Abstract
Apoptosis, the intrinsic programmed cell death process, is mediated by the Bcl-2 family members Bak and Bax. Activation via formation of symmetric core dimers and oligomerization on the mitochondrial outer membrane (MOM) leads to permeabilization and cell death. Although this process is linked to the MOM, the role of the membrane in facilitating such pores is poorly understood. We recently described Bak core domain dimers, revealing lipid binding sites and an initial role of lipids in oligomerization. Here we describe simulations that identified localized clustering and interaction of triacylglycerides (TAGs) with a minimized Bak dimer construct. Coalescence of TAGs occurred beneath this Bak dimer, mitigating dimer-induced local membrane thinning and curvature in representative coarse-grain MOM and model membrane systems. Furthermore, the effects observed as a result of coarse-grain TAG cluster formation was concentration dependent, scaling from low physiological MOM concentrations to those found in other organelles. We find that increasing the TAG concentration in liposomes mimicking the MOM decreased the ability of activated Bak to permeabilize these liposomes. These results suggest that the presence of TAGs within a Bak-lipid membrane preserves membrane integrity and is associated with reduced membrane stress, suggesting a possible role of TAGs in Bak-mediated apoptosis.
Significance
Bak and Bax regulate the intrinsic apoptosis pathway principally via pore formation on the mitochondrial outer membrane (MOM). Using a combination of atomistic and coarse-grain molecular dynamics simulations supported by liposomal assays, we demonstrate how triacylglycerides (TAGs) in the MOM aggregate and co-localize with membrane-bound Bak core domain dimers. The presence of TAGs reduces membrane curvature and thinning and the ability of activated Bak to permeabilize liposomes in steps preceding pore formation. This suggests an associated protective or correlated role of TAGs with Bak. This study provides molecular insights into the role of certain membrane lipid components in facilitating Bak-mediated apoptosis, which may assist with therapeutic targeting of Bak in certain disease states.
Introduction
The Bcl-2 family of proteins regulates the programmed cell death process of intrinsic apoptosis. As a result of cell stress, permeabilization of the mitochondrial outer membrane (MOM) leads to subsequent release of cytochrome c and commencement of the apoptotic cascade, a step along the pathway leading to cell death (1). Deregulation and disruption of this process, which is highly regulated by the dynamic interplay of pro-survival (e.g., Bcl-2, Bcl-xL, and Bcl-w) and pro-apoptotic proteins (Bak and Bax) of the Bcl-2 family, have been implicated in many pathological conditions, including cancer (2) and autoimmune diseases (3).
Comprised of nine α helices (α1–α9) arranged around a central core helix (α5) and sharing all four Bcl-2 homology domains (BH1–BH4), the pro-apoptotic effector proteins Bak and Bax oligomerize on the MOM to form the apoptotic pore responsible for permeabilization (1,4,5). In healthy cells, Bax, which is primarily cytosolic, shuttles from the cytosol to the membrane, whereas Bak is primarily membrane bound. Associated with the membrane via insertion of α9, activation of Bak and Bax occurs via transient engagement of BH3-only proteins (4,6). As a result of this engagement, partial unfolding and dissociation of α1 leads to exposure of the core (α2–α5) and latch (α6–α8) domains, facilitating homodimerization and formation of an amphipathic symmetric core domain dimer (the α2–α5 domains of one monomer engage the α2′–α5′ domains of another [where prime denotes the corresponding domains of the alternate monomer]) (Fig. 1 A) (4). Further oligomerization of Bak and Bax dimers occurs via a poorly understood mechanism, leading to formation of higher-order proteinaceous or, more likely, toroidal lipidic pore structures (7,8). The process of dimer clustering on membranes has recently been suggested to be facilitated directly by the membrane (9), suggesting a specific role of the lipid bilayer interacting with pro-apoptotic members and promoting MOM permeabilization (MOMP).
Figure 1.
Description of the Bak dimer and interactions with the membrane. (A) Crystal structure of the Bak α2-α5 symmetric core dimer (PDB: 6UXM) with a transparent surface overlaid. Residues of one Bak monomer that are orientated toward and buried within the membrane are shown in stick representation (top). Also shown is a sequence domain description of Bak, indicating positioning of the BH domains (BH1–BH4) and the transmembrane domain in relation to α helices (α1–α9). The core and latch domain descriptors are indicated above. (B) Top-down view of the CG Bak dimer (blue) embedded in a representative CG MOM bilayer. Lipid species are shown in stick representation: DOPC (gray), FA (purple), CHOL (shown in surface view for clarity; pink), PE (light green), SM (orange), CL (cyan), PIP (mauve), and TAG (red). (C) Density profile of the DOPC headgroup (PO4 bead, gray), CHOL (pink), Bak dimer (blue), and TAG (red) across the membrane. Distances are measured relative to the bilayer center, separating upper and lower leaflets. The density of the DOPC PO4 bead is marginally reduced in the upper leaflet (approximate relative z position, 2 nm) compared with the lower leaflet (approximate z position, −2 nm) because of displacement by the Bak dimer and TAG. (D) Side view of the CG Bak dimer embedded in the MOM, demonstrating a lipid binding event to the α5α5′ binding site via the sn3 lipid headgroup (interactions are indicated by broken black lines). All other lipids were removed for clarity.
In general, significant experimental and computational investigations of changes in bilayer composition have identified effects on membrane characteristics and on embedded protein interactions. Variation in bulk membrane composition has demonstrated altered membrane recruitment and subsequent modulation of protein function after membrane association. Functional effects are attributed to a change in direct lipid-protein interaction (e.g., binding of membrane cholesterol to a protein site, stabilizing conformations, as in the case of some G-protein-coupled receptors) (10), and/or from direct or indirect induced membrane remodeling, including changes in membrane curvature, lipid localization, and bilayer thickness (11). This feature has been observed in numerous X-ray crystal and molecular dynamics (MD) studies, exemplified initially with studies of anti-microbial peptides (12,13), and subsequently within the G-protein-coupled receptor family (11,14), where cholesterol binds to and modulates the conformation of the receptor. A comprehensive review of simulation techniques used in studying model and multicomponent membranes has been compiled by Marrink et al. (15).
Mounting evidence within the literature over the past two decades points to MOM lipids playing an active role in facilitating Bcl-2-family-mediated permeabilization. The heterogeneous composition of the MOM has significant implications for determining the recruitment of Bcl-2 family members and in subsequent mediated interactions affecting the overall size and shape of the bilayer (16,17). More compelling, the specific MOM composition is enough to promote recruitment of Bak and Bax and to facilitate activation in the absence of BH3-only activators, suggesting that MOM composition may indeed be key in facilitating apoptotic pores (18). Furthermore, lysosomes mimicking the lipidic composition of the endoplasmic reticulum (ER) are not permeabilized by tBid and monomeric Bax, necessitating an environment specific to mitochondria (19).
Although a conclusive mechanistic model detailing the role of membrane components affecting pro-apoptosis-mediated permeabilization has not been presented, numerous lipids are known to regulate permeabilization. For example, cardiolipin (CL), which is present at relatively high concentrations within the inner (∼15%–20%) (20,21) and debatably at lower concentrations (≤3%) (22) in the outer mitochondrial membrane, promotes translocation and membrane recruitment of tBid (23) and has been suggested as an essential membrane component in promoting active pore-forming oligomers in giant unilamellar vesicles (24). However, more recent studies have demonstrated that neither deletion of CL synthase in yeast expressing Bax (25) nor tafazzin knockdown cells leading to CL-deficient mitochondria (26) negated Bax and Bak pore formation, suggesting that CL content is not essential for Bak/Bax insertion or oligomerization.
Other lipids, including ceramides and cholesterol (CHOL), have been proposed to regulate apoptotic permeabilization. The presence of ceramides is sufficient to form pores and induce permeabilization independent of Bax; however, they can additionally translocate and activate Bax-mediated permeabilization when the concentration of each individually is insufficient to cause MOMP (27) in a synergistic process. In contrast, CHOL has been proposed as a negative and positive regulator of Bax permeabilization, dependent on concentration, via changes in the fluidity of the membrane (28,29) and/or altering recruitment kinetics of Bax insertion into membranes (28). Broadly, the role of individual lipids in MOMP is not well understood.
