Summary
Background
Health care-associated infections (HCAI) in neonatal units in low- and middle-income countries (LMIC) are a major cause of mortality. This scoping review aimed to synthesise published literature on infection prevention and care bundles addressing neonatal HCAI in LMICs and to construct a Classification Framework for their components (elements).
Methods
Five electronic databases were searched between January 2001 and July 2020. A mixed-methods approach was applied: qualitative content analysis was used to build a classification framework to categorise bundle elements and the contents of the classification groups were then described quantitatively.
Findings
3619 records were screened, with 44 eligible studies identified. The bundle element Classification Framework created involved: (1) Primary prevention, (2) Detection, (3) Case management, and Implementation (3 + I). The 44 studies included 56 care bundles with 295 elements that were then classified. Primary prevention elements (128, 43%) predominated of which 71 (55%) focused on central line catheters and mechanical ventilators. Only 12 elements (4%) were related to detection. A further 75 (25%) elements addressed case management and 66 (88%) of these aimed at outbreak control.
Interpretation
The 3 + I Classification Framework was a feasible approach to reporting and synthesising research for infection-relevant bundled interventions in neonatal units. A shift towards the use in infection prevention and care bundles of primary prevention elements focused on the neonate and on commonly used hospital devices in LMIC (e.g., self-inflating bags, suctioning equipment) would be valuable to reduce HCAI transmission. Detection elements were a major gap.
Funding
This work was made possible in part by the John D. and Catherine T. MacArthur Foundation, the Bill & Melinda Gates Foundation, ELMA Philanthropies, The Children's Investment Fund Foundation UK, The Lemelson Foundation, and the Ting Tsung and Wei Fong Chao Foundation under agreements to William Marsh Rice University. The project leading to these results has also received the support of a fellowship from the “la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/EU19/11710040. EJAF is an Academic Clinical Fellow whose salary is funded by the UK National Institute for Health Research (NIHR). NES receives a Research Training Program Scholarship (Australian Commonwealth Government).
Keywords: scoping review, health care-associated infections, care bundles, neonatal units, infection prevention and control, low- and middle-income countries
Abbreviations: 3 + I Framework, (1) Primary Prevention (2) Detection (3) Case Management + Implementation; ERIC, Expert Recommendations for Implementing Change; HCAI, Health Care-Associated Infections; LMIC, Low- and Middle-Income Countries; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PRISMA-ScR, Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews
Research in context.
Evidence before this study
Health care-associated infections (HCAI) are a major cause of neonatal morbidity and mortality in low- and middle-income countries (LMIC) and of increasing concern owing to their associated antimicrobial resistance. Infection prevention and care bundles have expanded to neonatal care settings and proven effective in reducing HCAI. Consequently, the creation of a Classification Framework for the care bundles’ components (elements) will provide a basis for a uniform nomenclature for the creation of future care bundles. EMBASE, Pubmed, Global Health, CINAHL, and Web of Science were searched for studies published between January 2001 and July 2020 with the search terms: “neonate”, “care bundles”, “health care-associated infections”, and “low- and middle-income countries”.
Added value of this study
This is the first scoping review synthesising all published literature on neonatal infection prevention and care bundles addressing HCAI in LMIC. From 56 bundles 295 individual elements were identified. A novel 3 + I Classification Framework was created into which these elements were classified, covering: (1) Primary prevention, (2) Detection, (3) Case management + Implementation (3 + I). Almost half the bundle elements were for infection primary prevention, notably targeting central line catheters and mechanical ventilators. Importantly we found almost a total lack of elements aimed at HCAI detection. Case management elements focused on the supportive care of neonates with HCAI were scarce.
Implications of all the available evidence
The 3 + I Classification Framework provided a systematic way to organise the elements of infection prevention and care bundles. Further research is required on infection detection elements in care bundles. We highlight the need for innovations in detection and surveillance systems, especially in LMIC where laboratory services are limited yet the burden of HCAI is highest. This detection gap could be further simplified with advances in point-of-care testing.
Alt-text: Unlabelled box
Introduction
Around the world, an estimated 2.4 million neonates die every year. Almost 80% of these deaths occur in sub-Saharan Africa and Southern Asia.1 The Sustainable Development Goals set by the United Nations include a target to reduce national neonatal death rates to less than 12 per 1000 live births by 2030.2 To deliver this target, a higher coverage and quality of health care is needed for the 30 million newborns requiring hospital care annually.3
The emergence of antimicrobial resistance associated with the widespread but often unreported health care-associated infections (HCAI) is a significant threat to progress for ending preventable neonatal deaths.4,5 In low- and middle-income countries (LMIC) the HCAI incidence in inpatient newborn care units is estimated to be 15.2 to 62.0 per 1000 patient-days; nine times higher than observed in some high-income settings.6 Infection prevention and control interventions need to be implemented into daily neonatal care to reduce neonatal mortality and improve health care quality.
Care bundles are a strategy developed by the Institute for Healthcare Improvement to strengthen the quality of care in adult intensive care units in 2001.7 Each care bundle is a collection of evidence-based practices (called bundle ‘elements’) implemented together to improve patient outcomes. The use of infection prevention and care bundles has rapidly extended to neonatal care settings and proven effective in reducing adverse clinical outcomes, including HCAI.8, 9, 10 To provide consistency in their formulation and implementation for reducing neonatal HCAI in LMIC, a classification system is needed. The creation of a Classification Framework will also provide common and consistent terminology and definitions for the care bundle element categories. These element categories can be used by neonatal health services as potential building blocks for the construction of infection prevention and care bundles, proposing a holistic approach in their design to address the different causes (e.g., contact transmission through health care staff, lack of hand hygiene equipment, or inappropriate use of antibiotics) and stages of infection (e.g., prevention, detection, or control).11, 12, 13 This taxonomy will also highlight evidence gaps for future research to tackle HCAI.
Previous systematic reviews on care bundles in neonatal settings have focused on evaluating their effectiveness in reducing central line- or ventilator-associated infections.8,9 However, no published review has assembled the infection prevention and care bundles addressing neonatal HCAI in LMIC, and there is no existing framework to categorise them and their care bundle elements.
This scoping review aimed to search and synthesise published literature on infection prevention and care bundles addressing neonatal HCAI in LMIC. The objectives were to build a bundle element Classification Framework based on identified care bundles and to quantitatively analyse its content.
Methods
This scoping review was based on the guidance framework for conducting scoping reviews developed by the Joanna Briggs Institute.14 It is reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR).15 The study protocol was registered with the Open Science Framework (Fig. 1 in Appendix).16
Information sources and search strategy
The literature search was performed across the databases EMBASE, Pubmed, Global Health, CINAHL, and Web of Science. The search strategy included English keywords and medical subject headings for four concepts: neonates, care bundles, HCAI, and LMIC (Table 1 in Appendix). In 2001, the Institute for Healthcare Improvement developed the concept of care bundles,7 therefore, searches were limited to studies published from 2001 until July 3rd, 2020 in English, Spanish, and French languages.
Eligibility criteria
The main inclusion criteria were aligned with the PCC mnemonic (Population, Concept, Context), as follows (Table 2 in Appendix): For the first mnemonic term (‘Population’), studies were included if neonates (infant less than 28 days of life) were the target study population. Concerning the second term (‘Concept’), studies were eligible for inclusion if reporting on: (1) care bundles (as defined by the Institute for Healthcare Improvement as: "A small set of evidence-based interventions for a defined patient segment/population and care setting that, when implemented together, will result in significantly better outcomes than when implemented individually")7 and (2) any measures of HCAI disease frequency, exposure effects, or any neonatal outcomes. To meet this second inclusion criteria, care bundles had to incorporate at least two elements related to HCAI prevention, detection, control, or management after birth. Thirdly, included studies were set in inpatient newborn care units (all levels of care) in LMIC (World Bank, 2020) (‘Context’).17
All study designs were included. Exclusion criteria were: studies set outside of newborn care wards, studies with results including infants older than 28 days, studies reporting on guidelines, single interventions, management protocols, conference abstracts, editorials, reviews, research protocols, opinion articles, or publications where the full-text could not be accessed.
Selection of sources of evidence
Duplicates were removed from the identified records. In the first stage, all studies were screened by one reviewer (AMG) by title and abstract. A second reviewer (EJAF), who was blinded to the screening results of the first reviewer, screened a random sample of 20 of these studies, with 100% agreement between reviewers. In the second stage, all full-text articles were assessed for eligibility independently by both reviewers, with 93% agreement on the articles to be included. The disagreements in this stage were resolved by consensus between the two reviewers.
Data charting process
T2he data charting form was piloted by AMG and EJAF on two articles. The following data were extracted by AMG: study characteristics (i.e., first author, year of publication, aim, country of study, study design, level of inpatient newborn care units and terminology found to describe the care bundles). The number and description of bundle elements were extracted independently by both reviewers, blinded to each other's findings. Both reviewers agreed on their identification on 73% of studies. The bundle elements of the rest of the studies were resolved by consensus between reviewers. Within included studies, any bundle elements implemented in settings other than inpatient newborn care units (i.e., labour ward) were not included.
