Abstract
Introduction:
Testicular germ cell tumors, particularly nonseminomatous germ cell tumors (NSGCT), comprise the most common solid malignancy in male children and younger adults. While these patients experience excellent survival outcomes, few studies have characterized their survival by age. Thus, we aimed to characterize the relative survival of NSGCT by age, stratifying patients by stage group.
Methods:
Using the Surveillance Epidemiology and End Results (SEER) database, we divided patients with NSGCT into pediatric patients and adolescents (<19 years), young adults (19–30 years), and older adults (>30 years). Survival analysis, using Cox proportional hazards models and Kaplan Meier curves, described overall and cancer-specific survival (CSS) of each age category for Stage I-III NSGCT by stage group.
Results:
A total of 14,786 patients met inclusion criteria and comprised the age groups <19 years (N=1,287), 19–30 years (N=7,729), and >30 years (N=5,770). Stage group distribution at presentation was similar between each group. Survival analysis demonstrated no differences in cancer-specific survival (CSS) among Stage I or II NSGCT. However, among Stage III tumors, multivariable models noted worse CSS in patients >30 years (HR=3.35 (95%CI: 1.45–7.73), p=0.005) and those 19–30 years (HR=2.28 (95%CI: 0.99–5.21), p=0.053) compared to pediatric and adolescent patients.
Conclusions:
Younger NSGCT patients experience excellent oncologic outcomes compared to their older counterparts. These survival differences by age group are largely driven by differential survival among Stage III neoplasms. Furthermore, our report lends additional evidence that age is an important prognostic factor in advanced NSGCT, including pediatric and adolescent patients.
Keywords: testicular cancer, nonseminomatous germ cell tumor, pediatric oncology
Introduction
While testicular cancer accounts for only roughly 1% of male tumors, it remains the most common solid malignancy in younger men and adolescents. Among the over 9,000 new cases in 2020, the majority will have nonseminomatous germ cell tumor (NSGCT), particularly in patients under 30 years old.1, 2, 3 Prior analyses often cite 5-year overall survival (OS) and cancer-specific survival (CSS) >80% among all patients.4 However, a relative paucity of data regarding survival in pediatric (<19 years) and younger adult (19–30 years) patients remains.
Prior studies have leveraged large administrative databases, such as the Surveillance, Epidemiology, and End Results Registry (SEER) and National Cancer Database, to better characterize testicular cancer survival among these populations. These studies have noted improved OS and CSS among adolescent patients compared to young adults (≥ 20 years).5 However, no prior studies have stratified patients by the American Joint Committee on Cancer (AJCC) staging grouping. As a result, it remains unclear if age applies a homogenous effect on testicular cancer outcomes across stages or is more impactful in particular cancer stages. More recently, a contemporary analysis of advanced NSGCT from the International Germ Cell Cancer Collaborative Group (IGCCCG) found increasing age to portend worse progression-free survival among advanced NSGCT.6 Thus, we hypothesize that age is associated poorer survival outcomes, particularly in advanced disease. To examine this, we studied the survival of males with NSGCT in a stage-stratified approach among pediatric and adolescent patients (<19 years), young adults (19 – 30 years), and older adults (>30 years).
Methods
Data Collection and Source
The National Cancer Institute populates and maintains the Surveillance, Epidemiology, and End Results (SEER) program. The database covers approximately 34% of the United States population. It incorporates data regarding OS, CSS, patient demographics, insurance status follow up time, and tumor characteristics (such as tumor size, pathologic features, histology, TMNS staging, and site of metastasis).7
Study Population
Patients with Stage I-III NSGCT of testicular or mediastinal origin from 1988–2016 were included. Staging information directly from SEER was incorporated when grouping patients into Stage Group I, II, or III. Additionally, staging data was based on variables from the AJCC 7th Edition. Though SEER does not list IGCCCG risk groups (good, intermediate, and poor), we constructed these groups using available TMNS (including post-orchiectomy tumor marker data) information. Patients with incomplete staging, histology or age data were excluded from analysis.
Statistical Analysis
Patients meeting inclusion criteria were stratified into three age groups: pediatric (0–18 years), young adults (19–30 years), older adults (>30 years). The pediatric group was further divided into pre-pubertal (0–10 years) and pubertal (11–18 years). Means and medians assessed continuous variables. Proportions and frequencies characterized categorical variables. A multipart histogram illustrated frequency of different NSGCTs at age of presentation within each histology (yolk sac, embryonal, teratoma, mixed germ cell tumor, choriocarcinoma).
Survival analysis was stratified into Stage I, II and III NSGCT. To preserve sample size within the main analysis, pre-pubertal and pubertal patients grouped together. Within each stage strata, we calculated relative survival using Cox proportional hazards models (univariable and multivariable) and Kaplan Meier Curves. Covariates in multivariable analysis were selected based on relevant pathology data available in SEER and prior literature examining testicular cancer outcomes. To assess for collinearity between covariates, variance inflation factor was calculated for regression analyses. Additionally, among Stage III NSGCT our multivariable analysis controlled for IGCCCG risk category as well.
To distinguish pre-pubertal and pubertal NSGCT, a sub-analysis was performed. First, CSS and OS were assessed between those 0–10, 11–18, 19–30, >30 years, not stratified by stage. Another survival analysis with these age groups was conducted among Stage I patients, stratified by age group. However, the limited sample size of pre-pubertal patients precluded their analyses among Stage II (N=8) and Stage III (N=5) patients. To preserve data granularity, stage-stratified survival analyses also assessed age as a continuous variable. Lastly, a sub-analysis of only testicular germ cell tumors (excluding mediastinal tumors) was performed.
All statistical analyses, including figure generation, were performed using STATA software (version 15.0, StataCorp, College Station, Texas) and 2-sided α was set to 0.05.
