Abstract
Seed dispersal has received much research attention. The plant canopy can intercept diaspores, but the effect of the plant canopy (the aboveground portion of a plant consisting of branches and leaves) on dispersal distance has not been explored empirically. To determine the effect of plant canopy on seed dispersal distance, a comparison of diaspores falling through open air and through plant canopy was made in a wind tunnel using three wind speeds and diaspores with various traits. Compared with diaspores falling through open air, the dispersal distance of diaspores falling through plant canopy was decreased or increased, depending on wind speed and diaspore traits. When falling through a plant canopy, dispersal distance of diaspores with thorns or those without appendages was promoted at low wind speed (2 m s−1), while that of diaspores with low wing loading (0.5 mg mm−2) and terminal velocity (2.5 m s−1) was promoted by relatively high (6 m s−1) wind speed. A plant canopy could increase seed dispersal distance, which may be due to the complicated updraft generated by canopy. The effect of maternal plants on seed dispersal regulates the distribution pattern and the species composition of the community.
Subject terms: Ecology, Conservation biology
Introduction
Seed/diaspore dispersal can influence the spatial pattern and dynamics of a plant species at the population and metapopulation levels1–5, and it is an important research topic6,7. Seeds can be dispersed in multiple ways, and, on average, 10–30% of seeds and up to 70% of the plant species in temperate plant communities are more conductive to wind dispersal8. Any seed will be affected by wind9, which may further affect seed dispersal. Wind dispersal of diaspores occurs in all types of vegetation3, Wind can promote lateral dispersal speed of diaspores, and diaspore dispersal time/distance can be prolonged by strong horizontal wind2,10. The common wind levels in open xerophytic forest are 3–7 of Beaufort scale, occasionally can reach 8 levels, and wind speeds above 8 levels are rare8. Since wind speed and direction are variable, it is difficult to collect data on diaspore dispersal by wind in the field11. Thus, wind tunnels make it possible to conduct controlled experiments on the effects of wind on diaspore dispersal12–15.
Dispersal distance means how far a seed is moved away from the mother plant3, which affects the distribution pattern of seeds10,16,17. The pattern of diaspore dispersal will determine if the new plant becomes established in a habitat where the level of competition is low enough to be favorable for establishment and growth or if there is a high level of competition17. Thus, dispersal of diaspores may increase regional biodiversity18, and it can affect the management of weeds and endangered species19. Research on seed dispersal distance can facilitate an accurate prediction of population dynamics and distribution and improve the theoretical basis for vegetation restoration and biodiversity conservation.
Seeds are often the diaspores that are dispersed9, but the dispersed diaspores of angiosperms can be fruits or fruits plus appendages such as the bracts, perianth or parts of plants, tumbleweeds dispersal through whole plants3,20–22. The type of diaspore appendages will affect the way of seed dispersal23,24, and the diaspores with hairy or wing will be subjected to greater updraft9, which is more conducive to wind dispersal. Winged diaspores often fall in a rotating manner through the air, and the speed at which the size of the wing decreases is significantly correlated25. Diaspore traits, such as the maximum speed when air resistance exerted on the seed is equal to its pull of gravity during free fall in motionless air (terminal velocity) and the ratio of mass to projected area (wing loading), are important indicators to evaluate seed wind dispersal14.
The assumption in most theoretical studies, especially models on diaspore dispersal distance, is that diaspores are released through open air, and these studies have considered the movement of diaspores from the release point20,26,27. Although Cousens and Rawlinson (2001) considered the shape of the canopy into the seed dispersal model, they did not explore the influence of the morphological properties of the diaspore28. Previous field studies indicated that shrub canopy can intercept diaspores with appendages (hair, samara, wing, balloon) during low wind speeds29, however, the effect of seeds passing through the canopy on their dispersal distance is still unclear30–34. Pounden et al.35 found that winged diaspores had significantly reduced dispersal following boles collision, the hair diaspores did not, but how the plant canopy (i.e. aboveground portion of a plant consisting of branches and leaves) affects other types of diaspores is still unknown36,37. So, we hypothesized that the plant canopy can reduce diaspore dispersal distance, which is controlled by diaspore traits and wind speed. To test our hypothesis, controlled experiments were conducted in a wind tunnel, using 29 species with different diaspore traits.
Materials and methods
Diaspore selection and trait measurements
To determine the relationship between diaspore traits and dispersal distance of diaspores passing through plant canopy versus through open air at different wind speeds, we selected diaspores of 29 plant species that differ in appendage type, mass, projected area, shape index, wing loading, and terminal velocity (Table 1), differences in traits of the selected species were not restricted by phylogeny, and each diaspore was only considered as a representation of its own morphological attributes. Firstly, diaspores with different types of appendages (samara, wing, thorn, hair and balloon) were selected, and then the gradient of the same appendage diaspores was set according to the quality.
Table 1.
