Abstract
Context
Parathyroid hormone (PTH) gene mutations represent a rare cause of familial isolated hypoparathyroidism (FIH). These defects can cause hypoparathyroidism with increased or decreased serum levels of PTH through 1) impaired PTH synthesis; 2) induction of parathyroid cell apoptosis; or 3) secretion of bioinactive PTH molecules. Eight pathogenic mutations of this gene have been described previously.
Objective
Through describing 2 novel mutations of the PTH gene, we aim to extend the molecular basis for FIH and further refine the proposed mechanisms by which PTH mutations cause hypoparathyroidism.
Methods
Proband case reports were compiled with extended family analysis. The probands in both kindreds presented before age 10 days with hypocalcemia and elevated phosphate levels. Proband A had low PTH levels, whereas these levels were elevated in Proband B. Proband B was initially diagnosed with pseudohypoparathyroidism. Methylation analysis was performed of CpG dinucleotides within 3 GNAS differentially methylated regions; whole-genome sequencing; and PTH infusion with analysis of nephrogenous 3′,5′-cyclic adenosine 5′-monophosphate.
Results
Proband A had a novel heterozygous sequence change in exon 2 of the PTH gene, c.46_47delinsAA (p.Ala16Lys), and proband B had a novel homozygous nucleotide transition in PTH exon 3 (c.128G > A; p.G43E) that led to replacement of glycine by glutamic acid at position 12 of PTH 1-84. PTH 1-34 infusion demonstrated that renal responsiveness to PTH was intact and not antagonized by circulating bioinactive PTH.
Conclusion
PTH gene mutations are uncommon causes of hypoparathyroidism, but can be misdiagnosed as disorders of gland development or receptor function if PTH levels are decreased or elevated, respectively. Genetic testing should be considered early in the diagnostic approach to these presentations.
Keywords: parathyroid, hypoparathyroidism, hypocalcemia, genetic, bioinactive, PTH
Familial isolated hypoparathyroidism (FIH) is an uncommon condition that is associated with mutations in genes that are required for structural or functional integrity of the parathyroid glands. The most common molecular pathology in FIH is related to genetic defects that lead to activation of the calcium-signaling pathway and that inhibit secretion of parathyroid hormone (PTH) from otherwise normal parathyroid glands. This condition has been termed autosomal dominant hypocalcemia and is usually associated with gain-of-function mutations in the genes encoding the calcium sensing receptor (CASR, autosomal dominant hypocalcemia type 1; OMIM 601 198) (1) or the alpha subunit of the G11 protein (GNA11, autosomal dominant hypocalcemia type 2; OMIM 615 361) (2-4) that couples the CASR to intracellular signaling (5). A second and less common mechanism is the lack of development or survival of parathyroid cells, a condition that is most commonly associated with recessive loss of function (6-9) or dominant negative (10-12) mutations (OMIM 618 883) in the glial cells missing-2 (GCM2) gene, which encodes a transcription factor that has been termed the master regulator of parathyroid development (13, 14).
A third form of FIH is due to dominant or recessive mutations in the PTH gene (OMIM 168 450) located at 11p15.3 (15) that impair secretion of bioactive PTH molecules. Although only 8 mutations have been described in the PTH gene (16) to date, these genetic defects lead to hypoparathyroidism through at least 3 different pathophysiological mechanisms, including impaired synthesis of PTH (17), induction of an endoplasmic reticulum (ER) stress response that leads to apoptosis of the parathyroid cells (18-21), or secretion of bioinactive PTH molecules (22, 23). Here we describe 2 novel mutations in the PTH gene that extend the molecular basis for FIH and further refine the proposed mechanisms by which PTH mutations cause hypoparathyroidism.
Materials and Methods
This study was approved by the institutional review board of The Children’s Hospital of Philadelphia (CHOP). Written informed consent, and assent as appropriate, was obtained from all participants.
We recruited 2 individuals and all available family members and collected clinical and molecular data. The diagnosis of functional hypoparathyroidism was based on the presence of hypocalcemia and hyperphosphatemia; serum levels of PTH were low in the individual from family A and elevated in affected members of family B, who were initially suspected to have pseudohypoparathyroidism (PHP) type 1B (PHP1B). Not all participants were able to undergo all analyses.
Genetic Sequence Analyses
Peripheral blood DNA was extracted from all participants using standard techniques. Genomic DNA from proband of family A (Fig. 1A) was subjected to targeted genetic analysis using a commercial gene panel (University of Chicago Genetic Services Laboratory Hypoparathyroidism Panel) that enabled assessment of 20 genes (AIRE, AP2S1, CASR, CHD7, CYP24A1, FAM111A, GATA3, GCM2, GNA11, GNAS, HADHA, HADHB, PDE4D, PRKAR1A, PTH, PTH1R, SOX3, STX16, TBCE, and TBX1) that have been linked to parathyroid disturbances using next-generation sequencing.
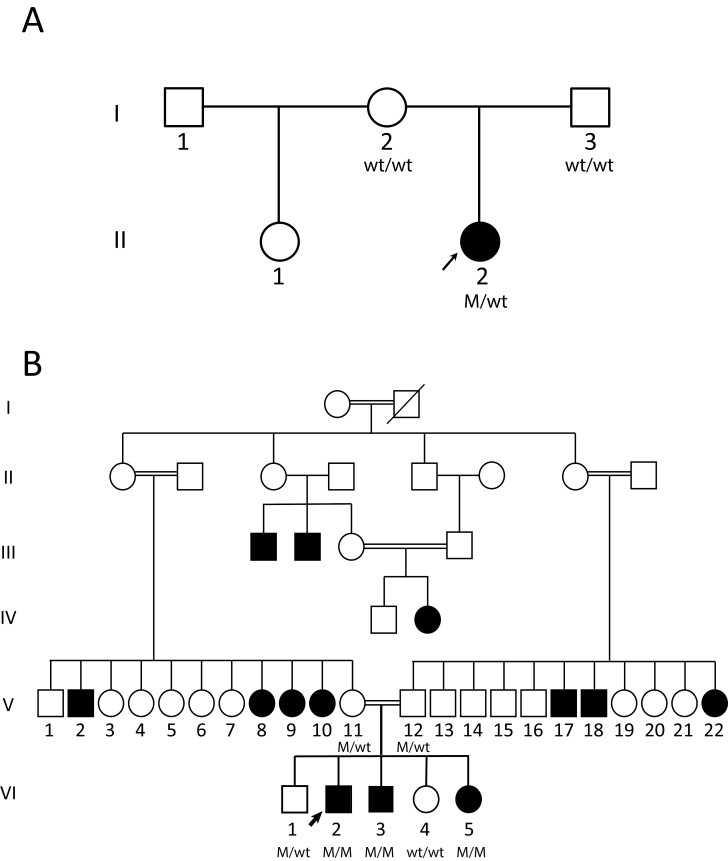
Figure 1.
