ABSTRACT
Novel β-lactam–β-lactamase inhibitor combinations currently approved for clinical use are poorly active against metallo-β-lactamase (MBL)-producing strains. We evaluated the in vitro activity of cefepime-taniborbactam (FTB [formerly cefepime–VNRX-5133]) and comparator agents against carbapenemase-producing Enterobacterales (n = 247) and carbapenem-resistant Pseudomonas species (n = 170) clinical isolates prospectively collected from different clinical origins in patients admitted to 8 Spanish hospitals. FTB was the most active agent in both Enterobacterales (97.6% MICFTB, ≤8/4 mg/L) and Pseudomonas (67.1% MICFTB, ≤8/4 mg/L) populations. The MICFTB was >8 mg/L in 6/247 (2.4%) Enterobacterales isolates (3 KPC-producing Klebsiella pneumoniae isolates, 1 VIM-producing Enterobacter cloacae isolate, 1 IMP-producing E. cloacae isolate, and 1 NDM-producing Escherichia coli isolate) and in 56/170 (32.9%) Pseudomonas isolates, 19 of them carbapenemase producers (15 producers of VIM, 2 of GES, 1 of GES+VIM, and 1 of GES+KPC). Against the Enterobacterales isolates with meropenem MICs of >2 mg/L (138/247), FTB was the most active agent against both serine-β-lactamases (107/138) and MBL producers (31/138) (97.2 and 93.5% MICFTB, ≤8/4 mg/L, respectively), whereas the activity of comparators was reduced, particularly against the MBL producers (ceftazidime-avibactam, 94.4 and 12.9%, meropenem-vaborbactam, 85.0 and 64.5%, imipenem-relebactam, 76.6 and 9.7%, ceftolozane-tazobactam, 1.9 and 0%, and piperacillin-tazobactam, 0 and 0%, respectively). Among the meropenem-resistant Pseudomonas isolates (163/170; MIC, >2 mg/L), the activities of FTB against serine-β-lactamase (35/163) and MBL (43/163) producers were 88.6 and 65.1%, respectively, whereas the susceptibilities of comparators were as follows: ceftazidime-avibactam, 88.5 and 16.0%, meropenem-vaborbactam, 8.5 and 7.0%, imipenem-relebactam, 2.9 and 2.3%, ceftolozane-tazobactam, 0 and 2.3%, and piperacillin-tazobactam, 0 and 0%, respectively. Microbiological results suggest FTB as a potential therapeutic option in patients infected with carbapenemase-producing Enterobacterales and carbapenem-resistant Pseudomonas isolates, including MBL producers.
KEYWORDS: cefepime-taniborbactam susceptibility, carbapenemase-producing Enterobacterales, carbapenemase-producing Pseudomonas aeruginosa
INTRODUCTION
β-Lactams (penicillins, cephalosporins, monobactams, and carbapenems) represent the most important and diverse group of antimicrobials and are the most widely used in clinical practice, especially carbapenems in the hospital setting (1). Resistance to carbapenems is mediated by several mechanisms, including transferable carbapenemase enzymes, many of which also confer resistance to most penicillins and cephalosporins. Carbapenemases are classified into different molecular classes: class A (e.g., KPC and GES); class B, or metallo-β-lactamases (MBLs) (e.g., VIM, IMP, and NDM); and class D, or oxacillinases (e.g., OXA-23, -40, -58, or -48 types) (2). MBLs are the most structurally diverse group of carbapenemases and are capable of hydrolyzing all β-lactams, with the exception of monobactams, conferring an almost pan-β-lactam-resistant phenotype that usually leaves few treatment options (3). Overall, carbapenemases are commonly plasmid mediated and are mainly reported in multidrug-resistant (MDR) Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter species isolates (4).
Carbapenem resistance represents an increasing clinical challenge due to the limited therapeutic options and the high rate of associated morbidity and mortality. Previous studies have demonstrated enhanced activity of β-lactams in combination with β-lactamase inhibitors (e.g., ceftazidime-avibactam, imipenem-relebactam, or meropenem-vaborbactam) against both Enterobacterales and P. aeruginosa isolates expressing serine-β-lactamases, such as KPC and occasionally OXA-48 (5). Nevertheless, treatment options for infections caused by MBL producers are still limited, and new therapies are needed.
Taniborbactam (formerly VNRX-5133; Venatorx Pharmaceuticals, Inc., Malvern, PA, USA) is a bicyclic boronate with a selective pan-spectrum β-lactamase inhibitory activity against serine-β-lactamases (KPC or GES and OXA-48), but also MBLs, including VIM and NDM enzymes, but not IMP (6–8). Taniborbactam is in clinical development combined with cefepime, a fourth-generation cephalosporin, in patients with complicated urinary tract infections (ClinicalTrials.gov identifier NCT03840148). The combination has activity against carbapenem-resistant Enterobacterales (CRE) and carbapenem-resistant P. aeruginosa (CRPA) isolates, including MBL-producing strains (6, 8).
In this work, we evaluated the in vitro activities of cefepime-taniborbactam (FTB) (formerly cefepime–VNRX-5133) and comparator agents against carbapenemase-producing Enterobacterales and carbapenem-resistant Pseudomonas species clinical isolates prospectively collected from different infection sources in patients admitted to 8 Spanish hospitals.
RESULTS
Bacterial isolates.
Overall, 417 carbapenemase-producing Enterobacterales (n = 247) and carbapenem-resistant Pseudomonas species (n = 170) nonreplicate clinical isolates were recovered from urinary tract infection (UTI) (42.2% [176/417]), respiratory tract infection (22.8% [95/417]), surgery site infection (17.3% [72/417]), blood (12.7% [53/417]), and intra-abdominal infection (IAI) (5.0% [21/417]).
Among the Enterobacterales species, Klebsiella pneumoniae (75.3% [186/247]) was the most frequent, followed by Enterobacter cloacae complex (9.7% [24/247]) and Escherichia coli (6.1% [15/247]). Other Enterobacterales species (n = 22) isolates were also found (see Table S1 in the supplemental material). Among the Pseudomonas isolate collection, Pseudomonas aeruginosa was predominant (95.9% [163/170]), although other minority species were also included (Table S1).
Molecular characterization of carbapenemase genes.
Carbapenemase production was confirmed in all Enterobacterales isolates. The OXA-48 type was the most frequent carbapenemase detected (46.1% [114/247]), followed by KPC (35.6% [88/247]) and MBLs (18.2% [45/247]). The following MBL enzymes were encountered: VIM (86.7% [39/45]), NDM (8.9% [4/45]), and IMP (4.4% [2/45]) (see Table S2 in the supplemental material). Moreover, 59.9% of the Enterobacterales isolates (148/247) were also extended-spectrum β-lactamase (ESBL) coproducers (55.6% [25/45] of MBL producers, 54.4% [62/114] of OXA-48-type producers, and 69.3% [61/88] of KPC producers).
Among the Pseudomonas species collection, the presence of carbapenemase genes was confirmed in 47.1% (80/170) of the isolates (blaVIM, 45/170 [26.5%]; blaGES, 30/170 [17.6%]; blaGES+blaVIM, 3/170 [1.8%]; and blaGES+blaKPC, 2/170 [1.2%]). In the remaining Pseudomonas species isolates (52.9% [90/170]), 41.8% (71/170) were classified as multidrug-resistant (MDR) (32.9% [56/170]), extremely drug resistant (XDR) (8.8% [15/170]), and non-MDR (6.5% [11/170]). The distributions of Enterobacterales and Pseudomonas species isolates by species and the carbapenemase group detected are shown in Tables S2 and S3, respectively, in the supplemental material.
