Abstract
Esophageal carcinoma (EC) is one of the most pervasive cancers in the world, with upwards of 500,000 new diagnoses, annually. Despite its prominence, advancements in the detection and treatment of EC have been marginal over the past 30 years and the survival rate continues to stay below 20%. This is due to the uncommonly heterogeneous presentation of EC which presents unprecedented challenges in improving patient survival and quality of care. However, distinct epigenetic alterations to the DNA methylome may provide an avenue to drastically improve the detection and treatment of EC. Specifically, the creation of novel biomarker panels that consist of EC-specific methylation markers have shown promise as a potential alternative to the more invasive, contemporary diagnostic methods. Additionally, growing insight into the biological and clinical properties of EC-specific methylation patterns have opened a window of opportunity for enhanced treatment; of growing interest is the application of “DNMT inhibitors” - a class of drugs which inhibit excessive methylation and have been shown to re-sensitize chemoresistant tumors. Here we provide a comprehensive review of the current advancements in EC DNA methylation to underscore a potential approach to its detection and treatment.
Keywords: Esophageal carcinoma, DNA methylation, DNMT inhibitors, cancer precursor lesion
Introduction
Every year, over 500,000 people worldwide are diagnosed with esophageal carcinoma (EC), with approximately the same number of EC-related deaths. The two major subtypes of EC are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). While ESCC, commonly seen in Africa, Asia and South America, is the predominant form of EC, the number of EAC cases has increased 600-800% in developed, western countries over the past 30 years. Due to this increase, EC has become the 6th most common cause of cancer-related deaths in the United States [1,2]. Both ESCC and EAC are markedly prevalent in men over women, with men accounting for 70% of all EC cases worldwide [3]. In contrast, obesity and gastroesophageal reflux disease (GERD) has been strongly linked to an increased risk for EAC, while smoking and alcohol consumption pose an increased risk for ESCC [4].
Currently, given the high mortality rate of EC and the inefficacy of existing treatments, prevention and early detection continue to yield the best chances of survival. The defining precursors for EAC and ESCC are Barrett’s Esophagus (BE) and squamous dysplasia, respectively, with BE representing a 50 to 100-fold increased risk for carcinogenesis [5]. Moreover, these pre-malignant lesions provide an opportunity for the early detection and treatment of ECs, given their locations. Both BE and squamous dysplasia occupy the same regions as their associated malignancies: BE is found to be at the gastroesophageal junction (GEJ) and in the distal third of the esophagus, while squamous dysplasia is found in the proximal two-thirds of the esophagus [3,6]. At present, the gold standard for EC screening is endoscopy, with biopsy for iodine staining [1]; however, while screening for these pre-malignant lesions offer some forewarning of subsequent EC, BE alone is not an absolute indicator of malignancy. Moreover, such invasive and financially burdensome procedures call into question the feasibility of widespread endoscopic screening for EC. Specifically, given the large proportion of ESCC patients residing in impoverished parts of the world, labor-intensive and costly screening methods will only marginally help reduce the mortality rate of EC. For this reason, EC typically remains undiagnosed until advanced stage disease, when the overall survival is typically ≤1 year [1]. Despite significant advancements in medicine and cancer biology over the past several decades, treatment outcomes for EC have only seen marginal improvements, keeping the 5-year survival rate at 15-20% [1].
Despite our understanding of pre-existing conditions which pose increased risks for EC carcinogenesis, the absence of robust molecular markers have largely contributed to the marginal advancements in EC treatments. EC’s continuously low survival rate can also be attributed to its exceptionally heterogenous presentation. Thousands of genomic aberrations have been linked to EC [7,8]. In fact, the extent of EC’s heterogeneity is not only seen among different patients, but also, differing genomic alterations have been reported to be present in different sections of the same tumor [9-11]. This degree of heterogeneity has remained a foremost impediment for researchers who try to stratify risk levels associated with disease progression and identify reliable prognostic indicators. Therefore, the establishment of non-interventional, economically feasible screening methods in conjunction with dependable biomarkers are of paramount importance in mitigating EC’s lethality.
DNA methylation
Epigenetic markers on DNA are heritable modifications that alter gene function or activity, yet do not change the underlying DNA sequence. One prominent marker in EC is DNA methylation - the process by which Cytosine residues are methylated at the C5 position, therein becoming 5-methylcytosine. DNA methylation is essential for myriad functions, including mammalian development, and is catalyzed by several different proteins, termed epigenetic writers [12], in the DNA methyltransferase (DNMT) family of enzymes. In general, DNMT3a/b are responsible for de novo methylation, while DNMT1 maintains existing DNA methylation by copying methylation patterns onto newly synthesized strands during DNA replication [13]. While DNA methylation is paramount in modulating gene expression across a wide variety of cells, its dysregulation has been linked to carcinogenesis, and is classified into two categories: hypermethylation and hypomethylation. Hypermethylation is associated with gene repression, while hypomethylation is correlated to increased gene expression [14]. DNA methylation changes alter the recruitment of epigenetic regulators and transcription factors to their binding sites [15]. In EC, the two predominant forms of dysregulated methylation patterns are global hypomethylation, and CpG-specific hypermethylation [16,17] (Figure 1).
Figure 1.

Aberrant DNA methylation in carcinogenesis. DNA methylation, catalyzed by DNMT family enzymes, alter gene function or activity in esophageal cancer. DNA methylation, mainly classified into hypermethylation and hypomethylation, in esophageal cancer alter different gene function or activity. After the interaction of DNMT and DNA binding sites, global hypomethylation causes the enhanced activity of the genes, such as LINE-1, and CpG-specific hypermethylation is correlated to gene expression reduction, such as MGMT and CDKN2A.
Over the past several decades, multiple studies have shown that global hypomethylation plays a significant role in carcinogenesis, through several Mechanisms [18-21] (Figure 1). Namely, the hypomethylation of parasitic, repetitive DNA sequences, such as retrotransposons, or of proto-oncogenes has pronounced deleterious effects on chromosomal stability and cellular function [22]. Further, excessive hypomethylation of centromeric and pericentromeric satellite sequences is commonly seen in a variety of tumors, and its consequential effects on chromosomal stability has been suggested to lead to aneuploidy [23]. Following its established significance in carcinogenesis, meta-analyses of global DNA hypomethylation in various cancers have suggested a link between the extent of hypomethylation and cancer stage [22,24].
In contrast to its activating counterpart, extensive DNA hypermethylation of promoter-region CpG islands has been reported to largely facilitate tumorigenesis by repressing tumor suppressor genes [25]. Numerous studies have since shown the degree to which hypermethylation promotes tumorigenesis. One example of this was shown in the promoter region of the cyclin-dependent kinase (CDK) inhibitor gene, p16INK4A, whereby hypermethylation is observed to occur in the pre-cancerous stages of tumorigenesis [26-29]. Another mechanism by which DNA hypermethylation results in aberrant gene expression is through C>T point mutations, via spontaneous deamination of 5-methylcytosine which ultimately is replaced by thymine residues, if not repaired [30]. Such reactivating hypermethylation mutagenesis patterns are often seen in the promoter region of Telomerase Reverse Transcriptase (TERT) complex, which is found in approximately 90% of human cancers, including gastric, pancreatic, and cervical cancers [31-38]. Therefore, given the extent to which aberrant methylation patterns - both of hypo and hypermethylation - influence the onset of carcinogenesis, developing better methods for monitoring alterations to the methylome will play a pivotal role in preventing cancer progression.
