Abstract
Introduction
ROS1-rearranged (ROS1+) non-small-cell lung cancer (NSCLC) is a rare lung cancer with limited treatment options. Phase I-II studies with ROS1-tyrosine kinase inhibitors (TKIs) included small numbers of patients and real-world data are lacking. We investigate the efficacy and safety of lorlatinib, a third-generation TKI targeting ALK and ROS1, in patients with ROS1+ NSCLC treated through an expanded access program.
Methods
Consecutive patients with advanced ROS1+ NSCLC treated with lorlatinib between October 2015 and June 2019 were included. Data were collected from medical records. The primary endpoint was progression-free survival.
Results
Out of the 80 patients included, 47(59%) were female, 49(62%) never smokers (less than 100 cigarettes over the lifetime), and 68(85%) had stage IV NSCLC at diagnosis. Most frequent histology was adenocarcinoma (95%) and median age was 58.2 years. At the time of lorlatinib initiation, 51(64%) patients had brain metastases and 55(81%) were PS 0-1. Lorlatinib was administered as second/third/fourth/fifth+ line in 29%/28%/18%/26% of patients. All patients previously received at least one ROS1 TKI, and 55(69%) previously received chemotherapy. Median follow-up from lorlatinib initiation was 22.2 months. Median progression-free survival and overall survival from lorlatinib initiation were 7.1 months [95% confidence interval (CI) 5.0-9.9 months] and 19.6 months (95% CI 12.3-27.5 months). Median duration of treatment with lorlatinib was 7.4 months (95% CI 6.5-13.1 months). Overall response and disease control rates were 45% and 82%, respectively. The central nervous system response rate was 72%. Treatment was stopped due to toxicity in 10 patients (13%). The safety profile was consistent with previously published data.
Conclusions
Lorlatinib is a major treatment option for advanced refractory ROS1+ NSCLC in treatment strategy.
Key words: NSCLC, ROS1, chemotherapy, brain metastases
Highlights
-
•
Data are lacking on lorlatinib efficacy in advanced refractory ROS1+ NSCLC.
-
•
Lorlatinib median progression-free survival and objective response rate were 7.1 months and 45%, respectively.
-
•
Lorlatinib represents a major treatment option for patients with a ROS1+ NSCLC.
Introduction
Rearrangements of the ROS1 receptor tyrosine kinase gene are observed in 1%-2% of non-small-cell lung cancers (NSCLCs), mainly in never-smoking (patients who smoked less than 100 cigarette in their lifetime) patients.1,2 This alteration leads to a fusion protein carrying the constitutively activated ROS1 tyrosine kinase domain. Several tyrosine kinase inhibitors (TKIs) are active against ROS1, including crizotinib, ceritinib, or entrectinib,3, 4, 5 based on data from phase I-II studies with limited cohorts of patients. As per current guidelines (European Society for Medical Oncology, National Comprehensive Cancer Network), management of patients with metastatic ROS1-rearranged (ROS1+) NSCLC relies on first-line crizotinib, ceritinib, or entrectinib which provide median progression-free survival (PFS) ranging from 6 to 20 months.4, 5, 6, 7, 8, 9 All ROS1+ NSCLC patients, however, eventually show tumor progression due to resistance mechanisms such as kinase domain mutations. The most common of these alterations is the G2032R mutation.10,11
Lorlatinib is a brain-penetrant third-generation ATP competitive reversible TKI of ALK and ROS1 retaining activity in vitro on several crizotinib-resistant ROS1 mutations.12 The clinical efficacy of lorlatinib in metastatic ROS1+ NSCLC after ROS1 TKI failure was evaluated in one single non-comparative phase I-II trial13; among the 40 patients pretreated with crizotinib in this study, the objective response rate (ORR) was 35% and the median PFS was 8.5 months. Real-life evidence regarding the efficacy and safety of lorlatinib in this setting, however, is still lacking as the number of ROS1+ patients is limited in previous reports.14,15 Meanwhile, treatment sequencing in ROS1+ NSCLC is still to be assessed in a routine practice setting.
