Abstract
The purple mangosteen (Garcinia mangostana) is a popular Southeast Asian fruit that has been used traditionally for its health promoting benefits for years. Unique to the mangosteen are a class of phytochemicals known as xanthones that have been reported to display significant anti-cancer and anti-tumor activities, specifically through the promotion of apoptosis, targeting of specific cancer-related proteins, or modulation of cell signaling pathways. α-Mangostin, the most abundant xanthone isolated from the mangosteen, has received substantial attention as it has proven to be a potent phytochemical, specifically as an anticancer agent, in numerous different cancer cell studies and cancer animal models. While the mechanisms for these anticancer effects have been reported in many studies, lesser xanthones, including gartanin, β-mangostin, γ-mangostin, garcinone C, and garcinone E, and mangosteen extracts from the pericarp, roots, rind, and stem show promise for their anticancer activity but their mechanisms of action are not as well developed and remain to be determined. Mangosteen products appear safe and have been well tolerated in human clinical trials where they show antioxidant activity, though their clinical anticancer activity has not yet been evaluated. This review summarizes the work that has been done to explore and explain the anticancer and antitumor activities of α-mangostin, lesser xanthones, and mangosteen extracts in vitro, in vivo, and in humans in various cancers.
Keywords: Xanthones, Garcinia mangostana, mangosteen, chemoprevention, anticancer, prostate cancer
Graphical Abstract

1. Introduction
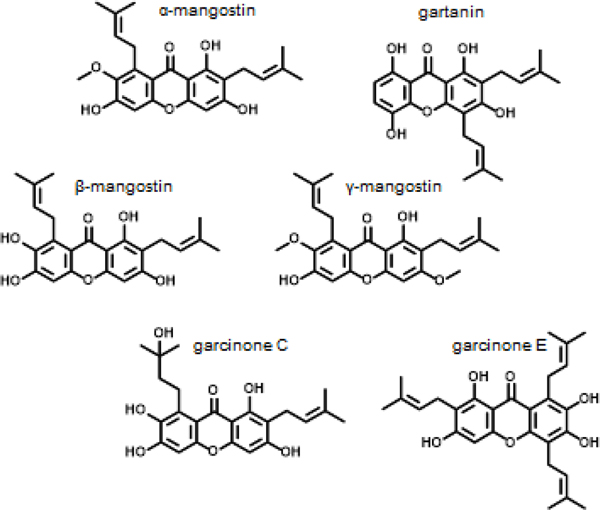
The purple mangosteen (Garcinia mangostana) is a tropical tree most famous for its dark purple mangosteen fruit. The origin of the mangosteen tree is not clearly understood however, it may have possibly originated in Malaysia and can now be found in hot and humid regions throughout Southeast Asia, Central America, and Africa [1, 2]. A notable feature of the mangosteen fruit is its sweet taste and different formulations of the mangosteen, including teas, ointments, tinctures, and other preparations have been used in traditional Eastern medicine to treat skin infections, urinary tract infections, dysentery, inflammation, abdominal pain, diarrhea, and fevers [3, 4]. The mangosteen plant, including the fruit, rind, roots, and leaves, contains a class of phytochemicals called xanthones, and more than 70 different xanthones have been isolated from the mangosteen Figure 1 [5]. These naturally occurring compounds have a flat planar structure that includes tricyclic aromatic ring systems with different functional groups attached to the A and C rings (commonly methoxy, hydroxy, or isoprenyl groups) [6, 7]. Xanthones including isoprenylated, oxygenated xanthones, xanthone glycosides, xanthonolignoids, bisxanthones, and other miscellaneous xanthones are most commonly associated with the mangosteen fruit, however, a few other types of xanthones have been found in other plants [8]. More recently, studies have used mangosteen xanthones, specifically α-mangostin, the most abundant xanthone, as well as some of the less abundant isoprenylated xanthones and reported them to have antioxidant, anti-inflammatory, antibacterial, antifungal, anticancer, and antitumor activities Figure 2 [9–11]. Pharmacological evaluations have focused on elucidating the cell signaling networks modulated by mangosteen xanthones, including the PI3K/AKT, AMPK, and MAPK pathways, that are commonly upregulated or overdriven in cancer programs. Multiple studies have suggested that mangosteen xanthones modulate reactive oxygen species (ROS) levels and their related signaling, as well as specific protein targets including STAT3, cyclin dependent kinases (CDKs), and matrix metalloproteinases (MMPs) [5, 9, 12, 13]. Herein we evaluate these studies, their data, and the suggested mechanism of how xanthones from the mangosteen display anticancer and antitumor activities.
Fig 1.
Mangosteen xanther structures
Figure 2:
Mangosteen xanthones have in vitro and in vivo anticancer activity and are safe in humans.
2.1. α-Mangostin
α-Mangostin (1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-9H-xanthen-9-one) is the most abundant isoprenylated xanthone in G. mangostana that is produced in the pericarp, arils, stem, and seeds of the fruit [5]. α-Mangostin has an empirical formula of C24H26O6 and was first isolated from the dried skin of a mangosteen fruit in 1855 by W. Schmid [14]. α-Mangostin has a typical xanthone tricyclic core with two isoprenyl groups at positions 2’ and 8’ that have been suggested to have biological activity [6, 15, 16]. Multiple formulations contain α-mangostin in different commercial products including capsules, supplements, juices, and skin creams and lotions, are currently popular with consumers for their various health promoting indications.
2.2. In vitro studies
The majority of studies evaluating xanthones from the mangosteen have been with α-mangostin with a particular emphasis on cancer chemoprevention and anticancer properties. Many of the anticancer studies report apoptosis in cancer cells [17–20], and there have also been a significant number of pharmacological studies that provide evidence that α-mangostin modulates specific cell signaling pathways and acts on specific targets. The reports that are summarized have found α-mangostin to target different proteins, including CDKs, MMPs, and STAT3, and cell signaling pathways, including PI3K/AKT, AMPK, MAPK, and ROS signaling.
PI3K/AKT, AMPK, and MAPK
Breast cancer
Kinases including PI3K/AKT, AMPK, and MAPK are important to cellular metabolism and proliferation considering their regulation of signaling pathways and the ubiquity of the proteins involved in these signaling cascades. There has been significant effort in identifying agents that modulate these kinases and as a result, there are multiple studies evaluating α-mangostin in cancer cells 25812084. α-Mangostin inhibited the growth of T47D breast cancer cells at a half maximal inhibitory concentration (IC50) of 7.5 μM, inhibited colony formation, and induced apoptosis by modulating the PI3K/AKT and MAPK signaling proteins [21]. Currently, the most used drugs for breast cancer are estrogen receptor alpha (ERα) antagonists, indicating the importance of this target. After a 6 hour treatment with α-mangostin, phosphorylation of ERα in T47D cells decreased, suggesting a decrease in ERα activity [22]. α-Mangostin decreased the phosphorylation of AKT at two different amino acids (Thr308 and Ser473) leading to the decreased expression of MAPK signaling proteins including NF-κB, p 65, c-Rel, and c-Myc [21]. Another study confirmed that α-mangostin can target ERα by showing that the viability of MCF-7 cells (ERα-positive) decreased more significantly than the viability of MDA-MB-231 cells (ERα-negative) and that the levels of ERα decreased in MCF-7 cells [23]. Lee et al also showed that α-mangostin affected the viability of MCF-7 breast cancer cells at concentrations > 6 μM and that it displayed chemoprotective effects by reversing the invasion and migration of MCF-7 cells treated with 12-O-Tetradecanoylphorbol-13-acetate (TPA; a compound used to induce carcinogenesis) [24]. ERK activation was induced by TPA but this was reversed and the expressions of p-JNK, p-ERK, and MMP-2/9 were decreased after treating the cells with α-mangostin [24]. Again, in MCF-7 and MDA-MB-231 cells, IC50 values of α-mangostin were reported to be between 2–3.5 μM for 24- and 48-hour treatments [25]. α-Mangostin induced apoptosis and decreased the expressions of fatty acid synthase and fatty acid kinase, both highly upregulated targets in breast cancer that are commonly regulated by PI3K/AKT and MAPK. α-Mangostin decreased p-AKT (Ser473) while it increased the levels of ERK1/2. These results demonstrate that α-mangostin may decrease the viability of MCF-7 and MDA-MB-231 cells by inhibiting the PI3K/AKT and fatty acid synthase pathways but not through the MAPK pathway [25]. The results from these studies indicate that α-mangostin may have clinical relevance for breast cancer patients, since current breast cancer pharmacotherapies are known to inhibit the PI3K/AKT pathway, and because the estrogen receptor remains an important target in breast cancer.
Colon cancer
Multiple xanthones decreased the viability of DLD-1 colon cancer cells, though α-mangostin was the most potent with an IC50 of 7.5 μM [26, 27]. A time course study with α-mangostin treatments showed normal cellular functions were disrupted and that the expressions of MAP and AKT kinases and pERK changed over time. The time course also revealed that ERK was activated at early timepoints but then deactivated over a span of 48 hours while the phosphorylation of JNK consistently increased and the phosphorylation of AKT consistently decreased. α-Mangostin in combination with 5-fluorouracil, a component of both the FOLFOX and FOLFIRI regimens for colon cancer, decreased cell viability and MAP and AKT activity had a more pronounced effect than either of these agents individually [27]. In another colon cancer cell line, HCT 116 cells responded to combination treatments of α-mangostin and betulinic acid and their cell viability was decreased more significantly with the combination treatment than with either of these two agents individually [28]. This combination also induced apoptosis and increased tumor suppressor p53 levels, DNA damage, and MAPK/ERK signaling pathways [28]. α-Mangostin promoted apoptosis in COLO 205, MIP-101, and SW 620 colon cancer cells and displayed IC50s of 23.7, 27.6, and 47.6 μM after 24 hours, respectively [29]. Watanapokasin et al reported increases in phospho-p53 and Bax, two proteins involved in the regulation of AKT [29, 30].
Hepatocarcinoma
The IC50s of α-mangostin in SK-HEP-1 hepatocarcinoma cells were reported to be 24.8 and 19.6 μM at 24 and 48 h, respectively, and further evaluation determined that α-mangostin inhibited the p38 MAPK pathway and promoted mitochondrial apoptosis [31]. A study using sorafenib-resistant HepG2 hepatocarcinoma cells evaluated the effect of α-mangostin on the TGF-β/Smad pathways, which have been shown to be inversely related to the PI3K/AKT pathways, and found that there was an increase in the activation of these pathways [32, 33]. At 23.74 μM, α-mangostin reduced the cell viability of sorafenib-resistant HepG2 cells and combination treatments of 10 μM sorafenib (i.e. a RAF/MEK/ERK, VEGFR, and PDGFR inhibitor) and 10 μM α-mangostin induced partial epithelial to mesenchymal transition, instead of a complete transition, and upregulated TGF-β/Smad pathway signaling and expression [32, 34].
Chondrosarcoma
In a study evaluating the effect of α-mangostin in SW1353 chondrosarcoma cells, cell toxicities were reported to be 10 μg/mL and apoptosis was induced via caspases-3, −6, and −9 [35]. There was a decrease in the phosphorylation of ERK1/2 and JNK while there was no change in the levels of p38 MAPK [35].
Tongue mucoepidermoid carcinoma
YD-15 tongue mucoepidermoid carcinoma cells treated with 0–30 μM of α-mangostin also showed decreases in cell proliferation, production of apoptotic bodies, and decreases in the phosphorylation of ERK1/2 and p38 [36].
Melanoma
In SK-MEL-2 and SK-MEL-30 melanoma cells, α-mangostin treatments resulted in IC50s of 8.14 and 7.78 μM, respectively [37]. Xia et al used the kinase inhibitor Sorafenib in combination with α-mangostin and observed that the combination more potently inhibited colony formation and the phosphorylation of AKT and ERK than either agent alone [37].
Glioblastoma
The AMPK pathway was also activated in glioblastoma cell lines GBM8401 and DBTRG-05MG with reported IC50 values of α-mangostin that included 6.4 and 7.3 μM, respectively [38]. While apoptosis was not induced in these cells, autophagy was induced and an increase in phosphorylated AMPK but not in the levels of PI3K, AKT, PTEN, or ERK was observed. AMPK was hypothesized to be the main driver of autophagy in α-mangostin-treated-cells [38].
Leukemia
In HL60 leukemia cells, α-mangostin induced apoptosis and was cytotoxic to cells at a concentration of 4 μM [39]. Matsumoto et al demonstrated that mitochondrial dysfunction was induced and that there was a slight induction of MAPKs after 6 h of treatment, indicating that this signaling was essential for the caspase activation they previously observed [39].
Renal carcinoma
The migration and metastasis of A-498 and 786-O renal carcinoma cells were inhibited by α-mangostin via inhibition of the phosphorylation of ERK but not p38 [40]. This study did not observe any cell toxicity or changes in cell viability in renal carcinoma cells treated with α-mangostin, which may be explained by the low concentrations tested in this study (>12 μM) [40].
Prostate cancer
Our lab recently published results showing that 22Rν1 prostate cancer cells underwent apoptosis after being treated with α-mangostin and that PERK signaling, along with related endoplasmic reticulum (ER) stress signaling pathways, were both increased [41].
Matrix metalloproteinases
Pancreatic cancer
Many studies have highlighted that α-mangostin affects the ability of cancer cells to migrate and invade other cells, which has implications in promoting the epithelial to mesenchymal transition of cells and is often regulated by the expression of matrix metalloproteinases (MMPs), specifically MMP-2 and MMP-9, and the epithelial marker protein E-cadherin. BxPC-3 and MIAPaCa-2 pancreatic cancer cells treated with 5 μM of α-mangostin showed decreased cell viability and decreased migratory, invasive, and proliferative abilities [42]. MMP-2 and MMP-9 mRNA and protein expression levels were increased after treatments with α-mangostin while the E-cadherin levels were decreased, as is common when the migratory and invasive capabilities of cancer cells are affected [42]. A later study reported consistent results showing that α-mangostin decreased the expressions of MMP-2 and MMP-9 in BxPc-3 and Panc-1 cells, that G0/G1 cell cycle arrest was induced, and that the PI3K/AKT pathway was suppressed [43].
