Abstract
Mutations in in fms-like tyrosine kinase 3 (FLT3) gene are common genomic alterations in acute myeloid leukemia (AML). FLT3 internal tandem duplication mutations (FLT3-ITD) have consistently been shown to be adversely prognostic, particularly those with high allelic ratio. Current AML treatment strategies including high dose cytarabine, purine analogs, FLT3 inhibitors (FLT3i), and with or without allogeneic stem cell transplant (SCT) have been shown to improve the outcomes in patients with FLT3 mutations. We analyzed a consecutive cohort of newly diagnosed patients with AML treated at a large academic medical center from January 2012 through January 2020. A total of 1576 patients with a new diagnosis of AML were reviewed. Among these, 1,438 (91%) had molecular testing for FLT3 mutations and 21% (304/1,438) had a FLT3 mutation, including 17% with a FLT3-ITD mutation. We show that FLT3-ITD high allelic ratio with NPM1 wild-type have significantly improved survival compared with other European LeukemiaNet (ELN) adverse risk disease. In multivariable cox proportional hazards model of patients receiving intensive or low intensity induction regimens, FLT3 mutations did not have prognostic significance. The use of allogeneic stem cell transplant in CR1 for patients with FLT3 mutations appears to improve survival, particularly in those with ELN adverse risk disease. Overall, this data highlights the changing prognostic impact of FLT3 mutations in a contemporary era with appropriate use of induction therapy combined with targeted agents and allogenic stem cell transplant.
Keywords: AML, AML- molecular diagnosis & therapy, Leukemia
Introduction
Mutations in the fms-like tyrosine kinase 3 (FLT3) gene are common genomic alterations in AML, identified in approximately 30% of cases 1–3. FLT3 mutations occur commonly in two regions: the juxta membrane domain leading to internal tandem duplication (FLT3-ITD), representing the most common type of FLT3 mutation, seen in approximately 25% of all cases; and the tyrosine kinase domain (FLT3-TKD), which occurs in approximately 7–10% of cases 1, 2. FLT3-ITD mutations have been found to be adverse in AML 4–10. The prognostic impact of FLT3-ITD mutations appears to be dependent on the allelic ratio (high allelic ratio ≥ 0.5) and the presence of NPM1 co-mutations. The current European LeukemiaNet (ELN) guidelines categorizes FLT3-ITD mutations into favorable (mutated NPM1 with either wild-type (WT) FLT3 or FLT3-ITD low allelic ratio), intermediate (FLT3-ITD high allelic ratio with mutated NPM1 or WT NPM1 with FLT3-ITD low allelic ratio), and adverse (FLT3-ITD high allelic ratio with WT NPM1) risk groups depending on allelic ratio and NPM1 co-mutational status 11. FLT3-TKD mutations do not appear to confer strong prognostic significance 12–15.
Development of small molecule FLT3 inhibitors (FLT3i) that inhibit the aberrantly active FLT3 signaling have been shown to improve leukemia-specific clinical outcomes 16–19. In the treatment of newly diagnosed patients with AML, both midostaurin and sorafenib have shown improvements in clinical outcomes in the frontline setting. In the phase 3 trial RATIFY study, midostaurin combined with cytarabine and daunorubicin (7+3) improved overall survival (OS) compared with placebo in patients <60 years of age, regardless of allelic ratio or type of mutation (ITD vs TKD) 18. Similarly, sorafenib combined with 7+3 in the SORAML study improved both event-free and relapse-free survival 16. There is also evidence that that use of fludarabine, cladribine, high-dose cytarabine, and idarubicin induction may overcome the negative impact of FLT3-ITD mutations 20, 21. Additionally, the use of allogeneic stem cell transplant (SCT) in first remission for FLT3-ITD mutated patients also appears to improve both relapse-free and overall survival22.
While the adverse prognostic impact of FLT3 mutations is clear in historical cohorts, it is not clear if the presence of FLT3 mutations is still as impactful when patients receive therapy that includes intensive induction regimens with high dose cytarabine, purine analogs, appropriate utilization of allogeneic stem cell transplant, and frequent use of FLT3i. In this study we analyzed a large, contemporary cohort of newly diagnosed AML patients treated at an academic center to determine the evolving prognostic impact of FLT3 mutations.
Methods
We analyzed data from all consecutive patients with a new diagnosis of AML presenting to and receiving at least induction treatment at MD Anderson Cancer Center from January 1, 2012, through December 31, 2019. Demographic, clinical, and molecular data was abstracted from the charts. Intensive induction therapy was defined as an induction regimen that included at minimum a combination of cytarabine and an anthracycline; high dose cytarabine was any dose that was greater than or equal to 1g/m2/day, and purine analogs used in the study period included cladribine, fludarabine, and clofarabine. All investigations were conducted under approval of the institutional review committee and in accordance with the declaration of Helsinki.
Baseline characteristics are summarized, and comparisons are tested using Wilcoxon rank sum test, Pearson’s Chi-squared test, and Fisher’s exact tests were performed where appropriate. p<0.05 was considered statistically significant. For univariate survival analysis, we utilized cox proportional hazards and log-rank to determine significance and Kaplan-Meier method for plotting. For survival analyses that investigated the role of stem cell transplantation (SCT), we utilized a landmark analysis that included patients that achieved a CR or CRi to induction therapy, survived at least as long as the median time to transplant within each group, and did not have core binding factor leukemia since these patients have good survival and cure rates without SCT.
Multivariable cox proportional hazard models were constructed using baseline variables with a p-value less than or equal to 0.15 in univariate analysis. The final multivariable model was selected through a forward and backward stepwise approach while keeping FLT3 mutations in the model. All analyses and graphs were created in R, version 4.1.