Antimicrobial susceptibility of Enterobacterales isolates.
Based on the percentage of susceptibility and the proposed FTB breakpoint, FTB was the most active agent in the Enterobacterales isolates (97.6% MICFTB, ≤8/4 mg/L; MIC50, 0.5/4 mg/L; MIC90, 4/4 mg/L), followed by meropenem-vaborbactam (MEV) (89.1 and 83.8% susceptible by EUCAST and CLSI, respectively; MIC50, 1/8 mg/L; MIC90, 16/8 mg/L) and amikacin (87.9 and 91.1% susceptible by EUCAST and CLSI, respectively; MIC50, ≤0.5 mg/L; MIC90, >64 mg/L) (Table 1).
TABLE 1.
Antimicrobial activities of cefepime-taniborbactam and comparators tested against Enterobacterales by major species
| Organism (no. tested) and antimicrobial(s) | MIC (mg/L) |
% of isolates S, I, or R by: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| EUCAST |
CLSI |
||||||||
| MIC50 | MIC90 | Range | S | I | R | S | I | R | |
| All Enterobacterales (n = 247) | |||||||||
| Cefepime | 32 | >32 | ≤0.25 to >32 | 9.7 | 6.1 | 84.2 | 12.1 | 6.9 | 81.0 |
| Cefepime-taniborbactama | 0.5/4 | 4/4 | ≤06/4 to 32/4 | 97.6 | 2.4 | 97.6 | 2.4 | ||
| Piperacillin-tazobactam | >128/4 | >128/4 | 1/4 to >128/4 | 2.0 | 0.8 | 97.2 | 2.8 | 3.6 | 93.5 |
| Meropenem | 4 | 64 | ≤0.5 to >128 | 44.1 | 28.7 | 27.1 | 25.5 | 18.6 | 55.9 |
| Meropenem-vaborbactam | 1/8 | 16/8 | ≤0.06/8 to >32/8 | 89.1 | 10.9 | 83.8 | 5.3 | 10.9 | |
| Ceftolozane-tazobactam | >32/4 | >32/4 | ≤0.25/4 to >32/4 | 11.7 | 88.3 | 11.7 | 5.3 | 83.0 | |
| Ceftazidime-avibactam | 1/4 | >32/4 | ≤0.25/4 to >32/4 | 80.6 | 19.4 | 80.6 | 19.4 | ||
| Imipenem-relebactam | 1/4 | 8/4 | ≤0.12/4 to >32/4 | 71.7 | 28.3 | ||||
| Levofloxacin | >4 | >4 | ≤0.25 to >4 | 12.1 | 5.7 | 82.2 | 12.1 | 5.7 | 82.2 |
| Amikacin | 4 | 16 | ≤0.5 to >64 | 87.9 | 12.1 | 91.1 | 4.0 | 4.9 | |
| Tigecycline | 1 | >1 | 0.12 to >1 | 36.8 | 63.2 | ||||
| K. pneumoniae (n = 186) | |||||||||
| Cefepime | >32 | >32 | ≤0.25 to >32 | 9.1 | 4.3 | 86.6 | 10.8 | 5.4 | 83.9 |
| Cefepime-taniborbactama | 0.5/4 | 4/4 | ≤0.06/4 to 16/4 | 98.4 | 1.6 | 98.4 | 1.6 | ||
| Piperacillin-tazobactam | >128/4 | >128/4 | 4/4 to >128/4 | 0.5 | 0.5 | 98.9 | 1.1 | 3.2 | 95.7 |
| Meropenem | 4 | 64 | ≤0.5 to >128 | 44.1 | 28.0 | 28.0 | 23.7 | 20.4 | 55.9 |
| Meropenem-vaborbactam | 1/8 | 8/8 | ≤06/8 to >32/8 | 93.0 | 7.0 | 87.6 | 5.4 | 7.0 | |
| Ceftolozane-tazobactam | >32/4 | >32/4 | ≤0.25/4 to >32/4 | 10.2 | 89.8 | 10.2 | 4.8 | 84.9 | |
| Ceftazidime-avibactam | 1/4 | 32/4 | ≤0.25/4 to >32/4 | 88.2 | 11.8 | 88.2 | 11.8 | ||
| Imipenem-relebactam | 1/4 | 4/4 | ≤0.12/4 to >32/4 | 80.1 | 19.9 | ||||
| Levofloxacin | >4 | >4 | ≤0.25 to >4 | 8.6 | 2.7 | 88.7 | 8.6 | 2.7 | 88.7 |
| Amikacin | 4 | 16 | ≤0.5 to >64 | 86.0 | 14.0 | 90.3 | 5.4 | 4.3 | |
| Tigecycline | 1 | >1 | 0.25 to >1 | 31.2 | 68.8 | ||||
| E. cloacae complex (n = 24) | |||||||||
| Cefepime | >32 | >32 | 2 to >32 | 0 | 8.3 | 91.7 | 8.3 | 8.3 | 83.3 |
| Cefepime-taniborbactama | 1/4 | 8/4 | 0.5/4 to 32/4 | 91.7 | 8.3 | 91.7 | 8.3 | ||
| Piperacillin-tazobactam | >128/4 | >128/4 | 8/4 to >128/4 | 4.2 | 0 | 95.8 | 4.2 | 0 | 95.8 |
| Meropenem | 8 | 32 | ≤0.5 to 64 | 37.5 | 29.2 | 33.3 | 12.5 | 25.0 | 62.5 |
| Meropenem-vaborbactam | 4/8 | 32/8 | 0.12/8 to >32/8 | 75.0 | 25.0 | 62.5 | 12.5 | 25.0 | |
| Ceftolozane-tazobactam | >32/4 | >32/4 | 2/4 to >32/4 | 4.2 | 95.8 | 4.2 | 4.2 | 91.7 | |
| Ceftazidime-avibactam | >32 | >32 | 0.5/4 to >32/4 | 29.2 | 70.8 | 29.2 | 70.8 | ||
| Imipenem-relebactam | 4/4 | 16/4 | ≤0.12/4 to >32/4 | 20.8 | 79.2 | ||||
| Levofloxacin | 4 | >4 | 0.5 to >4 | 16.7 | 25.0 | 58.3 | 16.7 | 25.0 | 58.3 |
| Amikacin | 2 | 8 | ≤0.5 to >64 | 95.8 | 4.2 | 95.8 | 0 | 4.2 | |
| Tigecycline | 0.5 | >1 | 0.5 to >1 | 58.3 | 41.7 | ||||
| E. coli (n = 15) | |||||||||
| Cefepime | 32 | >32 | ≤0.25 to >32 | 26.7 | 13.3 | 60 | 26.7 | 13.3 | 60 |
| Cefepime-taniborbactama | 0.25/4 | 8/4 | ≤0.06/4 to 16/4 | 93.3 | 6.7 | 93.3 | 6.7 | ||
| Piperacillin-tazobactam | >128/4 | >128/4 | 32/4 to >128/4 | 0 | 0 | 100 | 0 | 6.7 | 93.3 |