MD simulations, all-atom (AA; all atoms are discreetly simulated, including hydrogens) and coarse-grain (CG; groups of atoms are mapped to a single bead), provide an avenue to identify protein-protein and protein-lipid interactions that are difficult to determine experimentally (30,31). Notably, although simulations offer a unique avenue for studying membrane-bound proteins, reports of computational studies of Bcl-2-family proteins interacting with membranes are few. Despite the considerable field of research focusing on Bcl-2-family-mediated cell death, modeling and simulation of activated effector proteins are somewhat lacking, with recent computational approaches principally exploring the steps preceding activation. Exploring initial preliminary docked BH3-only peptides or small-molecule interaction partners to relevant pro- and anti-apoptotic Bcl-2 proteins has, for the most part, taken center stage; such as individual BH3-domain peptides binding to Bak (32,33) and Bax (34,35) and small molecules to Bak (36) and Bax (37,38). Few investigations have been presented that describe Bax and Bak associated with or embedded in a membrane or how the membrane is affected by Bax and Bak activation. Exceptions are the few studies of the Bax α9 transmembrane helix found to induce membrane curvature (39,40), monomeric Bax embedded in lipid nanodiscs (41), and a modeled Bax oligomeric proteolipidic pore with associated cytochrome c permeation (42,43). Such studies, however, have not explored specific lipid-protein interactions or the role of lipid composition in any significant detail.
In a previous article, we investigated crystallographic lipids that were identified to interact with and bridge multiple Bak dimers, providing insights into the role of the membrane in Bak oligomerization and formation of higher-order pores (9). In simulations of the Bak dimer embedded in a bulk 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) lipid membrane, phospholipid sn3 headgroups were found to localize to and interact with residues between the central α5α5′ helices and within a groove between helices, consistent with crystallographic structures. Simulations of embedded Bak dimers also demonstrated membrane stress resulting in local curvature and thinning directly beneath the Bak dimer.
Here we expand on that work by presenting further MD simulations of the Bak core domain dimer principally through use of CG simulations of Bak-membrane systems across the microsecond timescale. Where we previously described the preferential embedding of a Bak dimer into a single lipid bilayer leaflet during CG bilayer self-assembly, here we explore membrane dynamics because of Bak dimer engagement. We demonstrate the interactions of lipids at the defined α4α5α5′α4′ lipophilic binding site and identify the effect of lipid MOM composition. Our results, consistent with experiments, indicates specific and unique interactions of the individual components of the MOM with the Bak dimer, playing an integral role in modulation of the MOM on Bak activation.
Materials and methods
Molecular modeling
Models of Bak core domain dimers were prepared as described previously (9). Briefly, the Modeller software (v.9.14) (44) was used to generate models (residues Gly67–Thr148) of the Bak core domain dimer using the X-ray crystal structures of each of the dimers in the trimer-of-dimers structure as templates (PDB: 6UXM) (9). From 50 models, the model with the lowest Modeller objective function was used to seed all MD simulations.
CG MD simulation
The Gromacs (v.2019) simulation software (45) with the Martini 2.2 (46) force field was used in all CG simulation systems. Lipid systems of the MOM made use of up to eight different lipid components, broadly representing the composition of the MOM at relevant concentrations of each component determined experimentally, as detailed in Table S1. Lipids included 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-phosphoethanolamine (DOPE – PE), palmitic/stearic acid (PCA – FA), cholesterol (CHOL), phosphatidylinositol phosphate (POP1 – PIP), cardiolipin (CDL2; total charge −2 - CL), d18.1/18.0-sphingomyelin (DPSM – SM), and 1,3-dipalmitoyl-2-oleoylglycerol (TOG – TAG). The CG model of the Bak core domain dimer was generated using the martinize (47) python script, and simulations were set up using the same global elastic network as described previously (9). All components, including lipids and the CG Bak dimer where included, were placed randomly into a 130-Å cubic box, utilizing periodic boundary conditions and the Gromacs insert-molecules utility. The remaining volume was solvated with water molecules, and sodium and chloride ions were included to neutralize the system to a concentration of 0.15 M. Systems were initially minimized via a steepest descent protocol to a maximum of 10,000 steps, followed by 100-ns equilibration using isotropic pressure coupling, allowing the membrane to form spontaneously, and 100-ns semi-isotropic pressure coupling. All systems were allowed to continue using semi-isotropic pressure coupling for 15 μs in the NPT (isothermal-isobaric) ensemble. A temperature of 310 K coupled independently to protein, lipid, and solvent groups was maintained using a velocity rescaling thermostat (48) with a coupling of 1.0 ps. Berendsen (49) and Parrinello-Rahman (50) barostats were used to maintain isotropic and semi-isotropic pressure coupling to a reference pressure of 1 bar and a compressibility of 3.0 × 10−4 bar, respectively. A universal cutoff of 11 Å was used to account for non-bonded interactions and the reaction-field method (51) with a dielectric constant of 15 and 0 beyond the cutoff. As facilitated through use of the Martini force field, the integration time step was 30 fs. All simulations were conducted in five replicas.
AA MD simulation
AA simulation of the Bak dimer embedded in a pre-equilibrated membrane was carried out using the CHARMM-GUI server (52) as described previously described (9). Lipids included POPC, DOPC, DOPE, stearic acid (STE – FA), cholesterol (CHL1 – CHOL), 1-palmitoyl-2-oleoyl-inositol (POPI – PIP), d18.1/18.0-sphingomyelin (SSM – SM), cardiolipin (PMCL2; total charge −2 – CL), glyceryl trioleate/triolein (OOOTG – TAG). Parameters for TAG were derived from the Ligand Reader and Modeller utility, and the OOOTG parameters as part of the CHARMM-GUI server (52). Insertion and equilibration of the Bak dimer into membranes was performed as described previously using the Membrane Builder module in the CHARMM-GUI server (52). The resulting bilayer spanned an area of ∼150 × 150 Å2 with the number of lipids in the upper and lower leaflet detailed in Table S2. Simulations were conducted using the Gromacs (v.2019) software (45) and the CHARMM36m (53) force field. Initial minimization and equilibration followed established procedures outlined by the CHARMM-GUI via a steepest descent protocol, followed by short positionally restrained equilibration in the NVT (canonical) and NPT ensemble. Simulations were then allowed to progress in the NPT ensemble for 500-ns production calculations. The simulation protocol was otherwise as conducted previously (9). Simulations were conducted in three replicas.
Liposome permeabilization assay
Liposomes were prepared as described previously (9). Briefly, liposomes were made with mole percent mixtures of 43.8% POPC, 23.8% 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (PE), 10.5% L-α-phosphatidylinositol from bovine liver, 9.5% 1,2-dioleoyl-sn-glycero-3-phospho-L-serine, 7.6% 1′,3′-bis[1,2-dioleoyl-sn-glycero-3-phospho]-sn-glycerol (CL), 4.8% 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] nickel salt, and supplemented with none, 1%, or 5% TAG. All lipids were purchased from Avanti (Birmingham, AL, USA) except for TAG, which was purchased from Sigma (Sydney, Australia). Lipids were dissolved in chloroform and 0.01% butylated hydroxytoluene before mixing at the above ratios and drying under N2 gas. Lipid mixtures were resuspended in 50 mM self-quenching 5(6)-carboxyfluorescein. Resuspended lipids were frozen and thawed several times and then extruded an odd number of times (more than 20) through a membrane with a pore size of 100 nm and stored at 4°C. Excess dye was removed from the liposomes using a PD10 column just prior to use.
Liposome release experiments were performed by incubating 5 μM of liposomes with 50 nM recombinant human Bak ΔN22ΔC25 C166S 6His (produced as described previously (9)) with or without 1 μM human Bid BH3 peptide (BH3 interacting domain death agonist, purchased from Genscript, Hong Kong) in SUV buffer (10 mM HEPES (pH 7.5), 135 mM KCl), in a final volume of 150 μL. The His tag on this construct localizes the protein to the liposome membrane through binding to the 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] nickel salt lipid headgroup. Fluorescence was measured (excitation at 485 nm, emission at 535 nm) in a Chameleon V plate reader (LabLogic Systems, Sydney Australia) over 1 h at room temperature in Falcon 96-well U-bottom tissue culture plates (Corning, Melbourne Australia). Experiments were performed four independent times, with each independent experiment consisting of three technical replicates. Data were normalized to dye release from liposomes treated with CHAPS detergent (3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonate, 100%), and data were compared with unpaired two-tailed t-tests.
Molecular visualization
VMD was used along with the tachyon renderer (54) for visualization, and images were encoded to produced videos with ffmpeg.
Membrane remodeling analysis
Evaluation of local membrane curvature and thickness was performed using the g_lomepro (50) Gromacs extension utility on the sn3 phosphorus atom and phosphate (PO4) bead for AA and CG systems, respectively. Membrane thickness and curvature properties were independently mapped onto a grid along the plane of the membrane and divided using a bin resolution of 100 × 100. Individual lipid composition distribution was assessed using the gmx densmap Gromacs extension utility using a constant scale, discarding the first 5 μs of each simulation as equilibration (45).
Bak dimer-membrane interaction energy
The interaction energy (that is, the potential energy of the system between the protein and membrane) corresponding to only non-bonded interactions was calculated using the gmx energy utility. This involved the summing of short-range Coulomb and Lennard-Jones energies between protein and lipid atoms only.