Screening for esophageal carcinoma
Current techniques used for the screening of EC are similar and not sufficient for the diagnosis of precancerous lesions. One example being traditional white-light endoscopy (WLE), which is routinely used for the detection of invasive esophageal carcinomas, and even low-grade BE dysplasia. However, WLE is not capable of detecting esophageal squamous dysplasia [39,40]. A common and inexpensive alternative to WLE is chromoendoscopy, which is sensitive enough to detect precursor lesions, but has insufficient and inconsistent specificity (37%-82%) for squamous dysplasia [41-44]. Other conventional endoscopic methods include transnasal endoscopy, microendoscopy, and endocytoscopy - all of which consist of flexible probes surveying the esophagus (Table 1).
Table 1.
Comparison of the commonly used screen methods for early esophageal carcinomas
| METHOD | PROCEDURE | BENEFITS | DRAWBACKS | REF. |
|---|---|---|---|---|
| WHITE-LIGHT ENDOSCOPY | Endoscope with high-resolution imaging - may be done with endoluminal biopsy | Is sensitive enough to detect BE-LGD | Can’t detect ESCC precursor lesions; expensive; invasive | [116] |
| LUGOL CHROMOENDOSCOPY | Application of colored dyes to esophagus for targeted biopsy | Inexpensive; high sensitivity | Inconsistent specificity for squamous dysplasia | [117] |
| TRANSNASAL ENDOSCOPY | Nasal esophageal intubation with optic scope | Economical; less invasive; no need for general anesthesia | Conscious sedation poses increased risk for complication | [118] |
| MICROENDOSCOPY | Confocal laser endomicroscopy of targeted biopsy tissue | Higher sensitivity and specificity, relative to conventional microscopy methods | Not economically feasible - can cost thousands of dollars | [119] |
| ENDOCYSTOCOPY | Ultra-magnification of stained, or in-vivo, epithelial tissue | High sensitivity/specificity | Not readily available; expensive; lack of standardized criteria for diagnosis leads to ambiguous results | [120] |
| CYTOSPONGE | Ingested sponge capsule is retrieved from patient after 5 minutes wherein the apparatus scrapes cells from the epithelium | Very high sensitivity/specificity; non-invasive; inexpensive; convenient; can collect 500,000 cells for epigenetic analysis therein increasing reproducibility and robustness | Still requires traditional endoscopic methods, following a positive result; sensitivity is still not 100% | [90] |
| ESOPHACAP | Ingested sponge is retrieved after several minutes whereupon removal, cells are extracted | Highest sensitivity/specificity; 1,000,000 cells are collected; inexpensive; convenient; non-invasive | Still requires traditional endoscopic methods, following a positive result | [91] |
To detect aberrant methylation in patients with EC, typically one of the aforementioned endoscopic methods would be used for tissue biopsy, though newer, non-invasive methods such as the Cytosponge or Esophacap are being used to collect cells from the esophageal mucosa. Subsequently, a variety of assays, generally beginning with bisulfite conversion, could be performed to detect methylation [45]. Bisulfite conversion works by deaminating unmethylated cytosine residues to uracil while leaving methylate cytosine residues alone, therein highlighting the presence and location of DNA methylation [46,47]. Following bisulfite conversion, a number of different assays can be performed, including sanger or pyrosequencing [48]. Other popular methods of DNA methylation detection include array-based platforms and Methyl-cytosine based immunoprecipitation (IP) followed by sequencing [49,50] (Figure 2).
Figure 2.
Methylation-based screening for esophageal carcinoma. Tissue biopsy can be obtained by endoscopic methods. After sample collection, it will be directly used for DNA extraction and bisulfite conversion, which could highlight the presence and location of DNA methylation. Several assays can be performed for methylation detection, such as sanger-sequencing or pyrosequencing.
Potential applications of DNA methylation markers in EC
The importance of DNA methylation in cancer was first highlighted in 1983 when significant hypomethylation was observed in cancer cells, in contrast to the surrounding healthy cells [51]. Since then, deleterious epigenetic modifications in ECs have been investigated for a variety of reasons. At present, limitations in our understanding of EC biology has been a significant roadblock in developing effective treatment regimens, although existing therapeutics have yielded some degree of success. As a result, epigenetic signatures in EC have been the focus of biomarker research, offering the opportunity to serve not only in a diagnostic capacity, but also for the monitoring and prognostication of disease progression (progression markers), survival prognosis (prognostic markers), and likelihood of response to therapy (predictive markers). From scrutinizing methylomic changes in EC, several genes that present aberrant expression levels have been identified as preliminary inducers of carcinogenesis from BE to EAC, and can be seen before malignant histologic changes are observed [52]. Moreover, as the degree of DNA methylation becomes more prominent throughout the course of disease progression, clinicians are then able to more effectively implement an appropriate treatment plan that is specifically tailored to each patient.
While the primary focus of methylation markers in EC has been for surveillance purposes, the stratification of EC’s based upon distinct molecular signatures has shown promise in working towards delivering targeted therapeutics. Specifically, this classification of ECs into categorical subtypes has allowed clinicians to treat ESCC and EAC by addressing the root cause of abnormal gene activity [53]. For example, hypermethylated carcinomas appear to be sensitive to treatment with DNA methyltransferase and topoisomerase I inhibitors, while global hypomethylation may be sensitive to treatment with CDK2 inhibitors [54]. Although promising, further rigorous clinical validation is necessary to confirm the reliability of universal epigenetic alterations that are indicative of EC and its subsequent progression.
Ongoing methylation marker discovery and validation for esophageal carcinoma and precursor lesions
From data compiled by The Cancer Genome Atlas (TCGA) team, over 8,300 genes were found to be mutated across 165 cases of EAC [7]. Moreover, Bandla et al. reported the significance of mutational load in differentiating EAC from its precursor, BE [55]. Specifically, the buildup of epigenetic and genetic modifications in several genes - including TP53, CDKN2A, CTNNB1, and APC - that are commonly seen in EAC have also been reported to be present in BE, albeit less frequently [56-61]. The progressive accumulation of these mutations has therefore allowed for the stratification of disease into stages; this multistep progression starts with BE, then transitioning to low-grade dysplasia (LGD), subsequent high-grade dysplasia (HGD), and ultimately EAC [62,63] (Figure 3). However, given the level of heterogeneity and complexity in EC, a meticulous validation process is essential for confirming the robustness of molecular signatures which are indicative of the presence of ECs.
Figure 3.
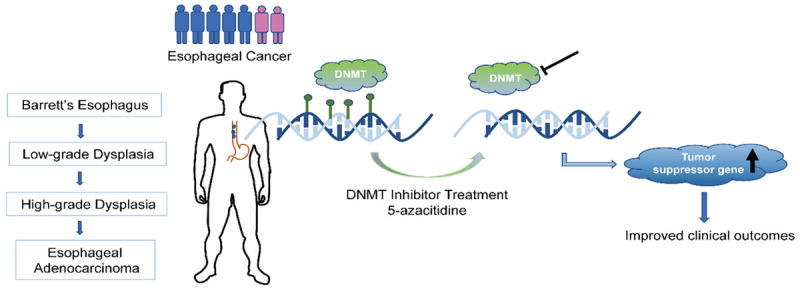
Application of DNMT inhibitor therapeutics in halting progression of EAC. During the progression of esophageal carcinoma, DNA methylation, catalyzed by DNMT, was modified on several genes, causing aberrant expression and activity. DNMT inhibitor treatment could reduce the degree/extent of DNA methylation and increase the expression of tumor suppressor genes, and thus improve clinical outcomes.
