Abstract
Aim
To detect the type and frequency of oral lesions and clinical conditions suggestive of saliva alterations in COVID‐19 patients in an intensive care unit (ICU), as well as to describe the patient´s management in each case
Methods
Information about oral conditions and mechanical ventilation was collected from oral medicine records of COVID‐19 patients in an ICU (n = 519)
Results
From the total collected, 472 patients (90.9%) were examined by the oral medicine staff. In 242/472 patients (51.3%), alterations in the oral cavity were noted. The most frequent changes were mechanical trauma (18.1%, derived mainly from intubation), vascular/coagulation disturbances (24.1%, petechiae, bruises, varicoses, and oral bleeding), and saliva alterations (24.4%, dry mouth, and sialorrhea). Infectious lesions were mentioned in the oral medicine records (16.9%), most associated with a viral infection (15.7%), mainly herpesvirus. Improved oral change protocols included oral hygiene, use of specific medications, and laser therapy
Conclusion
COVID‐19 patients in the ICU often showed dryness in the oral and mucosa oral lesions related to vascular/coagulation disturbances, and mechanical trauma derived from orotracheal tube. An oral medicine staff must be aligned with the ICU multidisciplinary team to manage COVID‐19 patients, as well as to establish diagnoses and oral cavity treatments.
Keywords: COVID‐19, intensive care unit, oral lesions, oral medicine, salivary flow changes
1. INTRODUCTION
The novel coronavirus disease, described in 2019 (COVID‐19), has been characterized as a high‐transmission infection, which can assume different clinical severity. COVID‐19 has the potential to induce high morbidity and long‐term hospitalization in intensive care units (ICUs). Particular signs and symptoms are associated with severe COVID‐19, mainly those involving epithelial cell injury and intense systemic inflammation. 1 Vascular disorders and immune system dysregulation are varieties of COVID‐19 that may be associated with alterations of tissues and organs, including the oral mucosa.
Some clinical reports described the presence of lesions in the oral mucosa, mainly in patients with severe COVID‐19. 2 , 3 , 4 However, these descriptions were restricted to very few ICU patients, and the diagnoses and treatment protocols were poorly addressed.
The aim of this retrospective study was to detect the type and frequency of oral lesions and clinical conditions suggestive of saliva alterations in COVID‐19 patients in an ICU, as well as to describe the patient´s management in each case.
2. MATERIALS AND METHODS
The care of the patients throughout the study period was conducted in accordance with the principles of the Helsinki Declaration of 1975, as revised in 2000. The methodology followed the STROBE guidelines for retrospective cohort studies.
2.1. Study design and settings
This study is a retrospective cohort study involving the collection of oral medicine records of patients enrolled in a COVID‐19 ICU. The study was conducted in the Hospital Israelita Albert Einstein (HIAE), Sao Paulo, Brazil. The period of record collection was from May 2020 to February 2021. The follow‐up reported in the oral medicine records started on the first day of the oral cavity examination to the ICU discharge.
2.2. Eligibility of oral medicine records
Consecutive oral medicine records from patients diagnosed with COVID‐19 in the ICU from May 2020 to February 2021, were selected with the following inclusion criteria: both sexes, age > 18 years, and adequate information of oral cavity conditions during ICU hospitalization. Exclusion criteria were oral medicine records without adequate information about sex, age, presence and type of mechanical ventilation, and oral conditions or information about. All the patients or their legal representatives were invited to sign a written informed consent, authorizing the publication of clinical data.
2.3. Data collection and variables
Data about clinical aspects of oral mucosa, presence and type of lesions, presence of dryness in the oral mucosa, tongue coating, drooling, dental health conditions, including dental mobility, and presence of infectious foci were recorded. The oral conditions were grouped in accordance with the possible etiology: a) nonodontogenic infections–when the clinical aspect of the lesions was suggestive of fungal, viral, or bacterial origin with or without diagnostic exam but with a topical or systemic therapeutic test with antifungal, antiviral, or antibiotics confirming the diagnosis hypothesis; b) vascular/coagulation disturbances–presence of varicoses or description of spontaneous bleeding and petechias/bruises associated with the use of anticoagulants; c) dental‐associated lesions–any change, such dental abscess, dental fracture, periodontal disease among others that are associated with the teeth; d) clinical conditions suggestive of saliva alterations and presence of sputum–saliva alterations were considered when dryness in the lips and oral mucosa or drooling (sialorrhea) were reported. Tongue coating was included in this group because was frequently associated to salivary flow reduction, and sputum present in the oral cavity was also collected; d) mechanical trauma—inflammatory conditions derived from the intubation process; e) unspecific lesions—idiopathic lesions presented in the oral mucosa without a possible etiology/diagnosis hypothesis.
