Abstract
Background
Existing therapeutic methods for reduction or removal of superficial vascular malformations and tumors have high risks of scarring and other complications that result in aesthetic appearance less favorable than the baseline. Patients are often cautioned against intervention, which can lead to psychosocial problems and low self‐esteem. Improved treatment modalities are therefore relevant from both medical and aesthetic perspectives.
Methods
Two volunteer subjects were treated with a medical 20 MHz high intensity focused ultrasound device developed for dermatological conditions. One patient was given three treatments to remove a superficial congenital hemangioma on the left middle cheek. The other patient was given a single treatment to remove seven cherry angiomas on the thighs. Handpieces with nominal focal depths of 0.8 – 1.8 mm below the skin surface were used to administer acoustic energy of 1.1 – 1.2 J/dose. An integrated dermoscope in the handpiece was used to monitor the treatment in real‐time.
Results
During treatment, blood in the capillary network of the lesions was coagulated immediately, and capillary walls were collapsed due to the thermal and mechanical effects of the high intensity focused ultrasound. During the healing phase, the areas regenerated a normal skin structure with very limited scar or dyspigmentation. At follow‐up, a clear aesthetic improvement was observed over the baseline for all treated targets with the exception of two cherry angiomas, where focal depth and/or dose coverage had not been optimal.
Conclusion
High intensity focused ultrasound is concluded to be a safe and efficient skin treatment for benign superficial vascular malformations and tumors.
Keywords: cherry angioma, congenital hemangioma, dermatology, focused ultrasound, HIFU, vascular tumor
1. INTRODUCTION
Virtually, all humans will experience various forms of benign vascular neoplasms during their lifetime. These can be congenital vascular malformations or develop as various vascular tumors at any later stage of life. 1 , 2
Congenital and infantile hemangiomas, colloquially termed "strawberry marks”, are common benign tumors of infancy. They may show a proliferative phase in the first month after childbirth (in case of infantile hemangiomas), but often involute spontaneously during early childhood. A subgroup of both congenital and infantile hemangiomas, however, persist or only partially involute, and remain as permanently visible lesions in adulthood. 3 Typically, the persisting lesions present as red or dark superficial telangiectasia of various capillary widening, and often with some atrophy and decreased skin elasticity. 3 , 4 , 5
In older life, vascular neoplasms, for example, in the form of cherry angiomas (senile angiomas, de Morgan spots), become extremely common. These tumors presents as round, slightly elevated, ruby red papules of up to 10 mm in diameter. Almost all humans at the age above 70 years will have at least one, and typically several, most often located on the trunk. 1 , 2 , 6
As both strawberry marks and cherry angiomas are predominantly benign and asymptomatic, they are often only treated for medical reasons in special situations where they ulcerate, cause pain, or in other ways impair the person's development or daily life. 1 , 2 , 3 , 4 , 5 , 6 While such treatments generally have low rates of adverse events from a strictly curative viewpoint, 7 , 8 treatments with the primary objective of improved aesthetic appearance are, however, much more problematic.
Ablative light‐based treatments using various methods, including IPL and CO2, Nd:YAG, and pulse‐dye lasers, have high risks of scarring. Likewise, surgical methods, such as excision, electrocautery, or cryotherapy, also frequently give a final appearance of the involved area that is less favorable than the baseline. In many cases, patients are therefore cautioned against intervention in consideration of the benign nature of the condition. 1 , 2 , 3 , 4 , 5 , 6 Persisting lesions, in particular originating from non‐involuting congenital/infantile hemangiomas in young people, may, however, cause significant psychosocial problems and low self‐esteem. 9
New modalities for removal and/or aesthetic improvements of such lesions are therefore relevant, and address large patient groups looking for solutions from both a medical and aesthetic perspective.
High Intensity Focused Ultrasound (HIFU) has been introduced over the last decade as a method for non‐invasive treatment for several critical indications, including a wide range of internal cancers of major organs, bone metastases, and cerebral pathologies. In these treatments, HIFU focal zones are positioned deep within the body or in the brain, with the anatomical location and the chosen focal point guided by MRI scanning or ultrasound imaging. In the focal zone inside the body, reproducible temperatures of about 60–80°C are reported, thus facilitating local cell necrosis without adversely affecting adjacent tissue. 10 , 11 , 12 , 13 These HIFU systems typically operate at frequencies from 500 kHz to approximately 3 MHz.