| Meropenem | ≤0.5 | 64 | ≤0.5 to 64 | 66.7 | 20 | 13.3 | 66.7 | 0 | 33.3 |
| Meropenem-vaborbactam | 0.5/8 | >32/8 | ≤0.06/8 to >32/8 | 86.7 | 13.3 | 86.7 | 0 | 13.3 | |
| Ceftolozane-tazobactam | 16/4 | >32/4 | 0.5/4 to >32/4 | 26.7 | 73.3 | 26.7 | 6.7 | 66.7 | |
| Ceftazidime-avibactam | ≤0.25/4 | >32/4 | ≤0.25/4 to >32/4 | 73.3 | 26.7 | 73.3 | 26.7 | ||
| Imipenem-relebactam | 1/4 | 32/4 | 0.25/4 to >32/4 | 80 | 20 | ||||
| Levofloxacin | >4 | >4 | ≤0.25 to >4 | 40 | 0 | 60 | 40 | 0 | 60 |
| Amikacin | 4 | 8 | 1 to 64 | 93.3 | 6.7 | 93.3 | 0 | 6.7 | |
| Tigecycline | 0.25 | 0.5 | 0.12 to 1 | 93.3 | 6.7 | ||||
| All other Enterobacterales spp. (n = 22) | |||||||||
| Cefepime | 32 | >32 | ≤0.25 to >32 | 13.6 | 13.6 | 72.7 | 18.2 | 13.6 | 68.2 |
| Cefepime-taniborbactama | 0.5/4 | 2/4 | ≤0.06/4 to 8/4 | 100 | 0 | 100 | 0 | ||
| Piperacillin-tazobactam | >128/4 | >128/4 | 1/4 to >128/4 | 13.6 | 4.5 | 81.8 | 18.2 | 9.1 | 72.7 |
| Meropenem | 4 | 128 | ≤0.5 to >128 | 36.4 | 40.9 | 22.7 | 27.3 | 9.1 | 63.6 |
| Meropenem-vaborbactam | 1/8 | >32/8 | ≤0.06/8 to >32/8 | 72.7 | 27.3 | 72.7 | 0 | 27.3 | |
| Ceftolozane-tazobactam | 32/4 | >32/4 | ≤0.25/4 to >32/4 | 22.7 | 77.3 | 22.7 | 9.1 | 68.2 | |
| Ceftazidime-avibactam | 1/4 | >32/4 | ≤0.25/4 to >32/4 | 77.3 | 22.7 | 77.3 | 22.7 | ||
| Imipenem-relebactam | 2/4 | >32/4 | ≤0.12/4 to >32/4 | 50 | 50 | ||||
| Levofloxacin | >4 | >4 | ≤0.25 to >4 | 18.2 | 13.6 | 68.2 | 18.2 | 13.6 | 68.2 |
| Amikacin | 4 | 8 | 1 to 32 | 90.9 | 9.1 | 90.9 | 0 | 9.1 | |
| Tigecycline | 1 | >1 | 0.25 to 1 | 22.7 | 77.3 | ||||
Cefepime-taniborbactam provisional breakpoint: S, ≤8/4 mg/L; R, >8/4 mg/L.
The activity of FTB against K. pneumoniae isolates (n = 186) was higher (98.4% MICFTB, ≤8/4 mg/L; MIC50, 0.5/4 mg/L; MIC90, 4/4 mg/L) than those of MEV (93 and 87.6% susceptible by EUCAST and CLSI, respectively; MIC50, 1/8 mg/L; MIC90, 8/8 mg/L) and ceftazidime-avibactam (CZA) (88.2% susceptible by EUCAST and CLSI; MIC50, 1/4 mg/L; MIC90, 32/4 mg/L). Against E. cloacae isolates (n = 24), amikacin showed a higher in vitro activity (95.8% susceptible by EUCAST and CLSI; MIC50, 2 mg/L; MIC90, 8 mg/L) than FTB (91.7% MICFTB, ≤8/4 mg/L; MIC50, 1/4 mg/L; MIC90, 8/4 mg/L) and MEV (75% susceptible by EUCAST and 62.5% by CLSI; MIC50, 4/8 mg/L; MIC90, 32/8 mg/L). Among the E. coli collection (n = 15), FTB (93.3% MICFTB, ≤8/4 mg/L; MIC50, 0.25/4 mg/L; MIC90, 8/4 mg/L), amikacin (93.3% susceptible by EUCAST and CLSI; MIC50, 4 mg/L; MIC90, 8 mg/L), and tigecycline (93.3% susceptible by EUCAST; MIC50, 0.25 mg/L; MIC90, 0.5 mg/L) were the most active antibiotics.
FTB showed elevated MIC values (2.4% MICFTB, 16/4 to 32/4 mg/L) against 6 Enterobacterales isolates (3 of K. pneumoniae, 2 of E. cloacae, and 1 of E. coli). In these isolates, production of KPC+CTX-M-1 (3/6 [K. pneumoniae]), VIM+CTX-M-9 (1/6 [E. cloacae]), NDM+CTX-M-1 (1/6 [E. coli]), and IMP (1/6 [E. cloacae]) was detected (see Table S4 in the supplemental material).
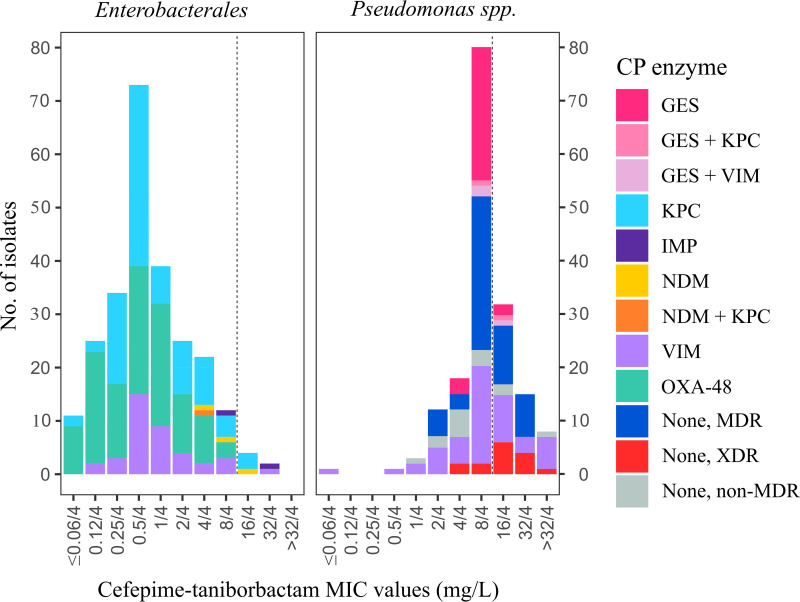
On the other hand, FTB showed an excellent activity against KPC (96 to 100%) and OXA-48-type (100%) producers independent of the bacterial species. Conversely, higher nonsusceptibility rates and MIC90 values were observed against MBL-producing strains, particularly in Enterobacter spp. (11.8% MICFTB, >8/4 mg/L; MIC90, 32/4 mg/L) and E. coli isolates (25% MICFTB, >8 mg/L; MIC90, 16/4 mg/L) (see Table S5 in the supplemental material). Note that the activities of CZA against KPC, OXA-48-type, and MBL producers were 93.2, 97.4, and 11.6%, respectively. The distribution of all Enterobacterales isolates by the FTB MIC value and carbapenemase genes detected is shown in Fig. 1.
FIG 1.
Distribution of Enterobacterales and Pseudomonas isolates by cefepime-taniborbactam MIC values and carbapenemase (CP) enzymes detected. MDR, multidrug resistance; XDR, extremely drug resistant. The dotted line represents the provisional cefepime-taniborbactam breakpoint used for comparative purposes only (S, ≤8/4 mg/L; R, >8/4 mg/L).