Sex, age, presence and type of mechanical ventilation, and diagnosis and treatment protocol for oral conditions were also collected.
2.4. Bias
The oral cavity examination was performed and reported in the medical records by the same group of dentists, who were previously calibrated for diagnosis and treatment of oral changes in patients who were in the COVID‐19 ICU. If discrepancies were detected during the collection of the data, the decision about the correct information was confirmed directly with the dentist responsible for the annotation. The classification of the oral changes was performed by the same researcher, which minimized possible bias related to frequency of oral lesions in each group.
2.5. Study size
The study size was based on Johnston et al., who calculated the probability and the expected precision of a categorical outcome in accordance with the sample size. Based on these calculations, in a sample size of n = 500, assuming a 50% probability of occurrence of an oral change in COVID‐19 ICU patients, it would be expected that at least 250 patients would present some oral alterations. 5 As the objective of the current study was to describe the frequency of oral lesions and clinical conditions suggestive of saliva alterations in patients with severe COVID‐19, the analysis of 250 patients would allow a robust description. Therefore, the target sample size of the current study was 500 medical records.
2.6. Statistical analysis
The results are shown in absolute and relative (%) frequencies. Comparisons between patients with or without oral changes were performed by means of the χ 2 test, corrected by Bonferroni´s test. Significance level was set at 5%.
3. RESULTS
3.1. Patient´s general characteristics
From May 2020 to February 2021, HIAE COVID‐19 ICU registered hospitalization of 519 patients. Information about oral conditions was collected from 472/519 records (90.94%). Forty seven patients were not included because they had a short ICU stay (less than 2 days), or did not need dental assistance so, there were no oral medicine records. At oral examination, the majority of patients were intubated (424/472, 89.8%); few patients received tracheostomy (24/472, 5.1%) or nasal catheter (18/472, 3.8%) (Table 1). There were more male patients (322/472, 68.2%) than females (150/472, 31.8%). Ages ranged from 51 to 60, 61 to 70, and 71 to 80 (Figure 1A). The median time of ICU hospitalization was 11 days (1–104 days range).
TABLE 1.
Frequency of sex and mechanical ventilation in COVID‐19 patients enrolled in intensive care unit showing or not oral changes
| Without oral changes | With oral changes | Total | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p valuea | |
| Sex | |||||||
| Male | 166 | 72.2 | 156 | 64.5 | 322 | 68.2 | .478 |
| Female | 64 | 27.8 | 86 | 35.5 | 150 | 31.8 | .229 |
| Total | 230 | 100.0 | 242 | 100.0 | 472 | 100.0 | |
| Ventilation | |||||||
| Orotracheal intubation | 228 | 99.1 | 196 | 81.0 | 424 | 89.8 | .149 |
| Tracheostomy | 2 | 0.9 | 22 | 9.1 | 24 | 5.1 | .003 |
| Nasal catheter | 0 | 0.0 | 18 | 7.4 | 18 | 3.8 | <.001 |
| Environment ventilation | 0 | 0.0 | 6 | 2.5 | 6 | 1.3 | .03 |
| Total | 230 | 100.0 | 242 | 100.0 | 472 | 100.0 | |
p value for χ2 test.
FIGURE 1.
