Abstract
Osler‐Weber‐Rendu disease is a genetic disease characterized by mucocutaneous and visceral telangiectasias. Pulmonary arteriovenous malformation is one of the main visceral complications revealing Osler‐Weber‐Rendu disease. The present case was a 34‐year‐old woman with exertional dyspnea and severe hypoxemia revealing pulmonary arteriovenous malformations on chest CT scan.
Keywords: arteriovenous malformations, Madagascar, Osler‐Weber‐Rendu disease, telangiectasias
The report of this case would allow the researchers in the domain of medicine to compare with the literature and could therefore constitute additional data on the diagnosis and management of Osler‐Weber‐Rendu disease.

1. INTRODUCTION
Osler‐Weber‐Rendu disease or hereditary hemorrhagic telangiectasia is an age‐related autosomal dominant constitutional vascular dysplasia. 1 The disease is rare and difficult to diagnose. 1 The diagnosis is clinical, based on the presence of spontaneous and recurrent epistaxis, multiple telangiectasias, typical localization (lips, oral cavity, fingers, and nose), or visceral localizations, and a family history in the first generation. 1 Its pathophysiology is linked to a perturbation of the balance between pro‐angiogenic factors, including the vascular endothelial growth factor, and anti‐angiogenic factors. 2 It is also a serious disease due to its complications and its locations, especially in the brain. A pulmonary arteriovenous malformation is one of the major visceral complications that reveal the disease. 3 We report the first Malagasy case of Osler‐Weber‐Rendu disease.
2. OBSERVATION
It was a 36‐year‐old woman hospitalized in the Department of Pneumology, University Hospital of Joseph Raseta Befelatanana. The symptoms started 3 weeks ago with exertional dyspnea associated with intermittent coughs. The symptoms worsened despite a well‐managed treatment, which motivated her hospitalization in the department. In this history, episodes of dyspnea were reported in September 2020, recurrent epistaxis with family context in the first generation on the maternal side. The examination at admission found a polypneic patient at 22 cycles per minute with a drop in pulsed oxygen saturation (SpO2) to 83% in ambient air. The respiratory and cardiovascular examinations were unremarkable. There was no cyanosis, no digital hippocratism, and no telangiectasia. The otorhinolaryngeal examination objectified bilateral small drip epistaxis without functional repercussions. Blood cells count showed a hemoglobin level of 8.9 g/dl, an average blood volume of 62.5 fl, an average corpuscular concentration of 27.3 g/dl, and a platelet count of 377 G/L. Renal (serum creatinine = 42 μmol/L), hepatic functions (an aspartate aminotransferase = 17 U/L, an alanine aminotransferase = 21 IU/L), and coagulation profile (prothrombin time = 13.5 s, prothrombin level = 100%) were normal. Arterial gas measurements showed deep hypoxemia (PaO2 = 40.4 mm Hg) with severe hypocapnia (PCO2 = 22 mm Hg). The genetic analysis was not performed. Imaging such as thoracic computed tomography with injection of contrast product showed multiple peripheral and central vascular malformations corresponding to sacciform vascular dilations by arteriovenous fistula (Figures 1 and 2). Cardiac Doppler ultrasound revealed pulmonary arterial hypertension at 37 mm Hg with dilation of the right chambers (right ventricle = 45 mm, right atrium = 18 mm), flattening of the interventricular septum and minimal mitral insufficiency on Doppler examination. Contrast transthoracic cardiac ultrasound with intravenous injection of microbubbles could have helped to discover a right‐to‐left shunt, but is not available in Madagascar. The abdominal ultrasound was unremarkable; the liver was homogeneous of normal size without dilation of the hepatic veins. The diagnosis of Osler‐Weber‐Rendu disease was suggested by the presence of epistaxis, similar case in the family in the first generation and bilateral sacciform vascular dilations of the pulmonary fields on the chest CT scan. The patient was put on oxygen therapy, and for the epistaxis, the hemostatic procedures were prolonged bidigital compression and the initiation of oral tranexamic acid. About the pulmonary arteriovenous malformations, arterial embolization was proposed to improve the dyspnea, arterial gazometry, and also preventing complications. However, this technique was not performed due to the patient's condition. The outcome was good with oxygen therapy alone, which was maintained at 2 L/min.
FIGURE 1.