Synthesis of results and methodology to construct the Classification Framework
The synthesis was displayed narratively, including qualitative and quantitative analysis. Qualitative inductive content analysis was carried out to build the Classification Framework for the bundle elements. An inductive approach was used as no previous care bundle element Framework was identified in the literature. It followed the three-step process proposed by Elo and colleagues: preparation, organisation and reporting (Figure 2 in Appendix).18 In the preparation step, care bundle elements (i.e., the single interventions composing a care bundle) were extracted from the bundles, exported, and read multiple times. In the organisation step, each bundle element was labelled with a coding heading to summarise their meaning. Coding headings that shared similar meaning were collated under higher order headings for the development of groups and subgroups. Following this, each group and subgroup was named based on the information it contained and was described narratively to provide a definition. If bundle elements were coded with headings related to implementation strategies, these were grouped after the categories created by Powell and colleagues in the Expert Recommendations for Implementing Change (ERIC) study.19 To further synthesise the results, once the bundle elements were grouped, whole bundles were also categorised according to the groups their elements were classified into. In the reporting step, descriptive and quantitative analyses (frequencies and percentages) of the content of the groups and subgroups of the Classification Framework were performed. This analysis was conducted by AMG and supported by JHC and JEL in the organisation phase if doubts arose concerning bundle element labelling, to reach consensus.
The focus of the review was to explore the breadth of the infection prevention and care bundles present in the literature for neonatal settings in LMIC and to map their elements in a Framework. Therefore, a critical appraisal of the studies was not performed as analysing the outcomes concerning infection prevention and care bundles was outside of the scope of the review.
Statistical analysis
Qualitative analysis was performed to construct the Classification Framework using Microsoft Excel (Microsoft, Redmond WA, USA). Quantitative analysis using frequencies and percentages were carried out for the synthesis of the results. No statistical tests were performed.
Ethics statement
The Research Ethics Committee at the London School of Hygiene and Tropical Medicine assessed this research project as not requiring ethical approval (Reference: 21720).
Role of funding sources
Funding agencies had no contributions to the study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit the paper. These agencies had no access to the dataset of this study. AMG, JHC, EJAF and JEL had access to the study dataset. AMG, JHC and JEL decided to submit the study for publication.
Results
A total of 5288 publications were identified (Figure 1). After duplicate removal, 3619 records remained for screening. After screening these records by title and abstract, 97 publications were selected for full-text screening. 59 of these publications were excluded according to the eligibility criteria, and the remaining 38 articles were selected for inclusion. Six other articles that fulfilled the inclusion criteria were identified through a backward snowballing technique of the 38 articles. Overall, 44 studies were included for analysis (Table 1).
Figure 1.
PRISMA flow diagram of study selection. Abbreviations: n = number of records; HCAI = health care-associated infections.
Table 1.
Characteristics of the 44 included studies.
| Author (Year) | Countryα | Aim of Study | Study Design | Level of INCU | Number of Care Bundles | Number of Bundle Elements | ||
|---|---|---|---|---|---|---|---|---|
| Type 1 - Primary Prevention Bundles | ||||||||
| Arora20 (2019) | India |
Evaluate the impact of management guidelines on neonatal morbidity and mortality of VLBW neonates | Uncontrolled, before-after | III | 3 | CL insertion bundle: 7 CL maintenance bundle: 6 CL hub care bundle: 5 |
||
| Azab37 (2015) | Egypt |
Evaluate the effectiveness of VAP prevention bundle on rates of neonatal VAP | Uncontrolled, before-after | III | 1 | 7 | ||
| Balla21 (2018) β | India |
Reduce neonatal CLABSI rates by 25% in three months and to sustain this over the next nine months | Uncontrolled, before-after | III | 1 | CL removal bundle: 2 | ||
| Hussain33 (2020)γ | Pakistan |
Design a CLABSI prevention package to decrease CLABSI rates | Uncontrolled, before-after | III | 1 | CL maintenance bundle: 5 | ||
| Resende34 (2011) | Brazil |
Reduce CLABSI rates using a care bundle | Uncontrolled, before-after | III | 1 | 5 | ||
| Resende35 (2015) | Brazil |
Evaluate the impact of an evidence-based bundle in LOS incidence rates | Uncontrolled, before-after | III | 1 | 7 | ||
| Rosenthal36 (2013)δ | El Salvador, Mexico, The Philippines, and Tunisia | Evaluate the impact of INICC multidimensional infection control programme to reduce CLABSI | Uncontrolled, before-after | III | 1 | CL: 7 | ||
| Tran38 (2018) | Vietnam |
Evaluate the impact of the EENC on clinical practices, NICU admissions, and adverse newborn outcomes | Uncontrolled, before-after | III | 1 | 8 | ||
| Wang27 (2015) | China |
Evaluate the effectiveness and feasibility of a CL bundle guideline with a standard checklist in the prevention of PICC-related infections in VLBW infants | Uncontrolled, before-after | III | 2 | CL insertion bundle: 5 CL maintenance bundle: 4 |
||
| Type 2 - Detection Bundles | ||||||||
| No detection bundles in the studies | ||||||||
| Type 3 - Case Management Bundles | ||||||||
| Bouallègue-Godet57 (2004) | Tunisia |
Report outbreak of S. enterica serotype Livingstone resistant to extended-spectrum cephalosporins Perform molecular subtyping |
Retrospective, descriptive | III | 1 | 3 | ||
| Indarso58 (2008) | Indonesia |
Report outbreak of S. worthington Report measures to control outbreak |
Retrospective, descriptive | NR | 1 | 5 | ||
| Jeena49 (2001) | South Africa |
Report outbreak of A. anitratus Determine the cause, source, and modes of transmission of the outbreak Report measures to control outbreak |
Retrospective, descriptive | III | 1 | 7 | ||
| Lithgow51 (2009) | Papua New Guinea |
Report outbreak of K. pneumoniae Determine the cause and source of the outbreak Report measures to control outbreak |
Retrospective, descriptive | NR | 1 | 4 | ||
| Moodley12 (2005) | South Africa |
Report outbreak of K. pneumoniae Determine the cause and source of the outbreak |
Retrospective, descriptive | III | 1 | 3 | ||
| Shanmuganathan22 (2004) | India |
Report outbreak of K. pneumoniae | Retrospective, descriptive | III | 1 | 3 | ||
| Type 4 - Implementation Bundles | ||||||||
| Balla21 (2018) β | India |
Reduce neonatal CLABSI rates by 25% in three months and to sustain this over the next nine months | Uncontrolled, before-after | III | 1 | CL insertion bundle: 3 | ||
| Cavicchiolo44 (2016)ε | Mozambique | To assess the effectiveness of interventions in terms of reduction of the neonatal mortality rate | Uncontrolled, before-after | NR | 2 | Structural bundle: 3 Equipment bundle: 5 |
||
| Gilbert48 (2014) | Brazil |
Develop an educational package and evaluate its impact on a range of neonatal outcomes | ITS | III | 1 | 6 | ||
| Gill39 (2009) | The Philippines | Evaluate the effectiveness of a package of infection control interventions | Uncontrolled, before-after | III | 1 | 4 | ||
| Picheansathian45 (2008) | Thailand | Identify the impact of a promotion programme on hand hygiene practices and its effect on nosocomial infection rates | Uncontrolled, before-after | NR | 1 | 6 | ||
| Villegas40 (2014) | Costa Rica |
Determine the BSI rate of a NICU Quantify the impact of preventive measures on the BSI rate |
Uncontrolled, before-after | III | 1 | 2 | ||
| Type 5 - Composite Bundles | ||||||||
| Agarwal25 (2007) | India | Evaluate the impact of simple interventions on neonatal mortality | Uncontrolled, before-after | III | 1 | 10 | ||
| Ahmed52 (2017) | Pakistan | Report outbreak of S. marcescens Determine the cause and source of the outbreak Report interventions to control the outbreak |
Retrospective, descriptive | III | 1 | 7 | ||
| Ávila53 (2011) | Cuba | Report outbreak of S. marcescens Determine cause of the outbreak Report interventions to control outbreak |
Retrospective, descriptive | NR | 1 | 7 | ||
