Abstract
Purpose
Breast cancer is mainly diagnosed using core needle biopsy (CNB), although other biopsy methods, including vacuum-assisted biopsy (VAB), may also be used. We compared differences in clinical characteristics and prognoses of patients with breast cancer according to biopsy methods used for diagnosis.
Methods
A total of 98,457 patients who underwent various biopsy methods (CNB, fine-needle aspiration [FNA], VAB, and excisional biopsy) for diagnosing breast cancer were recruited. Using CNB as a reference, related clinicopathological factors and prognostic differences between biopsy methods were analyzed retrospectively using large-scale data from the Korean Breast Cancer Society Registration System. The associations between biopsy methods and clinicopathological factors were compared using multinomial logistic regression analysis, and the prognoses of patients undergoing the different biopsy methods, as breast cancer-specific survival (BCSS) and overall survival (OS), were compared using the Kaplan-Meier method and Cox proportional hazard model.
Results
Univariate and multivariate analyses showed that unlike FNA, both VAB and excisional biopsy were significantly associated with tumor size, palpability, tumor stage, and histologic grade as relatively good prognostic factors compared to CNB. In particular, VAB showed lower odds ratios for these factors than excisional biopsy. In the univariate analysis, the prognosis of patients undergoing VAB was better than that of those undergoing CNB with respect to BCSS (hazard ratio [HR], 0.188, p < 0.001) and OS (HR, 0.359; p < 0.001). However, in the multivariate analysis, there were no significant prognostic differences from CNB in both BCSS and OS; differences were only evident for FNA.
Conclusion
In this study, we showed that the characteristics of breast cancer differed according to various biopsy methods. Although VAB is not a standard method for breast cancer diagnosis, it showed no prognostic differences to CNB.
Keywords: Biopsy, Breast, Carcinoma, Prognosis, Propensity Score
INTRODUCTION
Breast cancer is one of the most common cancers in women [1]. As early detection and treatment of breast cancer become increasingly important, the diagnostic technologies for breast cancer continue to evolve [2]. Biopsy is essential for breast cancer diagnosis. Until early 1990s, excisional biopsy after the localization of non-palpable lesions was the gold standard. However, its disadvantages include scarring, breast deformation, and reoperation when lesions are diagnosed as malignant [3,4].
Percutaneous biopsy is a non-surgical method for breast cancer diagnosis that involves using a needle to obtain suspicious tissues based on mammography or ultrasonography findings; the tissues are subsequently analyzed by pathologists to establish appropriate treatment plans. Percutaneous biopsy procedures include fine-needle aspiration (FNA), core needle biopsy (CNB), and vacuum-assisted biopsy (VAB). Along with clinical examination and mammography, FNA has been used as one of the “triple tests” in breast cancer diagnosis because it offers a fast, accurate, low-cost diagnosis with few side effects [5,6]. However, treatment planning after FNA is limited owing to a high non-diagnostic rate, difficulty in distinguishing between carcinoma in situ and invasive cancer, and inability to further implement immunohistochemistry [7,8]. CNB has been widely used since its first description in 1993 [9]. CNB, which can compensate for FNA disadvantages, such as the high rate of inadequate specimens for diagnosis and low sensitivity, became a new standard in the late 1990s [2,10] and has primarily been used as a biopsy method for suspicious breast lesions. However, owing to the limited amount of tissue obtained from CNB, diagnostic underestimation is possible, including the misdiagnosis of breast cancer as atypical ductal hyperplasia or invasive carcinoma as carcinoma in situ. These diagnostic limitations may lead to inappropriate or delayed treatments. To overcome this, VAB was introduced in 1995 [11] and has been widely used and popularized to the point that it is currently used in most South Korean hospitals and clinics. With a small incision, VAB can obtain a large amount of tissue with almost complete resection of most breast lesions, thereby improving the cosmetic outcome of resection.
When assessing the usefulness of biopsy methods for breast lesion diagnosis, previous studies have mainly compared diagnostic accuracies, such as diagnostic underestimations and false negatives. Furthermore, several comparative studies on the diagnostic accuracies of CNB and VAB have recently been reported [12,13]. The prognosis of breast cancer is a key indicator for diagnosing and treating breast cancer. However, few studies have investigated the relevance of biopsy methods in the prognosis of breast cancer. Therefore, an objective assessment of whether the choice of biopsy method can affect disease prognosis is needed. This study investigated differences in breast cancer characteristics and prognoses between various breast biopsy methods.
METHODS
Data collection
The data analyzed in this study were obtained from the Korean Breast Cancer Society Registration System, which is a database of breast cancer cases voluntarily registered by breast specialists at multiple institutions across the country since 1996. This has previously been described in detail [14]. In conjunction with the Korean Central Cancer Registry Ministry of Health and Welfare and the Korean National Statistical Office, the cause and date of death were updated until 2014. This study was approved by the Institutional Review Board of Daejeon St. Mary’s Hospital (No. DC20ZASI0080).
