Abstract
In the long-term safety testing of chemicals for carcinogenicity the toxicologist needs to be aware of a number of scenarios where renal tubule tumors, or their precursors, arise that are not due to a carcinogenic action of the test article. Situations producing false positive results in the kidney include exacerbation of chronic progressive nephropathy (CPN) in rats, confusion of atypical tubule hyperplasia (the obligate precursor of renal tubule tumor) with foci of benign CPN-related renal tubule cell proliferation, inclusion of spontaneous tumor entities, such as the amphophilic-vacuolar tumor, in the test article tumor count, the possibility of a link between spontaneous forms of tubule dilatation and renal tubule tumor formation in mice, and the supposed predictivity of chemically-induced karyomegaly for renal carcinogenicity in both rats and mice. Examples of these misleading situations are described and discussed.
Keywords: mouse, renal tubule tumors, modes of action, forchlorfenuron, cystic tubules
Introduction
In the long-term safety testing of chemicals for carcinogenicity, whether new pharmaceuticals, medical devices, industrial chemicals, agrochemicals, or food additives/ingredients, the toxicologist should be aware of a number of situations where renal tubule tumors, or their precursors, arise that are not due to a carcinogenic action of the test article. Situations with the potential to produce false positive results in the rodent kidney include: exacerbation of chronic progressive nephropathy (CPN) in rats; confusion of atypical tubule hyperplasia, the obligate precursor of renal tubule tumor, with foci of benign CPN-related renal tubule cell proliferation; inclusion of spontaneous tumor entities such as the amphophilic vacuolar tumor in the test article tumor count, including the possibility of a link between spontaneous forms of tubule dilatation and renal tubule tumor formation in mice; and the supposed predictivity of chemically-induced karyomegaly for renal carcinogenicity in both rats and mice.
One entity that will not be considered here is alpha-2u-globulin nephropathy in the rat. Many diverse chemicals can induce renal tubule tumors through the induction of increased expression and hepatic secretion of alpha-2u-globulin, which, when bound to the xenobiotic, accumulates in the proximal tubule to cause sustained epithelial cell damage and compensatory cell turnover. In this case, the tumors are genuinely induced by the chemical, but the mechanism underpinning the kidney tumor induction, namely non-covalent binding of the chemical to alpha-2u-globulin, does not apply to humans because humans do not have a similar protein to which the test article can bind.
The study that will be used for reference is the 1992 National Toxicology Program 2-year carcinogenicity bioassay of quercetin in rodents1. Quercetin is a widely distributed plant flavonol that is consumed by humans. In this study there was a low increase in foci of renal tubule hyperplasia and adenomas, and significantly, a single carcinoma, in the high-dose male rats. These results led the NTP to classify quercetin as showing some evidence of carcinogenic activity in male rats. This is in conflict with the numerous publications exploring the beneficial inhibitory effects of quercetin.
Exacerbation of chronic progressive nephropathy
Chronic progressive nephropathy is a spontaneous disease that commonly affects rat kidney. The disease commences at an early age, probably within a few months of birth being identified as sporadic foci (Fig. 1) of basophilic tubule cells with conspicuous basement membrane and crowded nuclei2. In male rats in particular it progresses relentlessly through mid-life to occupy more and more of the renal parenchyma, frequently causing end-stage chronic kidney disease by the time the rat becomes aged. Advanced, and in particular end-stage CPN, can represent a risk factor for the development of a low to marginal incidence of renal tubule tumors. This has been demonstrated in control rats by Hard et al3.
Fig. 1.