Melanoma and skin cancer
An in vitro proteomic study of B16-F10 melanoma cells treated with α-mangostin revealed that their proliferation was significantly affected at concentrations >5 μM and that their melanin content and cell differentiation abilities were decreased [44]. The metastatic potential of melanoma cells decreased in a dose-dependent manner and the levels of MMP-9 decreased. Proteomic analysis revealed proteins involved with regulation, biosynthesis, and transportation were upregulated in α-mangostin-treated cells [44]. α-Mangostin was also cytotoxic to SK-MEL-28 and A-431 skin cancer cells, increased caspase activity and promoted apoptosis, inhibited their migration, invasion, and adhesion, and decreased the levels of MMP-2, MMP-9, and AKT [45, 46].
Lung cancer
Shih et al reported that ERK phosphorylation is essential for α-mangostin to exert its anti-cancer effects on A549 lung cancer cells by using siRNA to silence ERK [47]. α-Mangostin was cytotoxic to A549 but not WI-38 normal lung cells, and inhibited adhesion, invasion, and migration and the expressions of MMP-2 and MMP-9 that were induced by Phorbol 12-myristate 13-acetate (PMA). In A549 cells with ERK siRNA and treated with PMA and α-mangostin, the modulations of the ERK and AKT pathways and changes in MMP-2 and MMP-9 levels were more pronounced than they were in wild type A549 cells [47].
Prostate cancer
In prostate cancer PC-3 cells, no cytotoxicity was observed below concentrations of 12.2 μM of α-mangostin, though cell viability was affected at higher concentrations [48]. MMP-2 and MMP-9 activities were inhibited by α-mangostin, PC-3 cell migration and invasion were reduced, and decreases in the phosphorylation of JNK1 and JNK2 but not ERK, p38, or AKT were observed [48].
Head and neck squamous carcinoma
Kaomongkolgit’s group has reported α-mangostin to have cytotoxic effects on HN-22, HN-30, and HN-31 head and neck squamous carcinoma cells at 7.32 μM and to dose dependently decrease MMP-2 and MMP-9 expressions and increase p53 expression, though further analyses of other related MAPK signaling proteins were not performed [49, 50].
STAT3
Hepatocellular carcinoma
STAT3 is a transcription factor that is upregulated in a variety of cancers modulating cell signaling related to tumor migration and cancer metastasis [51]. In hepatocellular carcinoma cell lines SK-Hep-1, HepG2, SMMC-7721, and Huh-7 HCC, 72-hour treatments of α-mangostin displayed IC50s of 9.44, 10.94, 13.22, and 14.49 μM, respectively [52]. Further bioassays were performed to identify the extent of STAT3’s involvement in the observed promotion of apoptosis, and results revealed suppression of Bcl2, survivin, cyclin D1, and c-Myc gene and protein expression. α-Mangostin also inhibited the phosphorylation STAT3 and levels of EGFR, Src, pERK, and p-AKT, suggesting that STAT3 signaling, and the phosphorylation of upstream proteins are involved in the promotion of apoptosis in hepatocellular carcinoma cells [52].
Interestingly, Hsieh et al, mentioned in the previous section, evaluated α-mangostin in the same cell lines and reported no change in the phosphorylation of ERK in SK-Hep-1 cells [31, 52]. This may be due to the different concentrations of α-mangostin used, different treatment times in which the cells were exposed to α-mangostin, or possibly even different passage rates of the cells used.
Gastric adenocarcinoma
STAT3 was also reported to play a major role in the promotion of apoptosis in gastric adenocarcinoma cells [53]. Cell viability was affected at concentrations between 12.2–24.4 μM of α-mangostin in BGC-823 and SGC-7901 cells and decreases in mitochondrial membrane potential, indicating mitochondrial disfunction, were observed. α-Mangostin also inhibited the phosphorylation of STAT3 and levels of Bcl-xL and Mcl-1, two anti-apoptotic proteins downstream of STAT3 [53].
Pancreatic cancer
The phosphorylation of STAT3 was also decreased by α-mangostin in PANC1 pancreatic cancer cells and the DNA binding activity of STAT3 was decreased after α-mangostin treatment [54]. NF-κB and the levels of reactive oxygen species were also decreased in PANC1 cells treated with α-mangostin [54]. Though none of these studies reported on the effect α-mangostin had on the expression of STAT3 itself, it appears that targets both up- and downstream of STAT3 were modulated by α-mangostin in hepatocellular carcinoma, gastric adenocarcinoma, and pancreatic cancer cells. Multiple STAT3 inhibitors are being evaluating in clinical trials with cancer immunotherapies, suggesting the importance of STAT3 as a target in cancer and emphasizing the significance that α-mangostin can modulate the STAT3 signaling pathway in vitro [51].
Reactive oxygen species
Lung cancer
G. mangostana xanthones have been reported to exhibit antioxidant activity in multiple studies, making their regulation of ROS another likely mechanism for their anti-cancer activities [13]. In non-small scale lung cancer cells, α-mangostin induced apoptosis in A549 cells but not in non-cancerous WI-38 and hPBMC cells [55]. Similarly, cell death was not observed in non-cancerous lung fibroblast cells while the IC50 of α-mangostin was calculated to be ~10 μM in A549 lung cancer cells. ROS were generated at a rate 6-fold higher in A549 cells treated with α-mangostin as compared to untreated cells and Zhang, Yu, and Shen were able to confirm ROS generation as the primary mechanism of α-mangostin-induced apoptosis using an antioxidant in combination with α-mangostin [55]. In A549 lung cancer cells, 14.6 μM of α-mangostin stimulated significant cell death and increased the intracellular accumulation of ROS as assessed by a fluorescent probe and flow cytometry [56]. Data showed that α-mangostin decreased mitochondrial membrane integrity and inhibited NAMPT signaling, thus depleting NAD production, upregulating ROS production, and promoting apoptosis [56].
Cervical cancer
α-Mangostin significantly decreased cell viability in HeLa and SiHa cervical cancer cells at concentrations ranging from 10–50 μM and induced apoptosis by increasing cleaved-PARP, -caspase-9, and -caspase-3 levels [57]. ROS levels were dramatically increased after treatments with α-mangostin and were determined to play an essential role in the phosphorylation of p38 and cleavage of caspases-3 and −9 and PARP, as well as in the promotion of apoptosis in cervical cancer cells [57].
Osteosarcoma
The viability of osteosarcoma cells but not normal hFOB osteoblasts was inhibited by treatments with α-mangostin in 143B and Saos-2 cells [58]. Yang et al reported that α-mangostin induced apoptosis, induced ER stress, and increased ROS levels associated with apoptosis and ER stress in osteosarcoma cells [58].
Breast cancer
Scolamiero et al generated multicellular tumor spheroids from breast cancer cell lines MDA-MD-231 and MCF-7 and reported that 1.70–3 μM of α-mangostin decreased viability, migration, and spheroid size, which was hypothesized to be due to decreases in oxidative stress and NF-κB machinery [59].
Ovarian cancer
In OVCAR-3 ovarian cancer cells, α-mangostin modulated both ROS levels and PI3K/AKT signaling, by increasing intracellular ROS levels and decreasing the phosphorylation of PI3K, AKT, and m-TOR [60]. Dose dependent concentrations of α-mangostin were seen to significantly decrease OVCAR-3 cell viability after 12, 24, and 48 hours, apoptosis was induced, and cell migration and colony formation were decreased by α-mangostin [60].
Pancreatic cancer
Another study induced hypoxia in pancreatic stellate cells to drive the induction of ROS and found that α-mangostin significantly reduced ROS levels, reduced IL-6, VEGF-A, and SDF-1 levels, and decreased cell viability of these cells [61]. α-Mangostin treatments also decreased the invasive and metastatic potential of Panc-1 pancreatic cancer cells, which are commonly associated with increased ROS levels [61]. While the relationship between ROS levels and α-mangostin reported by Lei et al were different than the previously mentioned studies, this may be attributed to the fact that pancreatic stellate cells derived from normal pancreas tissues were used instead of fully differentiated cancer cells and that increased ROS levels were induced prior to α-mangostin treatments since the cells were cultured under hypoxia conditions.
Cyclin dependent kinases
Prostate cancer
Cyclin dependent kinases (CDKs) have also been proposed as a target of α-mangostin in multiple studies performed in various cancers. α-Mangostin displayed inhibitory effects in four different prostate cancer cell lines (LNCaP, 22Rv1, DU145, and PC3) at concentrations of 5.9, 6.9, 22.5 and 12.7 μM, respectively [62]. Further experiments were conducted in 22Rν1 and PC3 cells and reported that α-mangostin inhibited colony formation, promoted cell cycle arrest in the G1 phase, and promoted apoptosis. Cell free and in vitro Western blot analyses revealed that α-mangostin inhibited cyclinD1/CDK4 and decreased the phosphorylation of retinoblastoma (p-Rb) protein, a key signaling and regulatory protein downstream of CDK4 [62].
Colon cancer
p-Rb and cyclinD1 were also seen to decrease in response to α-mangostin treatments in HCT 116 colon cancer cells, where G1 cell cycle arrest had occurred, and the proliferation of cells was significantly impacted [63]. The activation of p38α was attributed to the cytostatic effect α-mangostin had on HCT 116 cells [63].
Oral squamous carcinoma
α-Mangostin affected the viability of HSC-2, HSC-3, and HSC-4 oral squamous carcinoma cells at concentrations between 8–10 μM, induced apoptosis through mitochondrial dysfunction and caspase activation, and promoted G1 cell cycle arrest [64]. Further experimentation revealed that levels of CDKs 2 and 4 as well as their corresponding cyclin complex proteins cyclins E and D were decreased by α-mangostin treatments, both of which are CDK/cyclin complexes that are integral for the progression of the G1 phase [64].
Breast cancer
MDA-MB-231 breast cancer cells showed reduced viability in response to treatments of >12 μM α-mangostin and underwent apoptosis and G1 cell cycle arrest [65]. An increase in CHEK2, a cell cycle checkpoint regulator, was reported after α-mangostin treatment, while decreases in cyclin D1 and proliferating cell nuclear antigen (PCNA) were reported [65]. CDKs play integral roles in the proliferation of normal cells and are typically upregulated in many cancers, which has led to the development and FDA-approval of multiple CDK inhibitors for the treatment of breast cancer, though CDK inhibitors are also being investigated for their use in melanoma and multiple leukemias [66].
Cell-free assays
Both CDK 2 and CDK 4 are integrally involved in the G1 cell cycle checkpoint, therefore it is reasonable to hypothesize that the apoptotic effect that α-mangostin has on different cancer cell lines may be due to the inhibition of these CDKs [67]. Recently, our lab has published results reporting that α-mangostin inhibits CDKs 2 and 4 in cell free assays and has speculated that this inhibition is due to the isoprenyl and methoxy groups present at positions 2’ and 8’, respectively [6, 16]. Multiple xanthones from G. mangostana were evaluated in these studies for their anticancer and CDK inhibitory activities, leading us to believe that α-mangostin and other isoprenylated xanthones are promising anticancer compounds that may target CDK’s as well as the aforementioned STAT3 and ROS, PI3K/AKT, MAPK, and AMPK signaling pathways.
2.3. In vivo studies
Xenograft studies
Colon cancer
Multiple in vivo studies have reported the antitumor and anti-angiogenic effects of α-mangostin. To our knowledge, only one study has been conducted using rats to evaluate the in vivo efficacy of α-mangostin, while most other studies have been conducted in mice [68]. Nabandith et al stimulated preneoplastic colon lesions in rats with 1,2-dimethyldrazine and reported that rats who were fed diets containing either 0.02% or 0.05% α-mangostin developed significantly less aberrant crypt foci and dysplastic foci than the control group, both of which are markers of carcinogenic tissue progression [68]. Rats in this study did not develop tumors, even 5 weeks after lesions were stimulated, suggesting that α-mangostin has promise as a chemoprevention agent but will need to be evaluated in longer studies with this model to confirm this [68]. Mice xenografted with CT26 colon cancer cells displayed significantly smaller tumors after 20 mg/kg α-mangostin orally administered daily compared to control mice [69]. Autophagy was induced in vivo in α-mangostin-fed mice and was measured by observing puncta formation in transgenic mice with confocal microscopy. Kim et al confirmed that the promotion of autophagy was key to the mechanism of reducing tumor volumes [69]. Another colon cancer study in mice reported that mice xenografted with HT-29 cells whose diets were supplemented with 900 mg α-mangostin grew tumors that were 41% and 36% smaller in volume and 27% and 41% smaller in mass after 2 and 4 weeks, respectively [70]. Tumorigenic biomarkers BcL-2 and β-catenin decreased significantly in the tumor tissues of mice fed α-mangostin [70].
Glioblastoma
In nude mice subcutaneously injected with GBM8401 glioblastoma cells, mice who were intraperitoneally injected with 2 mg/kg of α-mangostin daily grew 50% smaller tumors than control mice [38]. An increase in the phosphorylation of AMPK in tumors was also reported [38].
Tongue mucoepidermoid carcinoma
Nude mice xenografted with YD-15 cells were intraperitoneally injected with α-mangostin and developed tumors that had significantly decreased volumes and weights and displayed increased rates of apoptosis after immunohistochemical analysis [36].
Hepatocellular carcinoma
Zhang et al continued their study of STAT3 and hepatocellular carcinoma and observed decreased tumor weights, volumes, and growth times in nude mice implanted with HepG2 or SK-Hep-1 cells that were injected with 50 mg/kg α-mangostin daily [52]. Treated mice also displayed decreases in body weight, though no other pathological changes were observed, and immunohistochemistry of tumor tissues from treated mice revealed decreases in p-STAT3 protein levels [52].
