Keywords: Cidea, Cidec, diabetes, fat metabolism, lipid droplets, lipids, obesity
Abstract
Fsp27 was previously identified as a lipid droplet-associated protein in adipocytes. Various studies have shown that it plays a role in the regulation of lipid homeostasis in adipose tissue and liver. However, its function in muscle, which also accumulate and metabolize fat, remains completely unknown. Our present study identifies a novel role of Fsp27 in muscle performance. Here, we demonstrate that Fsp27−/− and Fsp27+/− mice, both males and females, had severely impaired muscle endurance and exercise capacity compared with wild-type controls. Liver and muscle glycogen stores were similar among all groups fed or fasted, and before or after exercise. Reduced muscle performance in Fsp27−/− and Fsp27+/− mice was associated with severely decreased fat content in the muscle. Furthermore, results in heterozygous Fsp27+/− mice indicate that Fsp27 haploinsufficiency undermines muscle performance in both males and females. In summary, our physiological findings reveal that Fsp27 plays a critical role in muscular fat storage, muscle endurance, and muscle strength.
NEW & NOTEWORTHY This is the first study identifying Fsp27 as a novel protein associated with muscle metabolism. The Fsp27-knockout model shows that Fsp27 plays a role in muscular-fat storage, muscle endurance, and muscle strength, which ultimately impacts limb movement. In addition, our study suggests a potential metabolic paradox in which FSP27-knockout mice presumed to be metabolically healthy based on glucose utilization and oxidative metabolism are unhealthy in terms of exercise capacity and muscular performance.
INTRODUCTION
Cell death-inducing DNA fragmentation factor-like effector C (CIDE-protein family member, Cidec, aka Fsp27), plays an important role in regulating fat metabolism in adipocytes (1, 2). Fsp27 is an abundantly expressed lipid-droplet-associated protein in adipocytes, where it regulates fat storage and release (3–5). Fsp27 expression is lower in both visceral and subcutaneous adipose tissues of insulin-resistant, obese human subjects (6), and weight loss surgery improves insulin sensitivity while increasing Fsp27 expression (7). A nonsense mutation (E186X) in Fsp27 causes increased lipolysis, lipodystrophy, and insulin-resistant type 2 diabetes in humans (8), highlighting a positive association of Fsp27 with improved metabolic health. However, mouse models of Fsp27 have unveiled confusing results. Whole body Fsp27−/− mice have reduced levels of circulatory triglycerides (TGs) and show smaller lipid droplets in their white fat adipocytes. Fsp27−/− mice were protected from diet-induced obesity (DIO), resulting in improved glucose disposal and insulin sensitivity (9, 10). Furthermore, Fsp27-deficiency led to improved metabolic rates by upregulating genes involved in mitochondrial oxidative metabolism and browning of white adipose tissue (9, 10). Similarly, silencing of Fsp27 using intraperitoneal injection of antisense oligonucleotides in high-fat diet (HFD) fed mice and ob/ob mice resulted in decreased visceral adiposity, improved insulin sensitivity, and whole body glycemic control (11). In contrast, adipose-specific Fsp27−/− mice were resistant to HFD-induced weight gain, but impaired lipid storage in adipose tissue resulted in hepatosteatosis and systemic insulin resistance (12).
Given the fact that Fsp27−/− mice possess a higher mitochondrial oxidative metabolism, we hypothesized that they would outperform wild-type (WT) mice in muscle physiology tests. To test our hypothesis, we performed metabolic phenotype, endurance capacity, and grip strength in cohorts of WT, Fsp27+/−, and Fsp27−/− male and female mice. We show that Fsp27−/− led to marked deficiencies in fat storage and reduced muscle performance.
MATERIALS AND METHODS
Mouse Maintenance, Generation, and Dissection
Fsp27+/− mice were procured from Dr. Yoshikazu Tamori, Kobe University, Japan (9). These mice were in C57BL/6N background. FSP27-KO (Fsp27−/−) mice were obtained by crossing of heterozygotes. The details of genotyping the heterozygous and homozygous mice were published previously (9). Crossing of Fsp27+/− mice provided littermate controls, Fsp27+/− and Fsp27−/− mice. For the experiments in the present study, we used both male and female mice fed regular chow diet. All mice were 8–10 mo of age at the time of experimentation. Animals were housed at the Ohio University Animal Facility (Athens, OH) with access to mouse chow and water ad libitum and an automatic 12-h day/night cycle. Experimental protocols with mice were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. Ohio University is approved by the NIH-Office of Laboratory Animal Welfare (Animal Welfare Assurance number A3610-01). It also has a United States Department of Agriculture (USDA) license 31-R-0082, compliant with the USDA Animal Welfare Act Regulations and the Public Health Service Policy on Humane Care and Use of Laboratory Animals in accordance with the Guide for the Care and Use of Animals. All animals were euthanized by exposure to CO2 followed by cervical dislocation. Collected tissues were snap-frozen in isopentane and stored at −80°C. Sera were stored at −20°C.
Metabolic Profiling
Mice were housed individually at 22° to 23°C in transparent plastic cages (31 × 20 × 13 cm) placed in a light-controlled (12-h light/12-h dark cycle). For metabolic studies, mice were housed individually in metabolic chambers and acclimatized for 48 h. Over the subsequent 48 h, the metabolic parameters were measured every 15 min by a Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments). Oxygen consumption and carbon dioxide production of mice in the fed condition was measured with an indirect calorimetric system every 15 min for 24 h at 28°C. Every chamber has a dedicated electrochemical oxygen sensor and a Non-Dispersive Infrared (NDRI) carbon dioxide sensor for simultaneous measurements. Energy expenditure was calculated as the product of the calorific value of oxygen [3.815 + (1.232 × respiratory quotient)] and the volume of O2 consumed. Locomotor activity was recorded with an array of infrared photo beams that surround the cage. XY position of the animal was continuously recorded by the beams arranged in a grid pattern. Z-axis sensors were used to measure rearing events. Food intake was measured by indirect calorimetry. For food consumption, center feeder containing powdered chow was used. Water consumption was monitored by Volumetric Drinking Monitor system. Body composition (fat mass) was determined by NMR (LF50 BCA-Analyzer, Bruker) in live mice.
