Key Points
Question
Does a multimodal nonpharmacological approach prevent delirium in older patients undergoing elective surgical procedures?
Findings
This stepped-wedge cluster trial recruited 1470 patients 70 years and older who were randomized in 5 clusters to patient-centered evidence-based intervention (ie, personalized stimulation, company, relaxation) vs routine care. The intervention reduced delirium incidence after various major procedures, most significantly in patients undergoing noncardiac surgery; the intervention did not change cardiac surgery postoperative delirium incidence.
Meaning
Results of this stepped-wedge cluster trial suggest the implementation of this multimodal nonpharmacological delirium prevention program may improve delivery of targeted care and patient outcomes in older patients undergoing elective noncardiac surgical procedures.
This stepped-wedge cluster randomized clinical trial examines whether a multifaceted prevention intervention is effective in reducing postoperative delirium incidence and prevalence among older patients after various major surgical procedures.
Abstract
Importance
Delirium significantly worsens elective surgery outcomes and costs. Delirium risk is highest in elderly populations, whose surgical health care resource consumption (50%) exceeds their demographic proportion (15% to 18%) in high-resource countries. Effective nonpharmacologic delirium prevention could safely improve care in these vulnerable patients, but data from procedure-specific studies are insufficiently compelling to drive changes in practice. Delirium prevention approaches applicable to different surgical settings remain unexplored.
Objective
To examine whether a multifaceted prevention intervention is effective in reducing postoperative delirium incidence and prevalence after various major surgical procedures.
Design, Setting, and Participants
This stepped-wedge cluster randomized trial recruited 1470 patients 70 years and older undergoing elective orthopedic, general, or cardiac surgery from November 2017 to April 2019 from 5 German tertiary medical centers. Data were analyzed from December 2019 to July 2021.
Interventions
First, structured delirium education was provided to clinical caregivers at each site. Then, the study delirium prevention team assessed patient delirium risk factors and symptoms daily. Prevention was tailored to individual patient needs and could include: cognitive, motor, and sensory stimulation; meal companionship; accompaniment during diagnostic procedures; stress relaxation; and sleep promotion.
Main Outcomes and Measures
Postoperative delirium incidence and duration.
Results
Of 1470 included patients, 763 (51.9%) were male, and the median (IQR) age was 77 (74-81) years. Overall, the intervention reduced postoperative delirium incidence (odds ratio, 0.87; 95% CI, 0.77-0.98; P = .02) and percentage of days with delirium (intervention, 5.3%; control, 6.9%; P = .03). The effect was significant in patients undergoing orthopedic or abdominal surgery (odds ratio, 0.59; 95% CI, 0.35-0.99; P = .047) but not cardiac surgery (odds ratio, 1.18; 95% CI, 0.70-1.99; P = .54).
Conclusions and Relevance
This multifaceted multidisciplinary prevention intervention reduced postoperative delirium occurrence and days with delirium in older patients undergoing different elective surgical procedures but not cardiac procedures. These results suggest implementing this delirium prevention program will improve care and outcomes in older patients undergoing elective general and orthopedic procedures.
Introduction
Postoperative delirium is frequent in older patients. Its association with higher mortality,1,2 cognitive decline,3 loss of autonomy, and increased hospitalization and costs4,5 warrant preventive initiatives in frail or high-risk patients.4,5,6 The 16% of US individuals 65 years and older accounted for more than 40% of surgical interventions in 2019.7 This demographic/surgical health care utilization disproportion will worsen as the number of people 65 years and older is expected to double by 2050. In medical patients, one-third of delirium cases are considered preventable8,9,10 with multimodal nonpharmacological interventions.10,11,12 Once delirium occurs, no treatment changes its course or outcome,13 highlighting the importance of postoperative delirium prevention.
Patient frailty is related to age older than 65 years14 and best predicts delirium risk.15,16 Postoperative delirium rates vary (11% to 65%15,17,18); studies addressing risk,12,19,20,21,22 precipitating factors, and prevalence focus on single types of surgery.23 Hip fractures, an emergency procedure, represent most of the intervention literature. To our knowledge, no study stratifies for delirium risk or includes frail patients and/or patients with dementia. Large-scale studies incorporating different elective surgical interventions have not been published.11,15,23,24
We compared the efficacy of a multimodal, multidisciplinary, nonpharmacological intervention in patients 70 years and older undergoing various elective major surgical procedures. We hypothesized our multimodal individualized best practice–based intervention would reduce postoperative delirium incidence (our primary outcome) and shorten delirium duration.
Methods
Trial Oversight
The Patientensicherheit, Wirtschaftlichkeit und Lebensqualität (PAWEL; ie, patient safety, cost-effectiveness, and quality of life) study randomized 3 German university hospitals (Tübingen, Freiburg, and Ulm), and 2 German tertiary care (Stuttgart and Karlsruhe) center clusters. The first of the stepped-wedge model’s seven 12-week periods had no intervention. Then, every 12 weeks, 1 cluster was randomized to the prevention protocol. All 5 clusters thus implemented the intervention for at least 12 weeks by the end of the study. The Tübingen Faculty of Medicine’s Ethics Commission, Potsdam and Ulm Universities, and the District Physicians Chamber of Baden-Württemberg provided ethical approval. All patients or substitute decision-makers provided written informed consent. The trial protocol can be found in Supplement 1. This study followed the Consolidated Standards of Reporting Trials Extension (CONSORT Extension) reporting guideline for stepped-wedge cluster randomized trials.
Center eligibility was based on willingness and at least 900 major procedures per year in older adults. To balance procedures within and across centers, recruitment was capped in individual sites and in study intervals at 66% for: cardiac/vascular; orthopedic; and (combined) intra-abdominal, urological, or thoracic surgery procedures. Patients were assessed preoperatively, then daily up postoperative day 7 and on discharge.25
Participants
Patients 70 years and older undergoing major elective surgery with an expected cut-to-suture-time of 60 minutes or more were eligible. Recruitment was conducted by a site-based independent medical specialist. Patients requiring emergency surgery, patients who did not provide consent, and and patients with an expected survival of 15 months or less were excluded.25
Randomization
Cluster randomization sequences (5 clusters) were computer generated. Training teams coached local trainers and then clinical staff during 6 weeks prior to the center’s intervention implementation. Allocation was concealed both at the cluster level and the patient level; only the consortium leader and staff generating random sequences knew the randomization allocation. Participants and outcome assessors remained blinded.
Procedures
The intervention (AKTIVER [“More Active”]: Alltags- und Kognitions-Training & Interdisziplinarität verbessert Ergebnis und mindert das Risiko [“everyday skills and cognition training and interdisciplinarity improves outcome and mitigates risk”]) included 7 best-practice delirium prevention modules: cognitive, motor, and sensory stimulation; meal companionship; diagnostic test and operation room accompaniment; stress relaxation; and sleep promotion. Patient needs and preferences determined module deployment. Our AKTIVER manual provided explicit parameters for each module. The authors (J.S., C.B., and C.T.) constructed AKTIVER using evidence (Care of Confused Hospitalized Older Persons,26 Hospital Elderly Life Program,27 and others10), inspiration from other programs,28,29,30,31,32,33 and their clinical experience25 (Table 1).34,35,36,37,38,39,40,41,42,43
Table 1. AKTIVER Delirium Prevention Program.
