Summary
Background
Janus kinase (JAK)‐mediated cytokine signalling contributes to local and systemic inflammation in hidradenitis suppurativa (HS).
Objectives
To describe the safety and efficacy results from two multicentre phase II trials of the JAK1 inhibitor INCB054707 in patients with moderate‐to‐severe HS.
Methods
Patients received open‐label INCB054707 15 mg once daily (QD; Study 1) or were randomized to INCB054707 30, 60 or 90 mg QD or placebo (3 : 1 within each cohort; Study 2) for 8 weeks. Eligible patients were aged 18–75 years and had moderate‐to‐severe HS (Hurley stage II/III disease), lesions present in at least two anatomical locations, and a total abscess and inflammatory nodule count ≥ 3. The primary endpoint for both studies was safety and tolerability. Secondary endpoints included HS Clinical Response (HiSCR) and other efficacy measures.
Results
Ten patients were enrolled in Study 1 (15 mg INCB054707) and 35 in Study 2 (INCB054707: 30 mg, n = 9; 60 mg, n = 9; 90 mg, n = 8; placebo, n = 9). Overall, 70% of patients in Study 1 and 81% of patients receiving INCB054707 in Study 2 experienced at least one treatment‐emergent adverse event; 30% and 42% of patients, respectively, had at least one treatment‐related adverse event. Among the evaluable patients, three (43%) in Study 1 and 17 (65% overall: 30 mg, 56%; 60 mg, 56%; 90 mg, 88%) receiving INCB054707 vs. 4 patients (57%) receiving placebo in Study 2 achieved HiSCR at week 8.
Conclusions
INCB054707 was well tolerated, with responses observed in patients with moderate‐to‐severe HS. The safety and efficacy findings from these studies demonstrate proof of concept for JAK1 inhibition in HS. The studies are registered on ClinicalTrials.gov (NCT03569371 and NCT03607487).
What is already known about this topic?

Hidradenitis suppurativa (HS) is a chronic, painful, inflammatory skin condition that can ultimately lead to irreversible tissue damage and scar formation.
Adalimumab (anti‐tumour necrosis factor‐α) is currently the only approved therapy for HS; however, responses are varied and are achieved in only approximately one‐half of patients.
Janus kinase (JAK)‐mediated cytokine signalling contributes to inflammatory processes and is a potential drug target in HS.
What does this study add?
The JAK1 inhibitor INCB054707 was well tolerated and produced rapid and dose‐dependent clinical responses in two phase II trials of patients with moderate‐to‐severe HS.
INCB054707, compared with placebo, generally resulted in numerical improvements in HS Clinical Response, abscess and inflammatory nodule count, International HS Severity Scoring System score, and patient‐reported outcome measures including skin pain and quality of life.
Linked Comment: C. Sibbald and R. Alhusayen. Br J Dermatol 2022; 186:768–769.
Plain language summary available online
Hidradenitis suppurativa (HS), or acne inversa, is a chronic, inflammatory condition characterized by painful, deep‐seated nodules and abscesses of apocrine‐gland‐bearing skin. 1 , 2 In more severe disease, the development of pus‐discharging tunnels, known as sinus tracts, results in irreversible tissue destruction and scarring. 3 In European and US populations, the prevalence of HS is most likely between 0·7% and 1·2%. 4 Women are predominantly affected in Western countries, whereas reports from Asia describe a male majority. 5 , 6 , 7 , 8 , 9 , 10 Increased prevalence among black and biracial vs. white individuals has been described in the USA. 8 , 11 Disease onset typically occurs in young adulthood, and the most commonly affected areas include the groin/genitals, axillae, buttocks and breasts. 12 , 13 , 14 , 15 , 16 Patients with HS may experience painful lesions, impaired work ability or productivity, reduced sexual health, and feelings of shame and stigmatization, all of which contribute to markedly reduced quality of life (QoL). 17 , 18 , 19 , 20 Furthermore, patients are often burdened by psychological comorbidities that worsen with increasing disease severity. 21 , 22 , 23
Adalimumab [anti‐tumour necrosis factor (TNF)‐α] is the only agent approved by the US Food and Drug Administration and European Medicines Agency for the treatment of moderate‐to‐severe HS (Hurley stage II/III). 24 In two phase III trials, adalimumab treatment resulted in disease improvement based on HS Clinical Response (HiSCR) criteria in a statistically significant but relatively small proportion of patients vs. placebo (PIONEER I, 42% vs. 26%; PIONEER II, 59% vs. 28%). 25 Furthermore, response to adalimumab generally declines over time, 25 , 26 highlighting the need for additional therapeutic options.
The role of Janus kinase (JAK)/signal transducer and activator of transcription (STAT)‐mediated type I/II cytokine signalling in dermatological disease is becoming increasingly prominent. 27 , 28 Oral and topical JAK inhibitors have entered clinical testing in several inflammatory skin disorders, including alopecia areata, atopic dermatitis, psoriasis and vitiligo, with findings indicating reduced inflammation and improvement in disease severity and symptoms following treatment. 28 Literature suggests that HS pathogenesis is driven by multiple JAK/STAT‐mediated cytokines, including interleukin (IL)‐1β, IL‐17, IL‐23 and IL‐10 and, to a lesser extent, TNF‐α. 1 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 Thus, inhibiting JAK‐mediated pathways could address HS disease biology more broadly than agents targeting individual cytokines. A small case study of two patients with HS reported successful treatment with the pan‐JAK inhibitor tofacitinib; 38 however, clinical trials of JAK inhibitors in HS have not been previously published.
INCB054707 is an oral, small‐molecule JAK1 inhibitor with approximately 52‐fold greater selectivity for JAK1 vs. JAK2. 39 Specifically targeting JAK1, a critical regulator of proinflammatory cytokine signalling implicated in several immune‐related diseases, 40 may reduce cytokine signalling involved in HS pathogenesis while limiting JAK2‐mediated cytopenias. 41 Here we describe the safety and efficacy of INCB054707 in two multicentre phase II trials in patients with moderate‐to‐severe HS: Study 1 (NCT03569371) and Study 2 (NCT03607487).