The operation frequency of a HIFU system is always a compromise between the needed penetration depth and the size/resolution of the focal zone in the target. 11 , 14 Lower frequency ultrasound exhibits low attenuation, and thereby low loss of signal amplitude at targets deep inside the body. A low frequency, however, also means longer wavelength, and thereby larger and less well‐defined focal zones, which consequently leads to lower treatment accuracy and higher risk of inflicting damage to tissue outside the specific target.
High intensity focused ultrasound systems operating at higher frequencies for lower penetration depths, have been made widely commercially available during the same period where the medical systems were developed. These systems are almost exclusively used for aesthetic treatments, typically skin rejuvenation, and facial wrinkle reduction. The systems operates at 4–10 MHz, where focal zones are small enough to be confined to the lower dermis and subcutaneous layers located approximately 2–6 mm below the skin surface. 15 , 16 The systems are therefore primarily used to target and manipulate the adipose and collagen structures in these layers. The dimensions of the focal zones generated by such devices are, however, fundamentally close to, and most often higher than, the total thickness of the human dermis. 17 Very superficial therapeutic targets, for example, tumors localized in the epidermis, basement membrane, and upper dermis 0.5–1.5 mm from the skin surface, are thus out of scope for all such devices.
In recent publications, it has been demonstrated that HIFU operating at 20 MHz can produce confined and localized focal zones in the human dermis and epidermis. In the very small focal zone created at this high frequency, the clinical effect in the skin can furthermore be controlled and adjusted to obtain responses ranging from mild thermal activation to acute cell necrosis, due to combined thermal and mechanical effects. 18
The new treatment modality has been confirmed in both animal and humans, where pathological and clinical evaluations after healing only identified mild fibroplasia in cases of mid‐ and deep‐dermis focal points combined with high energy dosage. Treatments using more superficial focal points and/or lower energy dosages typically only showed minimal clinical change, with full recovery of the normal epidermal and dermal structures after a complete healing period of some 2–6 months. The 20 MHz HIFU system has therefore been concluded safe and relevant for a wide range of different dermatological indications. 18 , 19 Actinic keratosis, basal cell carcinoma, and Kaposi sarcoma were treated successfully as the first demonstration of the method applied to human medical indications. 20
With background in these results, the hypothesis for this report is that the effects from 20 MHz HIFU can selectively coagulate blood in the capillary network of various superficial vascular lesions. The soft tissue in the lesions will furthermore be denaturated and necrotized, and capillaries will be blocked for fresh blood supply. During healing and regeneration, all damaged tissue is expected to be replaced by normal skin cells. The trauma introduced by the proposed treatment can be concentrated and confined to the upper part of the dermis only, where scarring and other complications affecting the aesthetic outcome after healing are generally known to be mild.
The treatment of two cases investigating the above modality is the first application of dermatological 20 MHz HIFU to vascular lesions exemplified by congenital hemangioma and cherry angiomas.
2. MATERIALS AND METHODS
2.1. HIFU equipment
High intensity focused ultrasound treatment was performed using a commercial medical 20 MHz HIFU device (System ONE‐M, TOOsonix A/S). 21
The system has four different handpieces with nominal focal depths (NFD) of 0.8, 1.3, 1.8, and 2.3 mm. This corresponds to maximum focal zone depth location ranging from the epidermis to the deep dermis of normal human skin. HIFU is administered by foot‐switch activation of 50–500 ms doses having a maximum acoustic energy of up to 1.3 J/dose.
The system facilitates that treatment progress can be surveyed in real‐time via an integrated dermoscope in the handpiece. This allows the operator to selectively target and administer treatment to a general area, follow a precise contour of an individual feature, or apply high dose‐coverage to a particular target location.
Parker Aquasonic 100 ultrasound gel (Parker Laboratories Inc.) is used to provide acoustic coupling between the handpiece and skin. The system is shown in Figure 1.