Here, we took advantage of the French lorlatinib expanded access program (EAP), to assess treatment sequencing, and lorlatinib efficacy and safety, in patients with ROS1+ NSCLC.
Materials and methods
Study population and data collection
Consecutive adult patients, from 49 centers, with an advanced or metastatic ROS1+ NSCLC, treated from October 2015 to June 2019 with lorlatinib, 100 mg once daily, for at least 7 days, as part of the French lorlatinib EAP, were included in the present study. Patients were eligible for lorlatinib EAP if they had previously failed at least one ROS1 TKI. The EAP list of patients was provided by Pfizer.
Data and survival follow-up were extracted from medical records by independent research staff of the French Thoracic Cancer Intergroup (IFCT) at each center and documented in a standard case report form. The database is hosted by the IFCT that ensured the quality of the data collected by monitoring the centers with periodic visits of IFCT clinical research associates.
Study oversight
This non-interventional study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, approved by a national ethics committee, French Advisory Committee on Information Processing in Material Research in the Field of Health, and France’s national data protection authority (CNIL) in accordance with General Data Protection Regulation. All participating departments approved the study protocol and all included patients received information from their referring physician, with an opportunity not to participate.
Study endpoints
The primary endpoint was PFS measured from the date of first lorlatinib dose to the date of disease progression according to RECIST version 1.1 or death from any cause. Secondary endpoints included: ORR defined as the percentage of patients with partial or complete response to lorlatinib according to RECIST 1.1 evaluated by investigators; disease control rate (DCR) defined as the percentage of patients with partial or complete response or stable disease to lorlatinib according to RECIST 1.1 evaluated by investigators; overall survival (OS) calculated from the date of the lorlatinib first dose; OS calculated from the date of advanced or metastatic NSCLC diagnosis; duration of treatment (DOT) measured from the date of lorlatinib first dose to the date of treatment discontinuation or death from any cause during the study; DOT response measured from the date of first lorlatinib RECIST 1.1 tumor response to the date of disease progression or death from any cause; central nervous system (CNS) response rate defined as the rate of intracranial tumor response to lorlatinib according to RECIST 1.1 with baseline measurement only including CNS lesions evaluated by investigators among patients with brain metastasis; duration of CNS response defined as the time from the first documentation of objective cerebral response to the first documentation of cerebral response or death from any cause. ORR, PFS, and DOT were also collected for subsequent treatments after lorlatinib failure. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
Categorical variables are expressed as frequencies and percentages. Quantitative variables are expressed as median (range). The Kaplan–Meier method was used to estimate PFS and OS endpoints. The cut-off for survival analysis was 22 February 2020. All analyses were carried out using SAS software, Version 9.4 (SAS Institute, Cary, NC).
Results
Clinicopathological characteristics
Eighty patients out of the 343 listed in the lorlatinib EAP database were eligible and included in the present study (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100418). Baseline clinical features are provided in Table 1. Most patients were women (59%), never-smokers (62%), and displayed a stage IV NSCLC at diagnosis (85%). The median age was 58.2 years (range: 25.9-92.8 years). The most common histology was adenocarcinoma (95%). At lorlatinib initiation, most patients were PS 0 or 1 (81%) and had brain metastasis (64%). Overall, 71% of patients had previously undergone at least two lines of systemic therapy, and 34% had received brain radiation therapy. All patients in the cohort had previously been treated with a first generation ROS1 TKI (crizotinib), and 21% had received a second generation ROS1 TKI. Before lorlatinib, a total of 63 (79%)/13 (16%)/4 (5%) patients had received one/two/three or more ROS1 TKIs, respectively.
Table 1.