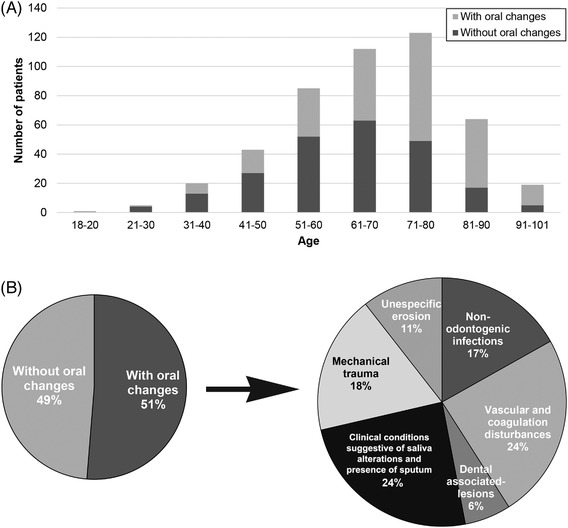
(A) Age frequency in patients with or without oral changes in a COVID‐19 intensive care unit. (B) Type and frequency of oral changes detected in COVID‐19 patients
Oral changes were detected in 242/472 patients (51.3%) (Figure 1B). In this group, frequency of patients from 71 to 80‐, 81 to 90‐, and 91 to 102‐years‐old was higher than the group without oral changes, who had significant differences in the 81–90 range (p = .001) (Figure 1A). Total occurrences in the oral cavity were 332, with 77 patients (77/242, 31.8%) showing two or three changes (Table 2).
TABLE 2.
Frequency of oral changes in the COVID‐19 patients (n = 242) enrolled in intensive care unit
| Occurrences (n = 332) | N | % |
|---|---|---|
| Saliva alterations and presence of sputum | 81 | 24.4 |
| Dryness ‐ oral mucosa | 33 | 9.9 |
| Dryness ‐ lips | 20 | 6.0 |
| Tongue coating | 10 | 3.0 |
| Sialorhea | 11 | 3.3 |
| Sputum | 7 | 2.1 |
| Vascular/coagulation disturbances | 80 | 24.1 |
| Petechia/Hematoma | 35 | 10.5 |
| Oral bleeding | 25 | 7.5 |
| Varicose | 8 | 2.4 |
| Edema not associated to mechanical trauma | 12 | 3.6 |
| Mechanical trauma | 60 | 18.1 |
| Non‐odontogenic infection | 56 | 16.9 |
| Suggestive for candidiasis | 18 | 5.4 |
| Suggestive for viral infection | 30 | 9.0 |
| Confirmed viral infection | 8 | 2.4 |
| Unespecific lesion | 35 | 10.5 |
| Dental associated‐lesions | 20 | 6.0 |
| Frequency of patients with concomitant oral alterations | N | % |
| Only one alteration | 165 | 68.2 |
| Two alterations | 69 | 28.5 |
| Three alterations | 8 | 3.3 |
| Total | 242 | 100.0 |
3.2. Frequency of oral lesions and saliva alterations
The two most frequent oral lesions were derived from mechanical trauma (60/332, or 18.1%), or vascular/coagulation disturbances (80/332, or 24.1%). Saliva alterations were found in 81/332 (24.4%) (Table 2 and Figure 1B).
Mechanical trauma was mainly comprised of ulcerations, edema, and hematomas in the upper lips, caused by orotracheal tube friction and compression (Figure 2A). Vascular/coagulation disturbances included petechiae/bruises, oral bleeding, varicoses, and edema not associated with trauma (Table 2). Petechiae and bruises were frequently found on the lateral border of the tongue, in the soft palate (Figure 2B), and the buccal mucosa. All the spontaneous oral bleeding occurrences were seen in patients on high doses of anticoagulants. Varicoses were found predominantly in the tongue's ventral surface and lateral border (Figure 2C), suggestive of microthrombi formation in the microvessels of the oral mucosa. Some cases of edema, largely those on the tongue, were linked to medications and vascular disturbances from COVID‐19.
FIGURE 2.
