-ILE N = 142 |
p-value |
| Age | Mean (Std Error) | 62.36 (1.26) | 62.31 (0.78) | 0.968 | 61.31 (1.47) | 62.51 (0.79) | 0.813 |
| Gender | Female | 6 (8.7%) | 24 (15.6%) | 0.163 | 11.28% | 10.40% | 0.868 |
| Male | 63 (91.3%) | 130 (84.4%) | 88.72% | 89.60% | |||
| Coronary artery disease | Yes | 25 (36.2%) | 44 (28.6%) | 0.253 | 29.80% | 30.81% | 0.891 |
| Diabetes | Yes | 18 (26.1%) | 42 (27.3%) | 0.854 | 22.07% | 25.44% | 0.612 |
| COPD | Yes | 9 (13.0%) | 14 (9.1%) | 0.370 | 10.50% | 9.90% | 0.897 |
| Cigarette smoking | Current smoker | 14 (20.3%) | 28 (18.2%) | 0.825 | 14.65% | 18.62% | 0.729 |
| Never smoked | 15 (21.7%) | 39 (25.3%) | 27.44% | 23.13% | |||
| Past Smoker | 40 (58.0%) | 87 (56.5%) | 57.91% | 58.25% | |||
| Body mass index | Mean (Std Error) | 26.96 (0.72) | 27.67 (0.45) | 0.394 | 27.33 (0.66) | 27.23 (0.44) | 0.831 |
| CT scan | Yes | 58 (84.1%) | 121 (78.6%) | 0.341 | 84.45% | 78.11% | 0.350 |
| Endoscopic ultrasound | Yes | 58 (84.1%) | 125 (81.2%) | 0.603 | 72.74% | 80.43% | 0.321 |
| PET scan | Yes | 68 (98.6%) | 154 (100.0%) | 0.309 | 98.97% | 100.00% | |
| ECOG status | 0 | 40 (58.0%) | 64 (41.6%) | 0.023 | 45.00% | 48.50% | 0.669 |
| 1/2 | 29 (42.0%) | 90 (58.4%) | 55.00% | 51.50% | |||
| Radiation dose | 41 Gray | 0 (0.0%) | 1 (0.7%) | > 0.999 | 0.00% | 0.70% | 0.754 |
| 45 Gray | 13 (18.8%) | 29 (18.8%) | 17.70% | 19.39% | |||
| 50.4 Gray | 56 (81.2%) | 124 (80.5%) | 82.30% | 79.95% | |||
| Time from CRT to surgery | Median Wks [IQR] | 8.57 [6.86–10.29] | 10.71 [9.43–13.43] | < 0.001 | 7.87 [5.83–9.81] | 10.52 [9.13–13.12] | < 0.001 |
IPTW Inverse probability of treatment weighting, O-ILE Open Ivor Lewis esophagectomy, M-ILE Minimally invasive Ivor Lewis esophagectomy, Std Standard, COPD Chronic Obstructive Pulmonary Disease, CT Computed Tomography, PET Positron Emission Tomograpy, CRT Chemoradiation therapy, IQR Interquartile range
After a 6–12 week interval, patients underwent Ivor-Lewis esophagectomy with an open or minimally invasive approach. For the abdominal phase of the M-ILE, a complete laparoscopic or robotic gastric mobilization was performed with an en-bloc resection of the celiac and peri-gastric lymph nodes. For the thoracic phase of the M-ILE, a thoracoscopic or robotic mobilization of the esophagus was performed and an intrathoracic esophagogastric anastomosis, which was routinely covered with omentum or mediastinal pleura. The O-ILE was performed with a mid-line laparotomy incision and a posterior-lateral right thoracotomy incision. The general conduct of the operation is the same as described above for the M-ILE technique. The surgical specimens were reviewed and processed in a standardized manner. The pathologic stage after neoadjuvant therapy (ypTNM) was based on the 8th edition of the AJCC/UICC staging manual [15]. The tumor regression grade is based on the Ryan scoring system [16]. Complete response (no viable cancer cells) is score 0; near complete response (single cancer cells or small clusters of cancer cells) is score 1; partial response (residual cancer with more than single cells) is score 2; poor or no response (extensive residual cancer with minimal evidence of tumor regression) is score 3. We also recorded perineural invasion, lympho-vascular invasion, tumor differentiation, signet ring cell features, and positive lymph nodes from the pathology reports.
