Abstract
Background and Aims
Many terrestrial orchids have an obligate dependence on their mycorrhizal associations for nutrient acquisition, particularly during germination and early seedling growth. Though important in plant growth and development, phosphorus (P) nutrition studies in mixotrophic orchids have been limited to only a few orchid species and their fungal symbionts. For the first time, we demonstrate the role of a range of fungi in the acquisition and transport of inorganic P to four phylogenetically distinct green-leaved terrestrial orchid species (Diuris magnifica, Disa bracteata, Pterostylis sanguinea and Microtis media subsp. media) that naturally grow in P-impoverished soils.
Methods
Mycorrhizal P uptake and transfer to orchids was determined and visualized using agar microcosms with a diffusion barrier between P source (33P orthophosphate) and orchid seedlings, allowing extramatrical hyphae to reach the source.
Key Results
Extramatrical hyphae of the studied orchid species were effective in capturing and transporting inorganic P into the plant. Following 7 d of exposure, between 0.5 % (D. bracteata) and 47 % (D. magnifica) of the P supplied was transported to the plants (at rates between 0.001 and 0.097 fmol h−1). This experimental approach was capable of distinguishing species based on their P-foraging efficiency, and highlighted the role that fungi play in P nutrition during early seedling development.
Conclusions
Our study shows that orchids occurring naturally on P-impoverished soils can obtain significant amounts of inorganic P from their mycorrhizal partners, and significantly more uptake of P supplied than previously shown in other green-leaved orchids. These results provide support for differences in mycorrhiza-mediated P acquisition between orchid species and fungal symbionts in green-leaved orchids at the seedling stage. The plant–fungus combinations of this study also provide evidence for plant-mediated niche differentiation occurring, with ecological implications in P-limited systems.
Keywords: 33P, Ceratobasidium, mineral nutrition, mycorrhizal networks, niche partitioning, orchid, phosphorus, Tulasnella, in vitro
INTRODUCTION
The Orchidaceae, with an estimated 28 000 species, is the largest flowering plant family (Christenhusz and Byng, 2016), and it is no surprise that they attract much attention for the explosive diversity and evolution of floral forms. Equally fascinating is that the many members of the Orchidaceae are dependent upon mycorrhizal fungi for their development and nutrition, namely nitrogen, phosphorus (P) and carbon (Smith, 1966; Rasmussen, 2002). However, despite many studies of mycorrhizal specificity and ecology in orchids, there are comparatively few studies of the role of these fungi in orchid mineral nutrition, particularly in comparison with the other major types (arbuscular mycorrhiza, ectomycorrhiza and ericoid mycorrhiza). While the mycorrhizal literature is dominated by considerations of mineral nutrient acquisition, particularly P (Smith and Read, 2008), the field of orchid mineral nutrition has remained largely unexplored. This is despite the recognition that many terrestrial orchids have simplified root systems (Brundrett, 2007), and that, as a result, they likely depend upon mycorrhizal fungi for the acquisition of minerals, especially in their germination and early developmental stages.
Whereas the pioneers of orchid mycorrhiza (ORM) research (e.g. Bernard, 1904; Burgeff, 1936) placed emphasis on the roles of ORM symbionts in the facilitation of seed germination solely through the provision of carbon, those in the fields of arbuscular mycorrhiza (e.g. Mosse, 1959; Baylis, 1975), ectomycorrhiza [e.g. Frank, 1885 (translated in Frank and Trappe, 2005); Harley and McCready, 1950; Melin and Nilsson, 1953] and ericoid mycorrhiza (e.g. Pearson and Read, 1973; Read and Stribley, 1973) emphasized the importance of mycorrhizas in the acquisition of mineral nutrients, in particular P. In an early exception to this dichotomy, Smith (1966) demonstrated, using split Petri-dish systems, that the extraradical mycelium of the European terrestrial green orchid Dactylorhiza purpurella was capable of capture and transport of P from the external environment to the developing protocorms. In the ensuing ~50 years, studies of orchid nutrition took the form of correlative studies of soil nutrients as drivers of orchid distribution and growth (Dijk and Olff, 1994; Silvertown et al., 1994; Hejcman et al., 2010; Tsiftsis et al., 2012), and, more recently, how soil nutrients affect ORM diversity (Mujica et al., 2016, 2020, 2021; Vogt-Schilb et al., 2020). However, the mechanism by which orchids and their ORMs obtained P from the soil remained unexplored. Despite a large increase in the number of analyses of the involvement of the other types of mycorrhizas (ectomycorrhizas and ericoid mycorrhizas) in P nutrition of non-orchid plants (Smith and Read, 2008) there have been only two further studies of P nutrition involving ORMs (Alexander et al., 1984; Cameron et al., 2007), both of which involved fully developed terrestrial green orchids of a single species from the northern hemisphere. Alexander et al. (1984) demonstrated P uptake by green plantlets of Goodyera repens colonized by the fungus Ceratobasidium cornigerum, where uptake was eliminated in plants from which the fungus had been removed by fungicide treatment. Subsequently, Cameron et al. (2007), applying in situ whole-system autoradiography to the same adult orchid–fungus combination, confirmed and quantified P transfer from the environment to the plant by way of extramatrical mycelium of the fungus. It remains the case, therefore, that the role of mycorrhizal fungi in the P nutrition of the ecologically diverse and numerous representatives of the southern hemisphere terrestrial orchid flora, or orchids in the seedling stage, remains largely unexplored.
Beyond the mechanistic uptake of nutrients, mycorrhizal associations have the potential to broaden a plant’s niche through more effective resource acquisition, allowing them to occupy ecological niches of lower nutrient availability (e.g. N or P) that may prove limiting for species that are non-mycorrhizal (Bever et al., 2010; Tedersoo et al., 2020). Plants with obligate mycorrhizal associations, such as ORMs, have the narrowest niches compared with other mycorrhizal types, and yet this extreme specialization can be key in allowing mycorrhizal-mediated niche differentiation (Gerz et al., 2018). The observed benefits of mycorrhiza-mediated niche differentiation include the alleviation of interspecific competition through soil nutrient partitioning and foraging strategies (Tedersoo et al., 2020). When functionality is shown in ORM associations, plant (host) specificity has been observed (Warcup, 1981; Bonnardeaux et al., 2007; Barrett et al., 2010; Roche et al., 2010; Smith et al., 2010). This observed specificity is also expected to enhance coexistence at the micro-habitat scale, which in part explains the high diversity and species richness of orchids that can be observed at the local scale, despite limiting soil nutrients (McCormick and Jacquemyn, 2014, 2015; Waud et al., 2016).