| Section of AKTIVER program | Content | Population trained and delirium risk factors addressed | Main indications | Model |
|---|---|---|---|---|
| Staff educationa | ||||
| Basic education (1.5 h) | Delirium primer, symptoms, outcome, prevention options, management | Nurses, physicians, aides, therapists, OR personnel, cleaning and transportation staff | Delirium symptom recognition, delirium awareness | CHOPs,b principle 6 |
| Delirium prevention level (10 h) | Delirium risk, adequate communication with patients with cognitive impairment | Nurses, therapists, physicians | Delirium risk and prevention, communication with patients with cognitive impairment | CHOPs, principle 6; PODc |
| Delirium advocacy level (30 h in addition to delirium prevention level) | Delirium screening, risk factors and modifiable risk, detection of depression and dementias, pain assessment (especially in patients with cognitive impairment), detection and management of psychotic symptoms, sleep-wake rhythm, hyperactive states and apathy | Nurses, therapists, physicians | Delirium management, risk evaluation, prevention | CHOPs, principle 6; POD |
| Tasks of delirium expert nurse | ||||
| Risk assessment (preadmission and daily) | Checking present unmodifiable and modifiable risk factors, minimizing polypharmacy and inappropriate medication (beginning and daily updates) | Age4,5,6,15,16,18,21,22,34 (SIGN evidence level, IIIb-IIb), comorbidity4,5,6,10,15,18,34 (SIGN evidence level, IIb-Ic), depression15,21 (SIGN evidence level, IIIb-IIb), polypharmacy4,10,15,34 (SIGN evidence level, IIb), infection4,16,18 (SIGN evidence level, IIb), electrolyte disturbance4,15,20 (SIGN evidence level, IIb), frailty4,10,12,15,16,18,34 (SIGN evidence level, IIa), sensory deficits4,5,6,10,12,15,16,18,34 vision and hearing (SIGN evidence level, Ia-IIb), cognitive dysfunction4,5,10,12,15,16,18,19,20,34 (SIGN evidence level, IIb-Ic), sleep15,16,22 (SIGN evidence level, IIa), delirium history16,18 | Planning of individual prevention schedule and frequency of modules | CHOPs, principles 1, 2, 3, and 5; DemDeld; HELPe; help+f |
| Rounds and patient visits (daily) | Patient assessment (pain, attention, orientation, psychomotor activity, psychotic symptoms), consultation with the interprofessional team on unit and family members, mobilization barriers (daily) | Pain4,5,10,12,15,16,18,34 (SIGN evidence level, Ia-IIb), cognition/cognitive dysfunction4,5,10,12,15,16,18,19,20,34 (SIGN evidence level, IIb-Ic), stress15,16,20,34 (SIGN evidence level, IV), medication4,10,15,18,19,34 (SIGN evidence level, Ib-IIa), sleep-wake cycle15,16,22 (SIGN evidence level, IIa), immobilization16,18,19 (SIGN evidence level, Ia-IIb), polypharmacy4,5,6,18,34 (SIGN evidence level, Ia-IIb) | Assessment of delirium symptoms, pain, new medication, training of case-based risk management, delirium awareness for interprofessional team36,37 | CHOPs, principles 1 to 7; DemDel; help+ |
| Module assignment (daily) | Collecting information from patient, family, and ward team, including preferences and aversions (Sunflower Tool); modules can be adapted during the hospital stay (beginning and daily updates) | See below | Individualization of delirium prevention according to risks | CHOPs, principles 4, 5, and 7; DemDel; HELP; help+ |
| Team handover (daily) | Discuss symptoms and daily well-being of patients, check their individual needs (pain, fluids, stress reduction) to optimize prevention, involving family if possible; microteaching and supervision (twice daily) | Pain4,5,10,12,15,16,18,34 (SIGN evidence level, Ia-IIb), stress16,18,19,34,38 (SIGN evidence level, IV), disorientation4,5,10,12,16,18,34 (SIGN evidence level, IIb-Ic), dehydration4,15,18 (SIGN evidence level, Ia-IIb), anxiety,15,21 apathy15 | Information sharing, module implementation planning, shift plans, teaching | DemDel; HELP; help+ |
| Intervention modules g | ||||
| Orientation visit | Naming the daily schedule, clock, calendar, bathroom sign, verbal orientation, put clean glasses on or insert hearing aids | Vision impairment4,5,6,18,34 (SIGN evidence level, Ic), hearing impairment4,5,6,18,34 (SIGN evidence level, Ia-IIb), cognitive dysfunction4,5,6,10,12,15,16,18,19,20,34 (SIGN evidence level, IIb-Ic), stress15,16,18,19,34 (SIGN evidence level, IV), anxiety15,21 | Reorientation, stress reduction | CHOPs, principles 4, 5, and 7; HELP; help+; mHELPh; POD |
| Mobilization | Motivation and activation to simple movement exercises, accompaniment during mobilization in bed or for walks | Immobilization16,18,19 (SIGN evidence level, Ia-IIb), frailty4,10,12,15,16,18,34 (SIGN evidence level, IIa) | Mobilization | DemDel; HELP; mHELP |
| Activation visit | Cognition promotion (eg, games, Sudoku, quiz, reading newspaper) | Cognitive dysfunction4,5,10,12,15,16,18,19,20,34 (SIGN evidence level, IIb-Ic) | Cognitive activation | CHOPs, principles 4, 5, and 7; HELP; help+ |
| Meal accompaniment | Company during meals, support with meal arrangement, fluid intake | Malnutrition15 (SIGN evidence level, IIb), dehydration6,10,12,16,18,34 (SIGN evidence level, IIb) | Nutrition, fluid intake | HELP; help+; mHELP; POD |
| Relaxation/sleep promotion | Music, warm drinks, acupressure, relaxation exercises | Anxiety,15,21 sleep promotion15,16,22 (SIGN evidence level, IIa), stress15,16,18,19,34 (SIGN evidence level, IV) | Stress reduction, sleep-wake rhythm | HELP; help+ |
| Diagnostic chaperonage | Accompanying patients to examinations (eg, CT, ECG), activation, orientation | Anxiety,15,21 stress15,16,18,19,34 (SIGN evidence level, IV), change of environment/transfer16,18 | Stress reduction, orientation | The Elderly in the ORi |
| Attendance to the OR | Accompanying to the OR sluice, first contact in the recovery room, reorientation, providing sensory aids | Anxiety,15,21 stress15,16,18,19,34 (SIGN evidence level, IV), change of environment/transfer16,18 (SIGN evidence level, IV), pain4,5,10,12,15,16,18,19,34 (SIGN evidence level, Ia-IIb), vision and hearing impairment4,5,6,10,12,15,16,18,34 (SIGN evidence level, Ia-IIb) | Provide familiarity, reorientation, stress reduction | The Elderly in the OR |
Abbreviations: CHOP, Care of Confused Hospitalised Older Persons; CT, computed tomography; DemDel, Basel Dementia and Delirium Prevention and Management Program; ECG, electrocardiography; HELP, Hospital Elder Life Program; help+, adapted Hospital Elder Life Program plus consultation/liaison physician; mHELP, modified Hospital Elder Life Program; OR, operating room; POD, Prevention of Delirium; SIGN, Scottish Intercollegiate Guidelines Network.
Tabet et al39 evaluated the efficacy of staff education to prevent delirium in a medical ward. The incidence of delirium was significantly lower on the intervention ward despite a wide confidence interval (odds ratio, 0.45; 95% CI, 0.26-0.96). Among single-component interventions, only staff education, reorientation protocol (GRADE evidence: very low), and Geriatric Risk Assessment MedGuide software (hazard ratio, 0.42; 95% CI, 0.35-0.52; GRADE evidence: moderate) were effective in preventing delirium.24
Implementation of a model of care for hospitalized older persons with cognitive impairment (the CHOPs) in 6 New South Wales hospitals.26 Settings included hospital-wide departments, surgery departments, medicine departments, intensive care units, and emergency departments in 22 hospitals in New South Wales, Australia, guided by 7 key principles.40
Settings for the POD program included medical and surgical departments in 8 National Health Service hospitals in Great Britain.28,29
The DemDel program is a before-and-after study of a nurse-led comprehensive delirium management program for older acute care inpatients with cognitive impairment.33 The program also used data from the interdisciplinary nurse-led Delirium Prevention and Management Program on nursing workload.32 Settings included hospital-wide rollouts, surgery departments, medicine departments, and emergency departments in a university hospital and a tertiary care hospital in Basel, Switzerland.