Bak dimer-lipid contact, H-bond, and cluster analysis
Lipid occupancy across all replica simulations, calculated as a percentage of the total simulation time where a lipid was in contact with a Bak dimer residue, was assessed using the PyLipID utility (55). A dual cutoff scheme between 0.55 nm and 1.0 nm, as recommended, was used to define lipid contacts and displayed colored from 0%–100% as a product of total cumulative simulation time. Protein-lipid hydrogen bond analysis was conducted using the Gromacs gmx hbond utility, using all default options of 3.5 Å between the H-bond donor and acceptor, with a 30° cutoff angle. Identifying clusters of TAG lipids was performed using an in-house Fortran utility. Clusters were defined as three or more lipids that shared contacts within 5 Å between constituent beads.
Membrane density
The Gromacs gmx density utility (45) was used to plot the 2D projection of CG lipid density, along the x-y plane of the simulation box. The PO4 headgroup bead was used to identify the extremities of the upper and lower leaflets, with all other membrane species, including all component beads, included in the calculation unless otherwise stated. The first 5 μs of each simulation was discarded as equilibration prior to analysis.
Results
Here we report CG and AA simulations of truncated Bak core domain dimers, embedded within simulated membranes, with details of each simulated system summarized in Table S1. Simulations consisted of AA or CG lipids together with the Bak dimer, as described recently (9). Models used here of the symmetric dimer were domain minimized, consisting of the α2–α5 region of each monomer, as demonstrated in our previous work (9). Bax domains α2–α5, forming the symmetric core, have been reported to be sufficient to cause oligomerization, with α9 required for targeting and insertion to the MOM (56). The α2–α5 core dimer inserts spontaneously into one leaflet of a membrane from a mélange of components (see below) without the need for α9 to target or insert into the membrane. We therefore focused on the interaction of the Bak core dimer with the membrane (in the absence of regions beyond, including α1 and α6–α9). Furthermore, our recent work demonstrates that symmetric α2-α5α5′-α2′ constructs are sufficient to probe changes to membrane properties, although likely not sufficient to probe pore structures, because of the presumed role of residues α6–α9 in mitochondrial association and for anchoring the dimer to the pore rim in a clamp-like mechanism, reducing the pore line tension (7,57,58). We therefore utilize only a single Bak dimer, focusing on the steps preceding higher-order oligomerization and formation of an activated pore.
To determine the role of certain lipid components on the membrane, we determined any clustering of lipids, enrichment or distribution of membrane components associated with the Bak dimer, and changes to membrane properties associated with changes in lipid concentration. We subsequently explored specific residue-lipid interactions of components that have been suggested previously to modulate Bak activity and/or that are extensive throughout the simulations observed here. We validate the most striking finding of triacylglyceride localization and membrane remodeling mitigation through use of a liposomal dye release assay, which further reinforced the correlated effect between this lipid and Bak.
Glycerides are sequestered under Bak core domain dimers in the MOM
Investigating the role, if any, of membrane composition on bilayer properties following Bak dimer insertion, we modeled a Martini (46) CG representative system mimicking the composition of the MOM. The MOM contains very high levels of phosphatidylcholine and phosphatidylethanolamine lipids, approximately 40% and 30%, respectively, with the remainder comprised of glycerides, fatty acids, CL, phosphatidylinositol, phosphatidylserine, sphingomyelin, and CHOL, ranging from 0.1% to 10% each (20, 21, 22). MOM lipids, detailed in Table S1, were inserted at random within a simulation box measuring 130 Å in all three Cartesian directions. Bilayer self-assembly of lipid species resulted in a heterogeneous bilayer forming over short, 100-ns simulations (Fig. S1; Video S1). All bilayers across five 15-μs simulations maintained a uniform membrane thickness of 40.5 ± 3.2 Å, with a roughly uniform distribution of most membrane components across the plane of the membrane (x and y axes) (Fig. S2), and a symmetric distribution between the upper and lower leaflets (Table S2; Fig. S3 A). Despite being distributed throughout the bilayer, triacylglycerides (TAGs; trioleoylglyceride) included at ∼1 mol% lipid composition (representing the approximate TAG composition of the MOM) saw formation of transient aggregates predominantly localizing to the bilayer center (Figs. S3 A and S2 B). Partitioning of TAG from the bilayer-water interface to explicit clusters and subsequent partial dissolving of clusters occurred frequently throughout each simulation. Cluster dynamics were similar to previous studies demonstrating coalescence of TAG (59) and diacylglycerol (DAG) (60) at concentrations above ⪆ 3 mol% (61) and likely at lower concentrations, depending on CHOL content (62).
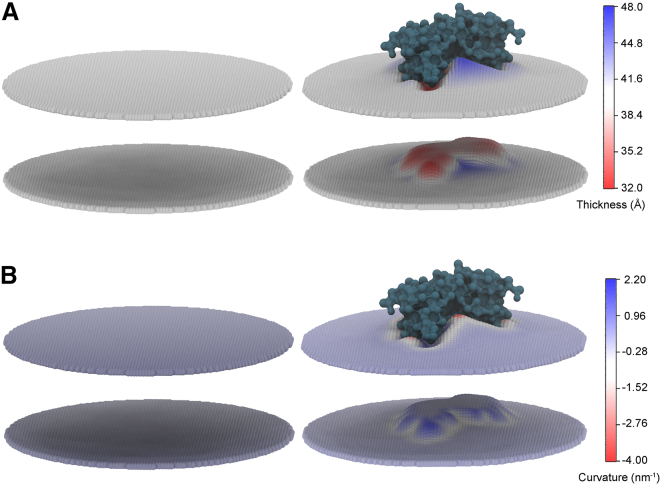
Assessing the effect on MOM dynamics because of Bak dimer insertion, a CG model of the Bak core domain dimer was inserted into a box of randomly placed MOM components; five independent simulations resulted in the Bak dimer preferentially embedding in a single leaflet of the self-assembled bilayer (Fig. 1 B; Video S2), congruent with previous findings (9). Plotting the two-dimensional density of protein, and PO4 lipid headgroup beads revealed that insertion of the dimer displaces lipids of the upper leaflet and leads to asymmetry in the number of lipids residing in each leaflet (Table S2). The dimer is clearly oriented above the bilayer interface, sitting within and extending above the boundaries of the upper leaflet (Fig. 1 C). In all systems extended over 15 μs, lipids contacted the previously identified α4α5α5′α4′ binding site via the phosphate headgroup, with no discernible bias for specific lipid types (Fig. 1 D). Comparable with previous findings, insertion of the dimer into a single leaflet resulted in pronounced membrane remodeling (changes in membrane thickness (Fig. 2 A) and local curvature (Fig. 2 B)). The average membrane thickness, assessed across x and y membrane lateral vectors for Bak-MOM and pure MOM systems, were indistinguishable (39.9 ± 3.3 Å (n = 4) and 40.5 ± 3.2 Å (n = 5) for Bak-MOM and pure MOM systems, respectively). Simulations including the Bak dimer displayed a modest but significant effect on local bilayer thickness directly beneath the Bak dimer (Fig. 2 A). The thickness directly beneath the dimer was reduced to 35.6 ± 2.8 Å (n = 4). The magnitude of the thinning in a MOM membrane was the same as what was observed in simulations of the dimer in a pure POPC membrane presented previously; the models of the MOM revealed slightly decreased although statistically insignificant local thinning compared with pure POPC as a result of Bak insertion (35.6 ± 2.8 Å (n = 4) and 33.5 ± 2.7 Å (n = 4) for Bak-MOM and Bak-POPC systems, respectively) (9).
Figure 2.
Simulation membrane thickness and curvature. (A and B) Thickness (A) and curvature (B) of representative CG MOMs without (left) and in the presence (right) of the Bak core dimer.