Results
In total, 1576 patients with a new diagnosis of AML were seen and treated at MD Anderson from January 1, 2012, through December 31, 2019. Of them, 1,438 (91%) had molecular testing for FLT3 mutations. FLT3 mutations were detected in 21% (304/1,438) of patients; 238/304 (78%) had a FLT3-ITD mutation, 92/304 (30%) had a FLT3-TKD mutation, and 26/304 (9%) had both a FLT3-ITD and FLT3-TKD mutations. Among patients with FLT3-ITD, the median allelic ratio of mutant FLT3 was 0.54 (IQR: 0.10 – 0.90).
FLT3 Mutations in the Context of ELN Risk Groups
We investigated the prognostic impact of FLT3 mutations in this cohort. Patients with FLT3-ITD with high allelic ratio (AR) (≥ 0.50) and wild-type (WT) NPM1 have historically had high-risk AML and this mutational pattern is defined as adverse risk by the European LeukemiaNet (ELN) classification. In the overall cohort of patients, we separated the FLT3-ITD high AR with WT NPM1 (n=61) from all other ELN adverse risk patients (n=632). Overall survival in patients FLT3-ITD high AR with WT NPM1 was significantly better than the other ELN adverse risk patients (HR 0.45, 95% CI: 0.32 to 0.63, p<0.001 compared with other ELN adverse risk disease) and was more similar to patients with ELN intermediate risk disease (Figure 1A). This finding was more pronounced in patients treated with intensive induction where FLT3-ITD high AR with WT NPM1 had a HR 0.33 (95% CI: 0.19 to 0.60, p<0.001) compared with other ELN adverse risk patients (Figure 1B). In patients treated with low intensity induction, the patients with FLT3-ITD high AR and WT NPM1 also had improved survival compared with the other ELN adverse risk patients; however, the hazard ratio or magnitude of benefit was not as high as the intensively treated patients (HR 0.63, 95% CI: 0.42 to 0.95, p=0.028). In a similar analysis, FLT3 defining ELN intermediate mutations (FLT3-ITD high AR/NPM1 mutated and FLT3-ITD low AR/NPM1 WT) did not separate significantly from other patients with ELN intermediate risk disease (Supplementary Figure 1A–C).
Figure 1: Prognostic Impact of FLT3-ITD high/NPM1 WT.
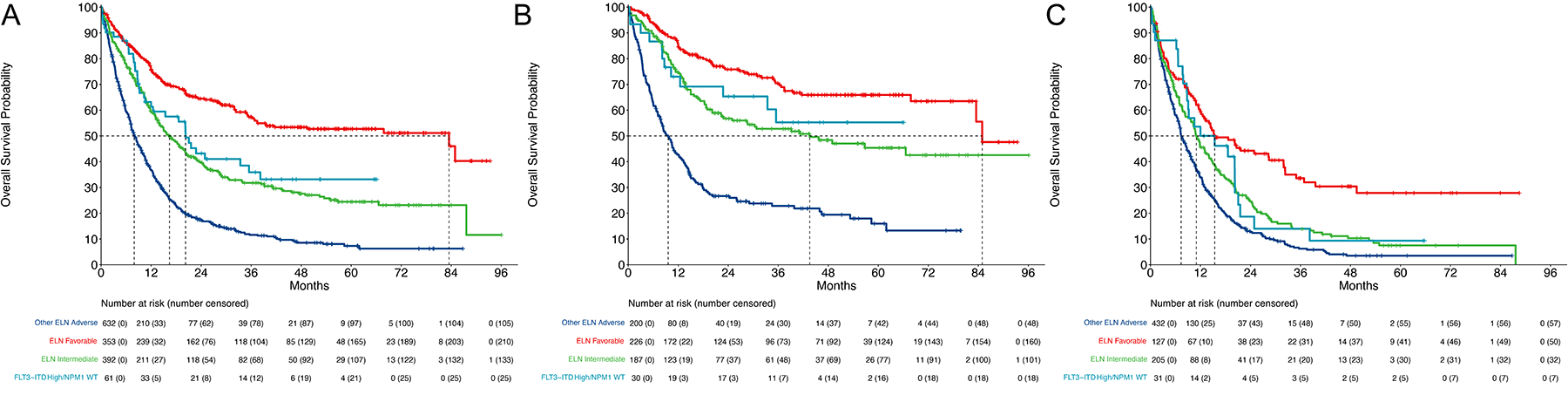
A. Overall survival of the full cohort stratified by ELN risk. ELN adverse risk patients were divided into FLT3-ITD high allelic ratio and NPM1 WT and all other ELN adverse risk patients. B. Overall survival of patients treated with intensive induction therapy stratified by ELN risk with FLT3-ITD high allelic ratio/NPM1 WT separated from other ELN adverse risk disease. C. Overall survival of patients treated with low intensity induction therapy stratified by ELN risk with FLT3-ITD high allelic ratio/NPM1 WT separated from other ELN adverse risk disease.
FLT3 Mutations in Patients Treated with Intensive Induction Regimens
In patients treated with intensive induction therapy (n=643) there were 172 patients with a FLT3 mutation. Among these there were 44 with a FLT3 TKD mutation and 128 with a FLT3-ITD mutation including 64 that had a high FLT3-ITD allelic ratio (AR) (≥ 0.50) and 64 that had a low FLT3-ITD AR (<0.50). Among patients with FLT3-ITD, the median allelic ratio of mutant FLT3 was 0.48 (IQR: 0.07 – 0.86).