Pancreatic cancer
In Hafeez et al’s study of STAT3 and pancreatic cancer, athymic nude mice inoculated with ASPC1 cells were intraperitoneally injected with 6 mg/kg α-mangostin [54]. Tumor volumes and weights were significantly reduced in the α-mangostin group and phosphorylation of STAT3 was also seen to decrease in pancreas tissues. Hafeez et al also performed an orthotopic xenograft study with primary human pancreatic cancer cells and found that mice treated with α-mangostin developed poorly nondifferentiated cancer, compared to the poorly differentiated cancer that the control mice developed, and grew tumors that weighed significantly less [54].
Osteosarcoma
Yang et al reported that mice xenografted with 143B osteosarcoma cells and injected with either 5 or 20 mg/kg α-mangostin suppressed tumor volumes and weights in a dose dependent manner [58]. Apoptosis and ER stress were induced in vivo as seen by the upregulation of cleaved-caspases-3/8 and ER stress proteins CHOP and ATF6 in tumors from treated mice [58].
Breast cancer
A metastatic mammary adenocarcinoma cell line that includes a p53 mutation that was designated BJMC3879luc2 was injected into female BALC/c mice that were exposed to either 0, 10, or 20 mg/kg/day α-mangostin through mini-osmotic pumps for six weeks [71]. Tumor volumes significantly decreased, mouse survival rates improved, angiogenesis decreased, and metastasis appeared less pronounced in the 10 and 20 mg/kg/day groups. In vivo phosphorylation of AKT in mammary tissues was decreased in the 20 mg/kg/day group [71].
Skin cancer
In a skin cancer mouse model induced by exposure to DMBA, female ICR mice were divided into control or 5 or 20 mg/kg α-mangostin groups and received daily intraperitoneal injections [72]. Treated groups showed significant inhibition of tumorigenesis, significantly decreased tumor incidence, and decreased skin hyperplasia [72]. Apoptosis and autophagy were induced and the phosphorylation of PI3K, AKT, and mTOR increased in tissues from the treated group, which was accompanied by an increase in anti-inflammatory factors and a decrease in pro-inflammatory factors [72].
Cervical cancer
Two different studies have evaluated the effect of α-mangostin in cervical cancer mouse models. Lee et al determined that the tumors in HeLa cell-inoculated nude mice were significantly smaller in size in mice that had been intraperitoneally injected with 20 or 40 mg/kg α-mangostin [57]. Pérez-Rojas et al showed that α-mangostin in combination with cisplatin was more effective than either agent individually at reducing tumor size in female BALB/c nude mice inoculated with HeLa cells [73].
Prostate cancer
Male athymic nude mice were xenografted with 22Rν1 prostate cancer cells and received 100 mg/kg α-mangostin by oral gavage and grew tumors that were 65% smaller than control mice [62]. Our group also performed a xenograft using 22Rν1 cells in athymic nude mice and found that mice that were orally administered different amounts of α-mangostin grew dose-dependently smaller tumors with increasing doses of α-mangostin [41].
Pharmacokinetics
The pharmacokinetic and ADME properties of any chemical compound are important in determining a safe and efficacious dose in the clinic. Han et al performed a pharmacokinetic study using single 5, 10, or 20 mg/kg doses of either orally or intramuscularly injected α-mangostin in ICR mice and determined the doses to be non-toxic and that they displayed dose-independent pharmacokinetics [74]. The major route of metabolism was reported to be non-renal and tissue distribution studies showed that α-mangostin had a high affinity for the liver, small intestine, fat, and lungs. Preliminary studies using MS/MS suggested that the major metabolites of α-mangostin are glucuronides and bis-glucuronides and hydrogenated, dehydrogenated, methylated, and oxidized conjugates [74]. Chitchumroonchokchai et al studied the distribution of xanthones and xanthone-metabolites in mice tissues after incorporating 900 mg/kg α-mangostin into their diets: α-mangostin was detected in mouse tumor tissues by LC-MS to be present in the highest amount, though multiple other xanthones were detected [70]. This is not surprising considering that the test diets were formulated with 94% α-mangostin and 6% other G. mangostana xanthones, though β-mangostin was also detected in a high quantity in tissue samples. α-Mangostin and other xanthones, as well as conjugated forms of α- and β-mangostin were still detectable in liver tissues and fecal pellets 2 and 4 weeks after feeding mice the formulated diets [70].
In a study using Wistar rats, a concentration-time profile of intravenously administered α-mangostin (2 mg/kg) was developed and determined its metabolism to be biphasic with a half-life of 2.97 h and plasma clearance of 1.54 L/h/kg [75]. Further experiments administered an oral dose of 20 mg/kg to the same rats after a washout period but could not quantify any levels of α-mangostin after 10 minutes; they posit that α-mangostin is not orally bioavailable in rats and undergoes extensive first-pass metabolism [75]. A study simulating the digestion of xanthones showed that α-mangostin was detectable in a significantly higher quantity before it had undergone small intestinal digestion [76]. Follow up experiments with Caco-2 cells confirmed that α-mangostin was mainly metabolized by Phase II metabolism enzymes, that its accumulation was proportional to the amount of media used in the experiment, that the uptake of α-mangostin was at a maximum after 1 h, and that transport of α-mangostin conjugates was more efficient across an apical membrane than across a basolateral membrane [76].
Our lab conducted a pharmacokinetic study with male C57BL/6 mice and detected levels of α-mangostin that had been administered as single 100 mg/kg oral doses for up to 24 hours [77]. The area under the curve was found to be 5,736 nmol/L/hr and the maximum plasma concentration was 1,382 nmol/L. We also detected monoglucuronide and diglucuronide metabolites of α-mangostin in plasma [77]. In another study, we reported that phase II metabolism accounts for the primary metabolism of α-mangostin in human and mouse microsomes and that the maximum concentration (Cmax) after a 35 mg/kg dose of α-mangostin in mice was 7.26 μM (similar to the dose that achieved biological effects in vitro), the time taken to reach Cmax (Tmax) was 1 h, and the area under the curve (AUC) was 8.14 μM m/mL [41]. Taken together, these studies provide evidence that α-mangostin has a good pharmacokinetic profile in mice and rats and that the doses of α-mangostin that exhibited anticancer effects in vitro and in vivo are achievable concentrations in plasma.
3.1. Less abundant xanthones
3.2. In vitro studies
Due to the limited availability of less abundant G. mangostana xanthones, fewer studies have been carried out with xanthones other than α-mangostin. Gartanin, β-mangostin, and γ-mangostin, the next most abundant xanthones, have been reported to have significant anticancer activity that is, in some cases, even more potent than that of α-mangostin [78, 79].
Gartanin
Upon testing 7 different isoprenylated xanthones in three malignant glioma cell lines, gartanin appeared to the most potent of the 7 and significantly affected cell viability at 10 μM in T98G, U87, and U251 glioma cells [80]. Gartanin did not appear to be cytotoxic in T98G cells, but decreased colony formation, caused G0/G1 cell cycle arrest, decreased migration, and induced autophagy through the inhibition of ERK and p38 phosphorylation [80]. In a study that investigated the effects of gartanin and α-mangostin against 7 different bladder cancer cell lines, gartanin was found to be the most potent in almost all 7 cell lines with IC50s ranging from 4.1–18.1 μM and from 7.0–9.8 μM for α-mangostin treatments [81]. It was emphasized that the 7 different cell lines tested had different genetic makeups and that α-mangostin and gartanin may promote non-specific cell death. The slight differences in IC50s may be explained by the structural differences between gartanin and α-mangostin: gartanin contains 2 hydroxyl groups where α-mangostin contains 1 methoxy and 1 hydroxyl group on the A ring, which may allow gartanin to form quinones in vitro, as our lab suggested in recent structure activity relationship papers [6, 16]. Liu et al continued their study on bladder cancer and reported that gartanin inhibited the mTOR pathway and induced both autophagy and apoptosis in multiple bladder cancer cell lines [81]. Gartanin inhibited the proliferation of Hep3B, HepG2, and Huh7 hepatocellular carcinoma cells at concentrations between 10–40 μM and promoted both autophagy and apoptosis, though the apoptotic effect appeared to be enhanced when autophagy was inhibited [82]. This may be significant as it has recently been shown that inhibiting autophagy in cancer cells may chemosensitize them, and a combination treatment of an autophagy inhibitor and gartanin may represent a novel treatment approach [83]. In a study looking at the effect of gartanin on the NEDDylation pathway in prostate cancer cells, it was reported that gartanin affected cell viability at a concentration of 8.32 μM in 22Rν1 cells and at 13.56 μM in PC3 cells, inhibited cellular NEDDylation, and induced autophagy [84]. Our lab has published data showing that gartanin decreases prostate cancer cell viability at concentrations of 14.9 μM in 22Rv1 cells and at 15.3 μM in LNCaP cells [85]. We determined that gartanin promoted apoptosis and targeted prostate cancer cells by interacting with and downregulating the expression of the androgen receptor while modulating ER stress proteins and chaperones [85].
β-Mangostin
β-Mangostin was identified as the strongest inhibitor of mammalian DNA polymerases and topoisomerases out of 8 xanthones isolated from G. mangostana leaves, both of which are common targets in cancer cells [86]. Further studies showed that β-mangostin specifically bound to DNA polymerases and topoisomerases but not DNA itself and that β-mangostin was also the most potent xanthone out of the 8 tested against HeLa cervical cancer cells with an IC50 of 27.2 μM [86]. Against glioma cell lines C6, U251, and T89G, β-mangostin affected cell viability at IC50s of 4.8, 5.8, and 10.3 μM [87]. β-Mangostin induced cell cycle arrest in the G1 and S phases, decreased colony formation and cell proliferation in a cell scratch assay, and induced apoptosis in C6 cells. Also, mitochondrial functions were impaired, intracellular levels of ROS were increased, and decreases in the levels of phosphorylated PI3K, AKT, and mTOR were observed, suggesting that β-mangostin affected glioma cell proliferation by inhibiting these signaling pathways [87]. In hepatocellular cells, β-mangostin was not found to be cytotoxic or induce cell cycle arrest in SK-Hep-1, Huh-7, or HA22T/VGH cell lines after 24 and 48 h treatments ranging from 0–10 μM, though cell migration and invasion were both inhibited [88]. The protein and mRNA expression levels of MMP-2 and MMP-9 decreased after β-mangostin treatments, which corresponded to the observed induction and activation of the ERK1/2 and JNK1/2 pathways [88]. Similarly, β-mangostin did not affect the cell viability of or cell cycle progression in HeLa and SiHa cervical cancer cells, but did inhibit cell migration and invasion [89]. The decrease in cell mobility was attributed to the inhibition of Snail and integrin αV/β3, proteins that are critically involved in cell mobility signaling, and was also attributed to the inhibition of the JNK pathway [89].
γ-Mangostin
U87 MG and GBM 8401 glioblastoma cell lines are representative of severe, high-grade cancer cells and were evaluated with γ-mangostin, which affected their cell viability in a dose dependent manner with IC50s of 74 and 64 μM, respectively [90]. γ-Mangostin induced apoptosis, increased ROS production, and induced mitochondrial dysfunction [90]. Balunas et al tested 2 different G. mangostana extracts and 12 pure xanthones for their aromatase inhibition and reported that γ-mangostin had strong inhibitory activity against aromatase in a microsomal activity and was cytotoxic to SK-BR-3 breast cancer cells that contain aromatase with an IC50 of 4.97 μM [91]. In a study using normal liver and hepatocellular carcinoma cells (Chang liver and HepG2 cells), both α-mangostin and γ-mangostin were tested for their effect on cell proliferation and Chang, Wu, and Yang determined that α-mangostin was toxic to both normal and cancer cells whereas γ-mangostin only affected proliferation of HepG2 cancer cells [92]. γ-Mangostin induced apoptosis and mitochondrial dysfunction, likely through a combination of an increase in ROS levels and free radical scavenging abilities [92]. In HT-29 colon cancer cells, γ-mangostin decreased cell viability most significantly at concentrations below 40 μM with an estimated IC50 of 68 μM [93]. γ-Mangostin promoted apoptosis in HT-29 cells by increasing ROS levels and inducing mitochondrial dysfunction [93]. Another study evaluated the effects of γ-mangostin on colon cancer cells and reported IC50 values to be between 10–15 μM in HCT116, SW480, RKO, HT29, DLD1, and LS174T cells [94]. The differences in reported IC50 values between this study and the prior colon cancer study may be due to the differences in treatment times with γ-mangostin, which were, respectively, 48 and 24 hours [93, 94]. Krishnamachary et al performed mechanistic studies with HCT116 and LS174T cells and reported that γ-mangostin induced apoptosis, induced cell cycle arrest in the G0/G1 phases, and inhibited the Wnt/β-catenin pathway, a hyperactivated signaling network in colon cancer, likely either by degrading TCF-4 and β-catenin or by binding to TCF-4 [94, 95]. In pancreatic cancer cell lines MIA PaCa-2 and PANC-1, γ-mangostin affected cell viability with IC50s 11.7 and 25 μM after 48 hours and 4.2 and 10.2 μM after 72 hours, respectively [96]. Cell death was promoted in both cell lines as evidenced by increases in pro-apoptosis and pro-autophagy protein expression levels as well as increases in levels of miRNAs involved in apoptosis, including miR-18a. Co-treatments with γ-mangostin and gemcitabine, a commonly used chemotherapy agent for pancreatic cancer, affected cell viability more strongly than gemcitabine treatments alone, indicating that γ-mangostin may sensitize pancreatic cancer cells to treatment [96].
Garcinone C
In a study of the Hedgehog pathway, a dysregulated signaling pathway in colon cancer, Garcinone C inhibited HT29 and HCT116 cell colony formation and induced cell cycle arrest at the G0/G1 phases [97]. Gl1, a key target involved in the Hedgehog signaling pathway, was blocked on a transcriptional level by Garcinone C, and downstream targets were also shown to be inhibited. The phosphorylation of AKT, an upstream target of Gl1, was also inhibited by Garcinone C [97]. Garcinone C has been isolated not only from G. mangostana but also from Garcinia oblongifolia and Xia et al used Garcinone C that had been extracted from G. oblongifolia to treat nasopharyngeal cancer cells [98]. In CNE1, CNE2, HK1 and HONE1 cells, the calculated IC50s of Garcinone C were 0.68, 13.24, 9.71, and 8.99 μM, respectively. Garcinone C reduced cell colony formation and induced apoptosis and necrosis through S phase cell cycle arrest, downregulation of cell cycle-related proteins, and inhibition of STAT3 [98].