Results
Cohort Demographics
The final cohort (N=14,786) encompassed patients <19 years (N=1,287), 19–30 years (N=7,729), and >30 years (N=5,770) with NSGCT. The median follow-up for all patients was 7.50 years (IQR: 3.33–12.50). Within the measured follow-up, 770 patients died from testicular cancer and 1,271 patients died from all causes, including testicular cancer. Mean age among pediatric patients, young adult, and older adult patients was 14.31 (SD=5.87), 24.66 (SD=3.29), and 39.56 (SD=8.13) years, respectively. Hispanic patients comprised a greater percentage of pediatric patients (39.08%) compared to young adults (28.99%) and older adults (14.99%) (p<0.001). Conversely, the two older groups had a higher proportion of White and uninsured patients (Table 1). Additionally, pediatric patients had a greater percentage of yolk sac tumors (13.68%, p<0.001) compared to older groups. Figure 1 further describes the histology distribution by age; mixed GCT was the most common histology, followed by embryonal histology. Lastly, we found no statistical differences in stage group (p=0.151) or lymphovascular invasion (p=0.601) at presentation (Table 1). Data regarding chemotherapy regimens, post-chemotherapy retroperitoneal lymph node dissection (RPLND), and salvage chemotherapy were not provided in our data. IGCCCG risk categories, constructed based on available TNMS staging data, are described in Supplement Table 1.
Table 1 –
Cohort Demographics
| Age <11 | Age 11–18 | Age 19 – 30 | Age >30 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Variables | Mean / Count | SD / Percentage | Mean / Count | SD / Percentage | Mean / Count | SD / Percentage | Mean / Count | SD / Percentage | p value | |
| N | 205 | 1082 | 7729 | 5770 | ||||||
| Follow-up | years | 9.13 | 7.18 | 8.64 | 6.04 | 8.58 | 6.55 | 8.78 | 6.51 | 0.001 |
| Age | years | 1.18 | 1.45 | 16.80 | 1.30 | 24.66 | 3.29 | 39.56 | 8.13 | <0.001 |
| Ethnicity | White | 72 | 35.12% | 585 | 54.07% | 4,839 | 62.61% | 4,473 | 77.52% | <0.001 |
| Black | 15 | 7.32% | 11 | 1.02% | 159 | 2.06% | 148 | 2.56% | ||
| Native American | 3 | 1.46% | 10 | 0.92% | 93 | 1.20% | 39 | 0.68% | ||
| Asian | 36 | 17.56% | 46 | 4.25% | 315 | 4.08% | 183 | 3.17% | ||
| Hispanic | 78 | 38.05% | 425 | 39.28% | 2,241 | 28.99% | 865 | 14.99% | ||
| Unknown | 1 | 0.49% | 5 | 0.46% | 82 | 1.06% | 62 | 1.07% | ||
| Insurance | Insured | 51 | 24.88% | 417 | 38.54% | 2,980 | 38.56% | 2,353 | 40.78% | |
| Medicaid | 59 | 28.78% | 201 | 18.58% | 871 | 11.27% | 425 | 7.37% | ||
| Uninsured | 0 | 0.00% | 24 | 2.22% | 551 | 7.13% | 270 | 4.68% | ||
| Unknown | 95 | 46.34% | 440 | 40.67% | 3,327 | 43.05% | 2,722 | 47.18% | ||
| Stage | Tis | 0 | 0.00% | 0 | 0.00% | 4 | 0.05% | 1 | 0.02% | <0.001 |
| 1A-B | 114 | 55.61% | 411 | 37.99% | 3,594 | 46.50% | 2,841 | 49.24% | ||
| 2A | 2 | 0.98% | 76 | 7.02% | 584 | 7.56% | 391 | 6.78% | ||
| 2B | 6 | 2.93% | 106 | 9.80% | 499 | 6.46% | 364 | 6.31% | ||
| 2C | 0 | 0.00% | 21 | 1.94% | 132 | 1.71% | 80 | 1.39% | ||
| 1S | 78 | 38.05% | 262 | 24.21% | 1,594 | 20.62% | 1,134 | 19.65% | ||
| 3NOS | 0 | 0.00% | 24 | 2.22% | 216 | 2.79% | 174 | 3.02% | ||
| 3A | 0 | 0.00% | 38 | 3.51% | 225 | 2.91% | 192 | 3.33% | ||
| 3B | 2 | 0.98% | 52 | 4.81% | 308 | 3.98% | 175 | 3.03% | ||
| 3C | 3 | 1.46% | 92 | 8.50% | 573 | 7.41% | 418 | 7.24% | ||
| Stage Group | I | 192 | 93.66% | 673 | 62.20% | 5,188 | 67.12% | 3,975 | 68.89% | <0.001 |
| II | 8 | 3.90% | 203 | 18.76% | 1,215 | 15.72% | 835 | 14.47% | ||
| III | 5 | 2.44% | 206 | 19.04% | 1,322 | 17.10% | 959 | 16.62% | ||
| Histology | Yolk Sac | 148 | 72.20% | 28 | 2.59% | 186 | 2.41% | 171 | 2.96% | <0.001 |
| Embryonal | 4 | 1.95% | 186 | 17.19% | 1,648 | 21.32% | 1,231 | 21.33% | ||
| Teratoma | 45 | 21.95% | 147 | 13.59% | 862 | 11.15% | 523 | 9.06% | ||
| Mixed GCT | 6 | 2.93% | 649 | 59.98% | 4,591 | 59.40% | 3,541 | 61.37% | ||
| Choriocarcionma | 2 | 0.98% | 72 | 6.65% | 442 | 5.72% | 304 | 5.27% | ||
| Tumor | ||||||||||
| Origin | Gonadal | 190 | 92.68% | 1,056 | 97.60% | 7,653 | 99.02% | 5,723 | 99.19% | |
| Mediastinal | 15 | 7.32% | 26 | 2.40% | 76 | 0.98% | 47 | 0.81% | <0.001 | |
| LVI | 15 | 36.59% | 147 | 40.16% | 1,133 | 42.45% | 792 | 42.17% | 0.601 | |
| Laterality | Right | 97 | 50.79% | 574 | 54.41% | 3,982 | 52.43% | 3,037 | 53.68% | 0.303 |
| Left | 94 | 49.21% | 481 | 45.59% | 3,613 | 47.57% | 2,621 | 46.32% | ||
SD = standard deviation; 3NOS = 3 Not otherwise specified; GCT = germ cell tumor; mm = millimeter; LVI = lymphovascular invasion
Figure 1:
Distribution of Histology and NSGCT by Age
Stage Stratified Survival Analysis
When examining CSS among Stage I NSGCT, we found no statistical association between age group and survival (19–30 years: HR=1.72 (95% CI: 0.39–7.62), p=0.476; >30 years: HR=1.52 (95% CI: 0.32–7.16), p=0.596). Similar results were noted when assessing OS (19–30 years: HR=1.60 (95% CI: 0.47–5.39), p=0.448); >30 years: HR=2.73 (95% CI: 0.80–9.25), p=0.108). Compared to White patients, multivariable analysis demonstrated worse CSS and OS among Native American and Black patients (Table 2). Patients on Medicaid or without insurance had worse survival than other insured patients. Within stage I NSGCT, all three age groups experienced 5-year CSS and OS >90% (Figure 2).