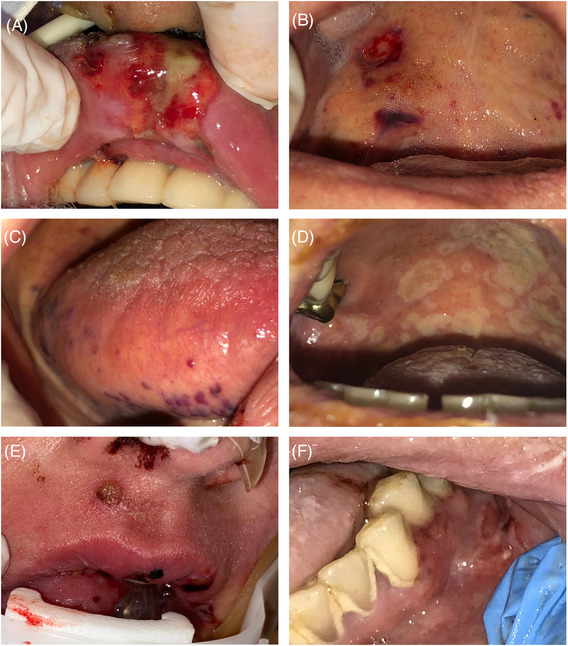
Clinical aspects of oral lesions in patients in a COVID‐19 intensive care unit. (A) Traumatic ulceration derived from the endotracheal tube friction in the upper lip mucosa. (B) Petechias and bruises in the soft palate. (C) Varicoses in the lateral border of the tongue, suggestive of microthrombi formation. (D) Multiple ulcerations in the hard and soft palate suggestive of viral infection. (E) Ulcerations in skin and lip vermillion and mucosa, suggestive of herpetic lesion. (F) Unspecific erosion with irregular borders in attached gingiva without a clear etiological factor [Colour figure can be viewed at wileyonlinelibrary.com]
Saliva alterations were related to clinical aspects of dryness in the lip vermilion and the oral mucosa, and excessive salivary flow (drooling) (Table 2), largely observed in patients with tracheostomy. Tongue coating was associated with salivary disturbances and COVID‐19 systemic conditions and medications. Sputum was observed in the oral cavity and oropharynx, sometimes resulting in difficult crusts to remove.
Nonodontogenic infections included fungal (suggestive of candidiasis) and viral infections (Table 2 and Figures 2D / 2E). With 30 cases suggestive of viral infection, eleven were confirmed by PCR analyses. The most common virus types were Herpes simplex types 1 and 2, human herpesvirus seven, and Epstein–Barr vírus. In samples of oral lesions collected from three cases, SARS‐CoV‐2 was associated with herpesviruses.
Unspecific lesions included erosion observed in the attached gingiva (Figure 2F). In some localized regions, the gingiva was atrophic and covered by a fibrinoid secretion; at times, red points in the erosive region were observed, suggestive of granulation tissue formation. The etiologic factor of lesions has been clinically unclear.
Dental associated‐lesions were less frequent (Table 2). These mainly included dental abscess, caries foci, dental fractures, periodontal disease with acute clinical signs, and dental mobility from periodontal disease.
3.3. Patient management
Table 3 shows general protocols adopted for each oral change in COVID‐19 patients. All patients, with or without oral changes, underwent periodic oral hygiene with antimicrobial solutions performed by dentists and nurses, focused on the prevention of mechanical ventilator‐associated pneumonia. The treatment protocols listed in the Table 3 were retrieved from the oral medicine records, but its effectiveness was not confirmed in the current study due to the lack of information about the treatment follow‐up.
TABLE 3.
General protocols for management of COVID‐19 patients enrolled in intensive care unit and with oral lesions and other manifestations
| Changes in the oral cavity | Management |
|---|---|
| Ulceration associated to mechanical trauma | |
| Petechia/hematoma |
|
| Oral bleeding |
|
| Varicose |
|
| Non‐traumatic edema |
|
| Dryness – lip vermillion |
|
| Dryness – oral mucosa |
|
| Tongue coating |
|
| Sialorrhea |
|
| Sputum removal |
|
| Oral candidiasis |
|
| Suggestive of viral infection |
|
| Confirmed viral infection |
|
| Unspecific erosion | |
| Dental associated‐lesions |
|
Oral hygiene was performed with topical lavage only with 0.12% chlorhexidine solution or with this solution associated with 1% hydrogen peroxide. The efficacy of hydrogen peroxide as an adjuvant antimicrobial agent for reducing oral contamination with bacteria and SARS‐CoV‐2 was been tested in a clinical trial research during the analyzed period in the current study.
Photobiomodulation for analgesia was performed with diode laser, 100 mW, 0.09 cm2 spot area, 660 nm and 808 nm simultaneously, 10 s, 1 J for each wavelength, 11.1 J/cm2 for each wavelength, with a total of 22.2 J/cm2 at each irradiation point. Photobiomodulation for tissue repair was done with diode laser, 100 mW, 0.09 cm2 spot area, 660 nm, 10 s, 1 J for each wavelength, 11.1 J/cm2 at each irradiation point.