| Balla21 (2018)β | India | Reduce neonatal CLABSI rates by 25% in three months and to sustain this over the next nine months | Uncontrolled, before-after | III | 2 | Main bundle: 4 CL maintenance bundle: 4 |
||
| Calil43 (2001) | Brazil | Evaluate the efficacy of measures to control colonisation and infection by multiresistant bacteria | Uncontrolled, before-after | III | 1 | 3 | ||
| Cavicchiolo44 (2016) ε | Mozambique | To assess the effectiveness of interventions in terms of reduction of the neonatal mortality rate | Uncontrolled, before-after | NR | 1 | Clinical bundle: 10 | ||
| Cetin61 (2015) | Turkey | Report outbreak of S. maltophilia Determine the cause and source of the outbreak Determine risk factors for infection Report outbreak management |
Retrospective, analytical (case-control) |
III | 1 | 5 | ||
| Chakrabarti24 (2001) | India | Report outbreak of P. anomala Determine the cause, source, and modes of transmission of the outbreak |
Retrospective, analytical (case-control) |
III | 1 | 4 | ||
| Chen26 (2015) | China | Evaluate the efficacy of different measures in preventing ICI in preterm infants < 33 weeks | Uncontrolled, before-after | III | 1 | 6 | ||
| Grey50 (2012) | Guatemala | Report outbreak ofK. pneumoniae Determine the cause, source, and modes of transmission of the outbreak Report measures to control outbreak |
Retrospective, descriptive | III | 1 | 4 | ||
| Hosoglu62 (2012) | Turkey | Report outbreak of A. baumanii Identify risk factors for A. baumanii Report measures to control outbreak |
Retrospective, analytical (case-control) |
III | 1 | 8 | ||
| Huang30 (2019) | China | Evaluate the efficacy of a bundle intervention on health care-associated MRSA infection | Uncontrolled, before-after | III | 1 | 6 | ||
| Hussain33 (2020)γ | Pakistan | Design a CLABSI prevention package to decrease CLABSI rates | Uncontrolled, before-after | III | 3 | Main bundle: 5 CL insertion bundle: 7 Prevention of fungal infections bundle: 3 |
||
| Irfan55 (2019) | Pakistan | Report outbreak of MRSA Report measures to control outbreak |
Retrospective, descriptive | II | 1 | 11 | ||
| Kulali41 (2019) | Turkey | Evaluate the effectiveness of bundled applications in the prevention of UVC-associated bloodstream infections | Uncontrolled, before-after | III | 1 | 7 | ||
| Landre-Peigne32 (2011) | Senegal | Evaluate the impact of a programme on the incidence of nosocomial bloodstream infections, neonatal mortality rates, the prevalence of drug-resistant strains and antimicrobial use | Uncontrolled, before-after | II | 1 | 4 | ||
| Mais42 (2015) | Lebanon | Evaluate the impact of quality improvement bundles on CLABSI rates | Uncontrolled, before-after | III | 1 | 3 | ||
| Miranda-Novales60 (2003) | Mexico | Report outbreak of S. marcescens Describe typing results using rapid pulsed-field gel electrophoresis and infection control measures |
Retrospective, descriptive | III | 1 | 4 | ||
| Moore56 (2005) | Egypt | Report outbreak of K. pneumoniae Determine the cause and source of the outbreak Determine effectiveness of control measures |
Retrospective, descriptive Uncontrolled, before-after |
III | 1 | 3 | ||
| Mshana59 (2011) | Tanzania | Report outbreak of a novel Enterobacter sp. Perform molecular subtyping |
Retrospective, descriptive | NR | 1 | 4 | ||
| Mwananyanda47 (2019) | Zambia | Evaluate the impact of an infection prevention control bundle on hospital-associated BSI and mortality | ITS | III | 1 | 5 | ||
| Narayan54 (2009) | Fiji | Report outbreak of E. aerogenes Determine the cause, source, and mode of transmission of the outbreak |
Retrospective, descriptive | III | 1 | 10 | ||
| Qi31 (2018) | China | Report outbreak of C. parapsilosis sensu stricto Determine the cause and source of the outbreak Report measures to control outbreak |
Retrospective, descriptive | III | 1 | 6 | ||
| Rahim46 (2009) | Malaysia | Implement education-based interventions to contribute to a reduction in nosocomial infections | Uncontrolled, before-after | III | 1 | 3 | ||
| Rosenthal23 (2012) | Argentina, Colombia, India, Mexico, Morocco, Peru, Philippines, El Salvador, Tunisia, Turkey | Evaluate the impact of the INICC multidimensional infection control programme on the reduction of VAP | Uncontrolled, before-after | III | 1 | 11 | ||
| Rosenthal36 (2013)δ | El Salvador, Mexico, The Philippines, and Tunisia | Evaluate the impact of INICC multidimensional infection control programme to reduce CLABSI | Uncontrolled, before-after | III | 1 | Main bundle: 6 | ||
| Zhou29 (2013) | China | Evaluate the effectiveness of an intervention programme in decreasing neonatal VAP rate, neonatal mortality and the prevalence of drug-resistant strains | Uncontrolled, before-after | III | 1 | 8 | ||
| Zhou28 (2015) | China | Characterise CLABSI in a Chinese NICU Evaluate the impact of a multifaceted EPIQ program on CLABSI reduction |
Uncontrolled, before-after | III | 1 | 5 | ||
Low- and middle-income countries where the included studies were performed as per World Bank definitions (2020).
Balla et al.21 contains four bundles in the following groups: two primary prevention, one implementation, and one composite.
Hussain et al.33 contains four bundles in the following groups: one primary prevention and three composite.
Rosenthal et al.36 contains two bundles in the following groups: one primary prevention and one composite.
Cavicchiolo et al.44 contains three bundles in the following groups: two implementation and one composite.
Abbreviations- BSI = bloodstream infections; CL = central line; CLABSI = central line-associated bloodstream infections; EENC = Early Essential Newborn Care; EPIQ = evidence-based practice for improving quality; ICI = invasive candida infections; INICC = International Nosocomial Infection Control Consortium; INCU = inpatient neonatal care units; ITS = interrupted time series; LOS = late-onset sepsis; MRSA = methicillin-resistant S. aureus; NICU = neonatal intensive care unit; NR = not reported; PICC = peripherally inserted central catheter; UVC = umbilical venous catheter; VAP = ventilator-associated pneumonia; VLBW = very low birth weight.
The studies reported data from 55 LMIC, with India and China being most commonly represented (Table 1).[20], 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 Regarding study design, 24 (55%) were uncontrolled before-after,20,21,23,25, 26, 27, 28, 29, 30,32, [33], 34, 35, 36, 37, 38, 39, 40, 41, 36, 43, 44, 45, 46 and two (5%) were interrupted time series.47,48 The rest were retrospective outbreak investigation studies.12,22,24,31,49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 36 (82%) and two (5%) studies were performed in tertiary or secondary inpatient neonatal care units, respectively. Six (14%) studies did not report their care level setting (Table 1).44,45,51,53,58,59
A total of 56 care bundles were identified (Table 1). Three studies contained various care bundles inside another main care bundle.21,33,36 Three other studies described more than one bundle.20,27,44 The rest of the studies (39, 89%) described one bundle. The most common terms used in the publications to name the care bundles were ‘measures’ (13, 30%),24,26,31,40,43,49,50,53,55,57, 58, 59,61 followed by ‘bundles’ (nine, 21%).20,23,27,30,34,35,37,41,47 (Figure 2).
Figure 2.
Word cloud with the terminology used to name the care bundles. The world cloud visually represents the names used to describe care bundles depicted in different sizes based on the frequency of their use in the 44 included studies: the higher the frequency of a name, the bigger its appearance in the cloud. Frequencies of the names: Measures = 13 (30%); Bundle = 9 (21%); Quality Improvement = 5 (11%); Programme = 4 (9%); Package = 3 (7%); Multifaceted Intervention = 2 (5%); Precautions = 1 (2%); Strategies = 1 (2%); Practices = 1 (2%); Options = 1 (2%); Multidimensional Approach = 1 (2%); Response = 1 (2%); Intervention = 1 (2%); Interventions = 1 (2%).
The 3 + I Classification Framework for care bundle elements
Qualitative content analysis to construct the Classification Framework
The four main element groups of the 3 + I Classification Framework were created according to the four main themes (coding headings) identified: (1) Primary prevention, (2) Detection (i.e., secondary prevention elements), (3) Case management (i.e., tertiary prevention elements), and Implementation (3 + I). A description of the main element themes and subthemes identified is represented in Table 2. A summary of the 3 + I Classification Framework is shown in Table 3 (for the complete taxonomy, see Table 3 in Appendix).
Table 2.