We selected three sympatric but phylogenetically distinct terrestrial green-leaved orchid species (Diuris magnifica, Microtis media subsp. media and Pterostylis sanguinea) from the biodiversity hotspot of the Southwest Australia Floristic Region (SWAFR), where terrestrial orchid species diversity is amongst the highest in the world (Parsons and Hopper, 2003). We also included the South African species Disa bracteata, which is an invasive alien species that co-occurs in habitats occupied by the other three study species. Terrestrial orchids of the south-west of Western Australia are not unusual in typically forming genus-specific associations with a range of symbiotic fungal taxa (Ceratobasidium, Tulasnella and Serendipita) (Warcup, 1981; Bonnardeaux et al., 2007; Brown et al., 2013). Pterostylis sanguinea has the narrowest fungal compatibility, producing viable seedlings only on Ceratobasidium isolated from adult P. sanguinea plants (Bonnardeaux et al., 2007). Diuris magnifica has a wider fungal compatibility, being able to produce viable seedlings on Tulasnella isolated from D. magnifica, Prasophyllum giganteum and a geographic spread of D. bracteata (Bonnardeaux et al., 2007). Notable exceptions in this study include M. media subsp. media and D. bracteata, which both have much broader fungal webs and can associate with Ceratobasidium sp., Tulasnella sp. and Sebacina sp. isolated from a wide generic range of orchid host plants (Bonnardeaux et al., 2007; De Long et al., 2012), and may be advantageous in niche exploitation. Interest in the P nutrition of these particular orchids is based on the fact that the soils in which they grow are amongst the most P-impoverished soils in the world [<10 mg kg−1 of total available P (McArthur, 1991; Lambers et al., 2010)]. The inclusion of the invasive generalist D. bracteata was made to provide insights into niche differentiation among orchids that do not naturally co-occur but are able to share mycorrhizal partners. Disa bracteata in its natural range grows in soils that are considered P-depauperate in a global context, but have ~40-fold higher P concentrations than the Australian soils in which it is able to co-occur with the other species of this study [338–422 mg kg−1 of total available P in the range of D. bracteata (Witkowski and Mitchell, 1987)]. The ability of D. bracteata to successfully compete for a recruitment niche in such P-impoverished soils would suggest it has the latent ability to forage effectively for P at much lower concentrations than in its natural range. Earlier molecular work investigating fungal specificity hypothesized that orchid hosts may compete for niche or recruitment space where overlapping compatibilities for mycorrhiza occur (McCormick et al., 2004; Bonnardeaux et al., 2007). More recent studies investigating ORM in isolation from host plants and in vitro have shown that there is niche differentiation under resource-limited scenarios, with ORMs isolated from different fungal genera able to access different forms of available nutrients from each other (Nurfadilah et al., 2013).
Here we investigate the uptake of inorganic P (orthophosphate) among co-occurring orchid–fungus combinations using plants in vitro in the orchid–fungal symbiosis at the green-leaf seedling stage of development. Specifically, we hypothesize that: (1) the extramatrical mycelium of orchid seedlings is responsible for the capture and rapid transport of P to orchid seedlings; (2) capture and transport of P is dependent on the size of the sink; (3) capture, transport and uptake differ between orchid species and fungal groups involved; and (4) furthermore, we hypothesize that orchids with a higher mycorrhizal specificity will exhibit greater P uptake efficiency. We also explored evidence for niche differentiation and exploitation in a P-limited system and the likelihood of fungal or host-mediated differentiation.
MATERIALS AND METHODS
Orchid seed and fungal material
Seed and fungal material of the orchids Diuris magnifica D.L. Jones, Pterostylis sanguinea D.L. Jones & M.A. Clem., Disa bracteata Sw. and Microtis media R.Br. subsp. media were collected from plants located in bushland on the Swan Coastal Plain, Western Australia. Seed collection occurred from September to November from naturally pollinated plants. Capsules were collected upon maturation from four adult plants of each species.
Fungal symbionts from four adult plants of each species were isolated onto fungal isolation medium with the addition of streptomycin (FIM + strep) following the procedure of Davis et al. (2015). Single growing hyphal tips emerging from pelotons were subcultured aseptically onto 6.8 g L−1 potato dextrose agar (Sigma; 6.8 g of potato dextrose agar, 6 g of agar, 1 L of reverse osmosis water; pH adjusted to 6.8 before autoclaving at 121 °C for 20 min) and hyphae were allowed to grow in thick culture across the dish.
DNA extractions were undertaken from isolates that produced viable seedlings, using the method detailed in Phillips et al. (2011). Molecular identification was then confirmed from these cultures using internal transcribed spacer (ITS) sequencing [using universal fungal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3) (White et al., 1990)], following the method of Phillips et al. (2011). PCR products were purified using the Agencourt AMPure PCR Purification System by Beckman Coulter (Brea, CA, USA). Sequencing was completed by Macrogen (Seoul, South Korea). Since this study was conducted, an enhanced primer combination and PCR protocol has proven effective for core Tulasnellaceae (Vogt-Schilb et al., 2020).
BLAST searches were conducted to establish the closest relatives (those sequences that had the highest percentage sequence identity) represented in the GenBank database.
Orchid seedlings were produced using standard symbiotic germination protocols for terrestrial orchids; no P was added to media. Seeds were sterilized in mesh bags (90-µm filter mesh) in 1 % (w/v) calcium hypochlorite for 15 min before rinsing in three successive washes of sterile deionized water. The mesh bags were opened in sterile conditions and ~100 seeds were transferred to each oatmeal agar plate (2.5 g of ground rolled oats and 8 g of agar per litre of reverse osmosis water, adjusted to pH 5.5). The agar was sterilized by autoclaving for 20 min at 121 °C and inoculated with fungal strains sourced from potato dextrose agar plates, following the protocol outlined in Warcup (1981), Ramsay et al. (1986) and Bonnardeaux et al. (2007). Seeds were inoculated with fungi isolated from adult plants of the same species. Cross-compatibility between species did not form part of this study. Plates were incubated in the dark at 18 °C for 6 weeks, by which time rhizoid formation was evident [equivalent of Stage 3 protocorms (following Batty et al., 2001)]. Plates were then exposed to artificial light for a 12-h photoperiod at 18 °C for a further 6 weeks, by which time seedlings had developed green leaves (equivalent of Stage 5 seedlings).