Patients with a FLT3 mutation were younger (median 52 vs 55, p=0.041) and more likely to receive an induction regimen that included high dose cytarabine (HiDAC) (97% vs 90%, p=0.011) and a purine analog (86% vs 77%, p=0.003) (Supplementary Table 1). Patients with FLT3 mutations were more likely to have de novo AML (93% vs 73%, p<0.001) and overall had fewer high risk cytogenetic and molecular alterations including higher percentage of diploid cytogenetics (58% vs 31%, overall p<0.001), lower percentage of ASXL1 mutations (1.4% vs 7.9%, p=0.006), and TP53 mutations (2.6% vs 15%, p<0.001). They were more likely to be co-mutated for DNMT3A (27% vs 16%, p=0.002) and NPM1 (48% vs 11%, p<0.001) (Supplementary Table 1).
Of the 172 patients with a FLT3 mutation, 78 (45%) were treated with a FLT3 inhibitor combined with intensive induction. 73 of the 128 (57%) with FLT3-ITD mutation received a FLT3i, including 67% (43/64) of those with FLT3-ITD high AR and 47% of those with FLT3-ITD low AR, while only 5 of the 44 (11%) with a FLT3-TKD mutation did. The most common FLT3i used was sorafenib, in 67 of 78 (86%) of patients receiving a FLT3i, followed by midostaurin in 8 (10%) patients, gilteritinib in 2 (2.5%), and quizartinib in 1 (1.5%). Limiting the analysis to patients without core binding factor (CBF) AML, survival was similar between patients whether they received a FLT3i with their induction regimen (HR 1.10 95% CI: 0.64 to 1.89, p=0.74, Supplementary Figure 2).
In a univariate analysis, patients with a FLT3 mutation had superior survival compared with those without a FLT3 mutation (HR 0.64, 95% CI: 0.49 – 0.84, p=0.001, Figure 2A). This held true when accounting for different types of FLT3 mutations, with both FLT3 TKD (HR 0.54 95% CI: 0.32 – 0.90, p=0.020) and FLT3 ITD high AR (HR 0.64 95% CI: 0.42 – 0.97, p=0.034) having significantly better OS compared with FLT3 WT patients (Figure 2B). When accounting for NPM1 co-mutational status, all groups had a HR <1 compared with FLT3 WT/NPM1 WT patients (Figure 2C). Patients with significantly improved survival compared with FLT3 WT/NPM1 WT patients were FLT3 WT/NPM1 mutated (HR 0.44 95% CI: 0.27 – 0.73, p=0.002), FLT3 TKD/NPM1 WT (HR 0.49 95% CI: 0.26 – 0.92, p=0.026), and FLT3 ITD high/NPM1 mutated (HR 0.56 95% CI: 0.32 – 0.98, p=0.041) (Figure 2C).
Figure 2: Prognostic value of FLT3 mutations in patients treated with intensive or low intensity therapy.
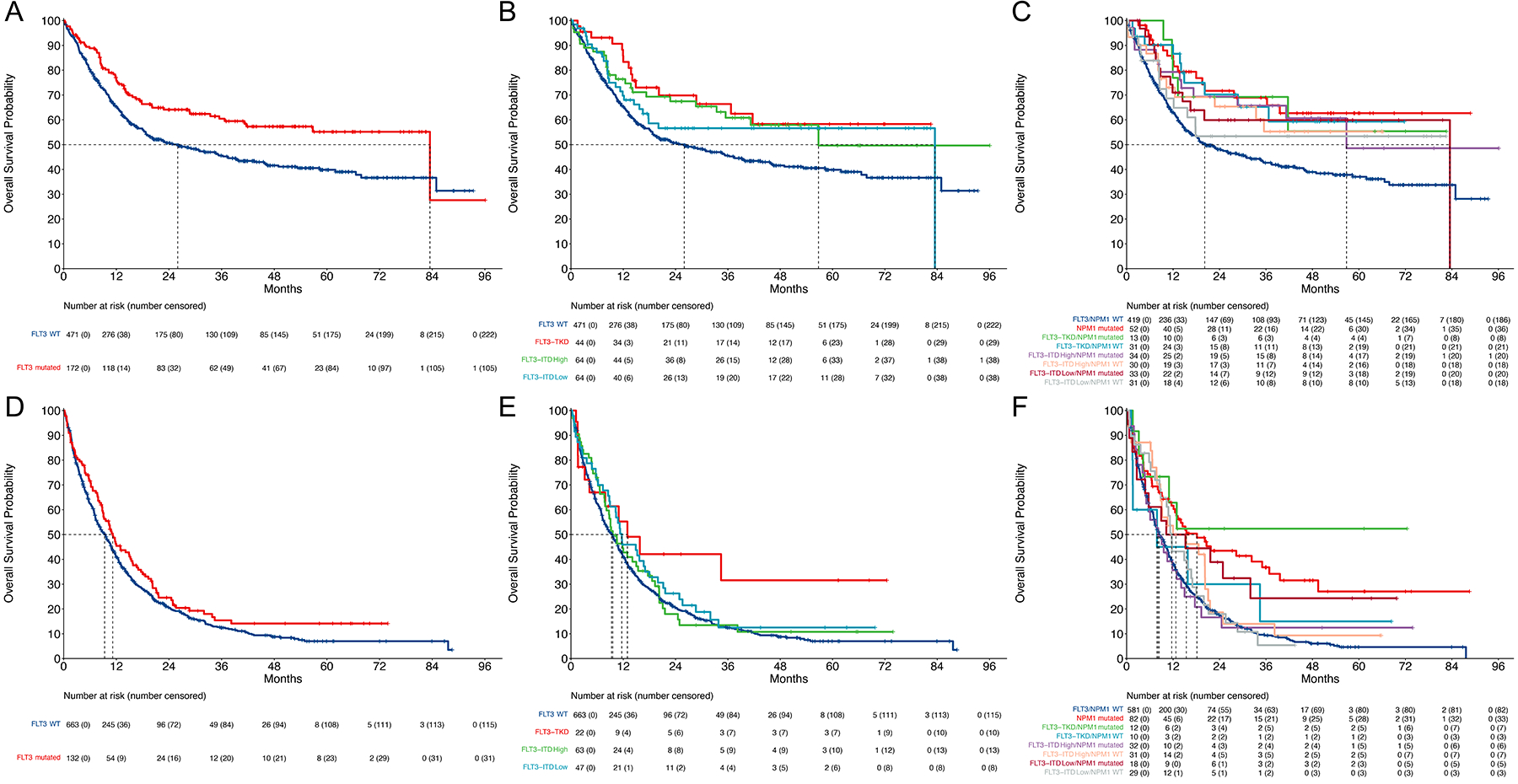
A. Overall survival of patients treated with intensive induction stratified by presence or absence of FLT3 mutation (ITD or TKD). B. Overall survival of patients treated with intensive induction stratified by of FLT3 mutation. FLT3-ITD high allelic ratio is ≥ 0.5. C. Overall survival of patients treated with intensive induction stratified by FLT3/NPM1 co-mutational status. D. Overall survival of patients treated with low intensity induction stratified by presence or absence of FLT3 mutation (ITD or TKD). E. Overall survival of patients treated with low intensity induction stratified by of FLT3 mutation. FLT3-ITD high allelic ratio is ≥ 0.5. F. Overall survival of patients treated with low intensity induction stratified by FLT3/NPM1 co-mutational status