Explanation of related terms.
| Terms | Explanation |
|---|---|
| Hair Diaspore | Diaspores partially or fully covered with hairs (trichomed) |
| Wing Diaspore | Flat diaspores with a common device for becoming airborne |
| Samara Diaspore | Spherical diaspores with a common device for becoming airborne |
| Balloon Diaspore | A layer of the seed coat is loose or seed may be surrounded by inflated parts |
| Thorn Diaspore | Diaspores with hard spines, which often attached to livestock and human bodies to dispersal |
| No Appendage Diaspores | Diaspores without appendage |
| Mass | The weight of the diaspore |
| Projected area | Horizontal projection area of the diaspore when it is placed naturally |
| Shape index | An index indicating the shape of the diaspore, The smaller the index, the closer to the spherical shape, the larger the closer to the flat |
| Wing loading | The ratio of mass to projected area |
| Terminal velocity | The constant falling velocity of a seed in still air |
To facilitate seed dispersal investigation, diaspores with samaras, wings, thorns, and without appendage were lightly sprayed with red aerosol paint, while diaspores with hairs were colored using red water-based markers. The dyed diaspores were naturally air-dried and placed in plastic boxes to ensure the integrity of morphological structure. Diaspore traits are measured after dyed and dried.
Twenty intact diaspores of each type were selected the same species for measurements of length, width, and thickness of each diaspore were measured with Vernier caliper (0.01 mm accuracy). Diaspores shape index (Vs) was calculated using an equation we developed from ideas in Thompson et al. (1993).
where N = 3, , , and .
Diaspore mass was determined using an electronic balance (0.0001 g), mass range of 29 diaspores were 1.12–316 mg. The projected area of diaspore was scanned with a digital scanner, and measured with analysis software (Motic Image Plus 2.0, Motic China Group Co., Xiamen, China), and project area range of 29 diaspores were 5–604 mm2. The wing loading was calculated as seed mass divided by projected area38,39, and wing loading range of 29 diaspores were 0.04–2.1 mg mm−2. Terminal velocity was measured with a camera described by Zhou et al. (2020), and terminal velocity range of 29 diaspores were 0.7–40 m s−1(Table 2).
Table 2.
Diaspore traits of the 29 study species (mean ± SE).
| Species | Abbreviation | Appendage type | Mass (mg) | Projected area (mm2) | Shape index | Wing loading (mg mm−2) | Terminal velocity (m s−1) |
|---|---|---|---|---|---|---|---|
| Atriplex canescens | Ac | Samara | 31.35 ± 8.569 | 77.276 ± 13.293 | 0.007 ± 0.005 | 0.409 ± 0.093 | 2.485 ± 0.228 |
| Caligonum leucocladum | Cl | Samara | 149.975 ± 25.142 | 218.669 ± 26.005 | 0.003 ± 0.003 | 0.686 ± 0.08 | 3.098 ± 0.138 |
| Caligonum rubicundum | Cr | Samara | 52.095 ± 4.929 | 166.598 ± 16.548 | 0.002 ± 0.003 | 0.314 ± 0.028 | 2.255 ± 0.164 |
| Haloxylon ammodendron | Ha | Samara | 6.776 ± 1.383 | 36.839 ± 4.368 | 0.063 ± 0.022 | 0.186 ± 0.04 | 1.685 ± 0.353 |
| Sympema regelii | Sr | Samara | 7.78 ± 1.891 | 54.844 ± 12.48 | 0.082 ± 0.021 | 0.145 ± 0.031 | 1.383 ± 0.225 |
|
Zygophyllum xanthoxylon (three-winged) |
Zx | Samara | 163.47 ± 43.58 | 558.871 ± 102.138 | 0.013 ± 0.007 | 0.291 ± 0.045 | 1.959 ± 0.244 |
| Acer saccharum | As | Wing | 35.799 ± 5.255 | 197.827 ± 20.144 | 0.16 ± 0.002 | 0.181 ± 0.015 | 0.78 ± 0.066 |
| Althaea rosea | Ar | Wing | 16.935 ± 0.613 | 44.744 ± 1.626 | 0.119 ± 0.013 | 0.379 ± 0.02 | 2.728 ± 0.473 |
| Ferula bungeana | Fb | Wing | 21.225 ± 2.766 | 50.52 ± 8.331 | 0.129 ± 0.008 | 0.428 ± 0.075 | 2.74 ± 0.223 |
| Syzygium aromaticum | Sa | Wing | 9.905 ± 1.807 | 25.481 ± 2.892 | 0.152 ± 0.011 | 0.394 ± 0.086 | 2.428 ± 0.27 |
| Ulmus pumila | Up | Wing | 10.22 ± 2.421 | 242.738 ± 34.34 | 0.17 ± 0.011 | 0.043 ± 0.012 | 0.908 ± 0.106 |