Microcosms
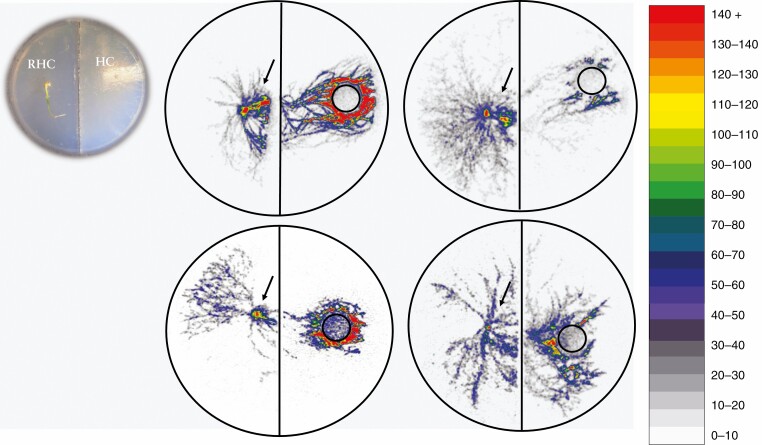
Seedlings of each species were placed in 90-mm two-compartment Petri dish (Sterilin) microcosms of the type employed by Cameron et al. (2007). The plants were placed on one side of the dish [root–hyphal compartment (RHC)] and incubated for a period of 2 weeks to enable the hyphae to cross the diffusion-proof barrier to the hyphal compartment [hyphal compartment (HC)]; both compartments contained 1.2 % (w/v) agar (water agar) (n = 5 microcosms per species) (see inset of Fig. 1). We used two-compartment Petri dishes that were made of polystyrene with a sealed, moulded wall providing two compartments. This system has been tested extensively by Cameron et al. (2006, 2007) for the risk of diffusion from the HC to the RHC compartment. No such diffusion was recorded by Cameron et al. (2006, 2007) or observed in this current study. A 0.8-cm diameter core of agar was removed from the HC of the microcosm and replaced with a core of agar containing 0.005 MBq of [33P]orthophosphoric acid in 0.85 mL, equating to 0.0011 ng of P, in molten 1.2 % water agar.
Fig. 1.
Digital autoradiographs of microcosms containing green-leaf orchid seedlings (left), 7 d after labelling the mycelial compartment (right) with 33P. Orchid species clockwise from top left are Pterostylis sanguinea, Diuris magnifica, Disa bracteata and Microtis media subsp. media. Inset image at top left shows microcosm with plant in RHC (on left) and hyphal network extending across the diffusion barrier to the HC (on right); the plug was removed for isotope source after the photograph was taken, and the species shown is D. magnifica. Position of orchid seedling is denoted on the left (arrow) and position of isotope plug is denoted on the right of each autoradiograph image (circle). The colour scale represents the number of counts of radioactivity detected in 1 h per 0.25-mm2 pixel.
Harvest and analysis
Orchid seedlings were harvested after 7 d of incubation (n = 5 per species). The agar in the RHC and HC was removed intact and imaged using digital autoradiography to determine the distribution of 33P throughout the hyphal network (Packard Instant Imager, Isotech, Chesterfield, UK).
The orchid seedlings were harvested intact and analysed as entire plants as these species do not produce roots of sufficient sample sizes at the seedling stage to examine intraradical hyphal uptake separately. Entire seedlings were oven-dried at 80 °C for 48 h, weighed and digested (modified from Bowman, 1988) in 1 mL of concentrated sulphuric acid and incubated at room temperature for ~3 h. Samples were then heated to 350 °C in a heating block for up to 15 min before being removed, cooled for several minutes prior to the addition of 0.2 mL of 30 % hydrogen peroxide and returned to the heating block to achieve oxidation to a colourless solution. After cooling for ~3 h, digests were made up to 10 mL with distilled water, and a 2-mL aliquot was mixed with 10 mL of emulsifying scintillant (Ecosafe, Fisher Biosciences, UK) for liquid scintillation counting of 33P (Packard Tri-Carb, 3100TR, Isotech). The 33P content of samples was corrected for background and isotope decay.
Non-radioactive P determinations were made using the molybdenum blue method (Murphy and Riley, 1962) (n = 5 per species).
Root and hyphal measurements
Hyphal length and absorptive surface area measurements were obtained using a modification of the line intersect method (Tennant, 1975). A 0.8-cm core of agar within the hyphal network was removed from the HC side of hyphal measurement plates (replicate two-compartment microcosms under the same incubation conditions, without 33P added) and macerated in an Eppendorf tube, ~200 µL of water was added and the Eppendorf content was melted to liquid in a beaker of hot water (~90 °C) (n = 5 hyphal measurement plates per species). Approximately 2 drops of 0.05 % w/v Trypan Blue in lactoglycerol were added to the solution and incubated for 15 min at ~90 °C. The solution was passed through a 45-µm filter paper using a vacuum pump. Ten random fields of view (at ×10 magnification) were viewed through a Whipple disc per cube of agar, with a total of five agar samples per fungal strain. Intercepts of hyphae with the grid of the Whipple disc were recorded for each fungal cube replicate and hyphal lengths were calculated as per Tennant (1975). Twenty-five random measurements were also taken at ×400 magnification to obtain hyphal diameters. Absorptive surface areas of the ORM were calculated using the equation for surface area of an open-ended cylinder (2πrh, where r is hyphal radius and h is hyphal length).
Seedlings from the hyphal measurement plates were harvested, and the root portion of the plants was transferred into a water-filled acrylic tray and scanned. Total root surface area was determined using WinRhizo (Regent Instruments, Quebec Canada) (n = 5 per species).
All data were analysed using ANOVA in SPSS (v21, IBM). The homogeneity of variance in the data was checked using Levene’s test. Tukey’s LSD (5 %) was used to further verify differences where significant differences were indicated between groups. Pearson correlation was used to compare surface area and biomass with P uptake (n = 5 per species).