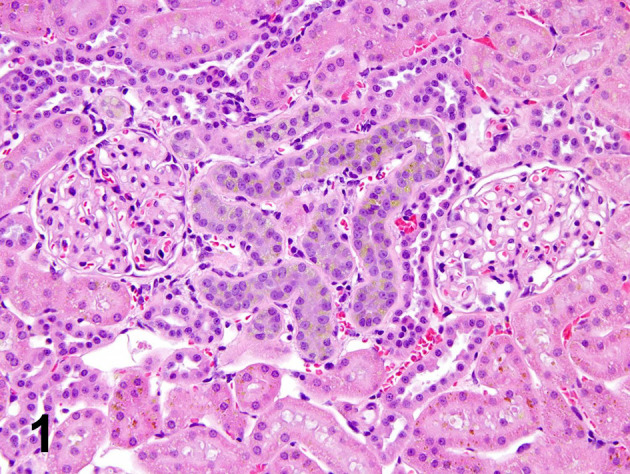
Early stage of CPN is a very good example of simple tubule hyperplasia. The convolutions of a single tubule are basophilic and the lining retains a single cell structure. The tubule cells are smaller than normal, crowded together, and with thickened basement membrane. Toxicologic pathologists also call this lesion regeneration.
The development of CPN is markedly affected by diet and sex hormones. High protein in the diet and androgens are both conducive for CPN severity. There appears to be no strict counterpart of CPN in humans, either pathologically or biologically. Because CPN is so easily affected by diet and sex hormones, it is debatable whether chemical enhancement of spontaneous disease severity can be regarded as an adverse reaction relevant to risk assessment.
Chemical treatment can exacerbate the development of CPN by increasing the rate at which severity of the disease progresses3. Because the number of rats with very advanced to end-stage CPN is increased in severe CPN exacerbation, accompanying this exacerbation of disease progression is a further increased risk of developing renal tubule adenomas. Traditionally the marginal increase in renal tubule tumors has been blamed on the test chemical but this overlooks the potential CPN-exacerbating properties of the test agent. If the severity of CPN is increased in the study to frequent cases of end-stage CPN, and the renal tubule tumor incidence is marginal to low, then a mode of action involving CPN exacerbation should be considered.
Re-examination of the kidney lesions in the NTP quercetin study by the author, including grading of CPN in each rat on a specialized scale of 0–8 where grade 8 was end-stage kidney, linked the foci of hyperplasia and adenomas to CPN exacerbation.
Another aspect of end-stage CPN that is frequently misunderstood is so-called transitional cell hyperplasia of the lining of the papilla, sometimes referred to as urothelial hyperplasia. This lesion is a consequence of CPN of advanced severity, and is not a direct result of administration of the test article. In Souza et al.4, we have reviewed this lesion and drawn attention to the facts that the lining of the papilla is not urothelium and the lesion itself appears not to be hyperplasia. The lesion seems to start as a vesicular outpouching of the papilla lining (Fig. 2) and we suggest that more accurate nomenclature would be “vesicular alteration of papilla lining”4. It is important to recognize that this lesion is only observed in advanced CPN.
Fig. 2.

So-called “transitional cell hyperplasia” of the renal papilla. This lesion occurs in advanced to end-stage CPN, and consists of vesicular outpouchings of lining epithelium. It is called urothelial cell hyperplasia by some authorities, but the renal papilla lining is not urothelium and the lesion does not satisfy the criteria for hyperplasia. More appropriate terminology is “vesicular alteration of renal papilla lining”.
Discrimination of tumor precursors from benign CPN tubule cell proliferation
In this Consultant’s experience, some long-term studies have quite frequently been confounded by a failure to distinguish between preneoplastic and benign types of renal tubule cell proliferation. For example, the Technical Report Series of the National Toxicology Program have used the single term hyperplasia, which is inclusive of simple tubule hyperplasia (Fig. 1), a benign type of tubule cell proliferation, and atypical tubule hyperplasia5, which is preneoplastic and an obligate precursor of renal tubule adenoma (Fig. 3). This problem is particularly evident in studies where cases of advanced to end-stage CPN are frequent because examples of non-neoplastic tubule cell proliferation with a misleading florid morphology are commonly encountered in the kidneys of rats with advanced CPN6.
Fig. 3.