Patients and methods
Study design and patients
Study 1 was an open‐label, single‐arm study in which patients received 15 mg INCB054707 once daily (QD) for 8 weeks, with a 4‐week safety follow‐up period at the end of treatment (Figure S1a; see Supporting Information). Study 2 was a placebo‐controlled, dose‐escalation study in which patients were randomized to INCB054707 QD (30‐, 60‐ or 90‐mg cohorts) or placebo QD (3 : 1 randomization within each cohort) for 8 weeks, with a 30‐day safety follow‐up period at the end of treatment (Figure S1b). A safety review was conducted at week 4 of treatment in each cohort to determine progression to the next dose cohort. Patients were randomized using an interactive web response system; patients and investigators were blinded to treatment. The studies were conducted at research centres and community dermatology practices in the USA (Study 1) and Canada, Denmark and Germany (Study 2).
For both studies, eligible patients were men and women aged 18–75 years with a diagnosis of moderate‐to‐severe HS (Hurley stage II/III) 42 and disease duration ≥ 6 months, stable course of HS for ≥ 90 days before screening per investigator assessment, HS lesions present in at least two distinct anatomical areas, total abscess and inflammatory nodule (AN) count ≥ 3 at screening and baseline, and willingness to avoid pregnancy or fathering children. Exclusion criteria for both studies included presence of more than 20 draining fistulas at screening and baseline, previous use of JAK inhibitors, previous use of adalimumab (or any other TNF‐α treatment) or experimental treatments within 12 weeks or 5 half‐lives (whichever was longer) before baseline, and use of systemic immunosuppressive or immunomodulating drugs or other systemic HS therapies within 4 weeks or 5 half‐lives (whichever was longer) before baseline.
Both studies were conducted in accordance with the principles embodied by the Declaration of Helsinki and were conducted in adherence to the study protocols approved by the central or local institutional review board or independent ethics committee at each participating centre (Appendix S1; see Supporting Information) and to the International Council for Harmonisation guidelines for good clinical practice. All patients provided written informed consent before initiation of any study‐related procedures. Both studies have been completed. The study protocols are detailed in Appendixes S2 and S3 (see Supporting Information).
Study endpoints and assessments
The primary endpoint in both studies was safety and tolerability, including the frequency, duration and severity of adverse events (AEs) and clinical laboratory test results. AE severity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4·03, or per investigator assessment if CTCAE classification was unavailable. Secondary and exploratory efficacy endpoints included the proportion of patients achieving HiSCR (defined as ≥ 50% reduction in AN count with no increase in either abscess or draining fistula counts relative to baseline) 43 at each visit, the proportion of patients achieving an AN count of 0–2 at each visit, and change from baseline in the International Hidradenitis Suppurativa Severity Score System (IHS4), defined as the number of inflammatory nodules (× 1), plus the number of abscesses (× 2), plus the number of draining fistulas (× 4). 44
Secondary and exploratory patient‐reported outcome measures included mean change from baseline in HS Quality of Life (HiSQoL) score at each visit, mean change from baseline in the HS skin pain numerical rating scale scores for worst skin pain during the past 24 h at each visit, the proportion of patients scoring within each Dermatology Life Quality Index (DLQI) category during the past week, and the change from baseline in DLQI at each visit. A preliminary version of the HiSQoL containing 45 questions was used to evaluate the impact of HS on domains such as daily activities, discomfort, symptoms, depression/anxiety, sexual function and work ability (scored on a five‐point scale ranging from ‘not at all’ to ‘extremely’ affected), with higher scores indicating greater impairment. 45 HS skin pain was measured using an 11‐point scale ranging from 0 (no skin pain) to 10 (worst imaginable skin pain) and was recorded in a daily diary. The DLQI is a 10‐item questionnaire evaluating symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment, in which patients answered each question on a scale of ‘not at all’ to ‘very much’, with higher summed scores indicating worse QoL. 46
Exploratory translational studies evaluated change from baseline in expression of select blood biomarkers at weeks 4 and 8 as measured with the Olink proteomics platform (Olink, Uppsala, Sweden). The data are reported as normalized protein expression values with arbitrary units on a log2 scale.
Statistical analyses
For Study 1, the sample size was based on the precedent of other preliminary safety studies and not on statistical power calculations. Sample‐size determination in Study 2 was dependent on safety findings; approximately nine patients were randomized at each dose level to provide > 85% chance of detecting at least one AE of interest (e.g. platelets, haemoglobin, absolute neutrophil count, liver function, and infections), assuming their occurrence in 20% of patients. For both studies, the safety‐ and efficacy‐evaluable study populations included all enrolled patients who received at least one dose of study drug. The studies were designed to identify safety signals and set dose–response expectations for larger studies and were not powered to show statistical differences in outcomes. Therefore, safety and efficacy data were summarized using descriptive statistics without formal between‐group comparisons. SAS software version 9·4 (SAS Institute Inc., Cary, NC, USA) was used for the generation of all tables, graphs and statistical analysis data. Analyses on proteomic data were performed in Omicsoft’s ArrayStudio (Qiagen, Redwood City, CA, USA) using two‐way repeated‐measures anova followed by post hoc testing. Differentially expressed proteins were identified using false discovery rate < 0·05 (Benjamini–Hochberg correction) 47 and absolute fold changes > 1·2.
Results
Patients
Study 1
Ten patients were enrolled in Study 1 (17 July 2018 to 22 April 2019). The mean (SD) age was 40·7 (14·4) years, 30% of patients were women, and 60% were white. Prior treatments for HS consisted of oral tetracyclines (n = 2), Oxycocet (n = 1) and an investigational drug (n = 1). Overall, 70% of patients had Hurley stage II HS at baseline. The mean (SD) AN and draining fistula counts were 7·3 (4·8) and 1·6 (2·4), respectively (Table 1). All 10 patients received INCB054707 15 mg QD. Three patients (30%) discontinued study treatment; reasons included an AE, loss to follow‐up and lack of efficacy (n = 1 each; Figure S2; see Supporting Information).
Table 1.