Demographics of the cohort
| Characteristics | N = 80 n (%) |
|---|---|
| Sex | |
| Male | 33 (41) |
| Female | 47 (59) |
| Median age (range), years | 58.2 (25.9-92.8) |
| Smoking status | |
| Current or former smokers | 30 (38) |
| Never smokers | 49 (62) |
| Unknown | 1 |
| Staging at diagnosis | |
| I-II | 4 (5) |
| III | 8 (10) |
| IV | 68 (85) |
| Brain metastasis at diagnosis | |
| Present | 17 (21) |
| Absent | 63 (79) |
| Histology | |
| Adenocarcinoma | 76 (95) |
| Squamous carcinoma | 1 (1) |
| Other | 3 (4) |
| PS at lorlatinib initiation | |
| 0-1 | 55 (81) |
| ≥2 | 13 (19) |
| Unknown | 12 |
| Previous lines of systemic therapy | |
| 1 | 23 (29) |
| 2 | 22 (28) |
| 3 | 14 (18) |
| ≥4 | 21 (26) |
| Previous systemic therapy | |
| Chemotherapy | 55 (69) |
| First-generation ROS1 TKI | 80 (100) |
| Second-generation ROS1 TKI | 17 (21) |
| Immune checkpoint inhibitors | 8 (10) |
| Previous lines of ROS1 TKI | |
| 1 | 63 (79) |
| 2 | 13 (16) |
| ≥3 | 4 (5) |
| Previous brain radiotherapy | 27 (34) |
| Brain metastasis at lorlatinib initiation | |
| Present | 51 (64) |
| Absent | 29 (36) |
Current smokers: ongoing smoking at baseline; Former smokers: quit more than 1 year before baseline; Never smokers: smoked less than 100 cigarettes before baseline.
TKI, tyrosine kinase inhibitor.
Efficacy
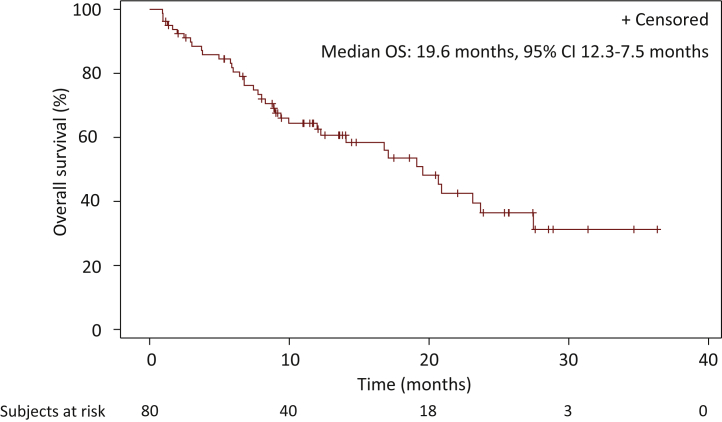
The median follow-up from lorlatinib initiation was 22.2 months (interquartile range: 13.6-31.3 months) (Table 2). Median PFS on lorlatinib was 7.1 months [95% confidence interval (CI) 5.0-9.9 months] and median OS from lorlatinib initiation 19.6 months (95% CI 12.3-27.5 months) (Figure 1 and 2). Median OS from advanced or metastatic NSCLC disease was 51.9 months (95% CI 41.8-74.5 months). ORR to lorlatinib and DCR were 45% and 82%, respectively. Median duration of lorlatinib treatment was 7.4 months (95% CI 6.5-13.1 months) and the median duration of response was 6.9 months (95% CI 5.1-20.6 months). CNS response rate was 72% and the median duration of CNS response was 16.7 months (95% CI 5.1-20.6 months). For patients with brain irradiation before lorlatinib initiation, the CNS response rate and the median duration of CNS response were, respectively, 75% and 9.0 months [95% CI 2.8 months-not reached (NR)]. Among the patients who did not receive brain radiotherapy before lorlatinib initiation, the CNS response rate and the median duration of CNS response were, respectively, 68% and 20.6 months (95% CI 1.9-22.7 months). For the 51 patients who experienced tumor progression, the main progression site was the thorax (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100418). When lorlatinib treatment was continued beyond progression, median DOT after progression was 0.9 months (95% CI 0.4-2.0 months). When patients had previously received one, two, or three or more ROS1 TKIs, median PFS and OS were 7.6 months (95% CI 6.2-12.9 months) and 20.7 months (95% CI 12.3-27.5 months), 2.8 months (95% CI 1.6-9.0 months) and 19.1 months (95% CI 2.9 months-NR), and 6.9 months (95% CI 0.5 months-NR) and 10 months (95% CI 1.0-14.1 months), respectively. ORR depending on whether patients had received one, two, or three or more previous ROS1 TKIs before lorlatinib initiation were 48%, 33%, and 25%, respectively.