In an effort to address this, Alvi et al. used an array-based approach and formed a four-marker panel (PIGR, RIN2, GJA12, and SLC22A18) that was able to distinguish between the presence of HGD/EAC and BE, and enhanced classification of disease-related risk, based upon the degree of methylation in this panel [64]. In a retrospective study, using a cohort consisting of 60 BE patients, 36 patients with dysplastic BE, and 90 with HGD/early EAC, this panel was validated by pyrosequencing and was shown to have a specificity of 97% and a sensitivity of 94%. In a broader approach, the Esophageal Cancer Clinical and Molecular Stratification (OCCAMS) consortium analyzed the results from whole-genome sequencing of 129 EAC cases and were able to categorize the mutational profiles into 3 distinct EAC groups based upon differing genomic mutations. These findings were substantiated in a subsequent study which analyzed the mutational profiles of another cohort, consisting of 87 patients [65]. This study served as the basis for Jammula et al.’s epigenetic analyses on over 300 BE and EAC cases; the results of which were consolidated with corresponding genomic and transcriptomic profiles to present a comprehensive analysis to confidently discern BE from EAC [54]. The data gathered allowed for the classification of EAC and BE tissues to be stratified into 4 subtypes; among the 4 subtypes, 3 involve aberrant methylation patterns that have been suggested to be clinically relevant for treatment regimen planning. Subtype 1 tissue displayed hypermethylation in the CpG islands of noncoding regions for genes necessary for DNA repair. Subtype 2 tissue likewise exhibited hypermethylation; however, also displayed hypomethylation in genes required for cell metabolism and ATP synthesis. The last subtype involving anomalous methylation signatures, subtype 4, presented extensive hypomethylation which resulted in significant structural variation.
Although having different etiologies, many of the aberrant methylation signatures found in EAC are also commonly seen in ESCC, further compounding the level of difficulty in biomarker validation. Moreover, ESCC presents the same multistep progression as is seen in EAC (non-dysplastic, LGD, HGD, carcinoma), wherein mutational load and accumulation of epigenetic alterations can assist with risk stratification for survivability and probability of carcinogenesis [66-71]. An example of this is the promoter hypermethylation of several tumor suppressor genes and DNA repair genes (MGMT, p14, p16) that have been found to be present in EAC as well as in dysplastic, ESCC precursor lesions [72-74]. Specifically, CDKN2A (official symbol for p16) hypermethylation is found to be present in up to 88% of ESCC tumors, and is indicative of an invasive phenotype [75-83]. Other well-known oncogenes such as KRAS, IGF1R, CDK6, and EGFR are also overexpressed in both EAC and ESCC, at similar frequencies. Additionally, the proximity in which these two malignancies can present, within the esophageal tract, may hinder the ability to accurately discern between histopathological differences.
To better discriminate between carcinomas in adjacent tissue, Pu et al. discovered and validated a 5-marker panel that showed significant CpG island hypermethylation in ESCC tissue, relative to EAC tissue. Three of these markers are in the promoter region of genes (STK3, ZNF418, and ZNF542) [84,85]. Additionally, Agrawal et al. highlighted distinct spectra in C:G>T:A mutation profiles in EACs (46%) vs. ESCCs (35%), with A:T>C:G substitutions more commonly found in EAC as opposed to a higher incidence of C:G>G:C substitutions and indels found in ESCC [86]. A more recent molecular distinction was shown in the overexpression of several genes, namely SOX2, CCND1, and/or TP63 are more frequently seen in ESCC, while GATA6, GATA4, and ERBB2 are typically representative of EAC [87]. In a different study, Lu et al. analyzed RNA-seq and methylation profiles in ESCC and found five candidate genes (ZNF608, SLC5A10, ZNF69, SPIN3, and ABCD1) with aberrant methylomic signatures that may potentially serve as prognostic makers for ESCC (See Table 2) [88]. Furthermore, as more molecular markers for EC are being discovered and validated, aberrant DNA methylation is a promising method by which both EAC and ESCC can be not only accurately diagnosed, but further categorized based upon a host of factors affecting clinical outcomes.
Table 2.
Promising Epigenomic Biomarkers for EC
| Marker | Function | Methylation Status | Malignancy | Reference |
|---|---|---|---|---|
| HER2 (ERBB2) | Cell differentiation; proliferation; suppresses apoptosis | Hypo | EAC | [110] |
| PD-L1 | Immune checkpoint, signals for apoptosis | Hypo | ESCC | [111] |
| CDKN2A | Tumor suppressor gene | Hyper | EAC/ESCC | [73] |
| ABCD1 | ABC transporter protein for fatty acids | Hypo | ESCC | [88] |
| SPIN3 | Tumor suppressor gene | Hyper | ESCC | [88] |
| TP63 | Antagonist of pro-apoptotic genes | Hypo | ESCC | [87] |
| GATA4/6 | Transcription factor | Hypo | EAC | [87] |
| SOX2 | Transcription factor; cell renewal | Hypo | ESCC | [87] |
| LINE-1 | Transposable element | Hypo | ESCC | [99] |
| KRAS | Part of RAS/MAPK pathway; cell proliferation | Hypo | EAC/ESCC | [84] |
Benefits and limitations of methylation markers in the detection and surveillance of EC
Although more progress still needs to be made, integrating methylation markers into routine EC screening has numerous potential benefits, including the potential for increased sensitivity and specificity over current methods [89]. Additionally, methylation marker panels that are currently being studied allow for the utilization of non-invasive methods of sample collection. In 2018, Chettouh et al. used a four-marker panel comprised of TFPI2, TWIST1, ZNF569, and ZNF345 to screen for BE, and were able to use an inexpensive, non-endoscopic Cytosponge collection apparatus [90]. Moreover, Wang et al. used a non-invasive Esophacap to collect esophageal mucosal cells and were able to discern healthy controls from BE samples using a five-marker panel (AKAP12, NELL1, HPP1, p16, and TAC1) with a specificity of 92.8% and a sensitivity of 78.6% [91]. Given the potential of reduced costs, reduced need for invasive endoscopic procedures, and increased sensitivity and specificity, the implementation of robust methylation-marker panels for the detection and surveillance of ECs shows promise as an alternative to conventional methods.
On the other hand, in spite of the progress made in identifying and validating methylation markers in EC, current methodologies also have some limitations. Of note, there is interobserver variability in diagnosing BE-LGD and its subsequent progression to carcinoma, which makes it difficult to standardize the results of biomarker studies and to identify a potential universal methylation marker panel. To help address this issue, investigation of the epigenomic alterations in esophageal cells during BE and EC carcinogenesis has shown increasing promise as a method through which objective analysis can be conducted. In doing so, many have used in-vitro cell, and 3D, culture models and in vivo animal models; however, these studies have received criticism for large discrepancies between these models and human EC pathobiology [92-98]. In addition to interobserver variations among clinicians and scientists, the exceptional degree of heterogeneity seen in ECs further complicates the task of finding reliable indicators of malignancy, or precursor lesions. For example, hypomethylation of the repetitive long interspersed transposable element (LINE), LINE-1, has been proposed as a biomarker for ESCC that is strongly correlated with high risk for progression and poor prognosis. However, studies on LINE-1 in EC vary greatly in their analyses of the proportion of hypomethylation required (25-92% increase) to exercise deleterious affect [99-102]. Therefore, it is paramount that improvements in methylation biomarkers be made before being put into practice.
Current directions in biomarker-related treatments for EC
Although many therapeutic interventions for EC are either still in development or have yielded modest success, new insight into EC methylomes have provided potential therapeutic targets. Of growing interest, the employment of DNMT inhibitors has shown promise in combination therapies for the treatment of chemoresistant tumors. Specifically, the methylation-induced repression of specific genes, that would otherwise sensitize tumor cells to chemotherapy, nulls the cytotoxic effects of conventional therapies, thereby rendering these treatments ineffective. Additionally, as previously discussed, many tumor suppressor genes (TSGs) are hypermethylated during EC carcinogenesis. This epigenetic repression has consequently led to recent investigation into the application of DNMT inhibitors (Figure 3).