In the subset of Enterobacterales isolates with a meropenem-resistant phenotype (MIC, >8 mg/L) (67/247), taniborbactam restored the activity of cefepime in 90% of strains, including KPC (94.3%), MBL (87.5%), and OXA-48-type (80%) producers (Table 2). Furthermore, FTB showed a higher activity (94.0% MICFTB, ≤8/4 mg/L) than CZA (73.1%), ceftolozane-tazobactam (CT) (3.0%), imipenem-relebactam (IMR) (53.7%), and MEV (62.7%) (Table 2). FTB was the most active agent against OXA-48-type (15/67) and MBL-producing isolates (16/67) (100 and 87.5% MICFTB, ≤8/4 mg/L, respectively) whereas the activities of comparators were reduced, particularly against the MBL producers (MEV, 6.7 and 31.2%, CZA, 93.3 and 12.5%, IMR, 0 and 12.5%, and CT, 13.3 and 0%, respectively) (Table 2).
TABLE 2.
Activities of cefepime-taniborbactam and comparators in meropenem-resistant isolates according to EUCAST breakpoints by the carbapenemase group detecteda
| Carbapenamase group | FEP |
FTB |
CZA |
CT |
IMR |
MEV |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
% S | MIC (mg/L) |
% S | MIC (mg/L) |
% S | MIC (mg/L) |
% S | MIC (mg/L) |
% S | MIC (mg/L) |
% S | |||||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |||||||
| Enterobacterales (n = 247) | 32 | >32 | 15.8 | 0.5/4 | 4/4 | 97.6 | 1/4 | >32/4 | 80.6 | >32/4 | >32/4 | 11.7 | 1/4 | 8/4 | 71.7 | 1/8 | 16/8 | 89.1 |
| MER-R (27.1% [67/247]) | >32 | >32 | 4.5 | 2/4 | 8/4 | 94.0 | 2/4 | >32/4 | 73.1 | >32/4 | >32/4 | 3.0 | 2/4 | >32/4 | 53.7 | 4/8 | >32/8 | 62.7 |
| KPC (35/67) | >32 | >32 | 0 | 2/4 | 8/4 | 94.3 | 2/4 | 8/4 | 94.3 | >32/4 | >32/4 | 0 | 0.25/4 | 2/4 | 97.1 | 0.5/8 | 4/8 | 100 |
| OXA-48 (15/67) | 16 | >32 | 20.0 | 4/4 | 8/4 | 100 | 1/4 | 2/4 | 93.3 | >32/4 | >32/4 | 13.3 | 32/4 | >32/4 | 0 | >32/8 | >32/8 | 6.7 |
| VIM, NDM, or IMP (16/67) | >32 | >32 | 0 | 2/4 | 16/4 | 87.5 | >32/4 | >32/4 | 12.5 | >32/4 | >32/4 | 0 | 8/4 | >32/4 | 12.5 | 16/8 | >32/8 | 31.2 |
| Pseudomonas spp. (n = 170) | 32 | >32 | 20.0 | 8/4 | 32/4 | 67.6 | 8/4 | >32/4 | 61.2 | 8/4 | >32/4 | 34.7 | 16/4 | >32/4 | 37.1 | 32/8 | >32/8 | 32.9 |
| MER-R (71.8% [122/170]) | 32 | >32 | 10.7 | 8/4 | 32/4 | 63.9 | 8/4 | >32/4 | 51.6 | 8/4 | >32/4 | 17.2 | 32/4 | >32/4 | 16.4 | >32/8 | >32/8 | 8.2 |
| GES (30/122)b | >32 | >32 | 0 | 8/4 | 8/4 | 93.3 | 4/4 | 8/4 | 96.7 | 8/4 | 8/4 | 0 | 32/4 | 32/4 | 0 | >32/8 | >32/8 | 0 |
| VIM (49/122)c | >32 | >32 | 13.0 | 8/4 | >32/4 | 61.2 | >32/4 | >32/4 | 14.3 | >32/4 | >32/4 | 0 | >32/4 | >32/4 | 0 | >32/8 | >32/8 | 4.1 |
| Non-carbapenemase (43/122) | 32 | >32 | 16.3 | 16/4 | 32/4 | 46.5 | 8/4 | >32/4 | 62.8 | 8/4 | >32/4 | 48.8 | 4/4 | 32/4 | 46.5 | 16/8 | >32/8 | 18.6 |
MER, meropenem (S, ≤2 mg/L; R, >8 mg/L); FTB, cefepime-taniborbactam (provisional breakpoint: S, ≤8/4 mg/L; R, >8/4 mg/L); CZA, ceftazidime-avibactam (S, ≤8/4 mg/L; R, >8/4 mg/L); CT, ceftolozane-tazobactam (Enterobacterales spp., S, ≤2/4 mg/L, and R, >2/4 mg/L; Pseudomonas spp., S, ≤4/4 mg/L, and R, >4/4 mg/L); IMR, imipenem-relebactam (S, ≤2/4 mg/L; R, >2/4 mg/L); and MEV, meropenem-vaborbactam (S, ≤8/8 mg/L; R, >8/8 mg/L).
The GES group includes 29 blaGES isolates and 1 blaGES+blaKPC isolate.
The VIM group includes 46 blaVIM and 3 blaVIM+blaGES isolates.
Antimicrobial susceptibility of Pseudomonas species isolates.
Among the Pseudomonas species collection (n = 170), FTB was also the most active agent (67.6% MICFTB, ≤8/4 mg/L; 86.5% MICFTB, ≤16/4 mg/L; MIC50, 8/4 mg/L; MIC90, 32/4 mg/L), followed by amikacin (64.7% susceptible by EUCAST and CLSI; MIC50, 8 mg/L; MIC90, 64 mg/L) and CZA (61.2% susceptible by EUCAST and CLSI; MIC50, 8/4 mg/L; MIC90, >32/4 mg/L). In the subset of P. aeruginosa isolates (n = 163), FTB showed a higher in vitro activity (67.5% MICFTB, ≤8/4 mg/L; MIC50, 8/4 mg/L; MIC90, 32/4 mg/L) than amikacin (64.4% susceptible by EUCAST and CLSI; MIC50, 8 mg/L; MIC90, 64 mg/L) and CZA (63.2% susceptible by EUCAST and CLSI, respectively; MIC50, 8/4 mg/L; MIC90, >32/4 mg/L) (Table 3).
TABLE 3.