Patients and clinicopathological factors
According to the database, 107,329 patients were diagnosed with breast cancer and underwent surgery between January 1982 and December 2014. Since it was necessary to minimize the change in breast cancer progression between the dates of diagnosis and surgery to analyze the associations between biopsy methods and clinicopathological characteristics of breast cancer, patients who received neoadjuvant or palliative chemotherapy (n = 6,511) and those who were diagnosed with stage IV breast cancer (n = 684) were excluded. In addition, patients who underwent incisional biopsy (n = 1,677) were excluded from the analysis because the biopsy method is rarely used for breast cancer diagnosis. Therefore, the analysis was performed for a final total of 98,457 patients.
The patients were categorized according to the biopsy method used for breast cancer diagnosis, namely CNB, FNA, VAB, and excisional biopsy. Clinicopathological characteristics and prognoses of patients were then compared among various biopsy methods, with CNB (the most commonly used biopsy method) as the reference.
Clinicopathological factors in patients included the year of operation, age, tumor size, palpability, pathological tumor stage, histologic grade, presence of estrogen receptor or progesterone receptor (ER or PR), presence of human epidermal growth factor receptor 2, breast surgery, axillary surgery, and adjuvant therapy (chemotherapy, radiotherapy, and hormonal therapy). The year of operation, age, and tumor size were quantitative variables, while the rest were categorical variables.
Propensity score matching
To minimize bias, all clinicopathological factors, including the year of operation, were matched using the propensity score matching function of SPSS version 22.0 (IBM Corp., Armonk, USA). All factors, except the tumor size, were standardized by setting the match tolerance to zero. For factors such as tumor size, which comprise continuous variables (including decimal values), the number of propensity score-matched patients may be too small to be analyzed. Thus, the categories were divided at 0.5 cm intervals only for tumor sizes below 5.0 cm. Those exceeding 5.0 cm were considered to have a similar prognosis based on the 7th edition of the American Joint Committee on Cancer staging system. They were, therefore, grouped into one category and converted into category variables for propensity score matching. Three cohorts were generated using 1:1 propensity score matching between the CNB group and each other biopsy method group, such as CNB-FNA (n = 1,484), CNB-VAB (n = 386), and CNB-Excisional biopsy (n = 1,456) cohorts. Thus, it was confirmed that the clinical characteristics of the biopsy methods were similar in each propensity score-matched cohort (Supplementary Table 1) [15].
Statistical analysis
The trends among the various biopsy methods were compared using annual frequency analysis based on the year of operation. Comparisons of clinicopathological characteristics, except for the year of operation factor, were made using the Student’s t-test, χ2 test, and ANOVA. The associations between the biopsy methods and clinicopathological factors were compared using multinomial logistic regression analysis. The prognoses among the biopsy methods were compared using the Kaplan-Meier method and Cox proportional hazard model for breast cancer-specific survival (BCSS) and overall survival (OS). The prognostic analyses of the entire and propensity score-matched cohorts were conducted in the same manner, and the results were compared. All statistical analyses, including propensity score matching, were performed using SPSS version 22.0 (IBM Corp.), and p-values < 0.05 were considered statistically significant.
RESULTS
Trends in breast biopsy
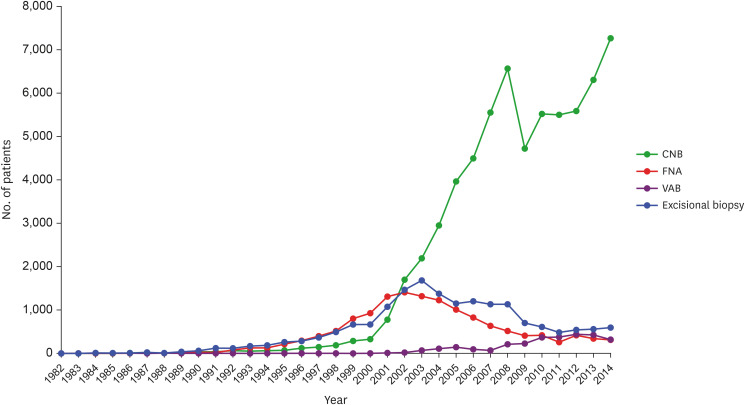
The overall number of CNBs performed increased between 1982 and 2014, and CNB has been the most used breast biopsy method since 2002. In contrast, the use of FNA had initially increased; however, it’s use has declined since 2002. The use of VAB has increased since 2007 but has declined slightly since 2012. After its initial decline in 2003, the use of excisional biopsy has increased slightly since 2011 (Figure 1). The number of patients who underwent CNB, FNA, VAB, and excisional biopsy were 64,535 (65.5%), 13,901 (14.1%), 2,850 (2.9%), and 17,171 (17.4%), respectively (Table 1).
Figure 1. Trends of various biopsy methods used in breast cancer diagnosis.
CNB = core needle biopsy; FNA = fine needle aspiration; VAB = vacuum-assisted biopsy.