Pedigrees of the 2 investigated families. Individuals with biochemical hypoparathyroidism are depicted by solid-colored symbols; unaffected individuals are depicted by open symbols. Those participants who underwent genetic testing have genotypes below the symbol; WT is a wild-type parathyroid hormone (PTH) gene allele and M is a mutant allele. Squares denote males and circles denote females. A, The proband, denoted by the arrow, is heterozygous for the PTH gene mutation and her parents are homozygous for WT PTH alleles. B, The proband, denoted by the arrow, and 2 siblings are homozygous for the PTH gene mutation, whereas his parents and 1 brother are heterozygous.
Whole-genome sequencing (WGS) was performed for members of kindred B (Fig. 1B) using previously described methods (24). Briefly, all the raw reads were aligned to the reference human genome using the Burrows-Wheeler Aligner (BWA-Mem) (25) and single-nucleotide variants and small insertions/deletions were captured using the Genome Analysis Tool Kit (26). The kinship coefficient was calculated between every 2 samples via KING (27) to confirm reported relationships.
All variants were confirmed by Sanger sequencing. The potential pathogenicity of all genetic variants that we identified was assessed using Polyphen-2 (28), SIFT (29), and MutationTaster (30). The classification of the genetic variants was performed using criteria recommended by the American College of Medical Genetics and Genomics (31). We analyzed the effect of amino acid replacements on secondary structure of PTH (1-84) using Chou-Fasman, Garnier-Osguthorpe-Robson, and Neural Network methods (http://cib.cf.ocha.ac.jp/bitool/MIX/) (32-34).
We used 2 publicly available databases, the Single Nucleotide Polymorphism Database (https://www.ncbi.nlm.nih.gov/projects/SNP/) and the Exome Aggregation Consortium (http://exac.broadinstitute.org/), to identify previous reports of sequence variants. We used the MUSCLE (multiple sequence comparison by log-expectation) online tool (http://www.ebi.ac.uk/Tools/msa/muscle/) to evaluate phylogenetic conservation of affected codons. The potential effect of missense mutations on signal peptide function was evaluated using SignalP, version 5 (https://services.healthtech.dtu.dk/service.php?SignalP-5.0) (35).
GNAS Gene Methylation Analysis
Bisulfite treatment of genomic DNA and pyrosequencing analysis was performed on DNA from patients VI-2 and VI-3 (see Fig. 1B) from kindred B using the PSQ 96HS system as previously described (24, 36). We also used assay ADS1410 to analyze methylation of the differentially methylated region (DMR) of the maternally imprinted gene NNAT encoding neuronatin on chromosome at 20q11.23 (20:g.36 149 607-36 152 092) as a control for global methylation defects. All experimental conditions are available on request. The amount of C relative to the sum of C and T at each CpG was determined, and we calculated the average of the percentage numbers for all sites in a particular DMR to determine the DMR methylation level (scale, 0-100).
Biochemical Analyses
All biochemical analyses were performed using standard clinical assays. Serum concentrations of 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) were measured by liquid chromatography–tandem mass spectrometry (CHOP). Serum intact PTH levels were determined by commercial immunoassays; for kindred A we used an immunoassay for intact PTH performed on a Siemens IMMULITE 2000; for kindred B we used the Roche e801 Elecsys assay in which a biotinylated monoclonal antibody reacts with epitopes in the amino acid regions 26 to 32 and a second antibody reacts with epitopes in amino acid regions 37 to 42 of the mature PTH 1-84. Fibroblast growth factor-23 levels were measured by Mayo Clinic Laboratories using an immunoassay that detects c-terminal fragments and intact fibroblast growth factor-23. Tubular maximum phosphate reabsorption per glomerular filtration rate was calculated using the Walton and Bijvoet nomogram (37). Concentrations of urinary 3′,5′-cyclic adenosine 5′-monophosphate (cAMP) were measured using the Enzo Life Sciences cAMP enzyme-linked immunosorbent assay kit (catalog No. ADI-900-067, RRID:AB_2814712; https://antibodyregistry.org/search.php?q=AB_2814712).
Parathyroid hormone infusion protocol
PTH infusion was performed using a protocol based on one previously described (38, 39). A dose of 40 μg of human recombinant PTH 1-34 (teriparatide) was administered subcutaneously at time 0. Urinary creatinine, phosphorus, calcium, and cAMP were measured at –60, –30, 0, 30, 60, 90, 120, 180, and 240 minutes. Serum creatinine, phosphorus, and intact PTH were measured at 0, 30, 60, 90, 120, 180, and 240 minutes.
Statistical Analyses
Results are presented as mean ± SD, and comparisons between groups were analyzed by one-way analysis of variance with Dunnett posttest using GraphPad InStat version 3.10 for Windows (GraphPad Software, www.graphpad.com).