Outcome measures
The demographic data, clinical data, and perioperative outcomes were extracted from our institutional Society of Thoracic Surgery Database (Table 1). The clinical and pathologic stages were recorded using the American Joint Committee on Cancer, 8th edition, staging manual for esophageal carcinoma (Table 2). The primary outcomes were overall survival (OS) and recurrence-free survival (RFS). Overall survival was calculated as the date of surgery to the last follow up visit (censored) or death (event). A recurrence-free survival event was defined as the interval from surgery to either biopsy-proven or radiographic evidence of disease recurrence or death. The follow-up protocol included an initial postoperative visit within 2–3 weeks after esophagectomy and surveillance visits with a history and physical and contrast-enhanced computed tomography scans every 3–6 months for the first 3 years. Patients who did not have at least one follow-up at 3 months or greater were classified as lost to follow-up and were excluded from the study. The other oncologic endpoint included the total number of lymph nodes removed during esophagectomy. The secondary outcomes included the rate of postoperative adverse events, 30-day and 90-day mortality rate, length of stay, and 30-day readmission rates (Table 5). Multivariate Cox regression models were used to assess independent clinical and pathologic variables that predicted overall and recurrence-free survival after trimodality therapy.
Table 2.
Staging and Pathologic Features
| Unadjusted | IPTW adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Levels | O-ILE N = 69 |
M-ILE N = 154 |
p-value | O-ILE N = 68 |
M-ILE N = 142 |
p-value |
| Clinical T-stage | 1/2 | 22 (31.9%) | 30 (19.5%) | 0.043 | 21.00% | 23.10% | 0.731 |
| 3 | 47 (68.1%) | 124 (80.5%) | 79.00% | 76.90% | |||
| Clinical N-stage | N0 | 16 (23.2%) | 66 (42.9%) | 0.001 | 41.46% | 37.01% | 0.868 |
| N1 | 50 (72.5%) | 71 (46.1%) | 51.38% | 56.05% | |||
| N2 | 3 (4.3%) | 17 (11.0%) | 7.17% | 6.95% | |||
| Histology | Squamous Cell Carcinoma | 3 (4.3%) | 11 (7.1%) | 0.426 | 3.07% | 7.26% | 0.166 |
| Adenocarcinoma | 66 (95.7%) | 143 (92.9%) | 96.93% | 92.74% | |||
| Pathologic T-stage | T0 | 19 (27.5%) | 41 (26.6%) | 0.703 | 18.89% | 27.22% | 0.674 |
| T1a/T1b | 12 (17.4%) | 28 (18.2%) | 17.78% | 17.71% | |||
| T2 | 11 (15.9%) | 34 (22.1%) | 24.09% | 22.50% | |||
| T3 | 27 (39.1%) | 51 (33.1%) | 39.24% | 32.57% | |||
| Pathologic N-Stage | N0/Nx | 43 (62.3%) | 99 (64.3%) | 0.399 | 60.83% | 66.18% | 0.394 |
| N1 | 13 (18.8%) | 37 (24.0%) | 20.31% | 22.92% | |||
| N2 | 7 (10.1%) | 12 (7.8%) | 10.22% | 7.87% | |||
| N3 | 6 (8.7%) | 6 (3.9%) | 8.64% | 3.03% | |||
| Differentiation | Moderate | 46 (66.7%) | 74 (48.1%) | 0.031 | 67.44% | 50.42% | 0.139 |
| Poor | 21 (30.4%) | 69 (44.8%) | 28.77% | 42.68% | |||
| Well | 2 (2.9%) | 11 (7.1%) | 3.79% | 6.90% | |||
| Lymphovascular invasion | Yes | 14 (20.3%) | 42 (27.3%) | 0.266 | 20.04% | 27.24% | 0.309 |
| Perineural invasion | Yes | 16 (23.2%) | 30 (19.5%) | 0.527 | 21.30% | 19.64% | 0.792 |
| Signet ring cell features | Yes | 4 (5.8%) | 19 (12.3%) | 0.138 | 9.93% | 11.97% | 0.741 |
| Tumor regression grade | Complete response | 18 (26.1%) | 38 (24.7%) | 0.043 | 18.60% | 25.58% | 0.274 |
| Near complete response | 24 (34.8%) | 58 (37.7%) | 42.41% | 36.52% | |||
| Partial response | 11 (15.9%) | 42 (27.3%) | 20.61% | 27.75% | |||
| No response | 16 (23.2%) | 16 (10.4%) | 18.38% | 10.14% | |||
| Lymph nodes removed | Median [IQR] | 18 [14–22] | 17 [14–21] | 0.956 | 16 [12–20] | 17 [13–21] | 0.822 |
IPTW Inverse probability of treatment weighting, O-ILE Open Ivor Lewis esophagectomy, M-ILE Minimally invasive Ivor Lewis esophagectomy, IQR Interquartile range
Table 5.