Patient demographics and baseline disease characteristics
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| Parameter | INCB054707, 15 mg QD (n = 10) | INCB054707, 30 mg QD (n = 9) | INCB054707, 60 mg QD (n = 9) | INCB054707, 90 mg QD (n = 8) | Placebo QD (n = 9) |
| Age (years), mean (SD) | 40·7 (14·4) | 41·0 (11·5) | 42·2 (12·0) | 42·8 (14·6) | 40·3 (16·7) |
| Sex, n (%) | |||||
| Male | 7 (70) | 2 (22) | 1 (11) | 3 (38) | 1 (11) |
| Female | 3 (30) | 7 (78) | 8 (89) | 5 (63) | 8 (89) |
| Race, n (%) | |||||
| White | 6 (60) | 7 (78) | 9 (100) | 7 (88) | 8 (89) |
| Black | 3 (30) | 0 | 0 | 1 (13) | 0 |
| American Indian/Alaska native | 0 | 2 (22) | 0 | 0 | 0 |
| Other | 1 (10) | 0 | 0 | 0 | 1 (11) |
| BMI (kg m−2), mean (SD) | 34·2 (9·3) | 42·4 (9·5) | 41·7 (10·0) | 31·8 (6·2) | 32·6 (7·8) |
| Nicotine users, n (%) | 1 (10) | 1 (11) | 2 (22) | 0 | 1 (11) |
| Time since first onset of HS (years), mean (SD) | 16·2 (13·2) | 16·8 (12·4) | 8·2 (12·5) | 13·3 (13·5) | 11·1 (13·1) |
| Select previous treatments for HS, a n (%) | |||||
| Oral tetracyclines | 2 (20) | 1 (11) | 2 (22) | 2 (25) | 2 (22) |
| Topical clindamycin | 0 | 2 (22) | 1 (11) | 3 (38) | 3 (33) |
| Benzoyl peroxide | 0 | 1 (11) | 0 | 3 (38) | 3 (33) |
| Oral clindamycin | 0 | 0 | 0 | 2 (25) | 2 (22) |
| Adalimumab | 0 | 0 | 1 (11) | 1 (13) | 1 (11) |
| Hurley stage at baseline, n (%) | |||||
| II | 7 (70) | 9 (100) | 5 (56) | 7 (88) | 4 (44) |
| III | 3 (30) | 0 | 4 (44) | 1 (13) | 5 (56) |
| AN count, mean (SD) | 7·3 (4·8) | 11·2 (6·7) | 16·7 (12·9) | 15·9 (15·1) | 17·1 (9·6) |
| Draining fistula count, mean (SD) | 1·6 (2·4) | 0·9 (1·1) | 3·1 (3·8) | 1·8 (3·8) | 4·8 (5·4) |
| IHS4 score, mean (SD) | 15·7 (13·7) | 16·4 (8·0) | 33·1 (31·2) | 25·0 (20·3) | 41·7 (36·0) |
| Platelet count (× 109 per L), mean (SD) | 319 (104) | 305 (51) | 362 (80) | 260 (59) | 334 (90) |
| Comorbidities, b n (%) | |||||
| Abdominal obesity | 1 (10) | 7 (78) | 5 (56) | 0 | 2 (22) |
| Hypertension | 4 (40) | 2 (22) | 2 (22) | 1 (13) | 2 (22) |
| Arthritis | 0 | 4 (44) | 3 (33) | 0 | 0 |
| Diabetes | 2 (20) | 2 (22) | 1 (11) | 0 | 1 (11) |
| Hyperlipidaemia | 2 (20) | 1 (11) | 1 (11) | 0 | 2 (22) |
| Psoriasis | 0 | 0 | 2 (22) | 1 (13) | 0 |
| PCOS | 0 | 2 (22) | 0 | 0 | 0 |
| Multiple sclerosis | NA | 0 | 1 (11) | 0 | 0 |
AN, abscess and inflammatory nodule; BMI, body mass index; HS, hidradenitis suppurativa; IHS4, International Hidradenitis Suppurativa Severity Score System; NA, not assessed; PCOS, polycystic ovary syndrome; QD, once daily.
Previous treatments of interest included adalimumab and other medications reported in ≥ 15% of patients in either study.
No patients enrolled in either study had Crohn disease, ulcerative colitis or spondyloarthropathy.
Study 2
Thirty‐five patients were enrolled in Study 2 (15 October 2018 to 14 August 2019). The mean (SD) age was 41·5 (13·3) years, 80% of patients were women and 89% were white. Prior HS treatments were highly variable (Table 1); agents reported in < 15% of patients included anilides, corticosteroids, glucocorticoids, cephalexin, IL inhibitors, triclosan and oral rifampicin. Overall, 71% of patients had Hurley stage II HS at baseline. The mean (SD) AN and draining fistula counts were 15·2 (11·1) and 2·7 (4·0), respectively (Table 1). Nine patients were randomized to placebo and 26 to INCB054707 (30 mg, n = 9; 60 mg, n = 9; 90 mg, n = 8). Two patients (22%) randomized to placebo discontinued treatment (both for reason of withdrawal by patient); no patients randomized to INCB054707 discontinued treatment (Figure S2).
Safety
Study 1
Overall, 70% of patients in Study 1 experienced at least one treatment‐emergent AE (TEAE; Table 2), whereas there were no serious TEAEs. The most common TEAE was upper respiratory tract infection (n = 3, 30%). Three patients (30%) experienced treatment‐related adverse events (TRAEs; Table 2), which included upper respiratory tract infection, headache and night sweats (n = 1 each). One patient discontinued INCB054707 treatment owing to TEAEs (upper respiratory tract infection and fibromyalgia), whereas no dose interruptions due to AEs were reported. Rescue incision and drainage was required by one patient the day before the week 1 visit (unscheduled). Rescue with intralesional triamcinolone acetate suspension was required on three occasions in one patient, occurring at week 1, prior to the week 6 visit (unscheduled) and at the week 8 visit. Laboratory values were generally within normal limits throughout the study.
Table 2.