Table 2.
Lorlatinib therapy clinical outcome
| Characteristics | N = 80 |
|---|---|
| Median follow-up (IQR), months | 22.2 (13.6-31.3) |
| Median PFS (95% CI), months | 7.1 (5.0-9.9) |
| Median OS (95% CI), months | 19.6 (12.3-27.5) |
| Median OS from advanced or metastatic NSCLC diagnosis (95% CI), months | 51.9 (41.8-74.5) |
| Best response overall response to lorlatinib, n (%) | |
| Number of patients with available data | 76 (95) |
| Complete response | 0 (0) |
| Partial response | 34 (45) |
| Objective response | 34 (45) |
| Stable disease | 28 (37) |
| Progression | 12 (16) |
| Not evaluable | 2 (3) |
| Median duration of response (95% CI), months | 6.9 (5.1-20.6) |
| CNS objective responsea (available data; %) | 33 (/46; 72) |
| CNS objective response in patients with prior brain radiotherapya (available data; %) | 18 (/24; 75) |
| CNS objective response in patients without prior brain radiotherapya (available data; %) | 15 (/22; 68) |
| Median duration of CNS response (95% CI), months | 16.7 (5.1-20.6) |
| Median duration of CNS response in patients with prior brain radiotherapy (95% CI), months | 9.0 (2.8-NR) |
| Median duration of CNS response in patients without prior brain radiotherapy (95% CI), months | 20.6 (1.9-22.7) |
| Median lorlatinib duration (95% CI), months | 7.4 (6.5-13.1) |
| Median lorlatinib duration beyond progression (95% CI), months | 0.9 (0.4-2.0) |
| Treatment discontinuation, n (%) | 53 (66) |
| Cause of treatment discontinuation, n (%) | |
| Disease progression | 37 (46) |
| Toxicity | 10 (13) |
| Death | 4 (5) |
| Investigator’s decision | 1 (1) |
| Patient’s decision | 1 (1) |
| Intercurrent disease | 1 (1) |
| PFS according to the number of previous ROS1 TKI lines | |
| 1 ROS1 TKI (95% CI), months(n=20, %) | 7.6 (6.2-12.9) |
| 2 ROS1 TKI (95% CI), months | 2.8 (1.6-9.0) |
| ≥3 ROS1 TKI (95% CI) months | 6.9 (0.5-NR) |
| OS according to the number of previous ROS1 TKI lines | |
| 1 ROS1 TKI (95% CI), months(n=20, %) | 20.7 (12.3-27.5) |
| 2 ROS1 TKI (95% CI), months | 19.1 (2.9-NR) |
| ≥3 ROS1 TKI (95% CI), months | 10 (1.0-14.1) |
| Objective response according to the number of previous ROS1 TKI lines | |
| 1 ROS1 TKI (n = 60) n (%)(n=20, %) | 29 (48) |
| 2 ROS1 TKI (n = 12) n (%) | 4 (33) |
| ≥3 ROS1 TKI (n = 4) n (%) | 1 (25) |
CI, confidence interval; CNS, central nervous system; IQR, interquartile range; NR, not reached; NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
Defined as the rate of intracranial tumor response according to RECIST v1.1.
Figure 1.
Progression-free survival.
Kaplan–Meier estimate of progression-free survival (PFS). Tick marks on the survival curves indicate censoring of data.
CI, confidence interval.
Figure 2.
Overall survival.