Garcinone E
Xu et al reported the IC50s of 24 different G. mangostana xanthones in HEY ovarian cancer cells and reported that Garcinone E had one of the most potent effects on cell viability [99]. Further mechanistic studies were carried out using Garcinone E in HEY, A2780, and A2780/Taxol (a paclitaxel-resistant line) cells and revealed that Garcinone E was able to induce apoptosis in all three cell lines, demonstrating that Garcinone E may be able to overcome multidrug-resistant cells. Garcinone E also induced ER stress, specifically by activating the IRE-1α pathway, and showed potential to inhibit ovarian cancer metastasis by inhibiting migration and cell invasion [99]. In a study of autophagy in ovarian cancer cells, Garcinone E was shown to inhibit autophagy in HEY cells by increasing the levels of LC3-II and p62 while impairing normal lysosomal functions and blocking autophagosome-lysosome binding [100]. In HeLa cervical cancer cells, Garcinone E reduced cell proliferation in a time and dose dependent manner, but not in normal cervical cells, and showed half maximal inhibitory activity around concentrations of 32 μM [101]. Garcinone E inhibited colony formation, induced apoptosis and cell cycle arrest at the G2/M phase, and inhibited cell migration and invasion of HeLa cells [101]. Garcinone E affected HSC-4 oral cancer cells at an IC50 of 4.8 μM and induced apoptosis [101]. Microscopy and cell staining experiments revealed that Garcinone E-treated cells grew smaller and fewer cell colonies and inhibited cell migration and invasion but did not show any changes in cell adhesion. The protein expression levels of IL-6, MMP-2, and MMP-9 were all increased in response to Garcinone E, while the level of IL-2 was decreased, indicating a potential for Garcinone E to promote antiinvasive activity and modulate inflammation in oral cancer cells [101]. 10 μM of Garcinone E was also tested in 14 different cancer cell lines and found affect proliferation by 50% in 13 of the cell lines (Hep3B, HCC36, TONG, HA22T, HepG2, SK-Hep-1, NCI-Hut 125, H2891, Calu-1, AZ521, NUGC-3, KATO-III, and AGC) [102]. While testing the cytotoxicity of Garcinone E and several hepatoma chemotherapeutic agents, it was determined that Taxol was the most potent drug and that Garcinone E had similar or more potent effects than mitoxantrone, methothrexate, vincristine, 5-Fu, and cisplatin in Hep G2, TONG, Hep 3B, and SK-HEp-1 cells. No mechanism was suggested in this study, though no changes in the cell cycle in response to Garcinone E treatments were observed [102].
3.3. In vivo studies
The limited availability of less abundant xanthones has made evaluating their efficacy in vivo difficult. To date, there have been 4 in vivo studies performed with single, isolated G. mangostana xanthones: β-mangostin in a glioma animal model [87], γ-mangostin in a colon cancer animal model [94], Garcinone C in a colon cancer animal model [103], and mangosenone F in a lung cancer animal model [104]. Male BALB/c nude mice xenografted with C6 glioma cells grew tumors that were significantly smaller in mice treated with β-mangostin (tail injections of 5 mg/kg) than in control mice and histopathological analysis revealed that tumor tissues contained decreased levels of caspase-3 and Nrf2 [87]. γ-Mangostin treatments injected intraperitoneally at 5 mg/kg daily for 21 days in male athymic nude mice harboring HCT-116 xenografts significantly decreased tumor sizes and displayed decreased levels of TCF4, β-catenin, Cyclin D1, and c-myc [87]. In an AOM/DSS-induced colon tumorigenesis mouse using male C57/BL6 mice, low and high doses of Garcinone C were injected intraperitoneally at 10 and 50 mg/kg [103]. Tumor incidence in both Garcinone C groups were significantly decreased and the expression of cell cycle proteins cyclin D1, cyclin E, and CDK6 as well as phosphorylated AKT and Gli1 were decreased [103]. Mangosenone F, a unique G. mangostana furanoxanthone with very little known about it, showed moderate cytotoxic in vitro activity against both melanoma and lung cancer cell lines and decreased tumor sizes in a lung xenograft study where NCI-H460 lung cancer cells were injected into nude mice [105] [104]. Mangosenone F was injected into mice at 1 mg/kg and 3 mg/kg and resulted in an 80% reduction in tumor volume and a 70% reduction in tumor weight [104].
4.1. Mangosteen extract
Due to the traditional use of the mangosteen fruit, leaves, and seeds for their medicinal benefits, there is a large interest in understanding the anticancer and antitumor activities of extracts from different parts of the plant. The most well-developed research has included extracts from the mangosteen pericarp, the outer white flesh of the fruit that is typically consumed, though recently there has been more interest in using extracts from the roots, rind, and stem.
4.2. In vitro studies
An extract from the mangosteen pericarp was tested in MCF-7 breast cancer cells and was reported to be cytotoxic to cells at a concentration of 45 μg/mL [106]. The pericarp extract induced apoptosis in cells and was evaluated for its disruption of ERα but was not reported to have any effect on its expression in an immunocytochemistry assay [106]. Both an ethanolic and methanolic extract from the pericarp of the mangosteen affected the viability of SK-BR-3 breast cancer cells with IC50s of 15 and 9.25 μg/mL, respectively [107, 108]. The methanolic extract changed cell morphology, promoted apoptosis, and increased ROS levels [108]. Another pericarp extract was reported to be cytotoxic to HepG2 hepatocarcinoma cell lines under normoxia and hypoxia conditions at concentrations of 50 and 109 μg/mL and was also evaluated in a zebrafish embryo model where it was reported to affect the survivability and development of the embryos [109]. HeLa and H357 cells, cervical and oral cancer cells, respectively, both showed decreases in proliferation and showed evidence that apoptosis was induced after treatments with a mangosteen pericarp extract [110]. The proliferation of A-431 and SK-MEL-28 skin cancer cells were significantly affected by treatments with a mangosteen pericarp extract at IC50s of 5.07 and 6.89 μg/mL [111]. Morphological differences were detected, the cell cycle was arrested at the G1 phase, apoptosis was induced, and the mRNA levels of cell cycle- and proliferation-related genes were decreased in cells treated with mangosteen extract [111].
Two different assays showed that a mangosteen pericarp extract affected NL-17 colon cancer cells by decreasing their proliferation and causing cell membrane damage with IC50s of 17 and 84 μg/mL in a WST-1 cytotoxicity assay and an LDH leakage assay, respectively [112]. In COLO 205, CX-1, MIP-101, and SW620 colon cancer cell lines, a mangosteen pericarp extract had anti-proliferative effects at IC50s of 7.5, 17.7, 10, and 16.1 μg/mL [113]. The extract induced apoptosis in colon cancer cells as seen by changes in their morphology, activation of caspases 3 and 8, and a release of cytochrome c into the cytosol after treatment [113]. Two other studies evaluated a mangosteen extract in colon cancer cells and used an extract from the rind of the fruit and HCT 116 colon cancer cells: IC50s of 9.2 and 6.5 μg/mL were reported to decrease cell viability [114]. Apoptosis was also induced via increases in caspase-3/7 activity, DNA fragmentation, loss of mitochondrial membrane potential, and the fragmentation and condensation of chromatin in extract-treated cells [115]. Considering Aisha et al detected and identified 5 xanthones in their root extract while Watanapokasin detected and identified 2 in their pericarp extract, it may be interesting to compare the phytochemical compositions of different G. mangostana extracts in the future to better understand the synergism of various xanthones involved in the promotion of apoptosis in colon cancer cells [113, 115]. There is also data showing that extracts from the root and stem bark of the mangosteen have anticancer properties against T-cell leukemia cell line CEM-SS (IC50s were 0.3, 14, and 17 μg/mL in a hexane stem bark extract, a hexane root bark extract, and a chloroform root bark extract, respectively) in which 6 different xanthones were identified, showing that extracts from many different parts of the mangosteen plant may have anticancer activity [116]. Our lab has published results showing that a mangosteen fruit extract (Avesthagen, Inc., Chatsworth, CA) promotes apoptosis in prostate cancer cells by upregulating ER stress: mangosteen fruit extract reduced the viability and proliferation of 22Rν1 and LNCaP cells, increased cleaved-caspase-3, and promoted apoptosis [41, 117]. ER stress specific proteins involved in the unfolded protein response pathway, including PERK, IRE1, CHOP, cleaved-caspase-4, spliced XBP-1, and ER chaperone proteins, were upregulated in response to mangosteen fruit extract, an effect that was observed specifically in cancer cells and not in non-cancerous prostate epithelial cells [117]. A treatment of α-mangostin that was thought to be an equivalent concentration to the amount of α-mangostin contained in the extract also upregulated some of these proteins, but the extract had a stronger effect in upregulating the expression of ER stress proteins in prostate cancer cells, providing evidence that there may be synergy amongst mangosteen xanthones in an extract [41].
4.3. In vivo studies
Out of the limited in vivo studies that have been conducted using mangosteen extracts, there have been consistent results showing the antitumor activity of mangosteen extracts in colon cancer. In a subcutaneous tumor model using COLO 205 colon cancer cells in male NCr nude mice, mangosteen pericarp extracts were administered intratumorally and initially decreased the tumor size and then stunted the re-growth of tumors in a dose dependent manner [113]. Mice administered extract also showed increased expressions of cleaved-caspases-3 and −8, promoting apoptosis in vivo [113]. Athymic NCR nude mice subcutaneously injected with HCT 116 colon cancer cells were given mangosteen rind extract mixed into their food and developed significantly reduced tumors compared to control mice [115]. Mangosteen pericarp extract dose dependently decreased tumor sizes by 50–70% and increased the lifespan of female BALB/c mice implanted with NL-17 colon cancer cells in their left footpad and then intraperitoneally injected with extract [112]. Another pericarp extract decreased the weight and size of tumors in a cholangiocarcinoma hamster allograft model using male Syrian hamsters, intradermal injections of Ham-1 cells, and orally administered extract [118]. Tumor tissues also revealed that those treated with extract had decreased expressions of cytokeratin 19 and proliferating cell nuclear antigen, which both have a positive correlation with cholangiocarcinoma [118]. Though not specified to be an extract, Panaxanthone, composed of varying amounts of α-mangostin and γ-mangostin, was supplemented into the diets of female BALB/c mice with established BJMC3879 mammary adenocarcinoma tumors [119]. Panaxanthone reduced tumor size, reduced cell proliferation and induced apoptosis via caspase signaling, and reduced lung and lymph node metastasis [119]. Our lab showed that a mangosteen pericarp extract that was intraperitoneally administered reduced the tumor size of nude mice xenografted with 22Rν1 prostate cancer cells by 88% [117].
A pharmacokinetic and metabolism study compared pure α-mangostin and mangosteen fruit extract (containing ~15% α-mangostin) in male ICR mice and reported that the extract was more tolerable than pure α-mangostin with LD50s of 231 and 150 mg/kg, respectively, that extract had a higher AUC and slower clearance than α-mangostin when injected intravenously, and that α-mangostin was detected in plasma more quickly after oral doses of both α-mangostin and mangosteen extract, suggesting it can be absorbed in the GI tract as both a pure compound and as part of an extract [120]. Evaluating the distribution of α-mangostin revealed that its concentration was high in the liver, small intestine, kidney, and lung when pure α-mangostin was intravenously delivered and that there were increases in the liver, stomach, spleen, brain, and heart and decreases in the small intestine, large intestine, mesentery, kidney, and lung when extract was intravenously delivered [120]. In male Sprague Dawley rats, a pharmacokinetic study was performed with a mangosteen pericarp extract after validating a method by analyzing both an intravenous and oral administration of α-mangostin and then γ-mangostin [121]. Based on those results, a concentration of 160 mg/kg extract was orally administered (equivalent to 20 mg/kg α-mangostin and 4.5 mg γ-mangostin) to rats and was reported to be metabolized and eliminated in one phase instead of the two phases that were observed with single compound administrations. The half-life of α-mangostin and γ-mangostin were much shorter when administered as part of an extract than as single administration and the Tmax was much longer (2 hours compared to 1 hour) for compounds administered as part of an extract, suggesting the combination of xanthones in the mangosteen extract may inhibit the conjugation of major xanthones present in the extract (glucuronidation or sulfation) [121]. Based on our lab’s previous study on the pharmacokinetics of α-mangostin, we established a method to monitor the pharmacokinetics of α-mangostin and its conjugates in a mangosteen extract standardized to α-mangostin that was delivered to C57/BL6 mice [122]. We observed 5 possible glucuronates of α-mangostin, a 75% increase in the Cmax of α-mangostin when delivered as part of an extract, and a 25% increase in the AUC of α-mangostin when delivered as part of an extract, providing more evidence that the combination of mangosteen xanthones present in the extract effects how individual xanthones can be metabolized and that mangosteen extracts may be more potent and bioavailable anticancer agents than xanthones individually [122].