Lipid Analysis and Glycogen Determination
Serum from WT and Fsp27−/− mice (n = 5) was analyzed by the Lipid Core at Vanderbilt University Medical Center for total free fatty acid (FFA), TG, and the lipid species composition. Liver and muscle glycogen content in fasted and unfasted WT and Fsp27−/− mice (n = 5), before and after exercise (n = 5, male), was determined with the Glycogen Assay Kit (Sigma-Aldrich), as per the manufacturer’s instructions.
Glucose Tolerance Test and Insulin Tolerance Test
Glucose tolerance test (GTT) was performed in 8 h fasted mice. Glucose (1.5 g/kg) was injected intraperitoneally, and blood glucose was monitored at the indicated time points using a GE100 blood glucose monitoring system glucometer (GE Healthcare). Insulin tolerance test was performed in 6 h fasted mice. Insulin (0.75 IU/kg; Humulin-N, Eli Lilly) was injected intraperitoneally, and blood glucose was monitored as mentioned earlier.
Treadmill Running Endurance
Muscle endurance was determined by a muscle fatigue test on the Exer 3/6 Metabolic Treadmill (Columbus Instruments). A fatigue zone was determined at the back end of the treadmill, which included the shock grid region and approximately one mouse body length of running track. Mice were trained and acclimated to use the treadmill at low speed (10–12 m/min) over a course of 3 days, with the speed increasing by 1 m/min each day. Mice which received an abnormal number of shocks were removed from training and were not utilized for the test. Treadmill fatigue testing was performed by placing the mice on the stationary treadmill and slowly increasing the speed to 12 m/min. The speed was then slowly increased to a maximum speed of 26 m/min (13). Six mice were tested simultaneously and allowed to run freely until spending 5 s consecutively in the fatigue zone (defined above) after which point the shock grid was disabled and the total distance run (km) by each mouse was determined and normalized to body weight (g).
Four-Limb Hanging Grid Test
Muscle strength was examined by four-limb hanging grid test with a custom made 38 cm by 30 cm grid with a 1-cm metal mesh. Mice were placed on the grid and allowed to explore the surface for 2 min before testing. The hanging grid was slowly inverted and placed onto an apparatus which suspended the mice ∼42 cm above a soft cushion. Mice were allowed to hang upside-down until their muscles could no longer support their body weight. The total time that each mouse spent inverted was recorded. Mice were tested a total of five tests. If a mouse showed signs of nonperformance (such as continually looking downward and then releasing from the grid intentionally), that mouse was removed from the study. Fsp27−/− mice were significantly lighter than WT and Fsp27+/− mice, so we accounted for this difference by converting hanging time into holding impulse. Holding impulse was calculated by converting the weight of the mouse (g) to Newtons and multiplying it by the hanging time (sec).
Muscle Fiber Typing and Microscopy
Gastrocnemius, transverse abdominis (TA), and extensor digitorum longus (EDL) muscles were cut on a cryostat (Leica CM 1950) into 10-µm sections and mounted on Superfrost Plus microslides (VWR). Staining was performed as described by Bloemberg and Quadrilatero (14), with some modifications. Immediately after cutting, slides were air-dried for 10 min. Sections were blocked with 10% goat serum and Mouse-on-Mouse blocking reagent (1:25, Vector Laboratories) in PBS for 1 h at room temperature. Primary antibodies targeting myosin heavy chains Type I, Type IIa, and Type IIb (BA-D5, SC-71, and BF-F3; Developmental Studies Hybridoma Bank, University of Iowa) were combined at a concentration of 1:200 per antibody in blocking buffer and applied to sections overnight at 4°C. Slides were washed three times in PBS for 5 min. Secondary antibodies targeting each specific primary antibody (BA-D5, Alexa Fluor 350 IgG2b; SC-71, Alexa Fluor 488 IgG1; BF-F3, Alexa Fluor 555 IgM; Thermo Fisher Scientific) were combined at a concentration of 1:500 per antibody in blocking buffer and applied to sections for 1 h at room temperature in the dark. Slides were washed three times in PBS for 5 min and then mounted in Vectashield mounting medium (Vector Laboratories). Confocal microscopy was performed on Nikon A1R (Nikon, Japan) confocal microscope using ×60 oil immersion objective. Images were processed using Nikon software. Fiber percentage for each type of fibers was calculated based upon total number of fibers in the field of each microscopic image.
RNA Isolation, Reverse Transcriptase-Polymerase Chain Reaction, and Quantitative PCR Analysis
Total RNA was isolated from muscle with TRIzol (Ambion, Life Technologies, Cat. No. 15596019). RNA (1 µg) was used to make cDNA using a RevertAidRT kit (Thermo Fisher Scientific, Cat. No. K1691). Quantitative (q)PCR was performed with PowerUp SYBR Master Mix (Applied Biosystems, Cat. No. 100029284).