Table 1. Clinicopathological characteristics of biopsy methods in the entire breast cancer cohort.
| Factors | Biopsy methods | p-value | ||||
|---|---|---|---|---|---|---|
| CNB (n = 64,535) | FNA (n = 13,901) | VAB (n = 2,850) | Excisional biopsy (n = 17,171) | |||
| Age (yr) | 50.4 ± 10.6 | 49.2 ± 10.8 | 48.0 ± 9.8 | 48.4 ± 10.8 | < 0.001 | |
| Tumor size (cm) | 2.1 ± 1.5 | 2.6 ± 1.7 | 1.3 ± 1.5 | 2.0 ± 1.8 | < 0.001 | |
| Palpability | < 0.001 | |||||
| No | 10,315 (16.0) | 680 (4.9) | 1,097 (38.5) | 3,480 (20.3) | ||
| Yes | 44,270 (68.6) | 11,605 (83.5) | 1,073 (37.6) | 11,343 (66.1) | ||
| Unknown | 9,950 (15.4) | 1,616 (11.6) | 680 (23.9) | 2,348 (13.7) | ||
| Tumor stage | < 0.001 | |||||
| 0 | 5,666 (8.8) | 586 (4.2) | 989 (34.7) | 3,474 (20.2) | ||
| I | 26,653 (41.3) | 3,828 (27.5) | 1,157 (40.6) | 5,760 (33.5) | ||
| II | 24,361 (37.7) | 7,030 (50.6) | 475 (16.7) | 5,850 (34.1) | ||
| III | 7,088 (11.0) | 2,278 (16.4) | 107 (3.8) | 1,349 (7.9) | ||
| Unknown | 767 (1.2) | 179 (1.3) | 122 (4.3) | 738 (4.3) | ||
| Histologic grade | < 0.001 | |||||
| Grade 1–2 | 34,587 (53.6) | 5,940 (42.7) | 1,079 (37.9) | 6,494 (37.8) | ||
| Grade 3 | 19,280 (29.9) | 4,822 (34.7) | 434 (15.2) | 2,890 (16.8) | ||
| Unknown | 10,668 (16.5) | 3,139 (22.6) | 1,337 (46.9) | 7,787 (45.3) | ||
| ER or PR | < 0.001 | |||||
| Negative | 16,690 (25.9) | 4,590 (33.0) | 549 (19.3) | 3,619 (21.1) | ||
| Positive | 46,063 (71.4) | 8,542 (61.4) | 2,187 (76.7) | 11,582 (67.5) | ||
| Unknown | 1,782 (2.8) | 769 (5.5) | 114 (4.0) | 1,970 (11.5) | ||
| HER2 | < 0.001 | |||||
| Negative | 40,626 (63.0) | 6,071 (43.7) | 1,703 (59.8) | 8,103 (47.2) | ||
| Positive | 12,845 (19.9) | 2,608 (18.8) | 498 (17.5) | 2,270 (13.2) | ||
| Unknown | 11,064 (17.1) | 5,222 (37.6) | 649 (22.8) | 6,798 (39.6) | ||
| Breast surgery | < 0.001 | |||||
| BCS | 37,047 (57.4) | 5,000 (36.0) | 1,928 (67.6) | 7,945 (46.3) | ||
| Mastectomy | 26,855 (41.6) | 8,700 (62.6) | 880 (30.9) | 8,752 (51.0) | ||
| Unknown | 633 (1.0) | 201 (1.4) | 42 (1.5) | 474 (2.8) | ||
| Axillary surgery | < 0.001 | |||||
| SLNB | 22,482 (34.8) | 1,432 (10.3) | 1,297 (45.5) | 1,984 (11.6) | ||
| AD | 37,104 (57.5) | 11,485 (82.6) | 1,123 (39.4) | 12,036 (70.1) | ||
| Unknown | 4,949 (7.7) | 984 (7.1) | 430 (15.1) | 3,151 (18.4) | ||
| Chemotherapy | < 0.001 | |||||
| No | 20,901 (32.4) | 2,546 (18.3) | 1,619 (56.8) | 6,312 (36.8) | ||
| Yes | 37,930 (58.8) | 9,700 (69.8) | 908 (31.9) | 8,943 (52.1) | ||
| Unknown | 5,704 (8.8) | 1,655 (11.9) | 323 (11.3) | 1,916 (11.2) | ||
| Radiotherapy | < 0.001 | |||||
| No | 20,044 (31.1) | 6,102 (43.9) | 868 (30.5) | 7,167 (41.7) | ||
| Yes | 36,767 (57.0) | 5,594 (40.2) | 1,651 (57.9) | 7,426 (43.2) | ||
| Unknown | 7,724 (12.0) | 2,205 (15.9) | 331 (11.6) | 2,578 (15.0) | ||
| Hormonal therapy | < 0.001 | |||||
| No | 19,199 (29.7) | 4,832 (34.8) | 765 (26.8) | 5,089 (29.6) | ||
| Yes | 38,633 (59.9) | 7,200 (51.8) | 1,748 (61.3) | 9,905 (57.7) | ||
| Unknown | 6,703 (10.4) | 1,869 (13.4) | 337 (11.8) | 2,177 (12.7) | ||
Values are presented as Mean ± standard deviation or number (%).
CNB = core needle biopsy; FNA = fine needle aspiration; VAB = vacuum-assisted biopsy; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth receptor 2; BCS = breast-conserving surgery; SLNB = sentinel lymph node biopsy; AD = axillary dissection.