Table 2A –
Cancer-specific Survival of Stage I NSGCT by Age Category
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.16 | 0.62 | 2.18 | 0.649 | 1.55 | 0.34 | 7.05 | 0.57 | |
| >30 | 1.44 | 0.76 | 2.71 | 0.259 | 1.49 | 0.31 | 7.21 | 0.619 | |
| Histology | Mixed GCT | REF | REF | REF | REF | REF | REF | REF | REF |
| Embryonal | 0.95 | 0.63 | 1.44 | 0.81 | 0.63 | 0.21 | 1.88 | 0.41 | |
| Teratoma | 1.15 | 0.73 | 1.81 | 0.535 | 0.59 | 0.08 | 4.42 | 0.605 | |
| Yolk Sac | 1.88 | 0.98 | 3.61 | 0.059 | 0.97 | 0.12 | 7.67 | 0.98 | |
| Choriocarcinoma | 1.21 | 0.58 | 2.49 | 0.612 | 1.81 | 0.42 | 7.85 | 0.425 | |
| Race | White | REF | REF | REF | REF | REF | REF | REF | REF |
| Black | 3.04 | 1.48 | 6.26 | 0.002 | 2.57 | 0.57 | 11.58 | 0.219 | |
| Native American | 1.57 | 0.39 | 6.37 | 0.527 | 5.34 | 1.16 | 24.54 | 0.031 | |
| Asian | 1.40 | 0.65 | 3.00 | 0.394 | 0.93 | 0.12 | 7.04 | 0.942 | |
| Hispanic | 1.71 | 1.20 | 2.42 | 0.003 | 1.19 | 0.51 | 2.77 | 0.689 | |
| Laterality | Left | 1.11 | 0.81 | 1.52 | 0.521 | 0.68 | 0.33 | 1.40 | 0.294 |
| Insurance | Insured | REF | REF | REF | REF | REF | REF | REF | REF |
| Medicaid | 2.53 | 1.29 | 4.97 | 0.007 | 2.93 | 1.06 | 8.14 | 0.039 | |
| Uninsured | 4.05 | 2.13 | 7.69 | <0.001 | 8.30 | 3.53 | 19.48 | <0.001 | |
| Unknown | 1.87 | 1.22 | 2.86 | 0.004 | 5.66 | 1.56 | 20.49 | 0.008 | |
| LVI | 1.79 | 0.89 | 3.57 | 0.101 | 2.05 | 1.00 | 4.21 | 0.05 | |
HR = hazard ratio; 95% CI = 95% confidence interval; LVI = lymphovascular invasion; GCT = germ cell tumor
Figure 2A:
Cancer-specific Survival of Stage I NSGCT by Age Group
Similarly, among the different age groups, Stage II patients did not experience inferior CSS or OS. Histology predicted survival among these patients, as those with yolk sac (CSS HR=6.61 (95% CI: 3.72–11.74), p<0.001) and choriocarcinoma (CSS HR=5.32 (95% CI: 2.96–9.56), p<0.001) experienced inferior outcomes. Conversely, those with embryonal histology (CSS HR=0.24 (95% CI: 0.10–0.56), p = 0.001) had improved oncologic outcomes. Additionally, poorer CSS and OS among Hispanic and Medicaid-receiving Stage II NSGCT patients was observed (Table 3). Similar to Stage I disease, Stage II patients experience CSS and OS >90%, regardless of age group – no statistical differences in survival were noted by Kaplan Meier estimates (Figure 3).