Results
Case Reports
Kindred A
The proband (Fig. 1A) is an African American boy who was born at term following an uncomplicated pregnancy. He had been formula-fed with appropriate weight gain but at age 8 days he was admitted to hospital after a prolonged generalized, tonic-clonic seizure, preceded by twitching of his upper and lower limbs the day of presentation. Biochemical investigation demonstrated a serum calcium concentration of 1.3 mmol/L (reference range, 1.9-2.6 mmol/L), ionized calcium concentration of 0.6 mmol/L (reference range, 1.1-1.4 mmol/L mmol/L), inorganic phosphate level of 4.1 mmol/L (reference range, 1.5-2.9), magnesium level of 0.7 mmol/L (reference range, 0.7-1 mmol/L), 25(OH)D concentration of 24.5 nmol/L (reference range, 75-250 nmol/L), and intact PTH level of 0.6 pmol/L (reference range, 1.1-6.9 pmol/L). Alkaline phosphatase and renal function were normal. Physical examination was notable for a high-pitched holosystolic murmur, hypertelorism, and an exaggerated Moro reflex that evolved into active convulsions. An echocardiogram demonstrated a small ventricular septal defect with left-to-right shunting and a very small patent foramen ovale. Genetic testing for DiGeorge sequence was negative, and a single-nucleotide variation microarray was normal. Intact PTH levels remained low. He has been treated with elemental calcium plus calcitriol.
Kindred B
The proband, patient VI-2 and his brother VI-3 each presented at age 10 days with hypocalcemia. They are White and from the United Arab Emirates. A sister (VI-5) is also affected. Their consanguineous parents and 2 other siblings are unaffected, but there are many maternal and paternal aunts and uncles who are also affected (see Fig. 1B and Table 1). The proband, now aged 11 years, presented with a seizure at age 10 days and was found to have a very low serum calcium level (1.4 mmol/L) with elevated levels of phosphorus and PTH. He also had severe vitamin D deficiency that was treated as well. His present treatment is elemental calcium plus alfacalcidol daily. Both brothers showed normal growth and development. All affected individuals had markedly elevated serum levels of PTH, hypocalcemia, and hyperphosphatemia (see Table 1). Based on these laboratory findings, the proband and his affected siblings were initially diagnosed as having a possible form of autosomal recessive PHP1B (40).
Table 1.
Clinical and biochemical characteristics of members of family B
| Family member | Clinical status | Current age, y | Sex | PTH genotype | sCa, mmol/L | sPhos, mmol/L | iCa, mmol/L | 25(OH)D, nmol/L | 1,25(OH)2D, pmol/L | Intact PTH, pmol/L |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference range | 2.15-2.55 | 1.05-1.8 | 1.1-1.3 | 50-150 | 48-192 | 1.6-6.9 | ||||
| V-8 | Affected | 40 | F | ND | 1.8 | 1.46 | 0.91 | 36.5 | 9.3 | |
| V-9 | Affected | 25 | F | ND | 1.65 | 2.06 | 36.2 | 19.1 | ||
| V-11 | Unaffected | 32 | F | Het | 2.39 | 0.82 | 1.22 | 33.7 | 6.4 | |
| V-12 | Unaffected | 35 | M | Het | 2.32 | 0.95 | 1.28 | 23.8 | 8.1 | |
| V-22 | Affected | 36 | F | ND | 1.76 | 1.52 | 66.2 | 32.2 | ||
| VI-1 | Unaffected | 12 | M | Het | 2.35 | 1.11 | 29 | 6.8 | ||
| VI-2 | Affected | 11 | M | Hom | 1.7 | 2.61 | 0.89 | 14 | 29 | 23.8 |
| VI-3 | Affected | 9 | M | Hom | 1.69 | 2.28 | 0.85 | 56 | < 12 | 74 |
| VI-4 | Unaffected | 5 | F | WT | 2.5 | 2.03 | 2.7 | |||
| VI-5 | Affected | 2 | F | Hom | 1.7 | 3.16 | 0.97 | 84.7 | 76.9 |
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; F, female; M, male; HET, heterozygous; HOM, homozygous; iCa, intact calcium; ND, not done; PTH, parathyroid hormone; sCa, serum calcium; sPhos, serum phosphorus; WT, wild-type.
Methylation Analysis
We used quantitative pyrosequencing to assess the methylation status of CpG dinucleotides within 3 GNAS DMRs that are associated with methylation defects in individuals with PHP1B (36) in members of family B. Our pyrosequencing analyses showed that both VI-2 and VI-3 had normal methylation at each DMR tested (Table 2).
Table 2.
Methylation analysis of GNAS differentially methylated regions in family B
| NESP55 DMR methylation, % | XL DMR methylation, % | A/B DMR methylation, % | |
|---|---|---|---|
| Controls | |||
| 45.9 | 38.2 | 50.1 | |
| 39.4 | 34.7 | 49.2 | |
| 41.2 | 39.1 | 51.9 | |
| Mean ± SD | 42.1 ± 3.4 | 37.3 ± 2.3 | 50.4 ± 1.4 |
| Participants | |||
| VI-2 | 42.3 | 39.6 | 51.6 |
| VI-3 | 40.8 | 45.7 | 51.8 |
| Mean ± SD | 41.5 ± 1.1 | 42.7 ± 4.3 | 51.7 ± 0.1 |
Abbreviations: DMR, differentially methylated region; GNAS, alpha subunit of the Gs protein.