Settings for the HELP program included predominantly medical (and geriatric) departments, some surgical departments, few hospital-wide rollouts, emergency departments, and rehabilitation facilities in more than 80 hospitals in the US, Canada, and Australia and in single sites in Europe.27,31,41
The help+ program has adapted the HELP program to the German health care system and included a psychogeriatric consultation/liaison physician.30,42 Settings included medical, surgical, and neurological departments in a tertiary care university hospital in Bielefeld, Germany.
The setting for the mHELP program was an abdominal surgery department in a tertiary care hospital in Taipeh, Taiwan.37
Settings for The Elderly in the OR program included surgical departments, ORs, and intensive care units in a tertiary care hospital in Münster, Germany, and in several other hospitals in Germany.43
At each site, more than 70% of hospital staff completed a 90-minute basic lesson in delirium detection, management, and prevention; 20% completed additional 10-hour and 10% completed 30-hour delirium advocacy courses.24,35,39,44 Local psychogeriatric nurse specialists received 80 hours of training in delirium risk detection, assessments, prevention, medication surveillance, daily evaluations, and prescribing individual prevention modules. After 40 hours of content/implementation training for 7 intervention modules, the independent delirium study prevention team observed patients throughout hospitalization and provided the intervention modules several times a day as needed. This team consisted primarily of 2 psychogeriatric nurses working 20 hours per week, supported by 3 to 5 volunteer aides (about 100 hours per week). Compliance and reliability across sites were ensured by one team training each center’s staff, a reference manual, unannounced on-site visits, and documentation reviews.
Outcomes
Trained clinicians assessed all outcomes. Blinding was ensured by suggesting these data would serve for delirium risk score validation.
Delirium, the primary outcome, was assessed daily with the validated Confusion Assessment Method (CAM),45 I-Confusion Assessment Method (I-CAM)46 between 1 and 6 pm (7 postoperative days), followed by a validated postdischarge medical record review. Delirium symptoms fluctuate47; medical record reviews capture findings absent during CAM assessments, as described by others.48 Individual study participant data, and not clusters, determined outcome measurements.
The German I-CAM46 was operationalized further; we assessed structured attention and logic and identified abnormal psychomotor activity to classify delirium subtype. These modifications harmonized CAM screening with International Statistical Classification of Diseases and Related Health Problems, Tenth Revision delirium diagnostic criteria. Delirium duration was assessed by medical record review. We tallied days with delirium in all patients, mean delirium days, and percentage of days with delirium in each study group.
Baseline data included cognitive function (Montreal Cognitive Assessment [MoCA]),49 subjective memory impairment (SMI),50 comorbidities (Charlson Comorbidity Index [CCI]),51 visual impairment,52 depression (4-item Patient Health Questionnaire [PHQ-4]),53 functional status,54 and frailty (Canadian Study of Health and Aging [CSHA] Clinical Frailty Scale [CFS]).55 Polypharmacy meant routine administration of 5 or more daily medications.56 Anesthesia duration was the time from induction until extubation.
We recorded adverse events, including falls, strokes, infections, and significant perioperative complications (death, reoperation, pneumonia, sepsis). An external Regional Ethics and Data Monitory Board assessed feasibility and serious adverse event occurrence every 3 months and performed yearly audits.
Statistical Analysis
The power analysis for the primary outcome, delirium incidence, assumed a 25% to 15%11 reduction after intervention. A Fisher exact test conventional analysis detected delirium proportion differences, given a power of 1 − β = 0.80 and an α error of 5%, showed that the study would require 514 patients with a 1:1 randomization. Woertmann adjustment57 for 5 crossing points in the stepped-wedge design, with 50 patients maximum per cluster per period and a 0.01 intracluster correlation, led to a correction factor of 2.63; this lead to a target population of 514 × 2.63 = 1351 patients. Assuming a 15% dropout rate increased the estimated sample to 1500 patients (750 per arm).25
Sample characteristics were summarized as frequencies and percentages for categorical variables and medians and IQRs for nonparametric continuous variables. Normality was established using Shapiro-Wilk tests. Baseline intervention and control group differences in bivariate analyses were examined using Mann-Whitney U tests for nonparametric continuous variables (age, education [years], Barthel Index score, MoCA score, CCI score, body mass index, CSHA-CFS score), multinomial regression for categorical variables (marital status, type of surgery), and χ2 tests for binary variables (sex, subjective memory impairment, visual impairment, dementia [CCI score], depression (PHQ-4 score), polypharmacy, sleeping medication use, current daily smoker, current alcohol misuse). We calculated risk ratios (RRs) for binary variables, relative RRs (RRRs) for categorical variables, and median differences for continuous variables. Median differences with 95% CIs of the difference were estimated using the Hodges-Lehman estimator. Significantly different variables (intervention vs control groups) were included as covariates in the generalized estimating equation (GEE) models. Odds ratios (ORs), RRs, and RRRs for delirium were calculated in intervention and control groups. An intraclass correlation coefficient was obtained for all clusters. Interrater reliability (IRR) was established for I-CAM assessments and medical record reviews across raters and centers.58
Primary outcome data were available for all participants, reducing the 1500 patient requirement to 1351. For GEE model covariates, missing data occurred in less than 5%, accounting for 0% to 3.7% (eTable 3 in Supplement 2). The Little test of missing completely at random was not significant (χ223 = 31.56; P = .11). For secondary analysis variables, data were missing in less than 0.5%. The GEE model examined the intervention’s effect on delirium, using the center as the subject variable. We assumed a binomial distribution, a logit link, and an exchangeable correlation structure.
For the first model, all variables with significantly different baselines (intervention vs control groups) were considered explanatory covariates (model 1). Four established major risk factors (age, MoCA score, dementia, and polypharmacy) were added in the second model (model 2). In model 3, we stratified the second model for cardiac and vascular surgery (hereafter referred to as cardiac surgery) (model 3a) vs noncardiac surgery (model 3b). The same GEE models with a Poisson distribution and log-link function were applied to obtain RRs and 95% CIs58 and to calculate RRRs.59
In secondary analyses, Mann-Whitney U testing assessed differences between intervention and control groups overall delirium days and percentages of days with delirium in the total sample and subgroups. We conducted the same GEE models in a subsample of patients with baseline frailty, ie, patients with CSHA-CFS60 scores of 5 or more.
Tests were 2-tailed, and statistical significance was set at a P value less than .05. Statistical analyses were performed using SPSS version 26 (IBM).