Treatment‐emergent adverse events (TEAEs) and treatment‐related adverse events (TRAEs) occurring in more than one patient in any treatment group
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| Parameter, n (%) | INCB054707, 15 mg QD (n = 10) | INCB054707, 30 mg QD (n = 9) | INCB054707, 60 mg QD (n = 9) | INCB054707, 90 mg QD (n = 8) | Placebo QD (n = 9) |
| Any TEAE | 7 (70) | 8 (89) | 6 (67) | 7 (88) | 4 (44) |
| Fatigue | 0 | 1 (11) | 2 (22) | 3 (38) | 1 (11) |
| Headache | 1 (10) | 0 | 2 (22) | 2 (25) | 2 (22) |
| Folliculitis | 0 | 2 (22) | 1 (11) | 0 | 1 (11) |
| Nasopharyngitis | 0 | 1 (11) | 2 (22) | 0 | 1 (11) |
| Thrombocytopenia | 0 | 0 | 0 | 4 (50) | 0 |
| Upper respiratory tract infection | 3 (30) | 0 | 0 | 1 (13) | 0 |
| Diarrhoea | 0 | 1 (11) | 0 | 0 | 2 (22) |
| Gastroenteritis | 0 | 0 | 2 (22) | 0 | 0 |
| Any TRAE | 3 (30) | 4 (44) | 1 (11) | 6 (75) | 2 (22) |
| Headache | 1 (10) | 0 | 1 (11) | 2 (25) | 1 (11) |
| Thrombocytopenia | 0 | 0 | 0 | 4 (50) | 0 |
| Any serious TEAE | 0 | 0 | 0 | 0 | 0 |
QD, once daily.
Study 2
Overall, 81% of patients treated with INCB054707 in Study 2 experienced at least one TEAE (Table 2). The most common TEAEs overall among patients treated with INCB054707 were fatigue (n = 6, 23%), headache (n = 4, 15%) and thrombocytopenia (n = 4, 15%; platelet count < 150 × 109 cells L−1; all at 90 mg). No serious TEAEs were observed. Among patients who received placebo, four (44%) had TEAEs, most commonly headache and diarrhoea (n = 2, 22% each). Eleven patients (42%) treated with INCB054707 experienced TRAEs (Table 2). There were no clinically meaningful changes in most laboratory values over the course of the study. The only TRAEs occurring in more than one patient in any treatment group were thrombocytopenia (n = 4 at 90 mg INCB054707) and headache (n = 2 at 90 mg INCB054707). There were no INCB054707 treatment discontinuations due to TEAEs. Four patients (all asymptomatic thrombocytopenia at 90 mg INCB054707) had dose interruptions up to 2 weeks; all platelet counts returned to levels > 100 × 109 cells L−1, and the drug was restarted without sequelae or further decreases in platelets requiring repeated drug interruptions.
Topical benzoyl peroxide was used in one patient in the placebo group, and topical benzoyl peroxide with clindamycin was used in one patient in the INCB054707 90 mg dose group at week 8. No patients in any dose group required rescue lesional treatment during Study 2.
Efficacy
Study 1
Three patients (43%) achieved HiSCR at week 8 (Figure 1). The proportions of patients achieving an AN count of 0–2 at week 8 and the safety follow‐up were 43% and 57%, respectively (Figure 2a). The mean (SD) change from baseline in IHS4 was −2·1 (7·5) at week 8.
Figure 1.
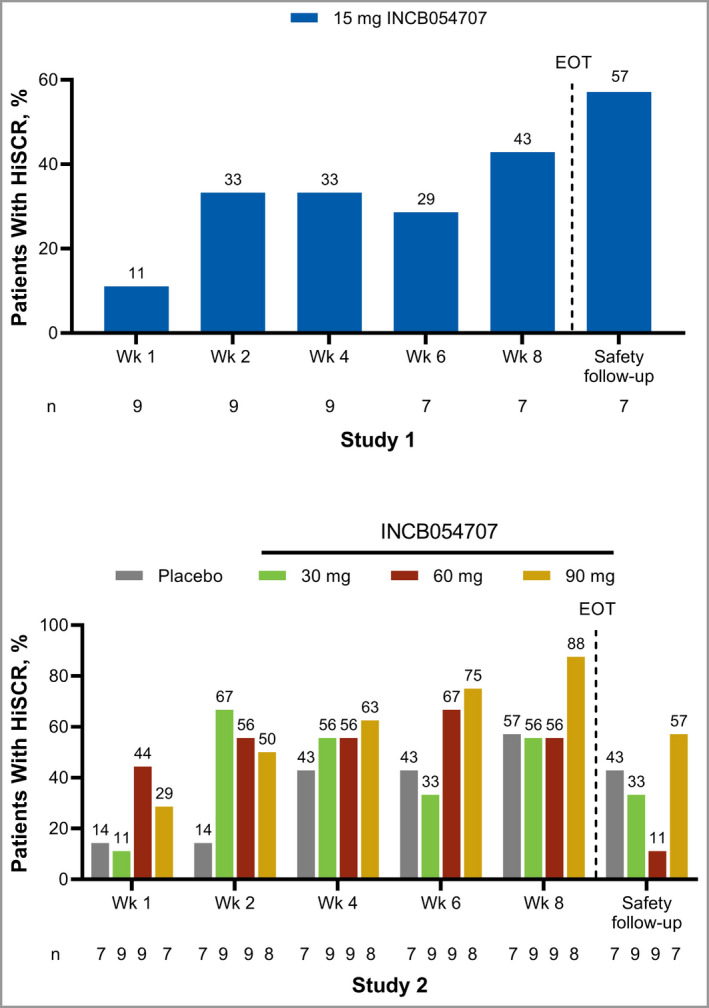
Hidradenitis Suppurativa Clinical Response (HiSCR) by study visit. EOT, end of treatment. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
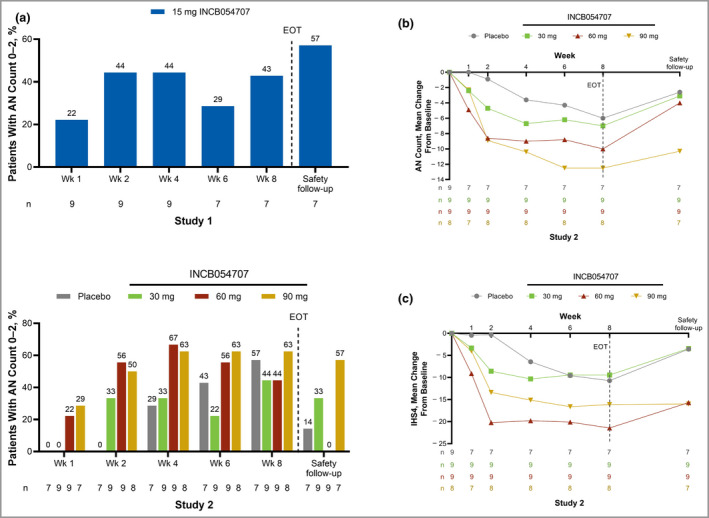
(a) Abscess and inflammatory nodule (AN) count 0–2. (b) Mean change from baseline in AN count. (c) Mean change from baseline in International Hidradenitis Suppurativa Severity Score System (IHS4) by study visit. EOT, end of treatment. [Colour figure can be viewed at wileyonlinelibrary.com]
Study 2
In Study 2, 17 patients (65%: 30 mg, 56%; 60 mg, 56%; 90 mg, 88%) receiving INCB054707 vs. four patients (57%) receiving placebo achieved HiSCR at week 8 (Figure 1). Half of the patients receiving INCB054707 (30 mg, 44%; 60 mg, 44%; 90 mg, 63%) at week 8 (vs. 57% for placebo) and 28% (30 mg, 33%; 60 mg, 0%; 90 mg, 57%) at safety follow‐up (vs. 14% for placebo) achieved an AN count of 0–2 (Figure 2a). Dose‐dependent improvements in mean change from baseline in AN count were also observed with INCB054707 treatment over time (Figure 2b). The mean (SD) changes from baseline at week 8 were −7·0 (4·6), −10·0 (8·9) and −12·5 (15·7) for patients treated with 30, 60 and 90 mg INCB054707, respectively, vs. −6·0 (8·2) for those who received placebo. After the active treatment period and through the 4‐week follow‐up, we observed a trend towards a return to baseline AN count among all treatment groups. For IHS4, the mean (SD) changes from baseline at week 8 were −9·4 (6·8), −21·4 (21·4) and −16·1 (22·9) for patients treated with 30 mg, 60 mg and 90 mg INCB054707, respectively, vs. −10·7 (21·0) for the placebo group (Figure 2c).