Gene Sequencing
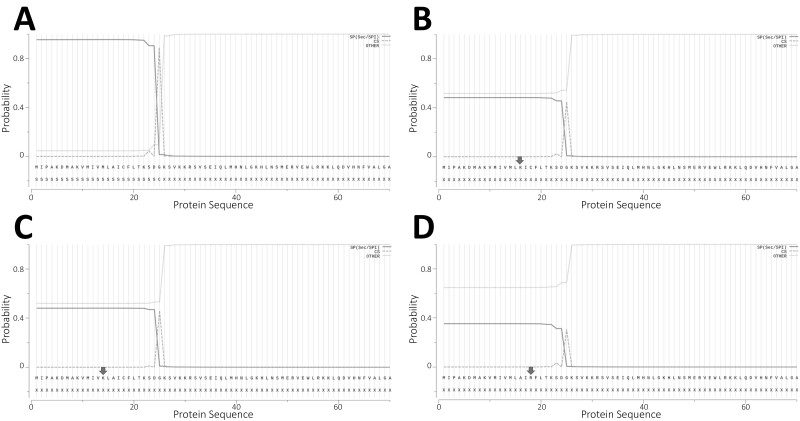
Genomic DNA from the proband in kindred A (see Fig. 1A) revealed a novel heterozygous sequence change in exon 2 of the PTH gene, c.46_47delinsAA (p.Ala16Lys). Analysis of parental DNA samples was negative for the variant. The p.Ala16Lys variant occurs within the hydrophobic core of the leader sequence of preproparathyroid hormone (preproPTH; Fig 2). The variant is not present in either the Single Nucleotide Polymorphism Database or Exome Aggregation Consortium database, making unlikely the possibility that the variant is a rare polymorphism. A phylogenetic analysis showed that alanine-16 is highly conserved among orthologs and therefore likely to have functional significance and its replacement is therefore considered to be pathogenic. The p.A16K substitution is flanked by 2 other similar heterozygous missense mutations, p.M14K and p.C18R, that have been linked to hypoparathyroidism through a mechanism in which defective processing of the leader sequence leads to intracellular retention of the nascent mutant protein, predominantly in the ER, with subsequent induction of the unfolded protein response and apoptosis (18-21). Similar to Cinque et al (19), we used the SignalP hidden Markov model program (version 5.0) to analyze the amino acid sequence of the p.A16K preproPTH proteins. The program correctly identified a signal sequence within the wild-type (WT) preproPTH protein (probability, 0.954) with a cleavage site between positions +25 and +26 (Sustainable Development Goals–Kolmogorov-Smirnov; probability of 0.889) (Fig. 3A). These analyses further predicted that the signal peptide has an n-region from amino acid 1 to 9, a hydrophobic core h-region from amino acid 10 to 20, and a c-region from amino acid 21 to 25. The SignaIP analysis of the preproPTH protein in which Ala-16 is replaced by Lys shows a markedly reduced probability (0.483) that the variant protein contains a signal peptide and fails to identify a functional cleavage site (Fig. 3B). Therefore, these in silico analyses for p.A16K, in the context of similar in silico results (Fig. 3C and 3D) and experimental data for p.M14K and p.C18R, suggest that defective processing of the leader sequence of these preproPTH molecules leads to ER stress-induced parathyroid cell death and provides an underlying mechanism for FIH due to these heterozygous missense mutations in the PTH gene.
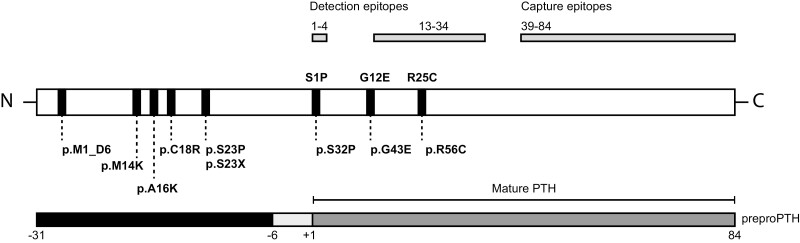
Figure 2.
Schematic representation of the parathyroid hormone (PTH) protein. The complete preproparathyroid hormone (preproPTH) molecule is shown in the center with positions of point mutations that affect the protein shown beneath the figure; positions of the 3 mutations that cause bioinactive PTH are also depicted above the figure in the mature, PTH 1-84 molecule. The lower figure shows preproPTH, which consists of 115 amino acids; the specific regions comprise a 25-amino acid pre sequence (–31 to –7), a 6-amino acid pro sequence (–6 to –1), and 84-amino acid mature PTH molecule (+1 to 84). The upper figure shows epitopes used for detection of whole PTH (1-4) or intact PTH (13-34) as well as the epitope used to capture PTH fragments (39-84) in conventional sandwich assays.
Figure 3.
Evaluation of the preproparathyroid hormone (preproPTH) as a signal peptide using the SignalP hidden Markov model program. A, Wild-type (WT) parathyroid hormone 1-84; B, p.A16K; C, p.M14K; D, p.C18R proteins; black arrows denote replaced amino acids. A highly relevant probability score was obtained for likelihood of N-terminal signal sequence (dotted red line) and likelihood is predicted for cleavage site (dotted green line). The WT protein (A) has a strongly predicted signal peptide cleavage site between amino acids 25 and 26. Residues are numbered conventionally, with the first residue (M) of the preproPTH polypeptide designated as +1. See text for “Materials and Methods”.
WGS revealed a novel homozygous nucleotide transition in PTH exon 3 (c.128G > A) in the proband of family B, V1-2 (see Fig. 1) that results in substitution of glycine at codon 43 to glutamic acid (p.G43E), which corresponds to amino acid 12 in the mature 1-84 PTH molecule (see Fig. 2). WGS did not disclose any other genetic variants in GNAS that could be disease causing (data not shown). The glycine at position 12 is highly conserved among the mature PTH proteins of all known species and is one of the few amino acids that is identical in PTH and PTH-related peptide (PTHrP), consistent with its importance in the interaction of these molecules with their shared receptor, the type 1 PTH receptor (PTHR1). The structural elements of PTH and PTHrP that are required for receptor binding and biological activity are 2 helical segments, one N-terminal and one C-terminal, connected by hinges or flexible points located around positions 12 and 19 (41, 42). The biological effects of structural modification of PTH and PTHrP at position 12 have been comprehensively evaluated with the finding that this position tolerates a wide range of substitutions without substantial weakening of receptor interaction (41-43). Moreover, introduction of hydrophobic amino acids at position 12 enhances antagonist activity for PTH analogues without substantially affecting binding affinity for the PTHR1 (42); in addition to competitively antagonizing cAMP signaling at PTHR1, these substituted ligands can also act as inverse agonists that can reduces basal cAMP signaling at certain constitutively active PTHR1 variants (44).