Postoperative complications
| Unadjusted | IPTW adjusted | |||||
|---|---|---|---|---|---|---|
| Variable | O-ILE N = 69 |
M-ILE N = 154 |
p-value | O-ILE N = 68 |
M-ILE N = 142 |
p-value |
| Postoperative event occurred | 36 (52.2%) | 62 (40.3%) | 0.098 | 53.39% | 39.19% | 0.089 |
| Acute respiratory distress syndrome | 0 (0.0%) | 1 (0.6%) | 0.00% | 0.74% | ||
| Acute myocardial infarction | 0 (0.0%) | 0 (0.0%) | 0.00% | 0.00% | ||
| Air leak ≥ 5 Days | 0 (0.0%) | 0 (0.0%) | 0.00% | 0.00% | ||
| Airway fistula | 0 (0.0%) | 5 (3.2%) | 0.00% | 3.08% | ||
| Anastomotic leak | 6 (8.7%) | 9 (5.8%) | 0.432 | 12.63% | 6.41% | 0.228 |
| Anastomotic leak requiring repair | 4 (5.8%) | 5 (3.2%) | 0.371 | 3.89% | 3.47% | 0.866 |
| Anastomotic leak requiring stent | 1 (1.4%) | 5 (3.2%) | 0.443 | 4.47% | 3.56% | 0.830 |
| Conduit necrosis requiring surgery | 2 (2.9%) | 2 (1.3%) | 0.405 | 2.13% | 1.22% | 0.576 |
| Atelectasis requiring bronchoscopy | 3 (4.3%) | 2 (1.3%) | 0.155 | 7.71% | 0.78% | 0.015 |
| Atrial arrhythmia requiring treatment | 10 (14.5%) | 25 (16.2%) | 0.741 | 16.67% | 15.34% | 0.828 |
| Anastomotic stricture | 4 (5.8%) | 7 (4.5%) | 0.690 | 5.06% | 5.27% | 0.948 |
| Respiratory failure | 11 (15.9%) | 10 (6.5%) | 0.026 | 18.12% | 5.29% | 0.006 |
| Pneumonia | 7 (10.1%) | 8 (5.2%) | 0.173 | 8.29% | 5.43% | 0.423 |
| Empyema requiring treatment | 1 (1.4%) | 0 (0.0%) | 0.93% | 0.00% | ||
| Ileus | 2 (2.9%) | 1 (0.6%) | 0.178 | 5.00% | 0.89% | 0.119 |
| Initial vent support 48 hour | 4 (5.8%) | 0 (0.0%) | 5.49% | 0.00% | ||
| Pleural effusion | 6 (8.7%) | 3 (1.9%) | 0.018 | 7.84% | 2.46% | 0.087 |
| Postoperative blood transfusion | 16 (23.2%) | 10 (6.5%) | < 0.001 | 24.17% | 6.28% | 0.001 |
| Pulmonary embolus | 1 (1.4%) | 1 (0.6%) | 0.558 | 1.26% | 0.58% | 0.570 |
| Renal failure | 1 (1.4%) | 1 (0.6%) | 0.558 | 1.10% | 0.00% | |
| Chylothorax | 1 (1.4%) | 0 (0.0%) | 1.26% | 0.00% | ||
| Deep venous thrombosis | 2 (2.9%) | 4 (2.6%) | 0.898 | 3.64% | 2.75% | 0.749 |
| Delirium | 7 (10.1%) | 14 (9.1%) | 0.803 | 9.36% | 8.61% | 0.859 |
| Sepsis | 3 (4.3%) | 3 (1.9%) | 0.306 | 3.45% | 2.13% | 0.555 |
| Tracheostomy | 2 (2.9%) | 1 (0.6%) | 0.178 | 3.07% | 0.74% | 0.214 |
| Urinary tract infection | 0 (0.0%) | 1 (0.6%) | 0.00% | 0.82% | ||
| Ventricular arrhythmia | 2 (2.9%) | 7 (4.5%) | 0.564 | 4.81% | 4.39% | 0.915 |
| Unexpected ICU admission | 20 (29.0%) | 13 (8.4%) | < 0.001 | 31.46% | 6.86% | < 0.001 |
|
Hospital length of stay (Days): Median [IQR] |
9 [7–13] | 8 [7–9] | 0.003 | 9 [7–13] | 7.5 [7, 8] | 0.010 |
| Readmission within 30 days | 6 (8.7%) | 16 (10.4%) | 0.695 | 5.88% | 9.75% | 0.293 |
| Death 30 days after surgery | 3 (4.4%) | 1 (0.7%) | 0.089 | 3.24% | 0.55% | 0.084 |
| Death 90 days after surgery | 3 (4.4%) | 5 (3.3%) | 0.705 | 3.24% | 3.61% | 0.883 |
IPTW Inverse probability of treatment weighting, O-ILE Open Ivor Lewis esophagectomy, M-ILE Minimally invasive Ivor Lewis esophagectomy, IQR Interquartile range
Statistical analysis