Atypical tubule hyperplasia, the obligate precursor of adenoma, consists of solid ingrowth of tubule cells into the tubule lumen. The lesion is encircled by a layer of flattened fibroblasts, indicating that it is expansile. The characteristic feature is particularly well shown in this example because of partial autolysis.
Rat kidneys with end-stage CPN have very little normal parenchyma. Most cortical tissue consists of strips of contracted, atrophic epithelium alternating with larger wedges of dilated tubules crammed with casts of eosinophilic, hyaline material. This pattern is particularly noticeable at the surface of the kidney. The tracts of dilated tubules cause the end-stage kidney in rat CPN to be larger than normal rat kidney, which is a major point of difference from end-stage kidney in humans7. End-stage kidney in humans is shrunken and smaller than normal human kidney.
Small florid epithelial proliferations within CPN-affected tubules of cortex and outer stripe of outer medulla involving the proximal tubule can be common in end-stage CPN rat kidney. These lesions have posed a diagnostic problem for study pathologists regarding discrimination from lesions on the pathway to tumor development, that is, atypical tubule hyperplasia. However, serial sectioning shows that such CPN-related tubule cell proliferations are not preneoplastic but peter out as atrophic tubules6. Florid CPN proliferative lesions tend to consist of small, bland cells with no nucleolar prominence, surrounded by conspicuous basement membrane thickening (Fig. 4). Encircling of the lesion by attenuated fibroblasts (implying expansion of the lesion), is absent8. CPN proliferative lesions can also take the form of mildly dilated tubules with modest, multicellular tubule lining in parts of the cross-sectioned tubule. There is usually some luminal desquamation of cells in these affected tubules. In many cases, the lesion represents a point where the tubule is making a turn into a convolution, where tubule cells tend to crowd together and pile up. Serial sectioning of end-stage CPN kidney shows these various, abnormal proliferative lesions to involve tubules undergoing atrophic change6. It could be speculated that these lesions represent attempts by the failing kidney to preserve some tubule epithelium, and perhaps maintain some kidney function. Some of the foci of hyperplasia in the NTP quercetin study were CPN-related tubule cell proliferations and were therefore not relevant for inclusion in the neoplasia/preneoplasia results.
Fig. 4.

CPN-related tubule cell proliferation. This lesion, occurring in end-stage CPN, is confused with atypical tubule hyperplasia, but it is not an obligate precursor of neoplasms. Distinguishing features are: small poorly defined epithelial cells, and the prominent band of basement membrane surrounding the lesion.
Inclusion of tumors of spontaneous origin in final tumor counts
The aim of conducting 2-year bioassays is to determine the long-term safety of a test article, in particular to eliminate the possibility of human exposure to agents with carcinogenic activity. In doing this it is also important to avoid inclusion of any neoplasms of spontaneous origin in the final tumor count, which would result in an inaccurate number because tumors unrelated to chemical treatment would be included. Accordingly, attention is drawn to a phenotypically distinctive tumor in rats, and a predisposition to cystic tubule formation that the author has encountered in CD-1 mice.
Amphophilic-vacuolar tumor of the rat
In rats there is a spontaneous renal tubule tumor that has been fully recognized only in the last 20 years. This neoplasm has distinctive epithelial morphology enabling its separation from kidney neoplasms that are induced by renal carcinogens. This spontaneous renal tubule tumor, named amphophilic-vacuolar (A-V) tumor, has been encountered in long-term studies conducted in the US, Europe, Great Britain, and Japan. It occurs in rats of various strains including the Fischer 344, Sprague-Dawley, and Wistar strains9, 10, and affects both genders. The author first became aware of this distinctive neoplasm in 1994 in 90-day toxicity studies11, although it had also been reported by other groups12. The Thurman et al.12 report was most significant because it identified that these neoplasms could occur in littermates, suggesting that this proliferation might have a genetic basis.