Table 3A –
Cancer-specific Survival of Stage II NSGCT by Age Category
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.03 | 0.49 | 2.19 | 0.93 | 1.42 | 0.66 | 3.05 | 0.37 | |
| >30 | 1.20 | 0.56 | 2.57 | 0.645 | 2.03 | 0.91 | 4.51 | 0.082 | |
| Histology | Mixed GCT | REF | REF | REF | REF | ||||
| Embryonal | 0.22 | 0.09 | 0.52 | 0.001 | 0.25 | 0.11 | 0.59 | 0.002 | |
| Teratoma | 0.94 | 0.44 | 1.99 | 0.873 | 1.11 | 0.52 | 2.37 | 0.787 | |
| Yolk Sac | 6.78 | 3.83 | 11.98 | <0.001 | 6.42 | 3.60 | 11.45 | <0.001 | |
| Choriocarcinoma | 5.15 | 2.88 | 9.23 | <0.001 | 5.40 | 3.00 | 9.71 | <0.001 | |
| Race | White | REF | REF | REF | REF | REF | REF | REF | REF |
| Black | 2.90 | 1.05 | 8.03 | 0.041 | 1.90 | 0.67 | 5.35 | 0.227 | |
| Native American | - | - | - | - | - | - | - | - | |
| Asian | 1.84 | 0.73 | 4.63 | 0.192 | 1.56 | 0.62 | 3.96 | 0.348 | |
| Hispanic | 2.17 | 1.39 | 3.39 | 0.001 | 1.79 | 1.11 | 2.88 | 0.017 | |
| Insurance | Insured | REF | REF | REF | REF | REF | REF | REF | REF |
| Medicaid | 2.37 | 1.37 | 4.13 | 0.002 | 1.96 | 1.11 | 3.47 | 0.021 | |
| Uninsured | 1.61 | 0.68 | 3.85 | 0.279 | 1.21 | 0.50 | 2.91 | 0.673 | |
| Unknown | 0.69 | 0.41 | 1.16 | 0.161 | 0.82 | 0.49 | 1.39 | 0.467 | |
| LVI | 0.22 | 0.05 | 1.05 | 0.058 | - | - | - | - | |
HR = hazard ratio; 95% CI = 95% confidence interval; LVI = lymphovascular invasion; GCT = germ cell tumor
Figure 3A:
Cancer-specific Survival of Stage II NSGCT by Age Group
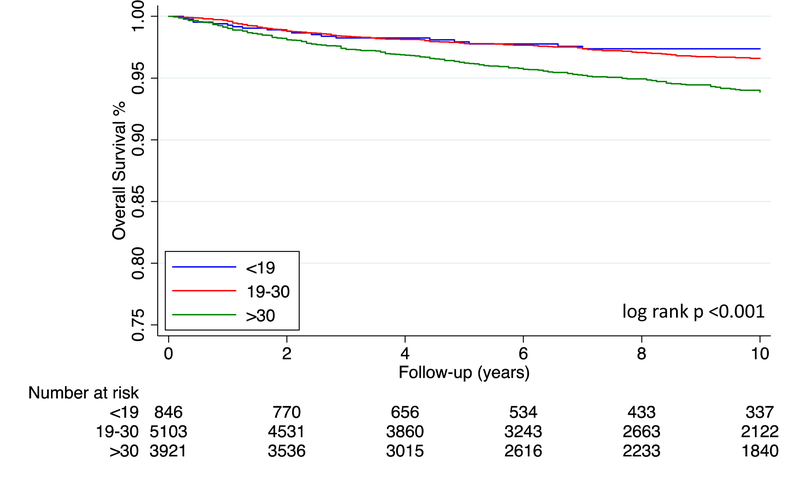
In contrast to Stage I-II NSGCT, multivariable analysis reveals inferior outcomes among Stage III patients with increasing age group (Table 4). When controlling for IGCCCG risk category, patients >30 years old had poorer CSS (HR=3.35 (95% CI: 1.45–7.73), p=0.005) Similar findings were noted those 19–30 years old (HR=2.27 (95% CI: 0.99–5.21), p=0.053) (Table 4A). Likewise, patients 19–30 years old (HR=2.50 (95% CI: 1.10–5.74), p=0.029) and those over 30 years old (HR=4.34 (95% CI: 1.90–9.94), p=0.001) experienced worse OS compared to patients <19 years old (Table 4B). Patients with choriocarcinoma, of Asian ethnicity, and insured by Medicaid had worse OS and CSS. These survival differences are further illustrated in Figure 4; 5-year CSS for those <19, 19–30, and >30 is 88.23%, 82.22%, and 74.13% (log rank p<0.001), respectively. Table 5 collates and summarizes CSS outcomes from the stage-stratified analysis detailed in Tables 2–4.