FIGURE 1.

TOOsonix System ONE‐M. 20 MHz HIFU is transmitted to target lesions in the epidermis and dermis of the skin. The system has an integrated high‐resolution color dermoscope that allows real‐time control of the treatment
2.2. Subject recruitment
Subjects were the volunteers of the Old Town Clinic, Wroclaw, Poland, requesting treatment for aesthetic reasons. Treatments were compliant to the labeling of the device. Thorough information about the treatment was given, and written consent for participation in the study and publication of anonymized photographs were obtained before any treatment was started.
3. CASE 1—CONGENITAL HEMANGIOMA
A female subject aged 43 years, presented a vascular lesion in the form of a non‐involuted congenital hemangioma of approximately 4–5 cm in total diameter on her right cheek. At frequent and continuous consultations by dermatologists since infancy, she had been advised against therapy. Lack of suitable therapeutic options and high risks of scarring, combined with the benign/aesthetic nature of the condition was given as reasons for withholding intervention.
Clinically, the lesion consisted of two major, almost separate, red and slightly elevated areas (upper and lower). Dermoscopy of the area using a Fotofinder Medicam 1000 (FotoFinder Systems GmbH) revealed a fine superficial capillary network with evenly distributed round junctions, typically less than 0.5 mm in diameter. A photograph and dermoscope picture of the lesion before treatment is shown in Figure 2.
FIGURE 2.
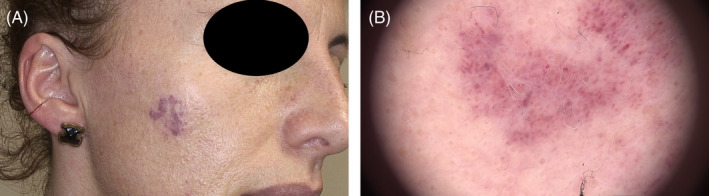
Congenital hemangioma on right cheek. (A): Photograph showing location of two closely spaced lesions. (B): Dermoscope picture of section in upper lesion showing a very fine capillary network connecting evenly distributed and round junctions of slightly larger capillary widening
3.1. HIFU treatment
The capillary network was very superficial and the tissue was soft, thus giving good confidence that a treatment with the most superficial handpiece with NFD 0.8 mm would be adequate to coagulate and stop the active blood flow with denaturation and collapse of the fine capillary walls. Risks for complications with the new HIFU method were thus considered minimal.
A pulse duration of 150 ms/dose at 1.1 J/dose was selected based on previous experience.
An initial HIFU treatment was applied to the upper and lower parts of the lesion, respectively, in two separate sessions two weeks apart. This was done to get an initial clinical evaluation of the healing dynamics and efficacy of the treatment. In the initial sessions, the upper and lower parts were treated with approximately 40 doses each, administered as shoulder‐by‐shoulder doses spaced approximately 2 mm apart at intervals of approximately 1 s. Treatment of remaining open capillaries and small capillary junctions in the overall lesion area was performed in a third session 8 weeks after the initial treatment. Approximately 40 doses was administered. The facility to accurately trace and target individual remaining open capillaries in real‐time via the integrated dermoscope in the handpiece was utilized to minimize the total number of doses.
The pain level during treatment was scored at approximately 2 on a 10‐point VAS scale, and reported to be present during the very short HIFU transmission only.
3.2. Immediate treatment effects after HIFU
As expected and reported elsewhere, 19 , 20 , 21 , 22 , 23 the immediate effects of HIFU treatment observed in the skin were localized whitening and edema in circular areas of approximately 1–2 mm in diameter around each HIFU target. Blood in the capillary junctions was coagulated as a result of the thermal and mechanical effect from HIFU, whereby the appearance of the area changed from red to black over a few seconds after each dose.
At end of treatment, the target area showed a blister‐like white swelling, where the epidermis sometimes had partially separated. The surrounding area furthermore showed an urticarial response with light redness due to histamine release, which disappeared over approximately one hour following treatment. Even if the basement membrane was broken during use of the very shallow focal depth of the selected handpiece, no bleeding was observed, and the treated area remained dry and intact.