The unadjusted baseline patient characteristics were compared between the M-ILE and O-ILE surgical approaches using chi-squared tests or Fisher exact tests where relevant for categorical variables and Student’s t-tests or Wilcoxon rank sum tests where relevant for continuous variables. The patient and disease characteristics were then balanced between the groups via propensity score methodology utilizing an inverse probability of treatment weighting (IPTW) technique to account for treatment selection bias [17]. The following differential variables were used to estimate the IPTW using binary logistic regression analysis: age, body mass index, gender, ECOG score, smoking status, coronary artery disease, COPD, and diabetes. IPTW-adjusted linear regression models were used to test for differences in the continuous variables. Overall survival and recurrence-free survival were estimated using IPTW-adjusted Kaplan–Meier analyses and multivariable Cox proportional hazards models including the same demographic characteristics used in the IPTW model listed above and compared using log-rank tests and Wald chi-square tests respectively.
IPTW-adjusted multivariable Cox proportional hazards models were fit for each of the overall and recurrence-free survival outcomes including patient baseline demographics and disease characteristic variables to assess potential confounding or effect modification of the relationship between surgery group and each of the OS and RFS outcomes. The proportional hazards assumption was assessed in each model by plotting the log of the negative log of the estimated survival density function vs log (time). Time dependent covariates were also tested and were not significant in the respective models. Hypothesis testing was conducted at an overall 5% type I error rate. The statistical analysis was designed and executed by an experienced biostatistician (MA) using the SAS, version 9.4 (SAS Institute, Cary NC).
Results
A total of 318 patients who underwent esophagectomy for esophageal carcinoma were reviewed initially, but 38 patients were excluded from the study because they underwent different esophagectomy approaches other than the Ivor Lewis technique and 57 patients were excluded who did not receive neoadjuvant chemoradiation (Fig. 1). After IPTW adjustment, there were 142 patients in the M-ILE trimodality group and 68 patients in the O-ILE trimodality group who analyzed for the study. After the IPTW-adjusted analysis, the patient demographics (Table1) and the disease characteristics (Table 2) were better balanced between the M-ILE and O-ILE trimodality groups, with a similar distribution of age, gender, medical comorbidities, smoking status, BMI, radiation doses, histology, and clinical TNM stages. The R0 resection rates were 100% for both groups. The median number of lymph nodes removed during the M-ILE and O-ILE procedures after IPTW adjustment was 17 nodes [IQR: 13–21] and 16 nodes [IQR: 12–20], respectively (p = 0.822). The median follow-up period for the O-ILE trimodlaity group was 87.9 months ([IQR]: 63.3–107.5) and 46.6 months ([IQR]: 27.6–60.6) for the M-ILE trimodality group. The tumor differentiation, lymphovascular invasion, and signet ring cell features were present at similar rates in the pathologic resection specimen (Table 2). The tumor regression grades (complete, near complete, partial, or no response to neoadjuvant chemoradiation) were also similar between the study groups (Table 2).