The distinctive morphology includes well developed, often large, epithelial cells with eosinophilic or amphophilic staining character, and cytoplasmic vacuoles. The vacuoles can be intracellular vacuoles, or represent minilumen formation where the perimeter of the vacuole is contributed by several neighboring cells. The nuclei are often quite large and can contain a hypertrophic nucleolus. The tumors can be adenomas or carcinomas. They appear to arise in the cortex, from foci of atypical hyperplasia. With growth, carcinomas develop as well-defined lobules of tumor cells, frequently with a central area of cell degeneration. Carcinomas protrude from the kidney surface, and their widest part is in the cortex. Typically, the tumor extends in tapering, wedge-shaped fashion down into the outer and inner stripes of outer medulla. Occasionally, basophilic lobules develop, but examination of all of the tumor area usually discloses the distinctive, staining and vacuolar character in parts. There is no record that this tumor phenotype is capable of metastasizing.
The occurrence of this tumor type was studied in detail in the archived renal tissue held in the NTP Archives9. This Archive had 150 long-term studies in which renal tubule tumors had been recorded, representing a pool of some 90,000 rats, predominantly Fischer 344 strain. Of these rats, 1,012 had been diagnosed as having a renal tubule tumor. Histological re-examination of each of these tumors showed that half of the studies with this diagnosis (74) had at least one tumor with the A-V phenotype. So the neoplasm is uncommon at approximately 0.1% incidence in 2-year studies, but is encountered relatively frequently in approximately 50% of long-term studies diagnosed with renal tubule tumors. None of the lesions in this survey of 74 studies had metastasized. Most studies with A-V tumors had a single neoplasm of this type, but the number of A-V tumors in a study varied from 1 to 5. This distribution again suggested that a single litter in the study may have been carrying a genetic defect. Statistical analysis of the data from this histological review showed that the distinctive tumor type was spontaneous and had no association with chemical exposure9 Therefore, tumors of this distinctive phenotype should not be included in kidney tumor counts in cancer bioassays, but should be recorded separately.
The single carcinoma in the high-dose males of the NTP quercetin study was unquestionably of the A-V phenotype (Fig. 5) and therefore of spontaneous origin. In summarizing the numbers of quercetin-related neoplastic lesions, excluding the CPN proliferative foci, and the A-V lesions, there was a modest increase in foci of preneoplasia and adenomas, but no carcinomas in high-dose male rats. That modest increase in foci of atypical tubule hyperplasia and adenomas was associated with CPN exacerbation.
Fig. 5.
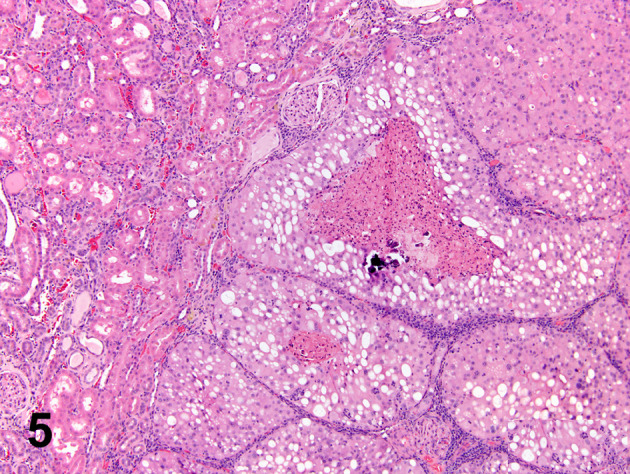
A typical cross–section of the carcinoma in the high-dose males of the quercetin study. The tumor is characterized by well-defined lobules of amphophilic and vacuolated cells with central degeneration. It is unquestionably an A-V tumor which should be excluded from the test article-induced tumor count. (Courtesy of, and adapted from, Hard et al., 2007, Food Chem Toxicol 45, 600–608).