Photographs of the upper and lower part of the lesion directly after the first HIFU treatments is shown in Figure 3.
FIGURE 3.

Upper (A) and lower (B) region of congenital hemangioma directly after HIFU Treatment. The two treatment sessions are 2 weeks apart. Whitening and edema can be observed at the location of each HIFU dose. An urticarial “wheal and flare” response in the general surrounding area can be observed
3.3. Follow‐up and results after treatment
After a few days, sporadic dry wound crusts formed on areas treated with closely spaced HIFU doses. The majority of the area that were treated with less dense dose‐coverage recovered naturally without external crust formation, and only inflammatory redness was visible from the surface after the immediate edema had ended.
The fields with crust formation gradually healed over approximately 2 weeks, with subsequent spontaneous loosening of the wound crust. After 6 weeks, the skin surface had normalized, and only a mild inflammatory redness was visible. After 15 weeks, the treated area was healed to an almost normal skin color and appearance. Dermoscope imaging confirmed a significant reduction in the capillary network and round junctions and indicated an almost fully recovered skin structure. Some minor capillaries exist in the periphery of the original lesion, but are difficult to distinguish from the immediate surrounding skin.
The clinical result and dermoscope picture 15 weeks after the first treatment is shown in Figure 4.
FIGURE 4.

Congenital hemangioma 15 weeks after first HIFU treatment. (A): Photograph showing treated area with decreased visual redness of area and generally good aesthetic result. (B): Dermoscope picture of section in upper lesion showing strongly decreased capillary network and hardly visible round junctions in the primary treatment area
4. CASE 2—CHERRY ANGIOMAS
A female subject aged 40 years presented several cherry angiomas on both thighs that she requested to be removed due to cosmetic considerations.
Clinically, all angiomas were characterized by the typical clearly elevated, ruby red rounded soft papules of 0.5–5.5 mm in diameter. The blood supply and demarcation of the edges were easy to identify visually, and thus good targets for coagulation of the soft tissue and collapse of the capillary walls by HIFU. Dermoscopy and clinical evaluation were used to characterize/categorize the capillary network and estimate the depth of each lesion.
The location and numbering of treated lesions is shown in Figure 5.
FIGURE 5.
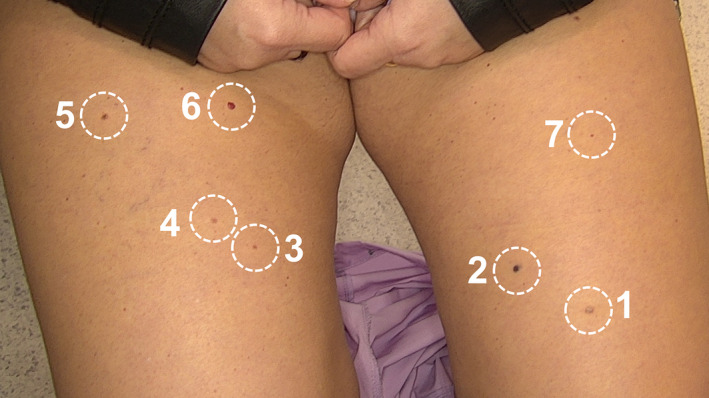
Distribution of seven cherry angiomas selected for treatment by HIFU
4.1. HIFU treatment
High intensity focused ultrasound was applied in a single session using a dose duration of 150 ms/dose and an acoustic energy of 1.2 J/dose.
Each angioma was treated with 5–10 doses placed closely in the center of the angioma, and with approximately 1 mm spacing between each dose around the periphery.
Handpieces with nominal focal depths of 0.8, 1.3, and 1.8 mm were used depending on the estimation of the depth of each individual lesion. For deep lesions, a “sandwich” treatment was chosen, where the center of the angioma was treated with a handpiece having a NFD of 1.8 mm followed directly by treatment with a handpiece having a NFD of 0.8 mm.
The patient scored the pain level associated with the procedure as 2–3 on a 10‐point VAS scale depending on the location of the lesion.