Results
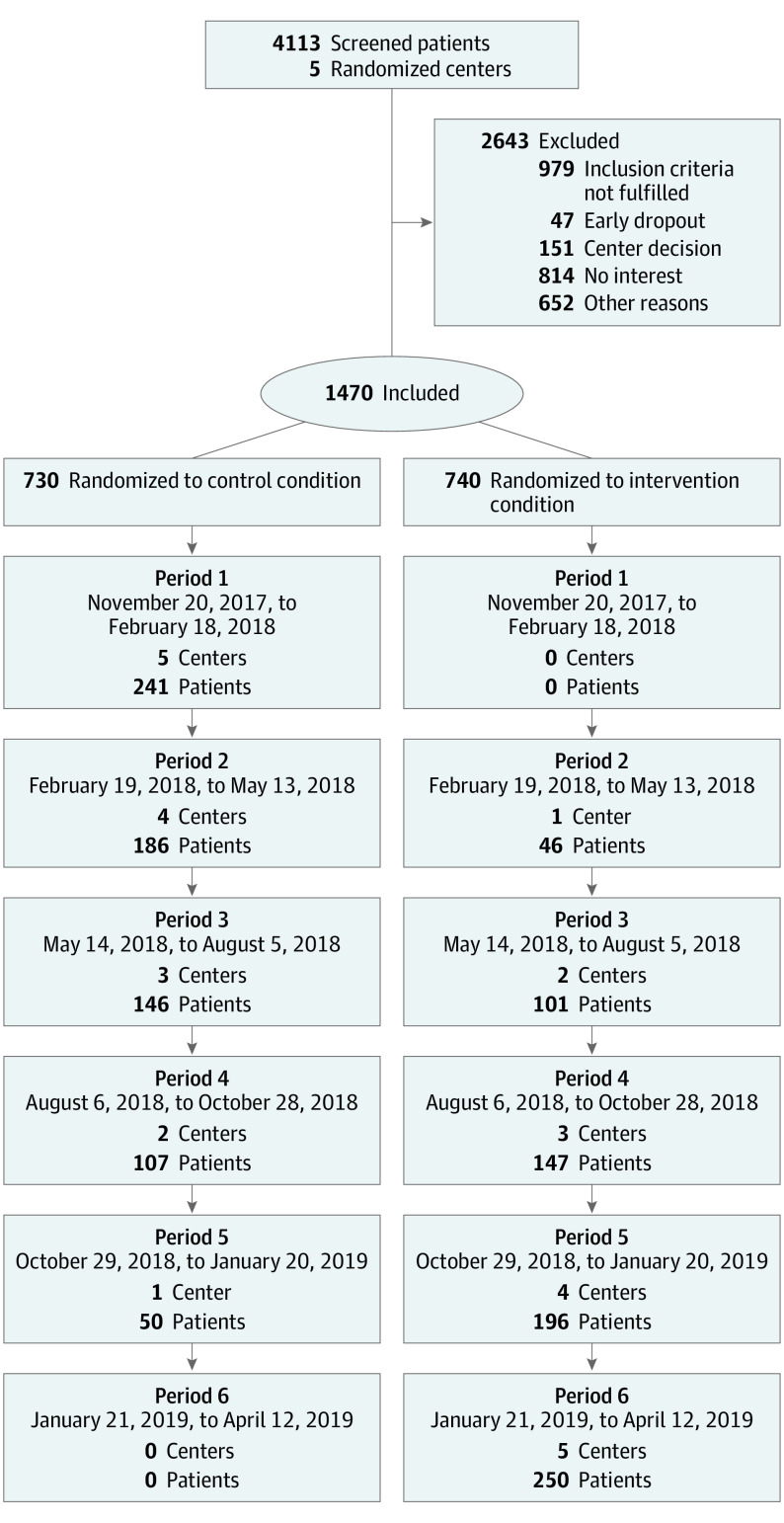
Between November 21, 2017, and April 12, 2019, 4113 patients were screened and 1470 recruited in the study (Figure). Of 1470 included patients, 763 (51.9%) were male, and the median (IQR) age was 77 (74-81) years.
Figure. Recruitment CONSORT Flowchart.
Group baseline demographic characteristics and surgical interventions appear in Table 2, and surgical procedures are shown in eTable 2 in Supplement 2. Age, marital status, visual impairment, and cognitive status (MoCA score) and functional status (Barthel Index score) were similar. While independent in everyday functioning, 1101 of 1470 patients (74.9%) presented at least mild baseline cognitive deficits. Compare with patients in the intervention group, those in the control group had fewer education years (12.5 vs 13.0; P < .001) and slightly higher frailty (mean CSHA-CFS score, 3.6 vs 3.4; P = .001; median difference, 0). The intervention group had more male patients (54.5% vs 49.3%; P = .048; RR, 1.10; 95% CI, 1.00-1.22), slightly more comorbidities (mean CCI score, 2.6 vs 2.1; P < .001; median difference, 0), and more subjective memory impairment (57.3% vs 49.2%; P = .005; RR, 1.15; 95% CI, 1.05-1.27). Patient populations differed by center before randomization (eTable 1 in Supplement 2); primary analyses were therefore conducted controlling for cluster effects.
Table 2. Baseline Characteristics of Participants.
| Characteristic | No./total No. (%) | P value | RR (95% CI) | RRR (95% CI) | Median difference (95% CI) | |
|---|---|---|---|---|---|---|
| Intervention group (n = 740) | Control group (n = 730) | |||||
| Age, median (IQR; range), y | 78 (74 to 81; 70 to 98) | 78 (74 to 81; 70 to 96) | .84 | NA | NA | 0 (−1.0 to 0) |
| Male | 403/740 (54.5) | 360/730 (49.3) | .048 | 1.10 (1.00 to 1.22) | NA | NA |
| Marital status | ||||||
| Married, living together | 466/739 (63.1) | 448/730 (61.4) | .78 | NA | 1.07 (0.85 to 1.34) | NA |
| Married, living separately | 45/739 (6.1) | 48/730 (6.6) | NA | 0.96 (0.62 to 1.50) | NA | |
| Single, divorced, or widowed | 228/739 (30.8) | 234/730 (32.0) | NA | 1 [Reference] | NA | |
| Education, median (IQR), y | 12 (12 to 15.5) | 12 (10 to 13) | <.001 | NA | NA | 0 |
| Barthel Index score, median (IQR) | 100 (95 to 100) | 100 (95 to 100) | .62 | NA | NA | 0 |
| MoCA score, median (IQR) | 24 (21 to 26) | 24 (21 to 26) | .26 | NA | NA | 0 (−1.0 to 0) |
| SMI | 422/736 (57.3) | 359/729 (49.2) | .003 | 1.15 (1.05 to 1.27) | NA | NA |
| Visual impairment | 327/677 (48.3) | 320/696 (46.0) | .39 | 1.05 (0.94 to 1.18) | NA | NA |
| CCI score, median (IQR) | 2 (1 to 4) | 2 (1 to 3) | <.001 | NA | NA | 0 (0 to 1.0) |
| Dementia (by CCI questionnaire) | 16/740 (2.2) | 12/730 (1.6) | .47 | 1.32 (0.63 to 2.76) | NA | NA |
| Depression (PHQ-4 score >3)53 | 131/696 (17.7) | 118/707 (16.2) | .30 | 1.13 (0.90 to 1.41) | NA | NA |
| BMI, median (IQR)a | 26.5 (24.1 to 29.3) | 26.6 (24.0 to 30.0) | .68 | NA | NA | −0.9 (−0.5 to 0.4) |
| CSHA-CFS score, median (IQR) | 3 (3 to 4) | 3 (3 to 4) | .001 | NA | NA | 0 |
| Polypharmacyb | 453/707 (64.1) | 481/709 (67.8) | .14 | 0.94 (0.88 to 1.02) | NA | NA |
| Sleeping medication use (last 4 wk) | 108/683 (15.8) | 138/706 (19.6) | .068 | .81 (0.64 to 1.02) | NA | NA |
| Current daily smokerc | 32/736 (4.3) | 31/730 (4.2) | .14 | 0.98 (0.80 to 1.20) | NA | NA |
| Current alcohol misused | 4/733 (0.5) | 2/727 (0.3) | .42 | 1.98 (0.36 to 10.78) | NA | NA |
| Type of surgery | ||||||
| Cardiac or vascular surgery | 273/740 (36.9) | 259/730 (35.5) | <.001 | NA | 0.34 (0.18 to 0.66) | NA |
| Orthopedic/spine surgery | 352/740 (47.6) | 390/730 (53.4) | NA | 0.29 (0.15 to 0.56) | NA | |
| Abdominal surgery | 75/740 (10.1) | 68/730 (9.3) | NA | 0.36 (0.18 to 0.73) | NA | |
| Other surgery | 40/740 (5.4) | 13/730 (1.8) | NA | 1 [Reference] | NA | |
| Length of anesthesia, median (IQR), min | 183 (145 to 276) | 185 (141 to 264) | .56 | NA | NA | 2.0 (−5.0 to 10.0) |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CSHA-CFS, Canadian Study of Health and Aging Clinical Frailty Scale; MoCA, Montreal Cognitive Assessment; NA, not applicable; PHQ-4, 4-item Patient Health Questionnaire; RR, risk ratio; RRR, relative risk ratio; SMI, subjective memory impairment.