|
Zygophyllum xanthoxylon (disc) |
Zxd | Wing | 90.685 ± 18.334 | 603.932 ± 83.549 | 0.142 ± 0.016 | 0.152 ± 0.031 | 1.646 ± 0.213 |
| Calligonum alaschanicum | Ca | Thorn | 44.42 ± 10.625 | 73.215 ± 16.064 | 0.007 ± 0.004 | 0.615 ± 0.122 | 3.285 ± 0.116 |
| Lappula intermedia | Li | Thorn | 6.775 ± 1.664 | 11.156 ± 1.702 | 0.004 ± 0.003 | 0.607 ± 0.12 | 2.225 ± 0.441 |
| Psilopeganum sinense | Ps | Thorn | 1.065 ± 0.496 | 5.125 ± 0.446 | 0.067 ± 0.023 | 0.211 ± 0.105 | 1.844 ± 0.416 |
| Tribulus terrestris | Tt | Thorn | 26.345 ± 13.79 | 24.244 ± 5.742 | 0.016 ± 0.008 | 1.167 ± 0.74 | 2.714 ± 0.239 |
| Xanthium sibiricum | Xs | Thorn | 74.66 ± 21.572 | 45.319 ± 3.998 | 0.027 ± 0.004 | 1.677 ± 0.576 | 3.729 ± 0.213 |
| Catalpa ovata | Co | Hair | 5.005 ± 1.227 | 73.216 ± 16.911 | 0.194 ± 0.004 | 0.069 ± 0.015 | 1.078 ± 0.173 |
| Clematis hexapetala | Ch | Hair | 4.095 ± 1.023 | 79.993 ± 21.214 | 0.083 ± 0.018 | 0.056 ± 0.023 | 1.008 ± 0.242 |
| Echinops gmelinii | Eg | Hair | 9.115 ± 1.86 | 40.598 ± 4.212 | 0.006 ± 0.004 | 0.227 ± 0.055 | 2.195 ± 0.225 |
| Reaumuria trigyna | Rt | Hair | 38.975 ± 9.163 | 111.218 ± 27.21 | 0.012 ± 0.008 | 0.482 ± 0.65 | 2.024 ± 0.257 |
| Carex lehmanii | Cle | None | 5.165 ± 0.403 | 8.218 ± 0.635 | 0.101 ± 0.005 | 0.631 ± 0.057 | 1.258 ± 0.461 |
| Euonymus maackii | Em | None | 40.865 ± 10.544 | 20.209 ± 2.554 | 0.041 ± 0.009 | 2.037 ± 0.526 | 2.47 ± 0.252 |
| Messerschmidia sibirica | Ms | None | 66.6 ± 7.452 | 34.095 ± 3.187 | 0.01 ± 0.006 | 1.961 ± 0.234 | 3.457 ± 0.262 |
| Nitraria tangutorum | Nt | None | 29.935 ± 7.056 | 20.489 ± 3.345 | 0.071 ± 0.015 | 1.455 ± 0.197 | 2.825 ± 0 |
| Platycladus orientalis | Po | None | 22.405 ± 10.257 | 14.758 ± 1.586 | 0.062 ± 0.011 | 1.537 ± 0.726 | 3.01 ± 0.39 |
| Thermopsis lanceolata | Tl | None | 18.185 ± 3.573 | 9.322 ± 0.781 | 0.019 ± 0.003 | 1.956 ± 0.378 | 2.858 ± 0.371 |
| Sect.arenicola | Sa | Balloon | 18.695 ± 6.681 | 69.533 ± 12.684 | 0.03 ± 0.012 | 0.269 ± 0.078 | 1.66 ± 0.165 |
| Sphaerophysa salsula | Ss | Balloon | 315.95 ± 56.919 | 406.716 ± 61.464 | 0.061 ± 0.013 | 0.779 ± 0.104 | 3.109 ± 0.499 |
Wind speed control
A wind tunnel (with a test section 2 m high × 2 m wide × 20 m long) was used to control wind speed. Wind speed was monitored inside the tunnel with a Pitot tube (160-96, Dwyer Instruments, Inc., Indiana, USA) connected with a Magnesense II Differential Pressure Transmitter (MS2-W102-LCD, Dwyer Instruments Inc., Indiana, USA) (Liang et al. 2020). In this study, wind speeds (measured 1 m above the flat sand surface) were set at 2, 4 and 6 m s−1, and they correspond to categories 3–5 of meteorological wind measurements, which is basically close to the common range of wind speeds under natural conditions (Mather, 1987). The underlying surface is set with a flat fixed sand surface in this experiment.
Model plant canopy setting
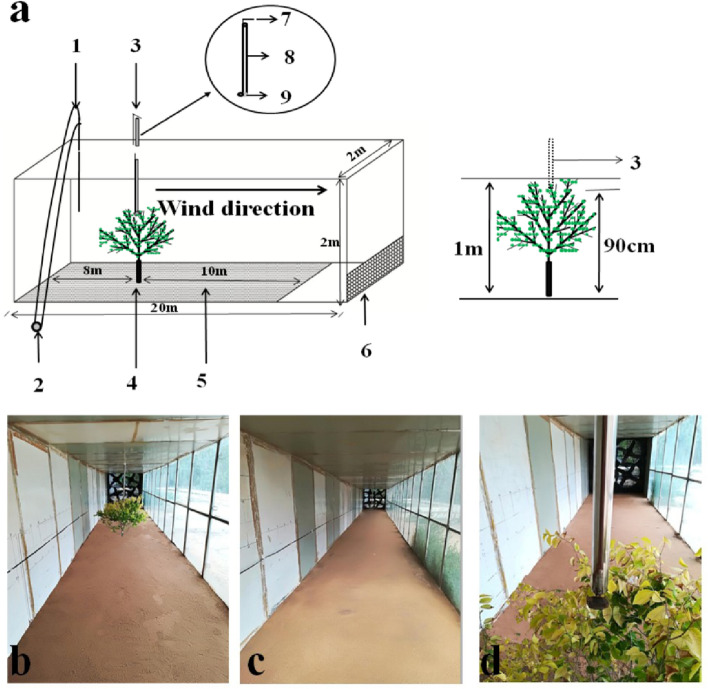
A young tree (sapling) of Ulmus parvifolia was selected for use as the model canopy because it has a high density of branches and many leaves. Further, this species is relatively easy to transplant and tolerant of high wind speed. Plant height was 1 m (Fig. 1), all leaves were present, and size of canopy was 1.0 m high × 1.2 m wide. The model plant selected was only used to explore the effect of canopy on seed dispersal distance and was not related to the maternal plants of the experimental diaspores. The model sapling was transplanted (transplanted with roots to ensure plant survival, and the root part is fixed with sand to keep the underlying surface flat) from the field to the front end of the wind tunnel test section,8 m away from the power section and in the middle of the two walls of the wind tunnel. The model plant grew well throughout the experiment.