Kaplan–Meier estimate of overall survival (OS) measured from lorlatinib initiation.
Tick marks on the survival curves indicate censoring of data.
CI, confidence interval; NR, not reached.
Safety
Grade 3 or more adverse events were reported in 33% of the patients (Table 3). The most common ≥grade 3 adverse events were elevated cholesterol levels (13%), cognitive effects (8%), elevated triglyceride levels (5%), mood effects (4%), peripheral neuropathies (3%), and renal failures (3%). Adverse events leading to treatment discontinuation occurred in 13% of the patients included in the study. They were mainly represented by renal failure, cognitive effects, and mood effects (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100418). The two renal failures observed in the study were, respectively, grade 3 and grade 4.
Table 3.
Serious adverse events in patients treated with lorlatinib (reported in >1% of patients)
|
N = 80, n (%) |
||||
|---|---|---|---|---|
| Grade 3-5 | Grade 3 | Grade 4 | Grade 5 | |
| Any adverse event | 26 (33) | 15 (19) | 11 (14) | 0 (0) |
| Hypercholesterolemia | 10 (13) | 6 (8) | 4 (5) | 0 (0) |
| Cognitive effect | 6 (8) | 5 (6) | 1 (1) | 0 (0) |
| Hypertriglyceridemia | 4 (5) | 1 (1) | 3 (4) | 0 (0) |
| Mood effect | 3 (4) | 2 (3) | 1 (1) | 0 (0) |
| Peripheral neuropathy | 2 (3) | 2 (3) | 0 (0) | 0 (0) |
| Renal failure | 2 (3) | 1 (1) | 1 (1) | 0 (0) |
| Edema | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Fatigue | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Arthralgia | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Pulmonary hypertension | 1 (1) | 0 (0) | 1 (1) | 0 (0) |
| Interstitial lung disease | 1 (1) | 0 (0) | 1 (1) | 0 (0) |
| Abdominal pain | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Amylase increase | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Cholestasis | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
Subsequent therapy
After lorlatinib treatment, 36 (46%) patients received at least one subsequent therapy, mainly chemotherapy and additional ROS1 TKI (Supplementary Table S2 and Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100418). Interestingly, a ROS1 TKI was used in 12 patients as first subsequent therapy after lorlatinib failure. For these patients, ORR and median PFS were 0% and 2.4 months (95% CI 0.7-4.7 months), respectively (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100418). The median OS from the initiation of ROS1 TKI as the first subsequent line after the lorlatinib was 8.2 months (95% CI 0.7-15.7 months).
Discussion
LORLATU represents the largest study describing the real-life efficacy and safety of lorlatinib in patients with ROS1+ NSCLC after the failure of at least one ROS1 TKI, while analyzing treatment sequences. We found an ORR of 45%, a CNS response rate of 72%, and a median PFS of 7.1 months. Despite the inclusion of patients treated with two or more ROS1 TKIs in our study (21% of patients), these results are comparable and even better to those obtained in the pivotal phase I-II study assessing lorlatinib efficacy in ROS1+ NSCLC. Indeed, this trial reports an ORR of 35%, an intracranial response of 50%, and a median PFS of 8.5 months in crizotinib pretreated ROS1+ NSCLC.13 Similar efficacy was also seen in smaller, real-life studies.14,16 Although lorlatinib is not yet approved for use in ROS1+ NSCLC, our results strongly support lorlatinib as a salvage therapy after the failure of at least one ROS1 TKI in these patients.