Clinicopathological characteristics
There were significant differences in all clinicopathological factors depending on the biopsy method (p < 0.001). Compared to the CNB group, the VAB and excisional biopsy groups had good clinical features, while the FNA group was characterized as having poor clinical features. Compared the other biopsy method groups, the VAB group was the youngest (mean ± SD = 48.0 ± 9.8), had the smallest tumor size (mean ± SD = 1.3 ± 1.5), the lowest proportion of palpable lesions (37.6%), the highest proportion of carcinoma in situ (34.7%), the highest proportion of ER- or PR-positive (76.7%), the highest proportion of breast-conserving surgery (67.6%) and sentinel lymph node biopsy (45.5%), and the lowest proportion of chemotherapy (31.9%) (Table 1).
Clinicopathological factors and prognoses associated with biopsy methods
When comparing associations between various biopsy methods and clinicopathological factors relative to those for CNB, only the use of VAB significantly increased over time (odds ratio [OR], 1.110; p < 0.001). Both VAB and excisional biopsy were significantly associated with tumor size, palpability, tumor stage, and histologic grade as relatively good prognostic factors compared to CNB in both univariate and multivariate analyses. This was contrary to FNA that was significantly associated with these factors (except tumor stage) as relatively poor prognostic factors compared to CNB. VAB showed lower OR values for these factors than excisional biopsy (Table 2).
Table 2. Clinicopathological characteristics of three biopsy methods in the entire breast cancer cohort, using core needle biopsy as a reference.
| Factors | Univariate analysis | Multivariate analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FNA | VAB | Excisional biopsy | FNA | VAB | Excisional biopsy | ||||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Year of operation | 0.770 (0.767–0.774) | < 0.001 | 1.110 (1.098–1.122) | < 0.001 | 0.796 (0.793–0.799) | < 0.001 | 0.746 (0.738–0.754) | < 0.001 | 1.064 (1.037–1.092) | < 0.001 | 0.766 (0.757–0.774) | < 0.001 | |
| Age (yr) | 0.989 (0.988–0.991) | < 0.001 | 0.978 (0.975–0.982) | < 0.001 | 0.982 (0.980–0.983) | < 0.001 | 1.007 (1.004–1.011) | < 0.001 | 0.966 (0.959–0.974) | < 0.001 | 0.999 (0.995–1.002) | 0.465 | |
| Tumor size (cm) | 1.181 (1.170–1.193) | < 0.001 | 0.589 (0.566–0.612) | < 0.001 | 0.971 (0.959–0.982) | < 0.001 | 1.032 (1.005–1.059) | 0.019 | 0.798 (0.730–0.874) | < 0.001 | 0.962 (0.931–0.993) | 0.017 | |
| Palpability | |||||||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Yes | 3.976 (3.670–4.309) | < 0.001 | 0.228 (0.209–0.249) | < 0.001 | 0.759 (0.727–0.793) | < 0.001 | 2.038 (1.761–2.358) | < 0.001 | 0.397 (0.338–0.466) | < 0.001 | 0.685 (0.622–0.756) | < 0.001 | |
| Tumor stage | |||||||||||||
| 0 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| I | 1.389 (1.267–1.522) | < 0.001 | 0.249 (0.227–0.272) | < 0.001 | 0.352 (0.335–0.371) | < 0.001 | 0.644 (0.412–1.007) | 0.053 | 0.304 (0.195–0.474) | < 0.001 | 0.476 (0.344–0.658) | < 0.001 | |
| II | 2.790 (2.552–3.050) | < 0.001 | 0.112 (0.100–0.125) | < 0.001 | 0.392 (0.372–0.412) | < 0.001 | 0.771 (0.492–1.208) | 0.256 | 0.272 (0.169–0.437) | < 0.001 | 0.397 (0.285–0.552) | < 0.001 | |
| III | 3.107 (2.819–3.425) | < 0.001 | 0.086 (0.071–0.106) | < 0.001 | 0.310 (0.289–0.334) | < 0.001 | 0.906 (0.573–1.433) | 0.672 | 0.287 (0.164–0.502) | < 0.001 | 0.409 (0.288–0.579) | < 0.001 | |
| Histologic grade | |||||||||||||
| Grade 1–2 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Grade 3 | 1.456 (1.397–1.519) | < 0.001 | 0.722 (0.645–0.808) | < 0.001 | 0.798 (0.762–0.837) | < 0.001 | 1.270 (1.183–1.365) | < 0.001 | 0.790 (0.657–0.952) | 0.013 | 0.846 (0.781–0.916) | < 0.001 | |
| ER or PR | |||||||||||||
| Negative | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Positive | 0.674 (0.648–0.702) | < 0.001 | 1.443 (1.312–1.588) | < 0.001 | 1.160 (1.113–1.208) | < 0.001 | 0.762 (0.678–0.856) | < 0.001 | 1.138 (0.832–1.557) | 0.417 | 0.898 (0.791–1.020) | 0.098 | |
| HER2 | |||||||||||||