Patient‐reported outcomes
Study 1
The mean (SD) change from baseline in HiSQoL was −15·3 (40·9) at week 8 in Study 1 (Figure 3a). At week 8, the mean (SD) change from baseline in worst skin pain score was −2·0 (1·8) (Figure 3b), and the mean (SD) change from baseline in DLQI was −4·4 (5·3) (Figure 3c). The proportion of patients reporting ‘no effect’ (DLQI 0–1) or a ‘small effect’ (DLQI 2–5) of HS on QoL increased from 10% at baseline to 43% at week 8, as the proportion of patients reporting ‘moderate’ to ‘extremely large’ effect (DLQI 6–30) decreased from 90% to 57%.
Figure 3.
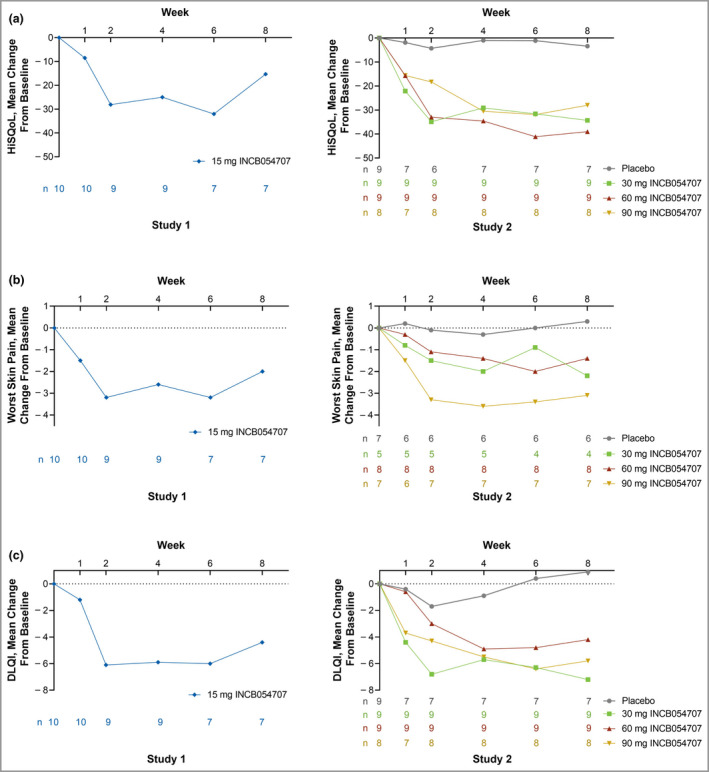
Mean changes from baseline in (a) Hidradenitis Suppurativa Quality of Life (HiSQoL), (b) worst skin pain and (c) Dermatology Life Quality Index (DLQI) by study visit. [Colour figure can be viewed at wileyonlinelibrary.com]
Study 2
In Study 2, the mean (SD) changes from baseline in HiSQoL were −34·3 (41·7), −39·0 (30·8) and −28·0 (21·4) for patients treated with 30, 60 or 90 mg INCB054707, respectively, at week 8, vs. −3·4 (16·1) for placebo (Figure 3a). At week 8, the mean (SD) changes from baseline in worst skin pain score were −2·2 (2·2), −1·4 (1·4) and −3·1 (3·3) for patients treated with 30, 60 and 90 mg INCB054707, vs. 0·3 (2·8) for placebo (Figure 3b). The mean (SD) changes from baseline to week 8 in DLQI were −7·2 (7·1), −4·2 (4·2) and −5·8 (4·7) among patients treated with 30, 60 and 90 mg INCB054707, vs. 0·9 (6·5) for placebo (Figure 3c). The proportion of patients reporting ‘no effect’ or a ‘small effect’ in DLQI was 15% at baseline, which increased to 54% among patients treated with INCB054707 at week 8 (vs. 22% to 29% for placebo), whereas the proportion reporting ‘moderate’ to ‘extremely large’ effect decreased from 85% to 46% for INCB054707 (vs. 78% to 71% for placebo).
Translational studies
Proteomic analysis was performed on serum samples collected before treatment and at weeks 4 and 8 following treatment. Of the proteins measured, select proteins of interest were preidentified for further assessment based on literature review and our previous analyses. 48 , 49 Differentially expressed proteins identified at weeks 4 and 8 vs. baseline for each dose were plotted together to illustrate dose dependency of responses to JAK1 inhibition (Figure 4a). JAK1 inhibition had little impact on relative expression of TNF‐α after 8 weeks of treatment (Figure 4b). In contrast, IL‐2 receptor α (IL2RA) was significantly reduced following 8 weeks of treatment with 60 or 90 mg INCB054707, with less pronounced reductions observed in sera from patients treated with lower doses (Figure 4c).
Figure 4.