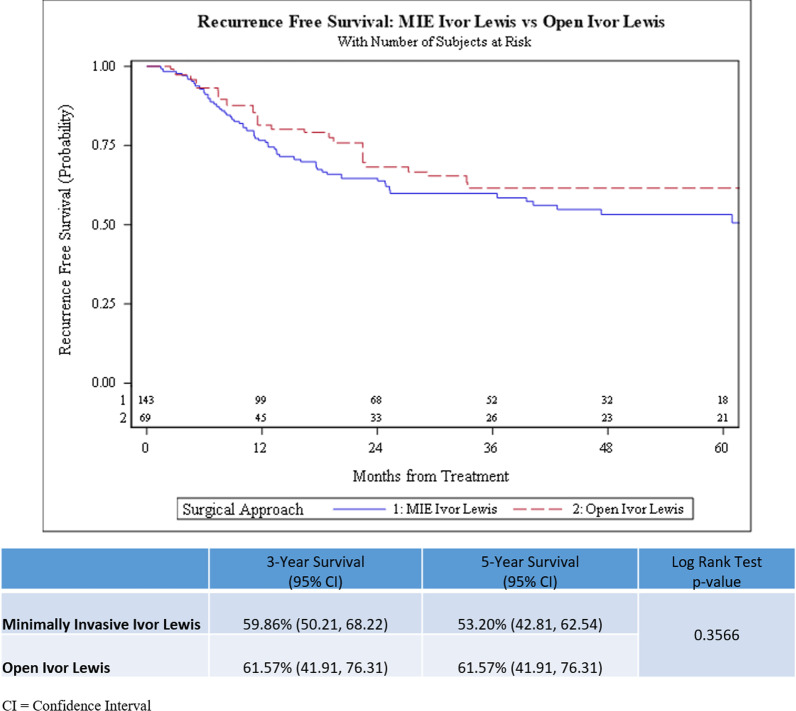
There was no statistically significant difference observed in the overall survival (log-rank test p = 0.699) and recurrence-free survival (log-rank test p = 0.357) in patients who underwent M-ILE compared to O-ILE after neoadjuvant chemoradiation (Figs. 2 and 3). The 3-year OS rate was 59.41% (95% CI, 49.82–67.76%) in the M-ILE trimodality group and 55.73% (95% CI, 39.16–69.44%) in the O-ILE trimodality group. The 5-year OS rate was 49.73% (95% CI, 38.28–60.16%) in the M-ILE trimodality group and 47.25% (95% CI, 30.58–62.22%) in the O-ILE trimodality group. The 3-year RFS rate was 59.86% (95% CI, 50.21–68.22%) for the M-ILE trimodality group and 61.57% (95% CI, 41.91–76.31%) for the O-ILE trimodality group. The 5-year RFS rate was 53.20% (95% CI, 49.81–62.54%) in the M-ILE trimodality group and 61.57% (95% CI, 41.91–76.31%) in the O-ILE trimodality group. In an IPTW adjusted multivariable Cox regression model, the variables age, body mass index, clinical N-stage, and tumor regression grade were independent predictors of overall survival (Table 3). In the RFS multivariable model, clinical N-stage, signet ring cell features, and tumor regression grade were independent predictors of recurrence-free survival (Table 4).
Fig. 2.
Kaplan Meier Curves for comparison of Overall Survival between minimally invasive (M-ILE) and open (O-ILE) Ivor Lewis esophagectomy after neoadjuvant chemoradiation
Fig. 3.
Kaplan Meier Curves for comparison of Recurrence-Free Survival between minimally invasive (M-ILE) and open (O-ILE) Ivor Lewis esophagectomy after neoadjuvant chemoradiation
Table 3.
IPTW multivariable overall survival model
| Parameter | HR (95% CI) | p-value |
|---|---|---|
| Study Group: MIE Ivor Lewis | 0.95 (0.59, 1.53) | 0.822 |
| Study Group: Open Ivor Lewis | Reference | |
| Age (Years) | 1.06 (1.03, 1.08) | < 0.0001 |
| Body Mass Index (kg/m2) | 0.95 (0.91, 0.98) | 0.006 |
| Clinical N stage: N0 | 0.41 (0.23, 0.73) | 0.003 |
| Clinical N stage: N1 | 0.83 (0.51, 1.37) | 0.467 |
| Clinical N stage: N2/N3 | Reference | |
| Tumor regression grade: complete response | 0.14 (0.07, 0.28) | < 0.0001 |
| Tumor regression grade: near complete response | 0.17 (0.09, 0.33) | < 0.0001 |
| Tumor regression grade: partial response | 0.39 (0.21, 0.74) | 0.004 |
| Tumor regression grade: no response | Reference |
IPTW Inverse probability of treatment weighting
Table 4.