Antimicrobial activities of cefepime-taniborbactam and comparators tested against Pseudomonas spp. by species and major phenotypes
| Organisms (no. tested) and antimicrobial(s) | MIC (mg/L) |
% of isolates S, I, or R by: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| EUCAST |
CLSI |
||||||||
| MIC50 | MIC90 | Range | S | I | R | S | I | R | |
| All Pseudomonas spp. (n = 170) | |||||||||
| Cefepime | 32 | >32 | 1 to >32 | 0.0 | 20.0 | 80.0 | 20.0 | 25.3 | 54.7 |
| Cefepime-taniborbactama | 8/4 | 32/4 | ≤0.06/4 to 32/4 | 67.6 | 32.4 | 67.6 | 32.4 | ||
| Piperacillin-tazobactam | 128/4 | >128/4 | 1/4 to >128/4 | 0 | 17.6 | 82.4 | 17.6 | 19.4 | 62.9 |
| Meropenem | 32 | 128 | ≤0.5 to >128 | 4.1 | 24.1 | 71.8 | 4.1 | 7.1 | 88.8 |
| Meropenem-vaborbactam | 32/8 | >32/8 | ≤0.06/8 to >32/8 | 32.9 | 67.1 | 14.7 | 2.4 | 67.1 | |
| Ceftolozane-tazobactam | 8/4 | >32/4 | ≤0.25/4 to >32/4 | 34.7 | 65.3 | 34.7 | 28.2 | 37.1 | |
| Ceftazidime-avibactam | 8/4 | >32/4 | ≤0.25/4 to >32/4 | 61.2 | 38.8 | 61.2 | 38.8 | ||
| Imipenem-relebactam | 16/4 | >32/4 | ≤0.12/4 to >32/4 | 37.1 | 62.9 | ||||
| Levofloxacin | >4 | >4 | ≤0.25 to >4 | 0.0 | 12.4 | 87.6 | 5.9 | 6.5 | 87.6 |
| Amikacin | 8 | 64 | ≤0.5 to >64 | 64.7 | 35.3 | 64.7 | 5.9 | 29.4 | |
| Tigecycline | >1 | >1 | 0.25 to >1 | 1.2 | 98.8 | ||||
| All P. aeruginosa (n = 163) | |||||||||
| Cefepime | 32 | >32 | 1 to >32 | 0 | 20.2 | 79.8 | 20.2 | 25.8 | 54.0 |
| Cefepime-taniborbactama | 8/4 | 32/4 | ≤0.06/4 to 32/4 | 67.5 | 32.5 | 67.5 | 32.5 | ||
| Piperacillin-tazobactam | 128/4 | >128/4 | 1/4 to >128/4 | 0.0 | 17.8 | 82.2 | 17.8 | 19.0 | 63.2 |
| Meropenem | 32 | 128 | ≤0.5 to >128 | 4.3 | 25.2 | 70.6 | 4.3 | 7.4 | 88.3 |
| Meropenem-vaborbactam | 32/8 | >32/8 | ≤0.06/8 to >32/8 | 33.7 | 66.3 | 15.3 | 1.8 | 66.3 | |
| Ceftolozane-tazobactam | 8/4 | >32/4 | ≤0.25/4 to >32/4 | 36.2 | 63.8 | 36.2 | 29.4 | 34.4 | |
| Ceftazidime-avibactam | 8/4 | >32/4 | ≤0.25/4 to >32/4 | 63.2 | 36.8 | 63.2 | 36.8 | ||
| Imipenem-relebactam | 8/4 | >32/4 | ≤0.12/4 to >32/4 | 38.7 | 61.3 | ||||
| Levofloxacin | >4 | >4 | ≤0.25 to >4 | 0 | 12.9 | 87.1 | 6.1 | 6.7 | 87.1 |
| Amikacin | 8 | 64 | ≤0.5 to >64 | 64.4 | 35.6 | 64.4 | 6.1 | 29.4 | |
| Tigecycline | 5 | 5 | 0.25 to >1 | 1.2 | 98.8 | ||||
| All other Pseudomonas spp. (n = 7) | |||||||||
| Cefepime | 4 to >32 | 0 | 14.3 | 85.7 | 14.3 | 14.3 | 71.4 | ||
| Cefepime-taniborbactama | 4/4 to >32/4 | 71.4 | 28.6 | 71.4 | 28.6 | ||||
| Piperacillin-tazobactam | 16/4 to >128/4 | 0 | 14.3 | 85.7 | 14.3 | 28.6 | 57.1 | ||
| Meropenem | 16 to >128 | 0 | 0 | 100 | 0 | 0 | 100 | ||
| Meropenem-varbobactam | 8/8 to >32/8 | 14.3 | 85.7 | 0 | 14.3 | 85.7 | |||
| Ceftolozane-tazobactam | 32/4 to >32/4 | 0 | 100.0 | 0 | 0 | 100 | |||
| Ceftazidime-avibactam | 8/4 to >32/4 | 14.3 | 85.7 | 14.3 | 85.7 | ||||
| Imipenem-relebactam | 32/4 to >32/4 | 0 | 100 | ||||||
| Levofloxacin | 2 to >4 | 0 | 0 | 100 | 0 | 0 | 100 | ||
| Amikacin | ≤0.5 to >32 | 71.4 | 28.6 | 71.4 | 28.6 | ||||
| Tigecycline | 1 to >1 | 0 | 100 | ||||||
Cefepime-taniborbactam provisional breakpoint: S, ≤8/4 mg/L; R, >8/4 mg/L.
FTB showed elevated MIC values in 32.5% (53/163) of the P. aeruginosa isolates (MICFTB, 16/4 to >32/4 mg/L). Production of carbapenemase genes was confirmed in 17 (32.1% [17/53]) of these isolates (VIM, 13/17; GES, 2/17; GES+VIM, 1/17; and GES+KPC, 1/17), suggesting the presence of additional resistance mechanisms. Among P. aeruginosa isolates with a MICFTB of ≤8/4 mg/L (67.5% [110/163]), carbapenemase production was also detected (50.9% [56/110]) (GES, 28/56; VIM, 25/56; GES+VIM, 2/56; and GES+KPC, 1/56). On the other hand, FTB had elevated MICs against 2/7 non-P. aeruginosa isolates (MICFTB, 16/4 and >32/4 mg/L) and production of VIM carbapenemase was confirmed in both strains.
Overall, in P. aeruginosa FTB was particularly active against GES producers (93.8% MICFTB, <8/4 mg/L), those non-carbapenemase-producers with non-MDR (81.8% MICFTB, <8/4 mg/L), and MBL-producing strains (63% to 66.7% MICFTB, <8/4 mg/L), while XDR P. aeruginosa isolates displayed the most elevated MIC values (73.3% MICFTB, 16/4 to >32/4 mg/L) (see Table S6 in the supplemental material). The distribution of Pseudomonas species isolates by the FTB MIC value and carbapenemase genes detected is represented in Fig. 1.
In the subset of Pseudomonas species isolates with a meropenem MIC value of >8 mg/L (122/170), taniborbactam restored the activity of cefepime in 53% of strains, especially in GES producers (93.3%), but also in VIM producers (48.2%). In addition, FTB (64% MICFTB, ≤8/4 mg/L) showed higher activity than CZA (52%), CT (17%), IMR (16%), and MEV (8%) (Table 2). Activities of FTB (MICFTB, ≤8/4 mg/L) against class A carbapenemase (n = 30) and MBL (n = 49) producers were 93.3 and 61.2%, respectively. The corresponding susceptibility rates of comparators in these isolates were as follows: CZA, 96.7 and 14.3%, MEV, 0 and 4.1%, IMR, 0 and 0%, and CT, 0 and 0%, respectively. Against carbapenem-resistant P. aeruginosa isolates in which carbapenemase production was not detected, the activity of CZA (62.8%) was higher than that of FTB (46.5%). In addition, FTB activity was comparable to those of CT (48.8%) and IMR (46.5%) and higher than that of MEV (18.6%) (Table 2).
On the other hand, among the Pseudomonas species isolates showing different resistant phenotypes, FTB was more active (39.4 to 64.5%) than other tested comparators (CZA, 43.2 to 55.7%; CT, 4.5 to 27.9%; IMR, 8.5 to 31.4%; and MEV, 5.1 to 25%) (Table 4).
TABLE 4.
