Abstract
Purpose:
To report the outcomes of pars plana insertion of Aurolab aqueous drainage implant (AADI) in adults with refractory glaucoma by the novel technique of making scleral tunnel instead of patch graft to cover the tube to prevent its migration.
Methods:
A retrospective study was done between April 2016 and April 2018 on patients with ≥12 months of follow-up. The main outcome measure was a surgical failure at 12 months. The failure was defined as intraocular pressure (IOP) >18 mmHg or IOP ≤5 mmHg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, loss of light perception vision, or implant explantation. Alternate definitions of failure including IOP >21 and IOP >15 mmHg were also considered.
Results:
The study included 32 eyes of 32 patients. The mean age was 46.2 ± 17.5 years. The most common etiology is traumatic glaucoma (12 eyes, 37.5%). The mean preoperative IOP and anti-glaucoma medications were 43.3 ± 10.3 and 3.4 ± 0.5 mmHg, respectively; both the parameters at the final follow-up were reduced to 15.2 ± 8.1 and 1.6 ± 0.5 mmHg. The Kaplan–Meier survival estimates demonstrated that the cumulative probability of failure was 15.6% (95% CI; 6.8–33.5%) at 3 months, 18.7% (95% CI; 8.9–37.0%) at 6 months, and 25.0% (95% CI; 13.4–43.8%) at 12 months.
Conclusion:
Pars plana AADI implantation with a newer modification technique is a useful procedure in reducing IOP and the number of anti-glaucoma medications in the eyes with refractory glaucoma. The visual acuity may be stabilized with the concurrent treatment of posterior segment pathology.
Keywords: Ahmed glaucoma valve, Aurolab aqueous drainage implant, Baerveldt glaucoma implant, glaucoma surgery, tube shunt
Glaucoma drainage devices (GDDs) have demonstrated a greater surgical success than trabeculectomy procedures in certain subsets of refractory glaucoma.[1] The previous studies have identified these groups as eyes with a history of prior conjunctival incisional surgery (e.g., trabeculectomy, extracapsular cataract extraction, pars plana vitrectomy, and scleral buckling surgery), history of the conjunctival cicatricial disease, or trauma and inflammatory conditions such as neovascular glaucoma, uveitic glaucoma, and iridocorneal endothelial syndrome.[2,3,4,5,6] The increased risk of fibroblast proliferation and episcleral scarring is postulated to lead to the obstruction of aqueous drainage, and subsequent trabeculectomy failure.[5,6] In these scenarios, GDDs are typically inserted in the anterior chamber, but may be placed in the ciliary sulcus or through the pars plana in the vitreous cavity in certain settings. These include the presence of corneal endothelial decompensation, anterior chamber angle abnormalities,[7,8,9,10,11] or coexisting posterior segment disease requiring vitrectomy.[12,13]
Since its introduction by Molteno in 1969,[14] GDD surgery has experienced significant growth in the variety of available implants; these differ in the surface area, shape, composition, and presence of a flow-regulating valve. In most developing countries, GDDs are not available or are imported, creating a substantial cost burden on the patient. Thus, there is a need for newer and affordable drainage implants to meet the ever-increasing demand. The Aurolab aqueous drainage implant (AADI; Aurolab, Madurai, India) is a low-cost, non-valved GDD, and has been commercially available in India since 2013. It is similar to the Baerveldt 350 glaucoma implant (BGI; Advanced Medical Optics, Santa Ana, CA, USA) in structure and function. Literature on the safety and efficacy of the AADI is evolving.[15,16,17,18,19,20,21] Articles have been published on its efficacy in the pediatric population,[15,16] comparison with valved and other non-valved GDDs,[17,18] its placement in the anterior chamber,[19] and comparison of its placement in the anterior chamber versus in vitreous cavity.[20,21] Although substantial published literature exists on pars plana implantation of BGI combined with Pars plana vitrectomy (PPV),[8,22,23,24,25,26] published work pertaining to AADI implantation through a pars plana approach is sparse with just two retrospective reports on this topic.[27,28] This retrospective review reports the outcomes after pars plana AADI implantation in the vitreous cavity including the reduction of intraocular pressure (IOP) and anti-glaucoma medication (AGM), as well as its associated complications and the need for additional interventions.
Methods
A retrospective review was performed with approval from the Institutional Ethics Committee of the Aravind Eye Care System. The study adhered to the principles of the Declaration of Helsinki. Medical records were evaluated for all patients older than 18 years with advanced or uncontrolled glaucoma who underwent implantation of AADI in the vitreous cavity between April 2016 and April 2018. A minimum of 12 months of follow-up and complete medical records were required for inclusion in the study. Only one eye from each patient was included in the study.