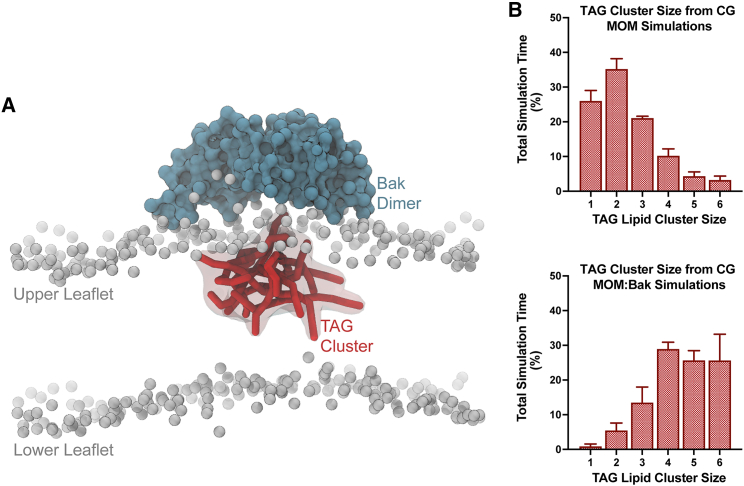
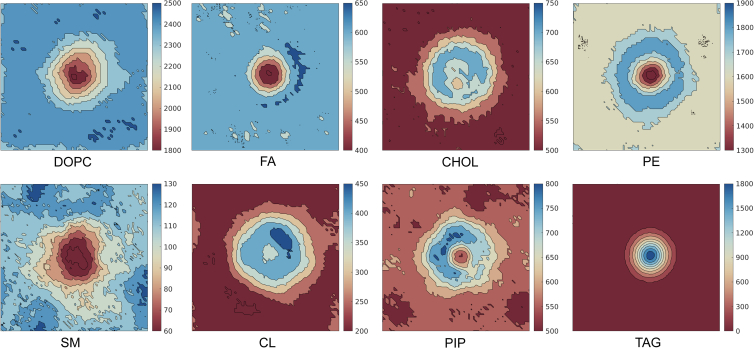
TAG partitioned to the bilayer interface, forming non-ordered aggregates early in simulations, but favoring regions associated with the upper leaflet (Fig. 1 C). Somewhat unexpectedly and perhaps most striking, clusters localized directly beneath the Bak dimer α4α5α5′α4′ lipophilic region (Fig. 3 A; Video S3). Clusters localized beneath sn1/sn2 acyl chains of alternate lipids that were bound to the sn3 α5α5′ binding site. Persisting over prolonged periods, aggregates demonstrated a reduced tendency for individual TAG to dissociate, implying greater aggregate stability (Fig. 3 B). Accordingly, TAG was enriched within the region corresponding to the Bak dimer (Fig. 4), with co-enrichment of CHOL, PE, CL, and PIP, although to a significantly lesser extent. The enrichment of CHOL associated with the TAG cluster is expected with CHOL and CHOL esters frequently found colocalized with TAG in lipid droplets (LDs) (63,64). Relative to the distribution of each lipid across the bilayer, PE, CL, and PIP are all observed enriched at the periphery of the Bak dimer, with concomitant de-enrichment observed centrally (Fig. 4). The headgroups of PE, CL, and PIP lipids are localized at the Bak periphery because of the electrostatic interactions between the phosphate, inositol, and ethanolamine functional headgroup beads with the Bak protein (Fig. S4). Accordingly, the aliphatic tails of these lipids are slightly enriched within the hydrophobic environment under the Bak dimer in the vicinity of the TAG cluster border (Fig. S4). The extensive apolar tails of CL result in a significant enrichment associated with TAG. The strongly defined enrichment and clustering of TAG consequently brought about a commensurate and compensatory dilution of all other MOM species directly beneath the Bak dimer (Fig. 4).
Figure 3.
TAG clustering from the CG Bak:MOM simulation. (A) Side view orientated along the x-y plane of CG Bak in the MOM bilayer, demonstrating TAG clustering (red, with a transparent surface). PO4 beads of lipid sn3 headgroups are included (as gray beads) to illustrate the relative position of the bilayer. (B) Clustering of CG TAG throughout simulation trajectories of the MOM (top) and MOM with the Bak dimer (bottom). Plots are presented as the total simulation time, as a percentage, where there are CG TAG clusters of varying sizes. Simulations without the Bak dimer demonstrate significantly less clustering and a decreased size of clusters compared with that of simulations including the Bak dimer. Error bars represent the standard deviation across all five replicates.
Figure 4.
Two-dimensional lateral density of CG membrane lipid components. Shown are plots in the plane of the membrane, demonstrating enrichment and dilution of lipid species. Plots are assembled from all replica simulation trajectories (five replicas of 15 μs, discarding the first 5 μs as equilibration) after centering the Bak dimer.
To quantify the degree of enrichment or dilution of lipids correlated with their interactions with Bak, mapping the occupancy of lipid interactions to protein residues broadly identified the significance associated with α4α5α5′α4′ hydrophobic side-chain beads (Fig. 5); this is likely due to the orientation of these residues toward the bilayer center. Unsurprisingly, lipids present at a greater abundance (i.e., DOPC, accounting for 33% of the bilayer) formed significantly more and longer duration contacts with buried α4α5 lipophilic face residues (Figs. 5 and S5), with solvent-exposed residues of domains α2α3 forming very few transient contacts. Notwithstanding being present at only 1%, TAG was consistently in close contact with residues Tyr108, Leu132, Phe111, and Tyr136, all situated within the core of the α4α5 helices; these residues were in contact (defined as any bead within 0.55 nm and 1.0 nm) more than 40% of the total simulation time (measured as the total number of frames) (Fig. 5). Similar non-polar bulky residues at the periphery of the α4α5 hydrophobic face, notably Phe119 and Tyr143 (Fig. 1 A), demonstrated reduced occupancy (33% and 29% of the total simulation time, respectively).
Figure 5.
Percentage of occupancy of individual lipid components on the Bak dimer. Shown is the occupancy of each lipid component from MOM + Bak dimer CG simulations, mapped onto the hydrophobic α4α5′α5α4′ face (embedded within the bilayer) of the Bak dimer. This was determined as a percentage of the total simulation time where a lipid was in contact with a Bak dimer residue; the color scale (white through red) details an absence of any contact (0%) through to 100% contact, respectively. Occupancy was calculated using the PyLipID utility, across all simulation replicas (5 × 15 μs), discarding the first 5 μs as equilibration.
To determine whether the presence of localized partitioned TAG within the membrane center correlated to changes in membrane thinning or curvature, we simulated the Bak dimer in a representative MOM excluding TAG. Consistent with previous findings, the absence of TAG resulted in a reduction of the thickness of the membrane directly beneath the dimer, 32.6 ± 2.7 Å compared with the average at the membrane periphery, 39.9 ± 2.7 Å (Fig. S6); this is in line with the membrane thinning observed for Bak-POPC discussed above (Bak-POPC = 33.5 ± 2.7 Å (n = 4)) (9). The induced mean local curvature of the membrane was also less pronounced in the absence of TAG (Bak-MOM = 0.096 nm−1 versus Bak-MOM (−) TAG = 0.295 nm−1), further suggesting that the presence of TAG mitigates changes in local membrane remodeling.
AA simulations of Bak core domain dimers in lipid membranes
The results of the simulations described above show that membrane thinning is comparable in pure POPC and MOM membranes despite the difference in membrane composition and effect of TAG aggregation beneath the Bak dimer. To help dissect the effect of TAG aggregates on membrane remodeling, we considered a membrane consisting only of POPC and TAG at 1 mol% using AA simulations. These AA simulations show the dimer remaining embedded within a single bilayer leaflet, as observed previously in simulations of the dimer in pure POPC bilayers (9) (Fig. 6 A), and addition of TAG does not adversely alter the structure of the dimer. Furthermore, there is no significant change to the protein-membrane environment, characterized by maintenance in the number of protein-lipid hydrogen bonds (average of 9 between the Bak dimer and POPC lipids across both systems), or a change in the protein-lipid potential energy (−2158.6 ± 70.2 kJ mol−1 and −2045.7 ± 101.6 kJ mol−1 for the Bak-POPC and Bak-POPC + TAG systems, respectively). Direct interactions between lipids at the α5α5′ and α4α5 lipid binding grooves were observed. POPC lipid phosphate headgroups formed sustained hydrogen bonds to the backbone amide nitrogen of Trp125, the phenolic hydroxyl group Tyr136′, and the guanidinium group Arg137′, as observed within the crystal structure of the Bak dimer and previous simulations (Fig. 6 B) (9). Lipids engaged at this site were generally retained throughout the simulation. TAG glycerol backbone groups formed negligible electrostatic interactions, instead transiently occupying the α4α5 binding site via apolar interactions between lipid acyl chains to α4 residues Phe111 and Phe119 and α5 residues Leu131 and Leu138 (Fig. 6 B). Consequently, occupation of TAG in the α4α5 groove was not as persistent as POPC lipids in the α5α5′ crevice. Broadly, very few specific interactions (including hydrogen bonds or electrostatic interactions) facilitate engagement of Bak within the membrane; the intereactions are instead underpinned by the hydrophobic contact of side chains of the resudues Tyr108, Phe119, Phe111, Ile123, Trp125, Leu131, Leu132, and Val142 with the aliphatic lipid tails. However, residues Tyr108 and Tyr103, which are located at the bilayer-solvent interface and distal from the dimer core, demonstrate infrequent cation-π and aromatic-choline interactions with the phosphcholine lipids. Such interactions, defined as the distance between the phenol ring and choline, were, however, highly transient.
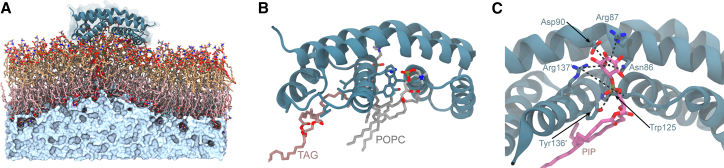
Figure 6.