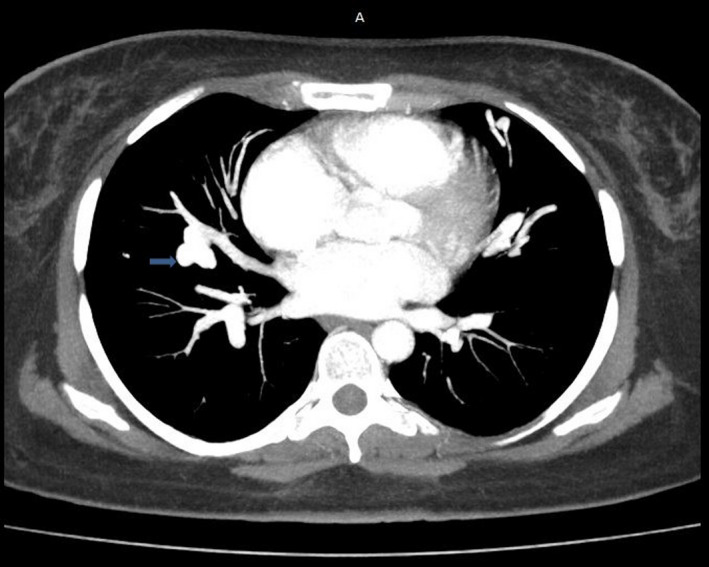
Mediastinal window chest CT scan with injection: pulmonary arteriovenous malformation of right pulmonary field
FIGURE 2.

Parenchymal window chest CT scan with injection: multiple pulmonary arteriovenous malformations
3. DISCUSSION
Osler‐Weber‐Rendu disease was described by Rendu in 1896, the description was supplemented by Osler in 1902 and by Weber in 1907. 4 The disease is due to a disturbance in the regulation of angiogenesis, linked to a genetic defect of autosomal dominant inheritance with variable expression. 2 Penetrance is related to age and is complete around age of 40–50 years old. 5 Three genes are associated with Osler‐Weber‐Rendu disease: ENG, ACVRL1, and MADH4 involved in the tumor growth factor signaling pathway. Mutation of only one of the two gene alleles is sufficient to cause the disease. 6 This disease is rare, but ubiquitous. To the best of our knowledge, this was the first case reported in Madagascar. A few cases have been reported in the African literature. 7 In France, the incidence of the disease was estimated at 1/6000 inhabitants. 8 The diagnosis is based on the clinical criteria of Curaçao (Table 1). 1 Recurrent epistaxis is the most common way of finding the disease. In the reported case, in front of marked respiratory signs with pseudoaneurysmal dilations of two pulmonary fields in chest CT scan, the pulmonary arteriovenous malformation was evoked. To sum up, our patient suffers from Osler‐Weber‐Rendu disease due to the presence of three Curaçao criteria, namely spontaneous epistaxis, reccurent epistaxis in the first generation family history and malformation arterioveinous pulmonary. In the literature, pulmonary arteriovenous malformation is one of the main visceral complications revealing Osler‐Weber‐Rendu disease, it is found in 15 to 33% of affected patients. 3 Arteriovenous malformation is an abnormal communication between a pulmonary artery and a pulmonary vein, resulting in the creation of a low‐pressure right‐to‐left shunt, which can lead, depending on the flow of the shunt, to chronic hypoxemia. The clinical and biological signs are consequently dyspnea, cyanosis, digital hippocratism, and rarely polycythemia. Cyanosis and polycythemia may be masked by pre‐existing anemia associated with epistaxis. Dyspnea on exertion is the most common symptom (26%–86%); it is generally moderate and is thought to be correlated with the level of the shunt and the size of the pulmonary arteriovenous malformations. 3 Chest radiography coupled with transthoracic cardiac contrast ultrasound with intravenous injection of microbubbles is excellent examinations for screening for arteriovenous malformation. Contrast transthoracic cardiac ultrasound can distinguish an intrapulmonary shunt from an intracardiac shunt and look for the presence of pulmonary arterial hypertension. 3 , 9 In the study by Cottin et al 10 , the combination of contrast ultrasound and chest X‐ray had a sensitivity of 100% and a specificity of 51% to diagnose a pulmonary arteriovenous malformation. If both examinations are positive, a chest CT scan is offered to confirm the diagnosis and specify the characteristics of the pulmonary arteriovenous malformation. 9 Pulmonary arterioveinous hypertension is a rare complication of osler weber rendu disease. There are two types, either idiopathic most often linked to a mutation in the ACVRL1 ; or linked to a caediac hyperflow. It was diagnosed on a cardiac Doppler ultrasound in our case. In published reviews, cardiac ultrasound is the gold standard for screening and diagnosis of this complication. 3 The treatment of pulmonary arteriovenous malformations is based on percutaneous arterial vaso‐occlusion (embolization), which improves dyspnea and prevents complications. 9
TABLE 1.