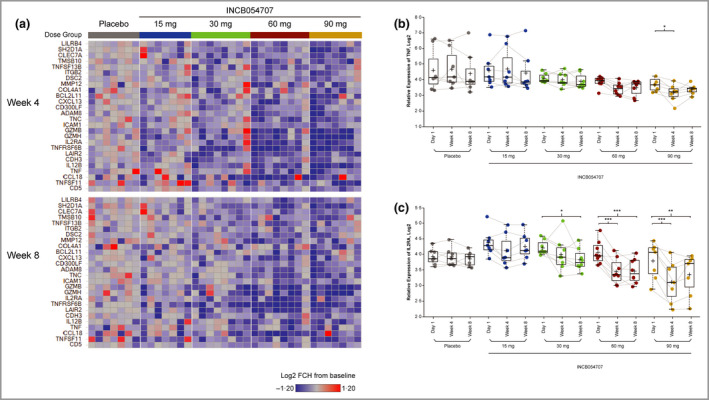
(a) Heat map from broad proteomic analysis showing fold change (FCH) from baseline in disease‐associated markers at weeks 4 and 8. Protein expression profiles of (b) tumour necrosis factor (TNF) and (c) interleukin‐2 receptor α (IL2RA). FDR, false discovery rate. *FDR < 0·05, **FDR < 0·01, ***FDR < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
The studies described here evaluated the orally administered JAK1 inhibitor INCB054707 for the treatment of moderate‐to‐severe HS. INCB054707 was well tolerated and demonstrated preliminary efficacy in both phase II studies. Thrombocytopenia was transient and dose dependent, which has been observed with other JAK1 inhibitors. 50 , 51 , 52 , 53 Compared with the other groups in Study 2, baseline platelet counts were lower in the 90‐mg group, which may have contributed to a higher proportion of these patients experiencing transient thrombocytopenia. Several reviews on the overall safety of JAK inhibitors have been published recently. 54 , 55 , 56
In both studies presented here, improvements in secondary and exploratory endpoints including HiSCR, AN count and patient‐reported outcomes of skin pain and QoL measures were observed over the course of INCB054707 treatment. Improvements compared with placebo were observed as early as week 1 for all efficacy endpoints with most doses of INCB054707.
Although IHS4 has not yet been used in pivotal trials as a primary endpoint, it provides certain advantages over HiSCR: it is a dynamic score that accounts for changes in draining fistulas in addition to abscesses and inflammatory nodules, further enabling disease severity classification. 57 Additionally, the use of IHS4 may be associated with a decreased placebo response compared with HiSCR. 58 In Study 2, INCB054707 at 60 mg demonstrated the highest change in IHS4 compared with the other two active doses, which may be attributed to its higher mean draining fistula count and consequentially higher baseline IHS4 score.
Adalimumab is currently the only approved therapy for HS; however, responses are achieved in only approximately one‐half of patients and are not durable. 24 , 25 , 26 Furthermore, there are no oral agents available for HS, as adalimumab administration requires weekly injections, which may be inconvenient for patients. 24 In contrast to adalimumab, which inhibits TNF‐α, JAK1 inhibition has the potential to regulate several downstream JAK1‐mediated proinflammatory cytokines implicated in the development of HS pathogenesis, including IL‐6 and TNF‐α. 30 , 32 Furthermore, these cytokines may act as biomarkers to assess disease severity. 31 Biomarker analysis demonstrated modulation of inflammatory mediators after treatment with INCB054707 after 4 and 8 weeks. TNF‐α is known to be elevated in HS sera and is a therapeutic target for this indication; 24 , 59 however, reduction of serum TNF‐α following treatment with INCB054707 was relatively minimal. In contrast, soluble IL2RA levels exhibited a striking dose‐dependent decrease. Although its regulation is complex, IL2RA can be induced by TNF signalling, and serum levels of soluble IL2RA are increased under chronic inflammatory conditions, including HS. 60 , 61
These phase II studies were limited by small sample sizes and a relatively short treatment duration (8 weeks). In addition, Study 1 was a single‐arm trial. In Study 2, patients were not stratified by disease severity, and a greater proportion of patients receiving placebo vs. INCB054707 had Hurley stage III HS at baseline. Although AN count was generally similar across treatment groups at baseline, heterogeneity was observed between groups in draining fistula count and time to first onset of HS, which may have contributed to differences in response to therapy. No formal statistical comparisons were conducted to evaluate differences between treatment groups.
In conclusion, the safety and efficacy findings from these two phase II studies establish proof of concept for the JAK1 inhibitor INCB054707 in the treatment of moderate‐to‐severe HS. Patients treated with INCB054707 reported improvements in QoL and skin pain as early as week 1 that were generally maintained over the 8‐week treatment period. Treatment with INCB054707 was associated with dose‐dependent AN count decrease, improvements in IHS4 score over time, and modulation of circulating inflammatory mediators. A phase II dose‐ranging, placebo‐controlled study exploring three dose levels and including approximately 200 patients is ongoing (NCT04476043) and is expected to provide additional evidence of the safety and efficacy profile of INCB054707 in patients with HS.
Author Contributions
Afsaneh Alavi: Investigation (equal); resources (equal); writing – review and editing (equal). Iltefat Hamzavi: Methodology (supporting); writing – review and editing (equal). Kurt Brown: Data curation (supporting); project administration (supporting); supervision (lead); validation (supporting); writing – review and editing (equal). Leandro L. Santos: Data curation (supporting); project administration (lead); supervision (supporting); validation (supporting); visualization (supporting); writing – review and editing (lead). Zhaoyin Zhu: Data curation (lead); formal analysis (lead); resources (equal); software (lead); validation (lead); visualization (lead); writing – review and editing (equal). Huiqing Liu: Data curation (supporting); formal analysis (equal); investigation (equal); visualization (supporting); writing – review and editing (equal). Michael D Howell: Data curation (supporting); formal analysis (equal); investigation (equal); visualization (supporting); writing – review and editing (equal). Joslyn S. Kirby: Investigation (equal); resources (equal); writing – review and editing (equal).
Supporting information
Appendix S1 Approving institutional review boards and independent ethics committees.
Appendix S2 Clinical study protocol for Study 1.
Appendix S3 Clinical study protocol for Study 2.
Figure S1 Study design and planned patient enrolment.