RESULTS
Fungal isolates from D. bracteata were identified as Tulasnella sp. (uncultured fungus), isolates from D. magnifica and M. media subsp. media were identified as Tulasnella calospora, and all P. sanguinea isolates were identified as Ceratobasidium sp. (Table 1). There was no discernable diversity of fungal species within a host plant species, with identical sequences being returned within a host plant species.
Table 1.
Plant dry weight, plant P content, mean hyphal surface area and corresponding orchid host root surface area (cm2). Different letters denote significant difference (n = 50 per orchid host species for hyphal area), (n = 5 per orchid species for plant dry weight, P content and below-ground plant area). Orchid species column shows GenBank accession number in brackets for fungal isolates from the current study. Fungal identity column shows GenBank accession number in brackets chosen as the accession with the highest identities (%).
| Orchid species | Fungal identity | Plant dry weight (mg per plant)* | P content (mg/g DW−1)† | Mean hyphal surface area (cm2)‡ | Mean below-ground plant surface area (cm2)¶ |
|---|---|---|---|---|---|
| Disa bracteata (KT601562) | Tulasnella sp. (KM211336.1) | 0.00045 ± 0.00005 | 0.16 ± 0.03a | 0.89 ± 0.05a | 0.05 ± 0.004b |
| Diuris magnifica (KT601561) | Tulasnella calospora (DQ388045.1) | 0.0005 ± 0.0001 | 0.40 ± 0.08b | 0.80 ± 0.03a | 0.05 ± 0.007b |
| Microtis media subsp. media (KT601563) | Tulasnella calospora (DQ388045.1) | 0.00069 ± 0.0001 | 1.03 ± 0.31b | 0.75 ± 0.02a | 0.20 ± 0.023a |
| Pterostylis sanguinea (KT601560) | Ceratobasidium sp. (GQ405561.1) | 0.00092 ± 0.0003 | 0.48 ± 0.10b | 2.94 ± 0.12b | 0.05 ± 0.005b |
*ANOVA: d.f. = 17; F = 0.82; P > 0.05.
†ANOVA: d.f. = 17; F = 4.64; P < 0.05.
‡ANOVA: d.f. = 199; F = 17.56; P < 0.05.
¶ANOVA: d.f. = 17; F = 1.02; P < 0.05.
Autoradiographs of all orchid species showed movement of P from the source in the HC to the plant in the RHC in the course of 7 d (Fig. 1). Phosphorus was transferred from the source, across the diffusion barrier via hyphal networks growing out of the orchid. The isotope was distributed both into the orchid and throughout the hyphal network, with highest counts visualized in the orchid tissue and in the growing ends of the hyphal fan (Fig. 1).
The amount of 33P recovered in the plants differed among species (Table 2). However, all species showed some degree of transfer of 33P from source to plant, confirming that P had moved via the fungal hyphae across the diffusion barrier. The amount of P recovered as a percentage of P supplied in D. magnifica was more than twice that recovered in P. sanguinea (although not significant, P > 0.05), and significantly more for M. media subsp. media (a 10-fold increase) and almost 100 times greater than that recovered in D. bracteata (100-fold increase) (P < 0.01) (Table 2). Diuris magnifica also had a significantly faster rate of transfer of 33P over the course of the experiment (0.097 fmol h−1; P < 0.01) (Table 2).
Table 2.
Mean rate of fungal-mediated transfer of 33P to the plant averaged over the 7-d incubation period (fmol h−1), and the percentage of 33P supplied assimilated by the fungus and transferred to the plant. Means are provided ± standard error; different letters denote significant differences.
| Orchid species | Mean transfer rate (fmol h−1)* | 33P assimilated (% of that supplied)† |
|---|---|---|
| Disa bracteata | 0.001 ± 0.00a | 0.51 ± 0.20a |
| Diuris magnifica | 0.097 ± 0.02b | 46.69 ± 13.46b |
| Microtis media subsp. media | 0.010 ± 0.00a | 5.19 ± 2.12a |
| Pterostylis sanguinea | 0.046 ± 0.02ab | 22.38 ± 10.54ab |
*ANOVA: d.f = 17; F = 5.23; P < 0.01.
†ANOVA: d.f = 17; F = 4.64; P < 0.01.
Of the 33P transferred from the source, 47 % was recovered in D. magnifica after the 7-d incubation period (Table 2). The percentage of P uptake was significantly higher in D. magnifica than in the other species (P < 0.01), representing a P uptake more than double that of any other species (Table 2).
The absorptive area of the orchid seedling roots did not differ among species (Table 1, P > 0.05); however, P. sanguinea had a significantly greater hyphal surface area (2.94 ± 0.12 cm2) than the other species as a result of a relationship with Ceratobasidium sp., which typically produce densely growing cultures, representing an absorptive area more than three times larger than that of the other species. However, the differences in absorptive surface area were not correlated with differences in P uptake among species (Pearson’s r = 0.21, P > 0.05) (Table 1). Orchid seedling biomass was not correlated with P uptake, negating a sink-size relationship in this study (biomass data not shown, Pearson’s r = 0.83, P > 0.05).
Discussion
This is the first study to quantify net P movement from source to developing orchid seedling in a phylogenetically diverse range of green-leaved terrestrial orchid species and diverse mycorrhizal partners. Here we conclusively demonstrate uptake of 33P by a range of ORMs and transfer along the mycelium to the developing green-leaved orchid seedlings in the order of 0.51–47 % of the amount of P supplied (Table 2). Pioneering work on European orchid species (e.g. Smith, 1966, 1967; Purves and Hadley, 1975; Hadley, 1984) has established the unidirectional movement of both carbon and P towards developing orchid seedlings via Rhizoctonia-type mycelial networks. However, only Smith (1966) demonstrated P uptake via the external mycelium of a single species of heterotrophic protocorms. Smith (1966) showed this to be a dynamic transfer, with radioactivity detected in the protocorm <72 h following the application of 33P to the established mycelium. A study by Cameron et al. (2007) on wild harvested adults of a single orchid species and its associated mycorrhizal partner (G. repens and C. cornigerum, respectively) provides the only comparable study to date on P uptake (1.1 % in plant material) in green-leaved terrestrial orchids. The majority of species in the current study showed a much greater uptake of P supplied to root and shoot (up to 47 times greater in D. magnifica; Table 2), demonstrating high variability of foraging capability between orchid–fungus combinations and plant life stages.