For ulcerations derived from mechanical trauma, the protocol included oral hygiene improvement, frequent position changes of the endotracheal tube, installation of tooth protector devices when appropriate, and photobiomodulation with low‐level laser therapy as adjuvant therapy for analgesia and tissue repair.
For situations of bleeding disturbances (petechiae / hematoma or oral bleeding) management was restricted to daily monitoring of the oral cavity, and alerting the medical staff about the condition. Oral bleeding demanded specific hemorrhage control by the dentist. Varicoses were reported to the medical staff as a clinical sign of a hypercoagulatory condition.
With dryness in the lip vermillion, topical application with vitamin E was indicated; for dryness in the oral mucosa, artificial saliva or enzymatic oral solution were prescribed. Tongue coating and sputum were controlled with improved oral hygiene; participation of physiotherapists for sputum removal was frequently cited in the records. Drooling was treated with propantheline gel.
For herpesvirus infection, systemic valacyclovir or acyclovir were prescribed. Therapeutic tests with these antiviral agents were done for patients who did not undergo the PCR or serological analyses.
Patient´s management with unspecific erosion in the oral cavity included daily monitoring of the lesions, and photobiomodulation as an adjuvant therapy for tissue repair. With this management, partial remission was frequently observed; however, in some cases, erosions evolved into ulcerations suggestive of viral infection. For these situations, a therapeutic test for viral infection, previously described, was then adopted. For dental‐associated lesions, management included removal of infectious foci, dental contention, or dental avulsion.
4. DISCUSSION
In this retrospective study, we found an expressive frequency of oral lesions and clinical conditions suggestive of saliva alterations in patients enrolled in an COVID‐19 ICU. This study is the first time that a large series of COVID‐19 cases, showing these situations, has been reported. Another study showed oral lesions in symptomatic patients, 4 but few involved ICU patients. Similar to our results, a high frequency of traumatic and infectious ulcerations, and lesions associated with coagulation disturbances were found in the cited study.
Most lesions in the current investigation were associated with the intubation process and drug‐related conditions, related to anticoagulant prescriptions and corticosteroid‐induced immunosuppression. The intubation process was responsible for all traumatic injuries. Besides topical application of antimicrobial and laser therapy for wound repair using previous validated protocols, 6 treatment for these injuries also included frequent tube repositioning to prevent further ulcerations. A multidisciplinary approach involving dentists, physiotherapists, and nursing staff was important for lesion repair and QoL maintenance.
The use of anticoagulants was associated with spontaneous bleeding, which was controlled by topical application of hemostatic agents. Petechiae and bruises were associated with anticoagulant drugs, and a possible SARS‐CoV‐2 role in the microvasculature of the oral mucosa. Varicoses were also noted in the oral mucosa, suggestive of microthrombi formation, in patients with a high risk of thrombosis. A previous case series demonstrated the dynamics of microthrombi formation in the tongue of intubated COVID‐19 patients; the authors related this condition to the systemic inflammation of COVID‐19. 7
Oral lesions with suspicious viral infections were found with relative high frequency in the current study. These lesions required careful diagnosis and prompt treatment with systemic antivirals. Photodynamic therapy was applied as an adjuvant treatment. 8 , 9 There is a hypothesis in the literature of a possible interaction with SARS‐CoV‐2 and herpesviruses, predisposing the replication of latent herpesvirus. 10 , 11 In addition, the lymphocytopenia induced by SARS‐CoV‐2 has been associated with herpetic lesions. 11 High dose corticosteroids have been related to viral infection in COVID‐19 patients. 12 Rapid diagnosis and treatment of viral infection in the oral cavity is important for COVID‐19 patients. The herpesvirus reactivation can predispose to viral pneumonia in patients on mechanical ventilation, increasing morbidity from COVID‐19, given the days of mechanical ventilation and the risk of sepsis. 13 A routine oral examination must be done in ICU patients with COVID‐19, focusing on the diagnosis and treatment of opportunistic oral infections.