Figure S2 Patient disposition.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
The authors thank the principal investigators for both studies, the study‐site personnel, and all study patients for their participation in this study. Additional thanks to Kathleen Butler, MD, and Fiona Kuo, PhD, of Incyte for initiating each study, Susan Smith, PhD, of Incyte for support with translational assays and analyses, and Annie Wang, PhD, of Incyte for support with biostatistical analyses. Writing assistance was provided by Jane Kovalevich, PhD, an employee of ICON (Blue Bell, PA, USA), and was funded by Incyte Corporation.
Funding sources These studies were funded by Incyte Corporation, who was involved in the study design, data collection, data analysis, manuscript preparation and publication decisions in collaboration with the authors.
Conflicts of interest A.A. has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRx and UCB; and received honoraria as an investigator for Boehringer Ingelheim and Processa. I.H. has served on an advisory board for AbbVie; received research funding from ChemoCentryx, Incyte Corporation and Pfizer; served as a consultant for Janssen, Novartis and UCB; and served as an uncompensated board member for the HS Foundation. K.B., L.L.S. and H.L. are employees and shareholders of Incyte Corporation. Z.Z. and M.D.H. were employees of Incyte Corporation at the time of the study. J.S.K. has served as a speaker for AbbVie and as a consultant for AbbVie, Bayer, ChemoCentryx, Incyte Corporation, InflaRx, Janssen, Novartis, Pfizer and UCB.
Data availability statement Access to individual patient‐level data is not available for this study. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical‐trial‐data‐sharing.pdf?ver=2020‐05‐21‐132838‐960.
Plain language summary available online
References
- 1. Vossen A, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol 2018; 9:2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scuderi N, Monfrecola A, Dessy LA et al. Medical and surgical treatment of hidradenitis suppurativa: a review. Skin Appendage Disord 2017; 3:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabat R, Jemec GBE, Matusiak L et al. Hidradenitis suppurativa. Nat Rev Dis Primers 2020; 6:18. [DOI] [PubMed] [Google Scholar]
- 4. Nguyen TV, Damiani G, Orenstein LAV et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol 2021; 35:50–61. [DOI] [PubMed] [Google Scholar]
- 5. Ingram JR, Jenkins‐Jones S, Knipe DW et al. Population‐based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018; 178:917–24. [DOI] [PubMed] [Google Scholar]
- 6. Calao M, Wilson JL, Spelman L et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population‐based cross‐sectional study. PLOS ONE 2018; 13:e0200683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delany E, Gormley G, Hughes R et al. A cross‐sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol 2018; 32:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg A, Kirby JS, Lavian J et al. Sex‐ and age‐adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol 2017; 153:760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayama K, Fujita H, Hashimoto T et al. 026 Nationwide investigation of hidradenitis suppurativa in Japan. J Invest Dermatol 2017; 137 (Suppl. 2):S197. [Google Scholar]
- 10. Cosmatos I, Matcho A, Weinstein R et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol 2013; 68:412–19. [DOI] [PubMed] [Google Scholar]
- 11. Garg A, Wertenteil S, Baltz R et al. Prevalence estimates for hidradenitis suppurativa among children and adolescents in the United States: a gender‐ and age‐adjusted population analysis. J Invest Dermatol 2018; 138:2152–6. [DOI] [PubMed] [Google Scholar]
- 12. Vossen A, van der Zee HH, Onderdijk AJ et al. Hidradenitis suppurativa is not associated with the metabolic syndrome based on body type: a cross‐sectional study. J Dermatol 2017; 44:154–9. [DOI] [PubMed] [Google Scholar]
- 13. Garg A, Neuren E, Cha D et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020; 82:366–76. [DOI] [PubMed] [Google Scholar]
- 14. Benhadou F, Van der Zee HH, Pascual JC et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross‐sectional study among 2465 patients. Br J Dermatol 2019; 181:1198–206. [DOI] [PubMed] [Google Scholar]
- 15. Peterson GC, Preston A, Frieder J et al. Analysis of characteristics and trends in treatment response of hidradenitis suppurativa patients: a Southern US cohort study. Dermatology 2020; 236:413–20. [DOI] [PubMed] [Google Scholar]
- 16. Jorgensen AR, Holm JG, Ghazanfar MN et al. Factors affecting quality of life in patients with hidradenitis suppurativa. Arch Dermatol Res 2020; 312:427–36. [DOI] [PubMed] [Google Scholar]
- 17. Mac Mahon J, Kirthi S, Byrne N et al. An update on health‐related quality of life and patient‐reported outcomes in hidradenitis suppurativa. Patient Relat Outcome Meas 2020; 11:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yao Y, Jorgensen AR, Thomsen SF. Work productivity and activity impairment in patients with hidradenitis suppurativa: a cross‐sectional study. Int J Dermatol 2020; 59:333–40. [DOI] [PubMed] [Google Scholar]
- 19. Alavi A, Farzanfar D, Rogalska T et al. Quality of life and sexual health in patients with hidradenitis suppurativa. Int J Womens Dermatol 2018; 4:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koumaki D, Efthymiou O, Bozi E et al. Perspectives on perceived stigma and self‐stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol 2019; 12:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kimball AB, Crowley JJ, Papp K et al. Baseline patient‐reported outcomes from UNITE: an observational, international, multicentre registry to evaluate hidradenitis suppurativa in clinical practice. J Eur Acad Dermatol Venereol 2020; 34:1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kouris A, Platsidaki E, Christodoulou C et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology 2016; 232:687–91. [DOI] [PubMed] [Google Scholar]
- 23. Hamzavi IH, Sundaram M, Nicholson C et al. Uncovering burden disparity: a comparative analysis of the impact of moderate‐to‐severe psoriasis and hidradenitis suppurativa. J Am Acad Dermatol 2017; 77:1038–46. [DOI] [PubMed] [Google Scholar]
- 24. Savage KT, Flood KS, Porter ML et al. TNF‐α inhibitors in the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis 2019; 10:2040622319851640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimball AB, Okun MM, Williams DA et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016; 375:422–34. [DOI] [PubMed] [Google Scholar]
- 26. Frew JW, Jiang CS, Singh N et al. Dermal tunnels influence time to clinical response and family history influences time to loss of clinical response in patients with hidradenitis suppurativa treated with adalimumab. Clin Exp Dermatol 2021; 46:306–13. [DOI] [PubMed] [Google Scholar]