Calculated as weight in kilograms divided by height in meters squared.
Polypharmacy defined as routine administration of 5 or more daily medications.
Defined as smoking 5 or more cigarettes per day.
Defined as the use of 3 or more drinks daily.
The intraclass correlation coefficient across centers revealed a value of −0.58, indicating random distribution. Comparing 86 I-CAM scores and 20 medical record reviews, the I-CAM score’s IRR (Krippendorff α = 0.73; 95% CI, 0.48-0.94) was satisfactory and the medical record review’s IRR (Krippendorff α = 0.85; 95% CI, 0.79-0.90) was good. Site visit checklist evaluation showed high adherence to recommended prevention measures and intervention fidelity within individual centers.61
Delirium occurred in 318 patients (21.6%) in the total sample, 190 (35.7%) of those undergoing cardiac procedures, and 128 (13.6%) of those undergoing noncardiac procedures. Preventive intervention led to lower new delirium proportions overall (19.9% vs 23.4%; RR, 0.85; 95% CI, 0.70-1.03; P = .10; RRR, 15.2%; 95% CI, −3.1 to 30.2). Striking outcome differences between cohorts led to surgery type-based stratification. Delirium rates in patients undergoing noncardiac surgery (n = 938) were significantly lower in the intervention group compared with the control group (10.9% vs 16.3%; RR, 0.67; 95% CI, 0.48-0.93; P = .008; RRR, 33.2%; 95% CI, 7.1-52.0).
The intervention and control groups were no different in patients undergoing cardiac surgery (Table 3). Delirium occurrence by period and center is depicted in eTable 4 in Supplement 2.
Table 3. Delirium Occurrence.
| Outcome | No. (%) | Odds ratio (95% CI) | RR (95% CI) | P value | RRR (95% CI), % | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Total | |||||
| Total sample, No. | 740 | 730 | 1470 | NA | NA | NA | NA |
| Delirium | 147 (19.9) | 171 (23.4) | 318 (21.6) | 0.81 (0.63 to 1.04) | 0.85 (0.70 to 1.03) | .10 | 15.2 (−3.1 to 30.2) |
| No delirium | 593 (80.1) | 559 (76.6) | 1152 (78.4) | ||||
| Cardiac surgery, No. | 273 | 259 | 532 | NA | NA | NA | NA |
| Delirium | 96 (35.2) | 94 (36.5) | 190 (35.7) | 0.95 (0.67 to 1.36) | 0.97 (0.77 to 1.22) | .79 | 3.1 (−21.7 to 22.9) |
| No delirium | 177 (64.8) | 165 (63.7) | 342 (64.3) | ||||
| Noncardiac surgery, No. | 467 | 471 | 938 | NA | NA | NA | NA |
| Delirium | 51 (10.9) | 77 (16.3) | 128 (13.6) | 0.63 (0.43 to 0.92) | 0.67 (0.48 to 0.93) | .008 | 33.2 (7.1 to 52.0) |
| No delirium | 416 (89.1) | 394 (83.7) | 810 (86.4) | ||||
Abbreviations: NA, not applicable; RR, risk ratio; RRR, relative risk ratio.
The effect of our multimodal intervention was demonstrated in the primary GEE analysis (model 1). Delirium is the dependent variable, and intervention the primary explanatory variable, while controlling for differing baseline characteristics in both the intervention and control groups and outcomes (Table 4). A significant inverse association between the intervention and delirium incidence was found (OR, 0.87; 95% CI, 0.77-0.98; P = .02), indicating the substantial delirium risk reduction predicted in the primary analysis.
Table 4. Generalized Estimating Equation Analysis (Model 1a) With Delirium as Dependent Variableb.
| Variable | Coefficient, β (SE) | Wald χ2 | OR (95% CI) | P value | RRR (95% CI), % |
|---|---|---|---|---|---|
| Constant | −2.52 (0.73) | 11.994 | 0.08 (0.02 to 0.33) | .001 | 91.6 (74.9 to 97.2) |
| Intervention (intervention group) | −0.14 (0.06) | 5.380 | 0.87 (0.77 to 0.98) | .02 | 9.2 (−1.9 to 19.1) |
| Male | 0.66 (0.14) | 21.298 | 1.93 (1.46 to 2.55) | <.001 | −61.2 (−106.7 to −25.8) |
| Education (years) | −0.05 (0.03) | 2.65 | 0.95 (0.89 to 1.01) | .10 | 3.6 (−1.0 to 8.1) |
| SMI | 0.13 (0.15) | 0.784 | 1.14 (0.85 to 1.54) | .38 | −9.7 (−35.8 to 11.3) |
| CCI score | 0.01 (0.04) | 0.104 | 1.01 (0.94 to 1.09) | .75 | −0.8 (−5.9 to 4.1) |
| CSHA-CFS score | 0.42 (0.06) | 56.085 | 1.52 (1.36 to 1.69) | <.001 | −34.4 (−45.6 to −24.1) |
| Type of surgeryc | |||||
| Cardiac or vascular | 0.77 (0.39) | 3.970 | 2.16 (1.01 to 4.61) | .046 | −77.3 (−192.7 to −7.4) |
| Orthopedic/spine | −0.71 (0.33) | 4.571 | 0.49 (0.26 to 0.94) | .03 | 39.7 (5.7 to 61.5) |
| Abdominal | −0.36 (0.17) | 4.608 | 0.70 (0.50 to 0.97) | .03 | 23.8 (1.6 to 40.9) |
Abbreviations: CCI, Charlson Comorbidity Index; CSHA-CFS, Canadian Study of Health and Aging Clinical Frailty Scale; RRR, relative risk ratio; SMI, subjective memory impairment.
Adjusted for all variables that were significant between the intervention group (n = 718) and control group (n = 708) at baseline.
Quasi-likelihood under independence model criterion = 1368.165. Corrected quasi-likelihood under independence model criterion = 1334.618.
Reference category was other surgery.
Frailty (as measured by CSHA-CFS score; OR, 1.52; 95% CI, 1.36-1.69; P < .001) or male sex (OR, 1.93; 95% CI, 1.46-2.55; P < .001) were significantly associated with delirium. Undergoing cardiac surgery was associated with higher delirium incidence (OR, 2.16; 95% CI, 1.01-4.61; P = .046). The variance inflation factor value for the explanatory variables included in GEE model 1 ranged from 1.04 to 1.12, indicating the multicollinearity assumption was not violated.
The second GEE model (model 2) integrated 4 major delirium risk factors (eTable 5 in Supplement 2), and the effects were similar to model 1. Of the major risk factors, age (OR, 1.03; 95% CI, 1.01-1.06; P = .01) and dementia (OR, 4.44; 95% CI, 1.61-12.28; P = .004) were associated with delirium, while MoCA scores were inversely associated with delirium (OR, 0.89; 95% CI, 0.85-0.93; P < .001). The variance inflation factor value for the explanatory variables included in GEE model 2 ranged from 1.04 to 1.34.
Model 3 stratified for cardiac vs noncardiac surgery (eTable 6A and 6B in Supplement 2). The intervention and delirium were unrelated in patients undergoing cardiac surgery (OR, 1.18; 95% CI, 0.70-1.99; P = .54). For patients undergoing noncardiac surgery, the intervention was significantly inversely associated with delirium incidence (OR, 0.59; 95% CI, 0.35-0.99; P = .047). Adding cardiopulmonary bypass as a covariate to the cardiac surgery group did not change the outcomes (OR, 2.15; 95% CI, 0.99-4.62; P = .05).