We developed a cox proportional hazard models to investigate the prognostic impact of FLT3 mutations. In univariate analyses we included baseline patient-intrinsic factors and biological characteristics. These factors are summarized in Supplementary Table 2. In the multivariate analysis including characteristics with a p-value ≤0.15 in the univariate analysis, FLT3 mutations (TKD, ITD high, ITD low) were not associated with survival, confirming the lack of prognostic significance of FLT3 mutations when correcting for other relevant prognostic factors. Adjusted overall survival Kaplan-Meier curve using this cox model confirm lack of prognostic significance of FLT3 mutations (Supplementary Figure 3). Factors associated with improved survival were higher pretreatment albumin (for each increase in 1 mg/dL increase in albumin: HR 0.70 95% CI: 0.55 – 0.89, p=0.003), favorable risk cytogenetics (compared with diploid, HR 0.44 95% CI: 0.26 – 0.74, p=0.002), and NPM1 mutation (HR 0.52 95% CI: 0.33 – 0.81, p=0.004). Factors associated with increased risk of death were increased age (for each increase in 10 years, HR 1.38 95% CI: 1.23 – 1.55, p<0.001), treated-secondary AML23 (HR: 2.13 95% CI: 1.36 – 3.35, p=0.001), impaired baseline renal function (for each increase in 1 mg/dL creatinine; HR: 1.40 95% CI: 1.03 – 1.90, p=0.030), adverse risk cytogenetics (HR 2.27 95% CI: 1.55 – 3.32, p<0.001), and TP53 mutation (HR 2.58 95% CI: 1.77 – 3.75, p<0.001) (Table 1).
Table 1:
Final multivariable model for patients treated with either intensive or low intensity therapy
| Intensive Therapy | Low Intensity Therapy | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.38 (1.23 to 1.55) | <0.001 | 1.25 (1.10 to 1.43) | <0.001 |
| Therapy related AML | ns | 1.32 (1.05 to 1.66) | 0.017 | |
| Secondary AML | ns | 1.73 (1.40 to 2.14) | <0.001 | |
| Treated secondary AML | 2.13 (1.36 to 3.35) | 0.001 | ns | |
| Pretreatment Platelet Count | ns | 0.98 (0.96 to 0.99) | 0.009 | |
| Pretreatment Peripheral Blasts | ns | 1.06 (1.02 to 1.11) | 0.002 | |
| Pretreatment Serum Creatinine | 1.40 (1.03 to 1.90) | 0.03 | 1.26 (1.03 to 1.55) | 0.027 |
| Pretreatment Total Bilirubin | ns | 1.50 (1.30 to 1.73) | <0.001 | |
| Pretreatment Albumin | 0.70 (0.55 to 0.89) | 0.003 | 0.78 (0.66 to 0.92) | 0.004 |
| Cytogenetic Groups | ||||
| Diploid | — | — | ||
| Favorable | 0.44 (0.26 to 0.74) | 0.002 | 1.40 (0.19 to 10.2) | 0.74 |
| Intermediate | 1.10 (0.71 to 1.71) | 0.67 | 1.35 (1.02 to 1.78) | 0.035 |
| 11q23 | 1.31 (0.68 to 2.50) | 0.42 | 1.62 (0.83 to 3.14) | 0.16 |
| Adverse | 2.27 (1.55 to 3.32) | <0.001 | 1.67 (1.26 to 2.21) | <0.001 |
| CEBPA Mutation | 0.71 (0.44 to 1.16) | 0.18 | 0.80 (0.56 to 1.14) | 0.22 |
| FLT3 Mutation | ||||
| WT | — | — | ||
| TKD | 1.21 (0.69 to 2.13) | 0.51 | 0.55 (0.29 to 1.06) | 0.074 |
| ITD High | 0.78 (0.46 to 1.32) | 0.36 | 0.79 (0.54 to 1.17) | 0.24 |
| ITD Low | 1.30 (0.77 to 2.19) | 0.33 | 1.11 (0.74 to 1.66) | 0.62 |
| IDH2 Mutation | ns | 0.54 (0.39 to 0.75) | <0.001 | |
| NPM1 Mutation | 0.52 (0.33 to 0.81) | 0.004 | 0.72 (0.53 to 0.99) | 0.041 |
| TP53 Mutation | 2.58 (1.77 to 3.75) | <0.001 | 1.44 (1.12 to 1.84) | 0.004 |
FLT3 Mutations in Patients Treated with Low Intensity Induction Regimens
In the patients treated with low intensity induction regimens (n=795) there were 132 patients with a FLT3 mutation. Among these there were 22 with a FLT3 TKD mutation and 110 with a FLT3-ITD mutation including 63 that had a high FLT3-ITD AR and 47 that had a low FLT3-ITD AR. Among patients with FLT3-ITD, the median allelic ratio of mutant FLT3 was 0.63 (IQR: 0.15 – 0.92).
