Abstract
VEXAS (vacuoles, E1 enzyme, X-linked, auto-inflammatory, somatic) syndrome is an inflammatory disorder with hematological and systemic features. A recent study demonstrated that the dermal infiltrate in neutrophilic dermatosis from VEXAS patients is derived from the pathological UBA1-mutated myeloid clone. Neutrophilic dermatosis is, however, only one of the various skin involvements observed in VEXAS syndrome. We analyzed 10 formalin-fixed paraffin-embedded skin biopsies from genetically confirmed VEXAS syndrome. UBA1 mutation was found in the biopsies related to neutrophilic dermatitis but in none of the other histological patterns (leukocytoclastic vasculitis and septal panniculitis). This could lead to a distinction between clonal and paraclonal cutaneous involvements in VEXAS syndrome, which could in turn improve therapeutic outcomes.
Keywords: Autoinflammatory diseases, Sweet syndrome, Vasculitis, Clonal hematopoiesis, Mutation
To the Editor,
VEXAS (vacuoles, E1 enzyme, X-linked, auto-inflammatory, somatic) syndrome is a recently described adult-onset inflammatory disorder with various involvements including arthritis, chondritis, cutaneous features, macrocytic anemia and myelodysplastic syndrome [1–4]. Zakine et al. recently performed molecular analyses on 8 paraffin-embedded skin tissue sections of neutrophilic dermatosis in patients with VEXAS syndrome [5]. They identified UBA1 mutations in all of these skin samples. This was the first study to demonstrate a strong link between the presence of UBA1-mutated cells in an involved tissue (except for bone marrow) and the related clinical manifestations. According to the author’s conclusion, this suggests that the dermal infiltrate in VEXAS skin lesions is derived from the pathological myeloid clone, which could be targeted to treat VEXAS patients with cutaneous involvement.
Neutrophilic dermatosis is, however, only one of the various skin involvements observed in VEXAS syndrome. Indeed, leukocytoclastic vasculitis, erythema nodosa and periorbital edema have also been reported in this rare and recently described disorder [1]. Consequently, we aimed to assess the presence and abundance of the UBA1-mutated clone in the different cutaneous involvements related to VEXAS syndrome.
We retrospectively analyzed the medical record of 6 patients with both genetically confirmed VEXAS syndrome (UBA1 mutation identified from blood samples, UBA1 variants detailed in Table 1) and related skin involvement with formalin-fixed paraffin-embedded skin biopsies performed between January 2017 and September 2021 in Angers University Hospital. Two methods of dewaxing and DNA extraction were used (Kit NucleoSpin Tissue and NucleoSpin DNA FFPE XS, Macherey Nagel, Düren, Germany). The quality and quantity of the extracted DNA samples were evaluated using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, USA). In case of sufficient DNA quantity and quality (260/280 nm absorbance ratio between 1.8 and 2.0), somatic mutations in UBA1 (NM_003334.3) [1, 6] were then screened by Sanger sequencing (BigDyeTM Terminator v3.1 Cycle Sequencing Kit with 3130xl genetic analyzer, Applied BiosystemsTM, USA), as previously described [7]. The minimal variant allele frequency (VAF) allowing UBA1 mutations to be detected with Sanger sequencing was determined to be 10% by diluting UBA1-mutated DNA samples with known VAF assessed by Next Generation Sequencing.
Table 1.