Western Blot
Total proteins were isolated from muscle using mammalian cell lysis buffer (Cell Signaling Technology, Cat. No. 786-180) in the presence of protease inhibitor (Roche, Cat. No. 11836153001) and phosphatase inhibitor (Roche, Cat. No. 04906837001). Samples were centrifuged twice to isolate the clear protein fraction. Protein was estimated and an equal amount of protein (µg) was loaded and resolved on 10% PAGE and subsequently transferred to the PVDF membrane. The membrane was then blocked and probed with primary antibody overnight followed by washing three times with Tris-buffered saline-Tween 20 (TBST) buffer and incubation with secondary antibodies (diluted in 4% BSA in TBST) for the next 2 h. Finally, the blots were washed three times with TBST and developed using horseradish peroxidase (HRP) substrate (Immobilon Western Chemiluminescent HRP substrate-Millipore, Cat. No. WBKLS0500) and visualized under chemiluminescence. Images were captured using BioRad Imager and blots were quantified using ImageJ (NIH) software.
Blood Lactate Measurement
Blood lactate was measured before and after exercise by tail bleed using lactate meter (Nova Biomedical).
RESULTS
Metabolic Characterization of Fsp27−/+ and Fsp27−/− Mice
We found no significant difference in body weight or body fat between WT and Fsp27+/− mice (Fig. 1, A and B). However, both male and female Fsp27−/− mice showed significantly reduced body weight than WT or Fsp27+/− mice (Fig. 1A). Male Fsp27−/− mice retained significantly less body fat than WT mice (Fig. 1B), whereas female Fsp27−/− mice retained less body fat than both WT and Fsp27+/− mice (Fig. 1B). Body weight and body fat of Fsp27+/− mice did not differ from WT mice (Fig. 1, A and B). Fsp27−/− mice showed increase food uptake (Fig. 1C). Gonadal white adipose tissue (WAT) in both male and female Fsp27−/− mice was significantly reduced compared to WT and Fsp27+/− mice (Fig. 1, D and E). Although, subcutaneous (SubQ) fat was also diminished in both male and female Fsp27−/−, it did not show significance, while brown adipose tissue (BAT) and liver mass remained unchanged (Fig. 1, D and E). Water consumption remained similar between WT, Fsp27+/− and Fsp27−/− mice (Fig. 1F). These data demonstrate that a single allele of Fsp27 is sufficient to maintain triacylglycerides (TAGs) levels in WAT and that complete loss of Fsp27 is necessary to reduce fat mass and increase food uptake significantly.
Figure 1.
Weight, food, and water consumption of mice. Body weight (A; in g) and body-fat percentage (B) of wild type, Fsp27+/− (Het-KO) and Fsp27−/− (Fsp27-KO) mice. Food consumption over 96 h (C) for each mouse genotype in male mice. The right panel shows the quantification of total food consumption in 4 days. Tissue mass was weighed immediately after dissection in male (D) and female (E) mice. Water consumption over 96 h (F) for each mouse genotype. The right panel shows quantification of total water consumption in 4 days (n = 6 mice for each group). Data are expressed as means ± SE. For statistical analysis, one-way ANOVA followed by Tukey’s multiple comparison test was performed, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Glucose and Insulin Tolerance Were Improved in Fsp27−/− Mice, but Not in Fsp27+/− Mice
Glucose homeostasis was tested by intraperitoneal glucose tolerance test (GTT) and intraperitoneal insulin tolerance test (ITT) in 8- to 10-mo-old mice. We found that male Fsp27−/− mice were more glucose tolerant and insulin sensitive than WT and Fsp27+/− mice (Fig. 2, A–D), while female Fsp27−/− mice showed improvement in GTT, but not insulin sensitivity (Fig. 2, E–H). No significant difference was observed in fasting blood glucose in male or female mice of either group (Fig. 2I). Overall, the results identify that Fsp27−/− mice are more glucose and insulin tolerant than WT or Fsp27+/− mice.
Figure 2.
Glucose and insulin tolerance testing in mice. Data are graphed over time for male and female mice. A: glucose insulin tolerance test (GTT) of males. B: the area under the curve (AUC) of GTT for males. C: insulin tolerance test (ITT) of males. D: AUC of ITT for males. E: GTT of females. F: AUC of GTT for females. G: ITT of females. H: AUC of ITT for females. I: fasted basal glucose, taken after overnight fasting, for both male and female mice. WT, wild type; Het-KO, Fsp27+/− mouse; Fsp27-KO, Fsp27−/− mouse (n = 6 mice for each group). Data are expressed as means ± SE. For statistics on A, C, E, and G, two-way ANOVA followed by Bonferroni post hoc analysis was performed; for statistics on B, D, F, and H, one-way ANOVA followed by Tukey’s multiple comparison test was performed, *P < 0.05, **P < 0.01, ***P < 0.001.
Fsp27−/− Mice Have Higher Energy Expenditure, While Fsp27+/− and Fsp27−/− Mice Had Reduced Locomotor Activity at Night
Energy expenditure and activity were assessed in metabolic cages over a 5-day period. There were no discernable differences in Respiratory Exchange Ratio (RER), oxygen uptake (V̇o2; maximum oxygen consumption), carbon dioxide output (V̇co2; carbon dioxide production), and heat released between Fsp27+/− and WT mice (Fig. 3, A–D). In contrast, we observed a significant increase in V̇o2 in Fsp27−/− mice, suggesting an increased metabolic rate (Fig. 3B). Similarly, V̇co2, respiratory exchange ratio, and heat released were also increased in Fsp27−/− mice than in WT (Fig. 3, A, C, and D). There were no observed differences in locomotor activity between the mice during the day cycle (Fig. 4, A–F). However, during the night cycle, Fsp27+/− mice showed reduced activity compared with WT mice in X-ambulatory movements (Fig. 4, A and B) as well as overall movement on the X axis and Z axis (Fig. 4, C–F). Likewise, Fsp27−/− mice exhibited reduced activity compared with WT and to Fsp27+/− mice on X axis and Z axis at night (Fig. 4, C–F).