Cystic tubules in the CD-1 mouse
On several occasions this author has encountered a predisposition in the CD-1 mouse for developing solitary or sporadic cystic renal tubules in the cortex (Hard GC, unpublished observations). This situation appears to lead to development of renal tubule adenomas and banal carcinomas. The tumors are faintly basophilic and initially located in the cortex, where the sporadic cystic tubules are located. In one study, there were intermediate stages of hyperplasia and early adenoma formation in cystic tubules, providing a definite link between cystic tubule and renal tubule neoplasia. However, incidence of cystic tubules did not correlate with exposure to the test article, but occurred spontaneously in all groups, including control mice. The tumors were most prevalent in the high-dose male mice, but this was a very high dose that exceeded regulatory authority guidelines for high dose selection. Such a high exposure would very probably result in saturation of metabolic pathways, resulting in very different metabolic profiles from those occurring under realistic conditions of exposure, thus predisposing to biologic stress13, 14, 15. In addition, the test article did not induce any pathologic indication of renal tubule cell injury at any time-point, including exposures at 4 weeks, 52 weeks, and 104 weeks. There was also no increased mitotic response in renal tubules, which would have occurred had there been any chemically-induced compensatory activity to replace chemically-damaged tubule epithelium. It was concluded that the renal tubule neoplasms in this mouse study were due to the spontaneous development of cystic tubules with abnormal cell lining, probably as a result of a genetic aberration. This case represents another example of spontaneous development of renal tubule tumors that should not be attributed to a test article response.
Karyomegaly
A markedly enlarged nucleus in a renal tubule cell (Fig. 6), known as karyomegaly, has long been viewed as an adverse finding that might predict the development of renal tubule tumors and identify the inducing chemicals as potential renal carcinogens16, 17, 18, 19, 20. A literature review of karyomegaly in laboratory animals and humans was conducted in 2018 by the author21. At least 50 chemicals/substances have been reported to induce this nuclear alteration in rats, but it is a much less common occurrence in mice and other laboratory animals21. A number of chemicals that induce this change in rats do not produce the same effect in other species. Of particular potency in the rat is the food component lysinoalanine, and the mycotoxin, ochratoxin A. Both of these compounds have been tested in other laboratory animal species, with negative results. Feeding an excessively high dose of lysinoalanine, 10,000 ppm to Swiss mice, was required to elicit a minimal response of karyomegaly in this species compared to 50 ppm in the rat22. Review of the literature indicates that the rat has a predisposition for developing karyomegaly as a response to chemical toxicity21. This literature review also demonstrated that karyomegaly in the rat kidney is not a reliable predictor of renal tumor development.
Fig. 6.

Renal tubule cell karyomegaly is an abnormally enlarged tubule cell nucleus. It is recommended that this diagnosis be reserved for nuclei of octaploidy or higher. (Courtesy of, and adapted from, Hard et al., 2000, Toxicol Sci 53: 237–244).
Modest increase in renal tubule nuclear size is not limited to chemical exposure, but also occurs in some physiological conditions. It has been known for many decades that, in general, nuclear volume doubles with each increase in ploidy level23. During the cell cycle, there is a change in ploidy from 2n to 4n in the DNA synthesis (S) phase, persisting into the G2 phase. There is also increase in nuclear size (up to doubling) in pathological conditions such as temporary renal ischemia24, and following unilateral nephrectomy25. Therefore, the threshold for diagnosing renal tubule karyomegaly needs to be set at a level of ploidy that discriminates the abnormal from the normal. It is suggested that this threshold be at least 4x normal tubule nucleus size (octaploidy) for diagnosing renal tubule karyomegaly.
Karyomegaly is rare in human kidney and the occasional cases observed in renal biopsy or autopsy tissue are from patients with a genetic condition termed karyomegalic interstitial nephritis (KIN). This condition was shown by Zhou and 43 co-authors26 to be caused by an autosomal recessive mutation of the FAN1 gene, the protein of which functions in repair of DNA interstitial crosslinks within the Fanconi anemia DNA damage response pathway. Karyomegaly in the human kidney has also been observed occasionally in HIV patients that have received the antiviral drug, tenofovir, but this association appears to be inconsistent27. Although the rat is uniquely predisposed to responding to chemically-induced toxic injury with renal tubule karyomegaly, renal tubule karyomegaly in the rat is not consistently associated with development of renal tubule tumors or their precursors21. Therefore, karyomegaly in the rat kidney is considered to be an inaccurate predictor of renal carcinogenic potential of chemicals.