The data collected included age, sex, diagnosis, visual acuity, IOP, previous ocular surgeries, number of AGMs used, complications, and period of follow-up. Preoperative IOP was determined as the mean of three measurements performed on the same visit prior to the operation. For all eyes, the placement of the tube in the vitreous cavity was chosen because anterior chamber placement was contraindicated or a simultaneous vitreoretinal procedure was required. The records were reviewed from postoperative day 1, 2 weeks, 1 month, 1.5 months, 3 months, 6 months, and 12 months after surgery. The visual acuity, IOP, and number of AGMs required were noted at each postoperative visit. Additionally, charts of patients who required more frequent examinations or underwent additional interventions were reviewed accordingly.
Surgical technique
This illustrated technique of AADI surgery is already described elsewhere.[29] Surgery was performed by experienced glaucoma (MAK) and retinal (VR) surgeons. In all cases, a fornix-based conjunctival flap was made in the inferonasal quadrant and Tenon’s capsule tissue was dissected. Inferior and medial recti muscles were isolated using muscle hooks and bridled with 4-0 silk sutures. Inferonasal quadrant placement of the implant was opted to minimize the loss of silicon oil, which is lighter than the aqueous and floats up. This quadrant was also associated with a lesser likelihood to demonstrate the impingement of adjacent inferior oblique muscle and offers better concealment of the tube/endplate in case of prominent eyes. In order to avoid the use of corneal or scleral patch grafts to cover the tube, a partial thickness scleral tunnel was fashioned using a crescent blade, approximately 4 mm in length and 2 mm in breadth. This tunnel was initiated from a distance of 3, 3.5, or 4 mm from the limbus depending on the lens status, whether aphakic, pseudophakic, or phakic, respectively.
The AADI implant tube patency was checked with a balanced salt solution to check for manufacturing defects. The endplate was positioned between the adjacent recti muscles, such that the anterior edge of the plate was approximately 10 mm from the limbus. The plate was then secured to the underlying sclera using the 7-0 vicryl suture, which was passed through the fixation holes of the implant. Temporary tube occlusion was achieved by ligation with a 7-0 vicryl suture. Complete closure was then confirmed by attempting to irrigate a balanced salt solution through the tube using a 27-gauge cannula. The retinal surgeon then performed a 3-port 23-gauge or 25-gauge pars plana vitrectomy and any additional vitreoretinal procedure. A thorough vitrectomy including the removal of the vitreous base was performed in order to prevent the occlusion of the tube by the vitreous. Upon conclusion of the vitrectomy, a max grip forceps was then passed through the tunnel, and the tube of the implant docked and then pulled through the tunnel using the forceps. The fenestration of the tube using the needle of 70 vicryl was done if an early reduction of IOP was desired. A tube length of 2 mm was measured beyond the limbus, and the extra tube was trimmed with a scissor in a bevelup fashion. A pars plana entry was made 3.5 mm away from the limbus at the scleral tunnel’s proximal end with a 23-G trocar. The tube’s trimmed end was held by the max grip forceps and inserted into the vitreous cavity. The position of the tube was evaluated, and the retina was examined to rule out peripheral breaks. All vitrectomy ports were closed with 7-0 vicryl. The conjunctiva and Tenon’s capsule were closed with 7-0 vicryl sutures, using both interrupted and running techniques.
The initial postoperative regimen included topical steroids prednisolone acetate 1% ophthalmic suspension used four times a day for 6 weeks as per the standard postoperative medication regimen for AADI and then tapered over 12 weeks; a topical antibiotic (ofloxacin 0.3%) was used three times a day for 15 days and topical cycloplegic (homatropine 2%) was used twice a day for 6 weeks and tapered. The continuation and number of AGMs were decided based on the IOP when the tube opened following spontaneous lysis of the ligating vicryl suture.
Primary outcome measure
The main outcome measure was the failure of the AADI at postoperative month 12. The failure was defined as IOP >18 mmHg or not reduced by at least 30% below baseline on two consecutive follow-up visits after 3 months; IOP ≤5 mmHg on two consecutive follow-up visits after 3 months, reoperation for glaucoma, loss of light perception vision or removal of the implant. Complete success was defined as achieving these IOP levels without AGMs, and qualified success was considered when IOP control was achieved with AGMs. The cumulative rates of complete and qualified success were also calculated for IOP ranging between 6 and 15 mmHg and between 6 and 21 mmHg for comparison with other studies. Additional surgeries that included cyclodestructive procedures were considered as reoperation for glaucoma. The complications leading to the loss of more than two lines of visual acuity on Snellen’s chart for two consecutive visits were defined as vision-threatening.
Statistical analysis
Statistical analysis was performed using the statistical software STATA, version 14.0 (Texas, USA). The baseline and clinical characteristics of the study participants were described as mean ± standard deviation or median with interquartile range for continuous variables. The categorical variables were presented as frequency and percentage. For statistical analysis, visual acuity was converted from Snellen’s equivalent to logarithm of the minimum angle of resolution (logMAR). The postoperative comparison of visual acuity, IOP, and AGM was compared using paired t-test or Wilcoxon sign-rank test. The survival analysis was performed and Kaplan–Meier curves were plotted to illustrate the cumulative probability of failure at various time points. The statistical significance was set as P value less than 0.05.