5. Clinical Safety and Efficacy
At present there have not been any clinical trials evaluating xanthones from the mangosteen in cancer patients, though there have been smaller pharmacokinetic studies in healthy volunteers. To evaluate the bioavailability and metabolism of mangosteen xanthones, a study monitored 10 participants that were given 60 mL of mangosteen juice and were fed relatively average nutrient-filled meals [123]. Over a 24-hour period, α-mangostin was the only xanthone to be detected in serum, where the average AUC was 1870 nmol/L x h for females and 1030 nmol/L x h for males, the average Cmax was 159 nmol/L for females and 66 nmol/L for males, and the average tmax was 3.8 h for females and 3.6 h for males. Multiple xanthones both conjugated and unconjugated were present in urine where the mean amount of xanthones was 6140 nmol/24 h in females and 5870 nmol/24 h in males and the ratio of free : conjugated xanthones was 49% : 51% in females and 58% : 42% in males [123]. In a randomized, double-blind, placebo-controlled study, 60 healthy males and females (30 and 30) were given 245 mL doses of either placebo (fructose liquid) or Verve energy drink containing mangosteen for 30 days [124]. The mangosteen group was reported to have a significantly higher peroxyl radical scavenging capacity (ORAC) of 630 μM compared to the 534 μM reported in the placebo group. This study also reported mangosteen significantly decreased in the level of C-reactive protein (CRP), an indicator of systemic inflammation, suggesting mangosteen provided protection against inflammation or that it reduced inflammation [124]. Another randomized, double-blind, placebo-controlled clinical trial was conducted using 20 healthy subjects who received 59 mL either Mangosteen Plus with Essential Minerals in the Vemma formulation or placebo liquid once and were monitored for 24 h [125]. There was a 16% increase in ORAC in the mangosteen group after 1 h and an 18% increase after 2 h that was continually observed until the study endpoint at 6 h. The Cmax of α-mangostin was 3.12 ng/mL with an observed tmax of 1 h. The levels of vitamins B2 and B5, both of which are essential for cell proliferation, were also monitored and were found to be significantly increased in the mangosteen group, with concentrations that were around double the values observed at 0 h before the treatment administration [125].
In a randomized, double-blind, placebo-controlled trial, 20 patients received 245 mL of either Verve liquid nutrition beverage containing mangosteen or placebo (fructose liquid) once and were monitored for 24 h [126]. A Cmax of 4.16 ng/mL of α-mangostin was observed at a tmax of 1 h and the levels of vitamins B2 and B5 were again monitored and found to increase most drastically at 1 h in the mangosteen group. The ORAC values of the mangosteen group increased by 60% at 1 h and then stayed at an increased level of 10% until the last time point at 6 h [126]. The immune function was reported to improve after administration of a mangosteen product in a randomized, double blind, placebo-controlled trial where subjects were given a 59 mL dose of either Mangosteen Plus with Essential Minerals in the Vemma Formulation or placebo (fructose liquid) once a day for 30 days [127]. Out of the 60 subjects, those who were given mangosteen had significantly increased levels of Th and DP cells (2.6% and 0.3% increases, respectively), IL-1α, and C3 and C4 complements, whereas there was a significant decrease in the levels of C-reactive protein, a marker of inflammation, though taken together these results indicated an improvement in immune function in the mangosteen group subjects [127]. Perhaps the longest study that has evaluated mangosteen products in humans was an 8-week randomized, double blind, placebo-controlled study where subjects were instructed to take 9 oz of their assigned liquid twice a day (containing either placebo, 3 oz XanGo Juice filled to 9 oz with placebo, 6 oz XanGo Juice filled to 9 oz with placebo, or 9 oz XanGo Juice) [128]. There was a significant decrease in the amount of high-sensitivity C-reactive protein in the mangosteen group compared to placebo and the levels of a few other markers of inflammation, including ENA-78, IP-10, IL-12p70, CCL5, and MIP-1 β, changed amongst individual treatment groups when comparing the 8-week measurement with the baseline measurement, though there were no significant differences in these levels when comparing them to placebo levels [128]. Overall, there is limited data regarding the anticancer activity of mangosteen products or xanthones in humans, though it is clear from the studies mentioned above that mangosteen products are safe and tolerable in humans, that xanthones are bioavailable, and that mangosteen products improve radical scavenging activity and immune function in humans, which may have indications for their anticancer activity.
7. Conclusions
Garcinia mangostana has long been used traditionally to treat a variety of health conditions and more recently has gained popularity as a juice or as an ingredient in health-promoting products. Mangosteen xanthones are reported to have multiple bioactivities and herein we have summarized the anticancer and antitumorigenic activities of mangosteen xanthones and extracts. There have been numerous studies evaluating α-mangostin, the most abundant mangosteen xanthone, suggesting that the likely mechanisms by which α-mangostin exerts its anticancer activity are by targeting specific proteins or cell signaling pathways, including CDKs, MMPs, and STAT3, or PI3K/AKT, AMPK, MAPK, and ROS signaling. α-Mangostin as well as other lesser xanthones including gartanin, β-mangostin, γ-mangostin, garcinone C, and garcinone E are all reported to induce apoptosis in various cancer cell lines, and some even promote cancer cell death in vivo. Mangosteen extracts have also been evaluated for their anticancer activity and may be even more potent than individual xanthones due to the synergy observed from the combination of xanthones present in the extracts, though further studies are required to validate these claims.
Overall, the evidence suggests that xanthones form the mangosteen are promising anticancer agents in in vitro and in vivo preclinical models. Mangosteen extracts containing xanthones appear to be safe and well-tolerated in several clinical trials. As there are limitations to the studies that have been performed to date, including small sample sizes used in animal studies and clinical trials, reproducibility of results within certain cell lines and animal models, and lack of information regarding the specific mangosteen extracts or xanthones used, future studies are warranted to address these concerns. Future studies should also focus on evaluating the anticancer capacity of G. mangostana either as individual xanthones or as extracts or mangosteen products in humans.
Table 1 –
in vitro activity of mangosteen xanthones
| Reference | Cell line | IC50 or concentration where change in viability was observed | α-Mangostin treatment for mechanistic studies | Targets |
|---|---|---|---|---|
| PI3K/AKT, AMPK, and MAPK | ||||
| [21] | T47D | 7.5 μM | 30 μM for 6 h | • P-p38, p-JNK, and pERK decreases • P-ERα decrease |
| [23] | MCF-7 | 5–10 μM | 1, 5, and 10 μM for 48 h | • Cytochrome c release • p53 increase • ERα decrease |
| [24] | MCF-7 | > 6 μM | 6 μM for 48 h | • Reversed the effect of TPA on invasion and migration • Decreased ERK, p-JNK, pERK, and MMPs-2/9 |
| [25] | MCF-7 and MDA-MB-231 | 2–3.5 μM | 4 μM for 24 h | • pAKT decrease • ERK increase • Fatty acid synthesis inhibited |
| [26, 27] | DLD-1 | 20 μM | 20 μM for 24 h | • Decreases in cyclin D1 • pAKT and p-p38 decreases |
| [28] | HCT 116 | 9.74 μM | 18.2 μM for 16 h | • Combination study with betulinic acid • Upregulation of p53 and MAPK pathways |
| [29] | COLO 205, MIP-101, and SW 620 | 23.7, 27.6, and 47.6 μM | 48.7 μM for 3 h | • p-p53 and Bax increases |
| [31] | SK-Hep-1 | 19.6–24.8 μM | 30 μM for 24 h | • P-p38 decrease |
| [32, 33] | HepG2surv | 23.74 μM | 10 μM for 24 h | • Combination study with Sorafenib • E-cadherin reduction • TGF-β1 and SMAD3 increases |
| [35] | SW1353 | 24.36 μM | 48.7 μM for 6 h | • pERK decrease |
| [36] | YD-15 | 10 μM | 15 μM for 24 h | • pERK and p-p38 decrease |
| [37] | SK-MEL-2 and SK- MEL-30 | 8.14 and 7.78 μM | 2 μM for 8 h | • Combination study with Sorafenib • pAKT and pERK decrease |
| [38] | GBM8401 and DBTRG-05MG | 6.4 and 7.3 μM | 10 μM for 12 h | • pAMPK increase |
| [39] | HL60 | 6.8 μM | 10 μM for 24 h | • Mitochondrial dysfunction • MAPK signaling increase |
| [40] | A-498 and 786-O | ≤12 μM | 12 μM for 24 h | • pERK decrease • MMP-9 decrease |
| [41] | 22Rv1 | 10 μM | 15 μM for 24 h | • pERK and p-pERK increases • ER stress signaling increase |
| Matrix metalloproteinases (MMPs) | ||||
| [42] | BxPC-3 and MIAPaCa | ≤5 μM-2 | 5 μM for 24 h | • MMP-2 and MMP-9 increases • E-cadherin decrease |
| [43] | BxPc-3, Panc-1 | 8μM | 8 μM for 24 h | • MMP-2 and MMP-9 decreases • pAKT decrease |
| [44] | B16-F10, SK-MEL-28 and A375 | 5, 10 and 15 μM | 15 μM for 24 h | • Increased melanin production • Decreased MMP-9 |
| [45, 46] | SK-MEL-28 and A-431 | 14.4 μM | 0–6.09 μM for 48 h | • G1 cell cycle arrest • Decreases in MMP-2, MMP-9, NF-κB, and AKT1 |
| [47] | A549 | 7.5–17.5 μM | 5 μM for 24 h | • MMP-2 and MMP-9 increases • pERK decrease • NF-κB decrease |
| [48] | PC3 | 12.2 μM | 12.2 μM for 24 h | • MMP-2 and MMP-9 decreases • pJNK decrease |
| [49, 50] | HN-22, HN-30, and HN-31 | 7.32 μM | 4.88 μM for 48 h | • MMP-2 and MMP-9 decreases • P53 increase |
| STAT3 | ||||
| [52] | SK-Hep-1, HepG2, SMMC-7721, and Huh-7 HCC | 9.44, 10.94, 13.22, and 14.49 μM | 20 μM for 24 h | • Cyclin D1 and c-myc decreases • pERK, pAKT, and p-STAT3 decreases |
| [53] | BGC-823 and SGC-7901 | 12.2–24.4 μM | 17.1 μM for 24 h | • p-STAT3, Bcl-xL, and Mcl-1 decreases |
| [54] | PANC1, BxPC3, and ASPC1 | 13–17 μM | 20–30 μM for 24 h | • ROS production decrease • NF-κB inhibition • P-STAT3 decrease |
| Reactive oxygen species (ROS) | ||||
| [55] | WI-38 and hPBMC | 10 μM | 10 μM for 24 h | • Increase in ROS generation |
| [57] | HeLa and SiHa | 10–50 μM | 30 μM for 24 h | • Increase in ROS production which increased p-p38 |
| [58] | 143B and Saos-2 | 20 μM | 30 μM for 24 h | • Increased ROS levels • Induced ER stress |
| [56] | A549 | 14.6 μM | 24.4 μM for 24 h | • Increased ROS production • Inhibited NAMPT signaling |
| [59] | MDA-MD-231 and MCF-7 spheroids | 1.70–3 μM | 36.5 μM for 48 h | • Decreased spheroid size due to oxidative stress upregulation |
| [60] | OVACAR-3 | 25 μM | 200 μM for 24 h | • ROS increase • Decreased p-PI3K, pAKT, and p-mTOR |
| [61] | Panc-1 | 16 μM | 16 μM for 24 h | • Decrease in hypoxia-induced ROS levels • Inhibits hypoxia-driven programs |
| Cyclin dependent kinases (CDKs) | ||||
| [62] | LNCaP, 22Rv1, DU145, | 5.9, 6.9, 22.5 and 12.7 μM | 15 μM for 48 h | • Decreased cyclinD1/CD K4 • Decreased p-Rb |
| [63] | HCT116 | 9.7 μM | 9.7 μM for 24 h | • G1 cell cycle arrest • Decreased cyclinD1 and p-Rb |
| [64] | HSC-2, HSC-3, and HSC-4 | 8–10 μM | 8 μM for 24 h | • CyclinE/CDK 2 and cyclinD1/CD K4 decreases |
| [65] | MDA-MB-231 | 12 μM | 20 μM for 24 h | • Cytochrome c release • p21cip1 and CHEK2 increases • Cyclin D1 decrease |
| [6, 16] | HCC 1937, HCT 116, 22Rv1, and MDA-MB-231 | 13.0, 23.4, 20.69, 19.52 μM | 0–40 μM | • CDK2 and CDK4 activities inhibited in cell free assay |
Table 2 –
in vivo activity of mangosteen xanthones
| Reference | Dose and route | Animal model (age in weeks) | Study type | In vivo efficacy |
|---|---|---|---|---|
| [68] | 0.02% and 0.05% crude α-mangostin diets |
Male F344 rats (4) | Colon preneoplastic lesions induced by subcutaneous injections of 1,2-dimethylhydrazine (40 mg/kg body weight) | • α-Mangostin groups developed lesions at a significantly slower rate • β-catenin accumulated crypts and dysplastic foci were significantly decreased in α-mangostin groups • The proliferating cell nuclear antigen (PCNA) labeling indices were dose-dependently decreased in • α-mangostin groups |
| [69] | 20 mg/kg administered orally 20 mg/kg administered orally for 3 days |
BALB/c mice (6) GFP-LC3 transgenic mice |
Subcutaneous injections of Her-2/CT26 colon carcinoma cells Evaluations of autophagy from LC3-II were made using confocal microscopy |
• Mice given α-mangostin had significantly smaller tumors • Autophagy was activated in the small intestine villi and crypt epithelial cells of mice given α-mangostin |
| [36] | 10 or 20 mg/kg dissolved in PBS administered 5 times per week intraperitoneally | Male BALB/c nude mice (5) | Subcutaneous injection of YD-15 oral squamous carcinoma cells | • Both α-mangostin groups had significantly smaller tumors in size and volume (89.9% smaller in the 20 mg/kg group) • Ki-67 protein expression was decreased in tumor tissues |