High frequency of clinical conditions suggestive of saliva alterations were noted in oral medicine records. Dry mouth is a common alteration in ICU patients, mainly with orotracheal intubation. 14 , 15 This condition was not necessarily derived from the SARS‐CoV‐2 action, although the presence of a novel coronavirus in the salivary glands was previously reported. 16 Fluid changes observed in COVID‐19′s systemic inflammation, zinc deficiency, and drug prescriptions may be associated with salivary flow reduction. 17 , 18 Sialorrhea was in the analyzed records, primarily in patients with tracheostomy. In these cases, rapid salivary flow control is mandatory to prevent aspiration pneumonia 19 and other complications, for example, improvement in SARS‐CoV‐2 transmission risk due to the high SARS‐CoV‐2 load in the saliva. 20 In addition, hypersalivation control with noninvasive therapies, 21 such as topical application of propantheline gel, may be more adequate for COVID‐19 patients in ICU.
Protocols for diagnosis and treatment of oral alterations in COVID‐19 patients in the current study were similar to those adopted for other clinical situations in the ICU. Some specific adaptations for the COVID‐19 condition were detected, such as an association of hydrogen peroxide and chlorhexidine for tube cleanliness and oral hygiene. 22 The patients who received this association were previously selected for a clinical trial conducted in the same ICU, focused in the efficacy analysis of antimicrobials solutions for reduction of salivary SARS‐CoV‐2 load. This finding suggests that control of SARS‐CoV‐2 and other infections in the oral cavity were an important concern for ICU staff. Another protocol adaptation was the need for frequent tube repositioning due to high frequency of traumatic lesions, necessitating a multidisciplinary approach (physiotherapists, nurses, and physicians). In addition, prone positioning complicated or induced some oral changes, such as dental mobility and secretion accumulation in the oral cavity; constant oral and dental examination was needed for prone patients. It is important to mention that the effectiveness of these treatment protocols was not addressed in the current study. Therefore, these therapies must be adopted with caution and with a daily follow‐up.
The current study contains major limitations. One of them was the absence of information about the systemic conditions of the patients, as well as of drug prescriptions. Difficulties in the access of medical records did not allow a reliable information collection of these variables, which could clarify the interference of potential confounders in the clinical outcomes, mainly in the diagnosis process of oral lesions.
In conclusion, the most frequent oral lesions found in COVID‐19 patients enrolled in the ICU were associated with vascular and coagulation disturbances followed by lesions derived from mechanical trauma caused by orotracheal tube. Nonodontogenic infections showed lower frequency, and the majority was suggestive of viral infection. Dryness in the oral mucosa and lips was also frequently reported and sometimes accompanied the oral lesions. Oral medicine staff are necessary in the multidisciplinary team for the management of COVID‐19 patients to optimally establish diagnosis and treatment.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS STATEMENT
This is a retrospective study, previously approved by the Ethical Committee in Human Research of the Hospital Israelita Albert Einstein (HIAE) (Proc. # 37760820.0.0000.0071).
AUTHOR CONTRIBUTIONS
All authors gave their final approval and agreed to be accountable for all aspects of the work.
Eduardo FdP, Bezinelli LM, Gobbi MF, Bergamin LG, de Carvalho DLC, Corrêa L. Oral lesions and saliva alterations of COVID‐19 patients in an intensive care unit: a retrospective study. Spec Care Dentist. 2022;00 1–9. 10.1111/scd.12705
REFERENCES
- 1. Bhatraju PK, Morrell ED, Zelnick L, et al. Comparison of host endothelial, epithelial and inflammatory response in ICU patients with and without COVID‐19: a prospective observational cohort study. Crit Care. 2021;25(1):148. 10.1186/s13054-021-03547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. La Rosa GRM, Libra M, De Pasquale R, Ferlito S, Pedullà E. Association of viral infections with oral cavity lesions: role of SARS‐CoV‐2 infection. Front Med (Lausanne). 2021;7:571214. 10.3389/fmed.2020.571214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID‐19: a living systematic review. J Dent Res. 2021;100(2):141‐154. [DOI] [PubMed] [Google Scholar]