Currently, the most well-known DNMT inhibitor, albeit still not fully understood, is 5-azacitidine which is an FDA approved nucleoside analog that is capable of inducing hypomethylation and has been shown to improve EC patient survivability [103,104]. In a cohort of 12 esophageal/gastric adenocarcinoma patients, Schneider et al. found that treatment with 5-azacitidine (V, 75 mg/m2) for 3-5 days prior to chemotherapy resulted in reactivation of hypermethylated TSGs, which may result in hypomethylation-induced chemoresensitization, ultimately improving clinical outcomes of subsequent chemotherapy and resection of residual tumors [105]. In a similar study, Fu et al. found 5-azacitidine (V, 75 mg/m2) for five days at least partially restored chemotherapeutic efficacy in chemoresistant ovarian cancers [106]. In addition to 5-azacitidine, the other FDA approved DNMT inhibitor is decitabine, the deoxy derivative of 5-azacitidine, which yields comparable results and works in similar fashion: incorporating itself onto DNMT DNA strands during replication wherein it acts as a chain terminator to inhibit activity [107-109]. Although there are currently not many therapeutics that directly act on the epigenomic changes caused by EC carcinogenesis, advancements in EC biology and epigenomics have allowed clinicians to better formulate personalized treatment regimens using current therapeutics.
Aside from the direct therapeutic targeting of epigenetic writers, significant advancements in biomarker-based immunotherapy have been instrumental in directing future research efforts. In particular, excessive hypomethylation of well-known biomarkers, such as PD-L1 and HER2 have contributed to their overexpression within the tumor microenvironment, and thus, its immunosuppressive properties [110,111]. Although further research needs to be done to improve efficacy, clinical trials on Trastuzumab, a humanized HER-2 monoclonal antibody, have improved progression-free survival in EAC patients, when combined with chemotherapy [112,113]. Moreover, myriad studies and clinical trials on the efficacy of a PD-L1 blockade therapy, such as Nivolumab and Keytruda, have yielded objective response rates of up to 20%, adding as much as 6 months to overall survival in ESCC patients [114,115]. It was also noted that overexpression of PD-L1 is correlated with microsatellite instability (MSI) and a DNA mismatch repair (MMR) deficiency. As a result, in conjunction with the PD-L1 and HER2 biomarkers, the National Comprehensive Cancer Network (NCCN) has also listed surveillance of MSI/MMR status to its pathologic biomarker testing guidelines. As previously mentioned, extensive DNA hypomethylation of centromeric and pericentromeric satellite sequences have significant deleterious effects on chromosomal stability and sequence integrity. Therefore, the combination of these suggested biomarkers, per NCCN guidelines, can serve in both diagnostic and therapeutic capacities, and are promising advancements in biomarker research for EC.
Conclusion
EC is one of the deadliest forms of cancer, with 5-year survival rate of less than 20%. This is largely due to asymptomatic patients who remain undiagnosed until late-stage carcinoma, when the survival rate is exceedingly low. This review addresses the significance of aberrant DNA methylation in ECs and the potential impact of advancing our understanding of methylation markers throughout the various stages of EC carcinogenesis, from non-dysplastic precursor lesions to invasive carcinoma. Moreover, studies over the past decade have revealed the applications of methylation markers in EC to be beneficial in diagnostic, prognostic, and therapeutic capacities. Of growing interest is the combination of distinct epigenomic markers to form “panels”, therein enhancing the accuracy of diagnostic and prognostic screening. Further, such panels have been helpful, thus far, in differentiating between different malignancies that would otherwise be difficult to discern due to proximity or histological presentation (See Table 2). Therefore, advancements in this field may not only improve overall survival, due to early detection, but may ultimately reveal novel therapeutic targets for improved treatment. However, the fact remains that despite improvements in methylome-based approaches for the detection, surveillance, and therapeutics for EC, challenges such as overcoming extensive heterogeneity and interobserver variability remain and underscore the importance of continuing to better understand DNA methylation in EC and to work towards improving this platform.
Disclosure of conflict of interest
None.
References
- 1.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141–1146. doi: 10.1111/jgh.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 3.Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010–1021. doi: 10.1007/s12328-020-01237-x. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Colquhoun A, Cook MB, Ferlay J, Forman D, Soerjomataram I. Obesity and the incidence of upper gastrointestinal cancers: an ecological approach to examine differences across age and sex. Cancer Epidemiol Biomarkers Prev. 2016;25:90–97. doi: 10.1158/1055-9965.EPI-15-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilson DH, van Hillegersberg R. Management of patients with adenocarcinoma or squamous cancer of the esophagus. Gastroenterology. 2018;154:437–451. doi: 10.1053/j.gastro.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 7.Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, Bandla S, Imamura Y, Schumacher SE, Shefler E, McKenna A, Carter SL, Cibulskis K, Sivachenko A, Saksena G, Voet D, Ramos AH, Auclair D, Thompson K, Sougnez C, Onofrio RC, Guiducci C, Beroukhim R, Zhou Z, Lin L, Lin J, Reddy R, Chang A, Landrenau R, Pennathur A, Ogino S, Luketich JD, Golub TR, Gabriel SB, Lander ES, Beer DG, Godfrey TE, Getz G, Bass AJ. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478–486. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaz AM, Grady WM, Stachler MD, Bass AJ. Genetic and epigenetic alterations in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am. 2015;44:473–489. doi: 10.1016/j.gtc.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junker JP, van Oudenaarden A. Every cell is special: genome-wide studies add a new dimension to single-cell biology. Cell. 2014;157:8–11. doi: 10.1016/j.cell.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Francies HE, Secrier M, Perner J, Miremadi A, Galeano-Dalmau N, Barendt WJ, Letchford L, Leyden GM, Goffin EK, Barthorpe A, Lightfoot H, Chen E, Gilbert J, Noorani A, Devonshire G, Bower L, Grantham A, MacRae S, Grehan N, Wedge DC, Fitzgerald RC, Garnett MJ. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun. 2018;9:2983. doi: 10.1038/s41467-018-05190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pectasides E, Stachler MD, Derks S, Liu Y, Maron S, Islam M, Alpert L, Kwak H, Kindler H, Polite B, Sharma MR, Allen K, O’Day E, Lomnicki S, Maranto M, Kanteti R, Fitzpatrick C, Weber C, Setia N, Xiao SY, Hart J, Nagy RJ, Kim KM, Choi MG, Min BH, Nason KS, O’Keefe L, Watanabe M, Baba H, Lanman R, Agoston AT, Oh DJ, Dunford A, Thorner AR, Ducar MD, Wollison BM, Coleman HA, Ji Y, Posner MC, Roggin K, Turaga K, Chang P, Hogarth K, Siddiqui U, Gelrud A, Ha G, Freeman SS, Rhoades J, Reed S, Gydush G, Rotem D, Davison J, Imamura Y, Adalsteinsson V, Lee J, Bass AJ, Catenacci DV. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 2018;8:37–48. doi: 10.1158/2159-8290.CD-17-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswas S, Rao CM. Epigenetic tools (The Writers, The Readers and The Erasers) and their implications in cancer therapy. Eur J Pharmacol. 2018;837:8–24. doi: 10.1016/j.ejphar.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 15.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 16.Ahrens TD, Werner M, Lassmann S. Epigenetics in esophageal cancers. Cell Tissue Res. 2014;356:643–655. doi: 10.1007/s00441-014-1876-y. [DOI] [PubMed] [Google Scholar]