The orchid species of this study grow in the highly weathered nutrient-impoverished soils of south-western Australia. The soils contain <10 mg kg−1 of total P (McArthur, 1991; Laliberté et al., 2012), representing some of the most P-depauperate soils in the world (Lambers et al., 2010). It has been hypothesized that terrestrial orchids develop mycelial networks as a means of overcoming their poorly developed root structures. This study quantitatively shows for the first time that a range of terrestrial orchid seedlings are able to access inorganic P sources via their ORM alone. The amount of P assimilated as a percentage of P supplied was greater in P. sanguinea (22 %), M. media (5.2 %) and D. magnifica (47 %) (Table 2) than in G. repens (1.1 %), despite a lower amount of total P being added in the current study (Cameron et al., 2007). The rate of transfer from P source to orchid seedling was also slower in the species of the present study (Table 2) than the 1.1 fmol h−1 recorded for G. repens (Cameron et al., 2007). In light of the lower provided P and naturally P-impoverished soils that the current study species are found in, the higher percentage P uptake may reflect the differing abilities of ORMs to forage, capture and utilize the small amounts of P that are available to them (Nurfadilah et al., 2013; Mujica et al., 2016), with tight coupling of P remobilization from senescing parts of these orchids enabling high levels of P conservation in planta (Pate and Dixon, 1982).
Phosphorus limitation increases plant diversity and has fuelled the proliferation of a variety of P-acquisition strategies in the SWAFR, although there has been a resulting decline in the abundance of mycorrhizal plants (Brundrett, 2009; Lambers et al., 2014; Zemunik et al., 2015, 2016). The terrestrial orchids of the SWAFR have retained their mycorrhizal lifestyle, becoming one of the few groups of plants that are obligately dependent on their fungal partners (Smith and Read, 2008), allowing niche differentiation (Nurfadilah et al., 2013). Despite P being the key limiting nutrient in the landscape (Lambers et al., 2010), this study shows that ORMs have the ability to successfully forage and transport P to their plant hosts. Both D. magnifica and M. media subsp. media utilized T. calospora as their fungal symbiont in this study, with very different abilities for P uptake. Despite T. calospora producing similar hyphal surface areas for D. magnifica and M. media subsp. media (Table 1), the former achieved significantly faster uptake (0.097 fmol h−1) and greater P uptake (46.7 % of P supplied) (Table 2) than the latter. Both species grow sympatrically and these results suggest niche differentiation may occur within an ORM species, where competitive advantage may be plant- or host-mediated.
Relative mycorrhizal specificity, inferred from previous studies, generally shows a decreasing ability for P uptake with increasing generalization of the host plant [specificity of P. sanguinea < D. magnifica < M. media subsp. media < D. bracteata (Bonnardeaux et al., 2007; De Long et al., 2012)], with the exception of D. magnifica being able to assimilate a greater proportion of the P supplied than the more specific P. sanguinea. While it was beyond the scope of this study, a suggested extension to this work where all orchid–fungus combinations are evaluated to further understand the variation in P transfer efficiency as a consequence of either fungus-mediated or host-mediated differentiation would be valuable. Indeed, extension to capture the broader ORM associations of more generalist species would also be very valuable in understanding orchid nutrition as a driver of niche partitioning. Ecological specificity has been inferred to be the driver of orchid rarity and distribution (Swarts et al., 2010); however, given the increasing evidence showing fungal sharing among the terrestrial orchid taxa (Roche et al., 2010; Reiter et al., 2020), host-mediated niche differentiation may be playing an as yet unknown role in orchid distribution in a resource-limited system, like the SWAFR.
The orchids of this study have poorly developed root structures and so a reduced absorptive area to take up nutrients without their fungal partners. Coupled with the nutrient-poor soils in which they are naturally found, obtaining sufficient nutrition presents a significant challenge. In these orchid species, the increase in absorptive surface area afforded by the ORM is between 3 and 60 times that of the root alone (Table 1). Hyphal length data for the fungi associated with these orchid species ranges between 360 and 1270 m m−1 (metre hyphal length per metre of root, data not shown), in comparison with hyphal lengths of ectomycorrhizas of Pinus sp. of 500–8000 m m−1 (Read and Boyd, 1986; Rousseau et al., 1994) and Salix viminalis of 289–308 m m−1 (Jones et al., 1991), arbuscular mycorrhizas of 3–3000 m m−1 and ericoid mycorrhizas of 30–8000 m m−1 (Smith and Read, 2008). Lineal investment in hyphal growth as a function of root length places orchids in a similar league to that of other mycorrhizal symbiosis types and highlights the increased foraging capability through mycorrhizal symbioses. In addition to the dependence on fungal symbionts to promote germination, which itself yields an associated increase in potential surface area achieved by the expanding protocorm, there is also the remarkable increase in absorptive surface area provided by the fast-growing hyphal networks, over which nutrient exchange can occur (Tedersoo et al., 2020). Orchids therefore show a remarkable investment in their hyphal networks in return for what is argued to be the most clear-cut case of survival-related dependence observed in mycorrhizal symbioses (Smith and Read, 2008).
In contrast to the current study, a study by Nurfadilah et al. (2013) that investigated axenic nutrient acquisition (varying sources of C, N and P) in a range of ORMs from the SWAFR growing in isolation from their orchid partners, Ceratobasidium sp. had a greater investment in growth (measured as biomass) than any of the Tulasnella sp. inferred to be involved in the acquisition of C, N and P. Specifically, orthophosphate [as was supplied in the study by Nurfadilah et al. (2013) and this current study], provided a similar biomass for all ORMs, except a significantly lower biomass from Drakaea elastica (Nurfadilah et al., 2013). Drakaea elastica is now known to associate with Tulasnella secunda (Linde et al., 2017), which has not been found to associate outside Drakaea and Paracaleana. The current study shows a greater investment in T. calospora (measured as hyphal area) when in combination with D. magnifica (Table 1), and also translated into greater P uptake (Table 2) than seen while in symbiosis with M. media subsp. media. While the study by Nurfadilah et al. (2013) did not include plant partners, both Nurfadilah et al. (2013) and the current study provide evidence for niche differentiation by a range of ORMs. Thus, the current study shows evidence of plant-mediated niche differentiation in P-foraging abilities and the need to consider the orchid–fungus symbiosis as a whole when making ecological interpretations of laboratory-based studies.