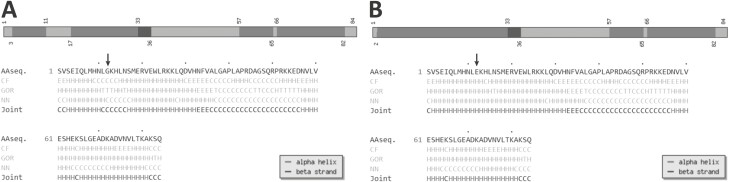
The p.G43E variant segregated with isolated hypoparathyroidism in this family. The unaffected parents were heterozygous, while 2 affected siblings were also homozygous. One unaffected brother was heterozygous for the same nucleotide change and an unaffected sister was homozygous for the WT nucleotide (see Fig. 1B). Serum levels of calcium were normal in the heterozygous participants but levels of intact PTH were at the upper limits of normal or slightly increased, possibly due to coexisting vitamin D deficiency as serum phosphate levels were low or low normal (see Table 1). The p.G43E variant is not present in either the Single Nucleotide Polymorphism Database or Exome Aggregation Consortium database, making unlikely the possibility that the variant is a rare polymorphism. Although in silico analyses classify p.G43E as a variant of unknown significance, when we ran a secondary structure prediction model on the WT and p.G43E proteins using the Chou and Fasman Secondary Structure Prediction Server (CFSSP, http://www.biogem.org/tool/chou-fasman/) (Fig. 4) we found that the amino acid replacement led to a critical disruption of the beta strand (45). Taken together, the data indicate that this variant is likely to be pathogenic.
Figure 4.
Secondary structure predicted by the Chou-Fasman algorithm. We used the CFSPP online server to analyze the predicted locations of alpha-helix and beta-strand from the amino acid sequence of A, wild-type (WT), and B, p.G43E parathyroid hormone proteins. In each panel, a black arrow denotes either the WT amino acid or the substituted amino acid at position 43. Alpha helices are shown in light grey and beta strands are shown in dark grey.
Parathyroid Hormone Infusion
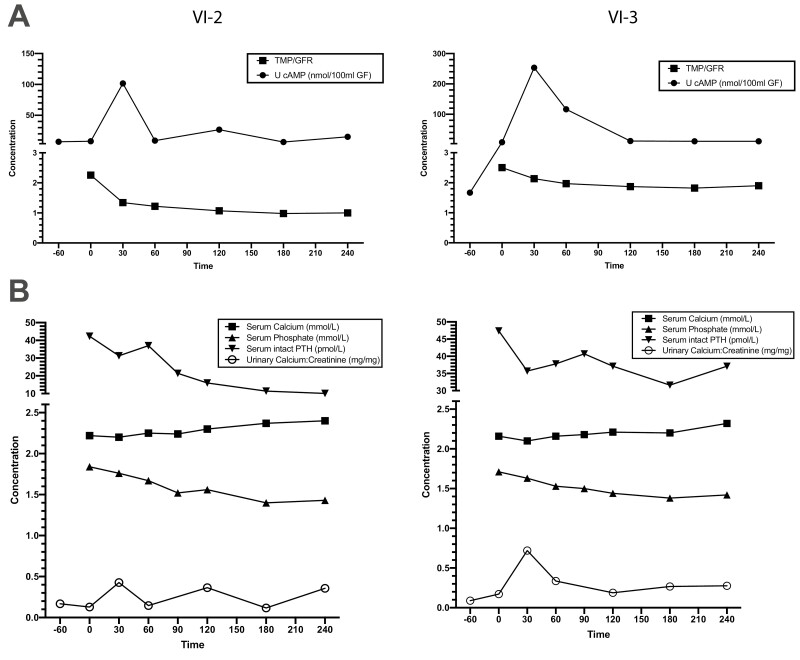
Subcutaneous administration of teriparatide to the proband (VI-2) and his affected brother (VI-3) from kindred B was performed when they were aged 8 and 10 years, respectively. This elicited normal (> 10-fold) increases in urinary excretion of nephrogenous cAMP followed by corresponding normal (> 20%) decreases in phosphate reabsorption (38, 39) (Fig. 5A) and serum phosphate concentrations (Fig. 4B) (46). In addition, the concentration of intact PTH (1-84) in serum fell appropriately after injection of teriparatide, presumably in response to increasing serum concentrations of total calcium that also led to increases in urinary calcium excretion (Fig. 5B) (46). These responses indicate that renal responsiveness to PTH was completely intact and was not antagonized by elevated circulating levels of bioinactive PTH (1-84).
Figure 5.
Teriparatide infusion in 2 affected individuals from family B. Effects of teriparatide on affected individuals VI-2 (left) and VI-3 (right) are shown. Teriparatide (40 µg) was administered subcutaneously at time 0. A, Upper panels show the changes in urinary excretion of 3′,5′-cyclic adenosine 5′-monophosphate (cAMP) and tubular maximum phosphate reabsorption per glomerular filtration rate (TMP/GFR) after infusion; B, lower panels show changes in serum levels of calcium, phosphate, and intact parathyroid hormone (PTH) as well as urinary calcium to creatinine ratio.
Discussion
Genetic defects are uncommon causes of hypoparathyroidism but should be purposefully considered in young children with early-onset or congenital hypoparathyroidism (47). The 2 families that we describe in this report provide relevant lessons about PTH biochemistry as well as the evolving approach to contemporary molecular diagnosis. Levels of serum intact PTH were low in the proband of family A, which led clinicians to initially consider embryological defects in parathyroid development as the basis for hypoparathyroidism. By contrast, serum levels of intact PTH were markedly elevated in affected individuals in family B, which prompted studies to evaluate a possible form of autosomal recessive PHP1B (40) as the basis. In both cases initial studies were noninformative, and ultimately the correct diagnoses were established through gene-agnostic molecular testing that used next-generation sequencing techniques. Thus, these cases argue for a diagnostic algorithm in which genetic testing occurs early during the evaluation of hypoparathyroidism, and which while perhaps less satisfying intellectually is ultimately more expedient (and cost-effective) functionally.