Qualitative data summary findings of the 3 + I Classification Framework.
| a. Groups identified | |
| Name | Description |
| Primary prevention | Elements aiming to avoid health care-associated infections in neonatal care units (e.g., promotion of kangaroo mother care or breastfeeding, reinforcement of the staff's hand hygiene). |
| Detection | Elements focused on secondary prevention such as the screening and surveillance of health care-associated infections in infected newborns admitted in inpatient neonatal care units (e.g., implementation and reinforcement of infection surveillance programmes). |
| Case management | Elements focused on tertiary prevention. They describe care of infected neonates or interventions to control the propagation of health care-associated infections in the neonatal units (e.g., cohorting of neonates, changes in antibiotic policy and stewardship, improvement to environmental and equipment disinfection protocols). |
| Implementation | Elements directed towards the methods of enhancing the adoption, implementation, or sustainability of interventions (e.g., provision of single use fluid vials or alcohol-based hand rub, conduct educational meetings on infection prevention and control measures, establishing audit and feedback mechanisms). |
| b. Subgroups identified | |
| Name | Description |
| Neonate | Elements aimed directly at the neonate. |
| Staff | Elements aimed at the health care staff. |
| Caretaker | Elements focused on the caretakers of the admitted newborn patients. |
| Environment | Elements directed towards the surroundings of the neonate in the inpatient neonatal care units and its organisation. |
| Device | Elements tackling nosocomial infections acquired through medical equipment. |
| Screening | Elements that encompass the detection of disease outbreaks and their risk factors. |
| Epidemiological surveillance | Bundle elements focused on the collection, analysis, and monitoring of data on health care-associated infections in neonatal care units. |
| Antibiotic prescription | Elements aimed at implementing or improving antibiotic policy and stewardship of inpatient neonatal care settings. |
| Outbreak control | Elements focused on controlling infectious outbreaks detected in the inpatient neonatal care units. |
| Audit and feedback | Elements focused on collecting clinical performance data to share with neonatal health care staff and managers to monitor, evaluate, and modify their behaviour*. |
| Change physical structure and equipment | Bundle elements that evaluate the existing set-up of the neonatal wards and adapt their physical structure and/or equipment to improve the quality of care*. |
| Conduct educational meetings | Bundle elements that teach all the interested groups about the health care intervention implemented in the neonatal ward through meetings*. |
| Create or change credentialing and/or licensure standards | Elements aiming at creating or changing a system that certifies staff's skills in the health care intervention and/or grants the health care system or unit with a license to implement an intervention*. |
| Create new clinical teams | Interventions that change health care staff members to ensure that the health care intervention is delivered by incorporating new skills and work profiles to the team*. |
| Develop educational materials | Elements focused on the creation of unit protocols, guidelines, tools, manuals, or other materials to improve staff's training and understanding of the health care innovation*. |
| Organise clinician implementation team meetings | Bundle elements that establish meetings for the clinicians responsible for implementing the health care intervention to ensure a time for reflection on the implementation process and for sharing lessons learnt*. |
| Recruit, designate and train for leadership | Elements that enrol, assign, and train the leaders of the clinical innovation in the neonatal units*. |
| Remind clinicians | Elements directed at creating reminder systems to promote the use of or provide information on a health care intervention in the neonatal wards*. |
| Revise professional roles | Bundle elements aiming at reviewing and changing the job profiles and responsibilities of the neonatal health care staff*. |
Table describing the different groups (a) and subgroups (b) identified after performing the qualitative data analysis to construct the Classification Framework for care bundle elements. If bundle elements were coded with headings related to implementation strategies, these were grouped after the categories created by Powell and colleagues in the Expert Recommendations for Implementing Change (ERIC) study.19Legend: *Adaptation of the category definitions proposed by Powell and colleagues in the Expert Recommendations for Implementing Change (ERIC) study to the neonatal care units.19
Table 3.
Frequency of the 295 bundle elements according to the 3 + I Classification Framework.
| Element classification | Bundle classification |
Total number of elements per row (%)β | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type 1 – Primary Prevention | Type 2 -Detectionα | Type 3 - Case Management | Type 4 -Implementation | Type 5 - Composite | |||||
| Primary Prevention | |||||||||
| 1. Neonate | 1.1. Feeding | 1 | ·· | ·· | ·· | 1 | 2 (1·6) | ||
| 1.2. Skin-to-skin contact | 1 | ·· | ·· | ·· | ·· | 1 (0·8) | |||
| 1.3. Skin disinfection | 4 | ·· | ·· | ·· | 3 | 7 (5·5) | |||
| 1.4. Drug prescription | 1 | ·· | ·· | ·· | 6 | 7 (5·5) | |||
| 1.5. Isolation | ·· | ·· | ·· | ·· | 1 | 1 (0·8) | |||
| 1.6. Reduction of handling | ·· | ·· | ·· | ·· | 1 | 1 (0·8) | |||
| 2. Staff | 2.1. HH | 10 | ·· | ·· | ·· | 8 | 18 (14·1) | ||
| 2.2. Use of protocols/policies | 1 | ·· | ·· | ·· | 1 | 2 (1·6) | |||
| 2.3. Organisation | ·· | ·· | ·· | ·· | 2 | 2 (1·6) | |||
| 2.4. Contact barrier precautions | 4 | ·· | ·· | ·· | 2 | 6 (4·7) | |||
| 3. Caretaker | 3.1. Empower mothers in routine care | ·· | ·· | ·· | ·· | 1 | 1 (0·8) | ||
| 4. Environment | 4.1. Areas & equipment disinfection | ·· | ·· | ·· | ·· | 3 | 3 (2·3) | ||
| 4.2. Waste disposal | ·· | ·· | ·· | ·· | 1 | 1 (0·8) | |||
| 4.3. General unit organisation | 1 | ·· | ·· | ·· | 4 | 5 (3·9) | |||
| 5. Deviceγ | 5.1. Catheter | 36 | ·· | ·· | ·· | 19 | 55 (42·9) | ||
| 5.2. Ventilator | 5 | ·· | ·· | ·· | 11 | 16 (12·5) | |||
| Total number of primary prevention elements | 64 | ·· | ·· | ·· | 64 | 128 (100) | |||
| Detection (Secondary Prevention) | |||||||||
| 1. Screening | 1.1. New screening programme | ·· | ·· | ·· | ·· | 2 | 2 (16·7) | ||
| 2. Epidemiological surveillance | 2.1. Implementation of new infection surveillance programme | ·· | ·· | ·· | ·· | 3 | 3 (25·0) | ||
| 2.2. Enhance existing surveillance programmes | ·· | ·· | ·· | ·· | 7 | 7 (58·3) | |||
| Total number of detection elements | ·· | ·· | ·· | ·· | 12 | 12 (100) | |||
| Case Management (Tertiary pPrevention) | |||||||||
| 1. Antibiotic prescription | 1.1. Antibiotic policy & stewardship | ·· | ·· | 1 | ·· | 8 | 9 (12·0) | ||
| 2. Outbreak control | 2.1. Neonate | 2.1.1 Skin disinfection | ·· | ·· | 1 | ·· | 1 | 2 (2·7) | |
| 2.1.2 Feeding | ·· | ·· | 1 | ·· | ·· | 1 (1·3) | |||
| 2.1.3 Drug prescription | ·· | ·· | 1 | ·· | ·· | 1 (1·3) | |||
| 2.1.4 Isolation | ·· | ·· | 2 | ·· | 3 | 5 (6·7) | |||
| 2.2. Staff | 2.2.1. HH | ·· | ·· | 4 | ·· | 4 | 8 (10·7) | ||
| 2.2.2. Use of protocols/policies | ·· | ·· | ·· | ·· | 4 | 4 (5·3) | |||
| 2.2.3. Organisation | ·· | ·· | ·· | ·· | 1 | 1 (1·3) | |||
| 2.2.4. Contact precautions | ·· | ·· | 3 | ·· | 4 | 7 (9·3) | |||
| 2.2.5. Treatment of staff | ·· | ·· | ·· | ·· | 1 | 1 (1·3) | |||
| 2.3. Environment | 2.3.1. Areas & equipment disinfection | ·· | ·· | 4 | ·· | 7 | 11 (14·7) | ||
| 2.3.2. General unit organisation | ·· | ·· | 7 | ·· | 17 | 24 (32·0) | |||
| 2.4. Device | 2.4.1. Catheter | ·· | ·· | ·· | ·· | 1 | 1 (1·3) | ||
| Total number of case management elements | ·· | ·· | 24 | ·· | 51 | 75 (100) | |||
| Implementation | |||||||||
| 1. Audit & feedback | ·· | ·· | ·· | 4 | 5 | 9 (11·3) | |||
| 2. Change physical structure & equipment | ·· | ·· | ·· | 12 | 8 | 20 (25·0) | |||
| 3. Conduct educational meetings | ·· | ·· | ·· | 10 | 25 | 35 (43·8) | |||
| 4. Create/change credentialing and/or licensure standards | ·· | ·· | ·· | 2 | 3 | 5 (6·3) | |||
| 5. Create new clinical teams | ·· | ·· | ·· | ·· | 1 | 1 (1·3) | |||
| 6. Develop educational materials | ·· | ·· | ·· | ·· | 3 | 3 (3·8) | |||
| 7. Organise clinician implementation team meetings | ·· | ·· | ·· | ·· | 1 | 1 (1·3) | |||
| 8. Recruit, designate & train for leadership | ·· | ·· | ·· | ·· | 1 | 1 (1·3) | |||
| 9. Remind clinicians | ·· | ·· | ·· | 1 | 1 | 2 (2·5) | |||
| 10. Revise professional roles | ·· | ·· | ·· | ·· | 3 | 3 (3·8) | |||
| Total of implementation elements | ·· | ·· | ·· | 29 | 51 | 80 (100) | |||
| Total number of elements per bundle group | 64 | ·· | 24 | 29 | 178 | 295 (100)δ | |||
Legend: α No detection bundles in the studies. β Percentages are calculated using the total number of elements for each group as the denominator. γ Two devices were the target of all the bundle elements found in the literature: central line catheters and mechanical ventilators. No bundle elements were found for other medical devices. δ 305 bundle elements were identified in the included studies. However, only 295 of them were coded into the 3 + I Classification Framework because two elements could not be categorised due to a lack of detail to interpret their meaning and eight other elements were implemented exclusively in labour wards and not in neonatal wards (and therefore excluded). (··) is a zero value.