4.2. Immediate treatment effects after HIFU
Due to the high blood content, most cherry angiomas reacted with an immediate color change from red to black following each HIFU dose. This was accompanied by a significant contraction of the angioma, following the collapse and denaturation of the very soft tissue in the affected focal volume. The periphery of the angioma, consisting mainly of normal skin, exhibited the same effects of whitening and edema as observed in previous treatments on normal skin. 19 , 20 , 21 , 22 , 23
The surrounding area around each lesion showed a mild urticarial response with light redness due to histamine release in the minutes following treatment. Due to the low number of dosing in each location, this effect was, however, less than that observed in the treatment of larger fields, for example, the treatment of the congenital hemangioma, where a higher number of doses were used to cover a larger coherent area.
4.3. Follow‐up after treatment
All treated areas generally developed a dry wound crusts over 1–3 days following treatment. After 7–10 days, the crusts spontaneously released from the healing skin below. The regenerated skin surface showed some inflammatory redness in the direct area of treatment, which faded to normal color approximately 2 mm from the treatment edges.
After healing, the treatment resulted in full removal of five out of seven treated cherry angiomas. A summary of the treatments and results is shown in Table 1.
TABLE 1.
Overview of treated cherry angiomas and results after 12 months
| Result 12 months after HIFU | |||||||
|---|---|---|---|---|---|---|---|
| Num | Diam (mm) | Capillaries | Depth | Nominal focal depth (mm) | Remaining capillaries | Fibrosis | De‐pigment |
| 1 | 5.5 | Fine | Deep | 1.8 + 0.8 | None | Mild | None |
| 2 | 4.0 | Very course | Deep | 1.8 + 0.8 | ~50% Reduced Heterogenous | Mild | N/A |
| 3 | 2.5 | Medium | Shallow | 0.8 | None | Mild | Mild |
| 4 | 3.5 | Fine | Shallow | 0.8 | None | None | None |
| 5 | 4.0 | Course | Shallow | 0.8 | None | Very mild | Mild |
| 6 | 5.0 | Very fine | Deep | 1.8 + 0.8 | ~10% Reduced Homogeneous | None | N/A |
| 7 | 2.0 | Medium | Medium | 1.3 | None | None | None |
The inflammatory redness in the successfully treated lesions gradually decreased over the week following treatment. After 12 weeks, the treatment sites were barely visible and showed no signs of tenderness or any other abnormal behavior according to the subject. At clinical follow‐up after 6 months, the surrounding inflammatory redness had fully disappeared.
The five angiomas that were fully removed thereby demonstrated the hypothesized immediate coagulation‐and‐contraction reaction during HIFU treatment. This therefore confirmed that the focal zones were located in the appropriate depth corresponding to the capillary network, and that the network was sufficiently fine to be fully collapsed by the HIFU doses. Very little fibrotic tissue could be observed after healing, and the original lesions were only visible as areas with very mild hypopigmentation, but a restored triangular skin pattern. Examples of successfully removed cherry angiomas are shown in Figures 6, 7, 8.
FIGURE 6.

Example of dermoscope pictures from successful removal of Cherry Angioma Number 4. (A): Before HIFU treatment. (B): Immediately after HIFU treatment. (C): 6 months after HIFU. (D): 12 months after HIFU treatment. A full removal without fibrotic tissue, remanent capillary network, or de‐pigmentation can be observed
FIGURE 7.

Example of dermoscope pictures from successful removal of Cherry Angioma Number 1. (A): Before HIFU treatment. (B): Immediately after HIFU treatment. (C): 6 months after HIFU treatment. (D): 12 months after treatment. A full removal of the angioma with minimal fibrotic change and no remanent capillary network or de‐pigmentation can be observed
FIGURE 8.