Patients with a FLT3 mutation were similar age (median 72 vs 72, p=0.45), were equally likely to receive a hypomethylating agent (HMA) containing regimen (73% vs 72%, p=0.72), a purine analog containing regimen (14% vs 19%, p=0.13), but less likely to receive venetoclax (18% vs 26%, p=0.047) (Supplementary Table 3). Like intensively treated patients, FLT3 mutated patients overall had less high risk cytogenetic and molecular alterations including higher percentage of diploid cytogenetics (58% vs 32%, overall p<0.001) and less TP53 mutations (6.6% vs 33%, p<0.001). FLT3 mutated patients were more likely to be co-mutated for DNMT3A (31% vs 16%, p<0.001) and NPM1 (48% vs 13%, p<0.001) (Supplementary Table 3).
Of the 132 patients with a FLT3 mutation, 91 (69%) were treated with a FLT3 inhibitor in the frontline setting. 87 of the 110 (79%) with FLT3-ITD mutation received a FLT3i, including 97% (61/67) of those with FLT3-ITD high AR and 55% of those with FLT3-ITD low AR, while only 4 of the 22 (18%) with a FLT3-TKD mutation did. The most common FLT3i used was sorafenib, in 54 of 91 (59%), followed quizartinib in 20 (22%), midostaurin in 12 (13%), and gilteritinib in 5 (6%). Limiting the analysis to patients without core binding factor (CBF) AML, survival was similar between patients whether they received a FLT3i with their induction regimen (HR 1.14 95% CI: 0.73 to 1.77, p=0.58, Supplementary Figure 4).
In patients treated with low intensity induction, FLT3 mutation did not affect OS in univariate analysis, with a trend toward improved OS in FLT3 mutated patients (HR 0.82 95% CI: 0.67 – 1.02, p=0.075) (Figure 2D). This trend was driven by patients with FLT3-TKD mutations (HR 0.60, 95% CI: 0.34 – 1.07, p=0.081) with FLT3-ITD high AR (HR 0.91, 95% CI: 0.68 – 1.21, p=0.51) and low AR (HR 0.82, 95% CI: 0.59 – 1.14, p=0.24) patients having similar survival compared with FLT3 WT patients (Figure 2E). When accounting for NPM1 co-mutational status, co-mutational pairs with improved survival compared with FLT3 WT/NPM1 WT patients were patients with FLT3 WT/NPM1 mutated (HR 0.49, 95% CI: 0.36 – 0.65, p<0.001), and FLT3-TKD/NPM1 mutation (HR 0.36 95% CI: 0.15 – 0.87, p=0.024) (Figure 2F).
Univariate analyses are summarized in Supplementary Table 4. In the multivariate analysis, FLT3 mutations (TKD, ITD high, ITD low) were not associated with survival in patients treated with low intensity induction. Adjusted overall survival Kaplan-Meier curve using this cox model confirm lack of prognostic significance of FLT3 mutations (Supplementary Figure 5). Factors associated with improved survival were increased platelet count (for each increase in 10K/μl; HR: 0.98 95% CI: 0.96 – 0.99, p=0.009), increased baseline albumin (for each increase in 1 mg/dL; HR: 0.78 95% CI: 0.66 – 0.92, p=0.004), IDH2 mutations (HR 0.54 95% CI: 0.39 – 0.75, p<0.001), and NPM1 mutation (HR: 0.72 95% CI: 0.53 – 0.99, p=0.041). Factors associated with increased risk of death were therapy related AML (HR 1.32 95% CI: 1.05 – 1.66, p=0.017), AML from an antecedent hematologic disease (HR: 1.73 95% CI: 1.40 – 2.14, p<0.001), increased baseline peripheral blasts (for each increase in 10%; HR 1.06 95% CI: 1.02 – 1.11, p=0.002), impaired renal function (for each increase in 1 mg/dL creatinine; HR: 1.26 95% CI: 1.03 – 1.55, p=0.027), impaired liver function (for each increase in 1 mg/dL total bilirubin; HR: 1.50 95% CI: 1.30 – 1.73, p<0.001), adverse risk cytogenetics (HR 1.67 95% CI: 1.26 – 2.21, p<0.001), and TP53 mutation (HR 1.44 95% CI: 1.12 – 1.84, p=0.004) (Table 1).