Figure 3.
Metabolic phenotyping of each mouse genotype. Mice were monitored through metabolic chambering for a total of 5 days. Measurements were taken during the day cycle (6:00 AM to 6:00 PM) and the night cycle (6:00 PM to 6:00 AM) were averaged within each respective time cycle. The respiratory exchange ratio for day and night cycles (A), V̇o2 (B), and V̇co2 (C). Heat generation through indirect calorimetry (D). Het-KO, Fsp27+/− mouse; Fsp27-KO, Fsp27−/− mouse (n ≥ 6 male mice/group). Data are expressed as means ± SE. For statistics, one-way ANOVA followed by Tukey’s multiple comparison tests was performed, *P < 0.05, ***P < 0.001, ****P < 0.0001.
Figure 4.
Locomotor activity of each mouse genotype. Mice were monitored through metabolic chambering for a total of 5 days. Measurements were taken during the day cycle (6:00 AM to 6:00 PM) and the night cycle (6:00 PM to 6:00 AM) were averaged within each respective time cycle. Mouse movement along the X-axis, either ambulatory (A and B). Total X-axis movement (C and D). Z-axis movement (E and F). Het-KO, Fsp27+/− mouse; Fsp27-KO, Fsp27−/− mouse (n ≥ 6 male mice/group). Data are expressed as means ± SE. n ≥ 6 male mice per group. For statistics, one-way ANOVA followed by Tukey’s multiple comparison tests was performed separately for day and night cycle, *P < 0.05, **P < 0.01, ***P < 0.001.
Muscle Performance is Compromised in Fsp27+/− and Fsp27−/− Mice
Treadmill running test and four-limb hanging grid test were used to measure exercise capacity and endurance. Both Fsp27+/− and Fsp27−/− male mice fatigued on the treadmill at a significantly shorter distance than WT mice (Fig. 5A). Fsp27−/− males fatigued significantly earlier than Fsp27+/− mice (Fig. 5A). Similar results were obtained when data were calculated as distance per body weight (not shown). Both WT and Fsp27+/− male mice outperformed Fsp27−/− mice on the four-limb hanging grid test (Fig. 5B). Female mice showed a similar trend, namely, WT and Fsp27+/− mice outperformed Fsp27−/− mice in endurance running (Fig. 5A), whereas only WT mice outperformed Fsp27−/− mice on the strength test.
Figure 5.
Muscle physiology testing and fiber typing. Treadmill running endurance was measured with an Exer 3/6 Metabolic Treadmill (A). After acclimation, mice (n >10 mice/group) were allowed to run until exhaustion as defined by spending over 5 consecutive s in the fatigue zone (see materials and methods). Results from the four-limb hanging grid test performed on mice (n >10 mice/group) with a minimum of 5 trials (B). Total hang time was used to calculate holding impulse after converting the weight of the mouse into Newtons. Fiber typing of the gastrocnemius (C), tibialis anterior (D), and extensor digitorum longus (E) muscle sections with multiplex immunofluorescent antibody staining (n = 3 male mice/group). WT, wild type; Het-KO, Fsp27+/− mouse; Fsp27-KO, Fsp27−/− mouse. Data are expressed as means ± SE. For statistics, one-way ANOVA followed by Tukey’s multiple comparison tests was performed separately for each fiber types, *P < 0.05, **P < 0.01, ***P < 0.001.
We then determined the fiber type of the gastrocnemius, TA, and EDL muscles by immunofluorescence. There was no difference in the amount of Type 1 fibers for any of the muscle types examined (Fig. 5, C–E). Gastrocnemius, TA, and EDL muscle had significant increase in Type 2a/b fibers in Fsp27−/− mice than in WT and Fsp27+/− mice (Fig. 5, C–E; Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.17354486). Fsp27−/− mice demonstrated a significant increase in Type 2a fibers and decrease in Type 2 b fibers in EDL as compared with WT (Fig. 5E; Supplemental Fig. S1). This was expected as these mice possess higher oxidative metabolism than WT mice. Type 2a muscle fibers are associated with higher endurance and less fatigability. Although Fsp27−/− mice possess an increased amount of oxidative Type 2a fibers in their EDL, this did not result in an improvement to running endurance.
Fsp27 Deletion Alters Lipid Profile without Impacting Liver and Skeletal Muscle Glycogen Content
A significant decline in muscle endurance and strength in Fsp27−/− mice was unexpected. To understand the underlying cause, we studied serum lipid profile and glycogen content in the liver and the muscle of WT and Fsp27−/− mice. Male Fsp27−/− mice showed a significant reduction in circulating TGs as compared with WT mice (Fig. 6A), while the amount of FFAs remained unchanged (Fig. 6A). Conversely, female Fsp27−/− mice had similar levels of circulating TGs but higher circulating FFAs than the WT (Fig. 6A). Closer examination of fatty acid species revealed that although WT males had increased circulating TGs, the percentages of each lipid species remained stable (Fig. 6, A and B). In females, while the total circulating TGs were similar, composition analysis revealed differences in unsaturated fatty acids: an increase in oleic acid (18:1w9, an omega-9 fatty acid) but a decrease in eicosapentaenoic acid (20:5) and docosahexaenoic acid (22:6), both of which are omega-3 fatty acids (Fig. 6C). Male and female mice showed changes in FFA composition which were largely similar between the sexes—a reduction in palmitic acid (16:0; saturated) in both male and female mice, but a reduction in palmitoleic acid (16:1; unsaturated) only in males as well as a consistent increase to oleic and linoleic acid for both sexes (18:2, an omega-6 fatty acid; Fig. 6, D and E). Glycogen stores in the liver and skeletal muscles were not affected under fed or fasted conditions in Fsp27−/− animals (Fig. 6, F and G). These data suggest that glycogen stores are not the cause for loss of muscle performance in Fsp27−/− mice.