Clinical and histological features of included patients and biopsies, and results of the DNA extraction and sequencing
| Skin biopsy specimen number | Treatment at the time of the biopsy | Clinical features | Histological pattern | DNA extraction of quality? | Sanger sequencing results |
|---|---|---|---|---|---|
| Patient #1 (male, 78 years-old, UBA1 mutation p.Met41Thr) | |||||
| No. 1 | None | Erythema nodosum | Septal panniculitis | Yes | UBA1-wild type |
| No. 2 | None | Erythema nodosum | Septal panniculitis | Yes | UBA1-wild type |
| No. 3 | Cortancyl | Papules | Neutrophilic dermatosis | Yes | UBA1-mutated |
| Patient #2 (male, 72 years-old, UBA1 mutation p.Met41Thr) | |||||
| No. 1 | None | Papules | Leukocytoclastic vasculitis | No | NA |
| Patient #3 (male, 63 years-old, UBA1 mutation p.Met41Leu) | |||||
| No. 1 | Methotrexate | Papules, nodules | Leukocytoclastic vasculitis | Yes | UBA1-wild type |
| No. 2 | Methotrexate, abatacept | Papules, purpura | Leukocytoclastic necrotizing vasculitis | Yes | UBA1-wild type |
| No. 3 | Etanercept | Papules | Neutrophilic dermatosis | Yes | UBA1-mutated |
| Patient #4 (male, 64 years-old, UBA1 mutation p.Met41Val) | |||||
| No. 1 | None | Erythema nodosum | Septal panniculitis | No | NA |
| No. 2 | None | Livedo | Subnormal | No | NA |
| Patient #5 (male, 87 years-old, UBA1 mutation p.Met41Val) | |||||
| No. 1 | None | Macules, purpura | Leukocytoclastic vasculitis | No | NA |
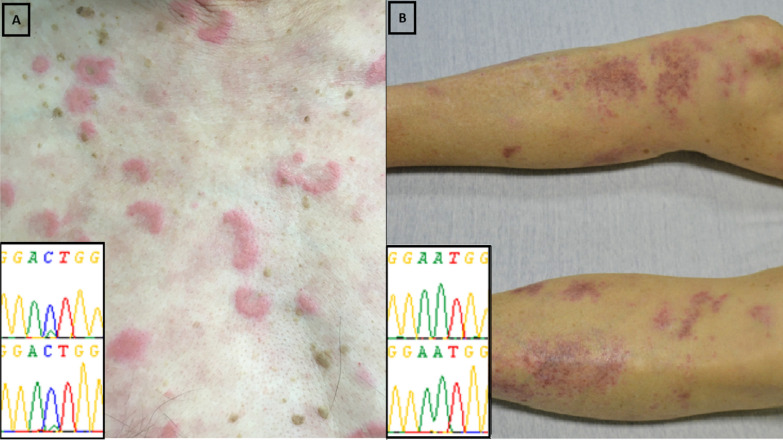
Ten skin biopsies were performed in the period of interest and involved either erythema nodosum, papules, nodules, purpuric macules or livedo. The clinical, molecular and pathological pattern of the included patients and biopsies were detailed in Table 1. The DNA extraction allowed sequencing in 6/10 samples from biopsies with the following histological patterns: 2 neutrophilic dermatosis (intense and diffuse neutrophilic infiltrate with no evidence of infection or vasculitis), 2 leukocytoclastic vasculitis (angiocentric segmental inflammation with fibrinoid necrosis and neutrophilic infiltrate with karyorrhexis in the small vessel walls), and 2 septal panniculitis (inflammatory cell infiltrate at the periphery of the hypodermal lobules). UBA1 mutation was found in the 2 biopsies related to neutrophilic dermatitis but in none of the other histological patterns (Figure 1). The mutational load was higher in neutrophilic dermatosis (> 50%) than in blood or bone marrow samples (< 50%) from the same patient. Associated hematological features were as follows: anemia (6/6), macrocytosis (4/6), thrombocytopenia (3/6), neutropenia (3/6), lymphopenia (3/6) and myeodysplastic syndrome (2/6, patients #1 and #4).
Figure 1.
Results of molecular analysis with Sanger sequencing according to the type of skin involvement. A presents the UBA1 mutation (p.Met41Leu, c.121A>C, mutational load >50%) observed with Sanger sequencing from a skin biopsy in a patient with VEXAS syndrome and neutrophilic dermatosis. B presents the UBA1-wild type gene observed in a skin biopsy in a patient with VEXAS syndrome and leukocytoclastic vasculitis
Previous studies specified the clinical contexts that should lead to searching for a UBA1 mutation in blood or marrow samples to confirm suspected VEXAS syndrome [4, 8], and demonstrated that the UBA1 mutated myeloid clone may also infiltrate the VEXAS-related skin lesions [5]. In our pilot study, we confirmed the presence of UBA1-mutated cells in skin tissues in cases of neutrophilic dermatosis. However, we did not identify any UBA1 variation in the four other biopsies with different histological patterns despite a sensitivity to identify the mutation in case of > 10% mutated cells. While we cannot rule out the idea that the absence of UBA1 mutation was related to a lower myeloid infiltrate in non-neutrophilic dermatosis lesions, we demonstrated that the UBA1-mutated clone is either absent or much less abundant in non-neutrophilic dermatosis skin lesions. We could hypothesize the distinction between “clonal” (neutrophilic dermatosis) and “paraclonal” (leukocytoclastic vasculitis and septal panniculitis) cutaneous involvements in VEXAS syndrome. This could result in different treatment options using clonal-depleting therapy in neutrophilic dermatosis, whereas paraclonal involvements could be treated with drugs targeting the inflammation and cytokine release. Indeed, Commont et al. recently showed promising results about the efficiency of azacitidine for treating patients with VEXAS syndrome [9]. This type of treatment could be particularly useful in patients with clonal involvements.