Impact of SCT on Survival in FLT3 Mutated Patients
We then further investigated the role of allogenic stem cell transplant (SCT) in first remission in patients in this cohort. For this analysis we performed a landmark analysis of patients that achieved a CR or CRi to induction therapy, who were alive at least as long as the median time to SCT and did not have CBF leukemia. There were 643 patients treated with intensive chemotherapy, of which 358 achieved a CR/CRi, did not have CBF leukemia and survived at least as long as the median time to SCT in (3.61 months) which were included in the landmark analysis. The receipt of SCT in CR1 in patients that responded to induction therapy was associated with increased survival (HR 0.57, 95% CI: 0.41 to 0.79, p<0.001) (Supplementary Figure 6A). When stratifying receipt of SCT in CR1 by FLT3 mutation status, patients with FLT3 mutation appeared to have a large benefit from SCT in CR1 (HR 0.43, 95% CI: 0.26 to 0.71, p<0.001) but also benefited FLT3 WT patients (HR 0.63 95% CI: 0.43 to 0.93, p=0.019) (Figure 3A).
Figure 3: Role of SCT.

A. Landmark survival analysis including patients treated with intensive induction, responded to induction therapy, were alive at the median time to SCT, and did not have core binding factor leukemia stratified by FLT3 mutation and receipt of SCT in first remission. B. Landmark survival analysis including patients treated with low intensity induction, responded to induction therapy, were alive at the median time to SCT, and did not have core binding factor leukemia stratified by FLT3 mutation and receipt of SCT in first remission. C. Overall survival of all patients with ELN adverse risk disease. Landmark survival analysis including patients that responded to induction therapy, were alive at the median time to SCT, and did not have core binding factor leukemia stratified by FLT3-ITD high allelic ratio and NPM1 WT and all other ELN adverse risk patients.
There were 795 patients treated with low intensity therapy, of which 369 achieved a CR/CRi, did not have CBF leukemia and survived at least as long as the median time to SCT in (4.01 months) which were included in the landmark analysis. The receipt of SCT in CR1 in patients that responded to induction therapy was associated with increased survival (HR 0.58 95% CI: 0.41 to 0.83, p=0.003) (Supplementary Figure 6B). When stratifying receipt of SCT in CR1 by FLT3 mutation status, patients with FLT3 mutation again had a large benefit from SCT in CR1 (HR 0.23 95% CI: 0.09 to 0.62, p=0.004) whereas the benefit in FLT3 WT patients was not as pronounced (HR 0.72 95% CI: 0.49 to 1.05, p=0.088) (Figure 3B).
Finally, focusing only on all ELN adverse risk patients that responded to induction therapy and survived at least as long as median time to transplant (n=321 of 693 total patients with ELN adverse risk disease), the receipt of SCT in CR1 had a striking impact on survival for patients that were FLT3 ITD high/NPM1 WT (78.7% survival rate at 3 years) compared with FLT3 ITD high/NPM1 WT (16.7% survival rate at 3 years) that did not receive SCT in CR1 (HR 0.13 95% CI: 0.04 to 0.39, p<0.001) and especially compared with other ELN adverse risk patients that received an SCT in CR1 (3-year survival rate of 41% in those that underwent SCT and 13.3% for those without SCT) HR 0.21 95% CI: 0.08 to 0.58, p=0.003) (Figure 3C).
Discussion
Newly diagnosed AML with a FLT3-ITD mutation has historically been associated with an adverse prognosis, characterized by higher risk of relapse and poor overall survival. The ELN AML risk classification system, accounting for co-mutations in NPM1 and the weight of mutant FLT3 allelic ratio, has categorized FLT3-ITD mutated, NPM1 WT as adverse risk. With evolving therapy standards, including the incorporation of FLT3 inhibitors, use of higher dose cytarabine during induction, use of a second nucleoside analogue, and the broader application of allogeneic stem cell transplant we have witnessed a significant improvement in the outcomes of patients with FLT3-ITD mutated AML. We aimed to review our experience with FLT3-ITD mutated AML to determine whether FLT3-ITD maintained its adverse prognostic significance, relative to other ELN risk groups, with current therapy. In a large, contemporary cohort of consecutively treated, newly diagnosed AML patients we observed that the presence of a FLT3-ITD mutation, at high or low AR, with or without NPM1 co-mutation no longer retained its adverse prognostic impact on overall survival. Among patients treated at our institution with FLT3 ITD high AR and NPM1 WT we observed improved overall survival compared with other ELN adverse risk disease patients, such that their survival was similar to that of ELN intermediate risk patients. This held true of patients treated with intensive or lower intensity induction regimens. Further investigation showed that, in univariate analyses, patients with FLT3 mutations had improved survival compared with FLT3 WT patients likely driven by enrichment for high-risk genomic features among FLT3 WT patients. In multivariable analysis with adjustments made for key clinical and molecular prognostic information the presence of FLT3 mutations did not have prognostic significance in patients treated with either intensive or lower intensity therapy. We did show that receipt of allogenic SCT in CR1 appears to have a dramatic effect on survival in patients with FLT3 mutated AML, particularly in those with high AR FLT3-ITD and NPM1 WT.
FLT3-ITD mutations did not show any significant survival disadvantage in our cohort. This is counter to previous data where FLT3-ITD has historically been associated with worse relapse free and overall survival24. Additionally, we also did not observe an effect of AR on outcomes, with patients with AR ≥ 0.5 and those <0.5 having similar survival. In line with previous reports, FLT3-TKD mutations were not associated with inferior survival15.