Figure 6.
Lipid and glycogen analysis. Serum collected from fasted wild type and Fsp27−/− mice (n = 5) was analyzed for total free fatty acids (FFAs) and triglycerides (TGs; A) as well as the lipid species composition (B–E). For A one-way ANOVA followed by Bonferroni post hoc analysis was performed. Data for the amount of each lipid species in the male TG fraction (B), lipid species in female TG fraction (C), lipid species in male FFA fraction (D), and lipid species in female FFA fraction (E). For B–E two-way ANOVA followed by Bonferroni post hoc analysis was performed. Liver (F) and muscle (G) glycogen content in fasted and unfasted wild-type and Fsp27−/− male mice (n = 3 mice per group). WT, wild type; Fsp27-KO, Fsp27−/− mouse. All data are expressed as means ± SE. For statistics, F and G one-way ANOVA followed by Tukey’s multiple comparison test was performed, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Fsp27−/− Mice Display Impaired Hepatic and Mitochondrial Function
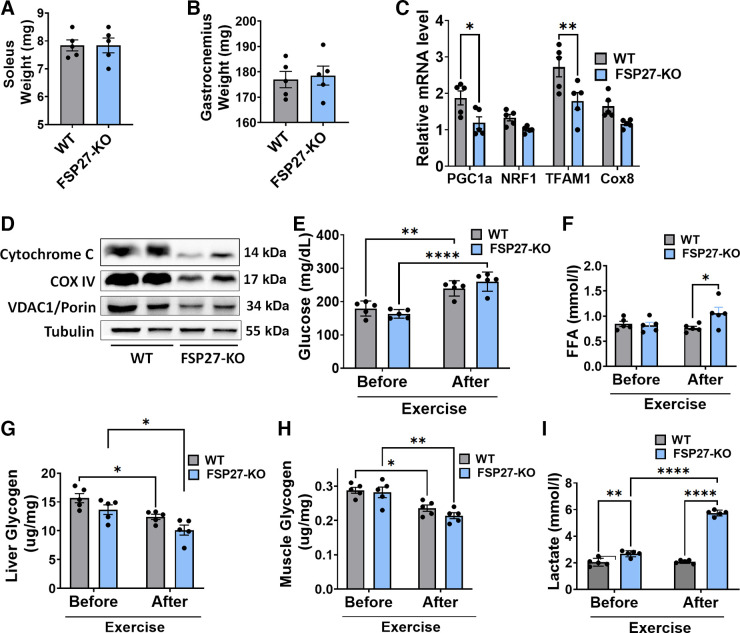
We next measured the muscle mass in these mice. Soleus or gastrocnemius muscle mass was similar in WT and Fsp27−/− mice (Fig. 7, A and B), ruling out the possibility of differences in muscle mass for the subpar performance of Fsp27−/− mice. Alternatively, we tested if reduced number of mitochondria in Fsp27−/− mice could have caused low energy production. Interestingly, skeletal muscle of Fsp27−/− mice showed significantly reduced level of transcription factor A, mitochondrial (TFAM) and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1-α gene expression) (Fig. 7C). TFAM and PGC1-α regulate mitochondrial transcription and mitochondrial biogenesis, respectively. Similarly, nuclear respiratory factor 1 (NRF1) and cytochrome c oxidase subunit 8 (COX8), that regulate mitochondrial DNA replication and electron transport chain/oxidative phosphorylation, respectively, also showed reduced expression in Fsp27−/− mice. Although the reduction in NRF1 and COX8 was not significant (Fig. 7C). We also found reduced mitochondrial density, measured by the protein expression of cytochrome C, cytochrome c oxidase IV (COX IV), and voltage-dependent anion selective channel 1 (VDAC1; aka Porin) in the muscle of Fsp27−/− mice (Fig. 7D).
Figure 7.
Energy reserves, fuel utilization, muscle mass, and mitochondrial/hepatic output during exercise in WT and FSP27−/− mice. Soleus muscle mass (A), gastrocnemius muscle mass (B). q-PCR analysis of mitochondrial genes normalized to β-actin (C). Western blot analysis of mitochondrial proteins: cytochrome C, COX IV, and voltage dependent anion selective channel 1 (D). Serum glucose levels (E), FFA levels (F), liver (G), and muscle (H) glycogen levels. Blood lactate levels before and after exercise in WT and FSP27−/− animals (I). WT, wild type; FSP27−/− male mice (n = 5 male mice/group). Data are expressed as means ± SE. For statistics, unpaired t test was performed for A and B, two-way ANOVA followed by Tukey’s multiple comparison test was performed for E–I. For C two-way ANOVA followed by Šídák’s multiple comparisons test was used to analyze the significance between the groups *P < 0.05, **P < 0.01, ****P < 0.0001.