In conclusion, the UBA1-mutated clone was observed in VEXAS-related skin involvement in cases of neutrophilic dermatosis but not in other histological patterns. This could lead to a distinction between clonal and paraclonal cutaneous involvements in VEXAS syndrome, with which could in turn improve therapeutic outcomes.
Acknowledgements
None.
Abbreviation
- VAF
variant allele frequency
Authors’ contributions
VL contributed to the study conception and design, analysis and interpretation of data, drafted the manuscript and critically revised the manuscript for important intellectual content. AB, LC, AC and AB contributed to the acquisition, analysis and interpretation of data and critically revised the manuscript for important intellectual content. GU and YLC contributed to the interpretation of data and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Angers University Hospital (2021-027) and was conducted in compliance with the Declaration of Helsinki.
Consent for publication
The two patients presented in Figure 1 gave their signed consent for the publication of these photographs.
Competing interests
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Valentin Lacombe and Annaelle Beucher contributed equally to this work
References
- 1.Beck DB, Ferrada MA, Sikora KA, Ombrello AK, Collins JC, Pei W, et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N Engl J Med. 2020;383:2628–38. doi: 10.1056/NEJMoa2026834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacombe V, Kosmider O, Prévost M, Lavigne C, Urbanski G. severe joint involvement in VEXAS syndrome: a case report. Ann Intern Med. 2021 doi: 10.7326/L21-0023. [DOI] [PubMed] [Google Scholar]
- 3.Georgin-Lavialle S, Terrier B, Guedon AF, Heiblig M, Comont T, Lazaro E, et al. Further characterization of clinical and laboratory features occurring in VEXAS syndrome in a large-scale analysis of multicenter case-series of 116 French patients. Br J Dermatol. 2021 doi: 10.1111/bjd.20805. [DOI] [PubMed] [Google Scholar]
- 4.Huang H, Zhang W, Cai W, Liu J, Wang H, Qin T, et al. VEXAS syndrome in myelodysplastic syndrome with autoimmune disorder. Exp Hematol Oncol. 2021;10:23. doi: 10.1186/s40164-021-00217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakine E, Schell B, Battistella M, Vignon-Pennamen M-D, Chasset F, Mahévas T, et al. UBA1 variations in neutrophilic dermatosis skin lesions of patients with VEXAS syndrome. JAMA Dermatol. 2021 doi: 10.1001/jamadermatol.2021.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulter JA, Collins JC, Cargo C, de Tute RM, Evans P, Ospina Cardona D. Novel somatic mutations in UBA1 as a cause of VEXAS syndrome. Blood. 2021 doi: 10.1182/blood.2020010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacombe V, Prevost M, Bouvier A, Thépot S, Chabrun F, Kosmider O, et al. Vacuoles in neutrophil precursors in VEXAS syndrome: diagnostic performances and threshold. Br J Haematol. 2021 doi: 10.1111/bjh.17679. [DOI] [PubMed] [Google Scholar]
- 8.Ferrada MA, Sikora KA, Luo Y, Wells KV, Patel B, Groarke EM, et al. Somatic mutations in UBA1 define a distinct subset of relapsing polychondritis patients With VEXAS. Arthritis Rheumatol. 2021 doi: 10.1002/art.41743. [DOI] [PubMed] [Google Scholar]
- 9.Comont T, Heiblig M, Rivière E, Terriou L, Rossignol J, Bouscary D, et al. Azacitidine for patients with Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic syndrome (VEXAS) and myelodysplastic syndrome: data from the French VEXAS registry. Br J Haematol. 2021 doi: 10.1111/bjh.17893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.