It should be noted that majority of our intensively treated patients received regimens that included the use of high dose cytarabine (92% overall) during induction as well as a purine analog (79% overall). Both have been shown to negate the negative prognostic impact of FLT3 mutations. For example, in an analysis of consecutively treated patients with fludarabine, high-dose cytarabine and idarubicin induction there was no significant difference in survival for patients with FLT3-ITD regardless of allelic ratio when there was an NPM1 mutation present20. In this analysis, 3-year OS was 71.8% and 74.1% in NPM1-mutated patients with or without low AR FLT3-ITD which was similar to NPM1-mutated patients with high AR FLT3-ITD (3-year OS 61.4%)20. The regimen included both HiDAC based induction as well as the use of fludarabine as a second nucleoside analogue. Similarly, in an analysis of patients treated with a frontline regimen containing cladribine added to 7+3 in normal karyotype patients, the addition of cladribine appeared to eliminate the adverse prognostic impact of FLT3-ITD mutations 21.
In addition to the choice of frontline therapy, the use of allogenic SCT in first remission for patients with FLT3-ITD mutations improves relapse-free and overall survival22, 25. The benefit of SCT in first remission has been shown to be independent of allelic ratio and NPM1 co-mutational status in those with FLT3-ITD mutations22. Additionally, there is evidence to suggest both FLT3-ITD and FLT3-TKD mutations derive benefit from SCT in first remission26. Our data suggests that SCT in first remission has a large effect on survival in both patients receiving intensive and low intensity induction regimens. This was particularly pronounced among patients with FLT3-ITD high AR, NPM1 WT (classified as ELN Adverse risk) that received SCT in first remission, with estimated three-year survival of 79%.
Incorporation of FLT3 inhibitors into induction and consolidation therapy for AML has shown improve outcomes and is becoming the standard of care for this subgroup of patients. The addition of sorafenib to intensive induction in the frontline setting demonstrated improved event and relapse-free survival in the SORAML study16. Midostaurin demonstrated an overall survival benefit when combined with 7+3 in newly diagnosed patients with FLT3 mutated AML in the RATIFY trial18. Additionally, the use of quizartinib with intensive induction was recently reported to improve overall survival27. Both quizartinib and gilteritinib have shown improvements in relapsed/refractory AML patients with FLT3 mutations19, 28. Overall, 45% of the patients with a FLT3 mutations that received intensive induction in our study received a concomitant FLT3i, including 57% with a FLT3-ITD mutation and 67% of those with FLT3-ITD high AR. Due to availability, 86% received sorafenib with smaller proportions receiving midostaurin, quizartinib, and gilteritinib. While survival was similar between those receiving and not receiving FLT3i in their induction regimen, more patients with FLT3-ITD and -ITD high AR were in the group receiving FLT3i. It should also be noted that there was great availability in our cohort of salvage therapies incorporating new and investigational FLT3i based combinations which may have also contributed to improved outcomes among those patients who did not receive FLT3i in the frontline and may be improving survival in those not undergoing SCT29. The incorporation of FLT3i into the frontline regimens may be playing an important role in improving outcomes with respect to the historically adverse prognostic significance.
There are several limitations of our study. The data are from a single academic center, but all consecutive patients treated during the designated time period were included to minimize selection bias. Nonetheless, replicating a similar study at other large institutions or cooperative study groups could help reproduce this data and help confirm improving outcomes in FLT3 mutated AML. Most of the patients treated with intensive chemotherapy received regimens that included high dose cytarabine and/or a purine analog which are known to overcome the negative prognostic impact of FLT3 mutations and could account for the significant improvement in outcomes; but improvements were also seen among patients treated with lower intensity approaches. Additional studies among cohorts primarily treated with 7+3 would also be insightful. Finally, while a large proportion of patients received a FLT3i in the frontline setting, most received sorafenib with only a minority of patients receiving midostaurin, quizartinib, and gilteritinib. With greater availability and wider use of these next generation FLT3 inhibitors, future studies will help evaluate their impact on AML outcomes.
In summary, our data highlights the need to reassess the prognostic impact of FLT3 mutations in the contemporary era. In this consecutive cohort of patients with newly diagnosed AML, FLT3 mutations did not retain the adverse prognostic impact that they previously have. This data suggests, that with the use of risk adapted and individualized therapy and greater accessibility to allogeneic SCT, we are improving the outcomes of patients with FLT3 mutations.
Supplementary Material
Funding:
P.K.R. is supported by a T32 training grant from the NIH (T32CA009666).
Conflicts of Interest Disclosure:
KS: Novartis: Consultancy, Research Funding; Daiichi-Sankyo: Membership on an entity’s Board of Directors or advisory committees; Pfizer: Membership on an entity’s Board of Directors or advisory committees.
HMG: Ascentage: Research Funding; Jazz: Research Funding; NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria; Aptitude Health: Honoraria; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astellas Health: Honoraria; Astra Zeneca: Honoraria; Ipsen Pharmaceuticals: Honoraria; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Amgen: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria.
NGD: Bristol Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Novartis: Consultancy; Abbvie: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Astellas: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Hanmi: Research Funding; Amgen: Consultancy, Research Funding; Novimmune: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding.
MY: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding.
CDD: Takeda: Honoraria; AbbVie: Consultancy, Research Funding; ImmuneOnc: Honoraria, Research Funding; Forma: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity’s Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity’s Board of Directors or advisory committees; Novartis: Honoraria; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding.
NJS: NGMBio: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Honoraria; AstraZeneca: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria.
GB: GSK: Consultancy; Takeda: Membership on an entity’s Board of Directors or advisory committees; Astex: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; Novartis: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Ryvu: Research Funding; ArgenX: Membership on an entity’s Board of Directors or advisory committees; Protagonist: Consultancy.