AA Bak dimer interactions with membranes including TAG. (A) AA Bak dimer embedded in a POPC membrane (yellow, top leaflet; pink, bottom leaflet) containing 1% TAG (red). The Bak dimer (ribbon representation with a transparent surface) is embedded in the outer leaflet of the membrane, causing induced local membrane curvature. The solvent, depicted as a surface representation (pale blue), is displayed only beneath the lower membrane leaflet for clarity. (B) AA representative snapshot of POPC (gray) and TAG (maroon) interacting with the Bak dimer. TAG is observed to interact with the α4α5 lipid binding crevice via ostensibly hydrophobic contacts, whereas POPC lipids are invariably found to be bound to the α5α5′ crevice via the sn3 phosphate to Trp125, Tyr136′, and Arg137′. All other lipids are not shown for clarity. (C) Binding of a PIP lipid, demonstrating binding of a PIP lipid to the α5α5′ crevice but showing interactions between the inositol group and the side chains of Asn86, Arg87, Asp90, and Arg137′.
We next generated AA MOM lipid systems similar to those investigated in CG simulations (Video S4), as detailed in Table S1. Unlike the CG simulations, no lipid species was noted as having a distribution distinctly different from that of a simulated membrane in the absence of the Bak dimer (Fig. S8). AA TAG lipids occupied each bilayer leaflet, in contrast to CG simulations, where occupation occurred essentially within the hydrophobic environment of the leaflet interface. In simulations of the MOM with the Bak dimer, neither the overall average membrane thickness (40.8Å ± 3.6 Å (n = 3) and 40.8Å ± 3.5 Å (n = 3)) nor the thickness directly beneath the Bak dimer (29.3Å ± 3.2 Å (n = 3) and 31.2Å ± 2.8 Å (n = 3)) was statistically significantly altered, with and without the inclusion of TAG, respectively.
Although there was no clearly defined phospholipid preference at the α4α5 lipid binding groove, with PE and PIP observed engaged throughout each of the simulation replicates, the binding of PIP formed extended electrostatic interactions (Fig. 6 C). Where binding of PIP to the lipid binding site is facilitated by the typical phosphate group interactions to Trp125, Tyr136′, and Arg137′ (as discussed previously), the extended solvent-exposed inositol group beyond the substituted phosphate group is positioned in proximity to the side chains of Asn86 Arg87, Asp90, and Arg137′. Throughout the simulation, the inositol hydroxyl oxygen was frequently found within 4 Å of the side chain amide, carboxyl groups, and guanidino groups of these residues, suggesting the presence of sustained hydrogen bonding.
Glyceride-Bak dimer co-localization is independent of MOM lipids and is tunable
TAG levels in LDs are significantly higher (63,65) than those found in the MOM and in localized high concentration in a number of physiological membranes, such as the ER (65). Although the concentration of TAG within the MOM is understood to be ∼1%, triglyceride levels in mitochondria are known to increase with age (66,67). Although it is not clear how elevated levels in mitochondria extend to levels specifically in the MOM, we sought to understand how different levels of TAG affected the membrane structure with the embedded Bak dimer. CG simulations of the Bak dimer in self-assembled pure POPC membranes and POPC membranes consisting of varying TAG concentrations of 0.5%, 1%, 5%, and 10% total lipid number were investigated. The periodic nature of the MD simulations does not permit sequestering of components beyond the periodic system. Thus, although a total TAG concentration of 10% is unlikely to be physiologically observed, it allows a larger number of TAG lipids to aggregate beneath the Bak dimer, which could be achieved physiologically.
Again, simulations in which TAG is present within the bilayer resulted in significantly decreased local curvature compared with bilayers without TAG, with more uniform membrane thickness under the bilayer compared with the periphery (Fig. 7 A). Increases in TAG concentration led to increased clustering (Fig. 7 B), increased membrane thickness, and decreased membrane curvature (Fig. 7 C) in a roughly concentration-dependent manner. Notably, at TAG concentrations below 1%, the membrane directly beneath the Bak dimer is thinner than that at the periphery (36.4 ± 3.0 Å beneath the dimer and 39.5 ± 4.2 Å at the periphery), whereas at concentrations of 5% and above, the thickness under the dimer is greater than at the periphery (46.0 ± 3.0 Å and 39.2 ± 3.0 Å, respectively). At a maximum assessed TAG concentration of 10% the membrane thickness is 54.2 ± 3.2 Å beneath the dimer and 38.6 ± 4.0 Å at the periphery. This is typical of previously identified oil lens “blisters” (59). Despite TAG concentrations modeled above and below the interfacial solubility limit, 3–5 mol% in comparable model membranes (59,61), TAG invariably formed persistent clusters at all concentrations considered here.
Figure 7.
CG Bak dimer interactions with POPC membranes at increasing concentration of TAG. (A) CG membrane thickness assessed at the center (blue) and the periphery (purple) of the bilayer at varying TAG concentrations; (B) the percentage of total CG TAG within each system, which coalesces into a cluster (of greater than three TAG lipids) at varying TAG concentrations; and (C) the lower leaflet CG membrane curvature at varying TAG concentrations. Error bars represent the standard deviation across all five replicates.
Bak liposome permeabilization is affected by the presence of TAGs
We next assessed the capacity of Bak to permeabilize liposomes with and without TAG (in this case 1,3-dipalmitoyl-2-oleoylglycerol) (Fig. 8). Liposomes were prepared with encapsulated self-quenching fluorescent dye and challenged with Bak protein with and without an activating Bid BH3 peptide. Activated Bak possessed robust permeabilization activity for liposomes without TAG, as reported previously (6). However, activated Bak displayed a reduced capacity to permeabilize liposomes possessing 5% TAG, consistent with TAG affecting the activity of the protein in these assays (Fig. S8).
Figure 8.
Liposome permeabilization by Bak in the presence of TAG. Liposome release experiments were performed with liposomes resembling the MOM and supplemented with triacylglyceride (TAG) at 1% and 5%. Activated Bak (+Bid BH3) had a reduced capacity to permeabilize liposomes containing 5% TAG compared with liposomes lacking TAG (p = 0.044 in an unpaired two-tailed t-test). Ni Mito are mitochondrial surrogate liposomes with a nickel salt used to coordinate the Bak dimer to the membrane. Data represent the 60-min time point of liposome time courses (Fig. S8) from four independent experiments and presented as mean with errors bars representing the standard error from all four replicates.
Discussion
We demonstrated that the presence of activated Bak core domain dimers embedded in a represented MOM results in significant and specific membrane remodeling, which is dependent on the composition of that membrane. Our results here build on and are consistent with previously demonstrated findings based on simulation and crystal structures of Bak dimers forming specific interactions with lipid species (9).
Importantly, we note the limitations of the α2–α5 core domain dimer used in exploring membrane remodeling and therefore briefly discuss this minimized construct. When targeted to the mitochondria via direct fusion of α9, Bak and Bax α2–α5 have been reported previously to be sufficient for inducing apoptosis in mouse embryonic fibroblasts (56); however, activated Bak α2–α5 fused to a C-terminal hexahistidine tag was not sufficient to elicit apoptosis in mouse-liver mitochondria or a model mitochondrial liposome (58). The absence of activity in this latter example is speculated to be due to the absence of the α6–α8 latch or due to the dimer (which forms a trimer of dimers) not being able to integrate into the membrane. Regardless, Bak α2–α5 domain constructs have been shown to be sufficient in exploring dimerization (5,9,58), and preferred engagement of lipid and detergent (9), with domains α6–α8 and α9 at least in part playing a role in targeting to the membrane. Certainly, Bak domains absent in this system likely engage the membrane, as identified in the recent structure of activated dimeric Bak, emphasizing that the amphipathic surface of the dimer extends through α6 and α7 (58). This additional hydrophobic surface likely engages the membrane and would also contribute to membrane remodeling. Because the simulated constructs presented here are initiated in proximity to membrane lipids, specific targeting of the dimers to the membrane is not necessary for facilitating membrane engagement or for identifying the initial membrane remodeling. Investigating the effect of the extended α6 and α7 surface is, however, an important future direction requiring attention.
We note limitations associated with, or inherent within, the simulation force fields used. The Martini2.2 force field has been used throughout this study, which allows a comparison with our previous work (9). However, the force field has been shown recently to underestimate cation-π interactions and, therefore, also aromatic-choline interactions, properties that have been addressed subsequently with the Martini2.3P (68) and, most recently, the refined Martini3 (69) force fields. We therefore replicated a subset of the simulations presented here making use of the Martini2.3P force field. We observed no substantial changes with regard to the results using the Martini2.2 force field; specifically, aggregation and retention of TAG beneath the Bak dimer are unchanged. Similarly, Bak dimer Phe residues on the surface of the hydrophobic face demonstrated no notable change because the Martini 2.3P force field improves the description of cation-π interactions. In contrast, the average number of transient interactions between Tyr residues at the Bak dimer periphery, specifically Tyr103 and Tyr108, and lipid headgroups increased with the use of the Martini2.3P force field, from 1.63 ± 2.0 using the Martini2.2 force field to 3.8 ± 3.1 (presumably because of the improved description of cation-π interactions).