Figure 1.
Diagram of wind tunnel used in the study. (a) 1, Pitot tube; 2, differential pressure transmitter; 3, diaspore release device (seed release point was 90 cm above the ground, and placed 10 cm in the center of the canopy); 4, leafy plant (plant height was 1 m); 5, experiment section; 6, diaspore-blocking net; 7, switch; 8, steel tube; 9, bottom flap. (b) Inside view of the wind tunnel showing position of the plant. (c) Inside view of the wind tunnel without the plant. (d) Close-up view of plant inside the wind tunnel showing position of diaspore release device. The photograph in this figure was taken by Xuanping Qin.
Diaspore release
To standardize our studies of the dispersal of diaspores through the canopy, we set the diaspore release point as the center of the canopy, 10 cm below the top of the canopy and 90 cm from the soil surface. A 4 cm diameter stainless steel tube was used as the release device. The release device was inserted into the canopy from the top of the wind tunnel. Diaspores were released from the upper part of the steel tube (which was controlled by a bottom flap to ensure that initial release rate was zero) when the target wind speed was reached. Five replicates of 20 diaspores of each type were used for each wind speed.
Effect of wind speed on diaspore dispersal through plant canopy
Five replicates of 20 diaspores of each type were used for each wind speed . The 20 diaspores were released simultaneously from the release device through the plant canopy, when the target wind speed was reached (2, 4 or 6 m s−1). We turned off the wind tunnel after 2 s of diaspore dispersal. The horizontal (dispersal) distance that each of the 20 diaspores was moved from the release point was measured. Mean dispersal distance was used to represent dispersal ability.
The control was the release of diaspores at a height of 90 cm from the ground through air at the three wind speeds, i.e. after the plant was removed from the wind tunnel. Diaspore dispersal distance was measured after the wind speed was maintained for 2 s.
Statistical analysis
The difference in the dispersal distance of each type of diaspore passing through the canopy versus through open air at different wind speeds was compared. Redundancy analyses (RDA) based on correlation matrices of diaspore dispersal distance and explanatory factors were conducted using Canoco 5.0 (version 5.0, Microcomputer Power, Ithaca, NY, USA; Tackenberg, 2003). We analyzed the contribution of diaspore mass, projected area, wing loading, terminal velocity, and shape index to dispersal distance and the relationship between the plant's effect on dispersal distance and diaspore traits. Statistical analyses were conducted using IBM SPSS Statistics 22.0 (IBM Corporation 1989, 2013, USA), and plots were drawn by Origin Pro 8.5 (Origin Lab Corporation 1991–2010, USA).
Results
Influence of plant canopy on diaspore dispersal distance at different wind speeds
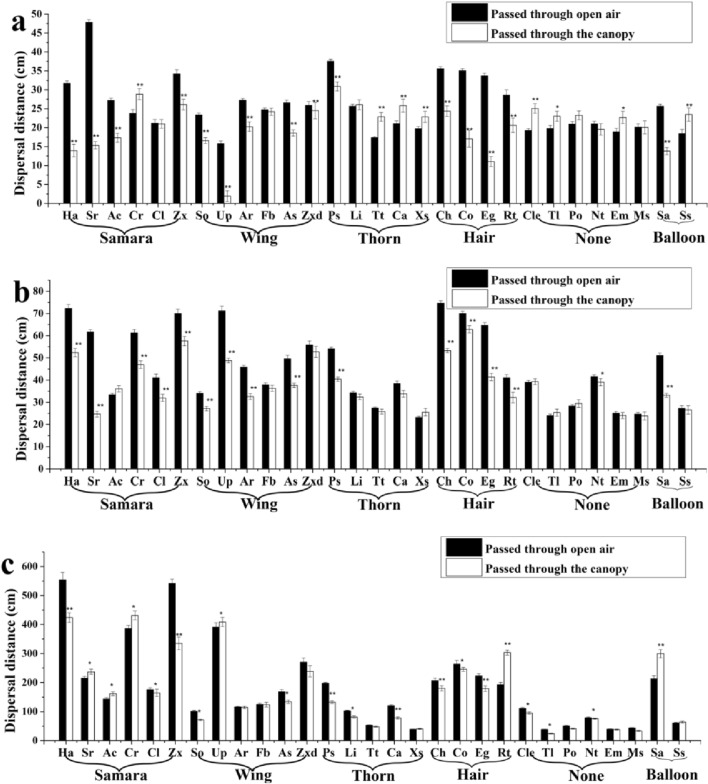
Plant canopy either increased or decreased diaspore dispersal distance, depending on diaspore traits and wind speed. Compared with dispersal through open air, dispersal distance of nine diaspore types (1 samara (of 7 tested), 3 thorn (of 5 tested), 4 without an appendage (of 7 tested), and 1 balloon (of 2 tested)) was significantly promoted by the plant canopy at a wind speed of 2 m s−1. Dispersal distance of diaspores with a thorn or those with no appendage was more likely to be promoted by the plant canopy than that of the other kinds of diaspores at a wind speed of 2 m s−1. However, dispersal distance of diaspores with a wing and those with hair was decreased by the plant canopy. The dispersal distance of all types of diaspore passed through the canopy were not significantly increased compared with that of diaspore passed through the open air when the wind speed was 4 m s−1. Dispersal distance of six diaspore types (3 samara (of 7 tested), 1 wing (of 7 tested), 1 hair (of 4 tested), and 1 balloon (of 2 tested)) was significantly increased by the plant canopy at a wind speed of 6 m s−1, and dispersal distance of diaspores with a thorn or those with no appendage was decreased by the plant canopy. At a wind speed of 6 m s−1, dispersal of diaspores with a wing was increased more by the canopy than that of the other kind of diaspores, and dispersal distance for diaspores without an appendage was significantly decreased (Fig. 2).