In our study, the safety profile of lorlatinib was broadly similar to that described in previous studies, with hypertriglyceridemia, hypercholesterolemia, mood disorders, cognitive effects, edema, and peripheral neuropathies being the most frequent side-effects. The rate of discontinuation due to toxicity, however, was actually higher in our study (12.5% versus 1%). This difference may be explained by the inclusion of 21 patients who were naive to any ROS1 TKI in the landmark trial, as well as a higher proportion of patients with a PS ≥2 in our study (19% versus 3%).13 In addition, two patients in our study experienced treatment discontinuations related to renal failure. Cases of renal toxicity have been described for other ALK inhibitors such as alectinib or crizotinib.17, 18, 19 To our knowledge, however, no renal toxicity has been reported with lorlatinib to date, except a case report describing proteinuria acquired under lorlatinib treatment and a case of lorlatinib discontinuation due to proteinuria in the CROWN trial, assessing lorlatinib as first-line treatment of ALK-rearranged NSCLC.20,21 These events may be related to the prior exposure to multiple lines of TKIs. Finally, as in other publications, several treatment discontinuations were observed following cognitive or mood disorders, highlighting the difficulty of managing this type of toxicity, which may also be exacerbated by the high frequency of brain metastases and prior radiation therapy.14,15
In our study, we were able to analyze the subsequent therapies received after lorlatinib progression. Although significant efficacy of pemetrexed-based chemotherapy has been reported in ROS1+ NSCLC, data are lacking regarding the value of systemic treatment after lorlatinib failure.22,23 Notably, the clinical efficacy of ROS1 TKI rechallenge after lorlatinib progression has not been previously described. Here, we observed an ORR of 0% and a median PFS of 2.4 months (95% CI 0.7-4.7 months) among patients treated with a ROS1 TKI as first subsequent therapy after lorlatinib. These results indicate a poor antitumoral efficacy of current ROS1 TKIs in this setting, which is consistent with the recently published description of lorlatinib resistance mechanisms in ROS1+ NSCLC.24 Indeed, ROS1 mutation occurred in 46% of post-lorlatinib patient samples, and these alterations led to resistance to ROS1 type I TKIs in preclinical models. Additionally, ROS1-independent mechanisms of resistance were also described, such as MET or KRAS amplification, KRAS mutation, NRAS mutation, MAP2K1 mutation, making efficacy of rechallenge with crizotinib, ceritinib, or entrectinib unlikely.
This study has several limitations mainly related to its retrospective nature. Thus, the modalities of patient follow-up and adverse events monitoring could not be harmonized. A central review of tumor response assessment and molecular analyses was also not feasible. We were also unable to collect data on ROS1 fusion partners or resistance mutations to crizotinib. It is therefore not possible to determine the impact of these alterations on the response to lorlatinib.
In conclusion, our study shows that lorlatinib stands for a major treatment option for patients with advanced ROS1+ NSCLC and is part of the sequential treatment strategy. Further studies are needed to evaluate the therapeutic options after progression. In addition, better characterization of resistance mechanisms would allow optimized decision making of treatment sequences.
Acknowledgments
Funding
This work was supported by the French Thoracic Cancer Intergroup (IFCT) and funded by an unrestricted grant from Pfizer (no grant number).
Role of the funder
The funding source had no role in the design, data collection, analysis, or interpretation of the study, or in the preparation of this manuscript.
Disclosure
SB reports non-financial support from Lilly, GlaxoSmithKline, Roche, Pfizer, personal fees from Roche, Boehringer Ingelheim, grants from Intergroupe Francophone de Cancérologie Thoracique. BB reports grants from Abbvie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, Bristol Myers Squibb (BMS), Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Inivata, Janssen, Onxeo, OSE Immunotherapeutics, Pfizer, Roche-Genentech, Sanofi, Takeda, Tolero Pharmaceuticals. DMS reports grants from Pfizer, Roche, AstraZeneca, BMS, Merck Sharp & Dohme (MSD), personal fees from Pfizer, Roche, Takeda, AstraZeneca, Lilly, BMS, MSD, Novartis, Amgen, Abbvie, Becton Dickinson, and non-financial support from Pfizer, Roche, Takeda, AstraZeneca, BMS, MSD. JC reports personal fees from Pfizer, Roche, Takeda, Novartis, AstraZeneca, MSD, BMS, and Boehringer Ingelheim. VW reports honoraria from Roche, AstraZeneca, BMS, MSD and non-financial support from Roche, Pfizer. BR reports grants or contracts from Chugai, consulting fees from BMS, AstraZeneca, Roche, support for attending meetings and/or travel from BMS, Amgen, MSD, Roche. JB reports personal fees for advisory boards and educational symposia from AstraZeneca, Bayer, BMS, MSD, Roche, Daichii, and Servier. All other authors have declared no conflicts of interest.