Results
Thirty-two eyes of 32 patients met the inclusion criteria. The demographic details and clinical characteristics of the study participants are shown in Table 1. The mean patient age ± standard deviation was 46.19 ± 17.5 years. The most common indication for the tube shunt placement was traumatic glaucoma (12/32, 37.5%). Two eyes had a diagnosis of glaucoma due to lens-related complications—one eye had secondary open-angle glaucoma due to sulcus placement of a foldable IOL and another eye had secondary angle-closure glaucoma due to anterior chamber intraocular lens. Table 2 displays the combined procedures that took place along with AADI implantation.
Table 1.
Demographic and clinical characteristics of the study participants
| Mean±SD or n (%) | Range | |
|---|---|---|
| Age, years | 46.1±17.5 | 18.9-72.2 |
| Male gender, n (%) | 27 (84.4) | - |
| Intraocular pressure (mmHg) | 43.3±10.3 | 26-60 |
| Best-corrected visual acuity (logMAR)* | 1.78±0.75 | 1.00-2.60 |
| Anti-glaucoma medications | 3.4±0.5 | 3-4 |
| Etiology of glaucoma, n (%) | ||
| Traumatic glaucoma | 12 (37.5) | - |
| Neovascular glaucoma | 7 (21.8) | |
| Post-vitreoretinal surgery, silicone oil-induced | 6 (18.8) | |
| Glaucoma secondary to lens-related complications | 2 (6.3) | |
| Post-keratoplasty glaucoma | 5 (15.6) | |
| Lens status, n (%) | ||
| Phakic | 13 (40.6) | - |
| Pseudophakic | 12 (37.5) | |
| PCIOL | 2 (6.3) | |
| SFIOL | 1 (3.1) | |
| ACIOL | 4 (12.5) | |
| Aphakic | ||
| Previous intraocular surgery†, n (%) | ||
| Vitreoretinal surgery | 12 (37.5) | - |
| Cataract extraction | 12 (37.5) | |
| Trabeculectomy | 4 (12.5) | |
| Keratoplasty | 3 (9.4) | |
| Lensectomy with SFIOL | 2 (6.3) | |
| Globe repair | 1 (3.1) |
logMAR - logarithm of minimal angle of resolution, PCIOL - posterior chamber intraocular lens, SFIOL - scleral fixated intraocular lens, ACIOL - anterior chamber intraocular lens. *BCVA was converted into logMAR and presented in the median and interquartile range. †An eye may have had one or more of these
Table 2.
Combined procedures with AADI insertion
| Procedures* | n (%) |
|---|---|
| Endolaser | 9 (28.1) |
| Cataract extraction† | 7 (21.8) |
| SFIOL implantation | 5 (15.5) |
| Lensectomy‡ | 3 (9.4) |
| Epiretinal membrane peeling | 2 (6.3) |
| Internal limiting membrane peeling | 2 (6.3) |
| Intraocular lens repositioning | 1 (3.1) |
| Silicone oil removal | 1 (3.1) |
SFIOL - Scleral fixated intraocular lens. *An eye may have had one or more of these. †For lenticular opacity. ‡For dislocated lens
Treatment outcome
The failure took place in nine eyes (28.1%) by 12 months after AADI implantation. The most common reason for failure was high IOP, which was seen in four eyes (44%) [Table 3]. The Kaplan–Meier estimates demonstrated that the cumulative probability of failure was 15.6% (95% CI; 6.8–33.5%) at 3 months, 18.7% (95% CI; 8.9–37.0%) at 6 months, and 25.0% (95% CI; 13.4–43.8%) at 12 months. Table 4 shows the cumulative rates of complete and qualified success at various time points based on different IOP criteria. The Kaplan–Meier plot for cumulative failure at various time points is shown in Fig. 1.
Table 3.
Treatment outcomes at 12 months
| Overall outcome | n (%) |
|---|---|
| Success | 23 (71.9) |
| Failure | 9 (28.1) |
| Reasons for failure | |
| Tube explantation | 3 |
| Hypotony (IOP ≤5 mmHg) | 1 |
| High IOP (IOP >18 mmHg) | 4 |
| Loss of light perception vision | 1 |
IOP - Intraocular pressure
Table 4.