Figure 2.
Dispersal distance of diaspores of the 29 species that passed through plant canopy or through open air at three wind speeds. (a–c) wind speed was 2, 4, and 6 m s−1, respectively. * (P < 0.05) and ** (P < 0.01) indicate significant differences between dispersal distance after passing through plant versus open air.
Influence of diaspore traits on dispersal distance by wind
The contribution (percentage) of diaspore traits to dispersal distance increased with wind speed (Table 3). Wing loading and terminal velocity had negatively correlations with diaspore dispersal distance for diaspores released through open air and through the plant canopy at wind speeds of 4 and 6 m s−1, and wing loading was more important than terminal velocity for dispersal distance. However, wing loading and terminal velocity did not have a significant effect on diaspore dispersal distance through the plant canopy at a wind speed of 2 m s−1. Neither shape index, projected area nor mass had a significant effect on diaspore dispersal distance either through the plant canopy or through open air at any of the three wind speeds. At a wind speed of 6 m s−1, the plant canopy increased dispersal-distance of diaspores with a small wing loading (≤ 0.5 mg mm−2) and terminal velocity (≤ 2.5 m s−1), and samaras had the greatest dispersal distance followed by diaspores with wing, hair, and balloon. The plant canopy decreased dispersal distance of diaspores with high wing loading and high terminal velocity (Fig. 3).
Table 3.
The relationship between diaspore traits and dispersal through open air and through plant canopy.
| Dispersal treatment | Wind speeds(m s−1) | E | WL | TV | SI | PA | MS |
|---|---|---|---|---|---|---|---|
| Release through open air | 2 | 43.70 | 36.0**0 | 23.50** | 0.90 | < 0.01 | 6.00 |
| 4 | 73.20 | 69.20** | 51.80** | 12.10 | 0.09 | 0.04 | |
| 6 | 83.50 | 73.90** | 46.30** | 4.90 | 17.20* | 0.80 | |
| Release through canopy | 2 | 17.40 | 7.20 | 12.60 | 12.20 | < 0.01 | 4.40 |
| 4 | 57.90 | 45.00** | 34.00** | 12.80* | 15.80* | 1.40 | |
| 6 | 70.60 | 53.90** | 48.50** | 1.50 | 11.40 | 0.00 |
E, percentage explained by diaspore traits; PA, projected area (mm2); WL, wing loading (mg mm−2); TV, terminal velocity (m s−1); SI, shape index; MS, diaspore mass (mg); *0.01 < p < 0.05; ** p < 0.01.
Figure 3.
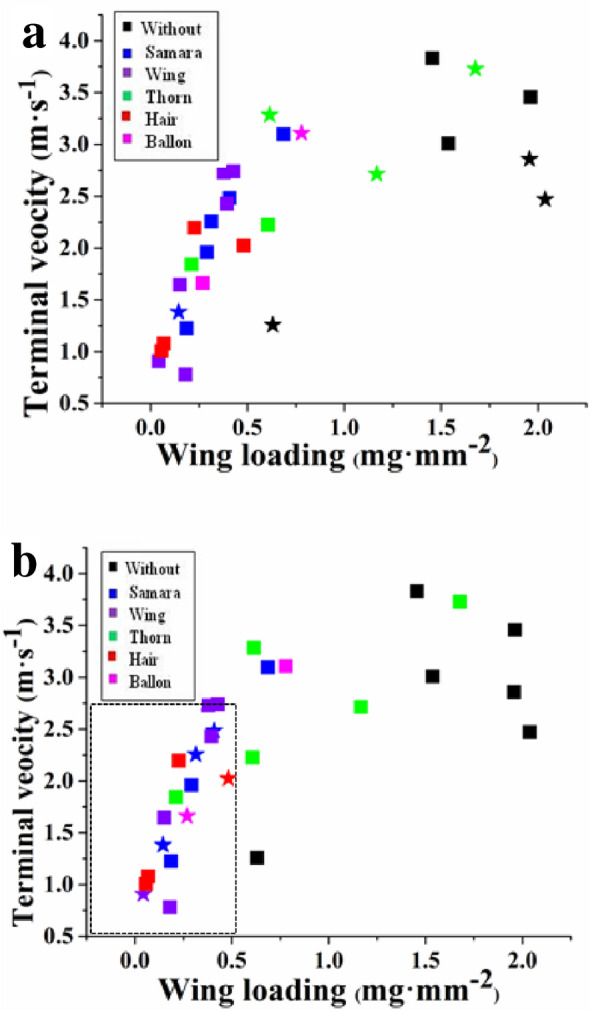
The effect of plant canopy on the dispersal distance of different diaspores. (a, b) Wind speed 2 and 6 m s−1. Squares represent diaspores for which plant canopy decreased dispersal distance, and star represent diaspores for which plant canopy increased dispersal distance. Colors represent diaspore with different appendage: black without appendage; blue, a samara; violet, with wing; green, with thorn; red, with hair; pink, with balloon.