Activities of cefepime-taniborbactam and comparators in P. aeruginosa isolates by resistance phenotypes according to EUCAST breakpointsa
| Isolate group | FTB |
CZA |
CT |
IMR |
MEV |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
S (%) | MIC (mg/L) |
S (%) | MIC (mg/L) |
S (%) | MIC (mg/L) |
S (%) | MIC (mg/L) |
S (%) | ||||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | ||||||
| All Pseudomonas spp. (n = 170) | 8/4 | 32/4 | 67.6 | 8/4 | >32/4 | 61.2 | 8/4 | >32/4 | 34.7 | 16/4 | >32/4 | 37.1 | 32/8 | >32/8 | 32.9 |
| Resistance group | |||||||||||||||
| PT-R (82.3% [140/170]) | 8/4 | 32/4 | 60.7 | 8/4 | >32/4 | 55.7 | 8/4 | >32/4 | 27.9 | 16/4 | >32/4 | 31.4 | >32/8 | >32/8 | 25.0 |
| FEP-R (80.0% [136/170]) | 8/4 | 32/4 | 59.6 | 8/4 | >32/4 | 52.2 | 8/4 | >32/4 | 23.5 | 16/4 | >32/4 | 27.9 | >32/8 | >32/8 | 23.5 |
| MER-R (71.8% [122/170]) | 8/4 | 32/4 | 63.9 | 8/4 | >32/4 | 51.6 | 8/4 | >32/4 | 17.2 | 32/4 | >32/4 | 16.4 | >32/8 | >32/8 | 8.2 |
| PT-FEP-R (76.5% [130/170]) | 8/4 | 32/4 | 57.7 | 8/4 | >32/4 | 53.1 | 8/4 | >32/4 | 23.1 | 16/4 | >32/4 | 27.7 | >32/8 | >32/8 | 22.3 |
| PT-FEP-MER-R (62.3% [106/170]) | 8/4 | 32/4 | 58.5 | 16/4 | >32/4 | 48.1 | 8/4 | >32/4 | 13.2 | 32/4 | >32/4 | 15.1 | >32/8 | >32/8 | 6.6 |
| CT-R (65.3% [111/170]) | 8/4 | 32/4 | 63.1 | 16/4 | >32/4 | 43.2 | 32/4 | >32/4 | 14.4 | >32/8 | >32/8 | 13.5 | |||
| CZA-R (38.8% [66/170]) | 16/4 | >32/4 | 39.4 | >32/4 | >32/4 | 4.5 | >32/4 | >32/4 | 18.2 | >32/8 | >32/8 | 15.1 | |||
| IMR-R (62.9% [107/170]) | 8/4 | 32/4 | 64.5 | 16/4 | >32/4 | 49.5 | 16/4 | >32/4 | 11.2 | >32/8 | >32/8 | 6.5 | |||
| MEV-R (67.1% [114/170]) | 8/4 | 32/4 | 62.3 | 8/4 | >32/4 | 50.9 | 8/4 | >32/4 | 15.8 | 32/4 | >32/4 | 12.3 | |||
| IMR-R or MEV-R (71.2% [121/170]) | 8/4 | 32/4 | 62.0 | 8/4 | >32/4 | 51.2 | 8/4 | >32/4 | 16.5 | 32/4 | >32/4 | 11.6 | >32/8 | >32/8 | 5.8 |
| CZA-R+IMR-R or MEV-R (34.7% [59/170]) | 16/4 | >32/4 | 42.4 | 8/4 | >32/4 | 5.1 | 32/4 | >32/4 | 8.5 | >32/8 | >32/8 | 5.1 | |||
FTB, cefepime-taniborbactam (S, ≤8/4 mg/L; R, >8/4 mg/L); CZA, ceftazidime-avibactam (S, ≤8/4 mg/L; R, >8/4 mg/L); CT, ceftolozane-tazobactam (S, ≤4/4 mg/L; R, >4/4 mg/L); IMR, imipenem-relebactam (S, ≤2/4 mg/L; R, >2/4 mg/L); and MEV, meropenem-vaborbactam (S, ≤8/8 mg/L; R, >8/8 mg/L).
DISCUSSION
Carbapenem resistance is a major global health problem that leaves limited therapeutic options to combat difficult-to-treat infections caused by multidrug-resistant pathogens. The efficacy of novel β-lactam–β-lactamase inhibitor combinations such as CZA, CT, IMR, or MEV for treating infections caused by carbapenem-resistant Enterobacterales (CRE) and carbapenem-resistant P. aeruginosa (CRPA) isolates has been demonstrated, including carbapenemase producers (5, 9, 10). Nevertheless, none of the currently approved β-lactam–β-lactamase inhibitor combinations has activity against MBL-producing Enterobacterales or P. aeruginosa strains (5, 9).
Taniborbactam is the first β-lactamase inhibitor with direct inhibitory activity against MBL enzymes, including both the NDM type and VIM type (7). At this time, the FTB combination is in phase 3 clinical trials to evaluate safety and efficacy in patients with complicated urinary tract infections (cUTIs). This novel, cyclic boronate β-lactam–β-lactamase inhibitor combination is targeted for the treatment of patients with cUTIs and hospital-acquired or ventilator-associated bacterial pneumonia (HABP/VABP) caused by MDR pathogens, including CRE and CRPA isolates (8).
In in vitro studies, FTB has demonstrated a higher bactericidal activity against both serine-β-lactamase- and MBL-producing Enterobacterales and P. aeruginosa compared to other approved combinations, such as CZA, CT, cefepime-tazobactam, and MEV (8, 11, 12). Concordantly, in our Enterobacterales collection, FTB was the most active agent (98% MICFTB, ≤8/4 mg/L), followed by MEV, amikacin, CZA and IMR. In addition, a higher inhibitory activity of FTB (94% MICFTB, ≤8/4 mg/L) was also demonstrated against the subset of strains with meropenem-resistant MIC values (EUCAST breakpoint) compared to other β-lactam–β-lactamase inhibitor combinations. Moreover, taniborbactam restored cefepime activity, including in most meropenem-resistant strains producing KPC, MBL, and OXA-48-type enzymes.
Among tested P. aeruginosa isolates, FTB was also the antimicrobial with the highest activity (68% MICFTB, ≤8/4 mg/L), followed by amikacin, CZA, IMR, and MEV. In addition, the susceptibility rate to FTB (64% MICFTB, ≤8/4 mg/L) was higher than that of the comparators in the subset of Pseudomonas species isolates with resistance to meropenem (EUCAST breakpoint) and also against P. aeruginosa strains resistant to other new β-lactam–β-lactamase inhibitor combinations. Notably, taniborbactam restored cefepime activity in most GES-producing P. aeruginosa isolates and about half of the VIM-producing strains.
CZA is indicated for treating cUTIs, complicated intra-abdominal infections (cIAIs), and HABP/VABP caused by carbapenemase-producing Enterobacterales, particularly KPC and some OXA-48-type strains, in addition to CRPA isolates (13, 14). According to our results, in the subset of meropenem-resistant Enterobacterales strains, FTB showed an equal provisional susceptibility rate to CZA against KPC-producing isolates, but a clearly increased activity against MBL producers, reinforcing targeted inhibition to these enzymes (5). Previous studies have also reported a broader spectrum of activity of FTB compared to CZA, especially against VIM- and NDM-producing Enterobacterales isolates (11, 15). Note that one of the most common resistance mechanisms against CZA in Enterobacterales, particularly in K. pneumoniae, is the emergence of novel KPC variants due to spontaneous mutations, frequently after or during antibiotic exposure (16, 17). FTB has also been reported to be effective against strains carrying novel blaKPC variants that compromise the activity of CZA (8, 18). Furthermore, in this study, the activity of FTB was slightly better than that of CZA against OXA-48-type producers. On the other hand, among the meropenem-resistant P. aeruginosa isolates, the activity of FTB was comparable to that of CZA against GES-producing strains, but evidently higher against MBL producers.