Disclosure of Potential Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Dunnick JK, and Hailey JR. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam Appl Toxicol. 19: 423–431. 1992. [DOI] [PubMed] [Google Scholar]
- 2.Travlos GS, Hard GC, Betz LJ, and Kissling GE. Chronic progressive nephropathy in male F344 rats in 90-day toxicity studies: its occurrence and association with renal tubule tumors in subsequent 2-year bioassays. Toxicol Pathol. 39: 381–389. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hard GC, Betz LJ, and Seely JC. Association of advanced chronic progressive nephropathy (CPN) with renal tubule tumors and precursor hyperplasia in control F344 rats from two-year carcinogenicity studies. Toxicol Pathol. 40: 473–481. 2012. [DOI] [PubMed] [Google Scholar]
- 4.Souza NP, Hard GC, Arnold LL, Foster KW, Pennington KL, and Cohen SM. Epithelium lining rat renal papilla: nomenclature and association with chronic progressive nephropathy (CPN). Toxicol Pathol. 46: 266–272. 2018. [DOI] [PubMed] [Google Scholar]
- 5.Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, and Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 40(Suppl): 14S–86S. 2012. [DOI] [PubMed] [Google Scholar]
- 6.Hard GC, and Seely JC. Histological investigation of diagnostically challenging tubule profiles in advanced chronic progressive nephropathy (CPN) in the Fischer 344 rat. Toxicol Pathol. 34: 941–948. 2006. [DOI] [PubMed] [Google Scholar]
- 7.Hard GC, Johnson KJ, and Cohen SM. A comparison of rat chronic progressive nephropathy with human renal disease-implications for human risk assessment. Crit Rev Toxicol. 39: 332–346. 2009. [DOI] [PubMed] [Google Scholar]
- 8.Hard GC, and Seely JC. Recommendations for the interpretation of renal tubule proliferative lesions occurring in rat kidneys with advanced chronic progressive nephropathy (CPN). Toxicol Pathol. 33: 641–649. 2005. [DOI] [PubMed] [Google Scholar]
- 9.Hard GC, Seely JC, Kissling GE, and Betz LJ. Spontaneous occurrence of a distinctive renal tubule tumor phenotype in rat carcinogenicity studies conducted by the national toxicology program. Toxicol Pathol. 36: 388–396. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo K, Hoshiya T, Nakazawa T, Saito T, Shimoyama N, Suzuki I, Tamura K, and Seely JC. Spontaneous renal tumors suspected of being familial in sprague-dawley rats. J Toxicol Pathol. 25: 277–280. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hard GC, Long PH, Crissman JW, Everitt JI, Yano BL, and Bertram TA. Atypical tubule hyperplasia and renal tubule tumors in conventional rats on 90-day toxicity studies. Toxicol Pathol. 22: 489–496. 1994. [DOI] [PubMed] [Google Scholar]
- 12.Thurman JD, Hailey JR, Turturro A, and Gaylor DW. Spontaneous renal tubular carcinoma in Fischer-344 rat littermates. Vet Pathol. 32: 419–422. 1995. [DOI] [PubMed] [Google Scholar]
- 13.Counts JL, and Goodman JI. Principles underlying dose selection for, and extrapolation from, the carcinogen bioassay: dose influences mechanism. Regul Toxicol Pharmacol. 21: 418–421. 1995. [DOI] [PubMed] [Google Scholar]