Discussion
Our study indicated that compared to dispersal through open air, diaspores with thorns or without an appendage were more strongly deflected by the canopy at low wind speed, thus their dispersal distance was also more likely to increase. Previous study found that the plant canopy had little effect on interception of diaspores with thorns and without appendages29, but it will disperse farther after passing through the canopy at low wind speed. Other diaspores (samara, hair, wing, and balloon) will be more easily intercepted by plant canopy at low wind speed29, and thus their dispersal distance was more likely to be decreased. Our study showed that the dispersal distance of samara diaspores were more easily increased by plant canopy at a higher wind speed than dispersal through air (Fig. 2), this may because samara diaspores were subjected to a strong updraft, The effect of wind on seed dispersal distance is complex in nature, resulting in different vertical and horizontal components of air flow including turbulence10,40,41, and complex updrafts potentially increasing wind dispersal within a canopy42.
The promotion of dispersal distance of some diaspores falling through the plant canopy (Fig. 3) is contrary to the branch interception of diaspores43,44. Increased dispersal distance may due to induction of an updraft by wind hitting the plant canopy or by change in direction of diaspores that collide with a plant part can change the original dispersal trajectory and increase dispersal distance. Wind conditions can be altered when wind passes through a plant canopy, such as by the generation of an updraft, increasing of vertical wind speed, and reducing of horizontal speed39,45,46. Low horizontal wind speed will decrease diaspore dispersal distance, but an updraft or vertical wind speed can increase dispersal time, thereby increasing diaspore dispersal distance. Thus, the plant canopy can increase or decrease diaspore dispersal distance compared to diaspores passing through open air at the same wind speed.
Tackenberg et al.47 found that updrafts are important for long-dispersal of dandelion in an open meadow environment. Our results showed that plant canopy not only promoted the dispersal of hairy diaspores, but also promoted the dispersal of diaspores with samara, wing, and balloon, which might be because of localised updrafts and eddies caused by plant canopy. Variety of canopy types will cause differences in turbulence, which may further affect the distance of seed wind dispersal. The canopy of branch and leaf density may produce complex updrafts, therefore, we speculate that a dense canopy of branches and leaves is more likely to promote seed wind dispersal, and wind-borne seeds are easier to dispersal over long distances in forests than in deserts, of course, this needs further research to confirm.
Diaspore dispersal distance is strongly correlated with wing loading and terminal velocity3,48,49. Our study indicated that the dispersal distance of diaspores with small wing loading and terminal velocity can be promoted by the plant canopy at high wind speed but not at a low wind speed (Fig. 3). This is because diaspores with low wing loading and terminal velocity are easily accelerated laterally by the drag of the wind following a collision with a solid object during high wind speed. Diaspores that are dispersed by wind generally have a smaller terminal velocity and wing loading, this means that wind-borne species are distributed more widely in vegetation-covered areas than in open landscapes.
In conclusion, a plant canopy may increase dispersal of diaspores with thorns or those without appendages during low wind speed, and dispersal of diaspores with small wing loading and terminal velocity at high wind speeds. An estimation of the plant canopy effect on diaspore dispersal distance can improve the accuracy of the assessment of dispersal distance of a cohort of diaspores in the real world. Accordingly to our results, we can predict that diaspores with small terminal velocity and wing loading are easier to dispersal long distances under hurricane or storm conditions.
Acknowledgements
The authors thank Ruibing Duan, Liang Tian, and Xinle Li for assistance with seed collecting and for help with the field experiment. We are especially grateful to all members of the Experimental Center of Desert Forestry for their full cooperation.
Author contributions
Z.L., M.L., Z.W., and X.Q. conceived the idea and designed the methodology; X.Q., Z.X., and Q.Z. collected the data. X.Q. analyzed the data and wrote the first draft of the manuscript. Z.L., W.L., C.B., and J.B. revised several drafts of the manuscript. All authors approved the manuscript for publication.
Funding
This study was financially supported by the National Natural Science Foundation of China (31770504, 41571270, 42007427 and 31971732).