The saturation of DAG and TAG, subsequent clustering, and oil lens formation of these neutral lipids has been studied previously, making use of CG and, to a much lesser degree, united-atom and AA simulations. Notably, AA simulations of triglyceride clustering has broadly necessitated a high mol% concentration (compared with the remaining phospholipids (70,71) or use of a pre-equilibrated glyceride layer inserted between phospholipid bilayers (70,72,73). To address this requirement, attention has been directed toward developing improved and accurate AA parameters that accurately reflect experimental properties (74). For example, current AA CHARMM36 parameters demonstrate excessive hydrophilicity at the glycerol-ester moiety (because of the phospholipid-derived parameterization process) (75). Accumulation and oil lens formation of TAG are understood to be facilitated in part by the favorable energetics of reducing interactions with polar membrane phospholipids and proteins (76), suggesting that overestimation of hydrophilicity may lead to attenuated clustering of TAG within the simulations presented here. Limited clustering of TAG in AA simulations presented here in a two-component (POPC with TAG) and multicomponent (MOM) membrane (Fig. S9) is likely in part attributable to this underestimated property. Importantly, however, comparable membrane thinning as a result of Bak insertion and binding of lipids to the Bak periphery and the α5α5′ lipid binding site was recapitulated across both AA and CG simulations.
The simulations and experiments explored here demonstrated the unanticipated role of TAG in Bak dimer interaction with the MOM. With low or no TAG present in the membrane, embedded Bak dimers caused membrane thinning and strong positive membrane curvature, hallmarks of increased cell stress and a step along the apoptosis pathway. Changes in membrane tension by Bak and Bax activation following MOM insertion is thought to destabilize the lamellar structure of the bilayer, leading to proteolipidic pores (1). Notwithstanding subsequent processes in which effector proteins likely line the rim and stabilize such pores, the initial localized cell stress observed in simulations here is consistent with expectation.
TAG, when modeled at relevant in vivo concentrations and well below the solubility limit, was found to undergo phase separation and aggregation only in the presence of the Bak core dimer; simulations without the protein saw only small transient cluster formation. Thus, the Bak dimer altered cluster stability, allowing spontaneous formation of TAG aggregations. TAG aggregation led to compensatory changes on the surrounding membrane.
The presence of other lipids, principally CHOL, is known to decrease the interfacial solubility of TAG (62), presumably because of changes in the hydrophobic environment of the interface between leaflets. We conducted simulations with and without CHOL and observed comparable results. Notably, our liposomal assay was performed without CHOL. The Bak dimer presents a large apolar surface, which likely alters the membrane environment and seeds TAG aggregates.
The physiochemical rationale for association of TAG aggregates to the dimer is not overtly clear. Clustering of TAG (59) and, more recently, DAG (60) with subsequent formation of blister-like aggregations has been recognized previously. Although the solubility of TAG in a pure phosphatidylcholine membrane is limited (approximately 3–5 mol%) (59,61), greater concentrations of TAG are found in certain natural biological lipidic membranes, including in the ER, lysosome membranes, LDs, and immune cells (63,65). A greater concentration of TAG has been suggested to be accommodated as a result of the coalescence of excess lipids and formation of disordered and persistent lipid aggregates between bilayer leaflets, a phenomenon demonstrated using MD simulations (59). Such aggregates have a striking macroscopic effect, resulting in “blistering” of the associated lipid bilayer. This process is associated with LD biogenesis from the ER, where LDs bud from the cytoplasmic leaflet after initial nucleation of TAG aggregates and accumulation of neutral lipids. TAG is therefore a significant component present at high concentrations in the ER and LD membranes (77). It is somewhat intriguing to speculate whether such a connection between Bak and the ER or LDs exists as a result of or is in some way linked to TAG aggregates.
Following this line of thought, although principally associated with regulation of cell death at the mitochondrial membrane, a proportion of Bcl-2 family members, including the effector proteins Bak and Bax, have been identified to shuttle between and localize to numerous subcellular organelles, including the ER, mitochondria, Golgi apparatus, peroxisomes, and lysosomes (78, 79, 80). At least at the ER, it has been reported that Bcl-2 proteins can regulate ER-mediated Ca2+ release via direct oligomerization and permeabilization by effector proteins (81) or via direct interactions with ER Ca2+ transporters (82). Although the mechanism by which Bcl-2 proteins localize to these different subcellular compartments is not well understood, shuttling between organelles is thought to occur via lysosomes or other intracellular vesicles (83). The association between TAG clusters and Bak may therefore serve as a mechanism to direct or facilitate trafficking of effector proteins to and from these organelles via LDs, peroxisomes, or lysosomes. In this proposed mechanism, the association of Bak dimers with TAG clusters in subcellular membranes prior to LD budding could facilitate localization to such intracellular vesicles and transportation to alternate membranes. Accumulation of TAG from LDs has been observed during apoptosis, advocating such a mechanism (84), but this would require extensive further investigation.
Conversely, TAG may have a protective effect against apoptosis by acting on effector proteins at the MOM, the ER, or both. The simulations presented here demonstrated that TAG aggregates are able to mitigate changes in membrane tension, both in curvature and thinning, although only at increased TAG concentrations. A significant effect on the membrane thickness beneath the Bak dimer was observed when TAG was included at 0.5%–1%, further indicating that these findings making use of a single or two component membrane (POPC or POPC with TAG) is sufficient to accurately illustrate this effect on membrane remodeling. At relevant in vivo concentrations, TAG inclusion in the membrane likely results in no physiological effects. In contrast, inclusion of TAG at 5%–10% results in significant lens formation and thickening of the membrane beneath the Bak dimer, possibly reducing the ability of Bak to form the initial pore structure. A high concentration of TAG in the membrane of cells with increased proliferation rates, such as cancer cells, may therefore hinder initiation of apoptosis from cell stress and delay the apoptotic cascade. LDs accumulate in certain cancer cells, providing a link for an increase in TAG content (85). The broad molecular link between the increase in TAGs and diseases associated with apoptosis similarly deserves further study.
Conclusions
In this study we investigated the interaction of core domain Bak dimers with the mitochondrial membrane, principally via use of CG computational techniques. CG TAGs, although present with relatively low abundance in the MOM, are found to coalesce at the bilayer center in the presence of membrane-inserted activated Bak dimers. These clusters, which associated with the lipophilic face of the Bak dimer, mitigated dimer-induced local membrane thinning and curvature, suggesting a possible role in altering Bak-induced apoptosis. We also identified morphological changes in the membrane that were dependent on TAG concentration. Confirming these observations, activated Bak dimers targeted to MOM-representative liposomes demonstrated a decreased ability to permeabilize these liposomes at TAG concentrations of 5%, a localized level present in numerous subcellular organelles.
There is a further requirement to fully understand the complete structure of activated Bak dimers when embedded in a membrane, giving rise to membrane permeabilization, and the role of the membrane composition in this process. Because of the significant effect of the membrane on facilitating apoptosis, it is of considerable future interest to understand the mechanism by which certain lipids alter the pore-forming effects of Bcl-2 effector proteins and the environment in which these lipids promote such pathways.
Our study confirms previous experimental observations of lipid binding sites in Bak core dimers and elucidates a role of certain membrane components in facilitating the initial steps of pore formation.
Author contributions
N.A.S., P.M.C., P.E.C., and B.J.S. conceptualized the research. N.A.S. conducted all molecular modeling, simulation, and visualization. A.Z.W. conducted liposome assay experiments. N.A.S., A.Z.W., and A.D.C. conducted all investigations. P.E.C., P.M.C., and B.J.S. provided supervision and direction. N.A.S. wrote the original draft, and all authors reviewed and edited the manuscript.
Acknowledgments
We acknowledge receipt of an Australian Postgraduate Award (to A.D.C.) and National Health and Medical Research Council fellowships 1079700 and 1116934 (to P.E.C. and P.M.C., respectively). This work is supported by NHMRC project grant 1079706, Ideas grant 2001406, and Program grant 1113133 (to P.E.C. and P.M.C.). Part of this work was undertaken using resources from the National Computational Infrastructure, which is supported by the Australian government and provided through Intersect Australia under LIEF grant LE170100032 and through the HPC-GPGPU Facility, which was established with the assistance of LIEF grant LE170100200.
Editor: Siewert Jan Marrink.
Footnotes
Angus D. Cowan present address is Division of Biological Chemistry and Drug Discovery, School of Life Sciences, University of Dundee, James Black Centre, Dundee, United Kingdom
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2021.12.043.