- 17.Baba Y, Watanabe M, Baba H. Review of the alterations in DNA methylation in esophageal squamous cell carcinoma. Surg Today. 2013;43:1355–1364. doi: 10.1007/s00595-012-0451-y. [DOI] [PubMed] [Google Scholar]
- 18.Bedford MT, van Helden PD. Hypomethylation of DNA in pathological conditions of the human prostate. Cancer Res. 1987;47:5274–5276. [PubMed] [Google Scholar]
- 19.de Capoa A, Musolino A, Della Rosa S, Caiafa P, Mariani L, Del Nonno F, Vocaturo A, Donnorso RP, Niveleau A, Grappelli C. DNA demethylation is directly related to tumour progression: evidence in normal, pre-malignant and malignant cells from uterine cervix samples. Oncol Rep. 2003;10:545–549. [PubMed] [Google Scholar]
- 20.Brothman AR, Swanson G, Maxwell TM, Cui J, Murphy KJ, Herrick J, Speights VO, Isaac J, Rohr LR. Global hypomethylation is common in prostate cancer cells: a quantitative predictor for clinical outcome? Cancer Genet Cytogenet. 2005;156:31–36. doi: 10.1016/j.cancergencyto.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Cadieux B, Ching TT, VandenBerg SR, Costello JF. Genome-wide hypomethylation in human glioblastomas associated with specific copy number alteration, methylenetetrahydrofolate reductase allele status, and increased proliferation. Cancer Res. 2006;66:8469–8476. doi: 10.1158/0008-5472.CAN-06-1547. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 23.Narayan A, Ji W, Zhang XY, Marrogi A, Graff JR, Baylin SB, Ehrlich M. Hypomethylation of pericentromeric DNA in breast adenocarcinomas. Int J Cancer. 1998;77:833–838. doi: 10.1002/(sici)1097-0215(19980911)77:6<833::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Smith IM, Mydlarz WK, Mithani SK, Califano JA. DNA global hypomethylation in squamous cell head and neck cancer associated with smoking, alcohol consumption and stage. Int J Cancer. 2007;121:1724–1728. doi: 10.1002/ijc.22889. [DOI] [PubMed] [Google Scholar]
- 25.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 26.Belinsky SA, Nikula KJ, Palmisano WA, Michels R, Saccomanno G, Gabrielson E, Baylin SB, Herman JG. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci U S A. 1998;95:11891–11896. doi: 10.1073/pnas.95.20.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong DJ, Foster SA, Galloway DA, Reid BJ. Progressive region-specific de novo methylation of the p16 CpG island in primary human mammary epithelial cell strains during escape from M(0) growth arrest. Mol Cell Biol. 1999;19:5642–5651. doi: 10.1128/mcb.19.8.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuovo GJ, Plaia TW, Belinsky SA, Baylin SB, Herman JG. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc Natl Acad Sci U S A. 1999;96:12754–12759. doi: 10.1073/pnas.96.22.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 30.Shen JC, Rideout WM 3rd, Jones PA. High frequency mutagenesis by a DNA methyltransferase. Cell. 1992;71:1073–1080. doi: 10.1016/s0092-8674(05)80057-1. [DOI] [PubMed] [Google Scholar]
- 31.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, Melo M, da Rocha AG, Preto A, Castro P, Castro L, Pardal F, Lopes JM, Santos LL, Reis RM, Cameselle-Teijeiro J, Sobrinho-Simoes M, Lima J, Maximo V, Soares P. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 32.Lee DD, Leao R, Komosa M, Gallo M, Zhang CH, Lipman T, Remke M, Heidari A, Nunes NM, Apolonio JD, Price AJ, De Mello RA, Dias JS, Huntsman D, Hermanns T, Wild PJ, Vanner R, Zadeh G, Karamchandani J, Das S, Taylor MD, Hawkins CE, Wasserman JD, Figueiredo A, Hamilton RJ, Minden MD, Wani K, Diplas B, Yan H, Aldape K, Akbari MR, Danesh A, Pugh TJ, Dirks PB, Castelo-Branco P, Tabori U. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J Clin Invest. 2019;129:223–229. doi: 10.1172/JCI121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 34.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliopoulos D, Oikonomou P, Messinis I, Tsezou A. Correlation of promoter hypermethylation in hTERT, DAPK and MGMT genes with cervical oncogenesis progression. Oncol Rep. 2009;22:199–204. doi: 10.3892/or_00000425. [DOI] [PubMed] [Google Scholar]
- 36.de Wilde J, Kooter JM, Overmeer RM, Claassen-Kramer D, Meijer CJ, Snijders PJ, Steenbergen RD. hTERT promoter activity and CpG methylation in HPV-induced carcinogenesis. BMC Cancer. 2010;10:271. doi: 10.1186/1471-2407-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumari A, Srinivasan R, Vasishta RK, Wig JD. Positive regulation of human telomerase reverse transcriptase gene expression and telomerase activity by DNA methylation in pancreatic cancer. Ann Surg Oncol. 2009;16:1051–1059. doi: 10.1245/s10434-009-0333-8. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Xu J, Geng X, Zhang W. Analysis of DNA methylation status of the promoter of human telomerase reverse transcriptase in gastric carcinogenesis. Arch Med Res. 2010;41:1–6. doi: 10.1016/j.arcmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Petit T, Georges C, Jung GM, Borel C, Bronner G, Flesch H, Massard G, Velten M, Haegele P, Schraub S. Systematic esophageal endoscopy screening in patients previously treated for head and neck squamous-cell carcinoma. Ann Oncol. 2001;12:643–646. doi: 10.1023/a:1011191720336. [DOI] [PubMed] [Google Scholar]
- 40.Roshandel G, Nourouzi A, Pourshams A, Semnani S, Merat S, Khoshnia M. Endoscopic screening for esophageal squamous cell carcinoma. Arch Iran Med. 2013;16:351–357. [PubMed] [Google Scholar]
- 41.Carvalho R, Areia M, Brito D, Saraiva S, Alves S, Cadime AT. Diagnostic accuracy of lugol chromoendoscopy in the oesophagus in patients with head and neck cancer. Rev Esp Enferm Dig. 2013;105:79–83. doi: 10.4321/s1130-01082013000200004. [DOI] [PubMed] [Google Scholar]
- 42.Dubuc J, Legoux J, Winnock M, Seyrig J, Barbier J, Barrioz T, Laugier R, Boulay G, Grasset D, Sautereau D, Grigoresco D, Butel J, Scoazec J, Ponchon T Société Française d’Endoscopie Digestive. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006;38:690–695. doi: 10.1055/s-2006-925255. [DOI] [PubMed] [Google Scholar]
- 43.Nagami Y, Tominaga K, Machida H, Nakatani M, Kameda N, Sugimori S, Okazaki H, Tanigawa T, Yamagami H, Kubo N, Shiba M, Watanabe K, Watanabe T, Iguchi H, Fujiwara Y, Ohira M, Hirakawa K, Arakawa T. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol. 2014;109:845–854. doi: 10.1038/ajg.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morita FH, Bernardo WM, Ide E, Rocha RS, Aquino JC, Minata MK, Yamazaki K, Marques SB, Sakai P, de Moura EG. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer. 2017;17:54. doi: 10.1186/s12885-016-3011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shames DS, Minna JD, Gazdar AF. Methods for detecting DNA methylation in tumors: from bench to bedside. Cancer Lett. 2007;251:187–198. doi: 10.1016/j.canlet.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gouil Q, Keniry A. Latest techniques to study DNA methylation. Essays Biochem. 2019;63:639–648. doi: 10.1042/EBC20190027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–150. doi: 10.2144/03351md01. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 50.Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schubeler D. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- 51.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 52.Kalatskaya I. Overview of major molecular alterations during progression from Barrett’s esophagus to esophageal adenocarcinoma. Ann N Y Acad Sci. 2016;1381:74–91. doi: 10.1111/nyas.13134. [DOI] [PubMed] [Google Scholar]