Complete and qualified success based on various success criteria and time points
| Complete success* (CI) | Qualified success* (CI) | Complete success† (CI) | Qualified success† (CI) | Complete success‡ (CI) | Qualified success‡ (CI) | |
|---|---|---|---|---|---|---|
| 1.5 month | 73.3%(43.6-89.1%) | 84.0%(62.8-93.7%) | 73.3%(43.6-89.1%) | 84.0%(62.8-93.7%) | 73.3%(43.6-89.1%) | 84.0%(62.8-93.7%) |
| 3 months | 66.7%(37.5-84.6%) | 80.0%(58.4-91.2%) | 66.7%(37.5-84.6%) | 80.0%(58.4-91.2%) | 66.7%(41.6-86.6%) | 78.2%(60.6-92.0%) |
| 6 months | 66.7%(37.5-84.6%) | 80.0%(58.4-91.2%) | 60.9%(31.8-79.6%) | 76.0%(54.2-88.4%) | 60.0%(33.8-80.5%) | 74.4%(58.2-86.4%) |
| 9 months | 66.7%(37.5-84.6%) | 80.0%(58.4-91.2%) | 58.2%(30.8-75.6%) | 72.8%(58.2-84.4%) | 54.0%(41.6-82.8%) | 70.8%(52.3-86.7%) |
| 12 months | 53.3%(26.3-74.4%) | 72.0% (50.1-85.6%) | 52.7%(28.3-72.8%) | 68.0%(46.1-82.5%) | 46.7%(21.2-68.7%) | 58.6%(40.1-70.8%) |
CI - Confidence Interval. *Success - IOP ≤21 and ≥5 mmHg or 20% reduction from baseline. †Success - IOP ≤18 and ≥5 mmHg or 30% reduction from baseline. ‡Success - IOP ≤15 and ≥5 mmHg or 40% reduction from baseline
Figure 1.
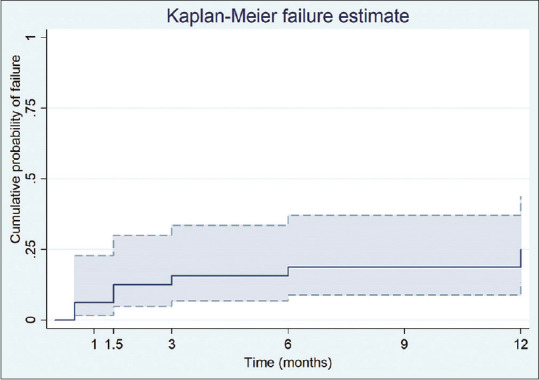
Kaplan–Meier curve demonstrating cumulative failure rate with 95% confidence interval through postoperative month 12.
Intraocular pressure
The AADI was effective in reducing the IOP. The mean IOP decreased from 43.3 ± 10.3 mmHg preoperatively to 15.2 ± 8.1 mmHg at 1 year (65% reduction, P ≤ 0.001) [Fig. 2]. The mean IOP decreased significantly up to 1.5 months post-surgery compared to baseline, after which it stabilized through the final follow-up at 12 months [Table 5].
Figure 2.
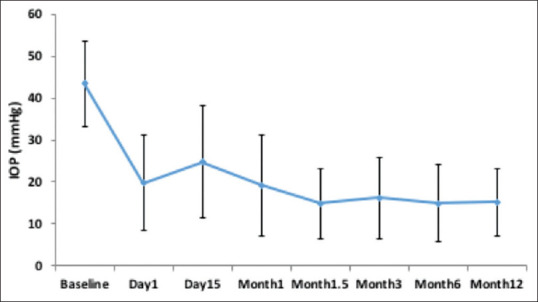
Distribution of intraocular pressure through postoperative month 12.
Table 5.
Comparison of intraocular pressure, anti-glaucoma medication, and best-corrected visual acuity during the follow-up period
| Intraocular Pressure | Anti-Glaucoma Medication | Best-Corrected Visual Acuity (logMAR) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean (SD) | P* | Mean (SD) | P † | Median (IQR) | P † | |
| Baseline | 43.3 (10.3) | - | 3.4 (0.5) | - | 1.7 (1.0-2.6) | - |
| Day 1 | 19.8 (11.3) | <0.001 | 2.2 (1.3) | 0.0001 | 2.6 (1.4-2.6) | 0.002 |
| Day 15 | 24.8 (13.3) | 0.066 | 2.1 (1.4) | 0.824 | 1.3 (0.8-2.6) | 0.001 |
| Month 1 | 19.1 (12.1) | 0.028 | 2.2 (1.2) | 0.982 | 1.0 (0.8-2.6) | 0.023 |
| Month 1.5 | 14.8 (8.4) | 0.034 | 1.7 (0.9) | 0.025 | 1.0 (0.6-2.6) | 0.112 |
| Month 3 | 16.2 (9.7) | 0.565 | 1.6 (0.8) | 0.257 | 0.78 (0.3-2.6) | 0.059 |
| Month 6 | 14.9 (9.1) | 0.110 | 1.4 (1.0) | 0.008 | 1.0 (0.3-2.6) | 0.979 |
| Month 12 | 15.2 (8.1) | 0.735 | 1.6 (0.5) | 0.096 | 1.0 (0.6-2.6) | 0.509 |
SD - standard deviation, IOP, and AGM were presented in mean and standard deviation, logMAR BCVA was presented in the median and interquartile range (IQR). P - compared with previous visits using paired t-test* and Wilcoxon sign-rank test†
Glaucoma medications
The AADI was effective in reducing the need for AGMs [Table 5]. The mean number of AGMs required decreased from 3.4 ± 0.5 preoperatively to 1.6 ± 0.5 at 1 year (52% reduction, P ≤ 0.001). The mean number of AGMs decreased significantly at 1.5 months post-surgery compared to baseline, after which it stabilized through the final follow-up at 12 months.