| Negative | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Positive | 1.359 (1.292–1.428) | < 0.001 | 0.925 (0.835–1.024) | 0.133 | 0.886 (0.842–0.932) | < 0.001 | 1.151 (1.070–1.239) | < 0.001 | 0.953 (0.790–1.149) | 0.612 | 0.837 (0.769–0.910) | < 0.001 | |
| Breast surgery | |||||||||||||
| BCS | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Mastectomy | 2.400 (2.310–2.494) | < 0.001 | 0.630 (0.581–0.683) | < 0.001 | 1.520 (1.469–1.573) | < 0.001 | 0.998 (0.895–1.111) | 0.965 | 0.973 (0.741–1.279) | 0.846 | 0.880 (0.782–0.991) | 0.035 | |
| Axillary surgery | |||||||||||||
| SLNB | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| AD | 4.860 (4.589–5.147) | < 0.001 | 0.525 (0.484–0.569) | < 0.001 | 3.676 (3.496–3.865) | < 0.001 | 0.838 (0.754–0.933) | 0.001 | 1.039 (0.865–1.249) | 0.679 | 1.059 (0.953–1.176) | 0.287 | |
| Chemotherapy | |||||||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Yes | 2.099 (2.003–2.200) | < 0.001 | 0.309 (0.284–0.336) | < 0.001 | 0.781 (0.753–0.810) | < 0.001 | 1.399 (1.265–1.548) | < 0.001 | 0.847 (0.706–1.015) | 0.073 | 1.199 (1.092–1.315) | < 0.001 | |
| Radiotherapy | |||||||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Yes | 0.500 (0.480–0.520) | < 0.001 | 1.037 (0.953–1.128) | 0.397 | 0.565 (0.544–0.586) | < 0.001 | 0.767 (0.690–0.851) | < 0.001 | 0.860 (0.654–1.131) | 0.281 | 0.737 (0.657–0.828) | < 0.001 | |
| Hormonal therapy | |||||||||||||
| No | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Yes | 0.741 (0.711–0.771) | < 0.001 | 1.136 (1.041–1.238) | 0.004 | 0.967 (0.931–1.005) | 0.086 | 1.239 (1.105–1.390) | < 0.001 | 0.655 (0.489–0.878) | 0.005 | 1.085 (0.959–1.229) | 0.196 | |
FNA = fine needle aspiration; VAB = vacuum-assisted biopsy; OR = odds ratio; CI = confidence interval; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth receptor 2; BCS = breast-conserving surgery; SLNB = sentinel lymph node biopsy; AD = axillary dissection.
The median observation period for the entire breast cancer cohort was 74 (range: 0–383) months, while that for the CNB, FNA, VAB, and excisional biopsy groups was 60 (range: 0–383), 121 (range: 0–381), 43 (range: 0–191), and 112 (range: 0–378) months, respectively. The 5-year survival rate for each group differed significantly in terms of BCSS (p < 0.001), from 97.6% in the CNB group, 93.4% in the FNA group, 99.6% in the VAB group, and 96.5% in the excisional biopsy group (Figure 2A). In contrast, the 5-year survival rate did not differ significantly in terms of OS (p = 0.154), from 94.1% in the CNB group, 89.6% in the FNA group, 98.1% in the VAB group, and 93.9% in the excisional biopsy group (Figure 3A). No significant difference was observed in the survival curves of all propensity score-matched cohorts for both BCSS (Figure 2B-D) and OS (Figure 3B-D). In the entire breast cancer cohort, univariate analysis of the Cox proportional hazard model revealed that the prognosis of the VAB group was better than that of the CNB group in terms of BCSS (hazard ratio [HR], 0.188; p < 0.001) and OS (HR, 0.359; p < 0.001). However, multivariate analysis showed that all groups, except the FNA group, had no significant prognostic differences from the CNB group in terms of both BCSS and OS. In the same analysis using the Cox proportional hazard model in the propensity score-matched cohorts, all other biopsy methods had no significant prognostic differences from the CNB group in terms of both BCSS and OS (Table 3).
Figure 2. Breast cancer-specific survival curves of various biopsy methods in (A) the entire and (B-D) propensity score-matched breast cancer cohorts. (B) CNB-FNA cohort; (C) CNB-VAB cohort; and (D) CNB-Excisional biopsy cohort.
CNB = core needle biopsy; FNA = fine needle aspiration; VAB = vacuum-assisted biopsy.
Figure 3. Overall survival curves of various biopsy methods in (A) the entire and (B-D) propensity score-matched breast cancer cohorts. (B) CNB-FNA cohort; (C) CNB-VAB cohort; and (D) CNB-Excisional biopsy cohort.
CNB = core needle biopsy; FNA = fine needle aspiration; VAB = vacuum-assisted biopsy.