Example of dermoscope pictures from partially successful removal of Cherry Angioma Number 5. (A): Before HIFU treatment. (B): Immediately after HIFU treatment. (C): 6 weeks after HIFU treatment. (D): 12 months after HIFU treatment. A full removal with, but with some fibrotic change and de‐pigmentation can be observed
The two angiomas that had unsuccessful removal were both large in size and estimated to extend deeper into the dermis than the smaller lesions. One angioma, number 2 shown in Figure 9, had a heterogeneous and incomplete removal of the lesion only, indicating that treatment depth and dose coverage were only partially correct. The second unsuccessful result, angioma number 6 shown in Figure 10, had a much finer capillary structure with significantly higher blood perfusion, and therefore also showed a different reaction to HIFU than the other six lesions. Only a small reduction in size was observed after healing, but the color and tissue structure was unchanged compared to the baseline.
FIGURE 9.

Example of dermoscope pictures from unsuccessful removal of Cherry Angioma Number 2. (A): Before HIFU treatment. (B): 6 months after HIFU treatment. (C): 12 month after HIFU treatment. A partial reduction of the angioma can be observed, but parts of the original angioma has regenerated a capillary network and is supplied with fresh blood. The capillaries can be observed to be relatively deep and course, and the original HIFU treatment has thus not been sufficiently deep and densely administered
FIGURE 10.
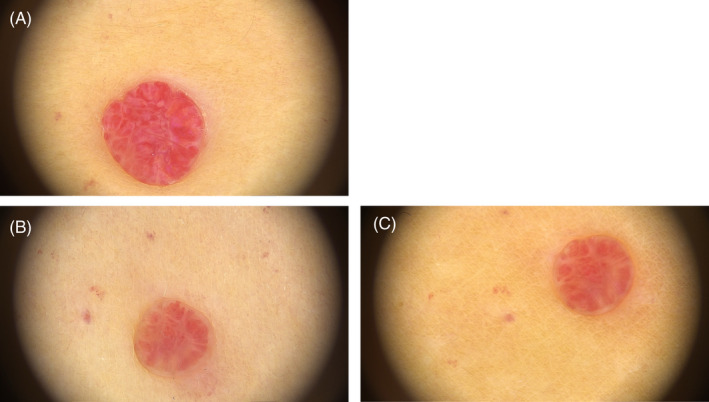
Example of dermoscope pictures from unsuccessful removal of Cherry Angioma Number 6. (A): Before HIFU treatment. (B): 6 months after HIFU treatment. (C): 12 months after HIFU treatment. A reduction in the size and capillary network can be observed, but the angioma has regenerated most capillary network and the soft tissue and is supplied with fresh blood
A comparison‐photograph of the subject before and after treatment is shown in Figure 11.
FIGURE 11.

Macroscopic photographs. (A): Before HIFU treatment. (B): 12 months after HIFU treatment. Five of the seven cherry angiomas are not clinically visible. Two cherry angiomas (number 2 and 6) have been reduced in sized but are still clearly visible and would need re‐treatment to be fully removed
5. DISCUSSION/CONCLUSION
The two cases illustrate that 20 MHz HIFU is a relevant new treatment modality for superficial vascular lesions, where patients have previously been advised against therapy due to an unfavorable risk‐benefit profile for the aesthetic outcome.
The hypothesized effects of HIFU‐generated blood coagulation in the capillary network could be observed immediately after treatment in both cases, where treated areas changed from red to a darker black/blue color. Immediate denaturation and collapse of the capillary walls were clearly observed as a whitening effect and edema appearing on the surface of the skin in an area of approximately 1–2 mm around each HIFU target center. The collapse was furthermore often very clearly visible as a contraction of the tissue, particularly in the treatment of the smaller protruding cherry angiomas.
In the case of the congenital hemangioma, two sessions were performed as initial partial treatments of separate areas followed by a third combined treatment. The full treatment result could therefore potentially have been reached in two sessions only.