Table 4A –
Cancer-specific Survival of Stage III NSGCT by Age Category
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.53 | 1.01 | 2.32 | 0.044 | 2.28 | 0.99 | 5.24 | 0.052 | |
| >30 | 2.35 | 1.55 | 3.55 | <0.001 | 3.55 | 1.53 | 8.23 | 0.003 | |
| IGCCCG Risk Category | Good | REF | REF | REF | REF | REF | REF | REF | REF |
| Intermediate | 1.92 | 0.90 | 4.10 | 0.091 | 1.96 | 0.91 | 4.22 | 0.084 | |
| Poor | 5.22 | 2.83 | 9.63 | <0.001 | 4.92 | 2.63 | 9.19 | <0.001 | |
| Histology | Mixed GCT | REF | REF | REF | REF | REF | REF | REF | REF |
| Embryonal | 0.78 | 0.59 | 1.03 | 0.081 | 1.28 | 0.81 | 2.02 | 0.29 | |
| Teratoma | 1.37 | 0.98 | 1.92 | 0.066 | 1.03 | 0.55 | 1.94 | 0.921 | |
| Yolk Sac | 1.79 | 1.22 | 2.61 | 0.003 | 0.94 | 0.49 | 1.83 | 0.865 | |
| Choriocarcinoma | 2.30 | 1.82 | 2.92 | <0.001 | 1.77 | 1.21 | 2.58 | 0.003 | |
| Race | White | REF | REF | REF | REF | REF | REF | REF | REF |
| Black | 1.22 | 0.73 | 2.06 | 0.449 | 0.81 | 0.35 | 1.85 | 0.61 | |
| Native American | 1.52 | 0.75 | 3.08 | 0.243 | 1.27 | 0.50 | 3.22 | 0.613 | |
| Asian | 1.90 | 1.29 | 2.78 | 0.001 | 1.71 | 0.96 | 3.04 | 0.067 | |
| Hispanic | 1.20 | 0.98 | 1.47 | 0.071 | 0.74 | 0.52 | 1.04 | 0.085 | |
| Insurance | Insured | REF | REF | REF | REF | REF | REF | REF | REF |
| Medicaid | 1.91 | 1.51 | 2.40 | <0.001 | 1.71 | 1.24 | 2.38 | 0.001 | |
| Uninsured | 1.40 | 1.00 | 1.97 | 0.053 | 1.02 | 0.59 | 1.76 | 0.945 | |
| Unknown | 1.25 | 0.98 | 1.58 | 0.068 | 0.66 | 0.09 | 4.79 | 0.679 | |
| LVI | 0.94 | 0.68 | 1.30 | 0.69 | - | - | - | - | |
HR = hazard ratio; 95% CI = 95% confidence interval; LVI = lymphovascular invasion; GCT = germ cell tumor
Table 4B –
Overall Survival of Stage III NSGCT by Age Category
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.58 | 1.08 | 2.32 | 0.019 | 2.50 | 1.09 | 5.72 | 0.031 | |
| >30 | 2.68 | 1.83 | 3.92 | <0.001 | 4.48 | 1.95 | 10.28 | <0.001 | |
| IGCCCG Risk Category | Good | REF | REF | REF | REF | REF | REF | REF | REF |
| Intermediate | 0.96 | 0.51 | 1.81 | 0.901 | 1.00 | 0.53 | 1.90 | 0.992 | |
| Poor | 3.02 | 1.94 | 4.71 | <0.001 | 2.89 | 1.83 | 4.57 | <0.001 | |
| Histology | Mixed GCT | REF | REF | REF | REF | REF | REF | REF | REF |
| Embryonal | 0.83 | 0.65 | 1.05 | 0.114 | 1.19 | 0.78 | 1.81 | 0.422 | |
| Teratoma | 1.24 | 0.91 | 1.69 | 0.176 | 1.10 | 0.63 | 1.92 | 0.747 | |
| Yolk Sac | 1.91 | 1.37 | 2.66 | <0.001 | 1.11 | 0.63 | 1.95 | 0.730 | |
| Choriocarcinoma | 2.15 | 1.73 | 2.66 | <0.001 | 1.65 | 1.16 | 2.35 | 0.005 | |
| Race | White | REF | REF | REF | REF | ||||
| Black | 1.47 | 0.96 | 2.24 | 0.075 | 1.04 | 0.53 | 2.07 | 0.906 | |
| Native American | 1.50 | 0.80 | 2.81 | 0.207 | 1.20 | 0.48 | 3.02 | 0.691 | |
| Asian | 1.64 | 1.14 | 2.35 | 0.008 | 1.67 | 0.98 | 2.85 | 0.058 | |
| Hispanic | 1.12 | 0.94 | 1.35 | 0.203 | 0.75 | 0.55 | 1.04 | 0.082 | |
| Insurance | Insured | REF | REF | REF | REF | REF | REF | REF | REF |
| Medicaid | 1.73 | 1.40 | 2.13 | <0.001 | 1.60 | 1.19 | 2.16 | 0.002 | |
| Uninsured | 1.37 | 1.01 | 1.86 | 0.042 | 1.14 | 0.71 | 1.82 | 0.587 | |
| Unknown | 1.23 | 1.00 | 1.52 | 0.051 | 0.54 | 0.07 | 3.96 | 0.547 | |
| LVI | 0.94 | 0.70 | 1.27 | 0.701 | - | - | - | - | |
HR = hazard ratio; 95% CI = 95% confidence interval; LVI = lymphovascular invasion; GCT = germ cell tumor
Figure 4A:
Cancer-specific Survival of Stage III NSGCT by Age Group
Table 5 –
Cancer-specific Survival of NSGCT by Age Category and Stage Group
| STAGE I | |||||||||
| Univariable | Multivariable * | ||||||||
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.16 | 0.62 | 2.18 | 0.649 | 1.55 | 0.34 | 7.05 | 0.57 | |
| >30 | 1.44 | 0.76 | 2.71 | 0.259 | 1.49 | 0.31 | 7.21 | 0.619 | |
|
| |||||||||
| STAGE II | |||||||||
| Univariable | Multivariable * | ||||||||
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.03 | 0.49 | 2.19 | 0.93 | 1.42 | 0.66 | 3.05 | 0.37 | |
| >30 | 1.2 | 0.56 | 2.57 | 0.645 | 2.03 | 0.91 | 4.51 | 0.082 | |
|
| |||||||||
| STAGE III | |||||||||
| Univariable | Multivariable * | ||||||||
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.53 | 1.01 | 2.32 | 0.044 | 2.28 | 0.99 | 5.24 | 0.052 | |
| >30 | 2.35 | 1.55 | 3.55 | <0.001 | 3.55 | 1.53 | 8.23 | 0.003 | |
| IGCCCG Risk Category | Good | REF | REF | REF | REF | REF | REF | REF | REF |
| Intermediate | 1.92 | 0.9 | 4.1 | 0.091 | 1.96 | 0.91 | 4.22 | 0.084 | |
| Poor | 5.22 | 2.83 | 9.63 | <0.001 | 4.92 | 2.63 | 9.19 | <0.001 | |
Adjusted for histology, race, insurance status, and lymphovascular invasion
HR = hazard ratio; 95% CI = 95% confidence interval; GCT = germ cell tumor
Pediatric NSGCT Sub-analysis
When dividing pediatric patients into pre-pubertal and pubertal, we noted that among all NSGCT stages, pre-pubertal patients (<11 years) had the most favorable survival outcomes (CSS HR=0.05 (95%CI: 0.01–0.20), p<0.001) (Supplemental Table 2 and Figure 1). When focusing only on Stage I NSGCT, however, statistical differences in CSS or OS were not observed (Supplemental Table 3 and Figure 2).