- 4. Favia G, Tempesta A, Barile G, et al. Covid‐19 symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the University Hospital Policlinic of Bari with a Purpose of a new classification. J Clin Med. 2021;10(4):757. 10.3390/jcm10040757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnston KM, Lakzadeh P, Donato BMK, Szabo SM. Methods of sample size calculation in descriptive retrospective burden of illness studies. BMC Med Res Methodol. 2019;19(1):9. 10.1186/s12874-018-0657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bezinelli LM, Eduardo FP, Neves VD, et al. Quality of life related to oral mucositis of patients undergoing haematopoietic stem cell transplantation and receiving specialized oral care with low‐level laser therapy: a prospective observational study. Eur J Cancer Care (Engl). 2016;25(4):668‐674. 10.1111/ecc.12344. [DOI] [PubMed] [Google Scholar]
- 7. do Espírito Santo DA, Lemos ACB, Miranda CH. In vivo demonstration of microvascular thrombosis in severe COVID‐19. J Thromb Thrombolysis. 2020;50(4):790‐794. 10.1007/s11239-020-02245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramalho KM, Cunha SR, Gonçalves F, et al. Photodynamic therapy and Acyclovir in the treatment of recurrent herpes labialis: a controlled randomized clinical trial. Photodiagnosis Photodyn Ther. 2021;33:102093. 10.1016/j.pdpdt.2020.102093. [DOI] [PubMed] [Google Scholar]
- 9. Conrado PCV, Sakita KM, Arita GS, et al. A systematic review of photodynamic therapy as an antiviral treatment: potential guidance for dealing with SARS‐CoV‐2. Photodiagnosis Photodyn Ther. 2021;34:102221. 10.1016/j.pdpdt.2021.102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ciccarese G, Parodi A, Drago F. SARS‐CoV‐2 as possible inducer of viral reactivations. Dermatol Ther. 2020;33(6):e13878. 10.1111/dth.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paolucci S, Cassaniti I, Novazzi F, et al. EBV DNA increase in COVID‐19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis. 2020;104:315‐319. 10.1016/j.ijid.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honore PM, Barreto Gutierrez L, Kugener L, et al. SARS‐CoV‐2 infection as a risk factor for herpesviridae reactivation: consider the potential influence of corticosteroid therapy. Crit Care. 2020;24(1):623. 10.1186/s13054-020-03349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Le Balc'h P, Pinceaux K, Pronier C, Seguin P, Tadié JM, Reizine F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID‐19 patients. Crit Care. 2020;24(1):530. 10.1186/s13054-020-03252-3. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dennesen P, van der Ven A, Vlasveld M, et al. Inadequate salivary flow and poor oral mucosal status in intubated intensive care unit patients. Crit Care Med. 2003;31(3):781‐786. 10.1097/01.CCM.0000053646.04085.29. [DOI] [PubMed] [Google Scholar]
- 15. Jang CS, Shin YS. Effects of combination oral care on oral health, dry mouth and salivary pH of intubated patients: a randomized controlled trial. Int J Nurs Pract. 2016;22(5):503‐511. 10.1111/ijn.12460. [DOI] [PubMed] [Google Scholar]
- 16. Huang N, Pérez P, Kato T, et al. Chertow D; NIH COVID‐19 autopsy consortium; HCA oral and craniofacial biological network, Frank K, Lee J, Boucher RC, Teichmann SA, Warner BM, Byrd KM. SARS‐CoV‐2 infection of the oral cavity and saliva. Nat Med. 2021. 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soto AP, Meyer SL. Oral implications of polypharmacy in older adults. Dent Clin North Am. 2021;65(2):323‐343. [DOI] [PubMed] [Google Scholar]
- 18. Tsuchiya H. Oral symptoms associated with COVID‐19 and their pathogenic mechanisms: a literature review. Dent J (Basel). 2021;9(3):32. 10.3390/dj9030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faria J, Harb J, Hilton A, Yacobucci D, Pizzuto M. Salivary botulinum toxin injection may reduce aspiration pneumonia in neurologically impaired children. Int J Pediatr Otorhinolaryngol. 2015;79(12):2124‐2128. 10.1016/j.ijporl.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 20. Fernandes LL, Pacheco VB, Borges L, et al. Saliva in the diagnosis of COVID‐19: a review and new research directions. J Dent Res. 2020;99(13):1435‐1443. 10.1177/0022034520960070. [DOI] [PubMed] [Google Scholar]
- 21. Paine CC 2nd, Snider JW 3rd. When saliva becomes a problem: the challenges and palliative care for patients with sialorrhea. Ann Palliat Med. 2020;9(3):1333‐1339. [DOI] [PubMed] [Google Scholar]
- 22. Carrouel F, Gonçalves LS, Conte MP, et al. Antiviral activity of reagents in mouth rinses against SARS‐CoV‐2. J Dent Res. 2021;100(2):124‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]