Visual acuity
In a comparison with the preoperative and final postoperative visual acuity at 1 year, there was a moderate improvement in Best corrected visual acuity (BCVA), although this was not statistically significant [Table 5].
Complications and interventions
During the study period, 22 complications were noted in 10 eyes (30%). The most common complication was choroidal detachment, which was seen in four eyes (13%), and was resolved with conservative management in two eyes. Tube explantation was required in three eyes: two eyes with retinal detachment requiring vitreoretinal intervention and one eye with tube exposure, hypotony, and endophthalmitis. Corneal edema developed in three eyes and hyphema developed in two eyes, all of which resolved without intervention. Vitreous hemorrhage developed in two eyes of which one required PPV. Tube-lens touch was noted in one eye; this resulted in cataract development and required cataract extraction. Conjunctival retraction developed in one case; this required re-suturing in the operation room. Repeat vitreoretinal procedures and AADI explantation were performed in three eyes each and were the most common interventions undertaken [Table 6].
Table 6.
Complications and interventions
| Complications* | n (%) | Interventions* | n (%) |
|---|---|---|---|
| Choroidal detachment | 4 (13) | Repeat vitreoretinal intervention | 3 (9) |
| Retinal detachment | 3 (9) | AADI explantation | 3 (9) |
| Cornea edema | 3 (9) | Vitreous lavage | 1 (3) |
| Vitreous hemorrhage | 2 (6) | Tube repositioning | 1 (3) |
| Hypotony | 2 (6) | Conjunctival re-suturing | 1 (3) |
| Hyphema | 2 (6) | Cataract extraction | 1 (3) |
| Endophthalmitis | 1 (3) | ||
| Conjunctival retraction | 1 (3) | ||
| Epiretinal membrane | 1 (3) | ||
| Tube-lens touch | 1 (3) | ||
| Cataract | 1 (3) | ||
| Tube exposure | 1 (3) | ||
| Total | 22 (69) | Total | 10 (31) |
*Interventions do not correspond to the complications provided in the same row of the table. AADI - Aurolab aqueous drainage implant
Discussion
The use of GDDs has significantly increased over the past decade,[30] and their IOP-lowering efficacy is well described.[31,32] The optimal location of GDD placement (anterior chamber, ciliary sulcus, or pars plana) is determined by each patient’s clinical profile; hence, the surgical technique must be individualized. This study on the 12-month outcomes of pars plana AADI implantation for refractory glaucoma revealed a significant reduction of IOP and the need for AGM. There was improvement noted in the BCVA through 1 month, and this was maintained up until the final follow-up at 12 months. The cumulative failure rate was 26.1% at 1 year. One-third of eyes had complications, with choroidal detachment being the most common. While the majority of complications were self-resolving, repeat vitreoretinal intervention and AADI explantation were the most common interventions. The AADI may be an effective, low-cost option with outcomes comparable to the Baerveldt 350 implant, even in the setting of complex disease requiring placement in the vitreous cavity.
Our study’s cumulative success rate at 12 months was 75%, which is comparable with 77% cumulative success at 12 months noted in a similar study of AADI in the vitreous cavity[27] and was in the lower range of 67–96% success rates previously described for AADI and Baerveldt implants.[20,26,33,34] This variation in the success may be due to a difference in the underlying severity of the disease, surgical technique, and length of follow-up. High IOP was the most common cause for failure in the present study, and all four eyes that failed for this reason had a diagnosis of neovascular glaucoma. With this finding in view, our group is performing a prospective study evaluating the efficacy of AADI with the adjunctive use of mitomycin-C in neovascular glaucoma.