While D. magnifica and T. calospora in combination produced a larger hyphal surface area and a greater P uptake than other T. calospora–orchid combinations, larger hyphal surface areas did not necessarily take up more 33P. No correlation was found between hyphal surface area and amount of P uptake (Pearson’s r = 0.21, P > 0.05) as shown for D. magnifica and D. bracteata, with significantly different rates and amounts of P uptake and similar hyphal surface areas (Tables 1 and 2). The size of the sink (orchid seedling biomass) was also not correlated with the amount of P uptake (Pearson’s r = 0.83, P > 0.05). This study showed no significant differences in sink size between species at the early seedling stage (P = 0.5), which is to be expected across a range of species barely beyond the protocorm life phase. The similarity in sink size at this early growth stage is likely driving the high variance and lack of correlation observed in this study. It is predicted that, as an orchid matures and tuber size and capacity for storage increase, the size of the sink may play a more active role in P uptake. It is suggested that the role of sink size be considered across a broader range of life stages and species in future nutrient uptake studies. The findings of the current study support the notion that P uptake in orchid seedlings is a rapid and active process, rather than one that depends on passive movement associated with sink strength. Nutrient uptake in heterotrophic protocorms occurs via a combination of direct cross-membrane transfer and also by lysis of pelotons (Kuga et al., 2014), although the route of uptake is yet to be demonstrated for P.
The current study suggests that some orchid–fungal symbiosis combinations may be more efficient at scavenging and transporting nutrients than others. For example, P uptake in D. magnifica is 10 times greater than that in M. media subsp. media, despite both orchids forming a symbiosis with the same fungal species, T. calospora. While the invasive D. bracteata is at times able to outcompete Australian native orchids for recruitment niches, the pairing of D. bracteata with a species of Tulasnella (Table 1) did not confer a competitive advantage that could be observed as increased P uptake efficiency, P transfer or investment in hyphal surface area (Tables 1 and 2). There are likely to be other interacting factors driving orchid recruitment and species composition, beyond P uptake abilities alone, as has been seen in other studies (McCormick and Jacquemyn, 2014: Mujica et al., 2020). While there was some evidence of higher fungal specificity conferring a greater rate of P transfer, further work across a range of taxa and life stages is required in this area. These results suggest that ORMs exhibit functional differences in contrasting plant–fungus combinations, as in arbuscular mycorrhiza systems (Klironomos, 2000; Smith et al., 2003). These differences may also be plant-mediated in ORM systems, by the provision of photosynthetically derived carbon to the mycorrhizal symbiont, which may fuel fungal growth and foraging capabilities. While this has been shown for one Ceratobasidium-associating orchid (Cameron et al., 2008), further investigations on a wider range of species are required to understand this plant–fungal exchange (Selosse and Martos, 2014; Lallemand et al., 2018). Further studies investigating the functional capacities of a range of ORMs associating with D. bracteata would be required to gain a greater understanding of how orchid nutrition may drive colonization in an invasive species.
Conclusions
The simple microcosm feeding experiments involving imaging and quantification in the current study show conclusively for the first time that a range of terrestrial orchids are utilizing their mycorrhizal partners to capture and transport P via hyphal uptake. This uptake is a dynamic process, occurring over a 7-d incubation period with differential efficiency among orchid–fungus combinations. Evidence of differential plant-mediated fungal foraging abilities shows strong evidence that niche partitioning takes place in natural systems. These findings are important in understanding the nutritional role of orchid mycorrhizas in the supply of the plant-growth-limiting nutrient P. Questions surrounding the exact pathway of P into the orchids and the mutualistic nature of the symbiosis remain to be resolved. Novel adaptations and optimization of existing microcosm and field experiment designs, integrated with molecular, radioisotopic and histological studies, aimed at a wide range of orchid–fungus symbioses will be required to further unravel the mystery of orchid P nutrition.
ACKNOWLEDGEMENTS
We thank Miss Irene Johnson (University of Sheffield) for technical support. We would like to thank two anonymous reviewers for their comments, which improved the final version. B.D., W-H.L, D.R, H.L. and K.D. planned and designed the research; B.D. and W.-H.L. collected data; B.D. performed statistical analyses; B.D. and W-H. L., drafted the manuscript; and all authors contributed to the final version.
FUNDING
This work was supported by an Australian Research Council (ARC) Linkage Grant (LP120200464) to B.D., and W-H. L. was supported by the Australian Orchid Foundation (grant number 286/2013).
DATA ACCESSIBILITY
GenBank accession numbers for the fungal isolates identified in this study: Disa bracteata (KT601562); Diuris magnifica (KT601561); Microtis media subsp. media (KT601563); Pterostylis sanguinea (KT601560).