- 14.Barton HA, Pastoor TP, Baetcke K, Chambers JE, Diliberto J, Doerrer NG, Driver JH, Hastings CE, Iyengar S, Krieger R, Stahl B, and Timchalk C. The acquisition and application of absorption, distribution, metabolism, and excretion (ADME) data in agricultural chemical safety assessments. Crit Rev Toxicol. 36: 9–35. 2006. [DOI] [PubMed] [Google Scholar]
- 15.Bus JS. “The dose makes the poison”: key implications for mode of action (mechanistic) research in a 21st toxicology paradigm. Curr Opin Toxicol. 3: 87–91. 2017. [Google Scholar]
- 16.Scheuer PJ. Histochemical changes in rat liver in Senecio and thioacetamide poisoning. J Pathol Bacteriol. 85: 507–516. 1963. [DOI] [PubMed] [Google Scholar]
- 17.Payne BJ, and Saunders LZ. Heavy metal nephropathy of rodents. Vet Pathol Suppl. 15: 51–87. 1978. [PubMed] [Google Scholar]
- 18.Dees JH, and Kramer RA. Sequential morphologic analysis of the nephrotoxicity produced in rats by single doses of chlorozotocin. Toxicol Pathol. 14: 213–231. 1986. [DOI] [PubMed] [Google Scholar]
- 19.Luijten M, Speksnijder EN, van Alphen N, Westerman A, Heisterkamp SH, van Benthem J, van Kreijl CF, Beems RB, and van Steeg H. Phenacetin acts as a weak genotoxic compound preferentially in the kidney of DNA repair deficient Xpa mice. Mutat Res. 596: 143–150. 2006. [DOI] [PubMed] [Google Scholar]
- 20.NTP. National Toxicology Program. Nonneoplastic Lesion Atlas. Research Triangle Park, NC. National Toxicology Program, NIEHS, NIH. 2015.
- 21.Hard GC. Critical review of renal tubule karyomegaly in non-clinical safety evaluation studies and its significance for human risk assessment. Crit Rev Toxicol. 48: 575–595. 2018. [DOI] [PubMed] [Google Scholar]
- 22.Feron VJ, van Beek L, Slump P and Beems RB. Toxicological aspects of alkali treatment of food proteins, In: Biochemical Aspects of New Protein Foods, FEBS vol. 44. P Schambye (ed). Pergamon Press, New York. 139. 1977.
- 23.Rather LJ. The significance of nuclear size in physiological and pathological processes. Ergeb Allg Pathol Pathol Anat. 38: 127–199. 1958. [PubMed] [Google Scholar]
- 24.Cain H, Fazekas S, and Ross W. Studien über die Folgen einer vorübergerhenden experimentellen Nierenischämie III. Karyometrie und Häufigkeitsanalyse des “bunten Kemmusters” während der regeneration. Virchows Arch Path Anat. 337: 53–64. 1963. [PubMed] [Google Scholar]
- 25.Schmeidt E. Zellkerngrösse und sogenannte kompensartorische Hypertrophie der Mäuseniere. Zeits Micr Anat Forsch. 57: 249–275. 1951. [PubMed] [Google Scholar]
- 26.Zhou W, Otto EA, Cluckey A, Airik R, Hurd TW, Chaki M, Diaz K, Lach FP, Bennett GR, Gee HY, Ghosh AK, Natarajan S, Thongthip S, Veturi U, Allen SJ, Janssen S, Ramaswami G, Dixon J, Burkhalter F, Spoendlin M, Moch H, Mihatsch MJ, Verine J, Reade R, Soliman H, Godin M, Kiss D, Monga G, Mazzucco G, Amann K, Artunc F, Newland RC, Wiech T, Zschiedrich S, Huber TB, Friedl A, Slaats GG, Joles JA, Goldschmeding R, Washburn J, Giles RH, Levy S, Smogorzewska A, and Hildebrandt F. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet. 44: 910–915. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karras A, Lafaurie M, Furco A, Bourgarit A, Droz D, Sereni D, Legendre C, Martinez F, and Molina JM. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 36: 1070–1073. 2003. [DOI] [PubMed] [Google Scholar]