NP: HemOnc Times/Oncology Times: Membership on an entity’s Board of Directors or advisory committees; MustangBio: Consultancy, Other; CareDx, Inc.: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Other, Research Funding; Dan’s House of Hope: Membership on an entity’s Board of Directors or advisory committees; Cellectis S.A. ADR: Other, Research Funding; Plexxicon: Other, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity’s Board of Directors or advisory committees; LFB Biotechnologies: Consultancy; DAVA Oncology: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Sager Strong Foundation: Other; Springer Science + Business Media: Other; Abbvie Pharmaceuticals: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Other, Research Funding; Roche Diagnostics: Consultancy; Celgene Corporation: Consultancy; Samus: Other, Research Funding; ASH Communications Committee: Membership on an entity’s Board of Directors or advisory committees; Aptitude Health: Consultancy; Affymetrix: Consultancy, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Incyte: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy.
RSM: Kadmon: Research Funding; Incyte: Research Funding; CSLBehring: Research Funding; Syndax: Research Funding.
JDK: Stemline Therapeutics: Research Funding; Angle: Research Funding; Kiromic: Research Funding.
MYK: Sanofi: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Agios: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Stemline Therapeutics: Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Ablynx: Other: grant support, Research Funding; Cellectis: Other: grant support; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; KisoJi: Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights.
EJ: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding.
FR: AstraZeneca: Honoraria; AbbVie: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Prelude: Research Funding; Novartis: Honoraria; Xencor: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding.
TMK: Genfleet: Other; Cure: Speakers Bureau; Agios: Consultancy; Liberum: Consultancy; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Astellas: Other; Ascentage: Other; Sanofi-Aventis: Consultancy; Pulmotech: Other; AbbVie: Consultancy, Other: Grant/research support; Novartis: Consultancy; Pfizer: Consultancy, Other; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Cellonkos: Other; Jazz: Consultancy; AstraZeneca: Other.
All other authors declare no competing interests.
References
- 1.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med. 2016;374: 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368: 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366: 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiyoi H, Naoe T, Nakano Y, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93: 3074–3080. [PubMed] [Google Scholar]
- 5.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98: 1752–1759. [DOI] [PubMed] [Google Scholar]
- 6.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61: 7233–7239. [PubMed] [Google Scholar]
- 7.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99: 4326–4335. [DOI] [PubMed] [Google Scholar]
- 8.Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100: 4372–4380. [DOI] [PubMed] [Google Scholar]
- 9.Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. 2005;19: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 10.Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114: 2386–2392. [DOI] [PubMed] [Google Scholar]
- 11.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129: 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno I, Martín G, Bolufer P, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88: 19–24. [PubMed] [Google Scholar]
- 13.Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110: 1262–1270. [DOI] [PubMed] [Google Scholar]
- 14.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111: 1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters--an analysis of 3082 patients. Blood. 2008;111: 2527–2537. [DOI] [PubMed] [Google Scholar]
- 16.Röllig C, Serve H, Noppeney R, et al. Sorafenib or placebo in patients with newly diagnosed acute myeloid leukaemia: long-term follow-up of the randomized controlled SORAML trial. Leukemia. 2021;35: 2517–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravandi F, Arana Yi C, Cortes JE, et al. Final report of phase II study of sorafenib, cytarabine and idarubicin for initial therapy in younger patients with acute myeloid leukemia. Leukemia. 2014;28: 1543–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med. 2017;377: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med. 2019;381: 1728–1740. [DOI] [PubMed] [Google Scholar]
- 20.Minetto P, Candoni A, Guolo F, et al. Fludarabine, High-Dose Cytarabine and Idarubicin-Based Induction May Overcome the Negative Prognostic Impact of FLT3-ITD in NPM1 Mutated AML, Irrespectively of FLT3-ITD Allelic Burden. Cancers (Basel). 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libura M, Giebel S, Piatkowska-Jakubas B, et al. Cladribine added to daunorubicin-cytarabine induction prolongs survival of FLT3-ITD+ normal karyotype AML patients. Blood. 2016;127: 360–362. [DOI] [PubMed] [Google Scholar]
- 22.Oran B, Cortes J, Beitinjaneh A, et al. Allogeneic Transplantation in First Remission Improves Outcomes Irrespective of FLT3-ITD Allelic Ratio in FLT3-ITD-Positive Acute Myelogenous Leukemia. Biol Blood Marrow Transplant. 2016;22: 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boddu P, Kantarjian HM, Garcia-Manero G, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017;1: 1312–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Port M, Böttcher M, Thol F, et al. Prognostic significance of FLT3 internal tandem duplication, nucleophosmin 1, and CEBPA gene mutations for acute myeloid leukemia patients with normal karyotype and younger than 60 years: a systematic review and meta-analysis. Ann Hematol. 2014;93: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 25.DeZern AE, Sung A, Kim S, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant. 2011;17: 1404–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaballa S, Saliba R, Oran B, et al. Relapse risk and survival in patients with FLT3 mutated acute myeloid leukemia undergoing stem cell transplantation. Am J Hematol. 2017;92: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quizartinib Added to Chemotherapy Demonstrates Superior Overall Survival Compared to Chemotherapy Alone in Adult Patients with Newly Diagnosed FLT3-ITD Positive AML. Available from URL: https://www.businesswire.com/news/home/20211118006328/en/Quizartinib-Added-to-Chemotherapy-Demonstrates-Superior-Overall-Survival-Compared-to-Chemotherapy-Alone-in-Adult-Patients-with-Newly-Diagnosed-FLT3-ITD-Positive-AML [accessed November 20, 2021.
- 28.Cortes JE, Khaled S, Martinelli G, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. The Lancet Oncology. 2019;20: 984–997. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz M, Alfayez M, DiNardo CD, et al. Outcomes with sequential FLT3-inhibitor-based therapies in patients with AML. Journal of hematology & oncology. 2020;13: 132–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