To account for a time effect in the model, an interaction term between the intervention and duration in intervention variables was added to models 3a and 3b. Similar intervention effects were found in those undergoing noncardiac procedures (OR, 0.50; 95% CI, 0.27-0.95; P = .03) and cardiac surgery procedures (OR, 1.48; 95% CI, 0.82-2.66; P = .20).
Among 300 patients with baseline frailty (CSHA-CFS scores of 5 or higher), the intervention reduced postoperative delirium risk compared with those in the control group (OR, 0.62; 95% CI, 0.41-0.95; P = .03). The benefit was significant in 260 frail patients undergoing noncardiac surgery (OR, 0.67; 95% CI, 0.50-0.89; P = .005). The association between the intervention and delirium was not significant in 40 frail patients undergoing cardiac surgery (OR, 3.01; 95% CI, 0.14-63.33; P = .48).
Compared with patients in the control group, patients in the intervention group experienced fewer delirium days (523 vs 699 days; mean, 0.7 vs 1.0; mean difference, 0.3 days; 95% CI, 0.1-0.6; P = .03; n = 1457) and lower percentage of days with delirium (5.3% vs 6.9%; P = .03) (Table 4); once delirium occurred, its length in days was no different between groups (median, 3 days; P = .84) In patients undergoing noncardiac procedures, delirium days (171 vs 310 days; mean, 0.4 vs 0.7; mean difference, 0.3 days; 95% CI, 0.1-0.5; P = .006; n = 929) and percentage of days with delirium (3.0% vs 4.7%; P = .007) were significantly lower in the intervention group compared with the control group. No differences were identified in patients undergoing cardiac procedures.
The mean length of stay was significantly lower in the intervention group compared with the control group (11.1 vs 11.4 days; P = .01) (eTable 7 in Supplement 2). This benefit was significant in those undergoing cardiac procedures (10.7 vs 11.2 days; P = .046; n = 528) but not in those undergoing noncardiac procedures.
Fewer postoperative transfers to a rehabilitation hospital (intervention group: 336; control group: 410) occurred following intervention (χ21 = 16.54; P < .001). The intervention influenced postoperative medication requirements. In patients undergoing noncardiac procedures, opiate administration (χ21 = 21.76; P < .001), benzodiazepines (χ21 = 11.49; P = .001), and newly dispensed neuroleptics (χ21 = 6.94; P = .008) were reduced in the intervention group compared with the control group. For those undergoing cardiac procedures, only postoperative opiate administration decreased (χ21 = 33.74; P < .001).
Most patients undergoing cardiac procedures (intervention group: 152; control group: 200) spent at least 1 postoperative day in the intensive care unit (χ21 = 27.03; P < .001). No difference in intensive care unit length of stay was found between groups (t350 = 1.53; P = .13), a caveat being that one center routinely monitored postoperative patients in intensive care unit for 3 days. The intervention caused no adverse events. Falls, strokes or brain hemorrhage, hemorrhage, embolism, or thrombosis, death, and surgical reintervention were similar in both groups.
Discussion
The clinical delirium syndrome is the final common pathway for many pathophysiological conditions. The significant baseline cognitive frailty we describe in this population is compounded by the physiologic stress and various biochemical cascades inherent to the perioperative and surgical context. The individual’s vulnerability and delirium risk profile,12,19,20,21,22 combined with surgery, make prevention in this population a challenging task. Our daily multicomponent intervention significantly reduced the relative risk of delirium by 33.2% and its duration by 139 days overall (171 vs 310 days with delirium; mean, 0.4 vs 0.7 days; P = .006) in patients undergoing many types of surgical procedures. It also reduced potentially harmful pharmacological exposure.
Earlier delirium prevention or duration reduction studies targeting smaller, more homogeneous populations either showed no effect, higher delirium rates with intervention,36 or lesser improvements in delirium prevalence.12 To our knowledge, none evaluated preoperative risk, frailty, or included effect on various medications.
Little evidence supports the effectiveness of currently recommended multicomponent delirium prevention approaches.15,18 A systematic review24 describing nonpharmacological delirium prevention in surgical patients included 3 hip fracture trials in its meta-analysis, suggesting elective surgery requires a clearer definition. A Taiwanese center37 assessed delirium prevention in patients undergoing abdominal procedures using cluster methodology resembling ours. Specialized nurses’ daily prevention based on 3 HELP modules reduced delirium by more than 50% (RR, 0.44; 95% CI, 0.23-0.83). In contrast, a Duke University pre-post study led by surgeons keenly interested in risk-reduction in intraabdominal surgery perioperative periods improved length of stay, shock, and ileus rates but worsened delirium rates.36
To our knowledge, this trial is the largest multicenter study showing effective delirium prevention in elective surgery in older adults and the only one to examine a wide range of surgical procedures. Our AKTIVER delirium prevention program combined evidence-based best practice components ranging from preadmission risk-stratifying ongoing assessments and follow-up at hospital discharge. We combined strategic education and knowledge dissemination techniques with daily prevention. To these interventions, adapted to individual needs as described in medical settings, we added caregiver presence, reassurance to minimize anxiety, and the provision of humane support in unknown surroundings, based on the German The Older Person in the Operating Room model.43 As in other examples of delirium prevention bundles,62 pain management, medication reduction, and human interactions were addressed daily and systematically by nurse specialists during daily rounds. Our experts mentored the trained volunteers who provided AKTIVER module care daily, tailoring it to individual needs and family engagement. Our ability to integrate diverse AKTIVER activities in previously inexperienced centers within the 6 weeks of operationalizing the study and our intervention fidelity metrics speaks to its feasibility. A consistent benefit was shown across different groups of vulnerable older surgical patients. Since AKTIVER delirium prevention integrated volunteers and family members, intervention delivery consistency is maintained while minimizing costs.
We controlled for various risk factors through extensive presurgical phenotyping of multidimensional clinical parameters and cognitive status. The delirium rates we observed (21.6% overall, 35.7% in those undergoing cardiac procedures, and 13.6% in those undergoing noncardiac procedures) were similar to other reports.11,15,34,63 Our elderly patients (age range, 70 to 98 years) represented high-risk populations with cognitive deficits, multiple comorbidities, and frailty. Including neuropsychiatric diseases maximizes our findings’ generalizability.23 As hospitals grapple with elective surgery delays because of the diversion of resources owing to the worldwide COVID-19 pandemic, reducing postoperative complications in older patients appears timely. Most interventions in our cohort, albeit elective (eg, colon cancer requiring resection), required rapid surgery.
Hospital-based professionals were supported by trained volunteers and aides, a potentially useful resource during the COVID-19 pandemic and postpandemic periods, which strained worldwide health care resources. Patients, health care workers, and relatives all benefit from delirium prevention, as delirium burdens them all.64 We are analyzing hospital and long-term care costs and benefits to identify the economic benefits of this delirium prevention approach.65 Whether pairing our approach with a prehabilitation stimulation program66 would further reduce delirium incidence merits testing.
Limitations
The following limitations should be considered. Our intervention had no effect on delirium occurrence in patients undergoing cardiac surgery. To our knowledge, no publication describes effective nonpharmacological delirium prevention in this population. A small pilot study67 showed no effect on delirium severity. Pharmacological agents, notably dexmedetomidine, may prevent delirium after cardiac surgery63 and in the critically ill68 but require hemodynamic monitoring. Risk factors common in cardiac surgery (age, low ejection fraction, kidney insufficiency, atrial fibrillation, and vasculopathy)69 are unmodifiable, and blood-brain barrier disruption70 characterizes delirium in older patients after cardiac surgery. Bispectral index-guided anesthesia and modulating cerebral oxygenation might be protective.71 The effectiveness of perioperative physiological neuromonitoring-driven interventions further differentiates delirium prevention after cardiac vs noncardiac surgery. These findings and our study’s dichotomous results suggest the postoperative delirium pattern in cardiac surgery differs from general surgery postoperative delirium in risks, potential interventions, and outcomes.