Abbreviations: HH = hand hygiene.
The primary prevention group was divided into five subgroups: neonate, staff, caretaker, environment, and device elements (Table 3). Two subgroups were found in the detection group: screening and epidemiological surveillance. Two subgroup themes were recognised in the case management group: antibiotic prescription and outbreak control initiatives. The outbreak control initiatives were further categorised using the same labels as those used in the primary prevention subgroups. Lastly, the implementation bundle elements found were grouped using ten of the 73 strategies compiled in the ERIC project (Table 4 in Appendix).19
After the bundle elements were categorised into the groups of the 3 + I Classification Framework, whole bundles were classified in different types according to the four element groups identified with an additional composite type for bundles containing a mixture of elements. Hence, there are a total of five bundle types: primary prevention (i.e. Type 1), detection (i.e. Type 2), case management (i.e. Type 3), implementation (i.e. Type 4), and composite (i.e. Type 5) (Table 3).
Quantitative analysis of the 3 + I Classification Framework
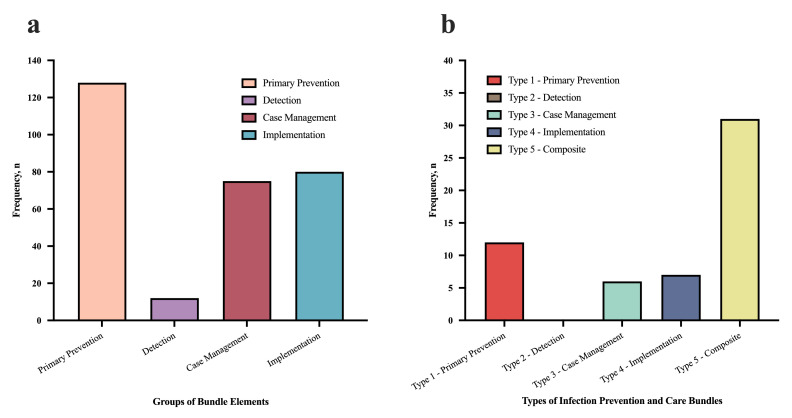
Across all the extracted publications, 305 bundle elements were identified. 295 elements were coded into the 3 + I Classification Framework (Table 3). Two elements from two bundles ("Control of risk factors" and "Taking meticulous care during invasive procedures") could not be categorised due to lack of detail to interpret their meaning.26,49 Two other bundles contained four bundle elements each implemented exclusively in labour wards, which were also excluded.38,44 Overall, the most common bundle elements detected were in the primary prevention group (128, 43%). 71(55%) of these primary prevention elements focused on advanced devices (i.e., central line catheters or mechanical ventilators). The detection element group had the least number of bundle elements within it (12, 4%). (Figure 3a).
Figure 3.
Frequency of the groups of bundle elements (3a) and frequency of the types of infection prevention and care bundles (3b). Colour legend: Figure 3a: Salmon = primary prevention; Violet = detection; Burgundy = case management; Blue = implementation. Figure 3b: Red = Type 1 – primary prevention; Grey = Type 2 – detection; Green = Type 3 – case management; Dark blue = Type 4 – implementation; Yellow = Type 5 – composite. After the creation of the four groups of the 3 + I Classification Framework using the bundle elements (i.e., prevention, detection, case management and implementation, represented in Figure 3a with their frequencies), whole bundles were also categorised into groups, according to the types of elements each one was made of (e.g., if one bundle contained four primary prevention elements, the whole bundle was categorised in primary prevention -Type 1- bundles. If one bundle contained a mixture of prevention, detection, case management, or implementation elements then the whole bundle was categorised into the composite -Type 5- group bundle). These are represented in Figure 3b.
12 (21%) of the bundles identified were classified as Type 1 bundles, six (11%) were Type 3 bundles, seven (13%) were Type 4 bundles, and 31 (55%) were Type 5. There were no bundles categorised as Type 2 (detection) (Figure 3b).
Primary prevention elements
A total of 128 (43%) elements were categorised as primary prevention (Table 3). The most frequent were device interventions (71, 55%), focused on central line catheters (55, 43%),20,21,27,28,[33], 34, 35, 36,41,42 and mechanical ventilators.23,29,37 No other elements directed to other devices were detected. 18 (14%) of the primary prevention elements were aimed at improving staff's hand hygiene. Only one element was found to promote breastfeeding,38 kangaroo mother care,38 or improve staff to patient ratios.44 64 (50%) of the primary prevention elements identified were contained in the Type 1 bundles (primary prevention).
Detection (secondary prevention) elements
A total of 12 (4%) elements were aimed at detection (Table 3). The most frequent interventions were to enhance existing surveillance programmes (7, 58%)43,52,53,59,60 and to implement new ones (3, 25%).36,62,63 Two elements were focused on introducing new screening programmes.30,46 No bundles were exclusively made up of detection elements. Subsequently, bundles containing some detection elements were allocated to Type 5 (composite).
Case management (tertiary prevention) elements
75 (25%) elements were categorised under case management (Table 3). These were focused on outbreak control (66, 88%) or on improving antibiotic policy and stewardship in the neonatal units (9, 12%). In the outbreak control subgroup, 35 (47%) elements were aimed at the environment of the neonatal units, such as disinfection of areas and equipment or improving the organisation of the unit (e.g., use of a temporary ward, cohorting, or overcrowding reduction) (Table 3 in Appendix).12,22,24,30,31,49, 50, 51, 52, 53, 54, 55, 56, 57,59, 60, 61, 62 Additionally, 21 (28%) elements targeted the unit staff such as improving their hand hygiene or their use of protocols and policies. 9 (12%) elements were directed to the neonate such as their isolation, improving neonatal nutrition or local skin disinfection for venipuncture.22,30,49,50,53,55,57,58 There were no infection prevention and care bundles directly focused on the management and treatment of neonatal HCAI (e.g. no sepsis bundles) (not shown).
Implementation elements
80 (27%) of the elements identified being part of infection prevention and care bundles were implementation strategies (Table 3). The most frequently used was conduct educational meetings (35, 44%). 20 (25%) of the implementation elements found were aimed to change physical structure & equipment of the neonatal units including the provision of new and basic equipment and drug supplies to control outbreaks (e.g., provision of small medication bottles, single use fluid vials, or alcohol-based hand rub) (Table 3 in Appendix).29,31,39,44,45,47,53,54,56,61
Discussion
This is the first scoping review to synthesise the published literature on infection prevention and care bundles addressing neonatal HCAI adverse outcomes in LMIC. 44 papers were found reporting 56 care bundles and categorising 295 elements. The 3 + I Classification Framework created in this study was useful in synthesising these into four mechanistic pathways. The majority of the elements were primary prevention interventions mostly focused on central line catheters and mechanical ventilators. There was a paucity of bundles and elements aimed at HCAI detection (4% of 295 elements). In addition, case management elements focused on the supportive care of neonates with HCAI were scarce. Although the United Nations targets the improvement of primary and secondary health care units for small and sick newborns to survive and thrive,3 only two (5%) of the studies were performed in secondary neonatal care units.
The absence of detection bundles and the fact that only a small number of detection elements were identified in the literature could support the evidence of a wide gap for HCAI detection and surveillance systems in LMIC hospitals reported in other studies.6,64 In LMIC, access to and quality of laboratory services are inconsistent and resource-constrained.65,66 Microbiological investigations such as blood cultures (a specimen sample prioritised by the World Health Organisation to launch routine epidemiological surveillance) are not usually performed as standard care.67,68,69 Health system factors that exacerbate this gap may include understaffing, uncovered training needs, and barriers to strengthening laboratory infrastructure and supply chains.70 Epidemiological detection and surveillance interventions are major milestones towards the reduction of HCAI attributed mortality and should be regarded as essential elements within the design of future care bundles.66 This gap is an opportunity to optimise the resources available and foster implementation research in the hospital services involved in HCAI detection and surveillance.71 For instance, research and development efforts focused on rapidpoint-of-care testing that can be performed in neonatal units could address these challenges in settings with limited laboratory capacity.72
Importantly, reported bundles in the literature consist mainly of primary prevention elements. However, these tended to focus on central line and mechanical ventilator devices which are exclusively used in neonatal intensive care units. No elements were orientated towards medical devices that reduce the most common causes of neonatal deaths in the levels of health care where most small and sick newborns are managed in LMIC (e.g., self-inflating bags, suctioning equipment, or incubators).3 These devices are also critical potential vectors of transmission of HCAI.73 Surprisingly, there was little or no mention of neonate subgroup elements in the primary prevention group such as breastfeeding or kangaroo mother care. These elements are simple, low-cost, evidence-based interventions that are proven to reduce HCAI in LMIC and are recommended by the World Health Organisation for infant care.74, 75, 76, 77 These elements can be easily introduced in all neonatal unit levels and income settings and could be the focus of future research efforts on bundles targeting HCAI primary prevention.