In the case of the cherry angioma, a single session was enough to remove five out of seven lesions. The two lesions that were not successfully removed were characterized by considerably different depth, blood perfusion, and capillary widening. It is therefore likely that adapted treatment settings and/or another handpiece selection would give better results for these lesions also. Such adaptations could be assisted by high‐frequency ultrasound imaging diagnosis before treatment. This has been demonstrated as highly beneficial in other treatments, where a 20 MHz ultrasound imaging system was used to diagnose the exact depths and extend of lesions before treatment. 22
Pain levels during all treatments were very mild, and of transient nature only. The reported pain scores of 2–3 on a 10‐point scale are significantly lower than the pain reported from, for example, treatment by laser, 20 , 23 and well within a tolerable range considering the very transient nature of the treatment. While use of topical anesthesia (EMLA®, AstraZeneca) has been shown previously to decrease pain significantly, 23 it was therefore not deemed necessary in these cases. For future treatment on other more pain‐sensitive anatomic locations or patient groups, it can, however, be prescribed as a standard self‐administered pre‐treatment option.
No bleeding, post‐treatment pain, or any other adverse effects were noted from any of the treatments. Directly after treatment, only a mild urticarial response was observed, which gradually disappeared over the first few hours. The subjects did not experience any down‐time following treatments. Some very mild inflammatory redness persisted in the exact target area some months after treatment in both cases, which gradually decreased over the following months.
In the case of the relatively superficial and flat congenital hemangioma, limited solid wound crust formation was observed, and the majority of transport of damaged and dead cells from the treatment were therefore done through the internal lymphatic and vascular systems. In the more concentrated treatments of protruding cherry angiomas, a dry wound crust was generated quickly after treatment, and transport of damaged and dead cells was thereby facilitated as an external separation.
Dermoscopy at the end of the study confirmed the overall positive result. Treated capillaries were closed and were no longer visible, with exception of two problematic cherry angiomas. The analysis could not detect any structures or features that could indicate potential recurrence.
Remaining untreated capillaries and round capillary junctions in the periphery of the original congenital hemangioma may be considered for follow‐up treatment after full healing.
Despite two persisting cherry angiomas, a clear overall aesthetic improvement was obtained without extraordinary visual scarring or dyspigmentation. Clinically, the overall final results of the treatments were therefore seen as positive by both doctor and subjects.
High intensity focused ultrasound is thereby concluded to have a future potential as a safe and efficient aesthetic treatment of benign vascular lesions, since the effect is targeted and highly controlled with limited damage of the surrounding tissue. The method shall, however, be studied in larger populations, both in terms of anatomic locations and the depth and size of the lesions. The two unsuccessful results on cherry angiomas underline the relevance of improved pre‐treatment diagnostics, for example, by high‐frequency ultrasound imaging or other methods for high‐resolution diagnostics of the skin structure.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of the manuscript. Authors take responsibility for the integrity of the work as a whole and grant final approval of the final publication.
ETHICAL APPROVAL
This study was conducted ethically and in accordance with the World Medical Association Declaration of Helsinki. Diagnostics and treatments were compliant to the labeling of the medical devices used. The subjects presented in the study signed a written informed consent regarding participation in treatment and publication of the case.
Calik J, Zawada T, Bove T. Treatment of superficial benign vascular tumors by high intensity focused ultrasound: Observations in two illustrative cases. J Cosmet Dermatol. 2022;21:3371–3379. doi: 10.1111/jocd.14682
Funding information
There were no funding sources for this work
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pariser RJ. Benign neoplasms of the skin. Med Clin North Am. 1998;82(6):1285‐1307. [DOI] [PubMed] [Google Scholar]
- 2. James WD, Berger TG, Elston DM, (editors). Andrews Diseases of the Skin. 12th ed. Elsevier; 2016. [Google Scholar]
- 3. Nasseri E, Piram M, McCuaig CC, Kokta V, Dubois J, Powell J. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. Am Acad Dermatol. 2014;70(1):75‐79. [DOI] [PubMed] [Google Scholar]
- 4. Léauté‐Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390(10089):85‐94. [DOI] [PubMed] [Google Scholar]
- 5. Holland KE, Drolet BA. Infantile Hemangioma. Pediatr Clin N Am. 2010;57:1069‐1083. [DOI] [PubMed] [Google Scholar]
- 6. Betz‐Stablein B, Koh U, Edwards HA, McInerney‐Leo A, Janda M, Soyer HP. Anatomic distribution of cherry angiomas in the general population. Dermatology. 2021;1‐9. Online ahead of print. https://www.karger.com/Article/FullText/517172 [DOI] [PubMed] [Google Scholar]
- 7. Cheng J, Liu B, Lee HJ. Outcomes of surgical treatment for hemangiomas. Pediatr Dermatol. 2019;36:207‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buslach N, Foulad DP, Saedi N, Mesinkovska NA. Treatment modalities for cherry angiomas: a systematic review. Dermatol Surg. 2020;46(12):1691‐1697. [DOI] [PubMed] [Google Scholar]
- 9. Vivar KL, Kruse L. The impact of pediatric skin disease on self‐esteem. Int J Womens Dermatol. 2018;4:27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Focused Ultrasound Foundation . State of the field 2020. http://www.fusfoundation.org/images/pdf/Focused_Ultrasound_Foundation_2020_State_of_the_Field_Report.pdf. Accessed October 6th.