Appendix
The LORLATU contributors, listed here, collaborated in this project and provided data for one patient or more, (not included in the list of authors):
Jennifer Arrondeau, Hôpital Cochin, Service Pneumologie, Paris
Fabrice Barlesi, Hôpital Nord, Service d’Oncologie Multidisciplinaire & Innovations Thérapeutiques, Marseille
Dominique Besson, Hôpital Privé des Côtes d’Armor, Cancérologie – Oncologie Médicale, Plerin
Clément Bonnet, Hôpital Saint-Louis, Service d’Oncologie Médicale, Paris
Arnaud Boutan-Laroze, Centre Hospitalier Victor Dupouy, Service de Pneumologie, Argenteuil
Quentin Bury, Béthune-Beuvry CH, Service de Pneumologie, Bethune
Eric Dansin, Centre Oscar Lambret, Département Oncologie Générale, Lille
Charles Dayen, Clinique de l’Europe, Service de Pneumologie, Amiens
Didier Debieuvre, Centre Hospitalier, Service de Pneumologie, Mulhouse
Renaud Descourt, CHU Morvan, Service d’Oncologie, Brest
Maxime Dewolf, CHU – Hôpital Maison Blanche, Service de Pneumologie, REIMS
Hélène Doubre, Hôpital Foch, Service de Pneumologie, Suresnes
Ludovic Doucet, Institut de Cancérologie de l’Ouest – René Gauducheau, Service d’Oncologie Médicale, Saint – Herblain
Pascale Dubray-Longeras, Centre Jean Perrin, Service de Radiothérapie, Clermont-Ferrand
Pierre Fournel, Institut de Cancérologie Lucien Neuwirth, Département d’Oncologie Médicale, Saint-Priest En Jarez
Thomas Gey, Clinique Médico-Chirurgicale Teissier Groupe A.H.N.A.C, Service de pneumologie, Valenciennes
Helen Homokos, Hôpital Privé de l’Estuaire, Service de Pneumologie, Le Havre
Sébastien Larive, Centre Hospitalier les Chanaux, Service de Pneumologie Unité R2, Macon
Hélène LE HO, Centre Hospitalier Général Yves Le Foll, Service de Pneumologie, Saint-Brieuc
Jeannick Madelaine, CHU Côte de Nacre, Service de Pneumologie, Caen
Anne Madroszyk, Institut Paoli Calmettes, Service d’Oncologie médicale 2, Marseille
Julien Mazieres, Hôpital Larrey, Service de Pneumologie, Toulouse
Bertrand Mennecier, Nouvel Hôpital Civil – Hôpitaux Universitaires de Strasbourg, Service de Pneumologie – Pôle de Pathologie Thoracique, Strasbourg
Patrick Merle, Hôpital Gabriel Montpied, Service d’Oncologie Thoracique – Hôpital de jour, Clermont-Ferrand
Lionel Moreau, Hôpital Louis Pasteur, Service de Médecine F, Colmar
Sophie Schneider, Centre Hospitalier de la Côte Basque, Service de Pneumologie, Bayonne
Andrei Seferian, Hôpital Bicêtre, Pneumologie, Le Kremlin-Bicetre
Claire Tissot Filippello, Hôpital Nord, Service de Pneumologie, Saint-Etienne
Pierre Vaillant, CHU de Brabois, Service de Pneumologie, Vandoeuvre-Les-Nancy
Rémi Veillon, Centre François Magendie – Hôpital du Haut-Lévèque, Service des Maladies Respiratoires, Pessac
Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
References
- 1.Bergethon K., Shaw A.T., Ou S.-H.I., et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies K.D., Le A.T., Theodoro M.F., et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S.M., Kim H.R., Lee J.-S., et al. Open-label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol. 2017;35(23):2613–2618. doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 4.Shaw A.T., Riely G.J., Bang Y.-J., et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drilon A., Siena S., Dziadziuszko R., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moro-Sibilot D., Cozic N., Pérol M., et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol. 2019;30(12):1985–1991. doi: 10.1093/annonc/mdz407. [DOI] [PubMed] [Google Scholar]