- 53.Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY, Zhao YD, Sun J, Zhou CC, Yao R, Wang SY, Wang P, Sun N, Zhang BH, Dong JS, Yu Y, Luo M, Feng XL, Shi SS, Zhou F, Tan FW, Qiu B, Li N, Shao K, Zhang LJ, Zhang LJ, Xue Q, Gao SG, He J. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 54.Jammula S, Katz-Summercorn AC, Li X, Linossi C, Smyth E, Killcoyne S, Biasci D, Subash VV, Abbas S, Blasko A, Devonshire G, Grantham A, Wronowski F, O’Donovan M, Grehan N, Eldridge MD, Tavaré S Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) consortium. Fitzgerald RC. Identification of subtypes of Barrett’s esophagus and esophageal adenocarcinoma based on DNA methylation profiles and integration of transcriptome and genome data. Gastroenterology. 2020;158:1682–1697. e1681. doi: 10.1053/j.gastro.2020.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bandla S, Peters JH, Ruff D, Chen SM, Li CY, Song K, Thoms K, Litle VR, Watson T, Chapurin N, Lada M, Pennathur A, Luketich JD, Peterson D, Dulak A, Lin L, Bass A, Beer DG, Godfrey TE, Zhou Z. Comparison of cancer-associated genetic abnormalities in columnar-lined esophagus tissues with and without goblet cells. Ann Surg. 2014;260:72–80. doi: 10.1097/SLA.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galipeau PC, Prevo LJ, Sanchez CA, Longton GM, Reid BJ. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. J Natl Cancer Inst. 1999;91:2087–2095. doi: 10.1093/jnci/91.24.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maley CC, Galipeau PC, Li X, Sanchez CA, Paulson TG, Blount PL, Reid BJ. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 2004;64:7629–7633. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 58.Maley CC, Galipeau PC, Finley JC, Wongsurawat VJ, Li X, Sanchez CA, Paulson TG, Blount PL, Risques RA, Rabinovitch PS, Reid BJ. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet. 2006;38:468–473. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- 59.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Galipeau PC, Sanchez CA, Blount PL, Maley CC, Arnaudo J, Peiffer DA, Pokholok D, Gunderson KL, Reid BJ. Single nucleotide polymorphism-based genome-wide chromosome copy change, loss of heterozygosity, and aneuploidy in Barrett’s esophagus neoplastic progression. Cancer Prev Res (Phila) 2008;1:413–423. doi: 10.1158/1940-6207.CAPR-08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaz AM, Wong CJ, Luo Y, Virgin JB, Washington MK, Willis JE, Leidner RS, Chak A, Grady WM. DNA methylation profiling in Barrett’s esophagus and esophageal adenocarcinoma reveals unique methylation signatures and molecular subclasses. Epigenetics. 2011;6:1403–1412. doi: 10.4161/epi.6.12.18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paulson TG, Reid BJ. Focus on Barrett’s esophagus and esophageal adenocarcinoma. Cancer Cell. 2004;6:11–16. doi: 10.1016/j.ccr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 63.Yu M, Maden SK, Stachler M, Kaz AM, Ayers J, Guo Y, Carter KT, Willbanks A, Heinzerling TJ, O’Leary RM, Xu X, Bass A, Chandar AK, Chak A, Elliott R, Willis JE, Markowitz SD, Grady WM. Subtypes of Barrett’s oesophagus and oesophageal adenocarcinoma based on genome-wide methylation analysis. Gut. 2019;68:389–399. doi: 10.1136/gutjnl-2017-314544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvi MA, Liu X, O’Donovan M, Newton R, Wernisch L, Shannon NB, Shariff K, di Pietro M, Bergman JJ, Ragunath K, Fitzgerald RC. DNA methylation as an adjunct to histopathology to detect prevalent, inconspicuous dysplasia and early-stage neoplasia in Barrett’s esophagus. Clin Cancer Res. 2013;19:878–888. doi: 10.1158/1078-0432.CCR-12-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Secrier M, Li X, de Silva N, Eldridge MD, Contino G, Bornschein J, MacRae S, Grehan N, O’Donovan M, Miremadi A, Yang TP, Bower L, Chettouh H, Crawte J, Galeano-Dalmau N, Grabowska A, Saunders J, Underwood T, Waddell N, Barbour AP, Nutzinger B, Achilleos A, Edwards PA, Lynch AG, Tavaré S, Fitzgerald RC Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) Consortium. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet. 2016;48:1131–1141. doi: 10.1038/ng.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin L, Lin DC. Biological significance of tumor heterogeneity in esophageal squamous cell carcinoma. Cancers (Basel) 2019;11:1156. doi: 10.3390/cancers11081156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin DC, Wang MR, Koeffler HP. Genomic and epigenomic aberrations in esophageal squamous cell carcinoma and implications for patients. Gastroenterology. 2018;154:374–389. doi: 10.1053/j.gastro.2017.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toh Y, Egashira A, Yamamoto M. Epigenetic alterations and their clinical implications in esophageal squamous cell carcinoma. Gen Thorac Cardiovasc Surg. 2013;61:262–269. doi: 10.1007/s11748-013-0235-3. [DOI] [PubMed] [Google Scholar]
- 69.You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawan C, Vaissiere T, Murr R, Herceg Z. Epigenetic drivers and genetic passengers on the road to cancer. Mutat Res. 2008;642:1–13. doi: 10.1016/j.mrfmmm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Teng H, Xue M, Liang J, Wang X, Wang L, Wei W, Li C, Zhang Z, Li Q, Ran X, Shi X, Cai W, Wang W, Gao H, Sun Z. Inter- and intratumor DNA methylation heterogeneity associated with lymph node metastasis and prognosis of esophageal squamous cell carcinoma. Theranostics. 2020;10:3035–3048. doi: 10.7150/thno.42559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin L, Cheng X, Yin D. Aberrant DNA methylation in esophageal squamous cell carcinoma: biological and clinical implications. Front Oncol. 2020;10:549850. doi: 10.3389/fonc.2020.549850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li JS, Ying JM, Wang XW, Wang ZH, Tao Q, Li LL. Promoter methylation of tumor suppressor genes in esophageal squamous cell carcinoma. Chin J Cancer. 2013;32:3–11. doi: 10.5732/cjc.011.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lima SC, Hernandez-Vargas H, Simao T, Durand G, Kruel CD, Le Calvez-Kelm F, Ribeiro Pinto LF, Herceg Z. Identification of a DNA methylome signature of esophageal squamous cell carcinoma and potential epigenetic biomarkers. Epigenetics. 2011;6:1217–1227. doi: 10.4161/epi.6.10.17199. [DOI] [PubMed] [Google Scholar]
- 75.Guo M, Ren J, House MG, Qi Y, Brock MV, Herman JG. Accumulation of promoter methylation suggests epigenetic progression in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2006;12:4515–4522. doi: 10.1158/1078-0432.CCR-05-2858. [DOI] [PubMed] [Google Scholar]