Diagnostic criteria according to the Curaçao consensus conference
| 1. Epistaxis |
|
| 2. Telangiectasias |
|
| 3. Visceral lesions |
|
| 4. Family history |
|
The diagnosis of Osler‐Weber‐Rendu disease is as follows: defined if three criteria are present; Possible or suspected if two criteria are present; Probable if there are less than two criteria present.
4. CONCLUSION
Osler‐Weber‐Rendu disease is a rare genetic disorder, diagnosed by the clinical criteria of Curaçao and which should be confirmed by genetic testing. After epistaxis, the pulmonary arteriovenous malformation is one of discovery modes of the disease. Management of the disease must be multidisciplinary involving pulmonologists and vascular surgeons. Genetic counseling in relatives would be necessary for early detection of asymptomatic arteriovenous malformations.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
HR followed up the patient, collected the clinical data, and drafted the report. FPPA, AMN, AZR, JNSR, and DA followed up the patient. HMT, JNSR, MIR, and JRK designed and critically revised the report. JLR and HMDV validated the report. All the authors have read and approved the final draft of the manuscript. All authors contributed to this work and approved the final version.
ETHICAL APPROVAL
The authors thank the patient who gave written permission for this report. The article does not contain any personal information that could identify the patient. The names and dates on the chest CT scan have been hidden. The authors have included only information necessary for scientific understanding.
CONSENT
The development of this article has been approved by the patient and the department of pneumology who have given written permission for this work.
ACKNOWLEDGEMENT
This work was carried out with the working group of the Society of Pneumology of Madagascar (SPMAD). The authors thank the patient who gave written permission for this report.
Ramanandafy H, Andriamahenina FP, Tiaray HM, et al. Pulmonary arteriovenous malformation revealing Osler‐Weber‐Rendu disease: A case report. Clin Case Rep. 2022;10:e05294. doi: 10.1002/ccr3.5294
Funding information
This research has not received any specific grant from any public, commercial, or for‐profit
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu‐Osler‐Weber syndrome). Am J Med Genet. 2000;91:66‐67. [DOI] [PubMed] [Google Scholar]
- 2. Dupuis‐Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307:948‐955. [DOI] [PubMed] [Google Scholar]
- 3. Cottin V, Blanchet AS, Cordier JF. Manifestations vasculaires pulmonaires de la maladie de Rendu‐Osler. Presse Med. 2005;34:1487‐1495. [DOI] [PubMed] [Google Scholar]
- 4. Rendu M. Épistaxis répétées chez un sujet porteur de petits angiomes cutanés et muqueux. Gaz Hop Paris. 1886;135:1322‐1326. [Google Scholar]
- 5. Plauchu H, Chadarevian JP, Bideau A, Robert JM. Age‐related clinical profi le of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet. 1989;32:291‐297. [DOI] [PubMed] [Google Scholar]
- 6. Duffaua P, Lazarrob E, Viallardb JF. Maladie de Rendu‐Osler. Rev Med Int. 2014;35:21‐27. [DOI] [PubMed] [Google Scholar]
- 7. Abdou S. La Maladie de Rendu Osler Weber:à propos de 3 cas [Thèse]. Médecine : Rabat ; 141.
- 8. Chinet T. Cas clinique à tendance diagnostique no 2 maladie de Rendu‐Osler. Rev Mal Respir Actual. 2012;4:49‐52. [Google Scholar]
- 9. Cottin V, Ben Kheder A, Israël‐Biet D, Montani D. Malformations artérioveineuses pulmonaires et maladie de Rendu‐Osler. Rev Mal Respir Actual. 2009;1:164‐167. [Google Scholar]
- 10. Cottin V, Chinet T, Lavole A, et al. Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine (Baltimore). 2007;86:1‐17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