The feasibility and effectiveness of this intervention should be tested, especially in smaller and nonacademic hospitals and in rural areas. Patient readmissions after hospital discharge were not documented and would have added valuable outcome-related information. Intensive care unit stay analyses must be interpreted cautiously, as hospital protocols differed in the clusters. The temporal change sensitivity analysis in our models suggests that 12-week intervals suffice to ascertain the effect of our intervention. We hope qualitative researchers will capture family and patient experience in future studies. Ongoing quality assurance initiatives could identify reproducibility in different medical cultures and languages.
Conclusions
The PAWEL trial successfully demonstrated that a structured, reliably reproducible, and safe nonpharmacological delirium prevention method is effective. The PAWEL protocol integrates a rigorously protocolized approach with individual patient’s needs. Its implementation significantly reduces postoperative delirium risk in patients 70 years or older undergoing noncardiac procedures. These results suggest that this delirium prevention program benefits patients undergoing elective general surgical and orthopedic procedures.
Trial protocol.
eTable 1. Baseline characteristics of participants in each centre.
eTable 2. List of surgical procedures performed.
eTable 3. Missing data for the main study variables.
eTable 4. Delirium rate (%) per period in each centre.
eTable 5. GEE analysis (model 2).
eTable 6. GEE analysis (model 3) of cardiac and noncardiac surgery group.
eTable 7. Effect of the intervention on overall delirium duration.
The PAWEL Study Group.
Data sharing statement.
References
- 1.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi: 10.1001/archinte.162.4.457 [DOI] [PubMed] [Google Scholar]
- 2.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi: 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 3.Bickel H, Gradinger R, Kochs E, Förstl H. High risk of cognitive and functional decline after postoperative delirium. a three-year prospective study. Dement Geriatr Cogn Disord. 2008;26(1):26-31. doi: 10.1159/000140804 [DOI] [PubMed] [Google Scholar]
- 4.Scottish Intercollegiate Guidelines Network . Risk reduction and management of delirium. Accessed October 6, 2021. https://www.sign.ac.uk/sign-157-delirium [DOI] [PubMed]
- 5.Australian Commission on Safety and Quality in Health Care . Delirium clinical care standard. Accessed October 6, 2021. https://www.safetyandquality.gov.au/our-work/clinical-care-standards/delirium-clinical-care-standard
- 6.National Institute for Health and Clinical Excellence . Delirium: prevention, diagnosis and management. Accessed October 6, 2021. https://www.nice.org.uk/guidance/cg103
- 7.Span P. The elderly are getting complex surgeries. often it doesn’t end well. The New York Times. June 7, 2019. Accessed February 8, 2021. https://www.nytimes.com/2019/06/07/health/elderly-surgery-complications.html
- 8.Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. doi: 10.1056/NEJM199903043400901 [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi: 10.1046/j.1532-5415.2001.49108.x [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;(3):CD005563. doi: 10.1002/14651858.CD005563.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. doi: 10.1001/jamainternmed.2014.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen TL, Alberts AR, Hooft L, Mattace-Raso F, Mosk CA, van der Laan L. Prevention of postoperative delirium in elderly patients planned for elective surgery: systematic review and meta-analysis. Clin Interv Aging. 2019;14:1095-1117. doi: 10.2147/CIA.S201323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldham MA, Flanagan NM, Khan A, Boukrina O, Marcantonio ER. Responding to ten common delirium misconceptions with best evidence: an educational review for clinicians. J Neuropsychiatry Clin Neurosci. 2018;30(1):51-57. doi: 10.1176/appi.neuropsych.17030065 [DOI] [PubMed] [Google Scholar]
- 14.Seib CD, Rochefort H, Chomsky-Higgins K, et al. Association of patient frailty with increased morbidity after common ambulatory general surgery operations. JAMA Surg. 2018;153(2):160-168. doi: 10.1001/jamasurg.2017.4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth. 2019;123(4):464-478. doi: 10.1016/j.bja.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watt J, Tricco AC, Talbot-Hamon C, et al. Identifying older adults at risk of delirium following elective surgery: a systematic review and meta-analysis. J Gen Intern Med. 2018;33(4):500-509. doi: 10.1007/s11606-017-4204-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults . American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142-150. doi: 10.1111/jgs.13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenther U, Riedel L, Radtke FM. Patients prone for postoperative delirium: preoperative assessment, perioperative prophylaxis, postoperative treatment. Curr Opin Anaesthesiol. 2016;29(3):384-390. doi: 10.1097/ACO.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 20.Gosselt AN, Slooter AJ, Boere PR, Zaal IJ. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care. 2015;19:346. doi: 10.1186/s13054-015-1060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wada S, Inoguchi H, Sadahiro R, et al. Preoperative anxiety as a predictor of delirium in cancer patients: a prospective observational cohort study. World J Surg. 2019;43(1):134-142. doi: 10.1007/s00268-018-4761-0 [DOI] [PubMed] [Google Scholar]
- 22.Simoncini M, Gatti A, Quirico PE, et al. Acupressure in insomnia and other sleep disorders in elderly institutionalized patients suffering from Alzheimer’s disease. Aging Clin Exp Res. 2015;27(1):37-42. doi: 10.1007/s40520-014-0244-9 [DOI] [PubMed] [Google Scholar]
- 23.Martin RC, DiBlasio CA, Fowler ME, Zhang Y, Kennedy RE. Assessment of the generalizability of clinical trials of delirium interventions. JAMA Netw Open. 2020;3(7):e2015080. doi: 10.1001/jamanetworkopen.2020.15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. the SENATOR project ONTOP Series. PLoS One. 2015;10(6):e0123090. doi: 10.1371/journal.pone.0123090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez A, Thomas C, Deeken F, et al. ; PAWEL Study group . Patient safety, cost-effectiveness, and quality of life: reduction of delirium risk and postoperative cognitive dysfunction after elective procedures in older adults-study protocol for a stepped-wedge cluster randomized trial (PAWEL Study). Trials. 2019;20(1):71. doi: 10.1186/s13063-018-3148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurrle S, Bateman C, Cumming A, Pang G, Patterson S, Temple A. Implementation of a model of care for hospitalised older persons with cognitive impairment (the Confused Hospitalised Older Persons program) in six New South Wales hospitals. Australas J Ageing. 2019;38(suppl 2):98-106. doi: 10.1111/ajag.12690 [DOI] [PubMed] [Google Scholar]
- 27.Inouye SK, Bogardus ST Jr, Baker DI, Leo-Summers L, Cooney LM Jr; Hospital Elder Life Program . The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48(12):1697-1706. doi: 10.1111/j.1532-5415.2000.tb03885.x [DOI] [PubMed] [Google Scholar]
- 28.Godfrey M, Green J, Smith J, et al. Process of implementing and delivering the Prevention of Delirium system of care: a mixed method preliminary study. BMC Geriatr. 2019;20(1):1-15. doi: 10.1186/s12877-019-1374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young J, Green J, Farrin A, et al. A multicentre, pragmatic, cluster randomised, controlled feasibility trial of the POD system of care. Age Ageing. 2020;49(4):640-647. doi: 10.1093/ageing/afaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singler K, Thomas C. HELP—Hospital Elder Life Program—multimodal delirium prevention in elderly patients. Internist (Berl). 2017;58(2):125-131. doi: 10.1007/s00108-016-0181-0 [DOI] [PubMed] [Google Scholar]