Mutations of the PTH gene are uncommon but they may be an underrecognized cause of hypoparathyroidism because of genetic defects that lead to low or undetectable levels of immunoreactive PTH in the serum. The human PTH gene contains 3 exons; exon 1 is noncoding, whereas exons 2 and 3 encode preproPTH (48-50) (see Fig. 2). The preproPTH messenger RNA encodes an amino-terminal 25-amino-acid leader or signal peptide that directs the nascent chain into the ER, where it is cotranslationally cleaved by the signalase enzyme. The 6-amino-acid propeptide is subsequently removed by proprotein convertases, after trafficking to the Golgi apparatus, thereby yielding the mature 84-amino-acid PTH polypeptide. Defects in the PTH gene can cause hypoparathyroidism through several different mechanisms (16). For example, mutations can lead to abnormal splicing of preproPTH messenger RNA (17), disrupt processing of the preproPTH protein (51-53), or generate bioinactive PTH molecules (22, 23).
In the proband of family A we identified a novel heterozygous missense mutation, p.A16K, in the hydrophobic core of the leader sequence that is flanked by 2 previously identified missense mutations, p.M14K (19) and p.C18R (18) that had been shown to cause autosomal dominant isolated hypoparathyroidism via inhibition of the normal processing of preproPTH to proPTH (19, 21). Functional assays in HEK293 cells demonstrated much greater intracellular retention of these mutant proteins, principally in the ER, with induction of ER stress and apoptosis that could be prevented by addition of the chemical chaperone 4-phenylbutyrate (4-PBA) to the cell culture medium (19, 20). These observations raise the possibility that 4-PBA, or another protein chaperone, might be useful as a rescue therapy (54) to restore PTH secretion and/or preserve parathyroid cell mass in patients with PTH gene mutations that impair function of the signal peptide.
In family B we identified a novel missense mutation, p.G43E, that replaces the highly conserved amino acid 12 in mature PTH with glutamic acid and results in a molecule that lacks PTH bioactivity in vivo but is recognized by second-generation immunoassays for intact PTH (see Fig. 2). Second-generation assays are sandwich immunoassays in which a capture antibody is directed against the C-terminal portion of mature PTH (residues 39-84) and a second detector antibody that is directed against epitopes within the N-terminal part of the molecule (13-34, 55). Although these assays were supposed to measure only full-length PTH, the later discovery of amino terminally truncated PTH fragments such as PTH (7-84) that can antagonize PTHR1 signaling led to the development of third-generation “whole-molecule” PTH assays that used detector antibodies directed against amino acids 1 to 4 (56). Two previous examples of bioinactive PTH molecules have been reported. In one case, replacement of serine by proline (p.S32P) at the position of the first amino acid in mature PTH (1-84) did not interfere with detection by second-generation assays (23). By contrast, the other mutation, p.R56C, which led to replacement of arginine by cysteine in position 25 of mature PTH, generated a PTH molecule that showed reduced reactivity when measured with an intact PTH assay (22). The discordant results between p.R56C and p.G43E (position 12 in mature PTH) in assays for intact PTH likely result from epitopes used in assays for intact PTH. Today, most assays for intact PTH are immunometric assays that use 2 distinct antibodies, a capture antibody usually directed against a carboxyl-terminal portion of PTH(1-84) and detector antibodies that are directed against an antigenic site located around amino acids 20 to 25 of mature PTH (57). The assay that we used to measure intact PTH in members of this family was a sandwich assay in which a biotinylated monoclonal capture antibody reacts with epitopes in the N-terminal amino acid regions 26 to 32, which would predict full reactivity of this assay with the bioinactive PTH molecules with replacement of residue 12 in kindred B and less predictable behavior with the bioinactive p.R56C PTH containing a substitution at residue 25. Unfortunately, it was not possible to assess the immunoreactivity of PTH pG43E in other assays, including assays for whole-molecule PTH.
The basis for the lack of bioactivity of p.G43E PTH is likely to be disruption of secondary structure. Gly43, which corresponds to amino acid 12 of mature PTH, is within the N-terminal region (residues 1-14) of the peptide that binds to the transmembrane helices and extracellular connecting loops (site 2) of PTHR1 and occupies the orthosteric pocket. Bimodal interaction of PTH with the PTHR1 is achieved through binding of a second sequence, residues 15 to 34, to an elongated hydrophobic groove on the extracellular domain of the receptor termed site 1 (58). Both of these binding regions form alpha helices that are connected by a beta-turn that provides a highly flexible midregion (59). Despite the flexible nature of glycine, Gly12 is in a strict helical conformation in the crystal structure of PTH 1-34 (59). Substitution of Gly12 with alanine, a helix promotor, in [Tyr34]hPTH-(1–34)NH2 was well tolerated, whereas substitution with proline, a helix breaker, decreased receptor binding affinity 840-fold and adenylyl cyclase–stimulating activity 3500-fold (42). Our analyses indicated that replacement of Gly12 by Glu leads to a loss of the beta-sheet in the PTH molecule, thereby disrupting the 2 distinct alpha helices needed to achieve bimodal binding to the PTHR1. Together, these findings indicate that Gly12 is essential for full biological activity of PTH and that replacement by polar glutamate disrupts the required secondary structure needed for binding and activation of the PTHR1.
In conclusion, we report 2 new mutations in the PTH gene that cause FIH through differing mechanisms. These mutations expand the number of pathogenic mutations in the PTH gene and emphasize the importance of considering the PTH gene as a cause of hypoparathyroidism in patients with either elevated or undetectable serum levels of immunoreactive PTH.
Acknowledgments
The authors gratefully acknowledge the family members who participated in these studies as well as the excellent technical assistance of Mr Harsh Kanwar. We also thank Dr Thomas Gardella, Massachusetts General Hospital, for thoughtful discussion of our work.
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- cAMP
3′,5′-cyclic adenosine 5′-monophosphate
- CASR
calcium-sensing receptor
- DMR
differentially methylated region
- ER
endoplasmic reticulum
- FIH
familial isolated hypoparathyroidism
- GCM2
glial cells missing-2
- GFR
glomerular filtration rate
- GNA11
G protein α11
- PHP
pseudohypoparathyroidism
- PHP1B
pseudohypoparathyroidism type 1B
- preproPTH
preproparathyroid hormone
- PTH
parathyroid hormone
- PTHR1
type 1 parathyroid hormone receptor
- PTHrP
parathyroid hormone–related peptide
- WGS
whole-genome sequencing
- WT
wild-type
Financial Support
This work was supported the CHOP Research Institute and the National Institutes of Health (grant No. R01 DK112955 to M.A.L.).