Next, we investigated if sufficient glucose as a fuel was in circulation in Fsp27−/− mice to meet energy demands of their exercising muscles. As shown in Fig. 7E, both WT and Fsp27−/− mice had comparable levels of blood glucose before or after exercise. As expected, blood glucose levels were significantly elevated after exercise in both groups, suggestive of effective hepatic glycogenolysis (Fig. 7E). No significant change was observed in the expression of genes involved in IMTG formation, diacylglycerol O-acyltransferase 1 (DGAT1) and diacylglycerol O-acyltransferase 2 (DGAT2), and in lipolysis, hormone sensitive lipase (HSL), and adipose triglyceride lipase (ATGL) (Supplemental Fig. S2). Next, we assessed circulating FFA levels in these animals. As shown in Fig. 7F, both groups had similar FFA profile before exercise. Rather Fsp27−/− animals had significantly elevated serum FFA levels after exercise suggesting elevated adrenergic response or impaired mitochondrial oxidation. The latter possibility is consistent with Fsp27−/− mice having slightly reduced mitochondrial number than WT animals (Fig. 7, C and D). Furthermore, we wanted to check glycogen levels in liver and muscles before and after exercise. As shown in Fig. 7, G and H, both WT and Fsp27−/− mice had similar levels of glycogen before and after exercise in liver as well as in muscles; however, glycogen levels dropped significantly in both organs in both groups of mice after exercise, suggesting that shortage of fuel supply could not account for poor muscle performance in Fsp27−/− mice. Also, these results rule out the incapacity to mobilize fat and glycogen reserves during running. Finally, we tested circulating lactate levels in WT and Fsp27−/− animals. Interestingly, high levels of circulating lactate were present before exercise in Fsp27−/− animals which were compounded further after exercise (Fig. 7I).
Fsp27 Deletion Alters Fat Content in the Muscle
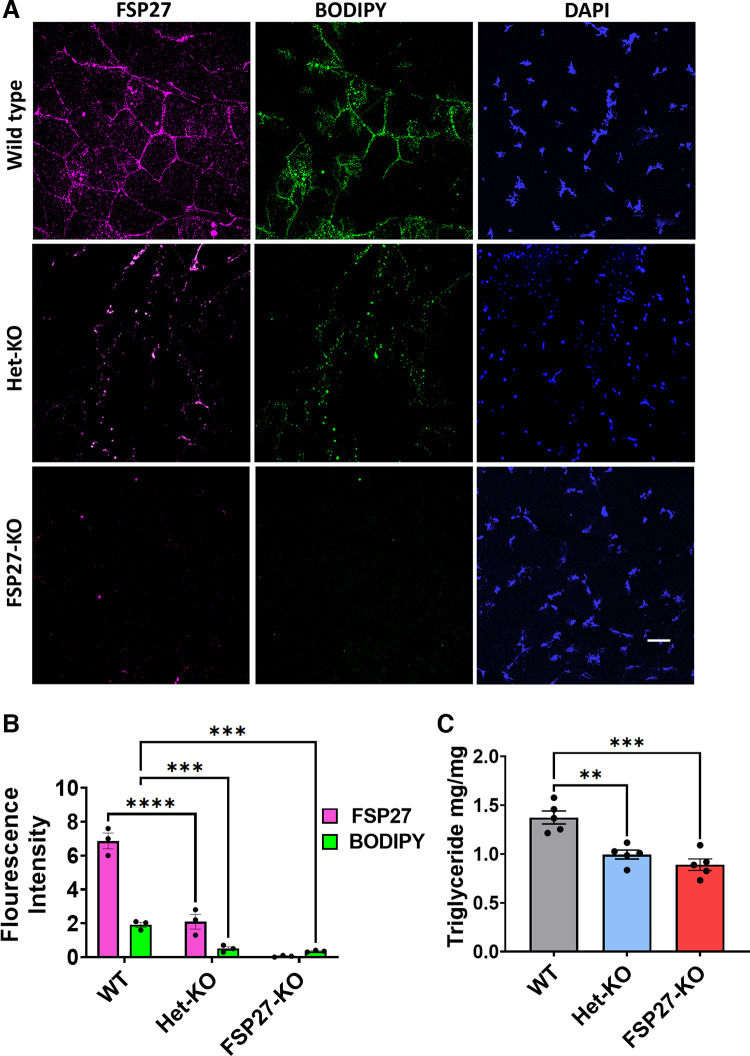
Since Fsp27 is a fat-associated protein that plays an important role in fat accumulation by regulating lipid droplet accumulation and enlargement (4, 5, 15–17), therefore, we hypothesized that it might be responsible for fat accumulation as a fuel source in the muscle. To test our hypothesis, we performed immunostaining of fat pads and gastrocnemius muscle of wild type, Fsp27+/− and Fsp27−/− mice for Fsp27 using Fsp27-antibodies. Bodipy dye was used to stain fat stores. Fsp27−/− mice showed negligible amounts of fat (Fig. 8, A and B). Interestingly, the Fsp27+/− mice showed a significantly lower amount of Fsp27 and fat in the gastrocnemius muscle (Fig. 8, B and C), suggesting that the severely compromised muscle performance in Fsp27−/− mice might be, at least partially, due to the lack of fat storage in muscle.
Figure 8.
Localization of fat and Fsp27 in muscle. A: confocal microscopy images representing the localization of Fsp27 (magenta; Cy5), neutral lipid (green; BODIPY), and nuclei (blue; DAPI) in the gastrocnemius of wild type, Fsp27+/− (Het-KO), and Fsp27−/− (FSP27-KO) mice. B: qualitative measurement of fat staining in the gastrocnemius of wild type, Fsp27+/−, and Fsp27−/− mice. C: intramuscular triglyceride contents. n = 5 male mice/group. Data are expressed as means ± SE. For statistics one-way ANOVA followed by Tukey’s multiple comparison test was performed **P < 0.01 ***P < 0.001, ****P < 0.0001.