IPTW multivariable recurrence-free survival model
| Parameter | HR (95% CI) | p-value |
|---|---|---|
| Study Group: MIE Ivor Lewis | 1.64 (0.93, 2.87) | 0.085 |
| Study Group: Open Ivor Lewis | Reference | |
| Clinical N-Stage: N0 | 0.22 (0.11, 0.43) | < 0.0001 |
| Clinical N-Stage: N1 | 0.53 (0.30, 0.92) | 0.025 |
| Clinical N-Stage: N2/N3 | Reference | |
| Signet ring cell feature: no | 0.50 (0.25, 0.99) | 0.047 |
| Signet ring cell feature: yes | Reference | |
| Anastomotic leak: no | 0.54 (0.27, 1.10) | 0.090 |
| Anastomotic leak: yes | Reference | |
| Tumor regression grade: complete response | 0.14 (0.06, 0.29) | < 0.0001 |
| Tumor regression grade: near complete response | 0.23 (0.12, 0.45) | < 0.0001 |
| Tumor regression grade: partial response | 0.30 (0.14, 0.62) | 0.001 |
| Tumor regression grade: no response | Reference |
IPTW Inverse probability of treatment weighting
The short-term postoperative surgical outcomes for esophagectomy by approach are listed in Table 5. After IPTW adjustment, the 30-day mortality rate was 0.55% for the M-ILE trimodality group and 3.24% for the O-ILE trimodality group (p = 0.084), which was not statistically significant. The 90-day mortality rates were similar between the two groups. The IPTW-adjusted median hospital length of stay was significantly shorter in the M-ILE trimodality group (7.5 days [IQR: 7–8]) versus (9 days [IQR: 7–13] in the O-ILE trimodality group (p = 0.01). The overall rate of postoperative adverse events was higher after IPTW adjustment in the O-ILE trimodality group, but the difference was not statistically significant (53.39% vs. 39.19%, p = 0.089). The anastomotic leak rate was lower in the M-ILE trimodality group compared to the O-ILE trimodality group [6.41% vs 12.63% (p = 0.228)], but the difference was not statistically significant. Respiratory failure and atelectasis requiring bronchoscopy occurred at significantly higher rates in the O-ILE trimodality group in the IPTW-adjusted analyses (Table 5). The IPTW-adjusted rate of unexpected ICU admission was significantly higher in the O-ILE trimodality group (31.46%) versus (9.75%) for the M-ILE trimodality group (p < 0.001). The 30-day readmission rates for the M-ILE and O-ILE trimodality groups were not significantly different (p = 0.293).
Discussion
Trimodality therapy (neoadjuvant chemoradiation followed by esophagectomy) is the current standard treatment for locally advanced esophageal carcinoma [2]. Ivor Lewis esophagectomy has become the procedure of choice for the resection of esophageal carcinoma involving the distal third of the esophagus and the gastroesophageal junction at many medical centers [18]. In an attempt to minimize the perioperative morbidity associated with O-ILE, many high volume centers have developed minimally invasive Ivor Lewis esophagectomy techniques. While the short-term postoperative outcomes for M-ILE have been extensively compared to O-ILE in multiple cohort studies and a small randomized clinical trial, the long-term oncologic outcomes for M-ILE after nedoadjuvant chemoradiation have been examined in only a single known study. Tapias and colleagues compared the long-term oncologic and short-term operative outcomes of M-ILE (N = 56) and O-ILE (N = 74) after neoadjuvant therapy in a single institution cohort study [19]. In the study, the overall survival rates at 5 years were similar between the two groups (open: 61% versus MIE: 50%, p = 0.933). The complete resection rates and the number of lymph nodes were similar for the M-ILE and O-ILE groups as well.
Our report describes a series of M-ILE cases compared to a cohort of O-ILE cases performed after neoadjuvant chemoradiation at a single institution. This is the largest propensity score adjusted series comparing the long-term oncologic outcomes of M-ILE and O-ILE performed after the completion of neoadjuvant chemoradiation. The oncologic results of the M-ILE approach were comparable to the O-ILE technique after neoadjuvant chemoradiation in this report. The median number of lymph nodes that were dissected in the M-ILE group (17 nodes) were similar to the O-ILE group (16 nodes). Both surgical approaches yielded a median number of lymph nodes that were comparable to other reports [20, 21]. All of the patients in the O-ILE and M-ILE group underwent a complete R0 resection with negative surgical margins. The 3-year and 5-year overall survival and recurrence-free survival rates were not significantly different for the M-ILE trimodality group compared to the O-ILE trimodality group. This finding was consistent with the results of a small randomized phase III clinical trial comparing open transthoracic esophagectomy (McKeown and Ivor Lewis) to minimally invasive transthoracic esophagectomy after neoadjuvant therapy. The long-term follow up of the TIME (Traditional Invasive versus Minimally Invasive Esophagectomy) trial demonstrated no observed differences for 3-year overall survival (MIE: 50.5% versus Open: 40.4%, p = 0.207) and disease-free survival (MIE: 40.2% versus Open: 35.9%, p = 0.602) in patients who underwent minimally invasive transthoracic esophagectomy compared to open transthoracic esophagectomy [22].