Table 3. Survival differences between biopsy methods.
| Breast cancer cohorts | Biopsy methods | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BCSS | OS | BCSS | OS | ||||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| The entire cohort | CNB | 1 | 1 | 1 | 1 | ||||
| FNA | 2.741 (2.543–2.955) | < 0.001 | 1.656 (1.575–1.741) | < 0.001 | 1.191 (1.039–1.364) | 0.012 | 1.183 (1.084–1.291) | < 0.001 | |
| VAB | 0.188 (0.101–0.351) | < 0.001 | 0.359 (0.273–0.474) | < 0.001 | 0.841 (0.348–2.031) | 0.700 | 0.982 (0.650–1.483) | 0.930 | |
| Excisional biopsy | 1.568 (1.443–1.704) | < 0.001 | 1.052 (0.997–1.111) | 0.064 | 0.974 (0.822–1.153) | 0.757 | 1.062 (0.958–1.179) | 0.253 | |
| Propensity score-matched cohorts | CNB | 1 | 1 | 1 | 1 | ||||
| FNA | 1.051 (0.586–1.887) | 0.866 | 0.958 (0.660–1.391) | 0.823 | 1.143 (0.632–2.066) | 0.659 | 1.010 (0.694–1.470) | 0.958 | |
| VAB | 0.332 (0.035–3.195) | 0.340 | 0.280 (0.058–1.347) | 0.112 | 0.530 (0.052–5.389) | 0.592 | 0.249 (0.050–1.228) | 0.088 | |
| Excisional biopsy | 1.102 (0.503–2.416) | 0.808 | 1.301 (0.824–2.052) | 0.258 | 1.072 (0.488–2.358) | 0.862 | 1.341 (0.850–2.118) | 0.207 | |
BCSS = breast cancer-specific survival; OS = overall survival; HR = hazard ratio; CI = confidence interval; CNB = core needle biopsy; FNA = fine needle aspiration; VAB = vacuum-assisted biopsy.
DISCUSSION
In this study, the characteristics of breast cancer were found to differ according to the biopsy method employed, with reference to CNB. In terms of prognosis, however, besides FNA, there was no difference among the various biopsy methods. One of the factors affecting the association between biopsy methods and prognosis is the displacement of tumor cells into the biopsy tract [16,17]. Kong et al. [17] demonstrated poor prognosis in patients with breast cancer who were diagnosed using CNB rather than using FNA if they did not undergo postoperative adjuvant radiotherapy, which could be associated with an increased risk of tumor cell displacement caused by using larger needles. However, Liebens et al. [18] performed a systematic review and showed that risk factors such as the interval period between biopsy and surgery [19] and histologic type [20], rather than the size of the biopsy needle, may have affected the tumor cell displacement. In this study, the prognoses of VAB and excisional biopsy did not differ from those of CNB; hence, the impact of tumor cell displacement on prognosis is unlikely to be significant. Regarding tumor cell displacement, the local recurrence rate between biopsy methods should be considered in the prognosis. However, this could not be analyzed because there were no local recurrence data. Although there have been almost no previous studies on the difference in local recurrence rates according to needle size, several studies have reported no significant differences in local recurrence rates between excisional biopsy (or needle-localized biopsy) and each percutaneous biopsy (FNA, CNB, or VAB) [21,22,23]. This may be because the biopsy tracts were excised together during surgery. Future studies on the relationship between needle size and local recurrence rate are necessary.
FNA exhibited a poorer prognosis than CNB in this study. The FNA group presented relatively worse clinical features than the CNB group, which is thought to be associated with the difference in prognosis. Although FNA was the first percutaneous biopsy method to diagnose breast cancer, it is known to have a high non-diagnostic rate [24]. Based on the trends in the development of diagnostic imaging equipment [25] and the increase in breast cancer screening rates [26], it is possible that many cases were diagnosed at a relatively advanced stage because FNA was commonly used in a period when early breast cancer detection was relatively rare.
Diagnostic methods are commonly performed via CNB owing to its diagnostic benefits for suspicious breast lesions. Excisional biopsy is primarily used for borderline breast lesions (B3) [27] or benign breast lesions [2]. In this study, the patients were diagnosed with relatively good clinical features after excisional biopsy because most breast cancers were clinically benign.
VAB is used under conditions similar to excisional biopsy [2,28]. Therefore, like those diagnosed by excisional biopsy, most breast cancers diagnosed via VAB are considered benign lesions in pre-diagnostic imaging. In addition, VAB is widely used for suspicious microcalcifications by stereotactic biopsy. This may explain why the VAB group included a large number of patients with ductal carcinoma in situ. Thus, it is not surprising that the characteristics of breast cancer diagnosed by these biopsy methods were better than those diagnosed by other biopsy methods, which seems to be associated with prognosis. However, the exact size of the breast lesions is unknown after resection via VAB because breast lesions are removed by making repeated cuts as thick as a needle [11]. Since part of the breast lesions can remain in the resection margin even after confirming complete resection through ultrasonography [29], it can affect the exact determination of the stage and subsequent treatment plans for breast cancer. We surmised that this was one of the reasons why the use of VAB increased slower than that of CNB and was less than that of excisional biopsy in this study.
As mentioned above, prognoses may vary depending on the biopsy method and the patient group. In this study, when the patient groups for each biopsy method were analyzed using propensity score matching, similar to CNB, there were no prognostic differences between biopsy methods in either multivariate or univariate analysis. This suggests that the prognosis was affected by differences in the characteristics of the patient group who underwent the biopsy methods rather than the biopsy methods themselves.