- 76.Lee EJ, Lee BB, Han J, Cho EY, Shim YM, Park J, Kim DH. CpG island hypermethylation of E-cadherin (CDH1) and integrin alpha4 is associated with recurrence of early stage esophageal squamous cell carcinoma. Int J Cancer. 2008;123:2073–2079. doi: 10.1002/ijc.23598. [DOI] [PubMed] [Google Scholar]
- 77.Fukuoka T, Hibi K, Nakao A. Aberrant methylation is frequently observed in advanced esophageal squamous cell carcinoma. Anticancer Res. 2006;26:3333–3335. [PubMed] [Google Scholar]
- 78.Wang J, Sasco AJ, Fu C, Xue H, Guo G, Hua Z, Zhou Q, Jiang Q, Xu B. Aberrant DNA methylation of P16, MGMT, and hMLH1 genes in combination with MTHFR C677T genetic polymorphism in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:118–125. doi: 10.1158/1055-9965.EPI-07-0733. [DOI] [PubMed] [Google Scholar]
- 79.Ling Y, Huang G, Fan L, Wei L, Zhu J, Liu Y, Zhu C, Zhang C. CpG island methylator phenotype of cell-cycle regulators associated with TNM stage and poor prognosis in patients with oesophageal squamous cell carcinoma. J Clin Pathol. 2011;64:246–251. doi: 10.1136/jcp.2010.082875. [DOI] [PubMed] [Google Scholar]
- 80.Li B, Wang B, Niu LJ, Jiang L, Qiu CC. Hypermethylation of multiple tumor-related genes associated with DNMT3b up-regulation served as a biomarker for early diagnosis of esophageal squamous cell carcinoma. Epigenetics. 2011;6:307–316. doi: 10.4161/epi.6.3.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taghavi N, Biramijamal F, Sotoudeh M, Khademi H, Malekzadeh R, Moaven O, Memar B, A’Rabi A, Abbaszadegan MR. p16INK4a hypermethylation and p53, p16 and MDM2 protein expression in esophageal squamous cell carcinoma. BMC Cancer. 2010;10:138. doi: 10.1186/1471-2407-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salam I, Hussain S, Mir MM, Dar NA, Abdullah S, Siddiqi MA, Lone RA, Zargar SA, Sharma S, Hedau S, Basir SF, Bharti AC, Das BC. Aberrant promoter methylation and reduced expression of p16 gene in esophageal squamous cell carcinoma from Kashmir valley: a high-risk area. Mol Cell Biochem. 2009;332:51–58. doi: 10.1007/s11010-009-0173-7. [DOI] [PubMed] [Google Scholar]
- 83.Maesawa C, Tamura G, Nishizuka S, Ogasawara S, Ishida K, Terashima M, Sakata K, Sato N, Saito K, Satodate R. Inactivation of the CDKN2 gene by homozygous deletion and de novo methylation is associated with advanced stage esophageal squamous cell carcinoma. Cancer Res. 1996;56:3875–3878. [PubMed] [Google Scholar]
- 84.Pu W, Wang C, Chen S, Zhao D, Zhou Y, Ma Y, Wang Y, Li C, Huang Z, Jin L, Guo S, Wang J, Wang M. Targeted bisulfite sequencing identified a panel of DNA methylation-based biomarkers for esophageal squamous cell carcinoma (ESCC) Clin Epigenetics. 2017;9:129. doi: 10.1186/s13148-017-0430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, Zhou F, Jiang C, Wang Y, Lu Y, Yang F, Wang N, Yang H, Zheng Y, Zhang J. Identification of a DNA methylome profile of esophageal squamous cell carcinoma and potential plasma epigenetic biomarkers for early diagnosis. PLoS One. 2014;9:e103162. doi: 10.1371/journal.pone.0103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agrawal N, Jiao Y, Bettegowda C, Hutfless SM, Wang Y, David S, Cheng Y, Twaddell WS, Latt NL, Shin EJ, Wang LD, Wang L, Yang W, Velculescu VE, Vogelstein B, Papadopoulos N, Kinzler KW, Meltzer SJ. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2012;2:899–905. doi: 10.1158/2159-8290.CD-12-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency; Brigham and Women’s Hospital; Broad Institute; Brown University; Case Western Reserve University; Dana-Farber Cancer Institute; Duke University; Greater Poland Cancer Centre; Harvard Medical School; Institute for Systems Biology; KU Leuven; Mayo Clinic; Memorial Sloan Kettering Cancer Center; National Cancer Institute; Nationwide Children’s Hospital; Stanford University; University of Alabama; University of Michigan; University of North Carolina; University of Pittsburgh; University of Rochester; University of Southern California; University of Texas MD Anderson Cancer Center; University of Washington; Van Andel Research Institute; Vanderbilt University; Washington University; Genome Sequencing Center: Broad Institute; Washington University in St. Louis; Genome Characterization Centers: BC Cancer Agency; Broad Institute; Harvard Medical School; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University; University of North Carolina; University of Southern California Epigenome Center; University of Texas MD Anderson Cancer Center; Van Andel Research Institute; Genome Data Analysis Centers: Broad Institute; Brown University:; Harvard Medical School; Institute for Systems Biology; Memorial Sloan Kettering Cancer Center; University of California Santa Cruz; University of Texas MD Anderson Cancer Center; Biospecimen Core Resource: International Genomics Consortium; Research Institute at Nationwide Children’s Hospital; Tissue Source Sites: Analytic Biologic Services; Asan Medical Center; Asterand Bioscience; Barretos Cancer Hospital; BioreclamationIVT; Botkin Municipal Clinic; Chonnam National University Medical School; Christiana Care Health System; Cureline; Duke University; Emory University; Erasmus University; Indiana University School of Medicine; Institute of Oncology of Moldova; International Genomics Consortium; Invidumed; Israelitisches Krankenhaus Hamburg; Keimyung University School of Medicine; Memorial Sloan Kettering Cancer Center; National Cancer Center Goyang; Ontario Tumour Bank; Peter MacCallum Cancer Centre; Pusan National University Medical School; Ribeirão Preto Medical School; St. Joseph’s Hospital &Medical Center; St. Petersburg Academic University; Tayside Tissue Bank; University of Dundee; University of Kansas Medical Center; University of Michigan; University of North Carolina at Chapel Hill; University of Pittsburgh School of Medicine; University of Texas MD Anderson Cancer Center; Disease Working Group: Duke University; Memorial Sloan Kettering Cancer Center; National Cancer Institute; University of Texas MD Anderson Cancer Center; Yonsei University College of Medicine; Data Coordination Center: CSRA Inc.; Project Team: National Institutes of Health. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu T, Chen D, Wang Y, Sun X, Li S, Miao S, Wo Y, Dong Y, Leng X, Du W, Jiao W. Identification of DNA methylation-driven genes in esophageal squamous cell carcinoma: a study based on The Cancer Genome Atlas. Cancer Cell Int. 2019;19:52. doi: 10.1186/s12935-019-0770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D, Iyer PG. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018;88:413–426. doi: 10.1016/j.gie.2018.04.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chettouh H, Mowforth O, Galeano-Dalmau N, Bezawada N, Ross-Innes C, MacRae S, Debiram-Beecham I, O’Donovan M, Fitzgerald RC. Methylation panel is a diagnostic biomarker for Barrett’s oesophagus in endoscopic biopsies and non-endoscopic cytology specimens. Gut. 2018;67:1942–1949. doi: 10.1136/gutjnl-2017-314026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z, Kambhampati S, Cheng Y, Ma K, Simsek C, Tieu AH, Abraham JM, Liu X, Prasath V, Duncan M, Stark A, Trick A, Tsai HL, Wang H, He Y, Khashab MA, Ngamruengphong S, Shin EJ, Wang TH, Meltzer SJ. Methylation biomarker panel performance in esophacap cytology samples for diagnosing Barrett’s esophagus: a prospective validation study. Clin Cancer Res. 2019;25:2127–2135. doi: 10.1158/1078-0432.CCR-18-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bus P, Siersema PD, van Baal JW. Cell culture models for studying the development of Barrett’s esophagus: a systematic review. Cell Oncol (Dordr) 2012;35:149–161. doi: 10.1007/s13402-012-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Das KM, Kong Y, Bajpai M, Kulkarni D, Geng X, Mishra P, Banerjee D, Hirshfield K. Transformation of benign Barrett’s epithelium by repeated acid and bile exposure over 65 weeks: a novel in vitro model. Int J Cancer. 2011;128:274–282. doi: 10.1002/ijc.25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Minacapelli CD, Bajpai M, Geng X, Cheng CL, Chouthai AA, Souza R, Spechler SJ, Das KM. Barrett’s metaplasia develops from cellular reprograming of esophageal squamous epithelium due to gastroesophageal reflux. Am J Physiol Gastrointest Liver Physiol. 2017;312:G615–G622. doi: 10.1152/ajpgi.00268.2016. [DOI] [PubMed] [Google Scholar]