- 31.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. doi: 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pretto M, Spirig R, Milisen K, Degeest S, Regazzoni P, Hasemann W. Effects of an interdisciplinary nurse-led Delirium Prevention and Management Program (DPMP) on nursing workload: a pilot study. Int J Nurs Stud. 2009;46(6):804-812. doi: 10.1016/j.ijnurstu.2009.01.015 [DOI] [PubMed] [Google Scholar]
- 33.Hasemann W, Tolson D, Godwin J, Spirig R, Frei IA, Kressig RW. A before and after study of a nurse led comprehensive delirium management programme (DemDel) for older acute care inpatients with cognitive impairment. Int J Nurs Stud. 2016;53:27-38. doi: 10.1016/j.ijnurstu.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 34.Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192-214. doi: 10.1097/EJA.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 35.Solberg LM, Campbell CS, Jones K, et al. Training Hospital Inpatient Nursing to Know (THINK) delirium: a nursing educational program. Geriatr Nurs. 2021;42(1):16-20. doi: 10.1016/j.gerinurse.2020.10.018 [DOI] [PubMed] [Google Scholar]
- 36.McDonald SR, Heflin MT, Whitson HE, et al. Association of integrated care coordination with postsurgical outcomes in high-risk older adults: the Perioperative Optimization of Senior Health (POSH) initiative. JAMA Surg. 2018;153(5):454-462. doi: 10.1001/jamasurg.2017.5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg. 2017;152(9):827-834. doi: 10.1001/jamasurg.2017.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173-178. doi: 10.1097/SLA.0b013e31818e4776 [DOI] [PubMed] [Google Scholar]
- 39.Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi: 10.1093/ageing/afi031 [DOI] [PubMed] [Google Scholar]
- 40.Agency for Clinical Innovation . Care of Confused Hospitalised Older Persons (CHOPS). Accessed November 13, 2021. https://aci.health.nsw.gov.au/chops
- 41.American Geriatrics Society . CoCare: HELP homepage. Accessed October 6, 2021. https://help.agscocare.org/
- 42.Evangelisches Klinikum Bethel . help+ Das Delir vermeiden. Accessed October 6, 2021. https://evkb.de/kliniken-zentren/besondere-angebote/delir-praevention-help/
- 43.Gurlit S, Möllmann M. How to prevent perioperative delirium in the elderly? Z Gerontol Geriatr. 2008;41(6):447-452. doi: 10.1007/s00391-008-0020-6 [DOI] [PubMed] [Google Scholar]
- 44.Lee SY, Fisher J, Wand APF, et al. Developing delirium best practice: a systematic review of education interventions for healthcare professionals working in inpatient settings. Eur Geriatr Med. 2020;11(1):1-32. doi: 10.1007/s41999-019-00278-x [DOI] [PubMed] [Google Scholar]
- 45.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the Confusion Assessment Method. a new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941 [DOI] [PubMed] [Google Scholar]
- 46.Thomas C, Kreisel SH, Oster P, Driessen M, Arolt V, Inouye SK. Diagnosing delirium in older hospitalized adults with dementia: adapting the confusion assessment method to International Classification of Diseases, Tenth Revision, Diagnostic Criteria. J Am Geriatr Soc. 2012;60(8):1471-1477. doi: 10.1111/j.1532-5415.2012.04066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312-318. doi: 10.1111/j.1532-5415.2005.53120.x [DOI] [PubMed] [Google Scholar]
- 48.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62(3):518-524. doi: 10.1111/jgs.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 50.Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiatry. 1999;156(4):531-537. [DOI] [PubMed] [Google Scholar]
- 51.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 52.Lachs MS, Feinstein AR, Cooney LM Jr, et al. A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med. 1990;112(9):699-706. doi: 10.7326/0003-4819-112-9-699 [DOI] [PubMed] [Google Scholar]
- 53.Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-621. doi: 10.1016/S0033-3182(09)70864-3 [DOI] [PubMed] [Google Scholar]
- 54.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61-65. [PubMed] [Google Scholar]
- 55.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489-495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? a systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woertman W, de Hoop E, Moerbeek M, Zuidema SU, Gerritsen DL, Teerenstra S. Stepped wedge designs could reduce the required sample size in cluster randomized trials. J Clin Epidemiol. 2013;66(7):752-758. doi: 10.1016/j.jclinepi.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 58.Gallis JA, Turner EL. Relative measures of association for binary outcomes: challenges and recommendations for the global health researcher. Ann Glob Health. 2019;85(1):137. doi: 10.5334/aogh.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ambrogi F, Biganzoli E, Boracchi P. Estimates of clinically useful measures in competing risks survival analysis. Stat Med. 2008;27(30):6407-6425. doi: 10.1002/sim.3455 [DOI] [PubMed] [Google Scholar]
- 60.Church S, Rogers E, Rockwood K, Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393. doi: 10.1186/s12877-020-01801-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy SL, Gutman SA. Intervention fidelity: a necessary aspect of intervention effectiveness studies. Am J Occup Ther. 2012;66(4):387-388. doi: 10.5014/ajot.2010.005405 [DOI] [PubMed] [Google Scholar]
- 62.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3-14. doi: 10.1097/CCM.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pieri M, De Simone A, Rose S, et al. Trials focusing on prevention and treatment of delirium after cardiac surgery: a systematic review of randomized evidence. J Cardiothorac Vasc Anesth. 2020;34(6):1641-1654. doi: 10.1053/j.jvca.2019.09.028 [DOI] [PubMed] [Google Scholar]
- 64.Sturm H, Wildermuth R, Stolz R, et al. Diverging awareness of postoperative delirium and cognitive dysfunction in German health care providers. Clin Interv Aging. 2019;14:2125-2135. doi: 10.2147/CIA.S230800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi: 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Humeidan ML, Reyes JC, Mavarez-Martinez A, et al. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the Neurobics randomized clinical trial. JAMA Surg. 2021;156(2):148-156. doi: 10.1001/jamasurg.2020.4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mailhot T, Cossette S, Côté J, et al. A post cardiac surgery intervention to manage delirium involving families: a randomized pilot study. Nurs Crit Care. 2017;22(4):221-228. doi: 10.1111/nicc.12288 [DOI] [PubMed] [Google Scholar]
- 68.Skrobik Y, Duprey MS, Hill NS, Devlin JW. Low-dose nocturnal dexmedetomidine prevents ICU delirium. a randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2018;197(9):1147-1156. doi: 10.1164/rccm.201710-1995OC [DOI] [PubMed] [Google Scholar]
- 69.Kotfis K, Szylińska A, Listewnik M, et al. Early delirium after cardiac surgery: an analysis of incidence and risk factors in elderly (≥65 years) and very elderly (≥80 years) patients. Clin Interv Aging. 2018;13:1061-1070. doi: 10.2147/CIA.S166909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abrahamov D, Levran O, Naparstek S, et al. Blood-brain barrier disruption after cardiopulmonary bypass: diagnosis and correlation to cognition. Ann Thorac Surg. 2017;104(1):161-169. doi: 10.1016/j.athoracsur.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 71.Eertmans W, De Deyne C, Genbrugge C, et al. Association between postoperative delirium and postoperative cerebral oxygen desaturation in older patients after cardiac surgery. Br J Anaesth. 2020;124(2):146-153. doi: 10.1016/j.bja.2019.09.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eTable 1. Baseline characteristics of participants in each centre.
eTable 2. List of surgical procedures performed.
eTable 3. Missing data for the main study variables.
eTable 4. Delirium rate (%) per period in each centre.
eTable 5. GEE analysis (model 2).
eTable 6. GEE analysis (model 3) of cardiac and noncardiac surgery group.
eTable 7. Effect of the intervention on overall delirium duration.
The PAWEL Study Group.
Data sharing statement.