Disclosures
The authors have nothing to declare.
Current Affiliation
Susan E. Tucker is currently affiliated with CHG Healthcare, Midvale, Utah 84047, USA.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Thakker RV. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium. 2004;35(3):275-282. [DOI] [PubMed] [Google Scholar]
- 2. Nesbit MA, Hannan FM, Howles SA, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368(26):2476-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li D, Opas EE, Tuluc F, et al. Autosomal dominant hypoparathyroidism caused by germline mutation in GNA11: phenotypic and molecular characterization. J Clin Endocrinol Metab. 2014;99(9):E1774-E1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mannstadt M, Harris M, Bravenboer B, et al. Germline mutations affecting Gα11 in hypoparathyroidism. N Engl J Med. 2013;368(26):2532-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roszko KL, Bi RD, Mannstadt M. Autosomal dominant hypocalcemia (hypoparathyroidism) types 1 and 2. Front Physiol. 2016;7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding C, Buckingham B, Levine MA. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest. 2001;108(8):1215-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yi HS, Eom YS, Park Ie B, et al. Identification and characterization of C106R, a novel mutation in the DNA-binding domain of GCMB, in a family with autosomal-dominant hypoparathyroidism. Clin Endocrinol (Oxf). 2012;76(5):625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SY, Eom YS, Choi B, et al. Genetic and clinical characteristics of Korean patients with isolated hypoparathyroidism: from the Korean Hypopara Registry Study. J Korean Med Sci. 2013;28(10):1489-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sticht H, Hashemolhosseini S. A common structural mechanism underlying GCMB mutations that cause hypoparathyroidism. Med Hypotheses. 2006;67(3):482-487. [DOI] [PubMed] [Google Scholar]
- 10. Mannstadt M, Bertrand G, Muresan M, et al. Dominant-negative GCMB mutations cause an autosomal dominant form of hypoparathyroidism. J Clin Endocrinol Metab. 2008;93(9):3568-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canaff L, Zhou X, Mosesova I, Cole DE, Hendy GN. Glial cells missing-2 (GCM2) transactivates the calcium-sensing receptor gene: effect of a dominant-negative GCM2 mutant associated with autosomal dominant hypoparathyroidism. Hum Mutat. 2009;30(1):85-92. [DOI] [PubMed] [Google Scholar]
- 12. Mirczuk SM, Bowl MR, Nesbit MA, et al. A missense glial cells missing homolog B (GCMB) mutation, Asn502His, causes autosomal dominant hypoparathyroidism. J Clin Endocrinol Metab. 2010;95(7):3512-3516. [DOI] [PubMed] [Google Scholar]
- 13. Kebebew E, Peng M, Wong MG, Ginzinger D, Duh QY, Clark OH. GCMB gene, a master regulator of parathyroid gland development, expression, and regulation in hyperparathyroidism. Surgery. 2004;136(6):1261-1266. [DOI] [PubMed] [Google Scholar]
- 14. Liu Z, Yu S, Manley NR. Gcm2 is required for the differentiation and survival of parathyroid precursor cells in the parathyroid/thymus primordia. Dev Biol. 2007;305(1):333-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tonoki H, Narahara K, Matsumoto T, Niikawa N. Regional mapping of the parathyroid hormone gene (PTH) by cytogenetic and molecular studies. Cytogenet Cell Genet. 1991;56(2):103-104. [DOI] [PubMed] [Google Scholar]
- 16. Lee JH, Davaatseren M, Lee S. Rare PTH gene mutations causing parathyroid disorders: a review. Endocrinol Metab (Seoul). 2020;35(1):64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parkinson DB, Thakker RV. A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism. Nat Genet. 1992;1:149-152. [DOI] [PubMed] [Google Scholar]
- 18. Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest. 1990;86(4):1084-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cinque L, Sparaneo A, Penta L, et al. Autosomal dominant PTH gene signal sequence mutation in a family with familial isolated hypoparathyroidism. J Clin Endocrinol Metab. 2017;102(11):3961-3969. [DOI] [PubMed] [Google Scholar]
- 20. Datta R, Waheed A, Shah GN, Sly WS. Signal sequence mutation in autosomal dominant form of hypoparathyroidism induces apoptosis that is corrected by a chemical chaperone. Proc Natl Acad Sci U S A. 2007;104:19989-19994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karaplis AC, Lim SK, Baba H, Arnold A, Kronenberg HM. Inefficient membrane targeting, translocation, and proteolytic processing by signal peptidase of a mutant preproparathyroid hormone protein. J Biol Chem. 1995;270(4):1629-1635. [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Mannstadt M, Guo J, et al. A homozygous [Cys25]PTH(1-84) mutation that impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015;30(10):1803-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gild ML, Bullock M, Luxford C, Field M, Clifton-Bligh RJ. Congenital hypoparathyroidism associated with elevated circulating nonfunctional parathyroid hormone due to novel PTH mutation. J Clin Endocrinol Metab. 2020;105(7):dgaa279. [DOI] [PubMed] [Google Scholar]
- 24. Li D, Bupp C, March ME, Hakonarson H, Levine MA. Intragenic deletions of GNAS in pseudohypoparathyroidism type 1A identify a new region affecting methylation of exon A/B. J Clin Endocrinol Metab. 2020;105(9):e3197-e3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25(14):1754-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26(22):2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40(Web Server issue):W452-W457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361-362. [DOI] [PubMed] [Google Scholar]
- 31. Richards S, Aziz N, Bale S, et al. ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qian N, Sejnowski TJ. Predicting the secondary structure of globular proteins using neural network models. J Mol Biol. 1988;202(4):865-884. [DOI] [PubMed] [Google Scholar]