- 7.Michels S., Massutí B., Schildhaus H.-U., et al. Safety and efficacy of crizotinib in patients with advanced or metastatic ROS1-rearranged lung cancer (EUCROSS): a European phase II clinical trial. J Thorac Oncol. 2019;14(7):1266–1276. doi: 10.1016/j.jtho.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger D.S., Wood D.E., Aisner D.L., et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–266. doi: 10.6004/jnccn.2021.0013. [DOI] [PubMed] [Google Scholar]
- 10.Gainor J.F., Tseng D., Yoda S., et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00063. PO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoach C.E., Le A.T., Gowan K., et al. Resistance mechanisms to targeted therapies in ROS1+ and ALK+ non-small cell lung cancer. Clin Cancer Res. 2018;24(14):3334–3347. doi: 10.1158/1078-0432.CCR-17-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou H.Y., Li Q., Engstrom L.D., et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci U S A. 2015;112(11):3493–3498. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw A.T., Solomon B.J., Chiari R., et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019;20(12):1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhu V.W., Lin Y.-T., Kim D.-W., et al. An international real-world analysis of the efficacy and safety of lorlatinib through early or expanded access programs in patients with tyrosine kinase inhibitor-refractory ALK-positive or ROS1-positive NSCLC. J Thorac Oncol. 2020;15(9):1484–1496. doi: 10.1016/j.jtho.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Frost N., Christopoulos P., Kauffmann-Guerrero D., et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: results from the German early access program. Ther Adv Med Oncol. 2021;13 doi: 10.1177/1758835920980558. 1758835920980558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochmair M.J., Fabikan H., Illini O., et al. Later-line treatment with lorlatinib in ALK- and ROS1-rearrangement-positive NSCLC: a retrospective, multicenter analysis. Pharmaceuticals (Basel) 2020;13(11):371. doi: 10.3390/ph13110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai K., Ono H., Matsuura M., et al. Progressive renal insufficiency related to ALK inhibitor, alectinib. Oxf Med Case Reports. 2018;2018(4):omy009. doi: 10.1093/omcr/omy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran P., Morcus R., Tahir M., Onukogu I., Spinowitz B., Wang J.C. Alectinib (Alecensa)-induced reversible grade IV nephrotoxicity: a case report and review of the literature. J Med Case Rep. 2018;12(1):303. doi: 10.1186/s13256-018-1849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijer-Schaap L., van Putten J.W.G., Janssen W.M.T. Effects of crizotinib on creatinine clearance and renal hemodynamics. Lung Cancer. 2018;122:192–194. doi: 10.1016/j.lungcan.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.-S., Wanchoo R., Seetharamu N. Lorlatinib induced proteinuria: a case report. J Oncol Pharm Pract. 2021;27(4):1037–1039. doi: 10.1177/1078155220961549. [DOI] [PubMed] [Google Scholar]
- 21.Shaw A.T., Bauer T.M., de Marinis F., et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 22.Mazières J., Zalcman G., Crinò L., et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 23.Park S., Ahn B.-C., Lim S.W., et al. Characteristics and outcome of ROS1-positive non-small cell lung cancer patients in routine clinical practice. J Thorac Oncol. 2018;13(9):1373–1382. doi: 10.1016/j.jtho.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Lin J.J., Choudhury N.J., Yoda S., et al. Spectrum of mechanisms of resistance to crizotinib and lorlatinib in ROS1 fusion-positive lung cancer. Clin Cancer Res. 2021;27:2899–2909. doi: 10.1158/1078-0432.CCR-21-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.