Additional Sub-analyses
To maximize data granularity, we assessed age as a continuous variable in a stage-stratified analysis – described in Supplemental Table 4. Age remained a statistically important predictor of CSS in Stage II and Stage III. Additionally, an analysis restricted only to those with testis-origin germ cell tumors (excluding mediastinal neoplasm) is described in Supplemental Table 5.
Discussion
Testicular cancer, the most common solid malignancy in young men, often has excellent survival. We present a large sample-size study of almost 15,000 patients and perform survival analysis of NSGCTs, stratifying by stage group. Upon dividing our patients into pediatric patients (<19 years), young adults (19–30 years), and older adults (>30 years), the stage group distribution was similar between each age category (Table 1). Patients <19 years more often had yolk sac tumors compared to older patients, but had a slightly lower percentage of mixed GCT, the most common histology in our cohort (Table 1, Figure 1). Higher rates of yolk sac tumors in the pediatric and young adult cohort are expected, since type 1 GCT would be included. Similarly, we noted excellent survival and no statistical differences in CSS or OS in each age group for Stage I and II NSGCT (Table 2 & 3). However, among Stage III patients we found that older patients had worse CSS and OSS, even after controlling for IGCCCG risk category (Table 4, Figure 4).
Prior studies have utilized large databases, such as SEER, to characterize oncologic outcomes among pediatric patients and young adults. While informative, these prior studies have not performed survival analysis with stage stratification.8, 9 In a recent analysis Amini et al., compared adolescents (13–19 years) to those >20 years with NSGCT and found similar CSS (p=0.139), but improved OS among adolescent patients (p=0.007). Additionally, their analysis implemented a logistic regression, finding that younger patients more often present with advanced disease (OR=1.16 (95% CI: 1.01–1.35), p=0.039). However, the raw proportions were similar between the two groups; 55.1% of adolescent patients had localized disease versus 58.1% of those >20 years. Furthermore, rather than group patients by AJCC stage group, the authors characterized patients as having localized, regional or distant disease and did not control for IGCCCG risk classification.5
In our grouped analysis, similar to Amini et al., we noted a small, yet statistically significant, decrease in OS among older patients in all NSGCTs. Our unstratified analysis also detected small differences in CSS by age group (Supplemental Figure 1). Of note, Amini et al. divided their cohort dichotomously into adolescents and those >20 years as opposed to our four-tier approach in the non-stage stratified analysis. When dividing NSGCTs by stage group, we only found significant survival differences by age categories in Stage III tumors (Figure 4), as Stage I and II NSGCT had similar survival in each age category. This differential survival persisted even after controlling for IGCCCG risk classification, which dictate primary chemotherapy regimens in advanced testicular cancer.10 Thus, our stratified approach allowed us to illustrate that Stage III NSGCT may largely explain the overall un-stratified differential survival by age group.
Our analysis adds to a growing body of evidence suggesting that age impacts oncologic outcomes in those with advanced and metastatic NSGCT.11 In a two-institution study, Necchi et al. identified age as a key predictor of relapse-free survival (age, per year: HR=1.02 (95%CI: 1.00–1.03), p=0.015). In a proposed IGCCCG reclassification, the authors subsequently identify age ≥ 30 years as an important risk factor among intermediate and poor risk NSGCT.12, 13 Our findings among Stage III NSGCT mirror these results, as those >30 years of age exhibit worse CSS and OS.
Furthermore, the prognostic value of age may extend into the adolescent population as well. Marina et al. examined 165 malignant GCTs, most of sacrococcygeal and thoracic origin. Survival analysis found age as the most predictive factor in event-free survival (EFS) (defined as disease progression, relapse, second malignancy, or death). Specifically, those patients ≥ 12 years old had distinctly worse EFS than their younger counter parts (48.9% vs. 84.1%, p<0.0001).14 Similarly, in a multi-institutional study, Frazier et al. assessed the survival of pediatric NSGCTs in reference to the applied IGCCCG risk classification. Compared to younger patients, those 15–18 years old have lower EFS (HR=2.2, p=0.0166) and OS (HR=2.7, p=0.0097), even after controlling for chemotherapy regimen.15 Though, with slightly different age partitions, we also found that adolescents and older teenagers have worse outcomes than the youngest NSGCT patients (Supplemental Figure 1). While, both groups experienced 5-year CSS >90%, the ability to tolerate chemotherapy regimens and higher-grade neoplasms may explain the differential survival. Age cutoffs, defining pre- and post-pubertal patients, may also influence outcomes reported in this paper and prior studies. However, our consistency with prior studies, allows us to confirm improved outcomes among pre-pubertal GCT patients.