Data availability
The data presented in this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Askew AP, Corker D, Hodkinson J, Thompson K. A new apparatus to measure the rate of fall of seeds. Funct. Ecol. 1997;11:121–125. doi: 10.1046/j.1365-2435.1997.00049.x. [DOI] [Google Scholar]
- 2.Bohrer G, Katul GG, Nathan R, Walko RL, Avissar R. Effects of canopy heterogeneity, seed abscission and inertia on wind-driven dispersal kernels of tree seeds. J. Ecol. 2008;96:569–580. doi: 10.1111/j.1365-2745.2008.01368.x:10.1111/j.1365-2745.2008.01368.x. [DOI] [Google Scholar]
- 3.Nathan R, et al. Mechanistic models of seed dispersal by wind. Thyroid Res. 2011;4:113–132. doi: 10.1007/s12080-011-0115-3. [DOI] [Google Scholar]
- 4.Abedi-Lartey M, Dechmann DKN, Wikelski M, Scharf AK, Fahr J. Long-distance seed dispersal by straw-coloured fruit bats varies by season and landscape. Global Ecol. Conserv. 2016;7:12–24. doi: 10.1016/j.gecco.2016.03.005. [DOI] [Google Scholar]
- 5.Garcıa C, Klein EK, Jordano P. Dispersal processes driving plant movement: Challenges for understanding and predicting range shifts in a changing world. J. Ecol. 2017;105:1–5. doi: 10.1111/1365-2745.12705. [DOI] [Google Scholar]
- 6.Sutherland WJ, et al. Identification of 100 fundamental ecological questions. J. Ecol. 2013;101:58–67. doi: 10.1111/1365-2745.12025. [DOI] [Google Scholar]
- 7.Beckman NG, Aslan CE, Rogers HS. The role of seed dispersal in plant populations: Perspectives and advances in a changing world. AoB Plants. 2020;12:plaa010. doi: 10.1093/aobpla/plaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willson MF, Rice BL, Westoby M. Seed dispersal spectra: a comparison of temperate plant-communities. J. Veg. Sci. 1990;1:547–562. doi: 10.2307/3235789. [DOI] [Google Scholar]
- 9.van Rheede K, Oudtshoorn V, van Rooyen MW. Dispersal Biology of Desert Plants. Springer; 1999. [Google Scholar]
- 10.Clark JS, Silman M, Kern R, Hillerislambers MJ. Seed dispersal near and far: patterns across temperate and tropical forests. Ecology. 1999;80:1475–1494. doi: 10.2307/176541:10.2307/176541. [DOI] [Google Scholar]
- 11.Maler A, Emig W, Leins P. Dispersal patterns of some Phyteuma species (Campanulaceae) Plant Biol. 1999;1:408–417. doi: 10.1111/j.1438-8677.1999.tb00723.x. [DOI] [Google Scholar]
- 12.Baker DV, Beck G. The weed tunnel: building an experimental wind tunnel. Weed Technol. 2008;22:549–552. doi: 10.1614/wt-07-162.1. [DOI] [Google Scholar]
- 13.Planchuelo G, Catalán P, Delgado JA. Gone with the wind and the stream: Dispersal in the invasive species Ailanthus altissima. Acta Oecol. 2016;73:31–37. doi: 10.1016/j.actao.2016.02.006. [DOI] [Google Scholar]
- 14.Liang W, et al. How do diaspore traits, wind speed and sand surface configuration interact to determine seed burial during wind dispersal? Plant Soil. 2019;440:357–368. doi: 10.1007/s11104-019-04071-4. [DOI] [Google Scholar]
- 15.Zhou Q, et al. Responses of secondary wind dispersal to environmental characteristics and diaspore morphology of seven Calligonum species. Land Degrad. Dev. 2020;31:842–850. doi: 10.1002/ldr.3489. [DOI] [Google Scholar]
- 16.Andersen MC. An analysis of variability in seed settling velocities of several wind-dispersed Asteraceae. Am. J. Bot. 1992 doi: 10.1002/j.1537-2197.1992.tb13702.x. [DOI] [PubMed] [Google Scholar]
- 17.Augspurger CK, Franson SE, Cushman KC, Muller-Landau HC. Intraspecific variation in seed dispersal of a Neotropical tree and its relationship to fruit and tree traits. Ecol. Evol. 2016;6:1128–1142. doi: 10.1002/ece3.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene DF, Quesada M. The differential effect of updrafts, downdrafts and horizontal winds on the seed abscission of Tragopogon dubius. Funct. Ecol. 2011;25:468–472. doi: 10.1111/j.1365-2435.2010.01788.x. [DOI] [Google Scholar]
- 19.Paice M, Day W, Rew J, Howard A. A simulation model for evaluating the concept of patch spraying. Weed Res. 1998;38:373–388. doi: 10.1046/j.1365-3180.1998.00108.x. [DOI] [Google Scholar]
- 20.Jongejans E, Telenius A. Field experiments on seed dispersal by wind in ten umbelliferous species (Apiaceae) Plant Ecol. 2001;152:67–78. doi: 10.1023/a:1011467604469. [DOI] [Google Scholar]
- 21.Augspurger CK. Morphology and dispersal potential of wind-dispersed diaspore of neotropical trees. Am. J. Bot. 1986;73:353–363. doi: 10.1002/j.1537-2197.1986.tb12048.x. [DOI] [Google Scholar]
- 22.Zhu J, Liu M, Xin Z, Liu Z, Schurr FM. A trade-off between primary and secondary seed dispersal by wind. Plant Ecol. 2019;220:541–552. doi: 10.1007/s11258-019-00934-z. [DOI] [Google Scholar]
- 23.Phartyal SS, Rosbakh S, Ritz C, Poschlod P, Bruun HH. Ready for change: Seed traits contribute to the high adaptability of mudflat species to their unpredictable habitat. J. Veg. Sci. 2020;31:331–342. doi: 10.1111/jvs.12841. [DOI] [Google Scholar]
- 24.Saatkamp A, et al. A research agenda for seed-trait functional ecology. New Phytol. 2019;221:1764–1775. doi: 10.1111/nph.15502. [DOI] [PubMed] [Google Scholar]