The limitations of this study include a selection bias in the biopsy method groups because biopsy methods with different indications were retrospectively analyzed. However, the results of differing clinicopathological characteristics according to the various biopsy methods revealed different indications. Since propensity score matching was only applied to factors observed in this study, there may be bias caused by hidden factors even after matching. In addition, there were relatively few propensity score-matched cases owing to some data defects. No cases were propensity score matched in the stage 0 category; therefore, it could not be reflected in the analysis after the matching. The size of the breast lesion is likely to be measured by imaging because it is cut into several pieces by VAB. Hence, the clinical tumor size may differ from the actual pathological tumor size. As the data used in this study were from a database on which multiple institutions were registered, the cohort was heterogeneous. Most of the cases recorded before the introduction of immunohistochemistry were excluded from the analysis owing to the absence of immunohistochemistry. In some rare cases, breast cancer can be diagnosed by biopsy of non-breast lesions such as lymph nodes; however, there were no available data to distinguish between breast and non-breast lesions. Finally, each biopsy method was introduced at different times, which suggests that CNB and VAB could have influenced the analysis of prognoses due to their relatively short observation periods.
Nevertheless, an objective analysis was possible based on large-scale data registered by multiple institutions. In addition, few studies on breast cancer prognostic comparisons between CNB and VAB have been reported previously; this study showed that the prognosis made using VAB was not different from that using CNB, despite the different characteristics of breast cancer reported for each biopsy method. These results were further highlighted using propensity score-matching analysis.
Biopsy methods for breast lesions have evolved toward being less invasive and more accurate. Although VAB is not a standard method for breast cancer diagnosis, our study revealed no prognostic differences between VAB and CNB. Further research is needed on the role of VAB in the diagnosis of breast cancer in the future.
ACKNOWLEDGMENTS
The data used in this study was provided by the Korean Breast Cancer Society.
Footnotes
Funding: No funding was received for this study.
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kim BK, Sun WY.
- Data curation: Kim BK, Ahn SG, Oh SJ, Kim H, Kang E, Jung Y, Byun KD.
- Formal analysis: Kim BK.
- Investigation: Kim BK.
- Methodology: Kim BK.
- Resources: Ahn SG, Oh SJ, Kim H, Kang E, Jung Y, Byun KD.
- Supervision: Lee J, Sun WY.
- Validation: Lee J.
- Writing - original draft: Kim BK.
- Writing - review & editing: Sun WY.
SUPPLEMENTARY MATERIAL
Clinical characteristics of biopsy methods in propensity score-matched breast cancer cohorts
References
- 1.Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett IC, Saboo A. The evolving role of vacuum assisted biopsy of the breast: a progression from fine-needle aspiration biopsy. World J Surg. 2019;43:1054–1061. doi: 10.1007/s00268-018-04892-x. [DOI] [PubMed] [Google Scholar]
- 3.Gisvold JJ, Goellner JR, Grant CS, Donohue JH, Sykes MW, Karsell PR, et al. Breast biopsy: a comparative study of stereotaxically guided core and excisional techniques. AJR Am J Roentgenol. 1994;162:815–820. doi: 10.2214/ajr.162.4.8140997. [DOI] [PubMed] [Google Scholar]
- 4.Jackman RJ, Nowels KW, Shepard MJ, Finkelstein SI, Marzoni FA., Jr Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology. 1994;193:91–95. doi: 10.1148/radiology.193.1.8090927. [DOI] [PubMed] [Google Scholar]
- 5.Thomas PA, Vazquez MF, Waisman J. Comparison of fine-needle aspiration and frozen section of palpable mammary lesions. Mod Pathol. 1990;3:570–574. [PubMed] [Google Scholar]
- 6.Kline TS, Joshi LP, Neal HS. Fine-needle aspiration of the breast: diagnoses and pitfalls. A review of 3545 cases. Cancer. 1979;44:1458–1464. doi: 10.1002/1097-0142(197910)44:4<1458::aid-cncr2820440440>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Pisano ED, Fajardo LL, Tsimikas J, Sneige N, Frable WJ, Gatsonis CA, et al. Rate of insufficient samples for fine-needle aspiration for nonpalpable breast lesions in a multicenter clinical trial: The Radiologic Diagnostic Oncology Group 5 Study. The RDOG5 investigators. Cancer. 1998;82:679–688. doi: 10.1002/(sici)1097-0142(19980215)82:4<679::aid-cncr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Rakha EA, Ellis IO. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol. 2007;60:1300–1306. doi: 10.1136/jcp.2006.045377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker SH, Jobe WE, Dennis MA, Stavros AT, Johnson KK, Yakes WF, et al. US-guided automated large-core breast biopsy. Radiology. 1993;187:507–511. doi: 10.1148/radiology.187.2.8475299. [DOI] [PubMed] [Google Scholar]