- 95.Whelan KA, Muir AB, Nakagawa H. Esophageal 3D culture systems as modeling tools in esophageal epithelial pathobiology and personalized medicine. Cell Mol Gastroenterol Hepatol. 2018;5:461–478. doi: 10.1016/j.jcmgh.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kapoor H, Lohani KR, Lee TH, Agrawal DK, Mittal SK. Animal models of Barrett’s esophagus and esophageal adenocarcinoma-past, present, and future. Clin Transl Sci. 2015;8:841–847. doi: 10.1111/cts.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nair DV, Reddy AG. Laboratory animal models for esophageal cancer. Vet World. 2016;9:1229–1232. doi: 10.14202/vetworld.2016.1229-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang M, Li H, Zhang Y, Yang Y, Lu R, Liu K, Lin S, Lan X, Wang H, Wu H, Zhu J, Zhou Z, Xu J, Lee DK, Zhang L, Lee YC, Yuan J, Abrams JA, Wang TC, Sepulveda AR, Wu Q, Chen H, Sun X, She J, Chen X, Que J. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature. 2017;550:529–533. doi: 10.1038/nature24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iwagami S, Baba Y, Watanabe M, Shigaki H, Miyake K, Ishimoto T, Iwatsuki M, Sakamaki K, Ohashi Y, Baba H. LINE-1 hypomethylation is associated with a poor prognosis among patients with curatively resected esophageal squamous cell carcinoma. Ann Surg. 2013;257:449–455. doi: 10.1097/SLA.0b013e31826d8602. [DOI] [PubMed] [Google Scholar]
- 100.Kawano H, Saeki H, Kitao H, Tsuda Y, Otsu H, Ando K, Ito S, Egashira A, Oki E, Morita M, Oda Y, Maehara Y. Chromosomal instability associated with global DNA hypomethylation is associated with the initiation and progression of esophageal squamous cell carcinoma. Ann Surg Oncol. 2014;21(Suppl 4):S696–702. doi: 10.1245/s10434-014-3818-z. [DOI] [PubMed] [Google Scholar]
- 101.Hoshimoto S, Takeuchi H, Ono S, Sim MS, Huynh JL, Huang SK, Marzese DM, Kitagawa Y, Hoon DS. Genome-wide hypomethylation and specific tumor-related gene hypermethylation are associated with esophageal squamous cell carcinoma outcome. J Thorac Oncol. 2015;10:509–517. doi: 10.1097/JTO.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 102.Baba Y, Watanabe M, Murata A, Shigaki H, Miyake K, Ishimoto T, Iwatsuki M, Iwagami S, Yoshida N, Oki E, Sakamaki K, Nakao M, Baba H. LINE-1 hypomethylation, DNA copy number alterations, and CDK6 amplification in esophageal squamous cell carcinoma. Clin Cancer Res. 2014;20:1114–1124. doi: 10.1158/1078-0432.CCR-13-1645. [DOI] [PubMed] [Google Scholar]
- 103.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 104.Issa JP, Kantarjian HM, Kirkpatrick P. Azacitidine. Nat Rev Drug Discov. 2005;4:275–276. doi: 10.1038/nrd1698. [DOI] [PubMed] [Google Scholar]
- 105.Schneider BJ, Shah MA, Klute K, Ocean A, Popa E, Altorki N, Lieberman M, Schreiner A, Yantiss R, Christos PJ, Palmer R, You D, Viale A, Kermani P, Scandura JM. Phase I study of epigenetic priming with azacitidine prior to standard neoadjuvant chemotherapy for patients with resectable gastric and esophageal adenocarcinoma: evidence of tumor hypomethylation as an indicator of major histopathologic response. Clin Cancer Res. 2017;23:2673–2680. doi: 10.1158/1078-0432.CCR-16-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu S, Hu W, Iyer R, Kavanagh JJ, Coleman RL, Levenback CF, Sood AK, Wolf JK, Gershenson DM, Markman M, Hennessy BT, Kurzrock R, Bast RC Jr. Phase 1b-2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer. 2011;117:1661–1669. doi: 10.1002/cncr.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020;5:229. doi: 10.1038/s41392-020-00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao L, Yao ZT, Fang WK, He QY, Xu WW, Li B. Epigenetics in esophageal cancer: from mechanisms to therapeutics. Small Methods. 2020;4:2000391. [Google Scholar]
- 109.Chen M, Nie J, Liu Y, Li X, Zhang Y, Brock MV, Feng K, Wu Z, Li X, Shi L, Li S, Guo M, Mei Q, Han W. Phase Ib/II study of safety and efficacy of low-dose decitabine-primed chemoimmunotherapy in patients with drug-resistant relapsed/refractory alimentary tract cancer. Int J Cancer. 2018;143:1530–1540. doi: 10.1002/ijc.31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Battaglin F, Naseem M, Puccini A, Lenz HJ. Molecular biomarkers in gastro-esophageal cancer: recent developments, current trends and future directions. Cancer Cell Int. 2018;18:99. doi: 10.1186/s12935-018-0594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. 2018;8:386. doi: 10.3389/fonc.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 113.Press MF, Ellis CE, Gagnon RC, Grob TJ, Buyse M, Villalobos I, Liang Z, Wu S, Bang YJ, Qin SK, Chung HC, Xu J, Park JO, Jeziorski K, Afenjar K, Ma Y, Estrada MC, Robinson DM, Scherer SJ, Sauter G, Hecht JR, Slamon DJ. HER2 status in advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma for entry to the TRIO-013/LOGiC trial of lapatinib. Mol Cancer Ther. 2017;16:228–238. doi: 10.1158/1535-7163.MCT-15-0887. [DOI] [PubMed] [Google Scholar]
- 114.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 115.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yip HC, Chiu PW. Endoscopic diagnosis and management of early squamous cell carcinoma of esophagus. J Thorac Dis. 2017;9(Suppl 8):S689–S696. doi: 10.21037/jtd.2017.06.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, Xu R, Liu M, Cai H, Cao C, Liu F, Li F, Guo C, Pan Y, He Z, Ke Y. Lugol chromoendoscopy detects esophageal dysplasia with low levels of sensitivity in a high-risk region of China. Clin Gastroenterol Hepatol. 2018;16:1585–1592. doi: 10.1016/j.cgh.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 118.Chung EJ, Rho YS, Jung KY, Kim JW, Lee SW. The role of transnasal esophagoscopy in ent office: a prospective, multicenter study in Korea. Clin Exp Otorhinolaryngol. 2014;7:123–125. doi: 10.3342/ceo.2014.7.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hur C, Choi SE, Kong CY, Wang GQ, Xu H, Polydorides AD, Xue LY, Perzan KE, Tramontano AC, Richards-Kortum RR, Anandasabapathy S. High-resolution microendoscopy for esophageal cancer screening in China: a cost-effectiveness analysis. World J Gastroenterol. 2015;21:5513–5523. doi: 10.3748/wjg.v21.i18.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abad MRA, Shimamura Y, Fujiyoshi Y, Seewald S, Inoue H. Endocytoscopy: technology and clinical application in upper gastrointestinal tract. Transl Gastroenterol Hepatol. 2020;5:28. doi: 10.21037/tgh.2019.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]