- 25.Green DS. The terminal velocity and dispersal of spinning samaras. Am. J. Bot. 1980;67:1218–1224. doi: 10.1002/j.1537-2197.1980.tb07754.x. [DOI] [Google Scholar]
- 26.Jongejans E, Pedatella NM, Shea K, Skarpaas O, Auhl R. Seed release by invasive thistles: the impact of plant and environmental factors. Proc R Soc B Biol Sci. 2007;274:2457–2464. doi: 10.1098/rspb.2007.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen M. Mechanistic models for the seed shadows of wind-dispersed plants. Am. Nat. 1991;137:476–497. doi: 10.1086/285178. [DOI] [Google Scholar]
- 28.Cousens RD, Rawlinson AA. When will plant morphology affect the shape of a seed dispersal "kernel”? J Thenr Biol. 2001;211:229–238. doi: 10.1006/jtbi.2001.2341. [DOI] [PubMed] [Google Scholar]
- 29.Qin X, et al. Shrub canopy interception of diaspores dispersed by wind. Seed Sci. Res. 2020;30:310–318. doi: 10.1017/S0960258520000410. [DOI] [Google Scholar]
- 30.Kelly N, Cousens RD, Taghizadeh MS, Hanan JS. Plants as populations of release sites for seed dispersal: A structural-statistical analysis of the effects of competition on Raphanus raphanistrum. J. Ecol. 2013;101:878–888. doi: 10.1111/1365-2745.12097. [DOI] [Google Scholar]
- 31.Donohue K. Maternal determinants of seed dispersal in cakile edentula: Fruit, plant, and site traits. Ecology. 1998;79:2771–2788. doi: 10.1890/0012-9658(1998)079[2771:MDOSDI]2.0.CO;2. [DOI] [Google Scholar]
- 32.Bullock JM, Moy IL. Plants as seed traps: inter-specific interference with dispersal. Acta Oecol. 2004;25:35–41. doi: 10.1016/j.actao.2003.10.005. [DOI] [Google Scholar]
- 33.Cousens R, Dytham C, Law R. Dispersal in plants. A population perspective. Ann. Bot. 2008 doi: 10.1111/j.1442-9993.2010.02216.x. [DOI] [Google Scholar]
- 34.Phillips ML. Dispersal in plants: A population perspective. Austral Ecol. 2011;36:e27. [Google Scholar]
- 35.Pounden E, Greene DF, Quesada M, Contreras Sánchez JM. The effect of collisions with vegetation elements on the dispersal of winged and plumed seed. J. Ecol. 2008;96:591–598. doi: 10.1111/j.1365-2745.2008.01380.x. [DOI] [Google Scholar]
- 36.Poggi D, Porporato A, Ridolfi L, Albertson JD, Katul GG. The effect of vegetation density on canopy sub-layer turbulence. BoLMe. 2004;111:565–587. [Google Scholar]
- 37.Greene DF. The role of abscission in long-distance seed dispersal by the wind. Ecology. 2005;86:3105–3110. doi: 10.1890/04-1430. [DOI] [Google Scholar]
- 38.Matlack GR. Diaspore size, shape, and fall behavior in wind-dispersed plant species. Am. J. Bot. 1987 doi: 10.1002/j.1537-2197.1987.tb08729.x. [DOI] [Google Scholar]
- 39.Zhou Q, et al. Relationship between seed morphological traits and wind dispersal trajectory. Funct. Plant Biol. 2019;46:1063–1071. doi: 10.1071/FP19087. [DOI] [PubMed] [Google Scholar]
- 40.Heydel F, Cunze S, Bernhardt-Römermann M, Tackenberg O. Long-distance seed dispersal by wind: Disentangling the effects of species traits, vegetation types, vertical turbulence and wind speed. Ecol. Res. 2014;29:641–651. doi: 10.1007/s11284-014-1142-5. [DOI] [Google Scholar]
- 41.Greene DF, Johnson EA. Long-distance wind dispersal of tree seeds. Can J Bot. 1995;73:1036–1045. doi: 10.1139/b95-113. [DOI] [Google Scholar]
- 42.Skarpaas O, Auhl R, Shea K. Environmental variability and the initiation of dispersal: turbulence strongly increases seed release. Proc. R. Soc. B Biol. Sci. 2006;273:751–756. doi: 10.1098/rspb.2005.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipoma ML, Cuchietti A, Gorne LD, Díaz SM, Zobel M. Not gone with the wind: Vegetation complexity increases seed retention during windy periods in the Argentine Semiarid Chaco. J. Veg. Sci. 2019;30:542–552. doi: 10.1111/jvs.12747. [DOI] [Google Scholar]
- 44.Soons MB, Heil GW, Ran N, Katul GG. Determinants of long-distance seed dispersal by wind in grasslands. Ecology. 2004;85:3056–3068. doi: 10.1890/03-0522. [DOI] [Google Scholar]
- 45.Soons MB, Bullock JM. Non-random seed abscission, long-distance wind dispersal and plant migration rates. J. Ecol. 2008;96:581–590. doi: 10.1111/j.1365-2745.2008.01370.x. [DOI] [Google Scholar]
- 46.Rotundo JL, Aguiar MR. Litter effects on plant regeneration in arid lands: A complex balance between seed retention, seed longevity and soil–seed contact. J. Ecol. 2005;93:829–838. doi: 10.1111/j.1365-2745.2005.01022.x. [DOI] [Google Scholar]
- 47.Tackenberg O, Poschlod P, Bonn S. Assessment of wind dispersal potential in plant species. Ecol. Monogr. 2003;73:191–205. doi: 10.1890/0012-9615(2003)073[0191:AOWDPI]2.0.CO;2. [DOI] [Google Scholar]
- 48.Schurr FM, Bond WJ, Midgley GF, Higgins SI. A mechanistic model for secondary seed dispersal by wind and its experimental validation. J. Ecol. 2005;93:1017–1028. doi: 10.1111/j.1365-2745.2005.01018.x. [DOI] [Google Scholar]
- 49.McGinley M, Brigham E. Fruit morphology and terminal velocity in Tragopogon dubious (L) Funct. Ecol. 1989;4:489–496. doi: 10.2307/2389623. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.