- 10.Britton PD, Flower CD, Freeman AH, Sinnatamby R, Warren R, Goddard MJ, et al. Changing to core biopsy in an NHS breast screening unit. Clin Radiol. 1997;52:764–767. doi: 10.1016/s0009-9260(97)80156-0. [DOI] [PubMed] [Google Scholar]
- 11.Park HL, Kim LS. The current role of vacuum assisted breast biopsy system in breast disease. J Breast Cancer. 2011;14:1–7. doi: 10.4048/jbc.2011.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Londero V, Zuiani C, Linda A, Battigelli L, Brondani G, Bazzocchi M. Borderline breast lesions: comparison of malignancy underestimation rates with 14-gauge core needle biopsy versus 11-gauge vacuum-assisted device. Eur Radiol. 2011;21:1200–1206. doi: 10.1007/s00330-010-2053-7. [DOI] [PubMed] [Google Scholar]
- 13.Sohn YM, Yoon JH, Kim EK, Moon HJ, Kim MJ. Percutaneous ultrasound-guided vacuum-assisted removal versus surgery for breast lesions showing imaging-histology discordance after ultrasound-guided core-needle biopsy. Korean J Radiol. 2014;15:697–703. doi: 10.3348/kjr.2014.15.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang SY, Kim YS, Kim Z, Kim HY, Kim HJ, Park S, et al. Breast cancer statistics in Korea in 2017: data from a breast cancer registry. J Breast Cancer. 2020;23:115–128. doi: 10.4048/jbc.2020.23.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalani N, Jimenez RB, Yeap B. Understanding propensity score analyses. Int J Radiat Oncol Biol Phys. 2020;107:404–407. doi: 10.1016/j.ijrobp.2020.02.638. [DOI] [PubMed] [Google Scholar]
- 16.Hoorntje LE, Schipper ME, Kaya A, Verkooijen HM, Klinkenbijl JG, Borel Rinkes IH. Tumour cell displacement after 14G breast biopsy. Eur J Surg Oncol. 2004;30:520–525. doi: 10.1016/j.ejso.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Kong YC, Bhoo-Pathy N, O’Rorke M, Subramaniam S, Bhoo-Pathy NT, See MH, et al. The association between methods of biopsy and survival following breast cancer: a hospital registry based cohort study. Medicine (Baltimore) 2020;99:e19093. doi: 10.1097/MD.0000000000019093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebens F, Carly B, Cusumano P, Van Beveren M, Beier B, Fastrez M, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas. 2009;62:113–123. doi: 10.1016/j.maturitas.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR Am J Roentgenol. 1999;173:1303–1313. doi: 10.2214/ajr.173.5.10541110. [DOI] [PubMed] [Google Scholar]
- 20.Nagi C, Bleiweiss I, Jaffer S. Epithelial displacement in breast lesions: a papillary phenomenon. Arch Pathol Lab Med. 2005;129:1465–1469. doi: 10.5858/2005-129-1465-EDIBLA. [DOI] [PubMed] [Google Scholar]
- 21.Taxin A, Tartter PI, Zappetti D. Breast cancer diagnosis by fine needle aspiration and excisional biopsy. Recurrence and survival. Acta Cytol. 1997;41:302–306. doi: 10.1159/000332516. [DOI] [PubMed] [Google Scholar]
- 22.King TA, Hayes DH, Cederbom GJ, Champaign JL, Smetherman DH, Farr GH, et al. Biopsy technique has no impact on local recurrence after breast-conserving therapy. Breast J. 2001;7:19–24. doi: 10.1046/j.1524-4741.2001.007001019.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen AM, Haffty BG, Lee CH. Local recurrence of breast cancer after breast conservation therapy in patients examined by means of stereotactic core-needle biopsy. Radiology. 2002;225:707–712. doi: 10.1148/radiol.2253011698. [DOI] [PubMed] [Google Scholar]
- 24.Gornstein B, Jacobs T, Bédard Y, Biscotti C, Ducatman B, Layfield L, et al. Interobserver agreement of a probabilistic approach to reporting breast fine-needle aspirations on ThinPrep. Diagn Cytopathol. 2004;30:389–395. doi: 10.1002/dc.20041. [DOI] [PubMed] [Google Scholar]
- 25.Moschetta M, Telegrafo M, Carluccio DA, Jablonska JP, Rella L, Serio G, et al. Comparison between fine needle aspiration cytology (FNAC) and core needle biopsy (CNB) in the diagnosis of breast lesions. G Chir. 2014;35:171–176. [PMC free article] [PubMed] [Google Scholar]
- 26.Song SY, Hong S, Jun JK. Digital mammography as a screening tool in Korea. J Korean Soc Radiol. 2021;82:2–11. doi: 10.3348/jksr.2021.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter-Ehrenstein C, Maak K, Röger S, Ehrenstein T. Lesions of “uncertain malignant potential” in the breast (B3) identified with mammography screening. BMC Cancer. 2018;18:829. doi: 10.1186/s12885-018-4742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn M, Krainick-Strobel U, Toellner T, Gissler J, Kluge S, Krapfl E, et al. Interdisciplinary consensus recommendations for the use of vacuum-assisted breast biopsy under sonographic guidance: first update 2012. Ultraschall Med. 2012;33:366–371. doi: 10.1055/s-0032-1312831. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Choi HY, Moon BI, Lee SN. Complete removal of a breast mass by US-guided mammotome biopsy: histologic assessment by marginal sampling. J Korean Radiol Soc. 2005;53:289–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical characteristics of biopsy methods in propensity score-matched breast cancer cohorts