There were no case management bundles directly focused on the management and treatment of neonatal HCAI (such as sepsis bundles), and only a small number contained elements that addressed this. Possible reasons for this gap could be that they are included in the management and treatment guidelines or protocols as single interventions (which were outside of the scope of this review), or these might have been already established in the neonatal wards when the care bundles of the studies were designed.
The implementation elements aimed at changing physical structure & equipment are evidence that neonatal units in LMIC still lack access to essential equipment to prevent HCAI (e.g., availability of soap, sinks or smaller volume medication and fluid supplies to allow for their disposal in less than 24 h and avoid their reuse), as reported in other studies.11 These basic supplies need to be guaranteed in hospital settings to provide a safe care environment before scaling up the level of health care in these units. In addition, implementation elements are present in many of the identified bundles, reflecting that there is crossover in the reporting of bundled interventions and the techniques used to strengthen their implementation. This has also been noted in bundles used in adult health care.78 Although some implementation strategies can be considered as bundle elements (e.g. conducting educational meetings), a separate implementation reporting system would help to delineate the two, so that interventions may be reliably replicated.79
Most bundles were implemented in tertiary care neonatal units that provide resource-intensive support to a highly selected, small, and sick infant population. Similar to other reviews of studies in LMIC, we found secondary level units to be underrepresented.80,81 While the risk of HCAI in small and sick newborns is higher compared to term and sick neonates, there is under appreciation of the threat of HCAI in the rapidly expanding primary and secondary level care provision for neonates in LMIC.
This review presents evidence from a comprehensive search in five electronic databases and incorporated a snowball technique to identify other possible publications for inclusion. However, this review has limitations. First, the inclusion of clinical guidelines, management protocols and conference abstracts in the eligibility criteria could have yielded more infection prevention and care bundles. Second, laboratory settings were not included in the search strategy together with neonatal units. This may have reduced the identification of Type 2 (detection) bundles and more detection elements implemented in hospital laboratory services and therefore narrowed the holistic approach of the review. Nevertheless, key steps in the detection and surveillance pathways also occur in neonatal units, such as the performance of microbiologic tests or collection of clinical evidence from patients’ charts. To obtain an equilibrium between the breadth of the scoping review, its feasibility, and the time available for its completion, these were excluded from the search strategy. This balancing challenge is also acknowledged in other scoping reviews.82,83 Third, despite 100% of the full texts were independently reviewed by two authors in the second screening stage, only a random sample of 20 abstracts were double screened independently in the first stage. Although there was 100% agreement on this screening stage, there may have been residual selection bias. Fourth, 15 different terms were used to refer to care bundles in the included studies, demonstrating the need for consistent terminology to describe these types of interventions. In addition, the definition of a care bundle proposed by the Institute for Healthcare Improvement is very broad.7 This increased the difficulty in extracting well-defined bundle elements. To mitigate information bias, two reviewers conducted the bundle element extraction process. Despite this strategy, both reviewers agreed on their identification on 73% of studies. Finally, although the preparation stage in the qualitative analysis was supported by two researchers, only one of them performed the organisation stage to construct the Classification Framework. This could have been a source for misclassification bias.
The purpose of this review was to identify the range of potential bundles and bundle elements published in the literature to reduce HCAI and not to capture single interventions. Therefore, our individual findings for the four element groups identified (i.e., primary prevention, detection, case management and implementation) may not be generalisable beyond published studies on infection prevention and care bundles. Nevertheless, the 3 + I Classification Framework could help other researchers or health practitioners from LMIC in the design and evaluation of infection prevention and care bundles, regardless of the health care level. The elements can serve as potential “ingredients” for the construction of future multifaceted, holistic infection prevention and care bundles.
HCAI are a major threat to neonatal survival in LMIC and in urgent need of more evidence-based strategies for their reduction, especially to address the epidemic of antimicrobial resistance. Infection prevention and care bundles may be a promising approach contributing to the reduction of these infections. Using a scoping review methodology, this review synthesises the published literature on infection prevention and care bundles for inpatient newborn care units in LMIC. Our novel 3 + I Classification Framework for the care bundle elements could provide a useful basis for designing subsequent care bundles in all hospital settings for health care practice. Future research efforts should be directed towards the inclusion of infection detection elements in infection prevention and care bundles, particularly focused on point-of-care testing and surveillance. This target will be critical in resource-limited settings, where detection and surveillance systems are inadequate, yet the burden of infection is highest.
Declaration of interests
We declare no competing interests.
Acknowledgments
Funding
This work was made possible in part by the John D. and Catherine T. MacArthur Foundation, the Bill & Melinda Gates Foundation, ELMA Philanthropies, The Children's Investment Fund Foundation UK, The Lemelson Foundation, and the Ting Tsung and Wei Fong Chao Foundation under agreements to William Marsh Rice University. The project leading to these results has also received the support of a fellowship from the “la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/EU19/11710040. EJAF is an Academic Clinical Fellow whose salary is funded by the UK National Institute for Health Research (NIHR). NES receives a Research Training Program Scholarship (Australian Commonwealth Government).
Contributors
AMG planned the study, designed and ran the search strategy, extracted data, performed the data analysis and wrote the first draft of the manuscript. JEL and JHC had the idea for the review, supervised the synthesis and critically revised the manuscript. AMG, JEL and JHC co-designed the 3 + I framework. AMG and EJAF performed the screening process of the articles, as described. EJAF was the second reviewer for data extraction of the bundle elements. All authors critically reviewed and provided inputs to the final version of the manuscript for publication. All the NEST360 Infection Prevention, Detection and Care Collaborative Group participated in the interpretation and reviewed the manuscript.
Data sharing agreement
No individual patient data are included in this study. The study protocol is available in the Open Science Framework (DOI osf.io/duw64). Search strategy and results of included papers are presented in the manuscript and are available at the corresponding author upon request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101259.
Contributor Information
James H. Cross, Email: james.cross@lshtm.ac.uk.
Joy E. Lawn, Email: joy.lawn@lshtm.ac.uk.
Appendix. Supplementary materials
References
- 1.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME) United Nations Children's Fund; New York: 2020. Levels & Trends in Child Mortality: Report 2020, Estimates Developed By the United Nations Inter-Agency Group for Child Mortality Estimation. [Google Scholar]
- 2.United Nations General Assembly . United Nations; 2015. Transforming Our world: the 2030 Agenda for Sustainable Development. [Google Scholar]
- 3.World Health Organisation . World Health Organisation; Geneva: 2019. Survive and Thrive: Transforming Care for Every Small and Sick Newborn. [Google Scholar]
- 4.Laxminarayan R., Bhutta Z. Antimicrobial resistance—a threat to neonate survival. Lancet Glob Health. 2016;4(10):e676–e6e7. doi: 10.1016/S2214-109X(16)30221-2. [DOI] [PubMed] [Google Scholar]
- 5.Sands K., Carvalho M.J., Portal E., Thomson K., Dyer C. Characterisation of antimicrobial-resistant Gram-negative bacteria that cause neonatal sepsis in seven low- and middle-income countries. Nat Microbiol. 2021;6(4):512–523. doi: 10.1038/s41564-021-00870-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allegranzi B., Nejad S.B., Combescure C., et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet N Am Ed. 2011;377(9761):228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 7.Resar R., Griffin F.A., Haraden C., Nolan T.W. Institute for Healthcare Improvement; Cambridge, Massachusetts: 2012. Using Care Bundles to Improve Health Care Quality. [Google Scholar]
- 8.Payne V., Hall M., Prieto J., Johnson M. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2018;103(5):F422–F4F9. doi: 10.1136/archdischild-2017-313362. [DOI] [PubMed] [Google Scholar]
- 9.Niedzwiecka T., Patton D., Walsh S., Moore Z., O'Connor T., Nugent L. What are the effects of care bundles on the incidence of ventilator-associated pneumonia in paediatric and neonatal intensive care units? A systematic review. J Spec Pediatr Nurs. 2019;24(4):e12264. doi: 10.1111/jspn.12264. [DOI] [PubMed] [Google Scholar]
- 10.Schlapbach L.J., Javouhey E., Jansen N.J.G. Paediatric sepsis: old wine in new bottles? Intensive Care Med. 2017;43(11):1686–1689. doi: 10.1007/s00134-017-4800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okomo U., Senghore M., Darboe S., et al. Investigation of sequential outbreaks of Burkholderia cepacia and multidrug-resistant extended spectrum β-lactamase producing Klebsiella species in a West African tertiary hospital neonatal unit: a retrospective genomic analysis. Lancet Microbe. 2020;1(3):e119–ee29. doi: 10.1016/S2666-5247(20)30061-6. [DOI] [PubMed] [Google Scholar]
- 12.Moodley P., Coovadia Y.M., Sturm A.W. Intravenous glucose preparation as the source of an outbreak of extended spectrum lactamase producing Klebsiella pneumoniae infections in the neonatal unit of a regional hospital in KwaZulu Natal. S Afr Med J. 2005;95(11):861–864. [PubMed] [Google Scholar]
- 13.Huynh B.T., Padget M., Garin B., et al. Burden of bacterial resistance among neonatal infections in low income countries: how convincing is the epidemiological evidence? BMC Infect Dis. 2015;15:127. doi: 10.1186/s12879-015-0843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aromataris E., Munn Z. Joanna Briggs Institute; 2020. JBI Reviewer's Manual. [Google Scholar]
- 15.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7) doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 16.Molina García A., Cross JH, Fitchett EJA, et al. Infection prevention and care bundles addressing hospital-acquired infections in neonatal care in low-middle income countries: a scoping review protocol. Open Science Framework. 2021 doi: 10.1016/j.eclinm.2021.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Bank. Low and middle income countries list. 2019. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed 01 April 2020).