DISCUSSION
Our present study highlights the previously unidentified role of Fsp27 in muscle performance. In accordance with previous reports (9, 10), we show that Fsp27−/− mice display improved insulin sensitivity, and glucose tolerance compared with WT and Fsp27−/+ mice. We found a shift toward hybrid type 2a/b muscle fibers in Fsp27−/− mice, which prompted us to hypothesize that Fsp27−/− mice would have higher muscle endurance. To our surprise, Fsp27−/− mice appeared to be less mobile during the day-night transition. Interestingly, Fsp27+/− and Fsp27−/− mice displayed significant differences in endurance at both physiological challenges compared with the WT mice. The loss of exercise capacity was dramatic, with both male and female Fsp27−/− mice becoming exhausted at ∼15% of the distance covered by wild-type controls. Similarly, Fsp27−/− mice were unable to perform comparably to controls on the four-limb hanging grid test, with a holding impulse that measured 30% of what was attainable by WT mice. We reasoned that reduced energy reserves or impaired capacity to mobilize fat and glycogen reserves might contribute to the poor performance in physiological challenge, but our data showed that liver and muscle glycogen contents remained unchanged before and after exercise while serum glucose and serum FFA levels were elevated in Fsp27−/− mice. The heterozygous Fsp27+/− mice underperformed in exercise, endurance, and strength tasks compared with WT mice, suggesting that Fsp27 haploinsufficiency compromises muscle performance. Sexual dimorphism was not observed. Overall, besides having higher glucose tolerance, Fsp27−/− caused marked deficiencies in fat storage and muscle performance.
The two main fuels for muscle metabolism are carbohydrates and fat. Various studies have highlighted the importance of fat as a support nutrient for energy during exercise (18–20). At low-to-moderate intensities of exercise, the primary fuel sources for skeletal muscle are glucose, derived from liver glycogen, and FFAs, primarily derived from adipose tissue lipolysis. During long-duration exercise, fatty acids are oxidized to be used as fuel for the muscle. A large portion of available fatty acids are stored in adipose tissue as triacylglycerols (TAGs), but a modest amount remains stored in the muscle itself and within blood plasma (21). Fsp27−/− mice are intriguing because they retain TAGs and FFAs within their blood plasma (Fig. 6A), but their ability to store fats within WAT (Fig. 1, D and E) and muscle is severely compromised (Fig. 8C). Their available fatty acids stores, therefore, come from the blood plasma only, which is minuscule in comparison with adipose and muscle. Despite having improved metabolic parameters and fatty acid oxidation rates (9), Fsp27−/− mice were unable to perform as well, as WT or Fsp27+/− mice on the running endurance and hanging grid challenge. Most likely the lack of fat stores in the WAT and muscle of Fsp27−/− mice caused the observed decline in running endurance. The amount of intramuscular fat in Fsp27+/− mice was lower than the WT mice but higher than Fsp27−/− mice, which related to their endurance capacity. Our study highlights that Fsp27 is expressed in muscles and plays an important role in fat storage in the muscle, as it does in adipocytes (4, 5, 15–17). It is likely that Fsp27 regulates lipid droplet dynamics to store triglycerides in the muscles, and regulates their breakdown via lipolysis, in a similar manner to its function in adipocytes (3, 22–24). Although glucose levels before and after exercise were similar in WT and Fsp27−/− mice, FFAs were higher in Fsp27−/− mice after the exercise. Perhaps Fsp27−/− mice could not utilize these FFAs due to reduced muscular mitochondrial mass. Alternatively, lactate build up in Fsp27−/− mice might also cause muscle fatigue and hence poor muscle performance. However, an alternate role of Fsp27 in muscle metabolism could also be a causative factor for subpar physiological performance of Fsp27−/− mice.
Our work shows that the insulin-sensitizing effects observed in young, male mice lacking Fsp27 (9, 25) are extended to adult (8–10 mo old) mice of both sexes. However, the enhanced absorption of glucose was the primary retained metabolic factor in our experiments, as we did not observe any significant differences during the insulin challenge. This is likely an age-related effect as the previous work was performed on a much younger set of mice that were highly metabolically active (9, 10). Interestingly, among both male and female mice, we did not detect a difference in the fasting blood glucose of the three genotypes. This suggests that the enhanced glucose metabolism seen in Fsp27−/− mice is the result of a necessity, as the increased systemic fatty acids are constantly being oxidized even at rest.
Previous studies have shown Fsp27’s role in lipid metabolism within the adipose tissue and liver. Our present study highlights a previously unidentified role of FSP27 in muscle performance. In addition, this study identifies a metabolic paradox in which Fsp27-KO mice presumed to be metabolically healthy based on glucose utilization and insulin sensitivity are in fact unhealthy in terms of exercise capacity and muscular performance.
SUPPLEMENTAL DATA
Supplemental Figs S1 and S2:https://doi.org/10.6084/m9.figshare.17354486.v1.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants RO1DK101711 (to V.P.), RO1HL140836 (to V.P.), RO1DK124126 (to V.P.), and funds from Osteopathic Heritage Foundation’s Vision 2020 to Heritage College of Osteopathic Medicine at Ohio University.
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., B.B., D.W.R., S.Y., V.M.S., and V.P. conceived and designed research; M.S., B.B., A.G., S.J., I.P., S.N., D.W.R., G.Y., V.M.S., and V.P. performed experiments; M.S., B.B., A.G., S.J., I.P., D.W.R., S.Y., V.M.S., and V.P. analyzed data; M.S., B.B., S.Y., V.M.S., and V.P. interpreted results of experiments; M.S., B.B., V.P. prepared figures; M.S. and V.P. drafted manuscript; V.M.S., B.B., and V.P. edited and revised manuscript; M.S., B.B., A.G., S.J., I.P., S.N., Y.T., D.W.R., G.Y., S.Y., V.M.S., and V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Carla Harris from the Vanderbilt University Medical Center Lipid Core for providing accurate and timely results.