- 27. Welsch K, Holstein J, Laurence A et al. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol 2017; 47:1096–107. [DOI] [PubMed] [Google Scholar]
- 28. Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol 2019; 10:2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Witte‐Handel E, Wolk K, Tsaousi A et al. The IL‐1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol 2019; 139:1294–305. [DOI] [PubMed] [Google Scholar]
- 30. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res 2018; 7:1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jimenez‐Gallo D, de la Varga‐Martinez R, Ossorio‐Garcia L et al. The clinical significance of increased serum proinflammatory cytokines, C‐reactive protein, and erythrocyte sedimentation rate in patients with hidradenitis suppurativa. Mediators Inflamm 2017; 2017:2450401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol 2019; 10:2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang XO, Panopoulos AD, Nurieva R et al. STAT3 regulates cytokine‐mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282:9358–63. [DOI] [PubMed] [Google Scholar]
- 34. Kelly G, Hughes R, McGarry T et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol 2015; 173:1431–9. [DOI] [PubMed] [Google Scholar]
- 35. Liu T, Li S, Ying S et al. The IL‐23/IL‐17 pathway in inflammatory skin diseases: from bench to bedside. Front Immunol 2020; 11:594735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schlapbach C, Hänni T, Yawalkar N et al. Expression of the IL‐23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol 2011; 65:790–8. [DOI] [PubMed] [Google Scholar]
- 37. Kelly G, Prens EP. Inflammatory mechanisms in hidradenitis suppurativa. Dermatol Clin 2016; 34:51–8. [DOI] [PubMed] [Google Scholar]
- 38. Savage KT, Santillan MR, Flood KS et al. Tofacitinib shows benefit in conjunction with other therapies in recalcitrant hidradenitis suppurativa patients. JAAD Case Rep 2020; 6:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fay B, He X, Margulis A et al. OP0280 The selective JAK1 inhibitor INCB054707 ameliorates cutaneous lesions in a spontaneous murine model of systemic lupus erythematosus. Ann Rheum Dis 2019; 78 (Suppl. 2):221–2. [Google Scholar]
- 40. Schwartz DM, Bonelli M, Gadina M et al. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 2016; 12:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akada H, Akada S, Hutchison RE et al. Critical role of JAK2 in the maintenance and function of adult hematopoietic stem cells. Stem Cells 2014; 32:1878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zouboulis CC, Del Marmol V, Mrowietz U et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015; 231:184–90. [DOI] [PubMed] [Google Scholar]
- 43. Kimball AB, Sobell JM, Zouboulis CC et al. HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo‐controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol 2016; 30:989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zouboulis CC, Tzellos T, Kyrgidis A et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017; 177:1401–9. [DOI] [PubMed] [Google Scholar]
- 45. Kirby JS, Thorlacius L, Villumsen B et al. The Hidradenitis Suppurativa Quality of Life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol 2020; 183:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc 2004; 9:169–80. [DOI] [PubMed] [Google Scholar]
- 47. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57:289–300. [Google Scholar]
- 48. Coates M, Mariottoni P, Corcoran DL et al. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLOS ONE 2019; 14:e0216249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rumberger BE, Boarder EL, Owens SL et al. Transcriptomic analysis of hidradenitis suppurativa skin suggests roles for multiple inflammatory pathways in disease pathogenesis. Inflamm Res 2020; 69:967–73. [DOI] [PubMed] [Google Scholar]
- 50. Silverberg JI, Simpson EL, Thyssen JP et al. Efficacy and safety of abrocitinib in patients with moderate‐to‐severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2020; 156:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gooderham MJ, Forman SB, Bissonnette R et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol 2019; 155:1371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmieder GJ, Draelos ZD, Pariser DM et al. Efficacy and safety of the Janus kinase 1 inhibitor PF‐04965842 in patients with moderate‐to‐severe psoriasis: phase II, randomized, double‐blind, placebo‐controlled study. Br J Dermatol 2018; 179:54–62. [DOI] [PubMed] [Google Scholar]
- 53. Simpson EL, Sinclair R, Forman S et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate‐to‐severe atopic dermatitis (JADE MONO‐1): a multicentre, double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet 2020; 396:255–66. [DOI] [PubMed] [Google Scholar]
- 54. Clarke B, Yates M, Adas M et al. The safety of JAK‐1 inhibitors. Rheumatology (Oxford) 2021; 60:ii24–ii30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chang PH, Huang SF, Chang PS et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol 2021; 48:1631–9. [DOI] [PubMed] [Google Scholar]
- 56. Yates M, Mootoo A, Adas M et al. Venous thromboembolism risk with JAK inhibitors: a meta‐analysis. Arthritis Rheumatol 2021; 73:779–88. [DOI] [PubMed] [Google Scholar]
- 57. Zouboulis CC, Gulliver W, Ingram J et al. Endpoints of clinical trials for hidradenitis suppurativa: proceedings of a round‐table session. Exp Dermatol 2020; 29 (Suppl. 1):67–72. [DOI] [PubMed] [Google Scholar]
- 58. Frew JW, Jiang CS, Singh N et al. Clinical response rates, placebo response rates, and significantly associated covariates are dependent on choice of outcome measure in hidradenitis suppurativa: a post hoc analysis of PIONEER 1 and 2 individual patient data. J Am Acad Dermatol 2020; 82:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matusiak L, Bieniek A, Szepietowski JC. Increased serum tumour necrosis factor‐alpha in hidradenitis suppurativa patients: is there a basis for treatment with anti‐tumour necrosis factor‐alpha agents? Acta Derm Venereol 2009; 89:601–3. [DOI] [PubMed] [Google Scholar]
- 60. Liao W, Lin JX, Leonard WJ. Interleukin‐2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Matusiak Ł, Bieniek A, Szepietowski JC. Soluble interleukin‐2 receptor serum level is a useful marker of hidradenitis suppurativa clinical staging. Biomarkers 2009; 14:432–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Approving institutional review boards and independent ethics committees.
Appendix S2 Clinical study protocol for Study 1.
Appendix S3 Clinical study protocol for Study 2.
Figure S1 Study design and planned patient enrolment.
Figure S2 Patient disposition.
Powerpoint S1 Journal Club Slide Set.