IMR and MEV are also indicated for the treatment of cUTIs, cIAIs, and HABP/VABP with limited or no alternative treatment options available. Both relebactam and vaborbactam display a potent inhibitory activity against class A (including ESBLs and KPCs) and class C (AmpC) β-lactamases in Enterobacterales (10, 19, 20). In addition, relebactam has also shown efficacy in restoring the activity of imipenem against CRPA by inhibiting the AmpC β-lactamase (20). In agreement with previous studies, in our meropenem-resistant isolates, IMR and MEV showed high susceptibility rates against KPC-producing Enterobacterales, but low or no activity against MBL- and OXA-48-type-producing Enterobacterales and against MBL- and GES-producing P. aeruginosa (21, 22).
Finally, CT is a reliable treatment option for CRPA isolates in which carbapenemase enzymes are not detected and against ESBL-producing Enterobacterales (5, 13, 23). In our collection, FTB displayed similar activity to CT against the subset of carbapenem-resistant, non-carbapenemase-producing P. aeruginosa isolates, but was clearly better across the entire collection.
In conclusion, among the antibiotics tested, our results place FTB as a potential therapeutic option for treating infections caused by carbapenem-resistant Enterobacterales and carbapenem-resistant P. aeruginosa isolates, including those that produce MBL enzymes, as well as serine-β-lactamases. Furthermore, in our collection, this novel β-lactam–β-lactamase inhibitor combination was one of the most potent antipseudomonal agents against MDR/XDR P. aeruginosa isolates, including those affecting β-lactam–β-lactamase inhibitor combinations currently used in clinical practice.
MATERIALS AND METHODS
Study design and bacterial isolates.
A multicenter study was designed to assess the in vitro activities of FTB and comparator antimicrobial agents against clinical isolates of Enterobacterales and P. aeruginosa prospectively recovered in Spanish hospitals between January 2020 and December 2020. Eight hospitals located in different regions participated in the study: Hospital Universitario Ramón y Cajal (coordinator testing laboratory) (Madrid), Complejo Hospitalario Universitario (A Coruña), Hospital Universitario Marqués de Valdecilla (Santander), Hospital Clínic (Barcelona), Consorcio Hospital General Universitario (Valencia), Hospital Universitario Son Espases (Palma de Mallorca), Hospital Universitario Virgen Macarena (Seville), and Hospital de Córdoba Reina Sofía (Cordoba). Species identification was carried out at each participant hospital and confirmed at the coordinator laboratory using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker-Daltonics, Bremen, Germany). Characterization of the carbapenemase production was preliminarily performed at the local participating centers using phenotypic methods following national and international guidelines (24, 25). The ethics committees of all participating sites approved the study.
Antimicrobial susceptibility testing.
MICs of FTB and comparators were determined by the standard broth microdilution method using 96-well plates (Lyophilized Sensititre panel; Thermo Fisher Scientific, Cleveland, OH, USA). The antimicrobial concentrations tested were as follows: amikacin (AMK), 0.5 to 64 mg/L; cefepime (FEP), 0.25 to 32 mg/L; cefepime-taniborbactam (FTB), 0.06/4 to 64/4 mg/L; ceftazidime-avibactam (CZA), 0.25/4 to 32/4 mg/L; ceftolozane-tazobactam (CT), 0.25/4 to 32/4 mg/L; imipenem-relebactam (IMR), 0.015 to 64 mg/L; levofloxacin (LEV), 0.25 to 4 mg/L; meropenem (MER), 0.5 to 128 mg/L; meropenem-vaborbactam (MEV), 0.06/8 to 32/8 mg/L; piperacillin-tazobactam (PT), 0.5/4 to 128/4 mg/L; and tigecycline (TGC), 0.015 to 1 mg/L. E. coli ATCC 25922, K. pneumoniae ATCC 700603, P. aeruginosa ATCC 27853, and K. pneumoniae ATCC BAA-1705 were used for quality control. The results were interpreted in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST-2021; https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf) and Clinical and Laboratory standards Institute (CLSI-2020, https://clsi.org/standards/products/microbiology/documents/m100/) guidelines. For comparison purposes, the definition of susceptible (S) (susceptible plus susceptible, increased exposure [I], formerly intermediate for EUCAST) was applied to both EUCAST and CLSI criteria. The proposed FTB breakpoint was used: susceptible (S) MICFTB of ≤8/4 mg/L and resistant (R) MICFTB of >8/4 mg/L. MIC50 and MIC90 values were also calculated.
In P. aeruginosa isolates, the following resistant phenotypes were also defined using the EUCAST interpretative criteria: PT-FEP-R (combined PT and FEP nonsusceptibility), PT-FEP-MER-R (combined PT, FEP, and MER nonsusceptibility), IMR-R or MEV-R (IMR nonsusceptibility, MEV nonsusceptibility, and IMR nonsusceptibility plus MEV nonsusceptibility), CZA-R+IMR-R or MEV-R (combined CZA nonsusceptibility and IMR nonsusceptibility plus combined CZA nonsusceptibility and MEV nonsusceptibility), MDR (multidrug resistant; nonsusceptibility to at least one agent in three or more antimicrobial categories), and XDR (extensively drug-resistant; nonsusceptibility to at least one agent in all but two or fewer antimicrobial categories).
Carbapenemase detection.
Carbapenemase production was confirmed in the coordinator testing laboratory using the KPC/MBL/OXA-48 Confirm kit (Rosco Diagnostica, Germany) and standard PCR as previously described (26). In the Enterobacterales isolates with an FTB nonsusceptible phenotype (MICFTB, >8/4 mg/L), carbapenemase genes were additionally confirmed using the Cepheid Xpert Carba-R assay (27).
ACKNOWLEDGMENTS
The study group includes the following members: Germán Bou and M. Carmen Fernández, Hospital Universitario A Coruña, A Coruña, Spain; Jorge Calvo, Jesús Rodríguez-Lozano, and María Siller-Ruiz, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Jordi Vila and Cristina Pitart, Hospital Clínic i Provincial, Barcelona, Spain; Luis Martínez-Martínez and Irene Gracia-Ahufinger, Hospital de Córdoba Reina Sofía, Córdoba, Spain; Antonio Oliver and Xavier Mulet, Hospital Universitario Son Espases, Palma de Mallorca, Spain; Álvaro Pascual and Elena Marín-Martínez, Hospital Universitario Virgen Macarena, Seville, Spain; Concepción Gimeno and Nuria Tormo, Consorcio Hospital General Universitario de Valencia, Valencia, Spain; and Marta Hernández-García, María García-Castillo, Patricia Ruiz-Garbajosa, and Rafael Cantón, Hospital Ramón y Cajal, Madrid, Spain.
R.C. has participated in educational programs organized by MSD and Pfizer and has received research support from MSD and Venatorx Pharmaceuticals.