In an IPTW adjusted multivariable Cox regression model, the variables age, body mass index, clinical N-stage, and tumor regression grade were independent predictors of overall survival. In the recurrence-free survival multivariable model, clinical N-stage, signet ring cell features, and tumor regression grade were independent predictors of recurrence-free survival. Tumor regression grade or the degree of pathologic response to neoadjuvant chemoradiation appeared to be a strong independent predictor for both overall and recurrence-free survival; whereas surgical approach (open versus MIE) did not demonstrate any prognostic significance. Takeda and colleagues performed a multivariable analysis on the varying degrees of pathologic response to neoadjuvant chemoradiation and demonstrated that tumor regression grade can reliably predict overall survival and systemic recurrence in patients with locally advanced esophageal cancer who were treated with trimodality therapy [23]. Similarly, Stiles and colleagues reviewed 238 patients who received neoadjuvant therapy followed by esophagectomy for locally advanced esophageal cancer. In a multivariable model, a poor clinical response to neoadjuvant therapy (HR 2.77; 95% CI: 1.14–7.15) was highly predictive of early mortality within a year of trimodality therapy [24]. These findings indicate that the tumor regression grade or response to neoadjuvant therapy has more prognostic significance than the surgical approach for esophagectomy.
Propensity score weighting and multivariable regression analysis allowed us to statistically balance baseline differences and control for confounders in the short-term perioperative outcomes and the long-term oncologic outcomes. Nonetheless, the present study has several limitations which should be considered when interpreting the results of study. The selection process for the surgical approach was determined by the surgeon based on individual preference and could include factors that were not captured in the study. Given the retrospective observational study design, we could not completely eliminate unmeasured selection bias; therefore, the results of the study are not generalizable. The follow up time for the M-ILE group was shorter, which decreased the accuracy of the survival data after the 3-year time point. However, the surveillance data for the first three years was robust and likely captured most of the recurrence cases and deaths.
Conclusions
In conclusion, OS and RFS rates were not significantly different between the M-ILE and O-ILE trimodality groups. Our analysis demonstrated that OS and RFS for locally advanced esophageal caracinoma are more likely determined by the degree of pathologic response to neoadjuvant chemoradiation as opposed to surgical approach for esophagectomy. The short-term surgical outcomes, such as respiratory failure and hospital length of stay, for patients undergoing M-ILE were significantly improved compared to patients undergoing O-ILE after IPTW adjustment for baseline demographic covariates. These results support the use of minimally invasive Ivor Lewis esophagectomy after neoadjuvant chemoradiation for surgical treatment of local advanced esophageal carcinoma at our institution.
Acknowledgements
None.
Abbreviations
- AJCC
American Joint Committee on Cancer
- BMI
Body Mass Index
- CI
Confidence Interval
- CRT
Chemoradiation
- CROSS
Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study
- COPD
Chronic obstructive pulmonary disease
- CT
Computed tomography
- ECOG
Eastern Cooperative Oncology Group
- IPTW
Inverse probability of treatment weighting
- IQR
Interquartile range
- M-ILE
Minimally invasive Ivor Lewis Esopahgectomy
- O-ILE
Open Ivor Lewis Esophagectomy
- OS
Overall survival
- PET
Positron emission tomography
- RFS
Recurrence-free survival
- SAS
Software analytics and solutions
- TNM
Tumor-node-metastasis
- UICC
Union of International Cancer Control
Authors' contributions
REM, MAR, KAP, and PJK designed the study. REM collected the data and performed data analysis. MAR performed the statistical analysis. REM and MAR wrote the manuscript. REM, MAR, DMD, PJK, and KAP edited the manuscript and approved the final version. All authors read and approved the final manuscript.
Funding
There was no funding source for this study.
Availability of data and materials
The dataset analyzed during the current study are not publicly available due to institutional restrictions on sharing patient data but can be made available from the corresponding author on reasonable request. Most of the data is included in the tables and figures.