Compared to baseline, a 65% reduction in IOP and a 52% decrease in AGM requirement was seen at the 12-month visit. A similar study by Campagnoli et al.[26] on GDD placement in the vitreous cavity found a slightly lower IOP reduction (62 and 50% for neovascular and non-neovascular glaucoma groups, respectively), and a much higher reduction in AGM requirement (82 and 72% for neovascular and non-neovascular glaucoma groups, respectively). Additionally, the Ahmed versus Baerveldt study[35] had findings similar to ours, with a 66% reduction in IOP and a 61% reduction in AGM requirement in the Baerveldt group. Moreover, a previous report on the pars plana placement of AADI demonstrated a slightly lower IOP reduction at 56% at 12 months.[27] Our findings were consistent with a report on AC placement of AADI, which demonstrated 60% IOP reduction at 12 months.[19] Furthermore, a report by Maheshwari et al.[21] found that AADI placement in the AC versus posterior segment demonstrated a similar IOP reduction of 38 and 35%, respectively. Our significantly higher reduction in IOP at 65% than that reported previously with AADI, suggests the non-inferiority of this approach in IOP reduction as compared to AC placement of AADI.
The hypertensive phase is known to occur in the early postoperative period[36] after GDDs, which was partially mitigated by the placement of venting slits in the drainage tube and the use of AGM. In our study, 36% of the cases developed a relatively high IOP, which required AGM until 6 weeks after surgery while the ligating material degraded. Additionally, a wide range of visual acuity outcomes has been reported with the pars plana placement of GDDs with stable or improved visual acuity being reported in the previous studies.[20,26,33] These findings are in sync with our study, in that moderate improvement in BCVA was noted at postoperative month 1, and was sustained over 12 months. Marginal improvement in visual acuity can be attributed to concomitant procedures, including cataract extraction and secondary IOL implantation.
The complication rate of 30% noted in this study was similar to the previous reports on AADI. A study by Babu N et al.[27] with pars plana placement of AADI described a complication rate of 30%. The most common complications were choroidal detachment, retinal detachment, and vitreous blocking of the AADI tip. Another study on AADI by Puthuran et al.[19] found a complication rate of 24%, with choroidal detachment being the most common complication between postoperative months 3 and 12. Of note, no eyes developed diplopia, which has been reported with the use of conventional GDDs at rates of 2%.[34]
The two other published reports on AADI implantation in the vitreous cavity were also retrospective in nature. Babu N, et al.[27] included 63 eyes and had a similar 60% IOP reduction as ours at 65%. Their cumulative probability of failure was 23% at 1 year also aligned with ours at 26.1%, and the complication rate was similar to ours (30%). Additionally, Rajamani M, et al.[28] evaluated the pars plana placement of AADI in six eyes, and had a short follow-up period of 2 months. This limited sample size and short follow-up period made the comparison challenging.
The limitations of the study include its retrospective nature, small sample size, and lack of a control group. The relative strengths include the participation of a single glaucoma surgeon (MAK) and retina surgeon (VR) in all procedures, and the use of a single type of GDD. The additional strengths include a relatively long follow-up period of 12 months in all the patients, and success/failure described at different IOP levels, as all eyes have a different target IOP. Given that this was a retrospective study, the addition of medications and need for additional procedures was based on the glaucoma surgeon’s clinical judgment. Although this was not standardized as in a randomized clinical trial. We believe this had no effect on the study outcomes.
This study further demonstrates that surgical success with the pars plana placement of the AADI may be comparable to other GDDs, which can be cost-prohibitive or not available in developing nations. The Ahmed glaucoma valve (New World Medical; Rancho Cucamonga, CA, USA) costs 650 USD when made available in India, and the Baerveldt glaucoma implant costs 750 USD and is not available in India. At 50 USD, the cost of the AADI is one-fifteenth that of the Baerveldt, and the AADI is available in several developing nations. These qualities, along with their comparable effectiveness, make the AADI a suitable option to be used more broadly.
Conclusion
This study adds to the literature on the outcomes associated with our modification over the conventional technique of pars plana implantation of the AADI device, as only two studies on this topic have been published.[27,28] Our encouraging results and the stabilization of visual acuity when concurrent posterior segment pathology is addressed offer glaucoma specialists a potentially effective and low-cost option for the management of refractory glaucoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge Mr. Mohammed Sithiq Uthuman for statistical analysis.