This project was sponsored by Venatorx Pharmaceuticals and has been funded in whole or in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract no. HHSO100201900007C. This study was also supported by Plan Nacional de I+D + i 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (RD16/0016/0001, RD16/0016/0004, RD16/0016/0006, RD16/0016/0007, RD16/0016/0008, RD16/0016/0010, and REIPI RD16/0016/0011), cofinanced by the European Development Regional Fund “A Way to Achieve Europe” (ERDF), operative program Intelligent Growth 2014–2020 and CIBER en Enfermedades Infecciosas (CIBERINF) (CB21/13/00084).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh TR. 2010. Emerging carbapenemases: a global perspective. Int J Antimicrob Agents 36:S8–S14. 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 4.Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen Seifert H, Woodford N, Nordmann P, Poirel L, Bogaerts P, Navon-Venezia S, Cornaglia G, European Network on Carbapenemases. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 5.Yahav D, Giske CG, Gramatniece A, Abodakpi H, Tam VH, Leibovici L. 2021. New β-lactam–β-lactamase inhibitor combinations. Clin Microbiol Rev 34:e00115-20. 10.1128/CMR.00115-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krajnc A, Brem J, Hinchliffe P, Calvopiña K, Panduwawala TD, Lang PA, Kamps JJAG, Tyrrell JM, Widlake E, Saward BG, Walsh TR, Spencer J, Schofield CJ. 2019. Bicyclic boronate VNRX-5133 inhibits metallo- and serine-β-lactamases. J Med Chem 62:8544–8556. 10.1021/acs.jmedchem.9b00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B, Trout REL, Chu GH, McGarry D, Jackson RW, Hamrick JC, Daigle DM, Cusick SM, Pozzi C, de Luca F, Benvenuti M, Mangani S, Docquier JD, Weiss WJ, Pevear DC, Xerri L, Burns CJ. 2020. Discovery of taniborbactam (VNRX-5133): a broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J Med Chem 63:2789–2801. 10.1021/acs.jmedchem.9b01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamrick JC, Docquier J-D, Uehara T, Myers CL, Six DA, Chatwin CL, John KJ, Vernacchio SF, Cusick SM, Trout REL, Pozzi C, de Luca F, Benvenuti M, Mangani S, Liu B, Jackson RW, Moeck G, Xerri L, Burns CJ, Pevear DC, Daigle DM. 2020. VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 64:e01963-19. 10.1128/AAC.01963-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vázquez-Ucha JC, Arca-Suárez J, Bou G, Beceiro A. 2020. New carbapenemase inhibitors: clearing the way for the β-lactams. Int J Mol Sci 21:9308. 10.3390/ijms21239308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karaiskos I, Galani I, Papoutsaki V, Galani L, Giamarellou H. 2021. Carbapenemase producing Klebsiella pneumoniae: implication on future therapeutic strategies. Expert Rev Anti Infect Ther 3:1–17. 10.1080/14787210.2021.1935237. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Zhao C, Wang Q, Wang Z, Liang X, Zhang F, Zhang Y, Meng H, Chen H, Li S, Zhou C, Li H, Wang H. 2020. In vitro activity of the novel β-lactamase inhibitor taniborbactam (VNRX-5133), in combination with cefepime or meropenem, against MDR Gram-negative bacterial isolates from China. J Antimicrob Chemother 75:1850–1858. 10.1093/jac/dkaa053. [DOI] [PubMed] [Google Scholar]
- 12.Avery LM, Vernacchio S, Mclaughlin L, Xerri L, Moeck G, Pevear D, Venatorx Pharmaceuticals. 2020. Assessment of cefepime (FEP)-taniborbactam (TAN) human exposures to suppress the emergence of resistance among serine (SBL)- and metallo-β-lactamase (MBL)-producing Gram-negative bacteria (GNB) in a hollow fiber infection model (HFIM). Open Forum Infect Dis 7(Suppl 1):S648. 10.1093/ofid/ofaa439.1447. [DOI] [Google Scholar]
- 13.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogue JM, Bonomo RA, Kaye KS. 2019. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 68:519–524. 10.1093/cid/ciy576. [DOI] [PubMed] [Google Scholar]
- 15.Piccirilli A, Segatore B, Brisdelli F, Amicosante G, Perilli M. 2021. Potent inhibitory activity of taniborbactam towards NDM-1 and NDM-1Q119X mutants, and in vitro activity of cefepime/taniborbactam against MBLs producing Enterobacterales. Int J Antimicrob Agents 57:106228. 10.1016/j.ijantimicag.2020.106228. [DOI] [PubMed] [Google Scholar]
- 16.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson CA, Bonacorsi S, Hervé J, Choudhury A, Magnan M, Cointe A, Bercot B, Tenaillon O, Birgy A. 2020. KPC beta-lactamases are permissive to insertions and deletions conferring substrate spectrum modifications and resistance to ceftazidime-avibactam. Antimicrob Agents Chemother 64:e01175-20. 10.1128/AAC.01175-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daigle D, Hamrick J, Chatwin C, Kurepina N, Kreiswirth BN, Shields RK, Oliver A, Clancy CJ, Nguyen M-H, Pevear D, Xerri L. 2018. Cefepime/VNRX-5133 broad-spectrum activity is maintained against emerging KPC- and PDC-variants in multidrug-resistant K. pneumoniae and P aeruginosa. Open Forum Infect Dis 5:S419–S420. 10.1093/ofid/ofy210.1201. [DOI] [Google Scholar]
- 19.Campanella TA, Gallagher JC. 2020. A clinical review and critical evaluation of imipenem-relebactam: evidence to date. Infect Drug Resist 13:4297–4308. 10.2147/IDR.S224228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhanel GG, Lawrence CK, Adam H, Schweizer F, Zelenitsky S, Zhanel M, Lagacé-Wiens PRS, Walkty A, Denisuik A, Golden A, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2018. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs 78:65–98. 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 21.Shields RK, McCreary EK, Marini RV, Kline EG, Jones CE, Hao B, Chen L, Kreiswirth BN, Doi Y, Clancy CJ, Nguyen MH. 2020. Early experience with meropenem-vaborbactam for treatment of carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 71:667–671. 10.1093/cid/ciz1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canver MC, Satlin MJ, Westblade LF, Kreiswirth BN, Chen L, Robertson A, Fauntleroy K, la Spina M, Callan K, Jenkins SG. 2019. Activity of imipenem-relebactam and comparator agents against genetically characterized isolates of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 63:e00672-19. 10.1128/AAC.00672-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haidar G, Philips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, Doi Y, Hao B, Press EG, Cooper VS, Clancy CJ, Nguyen MH. 2017. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 65:110–120. 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo J, Cantón R, Fernández Cuenca F, Mirelis B, Navarro F. 2011. Detección fenotípica de mecanismos de resitencia en gramnegativos. In Cercenado E, Cantón R (ed), Procedimientos en Microbiología Clínica Recomendaciones de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica. https://www.seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimientomicrobiologia38.pdf. [DOI] [PubMed]
- 25.Martínez-Martínez L, Cantón Spain R, Stefani S, Skov R, Glupczynski Y, Nordmann P, Wootton M, Miriagou V, Skov Simonsen G. 2017. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. J Infect 72:152–160. [Google Scholar]
- 26.Ruiz-Garbajosa P, Hernández-García M, Beatobe L, Tato M, Méndez MI, Grandal M, Aranzábal L, Alonso S, Lópaz MÁ, Astray J, Cantón R. 2016. A single-day point-prevalence study of faecal carriers in long-term care hospitals in Madrid (Spain) depicts a complex clonal and polyclonal dissemination of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 71:348–352. 10.1093/jac/dkv355. [DOI] [PubMed] [Google Scholar]
- 27.Tato M, Ruiz-Garbajosa P, Traczewski M, Dodgson A, McEwan A, Humphries R, Hindler J, Veltman J, Wang H, Cantón R. 2016. Multisite evaluation of Cepheid Xpert-Carba-R assay for the detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol 54:1814–1819. 10.1128/JCM.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 to S6. Download aac.02161-21-s0001.pdf, PDF file, 0.2 MB (222.4KB, pdf)