| [70] | 900 mg/kg α-mangostin supplemented in AIN-93G diets | Female athymic Balb/c mice (4) | Subcutaneous injection of HT-29 colon cells and serum metabolite study | • Tumor volumes were 41% and 36% smaller and tumor masses were 27% and 41% smaller in α-mangostin fed mice after 2 and 4 weeks • BcL-2 and β-catenin protein levels were decreased in tissues from α-mangostin mice • After incubating serum with enzymes, α-mangostin was found to be extensively conjugated and its total concentration was significantly greater in tumor-bearing mice vs nontumor-bearing mice |
| [38] | Intraperitoneal injections of 2 mg/kg α-mangostin in a 0.2 mL clear solution containing 25% polyethylene | Male nude BALB/cA-v (6) | Subcutaneous injections of GBM8401 brain cancer cells | • 50% tumor size reduction in mice injected with α-mangostin • Tumor tissues from treated mice had an increase in autophagocytic vacuoles, p-AMPK, and p-Raptor levels |
| [36] | Intraperitoneal injections of either 10 or 20 mg/kg α-mangostin | Male BALB/c nude mice (5) | Subcutaneous injections of YD-15 tongue mucoepidermoid carcinoma cells | • 89.9% reduction in tumor size in 20 mg/kg treated mice • Apoptotic cells were confirmed in tumor tissues from treated mice |
| [52] | Intraperitoneal injections of 50 mg/kg α-mangostin | Male BALB/c nude mice (6) | Subcutaneous injections of either HepG2 or SKHep-1 hepatocellular carcinoma cells | • Tumor weights and sizes of treated mice Were significantly less • Tumor tissues from treated mice showed a decrease of Bcl2 and p-STAT3 |
| [54] | 6 mg/kg of α-mangostin in 0.2 ml of PBS containing 25% polyethylene glycol (PEG) 6 mg/kg of α-mangostin in 0.2 ml of PBS containing 25% polyethylene glycol (PEG) |
Athymic nude mice (6) Athymic nude mice (6) |
Ectopic xenograft – subcutaneous injections of ASPC1 pancreatic cancer cells Orthotopic xenograft – PL-45 human primary pancreatic cancer cells were injected directly into the parenchyma of the pancreas after anesthetizing and incising the mice |
• The volume of ectopic tumors in treated mice were significantly lower • p-STAT3 and p-NF-κB protein levels were decreased in treated tumor tissues • Orthotopic xenograft tumors were significantly lighter in tumor weight in treated mice • α-Mangostin-treated mice only developed poorly nondifferentiated carcinoma |
| [58] | Intraperitoneal injections of 5 or 20 mg/kg α-mangostin (dissolved in olive oil) | Female athymic BALB/c nude mice (5) | Subcutaneous injections of 143B osteosarcoma cells | • Tumor volumes and weights were dose-dependently suppressed in treated groups • Cleaved-caspase-3/8 were upregulated in tissues from treated groups |
| [71] | Subcutaneously implanted mini-osmotic pumps delivered 0.5 μL solution (DMS0/100% ethanol (1:3, v/v) vehicle) per hour totaling 10 or 20 mg/kg α-mangostin per day | Female BALB/c mice | Subcutaneous injections of BJMC3879luc2 mammary carcinoma cells | • A statistically significant difference in survival rate was seen in the 20 mg/kg group • Tumor growth was significantly inhibited in both α-mangostin groups • There were significantly fewer metastasis-positive lymph nodes in the 20 mg/kg group • p-AKT-Thr308 levels were decreased in tumor tissues from the 20 mg/kg group |
| [72] | 5 mg/kg or 20 mg/kg α-mangostin dissolved in olive oil and injected intraperitoneally | Female ICR mice | DMBA (60 μg dissolved in 0.2 ml acetone) was topically applied to the shaved backs of mice, followed by a topical treatment of TPA (4 μg) one week later to induce skin tumorigenesis | • α-Mangostin-treated mice had significantly decreased skin tumor incidence rates and significantly reduced hyperplasia • In skin and tumor tissues of treated groups, there was inhibition of pro-inflammatory factors and promotion of anti-inflammatory factors • α-Mangostin promoted apoptosis and autophagy in vivo |
| [57] | Intraperitoneal injections of 20 or 40 mg/kg α-mangostin | Female nude BALC/c mice (5) | Subcutaneous injections of HeLa hepatocellular cells | • Treated groups showed decreases in tumor size, volume, and weights • Tumor tissues from treated groups showed increased levels of p-ASK1, p-p38, cleaved-PARP, and cleaved-caspase-3 |
| [73] | Orally administered α-mangostin (12.5 mg/kg suspended in 0.5% carboxymethyl-cellulose) alone or in combination with 3 mg/kg cisplatin | Female BALB/c mice | Subcutaneous injections of HeLa hepatocellular cells | • Tumor growth was significantly inhibited in combination groups and the cell doubling time was decreased by more than half |
| [62] | 100 mg/kg α-mangostin delivered via oral gavage | Male athymic (nu/nu) nude mice (7–8) | Subcutaneous injection of 22Rv1 prostate cancer cells | • Tumors in the α-mangostin group were significantly decreased by 65% |
| [41] | Xenograft - 35 or 70 mg/kg by intraperitoneal injection two times weekly | Male athymic nude mice (7–8) | Subcutaneous injection of 22Rv1 prostate cancer cells | • Significantly smaller tumors in size and volume in the 35 and 70 mg/kg groups |
Acknowledgements
Johnson JJ is supported by the National Institutes of Health (R37 CA227101). Mirielle Nauman is supported by the NCI training grant: T32CA057699. Figures were created with BioRender.com.
Abbreviations:
- CDKs
Cyclin dependent kinases
- MMPs
matrix metalloproteinases
- ROS
reactive oxygen species
- IC50
half maximal inhibitory concentration
- ERα
estrogen receptor alpha
- TPA
12-O-Tetradecanoylphorbol-13-acetate
- ER
endoplasmic reticulum
- PMA
phorbol 12-myristate 13-acetate
- Cmax
maximum concentration
- Tmax
the time to reach Cmax
- AUC
area under the curve
- ORAC
peroxyl radical scavenging capacity
- CRP
C-reactive protein
Footnotes
Conflicts of interest
The authors do not declare any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yahia EM Postharvest biology and technology of tropical and subtropical fruits. Volume 4, volume 4. 2011 [Google Scholar]
- 2.Osman M. b. M. A. R. W. J. T. Mangosteen : Garcinia mangostana l. Southampton, UK: Southampton Centre for Underutilised Crops, 2006, [Google Scholar]
- 3.Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M. and Pérez-Rojas JM Medicinal properties of mangosteen (garcinia mangostana). Food Chem Toxicol 2008. 46 3227–39. 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Ovalle-Magallanes B, Eugenio-Perez D. and Pedraza-Chaverri J. Medicinal properties of mangosteen (garcinia mangostana l.): A comprehensive update. Food Chem Toxicol 2017. 109 102–22. 10.1016/j.fct.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Aizat WM, Jamil IN, Ahmad-Hashim FH and Noor NM Recent updates on metabolite composition and medicinal benefits of mangosteen plant. PeerJ 2019. 7 e6324. 10.7717/peerj.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vemu B, Nauman MC, Veenstra JP and Johnson JJ Structure activity relationship of xanthones for inhibition of cyclin dependent kinase 4 from mangosteen (garcinia mangostana l.). Int J Nutr 2019. 4 38–45. [PMC free article] [PubMed] [Google Scholar]
- 7.Salman Z, Yu-Qing J, Bin L, Cai-Yun P, Iqbal CM, Atta-ur R. and Wei W. Antioxidant nature adds further therapeutic value: An updated review on natural xanthones and their glycosides. Digital Chinese Medicine 2019. 2 166–92. 10.1016/j.dcmed.2019.12.005. [DOI] [Google Scholar]
- 8.Cruz M. I. A. d. S. Xanthone and flavone derivatives as potential dual agents for alzheimer’s disease. Master in Pharmaceutical Chemistry. Porto: Universidade do Porto, 2015, 130. [Google Scholar]
- 9.Gutierrez-Orozco F. and Failla ML Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013. 5 3163–83. 10.3390/nu5083163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narasimhan S, Maheshwaran S, Abu-Yousef IA, Majdalawieh AF, Rethavathi J, Das PE and Poltronieri P. Anti-bacterial and anti-fungal activity of xanthones obtained via semi-synthetic modification of α-mangostin from garcinia mangostana. Molecules 2017. 22 10.3390/molecules22020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Thomas S. and Johnson JJ Polyphenols from the mangosteen (garcinia mangostana) fruit for breast and prostate cancer. Front Pharmacol 2013. 4 80. 10.3389/fphar.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shan T, Ma Q, Guo K, Liu J, Li W, Wang F. and Wu E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr Mol Med 2011. 11 666–77. 10.2174/156652411797536679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Li Y, Wang W. and Deng L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: A review. Expert Opin Ther Pat 2018. 28 415–27. 10.1080/13543776.2018.1455829. [DOI] [PubMed] [Google Scholar]
- 14.Schmid W. Ueber das mangostin. Justus Liebigs Annalen der Chemie 1855. 93 83–88. 10.1002/jlac.18550930105. [DOI] [Google Scholar]
- 15.Chi X-Q, Zi C-T, Li H-M, Yang L, Lv Y-F, Li J-Y, Hou B, Ren F-C, Hu J-M and Zhou J. Design, synthesis and structure–activity relationships of mangostin analogs as cytotoxic agents. RSC Advances 2018. 8 41377–88. 10.1039/C8RA08409B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nauman MC, Tocmo R, Vemu B, Veenstra JP and Johnson JJ Inhibition of cdk2/cycline1 by xanthones from the mangosteen (garcinia mangostana): A structure-activity relationship study. Nat Prod Res 2020. 1–5. 10.1080/14786419.2020.1777413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato A, Fujiwara H, Oku H, Ishiguro K. and Ohizumi Y. Alpha-mangostin induces ca2+-atpase-dependent apoptosis via mitochondrial pathway in pc12 cells. J Pharmacol Sci 2004. 95 33–40. 10.1254/jphs.95.33. [DOI] [PubMed] [Google Scholar]
- 18.Chen JJ, Long ZJ, Xu DF, Xiao RZ, Liu LL, Xu ZF, Qiu SX, Lin DJ and Liu Q. Inhibition of autophagy augments the anticancer activity of α-mangostin in chronic myeloid leukemia cells. Leuk Lymphoma 2014. 55 628–38. 10.3109/10428194.2013.802312. [DOI] [PubMed] [Google Scholar]
- 19.Markowicz J, Uram Ł, Sobich J, Mangiardi L, Maj P. and Rode W. Antitumor and anti-nematode activities of α-mangostin. Eur J Pharmacol 2019. 863 172678. 10.1016/j.ejphar.2019.172678. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, Iinuma M. and Nozawa Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod 2003. 66 1124–7. 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- 21.Kritsanawong S, Innajak S, Imoto M. and Watanapokasin R. Antiproliferative and apoptosis induction of α-mangostin in t47d breast cancer cells. Int J Oncol 2016. 48 2155–65. 10.3892/ijo.2016.3399. [DOI] [PubMed] [Google Scholar]
- 22.Sharma D, Kumar S. and Narasimhan B. Estrogen alpha receptor antagonists for the treatment of breast cancer: A review. Chem Cent J 2018. 12 107. 10.1186/s13065-018-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Won YS, Lee JH, Kwon SJ, Kim JY, Park KH, Lee MK and Seo KI Α-mangostin-induced apoptosis is mediated by estrogen receptor α in human breast cancer cells. Food Chem Toxicol 2014. 66 158–65. 10.1016/j.fct.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Lee YB, Ko KC, Shi MD, Liao YC, Chiang TA, Wu PF, Shih YX and Shih YW Alpha-mangostin, a novel dietary xanthone, suppresses tpa-mediated mmp-2 and mmp-9 expressions through the erk signaling pathway in mcf-7 human breast adenocarcinoma cells. J Food Sci 2010. 75 H13–23. 10.1111/j.1750-3841.2009.01407.x. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Tian W. and Ma X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol Cancer 2014. 13 138. 10.1186/1476-4598-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto K, Akao Y, Ohguchi K, Ito T, Tanaka T, Iinuma M. and Nozawa Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer dld-1 cells. Bioorg Med Chem 2005. 13 6064–9. 10.1016/j.bmc.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa Y, Iinuma M, Naoe T, Nozawa Y. and Akao Y. Characterized mechanism of alpha-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-g from mitochondria and increased mir-143 expression in human colorectal cancer dld-1 cells. Bioorg Med Chem 2007. 15 5620–8. 10.1016/j.bmc.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 28.Aisha AF, Abu-Salah KM, Ismail Z. and Majid AM Alpha-mangostin enhances betulinic acid cytotoxicity and inhibits cisplatin cytotoxicity on hct 116 colorectal carcinoma cells. Molecules 2012. 17 2939–54. 10.3390/molecules17032939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanapokasin R, Jarinthanan F, Nakamura Y, Sawasjirakij N, Jaratrungtawee A. and Suksamrarn S. Effects of α-mangostin on apoptosis induction of human colon cancer. World J Gastroenterol 2011. 17 2086–95. 10.3748/wjg.v17.i16.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Z. P53 regulation of the igf-1/akt/mtor pathways and the endosomal compartment. Cold Spring Harb Perspect Biol 2010. 2 a001057. 10.1101/cshperspect.a001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh SC, Huang MH, Cheng CW, Hung JH, Yang SF and Hsieh YH Α-mangostin induces mitochondrial dependent apoptosis in human hepatoma sk-hep-1 cells through inhibition of p38 mapk pathway. Apoptosis 2013. 18 1548–60. 10.1007/s10495-013-0888-5. [DOI] [PubMed] [Google Scholar]