References
- 1. Joshi AB, Parrish RK, Feuer WF. 2002 survey of the American Glaucoma Society:Practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma. 2005;14:172–4. doi: 10.1097/01.ijg.0000151684.12033.4d. [DOI] [PubMed] [Google Scholar]
- 2. Hyung SM, Kim SK. Mid-term effects of trabeculectomy with mitomycin C in neovascular glaucoma patients. Korean J Ophthalmol. 2001;15:98–106. doi: 10.3341/kjo.2001.15.2.98. [DOI] [PubMed] [Google Scholar]
- 3. Chow K, Mora J. Practice preferences for glaucoma drainage device implantation and cyclodestruction in Australia and New Zealand. J Glaucoma. 2012;21:199–205. doi: 10.1097/IJG.0b013e31820e2d08. [DOI] [PubMed] [Google Scholar]
- 4. Knape RM, Szymarek TN, Tuli SS, Driebe WT, Sherwood MB, Smith MF. Five-year outcomes of eyes with glaucoma drainage device and penetrating keratoplasty. J Glaucoma. 2012;21:608–14. doi: 10.1097/IJG.0b013e31821db3e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ceballos EM, Parrish RK, Schiffman JC. Outcome of Baerveldt glaucoma drainage implants for the treatment of uveitic glaucoma. Ophthalmology. 2002;109:2256–60. doi: 10.1016/s0161-6420(02)01294-0. [DOI] [PubMed] [Google Scholar]
- 6. Budenz DL, Gedde SJ, Brandt JD, Kira D, Feuer W, Larson E. Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology. 2004;111:2204–10. doi: 10.1016/j.ophtha.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 7. Chalam KV, Gandham S, Gupta S, Tripathi BJ, Tripathi RC. Pars plana modified Baerveldt implant versus neodymium:YAG cyclophotocoagulation in the management of neovascular glaucoma. Ophthalmic Surg Lasers. 2002;33:383–93. [PubMed] [Google Scholar]
- 8. Faghihi H, Hajizadeh F, Mohammadi S-F, Kadkhoda A, Peyman GA, Riazi-Esfahani M. Pars plana Ahmed valve implant and vitrectomy in the management of neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2007;38:292–300. doi: 10.3928/15428877-20070701-04. [DOI] [PubMed] [Google Scholar]
- 9. Scott IU, Alexandrakis G, Flynn HW, Smiddy WE, Murray TG, Schiffman J, et al. Combined pars plana vitrectomy and glaucoma drainage implant placement for refractory glaucoma. Am J Ophthalmol. 2000;129:334–41. doi: 10.1016/s0002-9394(99)00363-3. [DOI] [PubMed] [Google Scholar]
- 10. Malone PE, Herndon LW, Muir KW, Jaffe GJ. Combined fluocinolone acetonide intravitreal insertion and glaucoma drainage device placement for chronic uveitis and glaucoma. Am J Ophthalmol. 2010;149:800–6. doi: 10.1016/j.ajo.2009.12.009. e1. [DOI] [PubMed] [Google Scholar]
- 11. Tello C, Espana EM, Mora R, Dorairaj S, Liebmann JM, Ritch R. Baerveldt glaucoma implant insertion in the posterior chamber sulcus. Br J Ophthalmol. 2007;91:739–42. doi: 10.1136/bjo.2006.107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kono T, Shiga S, Takesue Y, Sakamoto T. Long-term results of pars plana vitrectomy combined with filtering surgery for neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2005;36:211–6. [PubMed] [Google Scholar]
- 13. Lima VC, de Moraes CG, Gentile RC, Sidoti PA, Prata TS, Liebmann JM, et al. Combined Baerveldt glaucoma implant and scleral buckling surgery for patients with retinal detachment and coexisting glaucoma. J Glaucoma. 2013;22:294–300. doi: 10.1097/IJG.0b013e318241bc37. [DOI] [PubMed] [Google Scholar]
- 14. Molteno AC. New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol. 1969;53:606–15. doi: 10.1136/bjo.53.9.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaushik S, Kataria P, Raj S, Pandav SS, Ram J. Safety and efficacy of a low-cost glaucoma drainage device for refractory childhood glaucoma. Br J Ophthalmol. 2017;101:1623–7. doi: 10.1136/bjophthalmol-2017-310276. [DOI] [PubMed] [Google Scholar]
- 16. Puthuran GV, Palmberg PF, Wijesinghe HK, Pallamparthy S, Krishnadas SR, Robin AL. Intermediate-term outcomes of Aurolab aqueous drainage implant in refractory paediatric glaucoma. Br J Ophthalmol. 2020;104:962–6. doi: 10.1136/bjophthalmol-2019-314399. [DOI] [PubMed] [Google Scholar]
- 17. Hafeezullah N, AlHilali S, Alghulaydhawi F, Edward DP, Ahmad S, Malik R. A preliminary comparison of the Aravind aurolab drainage implant with the Baerveldt glaucoma implant:A matched case-control study. Eur J Ophthalmol. 2021;31:445–52. doi: 10.1177/1120672120912383. [DOI] [PubMed] [Google Scholar]
- 18. Pandav SS, Seth NG, Thattaruthody F, Kaur M, Akella M, Vats A, et al. Long-term outcome of low-cost glaucoma drainage device (Aurolab aqueous drainage implant) compared with Ahmed glaucoma valve. Br J Ophthalmol. 2020;104:557–62. doi: 10.1136/bjophthalmol-2019-313942. [DOI] [PubMed] [Google Scholar]