- 11. Ellens NPK, Partanen A. Preclinical MRI‐guided focused ultrasound: a review of systems and current practices. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64(1):291‐305. [DOI] [PubMed] [Google Scholar]
- 12. Barile A, Arrigoni F, Zugaro L, et al. Minimally invasive treatments of painful bone lesions: state of the art. Med Oncol. 2017;34(4):53. [DOI] [PubMed] [Google Scholar]
- 13. Kim M, Jung NY, Park CK, Chang WS, Jung HH, Chang JW. Comparative evaluation of magnetic resonance‐guided focused ultrasound surgery for essential tremor. Stereotact Funct Neurosurg. 2017;95(4):279‐286. [DOI] [PubMed] [Google Scholar]
- 14. Ellens N, Hynynen K. Frequency considerations for deep ablation with high‐intensity focused ultrasound: a simulation study. Med Phys. 2015;42(8):4896‐4910. [DOI] [PubMed] [Google Scholar]
- 15. Ko EJ, Hong JY, Kwon TR, et al. Efficacy and safety of non‐invasive body tightening with High‐Intensity Focused Ultrasound (HIFU). Skin Res Technol. 2017;23:558‐562. [DOI] [PubMed] [Google Scholar]
- 16. Day D. Microfocused ultrasound for facial rejuvenation: current perspectives. Res Rep Focus Ultrasound. 2014;2:13‐17. 10.2147/RRFU.S49900 [DOI] [Google Scholar]
- 17. Park JH, Lim SD, Oh SH, Lee JH, Yeo UC. High‐intensity focused ultrasound treatment for skin: ex vivo evaluation. Skin Res Technol. 2017;23:384‐391. [DOI] [PubMed] [Google Scholar]
- 18. Bove T, Zawada T, Serup J, Jessen A, Poli M. High‐frequency (20‐MHz) High‐Intensity Focused Ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25(2):217‐228. [DOI] [PubMed] [Google Scholar]
- 19. Soegaard S, Aarup V, Serup J, et al. High frequency (20 MHz) High Intensity Focused Ultrasound (HIFU) system for dermal intervention: a 12 week local tolerance study in minipigs. Skin Res Technol. 2020;26(2):241‐254. [DOI] [PubMed] [Google Scholar]
- 20. Serup J, Bove T, Zawada T, Jessen A, Poli M. High frequency (20 MHz) High‐Intensity Focused Ultrasound (HIFU): treatment of actinic keratosis, basal cell carcinoma and Kaposi sarcoma. Skin Res Technol. 2020;26:824‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. TOOsonix A/S Denmark . System ONE‐M product brochure. https://www.toosonix.com/wp‐content/uploads/2020/08/TOOsonix‐DS‐S02‐ver2‐web.pdf. Accessed October 6th 2021.
- 22. Bove T, Zawada T, Jessen A, Poli M, Serup J. Removal of common warts by high‐intensity focused ultrasound: an introductory observation. Case Rep Dermatol. 2021;13:340‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serup J, Bove T, Zawada T, Jessen A, Poli M. High‐frequency (20 MHz) High‐Intensity Focused Ultrasound: new ablative method for color‐independent tattoo removal in 1–3 sessions. An open‐label exploratory study. Skin Res Technol. 2020;26:839‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