- 32.Adenina S, Louisa M, Soetikno V, Arozal W. and Wanandi SI The effect of alpha mangostin on epithelial-mesenchymal transition on human hepatocellular carcinoma hepg2 cells surviving sorafenib via tgf-β/smad pathways. Adv Pharm Bull 2020. 10 648–55. 10.34172/apb.2020.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Wu L, Wang J, Li G, Feng D, Zhang B, Li L, Yang J, Ma L. and Qin H. Opposing effects of pi3k/akt and smad-dependent signaling pathways in nag-1-induced glioblastoma cell apoptosis. PLoS One 2014. 9 e96283. 10.1371/journal.pone.0096283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adnane L, Trail PA, Taylor I. and Wilhelm SM Sorafenib (bay 43–9006, nexavar), a dual-action inhibitor that targets raf/mek/erk pathway in tumor cells and tyrosine kinases vegfr/pdgfr in tumor vasculature. Methods Enzymol 2006. 407 597–612. 10.1016/s0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 35.Krajarng A, Nakamura Y, Suksamrarn S. and Watanapokasin R. Α-mangostin induces apoptosis in human chondrosarcoma cells through downregulation of erk/jnk and akt signaling pathway. J Agric Food Chem 2011. 59 5746–54. 10.1021/jf200620n. [DOI] [PubMed] [Google Scholar]
- 36.Lee HN, Jang HY, Kim HJ, Shin SA, Choo GS, Park YS, Kim SK and Jung JY Antitumor and apoptosis-inducing effects of α-mangostin extracted from the pericarp of the mangosteen fruit (garcinia mangostana l.)in yd-15 tongue mucoepidermoid carcinoma cells. Int J Mol Med 2016. 37 939–48. 10.3892/ijmm.2016.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Y, Li Y, Westover KD, Sun J, Chen H, Zhang J. and Fisher DE Inhibition of cell proliferation in an nras mutant melanoma cell line by combining sorafenib and α-mangostin. PLoS One 2016. 11 e0155217. 10.1371/journal.pone.0155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao AC, Hsu YL, Liu CK and Kuo PL Α-mangostin, a dietary xanthone, induces autophagic cell death by activating the amp-activated protein kinase pathway in glioblastoma cells. J Agric Food Chem 2011. 59 2086–96. 10.1021/jf1042757. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto K, Akao Y, Yi H, Ohguchi K, Ito T, Tanaka T, Kobayashi E, Iinuma M. and Nozawa Y. Preferential target is mitochondria in alpha-mangostin-induced apoptosis in human leukemia hl60 cells. Bioorg Med Chem 2004. 12 5799–806. 10.1016/j.bmc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Chen CM, Hsieh SC, Lin CL, Lin YS, Tsai JP and Hsieh YH Alpha-mangostin suppresses the metastasis of human renal carcinoma cells by targeting mek/erk expression and mmp-9 transcription activity. Cell Physiol Biochem 2017. 44 1460–70. 10.1159/000485582. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Petiwala SM, Nonn L. and Johnson JJ Inhibition of chop accentuates the apoptotic effect of α-mangostin from the mangosteen fruit (garcinia mangostana) in 22rv1 prostate cancer cells. Biochem Biophys Res Commun 2014. 453 75–80. 10.1016/j.bbrc.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 42.Yuan J, Wu Y. and Lu G. Α-mangostin suppresses lipopolysaccharide-induced invasion by inhibiting matrix metalloproteinase-2/9 and increasing e-cadherin expression through extracellular signal-regulated kinase signaling in pancreatic cancer cells. Oncol Lett 2013. 5 1958–64. 10.3892/ol.2013.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Q, Ma J, Lei J, Duan W, Sheng L, Chen X, Hu A, Wang Z, Wu Z, Wu E, et al. Α-mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the pi3k/akt pathway. Biomed Res Int 2014. 2014 546353. 10.1155/2014/546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beninati S, Oliverio S, Cordella M, Rossi S, Senatore C, Liguori I, Lentini A, Piredda L. and Tabolacci C. Inhibition of cell proliferation, migration and invasion of b16-f10 melanoma cells by α-mangostin. Biochem Biophys Res Commun 2014. 450 1512–7. 10.1016/j.bbrc.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Wang JJ, Sanderson BJ and Zhang W. Significant anti-invasive activities of alpha-mangostin from the mangosteen pericarp on two human skin cancer cell lines. Anticancer Res 2012. 32 3805–16. [PubMed] [Google Scholar]
- 46.Wang JJ, Sanderson BJ and Zhang W. Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen (garcinia mangostana linn.) on human melanoma cells. Food Chem Toxicol 2011. 49 2385–91. 10.1016/j.fct.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 47.Shih YW, Chien ST, Chen PS, Lee JH, Wu SH and Yin LT Alpha-mangostin suppresses phorbol 12-myristate 13-acetate-induced mmp-2/mmp-9 expressions via alphavbeta3 integrin/fak/erk and nf-kappab signaling pathway in human lung adenocarcinoma a549 cells. Cell Biochem Biophys 2010. 58 31–44. 10.1007/s12013-010-9091-2. [DOI] [PubMed] [Google Scholar]
- 48.Hung SH, Shen KH, Wu CH, Liu CL and Shih YW Alpha-mangostin suppresses pc-3 human prostate carcinoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen expression through the jnk signaling pathway. J Agric Food Chem 2009. 57 1291–8. 10.1021/jf8032683. [DOI] [PubMed] [Google Scholar]
- 49.Kaomongkolgit R. Alpha-mangostin suppresses mmp-2 and mmp-9 expression in head and neck squamous carcinoma cells. Odontology 2013. 101 227–32. 10.1007/s10266-012-0081-2. [DOI] [PubMed] [Google Scholar]
- 50.Kaomongkolgit R, Chaisomboon N. and Pavasant P. Apoptotic effect of alpha-mangostin on head and neck squamous carcinoma cells. Arch Oral Biol 2011. 56 483–90. 10.1016/j.archoralbio.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Zou S, Tong Q, Liu B, Huang W, Tian Y. and Fu X. Targeting stat3 in cancer immunotherapy. Mol Cancer 2020. 19 145. 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Tan YP, Zhao L, Wang L, Fu NJ, Zheng SP and Shen XF Anticancer activity of dietary xanthone α-mangostin against hepatocellular carcinoma by inhibition of stat3 signaling via stabilization of shp1. Cell Death Dis 2020. 11 63. 10.1038/s41419-020-2227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shan T, Cui XJ, Li W, Lin WR, Lu HW, Li YM, Chen X. and Wu T. Α-mangostin suppresses human gastric adenocarcinoma cells in vitro via blockade of stat3 signaling pathway. Acta Pharmacol Sin 2014. 35 1065–73. 10.1038/aps.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hafeez BB, Mustafa A, Fischer JW, Singh A, Zhong W, Shekhani MO, Meske L, Havighurst T, Kim K. and Verma AK Α-mangostin: A dietary antioxidant derived from the pericarp of garcinia mangostana l. Inhibits pancreatic tumor growth in xenograft mouse model. Antioxid Redox Signal 2014. 21 682–99. 10.1089/ars.2013.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Yu G. and Shen Y. The naturally occurring xanthone α-mangostin induces ros-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J Biol Sci 2018. 25 1090–95. 10.1016/j.sjbs.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding YY, Luan JJ, Fan Y, Olatunji OJ, Song J. and Zuo J. Α-mangostin reduced the viability of a594 cells in vitro by provoking ros production through downregulation of nampt/nad. Cell Stress Chaperones 2020. 25 163–72. 10.1007/s12192-019-01063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH and Hsieh YH Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ask1/p38 signaling pathway in cervical cancer cells. Oncotarget 2017. 8 47425–39. 10.18632/oncotarget.17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang S, Zhou F, Dong Y. and Ren F. Α-mangostin induces apoptosis in human osteosarcoma cells through ros-mediated endoplasmic reticulum stress via the wnt pathway. Cell Transplant 2021. 30 9636897211035080. 10.1177/09636897211035080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scolamiero G, Pazzini C, Bonafè F, Guarnieri C. and Muscari C. Effects of α-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int J Med Sci 2018. 15 23–30. 10.7150/ijms.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Y, Fei Z. and Qin L. Anticancer effects of α-mangostin in ovacar-3 human ovarian carcinoma cells are mediated via involvement of reactive oxygen species, mitochondrial - mediated apoptosis, suppression of cell migration and invasion and m-tor/pi3k/akt signaling pathway. J buon 2020. 25 2293–300. [PubMed] [Google Scholar]
- 61.Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, Li X, Han L, Li W, Sun H, et al. Α-mangostin inhibits hypoxia-driven ros-induced psc activation and pancreatic cancer cell invasion. Cancer Lett 2014. 347 129–38. 10.1016/j.canlet.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM and Mukhtar H. Α-mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 2012. 33 413–9. 10.1093/carcin/bgr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korm S, Jeong H-C, Kwon O-S, Park J-R, Cho H, Kim Y-M, Chin Y-W and Cha H-J Α-mangostin induces g1 cell cycle arrest in hct116 cells through p38mapk-p16ink4a pathway. RSC Advances 2015. 5 34752–60. 10.1039/C5RA00780A. [DOI] [Google Scholar]
- 64.Kwak HH, Kim IR, Kim HJ, Park BS and Yu SB Α-mangostin induces apoptosis and cell cycle arrest in oral squamous cell carcinoma cell. Evid Based Complement Alternat Med 2016. 2016 5352412. 10.1155/2016/5352412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurose H, Shibata MA, Iinuma M. and Otsuki Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with α-mangostin extracted from mangosteen pericarp. J Biomed Biotechnol 2012. 2012 672428. 10.1155/2012/672428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang M, Zhang L, Hei R, Li X, Cai H, Wu X, Zheng Q. and Cai C. Cdk inhibitors in cancer therapy, an overview of recent development. Am J Cancer Res 2021. 11 1913–35. [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Lu C, Shen Q, Munoz-Medellin D, Kim H. and Brown PH Ap-1 blockade in breast cancer cells causes cell cycle arrest by suppressing g1 cyclin expression and reducing cyclin-dependent kinase activity. Oncogene 2004. 23 8238–46. 10.1038/sj.onc.1207889. [DOI] [PubMed] [Google Scholar]
- 68.Nabandith V, Suzui M, Morioka T, Kaneshiro T, Kinjo T, Matsumoto K, Akao Y, Iinuma M. and Yoshimi N. Inhibitory effects of crude alpha-mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1, 2-dimethylhydrazine in the rat. Asian Pac J Cancer Prev 2004. 5 433–8. [PubMed] [Google Scholar]
- 69.Kim SJ, Hong EH, Lee BR, Park MH, Kim JW, Pyun AR, Kim YJ, Chang SY, Chin YW and Ko HJ Α-mangostin reduced er stress-mediated tumor growth through autophagy activation. Immune Netw 2012. 12 253–60. 10.4110/in.2012.12.6.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chitchumroonchokchai C, Thomas-Ahner JM, Li J, Riedl KM, Nontakham J, Suksumrarn S, Clinton SK, Kinghorn AD and Failla ML Anti-tumorigenicity of dietary α-mangostin in an ht-29 colon cell xenograft model and the tissue distribution of xanthones and their phase ii metabolites. Mol Nutr Food Res 2013. 57 203–11. 10.1002/mnfr.201200539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shibata MA, Iinuma M, Morimoto J, Kurose H, Akamatsu K, Okuno Y, Akao Y. and Otsuki Y. Α-mangostin extracted from the pericarp of the mangosteen (garcinia mangostana linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med 2011. 9 69. 10.1186/1741-7015-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang F, Ma H, Liu Z, Huang W, Xu X. and Zhang X. Α-mangostin inhibits dmba/tpa-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating pi3k/akt/mtor signaling pathway in mice. Biomed Pharmacother 2017. 92 672–80. 10.1016/j.biopha.2017.05.129. [DOI] [PubMed] [Google Scholar]
- 73.Pérez-Rojas JM, González-Macías R, González-Cortes J, Jurado R, Pedraza-Chaverri J. and García-López P. Synergic effect of α-mangostin on the cytotoxicity of cisplatin in a cervical cancer model. Oxid Med Cell Longev 2016. 2016 7981397. 10.1155/2016/7981397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han SY, You BH, Kim YC, Chin YW and Choi YH Dose-independent adme properties and tentative identification of metabolites of α-mangostin from garcinia mangostana in mice by automated microsampling and uplc-ms/ms methods. PLoS One 2015. 10 e0131587. 10.1371/journal.pone.0131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li L, Brunner I, Han AR, Hamburger M, Kinghorn AD, Frye R. and Butterweck V. Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol Nutr Food Res 2011. 55 Suppl 1 S67–74. 10.1002/mnfr.201000511. [DOI] [PubMed] [Google Scholar]
- 76.Bumrungpert A, Kalpravidh RW, Suksamrarn S, Chaivisuthangkura A, Chitchumroonchokchai C. and Failla ML Bioaccessibility, biotransformation, and transport of alpha-mangostin from garcinia mangostana (mangosteen) using simulated digestion and caco-2 human intestinal cells. Mol Nutr Food Res 2009. 53 Suppl 1 S54–61. 10.1002/mnfr.200800260. [DOI] [PubMed] [Google Scholar]
- 77.Ramaiya A, Li G, Petiwala SM and Johnson JJ Single dose oral pharmacokinetic profile of α-mangostin in mice. Curr Drug Targets 2012. 13 1698–704. 10.2174/138945012804545524. [DOI] [PubMed] [Google Scholar]
- 78.Rohman A, Alam G, Muchtaridi M. and Windarsih A. Chemical composition and antioxidant studies of underutilized part of mangosteen ( garcinia mangostana l . ) fruit. Journal of Applied Pharmaceutical Science 2019. 9 10.7324/JAPS.2019.90807. [DOI] [Google Scholar]
- 79.Zarena AS and Udaya Sankar K. Xanthones enriched extracts from mangosteen pericarp obtained by supercritical carbon dioxide process. Separation and Purification Technology 2011. 80 172–78. 10.1016/j.seppur.2011.04.027. [DOI] [Google Scholar]