These survival differences may be a function of more than just tumor biology and instead reflect differences in healthcare delivery within the NSGCT cohort. The youngest patients, infants and young children, may more routinely have pediatrician visits. Furthermore, these patients undergo documented testicular examination by a provider – over 99% in a recent analysis.16 These rates drop in post-pubertal patients and even further so among adult patients. Minority patients or those without private insurance may also experience delays in care.17 Our results demonstrate that Medicaid patients experience worse outcomes than privately insured patients. This may have a larger magnitude among adult patients, where treatment may not be standardized between community and safety net hospitals. Conversely, pediatric patients with advanced disease will often be referred to high-volume centers, with expertise in pediatric surgery, advanced urologic oncology, and medical oncology. Integration of care for these patients can help overcome some of the sociodemographic factors in NSGCT outcomes.18
Our paper, powered by data from SEER, has several limitations to note, such as its retrospective nature. Due to data limitations, we could not incorporate precise comorbidity information – a key confounder when performing an age-stratified survival analysis. Additionally, staging data, especially regarding serum tumor markers and metastases site, are missing in some patients – especially those recorded prior to 2004. SEER defines S stage based on post-orchiectomy tumor markers – however, the precise timing with respect to tumor marker half-lives is not clear. Furthermore, only lung, liver, bone, and brain (the most common metastatic sites in testicular cancer) are recorded. Consequently, this affects our ability to accurately classify patients into their respective IGCCCG risk class. To mitigate this, we have excluded patients with missing staging and histology data. SEER also does not capture chemotherapy data, including number of cycles, which differentiates good and intermediate / poor risk NSGCT treatment. As a result, we do not have information regarding which patients receive and tolerate their recommended chemotherapy regimen. Data regarding rates of post-chemotherapy RPLND and salvage chemotherapy are also not provided.
Of note, as key pathology details (S stage, LVI, and metastasis site) are only available after 2004, multivariable survival analyses essentially contained data from 2004 onwards. However, this also means that conclusions drawn from our multivariable analysis are based on the most current data in our cohort. Also, treatment regimens and expertise in testicular cancer has evolved and this may slightly influence outcomes over time. Additionally, as long-term survival outcomes in testicular cancer are >90%, detecting survival differences among age groups may be challenging, even with our study’s large sample size. Lastly, our division of young adults (19–30 years) and older adults (>30 years) may influence some survival analysis results. While chosen based on prior literature and age distribution within SEER, we acknowledge that choosing different age cutoffs may result in slightly different survival results.
Despite these limitations, our analysis allows us to perform stage group-stratified analysis of NSGCTs among pediatric patients, young adults, and older adults. We find that previously reported survival differences, with improved outcomes in young patients, may be primarily driven by Stage III NSGCT. Furthermore, age may significantly impact outcomes in advanced NSGCT not only in adults, but also among pediatric patients.
Conclusion
This study presents a stage group stratified survival analysis of NSGCT, dividing patients into pediatric (<19 years), young adults (19–30 years), and older adults (>30 years) using the SEER registry. While CSS and OS are similar between the age subgroups for Stage I-II NSGCT, differential CSS and OS is seen among patients with Stage III NSGCT. Thus, age emerges as a key risk factor among Stage III tumors in both the adult and pediatric population. This not only explains previously described improved survival in younger NSGCT patients, but also suggests that age harbors key prognostic information in those with metastatic disease.
Supplementary Material
Figure 2B:
Overall Survival of Stage I NSGCT by Age Group
Figure 3B:
Overall Survival of Stage II NSGCT by Age Group
Figure 4B:
Overall Survival of Stage III NSGCT by Age Group
Table 2B –
Overall Survival of Stage I NSGCT by Age Category
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.18 | 0.76 | 1.84 | 0.465 | 1.56 | 0.46 | 5.33 | 0.478 | |
| >30 | 2.59 | 1.68 | 4.00 | <0.001 | 2.79 | 0.81 | 9.60 | 0.104 | |
| Histology | Mixed GCT | REF | REF | REF | REF | REF | REF | REF | REF |
| Embryonal | 1.03 | 0.81 | 1.30 | 0.836 | 0.66 | 0.29 | 1.48 | 0.311 | |
| Teratoma | 1.10 | 0.85 | 1.42 | 0.476 | 1.65 | 0.64 | 4.24 | 0.297 | |
| Yolk Sac | 1.27 | 0.81 | 2.00 | 0.304 | 0.65 | 0.09 | 4.86 | 0.672 | |
| Choriocarcinoma | 0.82 | 0.50 | 1.34 | 0.435 | 1.06 | 0.25 | 4.40 | 0.941 | |
| Race | White | REF | REF | REF | REF | REF | REF | REF | REF |
| Black | 2.20 | 1.35 | 3.58 | 0.002 | 3.38 | 1.17 | 9.74 | 0.024 | |
| Native American | 1.30 | 0.54 | 3.13 | 0.565 | 3.65 | 0.85 | 15.68 | 0.081 | |
| Asian | 1.11 | 0.68 | 1.81 | 0.669 | 1.52 | 0.46 | 5.01 | 0.490 | |
| Hispanic | 1.28 | 1.02 | 1.60 | 0.035 | 1.40 | 0.74 | 2.66 | 0.302 | |
| Laterality | Left | 0.96 | 0.79 | 1.15 | 0.647 | 1.04 | 0.62 | 1.75 | 0.884 |
| Insurance | Insured | REF | REF | REF | REF | REF | REF | REF | REF |
| Medicaid | 2.08 | 1.32 | 3.28 | 0.002 | 1.69 | 0.80 | 3.58 | 0.171 | |
| Uninsured | 2.23 | 1.36 | 3.66 | 0.001 | 3.19 | 1.62 | 6.27 | 0.001 | |
| Unknown | 1.46 | 1.11 | 1.91 | 0.006 | 2.56 | 0.90 | 7.33 | 0.079 | |