Supporting material
Analysis of leaflet occupation includes all CG simulations including the MOM, CG Bak dimer + POPC, and all-atom simulations. Analysis of lipid numbers for each replicate is detailed at the conclusion of each simulation. Lipid nomenclature is as in Table S1. Lipid leaflet occupation was defined using the position of the component headgroup or equivalent, bead/atom. For coarse-grain lipids – DOPC: PO4 bead, DOPE (PE): PO4 bead, PCA (FA): COO bead, CHOL: ROH, POP1 (PIP): PO4 bead, DPSM (SM): PO4 bead, CDL2 (CL): PO41 bead, TOG (TAG): GLY bead. For all-atom lipids – POPC: P atom, DOPC: P atom, DOPE (PE): P atom, STE (FA): C1 atom, CHL1 (CHOL): O3 atom, POPI (PIP): P atom, SSM (SM): P atom, PMCL2 (CL): P3 atom, OOOTG (TAG): C1 atom. Importantly, despite listed as occupied in either the upper or lower leaflet, CG TOG principally occupied the bilyer:bilayer interface, as indicated in Fig. S3A.
References
- 1.Czabotar P.E., Lessene G., et al. Adams J.M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 2.Campbell K.J., Tait S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018;8:1–11. doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czabotar P.E., Westphal D., et al. Colman P.M. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer J.M., Westphal D., et al. Czabotar P.E. Bak core and latch domains separate during activation, and freed core domains form symmetric homodimers. Mol. Cell. 2014;55:938–946. doi: 10.1016/j.molcel.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer J.M., Lan P., et al. Czabotar P.E. Conversion of Bim-BH3 from activator to inhibitor of Bak through structure-based design. Mol. Cell. 2017;68:659–672.e9. doi: 10.1016/j.molcel.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Bleicken S., Jeschke G., et al. Bordignon E. Structural model of active Bax at the membrane. Mol. Cell. 2014;56:496–505. doi: 10.1016/j.molcel.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian S., Wang W., et al. Huang H.W. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc. Natl. Acad. Sci. U S A. 2008;105:17379–17383. doi: 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan A.D., Smith N.A., et al. Czabotar P.E. BAK core dimers bind lipids and can be bridged by them. Nat. Struct. Mol. Biol. 2020;27:1024–1031. doi: 10.1038/s41594-020-0494-5. [DOI] [PubMed] [Google Scholar]
- 10.Gimpl G. Interaction of G protein coupled receptors and cholesterol. Chem. Phys. Lipids. 2016;199:61–73. doi: 10.1016/j.chemphyslip.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Sejdiu B.I., Tieleman D.P. Lipid-protein interactions are a unique property and defining feature of G protein-coupled receptors. Biophys. J. 2020;118:1887–1900. doi: 10.1016/j.bpj.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt N.W., Wong G.C.L. Antimicrobial peptides and induced membrane curvature: geometry, coordination chemistry, and molecular engineering. Curr. Opin. Solid State Mater. Sci. 2013;17:151–163. doi: 10.1016/j.cossms.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Koh J.J., et al. Beuerman R.W. Membrane active antimicrobial peptides: translating mechanistic insights to design. Front. Neurosci. 2017;11:1–18. doi: 10.3389/fnins.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ansell T.B., Song W., Sansom M.S.P. The glycosphingolipid GM3 modulates conformational dynamics of the glucagon receptor. Biophys. J. 2020;119:300–313. doi: 10.1016/j.bpj.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrink S.J., Corradi V., et al. Sansom M.S.P. Computational modeling of realistic cell membranes. Chem. Rev. 2019;119:6184–6226. doi: 10.1021/acs.chemrev.8b00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mignard V., Lalier L., et al. Vallette F.M. Bioactive lipids and the control of Bax pro-apoptotic activity. Cell Death Dis. 2014;5:e1266–e1268. doi: 10.1038/cddis.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peña-Blanco A., García-Sáez A.J. Bax, Bak and beyond — mitochondrial performance in apoptosis. FEBS J. 2018;285:416–431. doi: 10.1111/febs.14186. [DOI] [PubMed] [Google Scholar]
- 18.O’neill K.L., Huang K., et al. Luo X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 2016;30:973–988. doi: 10.1101/gad.276725.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwana T., Mackey M.R., et al. Newmeyer D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 20.Comte J., Maǐsterrena B., Gautheron D.C. Lipid composition and protein profiles of outer and inner membranes from pig heart mitochondria. Comparison with microsomes. Biochim. Biophys. Acta Biomembr. 1976;419:271–284. doi: 10.1016/0005-2736(76)90353-9. [DOI] [PubMed] [Google Scholar]
- 21.Ardail D., Privat J.P., et al. Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- 22.De Kroon A.I., Dolis D., et al. De Kruijff B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim. Biophys. Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 23.Lutter M., Fang M., et al. Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000;2:754–756. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 24.Lai Y.-C., Li C.-C., et al. Chiang Y.-W. The role of cardiolipin in promoting the membrane pore-forming activity of BAX oligomers. Biochim. Biophys. Acta Biomembr. 2019;1861:268–280. doi: 10.1016/j.bbamem.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Iverson S.L., Enoksson M., et al. Orrenius S. Cardiolipin is not required for bax-mediated cytochrome c release from yeast mitochondria. J. Biol. Chem. 2004;279:1100–1107. doi: 10.1074/jbc.M305020200. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalvez F., Schug Z.T., et al. Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J. Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganesan V., Perera M.N., et al. Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- 28.Lucken-Ardjomande S., Montessuit S., Martinou J.C. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ. 2008;15:484–493. doi: 10.1038/sj.cdd.4402280. [DOI] [PubMed] [Google Scholar]
- 29.Christenson E., Merlin S., et al. Schlesinger P. Cholesterol effects on BAX pore activation. J. Mol. Biol. 2008;381:1168–1183. doi: 10.1016/j.jmb.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller M.P., Jiang T., et al. Tajkhorshid E. Characterization of lipid-protein interactions and lipid-mediated modulation of membrane protein function through molecular simulation. Chem. Rev. 2019;119:6086–6161. doi: 10.1021/acs.chemrev.8b00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moqadam M., Tubiana T., et al. Reuter N. Membrane models for molecular simulations of peripheral membrane proteins. Adv. Phys. X. 2021;6:1932589. [Google Scholar]
- 32.Vila-Julià G., Granadino-Roldán J.M., et al. Rubio-Martinez J. Molecular determinants for the activation/inhibition of Bak protein by BH3 peptides. J. Chem. Inf. Model. 2020;60:1632–1643. doi: 10.1021/acs.jcim.9b01047. [DOI] [PubMed] [Google Scholar]
- 33.Ye K., Meng W.X., et al. Dai H. Characterization of an alternative BAK-binding site for BH3 peptides. Nat. Commun. 2020;11:3301. doi: 10.1038/s41467-020-17074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Z., Zhang H., Böckmann R.A. Allostery in BAX protein activation. J. Biomol. Struct. Dyn. 2016;34:2469–2480. doi: 10.1080/07391102.2015.1119731. [DOI] [PubMed] [Google Scholar]
- 35.Sinha S., Maity A., Ghosh Dastidar S. BIM binding remotely regulates BAX activation: insights from the free energy landscapes. J. Chem. Inf. Model. 2018;58:370–382. doi: 10.1021/acs.jcim.7b00628. [DOI] [PubMed] [Google Scholar]
- 36.Cao X., Yap J.L., et al. Smythe R.W. The novel BH3 α-helix mimetic JY-1-106 induces apoptosis in a subset of cancer cells (lung cancer, colon cancer and mesothelioma) by disrupting Bcl-xL and Mcl-1 protein–protein interactions with Bak. Mol. Cancer. 2013;12:42. doi: 10.1186/1476-4598-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritz J.R., Wachter F., et al. Walensky L.D. Allosteric sensitization of proapoptotic BAX. Nat. Chem. Biol. 2017;13:961–967. doi: 10.1038/nchembio.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garner T.P., Amgalan D., et al. Gavathiotis E. Small-molecule allosteric inhibitors of BAX. Nat. Chem. Biol. 2019;15:322–330. doi: 10.1038/s41589-018-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Z., Zhang H. Curvature effect and stabilize ruptured membrane of BAX derived peptide studied by molecular dynamics simulations. J. Mol. Graph. Model. 2019;88:152–159. doi: 10.1016/j.jmgm.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Liao C., Zhang Z., et al. Li J. Conformational heterogeneity of Bax helix 9 dimer for apoptotic pore formation. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep29502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López C.A., Swift M.F., et al. Gnanakaran S. Biophysical characterization of a nanodisc with and without BAX: an integrative study using molecular dynamics simulations and cryo-EM. Structure. 2019;27:988–999.e4. doi: 10.1016/j.str.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M., Zheng J., et al. Ma B. Oncogenic mutations differentially affect Bax monomer, dimer, and oligomeric pore formation in the membrane. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep33340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M., Zheng J., et al. Ma B. Release of cytochrome C from Bax pores at the mitochondrial membrane. Sci. Rep. 2017;7:2635. doi: 10.1038/s41598-017-02825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics. 2016;54:5.6.1–5.6.37. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham M.J., Murtola T., et al. Lindah E. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 46.Marrink S.J., Risselada H.J., et al. de Vries A.H. The MARTINI force field: coarse grained model for biomolecular simulations. J. Phys. Chem. B. 2007;111:7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 47.Monticelli L., Kandasamy S.K., et al. Marrink S.-J. The MARTINI coarse-grained force field: extension to proteins. J. Chem. Theory Comput. 2008;4:819–834. doi: 10.1021/ct700324x. [DOI] [PubMed] [Google Scholar]