Declarations
Ethics approval and consent to participate
This study protocol was approved Ohio State Wexner Medical Center Internal Review Board. The patient consent waivers were obtained. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests regarding the preparation of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Van Hagen P, Hulshof MC, Van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J, Van Lanshot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-tern results of a randomized controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 4.Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8(6):545–553. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- 5.Avendano CE, Flume PA, Silvestri GA, et al. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002;73:922–926. doi: 10.1016/S0003-4975(01)03584-6. [DOI] [PubMed] [Google Scholar]
- 6.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 7.Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;1:95–102. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biere SS, Van Berge Henegouwen MI, Bonavina K, et al. Minimally invasive versus open oesophagectomy for patients with oesopahgeal cancer: a multi-centre, open-label, randomized control trial. Lancet. 2012;379(9829):1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 9.Klevebro F, Scandavini CM, Kamiya S, et al. Single center consecutive series cohort study of minimally invasive versus open resection for cancer in the esophagus of gastroesophageal junction. Dis Esophagus. 2018;31:1–6. doi: 10.1093/dote/doy027. [DOI] [PubMed] [Google Scholar]
- 10.Kauppi J, Rasanen J, Sihvo E, et al. Open versus minimally invasive esophagectomy: clinical outcomes for locally advanced esophageal adenocarcinoma. Surg Endosc. 2015;29(9):2614–2619. doi: 10.1007/s00464-014-3978-8. [DOI] [PubMed] [Google Scholar]
- 11.Sihag S, Kosinski AS, Gasisert HA, et al. Minimally invasive versus open esophagectomy for esophageal cancer: a comparison of early surgical outcomes from the society of thoracic surgeons’ national database. Ann Thorac Surg. 2016;101(4):1281–1288. doi: 10.1016/j.athoracsur.2015.09.095. [DOI] [PubMed] [Google Scholar]
- 12.Shivo E, Helminen O, Gunn J, et al. Long-term outcomes following minimally invasive and open esophagectomy in Finland. Eur J Surg Onc. 2019;45:1099–1104. doi: 10.1016/j.ejso.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Mitzman B, Lufti W, Wang CH, et al. Minimally Invasive esophagectomy provides equivalent survival to open esophagectomy: an analysis of the national cancer database. Sem Thorac Cardiovasc Surg. 2017;29:244–253. doi: 10.1053/j.semtcvs.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Dantoc MM, Cox MR, Eslick GD. Does minimally invasive esophagectomy (MIE) provide for comparable oncologic outcomes to open techniques? A systematic review. J Gastrointest Surg. 2012;16(3):486–494. doi: 10.1007/s11605-011-1792-3. [DOI] [PubMed] [Google Scholar]
- 15.Rice TW, Ishwan H, Kelsen DP, et al. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:906–912. doi: 10.1111/dote.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–146. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabra MJ, Alwatari YA, Wolfe LG, et al. Ivor Lewis vs Mckeown esophagectomy: analysis of operative outcomes from the ACS NSQIP database. Gen Thorac Cardiovasc Surg. 2020;68(4):370–379. doi: 10.1007/s11748-020-01290-w. [DOI] [PubMed] [Google Scholar]
- 19.Tapias LF, Mathisen JM, Wright CD, et al. Outcomes with open and minimally invasive Ivor Lewis esophagectomy after neoadjuvant therapy. Ann Thorac Surg. 2016;101:1097–1103. doi: 10.1016/j.athoracsur.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 20.Shihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis esophagectomy at a single, high volume centre. Eur J Cardiothoracic Surg. 2012;42:430–437. doi: 10.1093/ejcts/ezs031. [DOI] [PubMed] [Google Scholar]
- 21.Schoppman SF, Prager G, Langer FB, et al. Open versus minimally invasive esophagectomy: a single-center case control study. Surg Endosc. 2010;24:3044–3053. doi: 10.1007/s00464-010-1083-1. [DOI] [PubMed] [Google Scholar]
- 22.Straatman J, Van der Wielen, Cuesta MA, et al. Minimally invasive versus open esophageal resection: Three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg 2017; 266:232–36. [DOI] [PubMed]
- 23.Takeda FR, Tustumi F, Obregon CDA, et al. Prognostic value of tumor regression grade based on ryan score in squamous cell carcinoma and adenocarcinoma of esophagus. Ann Surg Oncol. 2020;27:1241–1247. doi: 10.1245/s10434-019-07967-8. [DOI] [PubMed] [Google Scholar]
- 24.Stiles BM, Salzler GG, Nasar A, et al. Clinical predictors of early cancer-related mortality following neoadjuvant therapy and oesophagectomy. Eur J Cardiothoracic Surg. 2015;48:455–460. doi: 10.1093/ejcts/ezu479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed during the current study are not publicly available due to institutional restrictions on sharing patient data but can be made available from the corresponding author on reasonable request. Most of the data is included in the tables and figures.