| LVI | 1.54 | 0.92 | 2.58 | 0.101 | 1.78 | 1.04 | 3.03 | 0.034 | |
HR = hazard ratio; 95% CI = 95% confidence interval; LVI = lymphovascular invasion; GCT = germ cell tumor
Table 3B –
Overall Survival of Stage II NSGCT by Age Category
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value | |||
|
| |||||||||
| Age | <19 | REF | REF | REF | REF | REF | REF | REF | REF |
| 19–30 | 1.11 | 0.62 | 2.00 | 0.727 | 1.36 | 0.75 | 2.47 | 0.309 | |
| >30 | 1.40 | 0.78 | 2.54 | 0.262 | 2.01 | 1.09 | 3.71 | 0.025 | |
| Histology | Mixed GCT | REF | REF | REF | REF | REF | REF | REF | REF |
| Embryonal | 0.59 | 0.39 | 0.89 | 0.012 | 0.65 | 0.43 | 1.00 | 0.049 | |
| Teratoma | 0.82 | 0.47 | 1.44 | 0.49 | 0.93 | 0.53 | 1.64 | 0.812 | |
| Yolk Sac | 4.18 | 2.47 | 7.07 | <0.001 | 4.08 | 2.40 | 6.94 | <0.001 | |
| Choriocarcinoma | 3.47 | 2.08 | 5.79 | <0.001 | 3.72 | 2.22 | 6.23 | <0.001 | |
| Race | White | REF | REF | REF | REF | REF | REF | REF | REF |
| Black | 1.62 | 0.59 | 4.39 | 0.348 | 1.19 | 0.43 | 3.26 | 0.741 | |
| Native American | 0.80 | 0.11 | 5.73 | 0.823 | 0.82 | 0.11 | 5.95 | 0.848 | |
| Asian | 1.60 | 0.78 | 3.29 | 0.202 | 1.53 | 0.74 | 3.17 | 0.25 | |
| Hispanic | 1.76 | 1.24 | 2.50 | 0.002 | 1.54 | 1.06 | 2.24 | 0.025 | |
| Insurance | Insured | REF | REF | REF | REF | REF | REF | REF | REF |
| Medicaid | 2.75 | 1.72 | 4.41 | <0.001 | 2.50 | 1.54 | 4.05 | <0.001 | |
| Uninsured | 1.92 | 0.94 | 3.94 | 0.075 | 1.62 | 0.78 | 3.36 | 0.192 | |
| Unknown | 0.86 | 0.56 | 1.32 | 0.494 | 0.95 | 0.62 | 1.47 | 0.833 | |
| LVI | 0.45 | 0.17 | 1.19 | 0.107 | - | - | - | - | |
HR = hazard ratio; 95% CI = 95% confidence interval; LVI = lymphovascular invasion; GCT = germ cell tumor
Highlights.
Age, though not well explored, may predict oncologic outcomes in patients with NSGCT
We examined the survival impact of age in patients with Stage I-III NSGCT
Our analysis stratified patients by age and stage group
Age emerged as a key prognostic factor, most notably among Stage III neoplasms
Acknowledgments
Funding:
This work is supported by a grant from the National Cancer Institute (P30CA072720).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin, 70: 7, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Barr RD, Ries LA, Lewis DR et al. : Incidence and incidence trends of the most frequent cancers in adolescent and young adult Americans, including "nonmalignant/noninvasive" tumors. Cancer, 122: 1000, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Stokes W, Amini A, Maroni PD et al. : Patterns of care and survival outcomes for adolescent and young adult patients with testicular seminoma in the United States: A National Cancer Database analysis. J Pediatr Urol, 13: 386.e1, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Keegan TH, Ries LA, Barr RD et al. : Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer, 122: 1009, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Amini A, Waxweiler TV, Maroni PD et al. : Survival outcomes of adolescent and adult patients with non-seminomatous testicular germ-cell tumors: A population-based study. J Pediatr Urol, 12: 405.e1, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Gillessen S, Collette L, Daugaard G et al. : Redefining the IGCCCG classification in advanced non-seminoma. Annals of Oncology, 30: v357, 2019 [Google Scholar]
- 7.“SEER Incidence Database - SEER Data & Software.” SEER, Apr. 2020, seer.cancer.gov/data/. [Google Scholar]
- 8.Alanee S, Shukla A: Paediatric testicular cancer: an updated review of incidence and conditional survival from the Surveillance, Epidemiology and End Results database. BJU Int, 104: 1280, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Williams LA, Frazier AL, Poynter JN: Survival differences by race/ethnicity among children and adolescents diagnosed with germ cell tumors. Int J Cancer, 146: 2433, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol, 15: 594, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Fosså SD, Cvancarova M, Chen L et al. : Adverse prognostic factors for testicular cancer-specific survival: a population-based study of 27,948 patients. J Clin Oncol, 29: 963, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Necchi A, Pond GR, Nicolai N et al. : A Suggested Prognostic Reclassification of Intermediate and Poor-Risk Nonseminomatous Germ Cell Tumors. Clin Genitourin Cancer, 15: 306, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Rahman O: Incorporating age into International Germ Cell Consensus Classification (IGCCC): a time to move forward? Expert Rev Anticancer Ther, 18: 101, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Marina N, London WB, Frazier AL et al. : Prognostic factors in children with extragonadal malignant germ cell tumors: a pediatric intergroup study. J Clin Oncol, 24: 2544, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Frazier AL, Rumcheva P, Olson T et al. : Application of the adult international germ cell classification system to pediatric malignant non-seminomatous germ cell tumors: a report from the Children's Oncology Group. Pediatr Blood Cancer, 50: 746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber JA, Balasubramanian A, Jorgez CJ et al. : Do pediatricians routinely perform genitourinary examinations during well-child visits? A review from a large tertiary pediatric hospital. Journal of Pediatric Urology, 15: 374.e1, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Lerro CC, Robbins AS, Fedewa SA et al. : Disparities in stage at diagnosis among adults with testicular germ cell tumors in the National Cancer Data Base. Urol Oncol, 32: 23.e15, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Chertack N, Ghandour RA, Singla N et al. : Overcoming sociodemographic factors in the care of patients with testicular cancer at a safety net hospital. Cancer, 126: 4362, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.