REFERENCES
- 1.Chen FJ, Yin Y, Chua BT, Li P. CIDE family proteins control lipid homeostasis and the development of metabolic diseases. Traffic 21: 94–105, 2020. doi: 10.1111/tra.12717. [DOI] [PubMed] [Google Scholar]
- 2.Slayton M, Gupta A, Balakrishnan B, Puri V. CIDE proteins in human health and disease. Cells 8: 238, 2019. doi: 10.3390/cells8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grahn THM, Kaur R, Yin J, Schweiger M, Sharma VM, Lee MJ, Ido Y, Smas CM, Zechner R, Lass A, Puri V. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem 289: 12029–12039, 2014. doi: 10.1074/jbc.M113.539890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jambunathan S, Yin J, Khan W, Tamori Y, Puri V. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One 6: e28614, 2011. doi: 10.1371/journal.pone.0028614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech MP. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem 282: 34213–34218, 2007. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 6.Puri V, Ranjit S, Konda S, Nicoloro SMC, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, Perugini RA, Czech MP. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA 105: 7833–7838, 2008. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno-Navarrete JM, Ortega F, Serrano M, Rodriguez-Hermosa JI, Ricart W, Mingrone G, Fernandez-Real JM. CIDEC/FSP27 and PLIN1 gene expression run in parallel to mitochondrial genes in human adipose tissue, both increasing after weight loss. Int J Obes (Lond) 38: 865–872, 2014. doi: 10.1038/ijo.2013.171. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, Bottomley W, Vigouroux C, Magre J, Raymond-Barker P, Murgatroyd PR, Chawla A, Skepper JN, Chatterjee VK, Suliman S, Patch AM, Agarwal AK, Garg A, Barroso I, Cinti S, Czech MP, Argente J, O'Rahilly S, Savage DB; LD Screening Consortium. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med 1: 280–287, 2009. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest 118: 2808–2821, 2008. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, Yang H, Wen Z, Li P. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One 3: e2890, 2008. doi: 10.1371/journal.pone.0002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langhi C, Arias N, Rajamoorthi A, Basta J, Lee RG, Baldán Á. Therapeutic silencing of fat-specific protein 27 improves glycemic control in mouse models of obesity and insulin resistance. J Lipid Res 58: 81–91, 2017. doi: 10.1194/jlr.M069799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka N, Takahashi S, Matsubara T, Jiang C, Sakamoto W, Chanturiya T, Teng R, Gavrilova O, Gonzalez FJ. Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. J Biol Chem 290: 3092–3105, 2015. doi: 10.1074/jbc.M114.605980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougherty JP, Springer DA, Gershengorn MC. The treadmill fatigue test: a simple, high-throughput assay of fatigue-like behavior for the mouse. J Vis Exp 111: 54052, 2016. doi: 10.3791/54052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol 195: 953–963, 2011. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grahn THM, Zhang Y, Lee MJ, Sommer AG, Mostoslavsky G, Fried SK, Greenberg AS, Puri V. FSP27 and PLIN1 interaction promotes the formation of large lipid droplets in human adipocytes. Biochem Biophys Res Commun 432: 296–301, 2013. doi: 10.1016/j.bbrc.2013.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price AM, Doner NM, Gidda SK, Jambunathan S, James CN, Schami A, Yurchenko O, Mullen RT, Dyer JM, Puri V, Chapman KD. Mouse fat-specific protein 27 (FSP27) expressed in plant cells localizes to lipid droplets and promotes lipid droplet accumulation and fusion. Biochimie 169: 41–53, 2020. doi: 10.1016/j.biochi.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Bjorntorp P. Importance of fat as a support nutrient for energy: metabolism of athletes. J Sports Sci 9 Spec No: 71–76, 1991. doi: 10.1080/02640419108729867. [DOI] [PubMed] [Google Scholar]
- 19.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab 2: 817–828, 2020. [Erratum in Nat Metab 2: 990, 2020]. doi: 10.1038/s42255-020-0251-4. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr 72: 558S–563S, 2000. doi: 10.1093/ajcn/72.2.558S. [DOI] [PubMed] [Google Scholar]
- 22.Sharma R, Luong Q, Sharma VM, Harberson M, Harper B, Colborn A, Berryman DE, Jessen N, Jørgensen JOL, Kopchick JJ, Puri V, Lee KY. Growth hormone controls lipolysis by regulation of FSP27 expression. J Endocrinol 239: 289–301, 2018. doi: 10.1530/JOE-18-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma VM, Vestergaard ET, Jessen N, Kolind-Thomsen P, Nellemann B, Nielsen TS, Vendelbo MH, Møller N, Sharma R, Lee KY, Kopchick JJ, Jørgensen JOL, Puri V. Growth hormone acts along the PPARgamma-FSP27 axis to stimulate lipolysis in human adipocytes. Am J Physiol Endocrinol Metab 316: E34–E42, 2019. doi: 10.1152/ajpendo.00129.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh M, Kaur R, Lee MJ, Pickering RT, Sharma VM, Puri V, Kandror KV. Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase. J Biol Chem 289: 14481–14487, 2014. doi: 10.1074/jbc.C114.563080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Park SY, Xu L, Xia X, Ye J, Su L, Jeong KH, Hur JH, Oh H, Tamori Y, Zingaretti CM, Cinti S, Argente J, Yu M, Wu L, Ju S, Guan F, Yang H, Choi CS, Savage DB, Li P. Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nat Commun 6: 5949, 2015. doi: 10.1038/ncomms6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs S1 and S2:https://doi.org/10.6084/m9.figshare.17354486.v1.