- 18.Elo S., Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 19.Powell B.J., Waltz T.J., Chinman M.J., et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora S., Yadav P., Bajaj H., et al. Improving clinical outcomes of very low birth weight infants: implementation of standardized management guidelines in tertiary care hospital in Haryana. Int J Pediatr Adolesc Med. 2019;7(4):174–180. doi: 10.1016/j.ijpam.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balla K.C., Rao S.P.N., Arul C., et al. Decreasing central line-associated bloodstream infections through quality improvement initiative. Indian Pediatr. 2018;55(9):753–756. [PubMed] [Google Scholar]
- 22.Shanmuganathan C., Ananthakrishnan A., Jayakeerthi S.R., et al. Learning from an outbreak: ESBL - the essential points. Indian J Med Microbiol. 2004;22(4):255–257. [PubMed] [Google Scholar]
- 23.Rosenthal V.D., Rodriguez-Calderon M.E., Rodriguez-Ferrer M., et al. Findings of the international nosocomial infection control consortium (INICC), Part II: impact of a multidimensional strategy to reduce ventilator-associated pneumonia in neonatal intensive care units in 10 developing countries. Infect Control Hosp Epidemiol. 2012;33(7):704–710. doi: 10.1086/666342. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti A., Singh K., Narang A., et al. Outbreak of Pichia anomala infection in the pediatric service of a tertiary-care center in Northern India. J Clin Microbiol. 2001;39(5):1702–1706. doi: 10.1128/JCM.39.5.1702-1706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal R., Agarwal K., Acharya U., Christina P., Sreenivas V., Seetaraman S. Impact of simple interventions on neonatal mortality in a low-resource teaching hospital in India. J Perinatol. 2007;27(1):44–49. doi: 10.1038/sj.jp.7211620. [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Yu X., Zhou Y., et al. Integrated measures for prevention of invasive Candida infections in preterm infants in a Chinese neonatal intensive care unit. Am J Infect Control. 2015;43(12):1321–1325. doi: 10.1016/j.ajic.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang W., Zhao C., Ji Q., Liu Y., Shen G., Wei L. Prevention of peripherally inserted central line-associated blood stream infections in very low-birth-weight infants by using a central line bundle guideline with a standard checklist: a case control study. BMC Pediatr. 2015;15(1):69. doi: 10.1186/s12887-015-0383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q., Lee S.K., Hu X.J., et al. Successful reduction in central line–associated bloodstream infections in a Chinese neonatal intensive care unit. Am J Infect Control. 2015;43(3):275–279. doi: 10.1016/j.ajic.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q., Lee S.K., Jiang S.Y., et al. Efficacy of an infection control program in reducing ventilator-associated pneumonia in a Chinese neonatal intensive care unit. Am J Infect Control. 2013;41(11):1059–1064. doi: 10.1016/j.ajic.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Huang H., Ran J., Yang J., Li P., Zhuang G. Impact of MRSA transmission and infection in a neonatal intensive care unit in China: a bundle intervention study during 2014-2017. Biomed Res Int. 2019;2019 doi: 10.1155/2019/5490413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi L., Fan W., Xia X., et al. Nosocomial outbreak of Candida parapsilosis sensu stricto fungaemia in a neonatal intensive care unit in China. J Hosp Infect. 2018;100(4):e245–ee52. doi: 10.1016/j.jhin.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Landre-Peigne C., Ka A.S., Peigne V., Bougere J., Seye M.N., Imbert P. Efficacy of an infection control programme in reducing nosocomial bloodstream infections in a Senegalese neonatal unit. J Hosp Infect. 2011;79(2):161–165. doi: 10.1016/j.jhin.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Hussain A.S., Ahmed A.M., Arbab S., et al. CLABSI reduction using evidence based interventions and nurse empowerment: a quality improvement initiative from a tertiary care NICU in Pakistan. Arch Dis Child. 2020;106(4):394–400. doi: 10.1136/archdischild-2019-318779. [DOI] [PubMed] [Google Scholar]
- 34.Resende D.S., Moreira J., de Brito D.D., Abdallah V.O.S., Gontijo Filho P.P. Reduction of catheter-associated bloodstream infections through procedures in newborn babies admitted in a university hospital intensive care unit in Brazil. Rev Soc Bras Med Trop. 2011;44(6):731–734. doi: 10.1590/s0037-86822011000600015. [DOI] [PubMed] [Google Scholar]
- 35.Resende D.S., Peppe A.L.G., dos Reis H., Abdallah V.O.S., Ribas R.M., Gontijo Filho P.P. Late onset sepsis in newborn babies: epidemiology and effect of a bundle to prevent central line associated bloodstream infections in the neonatal intensive care unit. Braz J Infect Dis. 2015;19(1):52–57. doi: 10.1016/j.bjid.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal V.D., Duenas L., Sobreyra-Oropeza M., et al. Findings of the international nosocomial infection control consortium (INICC), part iii: effectiveness of a multidimensional infection control approach to reduce central line-associated bloodstream infections in the neonatal intensive care units of 4 developing countries. Infect Control Hosp Epidemiol. 2013;34(3):229–237. doi: 10.1086/669511. [DOI] [PubMed] [Google Scholar]
- 37.Azab S.F.A., Sherbiny H.S., Saleh S.H., et al. Reducing ventilator-associated pneumonia in neonatal intensive care unit using "VAP prevention Bundle": a cohort study. BMC Infect Dis. 2015;15(1):314. doi: 10.1186/s12879-015-1062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran H.T., Mannava P., Murray J.C.S., et al. Early essential newborn care is associated with reduced adverse neonatal outcomes in a tertiary hospital in da nang, viet nam: a pre- post- intervention study. EClinicalMedicine. 2018;6:51–58. doi: 10.1016/j.eclinm.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill C.J., Mantaring J.B.V., Macleod W.B., et al. Impact of enhanced infection control at 2 neonatal intensive care units in the Philippines. Clin Infect Dis. 2009;48(1):13–21. doi: 10.1086/594120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villegas M., Arias M., de Mezerville M. Acciones educativas dirigidas al personal médico y de enfermería para disminuir las infecciones del tracto sanguíneo relacionadas a catéteres venosos centrales. Enfermería Actual en Costa Rica. 2014;(27):1–11. [Google Scholar]
- 41.Kulali F., Çalkavur Ş., Oruç Y., Demiray N., Devrim İ. Impact of central line bundle for prevention of umbilical catheter–related bloodstream infections in a neonatal intensive care unit: a pre–post intervention study. Am J Infect Control. 2019;47(4):387–390. doi: 10.1016/j.ajic.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Mais A., Hajar F., Rajab M. A quality improvement program to reduce central line associated blood stream infections in neonates. Br J Med Med Res. 2015;7(8):638–646. [Google Scholar]
- 43.Calil R., Marba S.T.M., Nowakonski A., Tresoldi A.T. Reduction in colonization and nosocomial infection by multiresistant bacteria in a neonatal unit after institution of educational measures and restriction in the use of cephalosporins. Am J Infect Control. 2001;29(3):133–138. doi: 10.1067/mic.2001.114223. [DOI] [PubMed] [Google Scholar]
- 44.Cavicchiolo M.E., Lanzoni P., Wingi M.O., et al. Reduced neonatal mortality in a regional hospital in Mozambique linked to a Quality Improvement intervention. BMC Pregnancy Childbirth. 2016;16(1):366. doi: 10.1186/s12884-016-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Picheansathian W., Pearson A., Suchaxaya P. The effectiveness of a promotion programme on hand hygiene compliance and nosocomial infections in a neonatal intensive care unit. Int J Nurs Pract. 2008;14(4):315–321. doi: 10.1111/j.1440-172X.2008.00699.x. Wiley-Blackwell. [DOI] [PubMed] [Google Scholar]
- 46.Rahim R.H., Barnett T. Reducing nosocomial infection in neonatal intensive care: an intervention study. Int J Nurs Pract. 2009;15(6):580–584. doi: 10.1111/j.1440-172X.2009.01800.x. [DOI] [PubMed] [Google Scholar]
- 47.Mwananyanda L., Pierre C., Mwansa J., et al. Preventing bloodstream infections and death in Zambian neonates: impact of a low-cost infection control bundle. Clin Infect Dis. 2019;69(8):1360–1367. doi: 10.1093/cid/ciy1114. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert C., Darlow B., Zin A., et al. Educating neonatal nurses in Brazil: a before-and-after study with interrupted time series analysis. Neonatology. 2014;106(3):201–208. doi: 10.1159/000362532. [DOI] [PubMed] [Google Scholar]