- 33. Garnier J, Osguthorpe DJ, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978;120(1): 97-120. [DOI] [PubMed] [Google Scholar]
- 34. Prevelige P, Fasman GD. Chou-Fasman prediction of the secondary structure of proteins. In: Fasman GD, ed. Prediction of Protein Structure and the Principles of Protein Conformation. Springer; 1989:391-416. [Google Scholar]
- 35. Almagro Armenteros JJ, Tsirigos KD, Sønderby CK, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37(4):420-423. [DOI] [PubMed] [Google Scholar]
- 36. Danzig J, Li D, Jan de Beur S, Levine MA. High-throughput molecular analysis of pseudohypoparathyroidism 1B patients reveals novel genetic and epigenetic defects. J Clin Endocrinol Metab. 2021;106(11):e4603-e4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walton RJ, Bijvoet OL. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;2(7629):309-310. [DOI] [PubMed] [Google Scholar]
- 38. Mallette LE, Kirkland JL, Gagel RF, Law WM Jr, Heath H III. Synthetic human parathyroid hormone-(1-34) for the study of pseudohypoparathyroidism. J Clin Endocrinol Metab. 1988;67(5):964-972. [DOI] [PubMed] [Google Scholar]
- 39. Mallette LE. Synthetic human parathyroid hormone 1-34 fragment for diagnostic testing. Ann Intern Med. 1988;109(10): 800-804. [DOI] [PubMed] [Google Scholar]
- 40. Fernández-Rebollo E, Pérez de Nanclares G, Lecumberri B, et al. 2011 Exclusion of the GNAS locus in PHP-Ib patients with broad GNAS methylation changes: evidence for an autosomal recessive form of PHP-Ib? J Bone Miner Res. 1863;26(8):1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldman ME, McKee RL, Caulfield MP, et al. A new highly potent parathyroid hormone antagonist: [D-Trp12,Tyr34]bPTH-(7-34)NH2. Endocrinology. 1988;123(5):2597-2599. [DOI] [PubMed] [Google Scholar]
- 42. Chorev M, Goldman ME, McKee RL, et al. Modifications of position 12 in parathyroid hormone and parathyroid hormone related protein: toward the design of highly potent antagonists. Biochemistry. 1990;29(6):1580-1586. [DOI] [PubMed] [Google Scholar]
- 43. Peggion E, Mammi S, Schievano E, et al. Structure-function studies of analogues of parathyroid hormone (PTH)-1-34 containing beta-amino acid residues in positions 11-13. Biochemistry. 2002;41(25):8162-8175. [DOI] [PubMed] [Google Scholar]
- 44. Cheloha RW, Gellman SH, Vilardaga JP, Gardella TJ. PTH receptor-1 signalling—mechanistic insights and therapeutic prospects. Nat Rev Endocrinol. 2015;11(12):712-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kumar TA. CFSSP: Chou and Fasman secondary structure prediction server. Wide Spectrum. 2013;1:15-19. [Google Scholar]
- 46. Lindsay R, Nieves J, Henneman E, Shen V, Cosman F. Subcutaneous administration of the amino-terminal fragment of human parathyroid hormone-(1-34): kinetics and biochemical response in estrogenized osteoporotic patients. J Clin Endocrinol Metab. 1993;77(6):1535-1539. [DOI] [PubMed] [Google Scholar]
- 47. Gordon RJ, Levine MA. Genetic Disorders of parathyroid development and function. Endocrinol Metab Clin North Am. 2018;47(4):809-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goswami R, Mohapatra T, Gupta N, et al. Parathyroid hormone gene polymorphism and sporadic idiopathic hypoparathyroidism. J Clin Endocrinol Metab. 2004;89(10):4840-4845. [DOI] [PubMed] [Google Scholar]
- 49. Vasicek TJ, McDevitt BE, Freeman MW, et al. Nucleotide sequence of the human parathyroid hormone gene. Proc Natl Acad Sci U S A. 1983;80(8):2127-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reis A, Hecht W, Gröger R, et al. Cloning and sequence analysis of the human parathyroid hormone gene region. Hum Genet. 1990;84(2):119-124. [DOI] [PubMed] [Google Scholar]
- 51. Ertl DA, Stary S, Streubel B, Raimann A, Haeusler G. A novel homozygous mutation in the parathyroid hormone gene (PTH) in a girl with isolated hypoparathyroidism. Bone. 2012;51(3):629-632. [DOI] [PubMed] [Google Scholar]
- 52. Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S. A novel mutation of the signal peptide of the preproparathyroid hormone gene associated with autosomal recessive familial isolated hypoparathyroidism. J Clin Endocrinol Metab. 1999;84(10):3792-3796. [DOI] [PubMed] [Google Scholar]
- 53. Baran N, ter Braak M, Saffrich R, Woelfle J, Schmitz U. Novel activating mutation of human calcium-sensing receptor in a family with autosomal dominant hypocalcaemia. Mol Cell Endocrinol. 2015;407:18-25. [DOI] [PubMed] [Google Scholar]
- 54. Singh OV, Pollard HB, Zeitlin PL. Chemical rescue of deltaF508-CFTR mimics genetic repair in cystic fibrosis bronchial epithelial cells. Mol Cell Proteomics. 2008;7(6):1099-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Couchman L, Taylor DR, Krastins B, Lopez MF, Moniz CF. LC-MS candidate reference methods for the harmonisation of parathyroid hormone (PTH) measurement: a review of recent developments and future considerations. Clin Chem Lab Med. 2014;52(9): 1251-1263. [DOI] [PubMed] [Google Scholar]
- 56. Lepage R, Roy L, Brossard JH, et al. A non-(1-84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem. 1998;44(4):805-809. [PubMed] [Google Scholar]
- 57. Vieira JGH. PTH assays: understanding what we have and forecasting what we will have. J Osteoporos. 2012;2012:523246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ehrenmann J, Schöppe J, Klenk C, et al. High-resolution crystal structure of parathyroid hormone 1 receptor in complex with a peptide agonist. Nat Struct Mol Biol. 2018;25:1086-1092. [DOI] [PubMed] [Google Scholar]
- 59. Jin L, Briggs SL, Chandrasekhar S, et al. Crystal structure of human parathyroid hormone 1-34 at 0.9-A resolution. J Biol Chem. 2000;275(35):27238-27244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.