LITERATURE CITED
- Alexander C, Alexander IJ, Hadley G. 1984. Phosphate uptake by Goodyera repens in relation to mycorrhizal infection. New Phytologist 97: 401–411. [Google Scholar]
- Barrett CF, Freudenstein JV, Taylor DL, Koljalg U.. 2010. Rangewide analysis of fungal associations in the fully mycoheterotrophic Corallorhiza striata complex (Orchidaceae) reveals extreme specificity on ectomycorrhizal Tomentella (Thelephoraceae) across North America. American Journal of Botany 97: 628–643. [DOI] [PubMed] [Google Scholar]
- Batty AL, Diwon KW, Brundrett MC, Sivasithamparam K. 2001. Long-term storage of mycorrhizal fungi and seed as a tool for the conservation of endangered Western Australian terrestrial orchids. Australian Journal of Botany 49: 619–628. [Google Scholar]
- Baylis GTS. 1975. The magnolioid mycorrhiza and mycotrophy in root systems derived from it. In: Sander FE, Mosse B, Tinker PB, eds. Endomycorrhizas. London: Academic Press, 373–389. [Google Scholar]
- Bernard N. 1904. Récherches experimentale sur les orchidées. Revue Générale de Botanique 16: 405– 451. [Google Scholar]
- Bever JD, Dickie IA, Facelli E, et al. 2010. Rooting theories of plant community ecology in microbial interactions. Trends in Ecology and Evolution 25: 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K. 2007. Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycological Research 111: 51–61. [DOI] [PubMed] [Google Scholar]
- Bowman A. 1988. A rapid method to determine total phosphorus in soils. Soil Science Society of America Journal 52: 1301–1304. [Google Scholar]
- Brown A, Dixon KW, French C, Brockman G. 2013. Field guide to the orchids of Western Australia. Perth, Western Australia: Simon Nevill Publications. [Google Scholar]
- Brundrett MC. 2007. Scientific approaches to Australian temperate terrestrial orchid conservation. Australian Journal of Botany 55: 293–307. [Google Scholar]
- Brundrett MC. 2009. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil 320: 37–77. [Google Scholar]
- Burgeff H. 1936. Samenkeimung der Orchideen. Jena: Gustaf Fischer. [Google Scholar]
- Cameron DD, Leake JR, Read DJ. 2006. Mutualistic mycorrhiza in orchids: evidence from plant-fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytologist 171: 405–416. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Johnson I, Leake JR, Read DJ. 2007. Mycorrhizal acquisition of inorganic phosphorus by the green-leaved terrestrial orchid Goodyera repens. Annals of Botany 99: 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DD, Johnson I, Read DJ, Leake JR. 2008. Giving and receiving: measuring the carbon cost of mycorrhizas in the green orchid, Goodyera repens. New Phytologist 180: 176–184. [DOI] [PubMed] [Google Scholar]
- Christenhusz MJM, Byng JW. 2016. The number of known plant species in the world and its annual increase. Phytotaxa 261: 201–217. [Google Scholar]
- Davis BJ, Phillips RD, Wright M, Linde CC, Dixon KW. 2015. Continent-wide distribution in mycorrhizal fungi: implications for the biogeography of specialized orchids. Annals of Botany 116: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk E, Olff H. 1994. Effects of nitrogen, phosphorus and potassium fertilization on field performance of Dactylorhiza majalis. Acta Botanica Neerlandica 43: 383–392. [Google Scholar]
- Frank AB, Trappe JM. 2005. On the nutritional dependence of certain trees on root symbiosis with belowground fungi (an English translation of A.B. Frank’s classic paper of 1885). Mycorrhiza 15: 267–275. [DOI] [PubMed] [Google Scholar]
- Gerz M, Guillermo Bueno C, Ozinga WA, Zobel M, Moora M. 2018. Niche differentiation and expansion of plant species are associated with mycorrhizal symbiosis. Journal of Ecology 106: 254–264. [Google Scholar]
- Hadley G. 1984. Uptake of 14C glucose by asymbiotic and mycorrhizal orchid protocorms. New Phytologist 96: 263–273. [Google Scholar]
- Harley JL, McCready CC. 1950. Uptake of phosphate by excised mycorrhizal roots of the beech. New Phytologist 49: 388–397. [Google Scholar]
- Hejcman M, Schellberg J, Pavlu V. 2010. Dactylorhiza maculata, Platanthera bifolia and Listera ovata survive N application under P limitation. Acta Oecologica 36: 684–688. [Google Scholar]
- Jacquemyn H, Brys R, Merckx VSFT, Waud M, Lievens B, Wiegand T. 2014. Coexisting orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytologist 202: 616–627. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Waud M, Busschaert P, Lievens B. 2015. Mycorrhizal networks and coexistence in species-rich orchid communities. New Phytologist 206: 1127–1134. [DOI] [PubMed] [Google Scholar]
- Jones MD, Durall DM, Tinker PB. 1991. Fluxes of carbon and phosphorus between symbionts in willow ectomycorrhizas and their changes with time. New Phytologist 119: 99–106. [DOI] [PubMed] [Google Scholar]
- Kuga Y, Sakamoto N, Yurimoto H. 2014. Stable isotope cellular imaging reveals that both live and degenerating fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytologist 202: 594–605. [DOI] [PubMed] [Google Scholar]
- Klironomos J. 2000. Host-specificity and functional diversity among arbuscular mycorrhizal fungi. In: Bell CR, Brylinsky M, Johnson-Green P. eds. Microbial biosystems: new frontiers. Canada: Atlantic Society for Microbial Ecology, 845–851. [Google Scholar]
- Laliberté E, Turner BL, Costes T, et al. 2012. Experimental assessment of nutrient limitation along a 2-million-year dune chronosequence in the south-western Australia biodiversity hotspot. Journal of Ecology 100: 631–642. [Google Scholar]
- Lallemand F, Robionek A, Courty P-E, Selosse M-A. 2018. The 13C content of the orchid Epipactis palustris (L.) Crantz responds to light as in autotrophic plants. Botany Letters 165: 265–273. [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. 2010. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil 334: 11–31. [Google Scholar]
- Lambers H, Shane MW, Laliberté E, Swarts ND, Teste FP, Zemunik G. 2014. Plant mineral nutrition. In: Lambers H, ed. Plant life on the sandplains in Southwest Australia, a global biodiversity hotspot. Crawley, Western Australia: UWA Publishing, 101–127. [Google Scholar]
- Linde CC, May TW, Phillips RD, Ruibal M, Smith LM, Peakall R. 2017. New species of Tulasnella associated with terrestrial orchids in Australia. IMA Fungus 8: 28–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Long JR, Swarts ND, Dixon KW, Egerton-Warburton LM. 2012. Mycorrhizal preference promotes habitat invasion by a native Australian orchid: Microtis media. Annals of Botany 111: 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur WM. 1991. Reference soils of South-Western Australia. South Perth, Western Australia: Department of Agriculture. [Google Scholar]