- 80.Luo M, Liu Q, He M, Yu Z, Pi R, Li M, Yang X, Wang S. and Liu A. Gartanin induces cell cycle arrest and autophagy and suppresses migration involving pi3k/akt/mtor and mapk signalling pathway in human glioma cells. J Cell Mol Med 2017. 21 46–57. 10.1111/jcmm.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Z, Antalek M, Nguyen L, Li X, Tian X, Le A. and Zi X. The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mtor pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines. Nutr Cancer 2013. 65 Suppl 1 68–77. 10.1080/01635581.2013.785011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim MO, Lee HS, Chin YW, Moon DO and Ahn JS Gartanin induces autophagy through jnk activation which extenuates caspase-dependent apoptosis. Oncol Rep 2015. 34 139–46. 10.3892/or.2015.3948. [DOI] [PubMed] [Google Scholar]
- 83.Pérez-Hernández M, Arias A, Martínez-García D, Pérez-Tomás R, Quesada R. and Soto-Cerrato V. Targeting autophagy for cancer treatment and tumor chemosensitization. Cancers (Basel) 2019. 11 10.3390/cancers11101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pham V, Rendon R, Le VX, Tippin M, Fu DJ, Le TH, Miller M, Agredano E, Cedano J. and Zi X. Gartanin is a novel neddylation inhibitor for induction of skp2 degradation, fbxw2 expression, and autophagy. Mol Carcinog 2020. 59 193–201. 10.1002/mc.23140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li G, Petiwala SM, Yan M, Won JH, Petukhov PA and Johnson JJ Gartanin, an isoprenylated xanthone from the mangosteen fruit (garcinia mangostana), is an androgen receptor degradation enhancer. Mol Nutr Food Res 2016. 60 1458–69. 10.1002/mnfr.201600037. [DOI] [PubMed] [Google Scholar]
- 86.Onodera T, Takenaka Y, Kozaki S, Tanahashi T. and Mizushina Y. Screening of mammalian DNA polymerase and topoisomerase inhibitors from garcinia mangostana l. And analysis of human cancer cell proliferation and apoptosis. Int J Oncol 2016. 48 1145–54. 10.3892/ijo.2016.3321. [DOI] [PubMed] [Google Scholar]
- 87.Li K, Wu L, Chen Y, Li Y, Wang Q, Li M, Hao K, Zhang W, Jiang S. and Wang Z. Cytotoxic and antiproliferative effects of β-mangostin on rat c6 glioma cells depend on oxidative stress induction via pi3k/akt/mtor pathway inhibition. Drug Des Devel Ther 2020. 14 5315–24. 10.2147/dddt.S278414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang CF, Teng YH, Lu FJ, Hsu WH, Lin CL, Hung CC, Tung JN, Hsieh YH and Liu CJ Β-mangostin suppresses human hepatocellular carcinoma cell invasion through inhibition of mmp-2 and mmp-9 expression and activating the erk and jnk pathways. Environ Toxicol 2017. 32 2360–70. 10.1002/tox.22449. [DOI] [PubMed] [Google Scholar]
- 89.Lin CS, Lin CL, Ying TH, Chiou HL, Hung CH, Liao WS, Hsieh YH and Kao SH Β-mangostin inhibits the metastatic power of cervical cancer cells attributing to suppression of jnk2/ap-1/snail cascade. J Cell Physiol 2020. 235 8446–60. 10.1002/jcp.29688. [DOI] [PubMed] [Google Scholar]
- 90.Chang HF, Huang WT, Chen HJ and Yang LL Apoptotic effects of γ-mangostin from the fruit hull of garcinia mangostana on human malignant glioma cells. Molecules 2010. 15 8953–66. 10.3390/molecules15128953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balunas MJ, Su B, Brueggemeier RW and Kinghorn AD Xanthones from the botanical dietary supplement mangosteen (garcinia mangostana) with aromatase inhibitory activity. J Nat Prod 2008. 71 1161–6. 10.1021/np8000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang HF, Wu CH and Yang LL Antitumour and free radical scavenging effects of γ-mangostin isolated from garcinia mangostana pericarps against hepatocellular carcinoma cell. J Pharm Pharmacol 2013. 65 1419–28. 10.1111/jphp.12111. [DOI] [PubMed] [Google Scholar]
- 93.Chang HF and Yang LL Gamma-mangostin, a micronutrient of mangosteen fruit, induces apoptosis in human colon cancer cells. Molecules 2012. 17 8010–21. 10.3390/molecules17078010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krishnamachary B, Subramaniam D, Dandawate P, Ponnurangam S, Srinivasan P, Ramamoorthy P, Umar S, Thomas SM, Dhar A, Septer S, et al. Targeting transcription factor tcf4 by γ-mangostin, a natural xanthone. Oncotarget 2019. 10 5576–91. 10.18632/oncotarget.27159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schatoff EM, Leach BI and Dow LE Wnt signaling and colorectal cancer. Curr Colorectal Cancer Rep 2017. 13 101–10. 10.1007/s11888-017-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim M, Chin YW and Lee EJ Α, γ-mangostins induce autophagy and show synergistic effect with gemcitabine in pancreatic cancer cell lines. Biomol Ther (Seoul) 2017. 25 609–17. 10.4062/biomolther.2017.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J, Qiu S, Kim JT, Cho JS, Moon JH, Zhou Y, Auh J-H and Lee HJ Garcinone c suppresses colon tumorigenesis through the gli1-dependent hedgehog signaling pathway. Phytomedicine 2020. 79 153334. 10.1016/j.phymed.2020.153334. [DOI] [PubMed] [Google Scholar]
- 98.Xia Y, Liu X, Zou C, Feng S, Guo H, Yang Y, Lei Y, Zhang J. and Lu Y. Garcinone c exerts antitumor activity by modulating the expression of atr/stat3/4e-bp1 in nasopharyngeal carcinoma cells. Oncol Rep 2018. 39 1485–93. 10.3892/or.2018.6218. [DOI] [PubMed] [Google Scholar]
- 99.Xu XH, Liu QY, Li T, Liu JL, Chen X, Huang L, Qiang WA, Chen X, Wang Y, Lin LG, et al. Garcinone e induces apoptosis and inhibits migration and invasion in ovarian cancer cells. Sci Rep 2017. 7 10718. 10.1038/s41598-017-11417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu X-H, Chen Y-C, Xu Y-L, Feng Z-L, Liu Q-Y, Guo X, Lin L-G and Lu J-J Garcinone e blocks autophagy through lysosomal functional destruction in ovarian cancer cells. World Journal of Traditional Chinese Medicine 2021. 7 209–16. 10.4103/wjtcm.wjtcm_83_20. [DOI] [Google Scholar]
- 101.Yang L, Xu Z. and Wang W. Garcinone-e exhibits anticancer effects in hela human cervical carcinoma cells mediated via programmed cell death, cell cycle arrest and suppression of cell migration and invasion. AMB Express 2020. 10 126. 10.1186/s13568-020-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ho CK, Huang YL and Chen CC Garcinone e, a xanthone derivative, has potent cytotoxic effect against hepatocellular carcinoma cell lines. Planta Med 2002. 68 975–9. 10.1055/s-2002-35668. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Qiu S, Kim JT, Cho JS, Moon JH, Zhou Y, Auh JH and Lee HJ Garcinone c suppresses colon tumorigenesis through the gli1-dependent hedgehog signaling pathway. Phytomedicine 2020. 79 153334. 10.1016/j.phymed.2020.153334. [DOI] [PubMed] [Google Scholar]
- 104.Seo KH, Ryu HW, Park MJ, Park KH, Kim JH, Lee MJ, Kang HJ, Kim SL, Lee JH and Seo WD Mangosenone f, a furanoxanthone from garciana mangostana, induces reactive oxygen species-mediated apoptosis in lung cancer cells and decreases xenograft tumor growth. Phytother Res 2015. 29 1753–60. 10.1002/ptr.5428. [DOI] [PubMed] [Google Scholar]
- 105.Ryu HW, Jeong SH, Curtis-Long MJ, Jung S, Lee JW, Woo HS, Cho JK and Park KH Inhibition effects of mangosenone f from garcinia mangostana on melanin formation in b16f10 cells. J Agric Food Chem 2012. 60 8372–8. 10.1021/jf3015987. [DOI] [PubMed] [Google Scholar]
- 106.Setiawati A, Octa F, Riswanto D, Yuliani SH and Istyastono E. Anticancer activity of mangosteen pericarp dry extract against mcf-7 breast cancer cell line through estrogen receptor -α. Indonesian Journal of Pharmacy 2014. 25 119–24. 10.14499/indonesianjpharm25iss3pp119. [DOI] [Google Scholar]
- 107.Moongkarndi P, Kosem N, Luanratana O, Jongsomboonkusol S. and Pongpan N. Antiproliferative activity of thai medicinal plant extracts on human breast adenocarcinoma cell line. Fitoterapia 2004. 75 375–7. 10.1016/j.fitote.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 108.Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N. and Neungton N. Antiproliferation, antioxidation and induction of apoptosis by garcinia mangostana (mangosteen) on skbr3 human breast cancer cell line. J Ethnopharmacol 2004. 90 161–6. 10.1016/j.jep.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 109.Fazry S, Noordin MAM, Sanusi S, Noor MM, Aizat WM, Lazim AM, Dyari HRE, Jamar NH, Remali J, Othman BA, et al. Cytotoxicity and toxicity evaluation of xanthone crude extract on hypoxic human hepatocellular carcinoma and zebrafish (danio rerio) embryos. Toxics 2018. 6 10.3390/toxics6040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janardhanan S, Mahendra J, Mahendra L. and Devarajan N. Cytotoxic effects of mangosteen pericarp extracts on oral cancer and cervical cancer cells. Asian Pac J Cancer Prev 2020. 21 2577–83. 10.31557/apjcp.2020.21.9.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang JJ, Shi QH, Zhang W. and Sanderson BJ Anti-skin cancer properties of phenolic-rich extract from the pericarp of mangosteen (garcinia mangostana linn.). Food Chem Toxicol 2012. 50 3004–13. 10.1016/j.fct.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Kosem N, Ichikawa K, Utsumi H. and Moongkarndi P. In vivo toxicity and antitumor activity of mangosteen extract. J Nat Med 2013. 67 255–63. 10.1007/s11418-012-0673-8. [DOI] [PubMed] [Google Scholar]
- 113.Watanapokasin R, Jarinthanan F, Jerusalmi A, Suksamrarn S, Nakamura Y, Sukseree S, Uthaisang-Tanethpongtamb W, Ratananukul P. and Sano T. Potential of xanthones from tropical fruit mangosteen as anti-cancer agents: Caspase-dependent apoptosis induction in vitro and in mice. Appl Biochem Biotechnol 2010. 162 1080–94. 10.1007/s12010-009-8903-6. [DOI] [PubMed] [Google Scholar]
- 114.Majid AFAAMA-SDNJSIMSA. Antitumorigenicity of xanthones-rich extract from garcinia mangostana fruit rinds on hct 116 human colorectal carcinoma cells. Rev. bras. farmacogn 2011. 21 1025–34. 10.1590/S0102-695X2011005000164. [DOI] [Google Scholar]
- 115.Aisha AF, Abu-Salah KM, Ismail Z. and Majid AM In vitro and in vivo anti-colon cancer effects of garcinia mangostana xanthones extract. BMC Complement Altern Med 2012. 12 104. 10.1186/1472-6882-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Ee GC, Daud S, Izzaddin SA and Rahmani M. Garcinia mangostana: A source of potential anti-cancer lead compounds against cem-ss cell line. J Asian Nat Prod Res 2008. 10 475–9. 10.1080/10286020801948490. [DOI] [PubMed] [Google Scholar]
- 117.Li G, Petiwala SM, Pierce DR, Nonn L. and Johnson JJ Selective modulation of endoplasmic reticulum stress markers in prostate cancer cells by a standardized mangosteen fruit extract. PLoS One 2013. 8 e81572. 10.1371/journal.pone.0081572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aukkanimart R, Boonmars T, Sriraj P, Sripan P, Songsri J, Ratanasuwan P, Laummaunwai P, Boueroy P, Khueangchaingkhwang S, Pumhirunroj B, et al. In vitro and in vivo inhibitory effects of α-mangostin on cholangiocarcinoma cells and allografts. Asian Pac J Cancer Prev 2017. 18 707–13. 10.22034/apjcp.2017.18.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Doi H, Shibata MA, Shibata E, Morimoto J, Akao Y, Iinuma M, Tanigawa N. and Otsuki Y. Panaxanthone isolated from pericarp of garcinia mangostana l. Suppresses tumor growth and metastasis of a mouse model of mammary cancer. Anticancer Res 2009. 29 2485–95. [PubMed] [Google Scholar]
- 120.Choi YH, Han SY, Kim YJ, Kim YM and Chin YW Absorption, tissue distribution, tissue metabolism and safety of α-mangostin in mangosteen extract using mouse models. Food Chem Toxicol 2014. 66 140–6. 10.1016/j.fct.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 121.Li L, Han AR, Kinghorn AD, Frye RF, Derendorf H. and Butterweck V. Pharmacokinetic properties of pure xanthones in comparison to a mangosteen fruit extract in rats. Planta Med 2013. 79 646–53. 10.1055/s-0032-1328543. [DOI] [PubMed] [Google Scholar]
- 122.Petiwala SM, Li G, Ramaiya A, Kumar A, Gill RK, Saksena S. and Johnson JJ Pharmacokinetic characterization of mangosteen (garcinia mangostana) fruit extract standardized to α-mangostin in c57bl/6 mice. Nutr Res 2014. 34 336–45. 10.1016/j.nutres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chitchumroonchokchai C, Riedl KM, Suksumrarn S, Clinton SK, Kinghorn AD and Failla ML Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J Nutr 2012. 142 675–80. 10.3945/jn.111.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie Z, Sintara M, Chang T. and Ou B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci Nutr 2015. 3 342–8. 10.1002/fsn3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kondo M, Zhang L, Ji H, Kou Y. and Ou B. Bioavailability and antioxidant effects of a xanthone-rich mangosteen (garcinia mangostana) product in humans. J Agric Food Chem 2009. 57 8788–92. 10.1021/jf901012f. [DOI] [PubMed] [Google Scholar]
- 126.Xie Z, Sintara M, Chang T. and Ou B. Functional beverage of garcinia mangostana (mangosteen) enhances plasma antioxidant capacity in healthy adults. Food Sci Nutr 2015. 3 32–8. 10.1002/fsn3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang YP, Li PG, Kondo M, Ji HP, Kou Y. and Ou B. Effect of a mangosteen dietary supplement on human immune function: A randomized, double-blind, placebo-controlled trial. J Med Food 2009. 12 755–63. 10.1089/jmf.2008.0204. [DOI] [PubMed] [Google Scholar]
- 128.Udani JK, Singh BB, Barrett ML and Singh VJ Evaluation of mangosteen juice blend on biomarkers of inflammation in obese subjects: A pilot, dose finding study. Nutr J 2009. 8 48. 10.1186/1475-2891-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]