- 49.Jeena P., Thompson E., Nchabeleng M., Sturm A. Emergence of multi-drug-resistant Acinetobacter anitratus species in neonatal and paediatric intensive care units in a developing country: concern about antimicrobial policies. Ann Trop Paediatr. 2001;21(3):245–251. doi: 10.1080/02724930120077835. [DOI] [PubMed] [Google Scholar]
- 50.Gray J., Arvelo W., McCracken J., et al. An outbreak of Klebsiella pneumoniae late-onset sepsis in a neonatal intensive care unit in Guatemala. Am J Infect Control. 2012;40(6):516–520. doi: 10.1016/j.ajic.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 51.Lithgow A.E., Kilalang C. Outbreak of nosocomial sepsis in the special care nursery at port Moresby general hospital due to Multiresistant Klebsiella pneumoniae: high impact on mortality. P N G Med J. 2009;52(1/2):28–34. [PubMed] [Google Scholar]
- 52.Ahmed A., Waqar T., Ikram A., et al. Serratia marcescens outbreak causing septicemia in neonatal intensive care unit: substantiation of single source. Can J Infect Control. 2017;32(3):161–164. [Google Scholar]
- 53.Avila J.L. Practical method for diagnosis and control of an outbreak of nosocomial infection at the neonatology service. Metodo practico para el diagnostico y control de un brote de infeccion intrahospitalaria en un servicio de neonatologia. 2011;37(4):442–451. [Google Scholar]
- 54.Narayan S.A., Kool J.L., Vakololoma M., et al. Investigation and control of an outbreak of Enterobacter aerogenes bloodstream infection in a neonatal intensive care unit in Fiji. Infect Control Hosp Epidemiol. 2009;30(8):797–800. doi: 10.1086/598240. [DOI] [PubMed] [Google Scholar]
- 55.Irfan S., Ahmed I., Lalani F., et al. Methicillin resistant Staphylococcus aureus outbreak in a neonatal intensive care unit. East Mediterr Health J. 2019;25(7):514–518. doi: 10.26719/emhj.18.058. [DOI] [PubMed] [Google Scholar]
- 56.Moore K.L., Kainer M.A., Badrawi N., et al. Neonatal sepsis in Egypt associated with bacterial contamination of glucose-containing intravenous fluids. Pediatr Infect Dis J. 2005;24(7):590–594. doi: 10.1097/01.inf.0000168804.09875.95. [DOI] [PubMed] [Google Scholar]
- 57.Bouallegue-Godet O., Ben Salem Y., Fabre L., et al. Nosocomial outbreak caused by Salmonella enterica serotype Livingstone producing CTX-M-27 extended-spectrum beta-lactamase in a neonatal unit in Sousse, Tunisia. J Clin Microbiol. 2005;43(3):1037–1044. doi: 10.1128/JCM.43.3.1037-1044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Indarso F., Harianto A., Nada A., Aly H. Outbreak of neonatal cellulites and septicemia caused by Salmonella worthington. J Pediatr Infect Dis. 2008;3(4):241–244. [Google Scholar]
- 59.Mshana S.E., Gerwing L., Minde M., et al. Outbreak of a novel Enterobacter sp. carrying blaCTX-M-15 in a neonatal unit of a tertiary care hospital in Tanzania. Int J Antimicrob Agents. 2011;38(3):265–269. doi: 10.1016/j.ijantimicag.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Miranda-Novales G., Leanos-Miranda B., Diaz-Ramos R., et al. An outbreak due to Serratia marcescens in a neonatal intensive care unit typed by 2-day pulsed field gel electrophoresis protocol. Arch Med Res. 2003;34(3):237–241. doi: 10.1016/S0188-4409(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 61.Cetin B.S., Celebi S., Ozkan H., et al. Stenotrophomonas maltophilia outbreak in neonatal intensive care unit and outbreak management. J Pediatr Infect. 2015;9(4):147–152. [Google Scholar]
- 62.Hosoglu S., Hascuhadar M., Yasar E., Uslu S., Aldudak B. Control of an Acientobacter baumannii outbreak in a neonatal ICU without suspension of service: a devastating outbreak in Diyarbakir, Turkey. Infection. 2012;40(1):11–18. doi: 10.1007/s15010-011-0180-y. [DOI] [PubMed] [Google Scholar]
- 63.Rosenthal V.D., Alvarez-Moreno C., Villamil-Gomez W., et al. Effectiveness of a multidimensional approach to reduce ventilator-associated pneumonia in pediatric intensive care units of 5 developing countries: international nosocomial infection control consortium findings. Am J Infect Control. 2012;40(6):497–501. doi: 10.1016/j.ajic.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Alp E., Damani N. Healthcare-associated infections in intensive care units: epidemiology and infection control in low-to-middle income countries. J Infect Dev Ctries. 2015;9(10):1040–1045. doi: 10.3855/jidc.6832. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organisation . World Health Organisation; Geneva: 2011. Report on the Burden of Endemic Health Care Associated Infection Worldwide. [Google Scholar]
- 66.Ombelet S., Barbe B., Affolabi D., et al. Best practices of blood cultures in low- and middle-income countries. Front Med. 2019;6:131. doi: 10.3389/fmed.2019.00131. Lausanne. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organisation . World Health Organisation; Geneva: 2015. Global Antimicrobial Resistance Surveillance System. Manual for Early Implementation. [Google Scholar]
- 68.Okomo U., Dibbasey T., Kassama K. Neonatal admissions, quality of care and outcome: 4 years of inpatient audit data from The Gambia's teaching hospital. Paediatr Int Child Health. 2015;35:252–264. doi: 10.1179/2046905515Y.0000000036. [DOI] [PubMed] [Google Scholar]
- 69.Dailey P., Osborn J., Ashley E., et al. Defining system requirements for simplified blood culture to enable widespread use in resource-limited settings. Diagnostics. 2019;9(1):10. doi: 10.3390/diagnostics9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson M.L., Fleming K.A., Kuti M.A., Looi L.M., Lago N., Ru K. Access to pathology and laboratory medicine services: a crucial gap. Lancet N Am Ed. 2018;391(10133):1927–1938. doi: 10.1016/S0140-6736(18)30458-6. [DOI] [PubMed] [Google Scholar]
- 71.Sayed S., Cherniak W., Lawler M., et al. Improving pathology and laboratory medicine in low-income and middle-income countries: roadmap to solutions. Lancet N Am Ed. 2018;391(10133):1939–1952. doi: 10.1016/S0140-6736(18)30459-8. [DOI] [PubMed] [Google Scholar]
- 72.Land K.J., Boeras D.I., Chen X.S., Ramsay A.R., Peeling R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol. 2019;4(1):46–54. doi: 10.1038/s41564-018-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schabrun S., Chipchase L. Healthcare equipment as a source of nosocomial infection: a systematic review. J Hosp Infect. 2006;63(3):239–245. doi: 10.1016/j.jhin.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 74.Conde-Agudelo A., Belizán J.M., Diaz-Rossello J. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016;(8) doi: 10.1002/14651858.CD002771.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan J., Vesel L., Bahl R., Martines J.C. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity-a systematic review and meta-analysis. Matern Child Health J. 2015;19(3):468–479. doi: 10.1007/s10995-014-1526-8. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organisation . World Health Organisation; Geneva: 2015. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. [PubMed] [Google Scholar]
- 77.World Health Organisation. Breastfeeding. Available from: https://www.who.int/health-topics/breastfeeding#tab=tab_1; 2021, Accessed date: Feb 8 2021.
- 78.Gilhooly D., Green S.A., McCann C., Black N., Moonesinghe S.R. Barriers and facilitators to the successful development, implementation and evaluation of care bundles in acute care in hospital: a scoping review. Implement Sci. 2019;14(1):47. doi: 10.1186/s13012-019-0894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Proctor E., Silmere H., Raghavan R., et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murni I., Duke T., Triasih R., Kinney S., Daley A.J., Soenarto Y. Prevention of nosocomial infections in developing countries, a systematic review. Paediatr Int Child Health. 2013;33(2):61–78. doi: 10.1179/2046905513Y.0000000054. [DOI] [PubMed] [Google Scholar]
- 81.Garg P., Gogia S. Reducing neonatal mortality in developing countries: low-cost interventions are the key determinants. J Perinatol. 2009;29(1):74–75. doi: 10.1038/jp.2008.123. author reply 5. [DOI] [PubMed] [Google Scholar]
- 82.Levac D., Colquhoun H., O Brien K.K. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brien B.E., Lorenzetti D.L., Lewis S., Kennedy J., Ghali W.A. Overview of a formal scoping review on health system report cards. Implement Sci. 2010;5:2. doi: 10.1186/1748-5908-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.