- 48.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 49.Berendsen H.J.C., Postma J.P.M., et al. Haak J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 50.Gapsys V., De Groot B.L., Briones R. Computational analysis of local membrane properties. J. Comput. Aided. Mol. Des. 2013;27:845–858. doi: 10.1007/s10822-013-9684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Essmann U., Perera L., et al. Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 52.Lee J., Patel D.S., et al. Im W. CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theory Comput. 2019;15:775–786. doi: 10.1021/acs.jctc.8b01066. [DOI] [PubMed] [Google Scholar]
- 53.Huang J., Rauscher S., et al. MacKerell A.D. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods. 2017;14:71–73. doi: 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone J.E. An efficient library for parallel ray tracing and animation. University of Missouri (Masters Thesis 1747) 1998 [Google Scholar]
- 55.Song W., Corey R.A., Sansom M.S.P. Pylipid: A Python toolkit for analysis of lipid-protein interactions from MD simulations. bioRxiv, 2021 doi: 10.1101/2021.07.14.452312. [DOI] [Google Scholar]
- 56.George N.M., Evans J.J.D., Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basanez G., Nechushtan A., et al. Youle R.J. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. U S A. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birkinshaw R.W., Iyer S., et al. Czabotar P.E. Structure of detergent-activated BAK dimers derived from the inert monomer. Mol. Cell. 2021;81:2123–2134.e5. doi: 10.1016/j.molcel.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Khandelia H., Duelund L., et al. Ipsen J.H. Triglyceride blisters in lipid bilayers: implications for lipid droplet biogenesis and the mobile lipid signal in cancer cell membranes. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0012811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campomanes P., Zoni V., Vanni S. Local accumulation of diacylglycerol alters membrane properties nonlinearly due to its transbilayer activity. Commun. Chem. 2019;2:1–8. [Google Scholar]
- 61.Hamilton J.A. Interactions of triglycerides with phospholipids: incorporation into the bilayer structure and formation of emulsions. Biochemistry. 1989;28:2514–2520. doi: 10.1021/bi00432a025. [DOI] [PubMed] [Google Scholar]
- 62.Spooner P.J.R., Small D.M. Effect of free cholesterol on incorporation of triolein in phospholipid bilayers. Biochemistry. 1987;26:5820–5825. doi: 10.1021/bi00392a036. [DOI] [PubMed] [Google Scholar]
- 63.Gao Q., Goodman J.M. The lipid droplet—a well-connected organelle. Front. Cell Dev. Biol. 2015;3:49. doi: 10.3389/fcell.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zoni V., Khaddaj R., et al. Vanni S. Pre-existing bilayer stresses modulate triglyceride accumulation in the er versus lipid droplets. Elife. 2021;10:1–24. doi: 10.7554/eLife.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olzmann J.A., Carvalho P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flaquer A., Rospleszcz S., et al. Strauch K. Mitochondrial GWA analysis of lipid profile identifies genetic variants to be associated with HDL cholesterol and triglyceride levels. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0126294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollard A.K., Ortori C.A., et al. Chakrabarti L. Mouse mitochondrial lipid composition is defined by age in brain and muscle. Aging (Albany NY) 2017;9:986–998. doi: 10.18632/aging.101204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan H.M., Souza P.C.T., et al. Reuter N. Capturing choline-aromatics cation-πInteractions in the MARTINI force field. J. Chem. Theory Comput. 2020;16:2550–2560. doi: 10.1021/acs.jctc.9b01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Souza P.C.T., Alessandri R., et al. Marrink S.J. Martini 3: a general purpose force field for coarse-grained molecular dynamics. Nat. Methods. 2021;18:382–388. doi: 10.1038/s41592-021-01098-3. [DOI] [PubMed] [Google Scholar]
- 70.Bacle A., Gautier R., et al. Vanni S. Interdigitation between triglycerides and lipids modulates surface properties of lipid droplets. Biophys. J. 2017;112:1417–1430. doi: 10.1016/j.bpj.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caillon L., Nieto V., et al. Thiam A.R. Triacylglycerols sequester monotopic membrane proteins to lipid droplets. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-17585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim S., Swanson J.M.J. The surface and hydration properties of lipid droplets. Biophys. J. 2020;119:1958–1969. doi: 10.1016/j.bpj.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prévost C., Sharp M.E., et al. Walther T.C. Mechanism and determinants of amphipathic helix-containing protein targeting to lipid droplets. Dev. Cell. 2018;44:73–86.e4. doi: 10.1016/j.devcel.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S., Voth G.A. Physical characterization of triolein and implications for its role in lipid droplet biogenesis. J. Phys. Chem. B. 2021;125:6874–6888. doi: 10.1021/acs.jpcb.1c03559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campomanes P., Prabhu J., et al. Vanni S. Re-charging your fats: charmm36 parameters for neutral lipids triacylglycerol and diacylglycerol. bioRxiv. 2021 doi: 10.1016/j.bpr.2021.100034. 2021.09.29.462351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thiam A.R., Forêt L. The physics of lipid droplet nucleation, growth and budding. Biochim. Biophys. Acta. 2016;1861:715–722. doi: 10.1016/j.bbalip.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 77.Aon M.A., Bhatt N., Cortassa S. Mitochondrial and cellular mechanisms for managing lipid excess. Front. Physiol. 2014;5:1–13. doi: 10.3389/fphys.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dumitru R., Gama V., et al. Deshmukh M. Human embryonic stem cells have constitutively active Bax at the Golgi and are primed to undergo rapid apoptosis. Mol. Cell. 2012;46:573–583. doi: 10.1016/j.molcel.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hosoi K.I., Miyata N., et al. Fujiki Y. The VDAC2-BAK axis regulates peroxisomal membrane permeability. J. Cell Biol. 2017;216:709–721. doi: 10.1083/jcb.201605002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guan J.J., Zhang X.D., et al. Qin Z.H. DRAM1 regulates apoptosis through increasing protein levels and lysosomal localization of BAX. Cell Death Dis. 2015;6:1–12. doi: 10.1038/cddis.2014.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zong W.X., Li C., et al. Thompson C.B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonneau B., Prudent J., et al. Gillet G. Non-apoptotic roles of Bcl-2 family: the calcium connection. Biochim. Biophys. Acta. 2013;1833:1755–1765. doi: 10.1016/j.bbamcr.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 83.Popgeorgiev N., Jabbour L., Gillet G. Subcellular localization and dynamics of the bcl-2 family of proteins. Front. Cell Dev. Biol. 2018;6:13. doi: 10.3389/fcell.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al-Saffar N.M.S., Titley J.C., et al. Ronen S.M. Apoptosis is associated with triacylglycerol accumulation in Jurkat T-cells. Br. J. Cancer. 2002;86:963–970. doi: 10.1038/sj.bjc.6600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz A.L.S., Barreto E.D.A., et al. Bozza P.T. Lipid droplets: platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020;11:105. doi: 10.1038/s41419-020-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of leaflet occupation includes all CG simulations including the MOM, CG Bak dimer + POPC, and all-atom simulations. Analysis of lipid numbers for each replicate is detailed at the conclusion of each simulation. Lipid nomenclature is as in Table S1. Lipid leaflet occupation was defined using the position of the component headgroup or equivalent, bead/atom. For coarse-grain lipids – DOPC: PO4 bead, DOPE (PE): PO4 bead, PCA (FA): COO bead, CHOL: ROH, POP1 (PIP): PO4 bead, DPSM (SM): PO4 bead, CDL2 (CL): PO41 bead, TOG (TAG): GLY bead. For all-atom lipids – POPC: P atom, DOPC: P atom, DOPE (PE): P atom, STE (FA): C1 atom, CHL1 (CHOL): O3 atom, POPI (PIP): P atom, SSM (SM): P atom, PMCL2 (CL): P3 atom, OOOTG (TAG): C1 atom. Importantly, despite listed as occupied in either the upper or lower leaflet, CG TOG principally occupied the bilyer:bilayer interface, as indicated in Fig. S3A.