- 19. Puthuran GV, Palmberg P, Wijesinghe HK, Krishnadas SR, Robin A. Intermediate-term outcomes of an affordable aqueous drainage implant in adults with refractory glaucoma. Ophthalmol Glaucoma. 2019;2:258–66. doi: 10.1016/j.ogla.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 20. Rososinski A, Wechsler D, Grigg J. Retrospective review of pars plana versus anterior chamber placement of Baerveldt glaucoma drainage device. J Glaucoma. 2015;24:95–9. doi: 10.1097/IJG.0b013e31829d9be2. [DOI] [PubMed] [Google Scholar]
- 21. Maheshwari D, Dabke S, Rajagopal S, Kadar MA, Ramakrishnan R. Clinical outcome of a nonvalved Aurolab aqueous drainage implant in posterior segment versus anterior chamber. Indian J Ophthalmol. 2019;67:1303–8. doi: 10.4103/ijo.IJO_1341_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luttrull JK, Avery RL. Pars plana implant and vitrectomy for treatment of neovascular glaucoma. Retina. 1995;15:379–87. doi: 10.1097/00006982-199515050-00002. [DOI] [PubMed] [Google Scholar]
- 23. Luttrull J, Avery R, Baerveldt G, Easley K. Initial experience with pneumatically stented baerveldt implant modified for pars plana insertion for complicated glaucoma. Ophthalmology. 2000;107:143–9. doi: 10.1016/s0161-6420(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 24. Kolomeyer AM, Kim HJ, Khouri AS, Lama PJ, Fechtner RD, Zarbin MA, et al. Pars plana Baerveldt tube insertion with pars plana vitrectomy for refractory glaucoma. Oman J Ophthalmol. 2012;5:19–27. doi: 10.4103/0974-620X.94762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolomeyer AM, Seery CW, Emami-Naeimi P, Zarbin MA, Fechtner RD, Bhagat N. Combined pars plana vitrectomy and pars plana Baerveldt tube placement in eyes with neovascular glaucoma. Retina (Philadelphia, Pa) 2015;35:17–28. doi: 10.1097/IAE.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 26. Campagnoli TR, Kim SS, Smiddy WE, Gedde SJ, Budenz DL, Parrish RK, et al. Combined pars plana vitrectomy and Baerveldt glaucoma implant placement for refractory glaucoma. Int J Ophthalmol. 2015;8:916–21. doi: 10.3980/j.issn.2222-3959.2015.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Babu N, Baliga G, Wijesinghe HK, Puthuran GV. Intermediate-term outcomes of pars plana tube insertion of Aurolab aqueous drainage implant for refractory glaucoma. Br J Ophthalmol. 2020;104:1293–7. doi: 10.1136/bjophthalmol-2019-314639. [DOI] [PubMed] [Google Scholar]
- 28. Rajamani M, Ramamurthy C, Ramamurthy S, Chaya C, Puthuran G, Kumar S, et al. Outcome of a low-cost glaucoma drainage device with posterior chamber/pars plana insertion of the tube. Eye (Lond) 2021;35:901–12. doi: 10.1038/s41433-020-0994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah VJ, Abdul Khadar SM, Venugopal Reddy YC, Adeel SS, Kader MA, Ramakrishnan R, et al. Aurolab aqueous drainage implant in the vitreous cavity:Our modifications over the conventional technique of glaucoma implant surgery. Indian J Ophthalmol. 2021;69:1950–2. doi: 10.4103/ijo.IJO_3348_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122:1615–24. doi: 10.1016/j.ophtha.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 31. Christakis PG, Kalenak JW, Zurakowski D, Tsai JC, Kammer JA, Harasymowycz PJ, et al. The Ahmed Versus Baerveldt study:One-year treatment outcomes. Ophthalmology. 2011;118:2180–9. doi: 10.1016/j.ophtha.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 32. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol. 2007;143:9–22. doi: 10.1016/j.ajo.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 33. Qin VL, Kaleem M, Conti FF, Rockwood EJ, Singh A, Sood-Mendiratta S, et al. Long-term clinical outcomes of pars plana versus anterior chamber placement of glaucoma implant tubes. J Glaucoma. 2018;27:440–4. doi: 10.1097/IJG.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 34. Philip R, Chandran P, Aboobacker N, Dhavalikar M, Raman GV. Intermediate-term outcome of Aurolab aqueous drainage implant. Indian J Ophthalmol. 2019;67:233–8. doi: 10.4103/ijo.IJO_675_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christakis PG, Zhang D, Budenz DL, Barton K, Tsai JC, Ahmed IIK, et al. Five-year pooled data analysis of the ahmed baerveldt comparison study and the Ahmed Versus Baerveldt study. Am J Ophthalmol. 2017;176:118–26. doi: 10.1016/j.ajo.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 36. Siegner SW, Netland PA, Urban RC, Jr, Williams AS, Richards DW, Latina MA, et al. Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology. 1995;102:1298–307. doi: 10.1016/s0161-6420(95)30871-8. [DOI] [PubMed] [Google Scholar]