- McCormick MK, Jacquemyn H. 2014. What constrains the distribution of orchid populations? New Phytologist 202: 392–400. [Google Scholar]
- McCormick MK, Whigham DF, O’Neill J. 2004. Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytologist 163: 425–438. [DOI] [PubMed] [Google Scholar]
- Melin E, Nilsson H. 1953. Transport of labeled phosphorus to pine seedlings through the mycelium of Cortinus glaucopus (Schaeff. ex. Fr) Fr. Svensk Botanisk Tidskift 48: 555–558. [Google Scholar]
- Mosse B. 1959. Observations on the extramatrical mycelium of a vesicular-arbuscular endophyte. Transactions of the British Mycological Society 42: 439–448, IN4–IN5. [Google Scholar]
- Mujica MI, Saez N, Cisternas M, Manzano M, Armesto JJ, Perez F. 2016. Relationship between soil nutrients and mycorrhizal associations of two Bipinnula species (Orchidaceae) from Central Chile. Annals of Botany 118: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujica MI, Perez MF, Jakalski M, Martos F, Selosse MA. 2020. Soil P reduces mycorrhizal colonization while favors fungal pathogens: observational and experimental evidence in Bipinnula (Orchidaceae). FEMS Microbiology Ecology 96: fiaa178. [DOI] [PubMed] [Google Scholar]
- Mujica MI, Cisternas M, Claro A, Simunovic M, Perez F. 2021. Nutrients and fungal identity affect the outcome of symbiotic germination in Bipinnula fimbriata (Orchidaceae). Symbiosis 83: 91–101. [Google Scholar]
- Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36. [Google Scholar]
- Nurfadilah S, Swarts ND, Dixon KW, Lambers H, Merritt DJ. 2013. Variation in nutrient-acquisition patterns by mycorrhizal fungi of rare and common orchids explains diversification in a global biodiversity hotspot. Annals of Botany 111: 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RF, Hopper SD. 2003. Monocotyledonous geophytes: comparison of south-western Australia with other areas of Mediterranean climate. Australian Journal of Botany 51: 129–133. [Google Scholar]
- Pate JS, Dixon KW. 1982. Tuberous, cormous and bulbous plants – biology of an adaptive strategy. Perth: University of Western Australia Press. [Google Scholar]
- Pearson V, Read DJ. 1973. Movement of carbon compounds between the parents in orchid mycorrhiza. In: Sanders FE, Mosse B, Tinker PB, eds. Endomycorrhizas. London: Academic Press, 175–194. [Google Scholar]
- Phillips RD, Barrett MD, Dixon KW, Hopper SD. 2011. Do mycorrhizal symbioses cause rarity in orchids? Journal of Ecology 99: 858–869. [Google Scholar]
- Purves S, Hadley G. 1975. Movement of carbon compounds between the parents in orchid mycorrhiza. In: Sanders FE, Mosse B, Tinker PB, eds. Endomycorrhizas. London: Academic Press, 175–194. [Google Scholar]
- Ramsay RR, Dixon KW, Sivasithamparam K. 1986. Patterns of infection and endophytes associated with Western Australian orchids. Lindleyana 1: 203–214. [Google Scholar]
- Rasmussen HN. 2002. Recent developments in the study of orchid mycorrhiza. Plant and Soil 244: 149–163. [Google Scholar]
- Read DJ, Boyd R. 1986. Water relations of mycorrhizal fungi and their host plants. In: Ayres P, Boddy L, eds. Water, fungi and plants. Cambridge: Cambridge University Press, 287–303. [Google Scholar]
- Read DJ, Stribley DP. 1973. Effect of mycorrhizal infection on nitrogen and phosphorus nutrition of ericaceous plants. Nature New Biology 244: 81–82. [DOI] [PubMed] [Google Scholar]
- Reiter N, Phillips RD, Swarts ND, et al. 2020. Specific mycorrhizal associations involving the same fungal taxa in common and threatened Caladenia (Orchidaceae): implications for conservation. Annals of Botany 126: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche SA, Carter RJ, Peakall R, Smith LM, Whitehead MR, Linde CC. 2010. A narrow group of monophyletic Tulasnella (Tulasnellaceae) symbiont lineages are associated with multiple species of Chiloglottis (Orchidaceae): implications for orchid diversity. American Journal of Botany 97: 1313–1327. [DOI] [PubMed] [Google Scholar]
- Rousseau JVD, Sylvia DM, Fox AJ. 1994. Contribution of ectomycorrhiza to the potential nutrient-absorbing surface of pine. New Phytologist 128: 639–644. [Google Scholar]
- Selosse M-A, Martos F. 2014. Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon? Trends in Plant Science 19: 683–685. [DOI] [PubMed] [Google Scholar]
- Silvertown J, Wells DA, Gillman M, Dodd ME, Robertson H, Lakhani KH. 1994. Short-term effects and long-term after-effects of fertilizer application on the flowering population of green-winged orchid Orchis morio. Biological Conservation 69: 191–197. [Google Scholar]
- Smith SE. 1966. Physiology and ecology of orchid mycorrhizal fungi with reference to seedling nutrition. New Phytologist 65: 488–499. [Google Scholar]
- Smith SE. 1967. Carbohydrate translocation in orchid mycorrhizas. New Phytologist 66: 371–378. [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd edn. London: Academic Press. [Google Scholar]
- Smith SE, Smith AF, Jakobsen I. 2003. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiology 133: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZF, James EA, McClean CB. 2010. Mycorrhizal specificity of Diuris fragrantissima (Orchidaceae) and persistence in a re-introduced population. Australian Journal of Botany 58: 97–106. [Google Scholar]
- Swarts ND, Sinclair EA, Francis A, Dixon KW. 2010. Ecological specificity in mycorrhizal symbiosis leads to rarity in an endangered orchid. Molecular Ecology 19: 3226–3242. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Bahram M, Zobel M. 2020. How mycorrhizal associations drive plant population and community biology. Science 367: 867–875. [DOI] [PubMed] [Google Scholar]
- Tennant D. 1975. A test of a modified line intersect method of estimating root length. Journal of Ecology 63: 995–1001. [Google Scholar]
- Tsiftsis S, Tsiripidis I, Papaioannou A. 2012. Ecology of the orchid Goodyera repens in its southern distribution limits. Plant Biosystems 146: 857–866. [Google Scholar]
- Vogt-Schilb H, Tesitelova T, Kotilinek M, Suchacek P, Kohout P, Jersakova J. 2020. Altered rhizoctonia assemblages in grasslands on ex-arable land support germination of mycorrhizal generalist, not specialist orchids. New Phytologist 227: 1200–1212. [DOI] [PubMed] [Google Scholar]
- Warcup JH. 1981. The mycorrhizal relationships of Australian orchids. New Phytologist 87: 371–381. [Google Scholar]
- Waud M, Busschaert P, Lievens B, Jacquemyn H. 2016. Specificity and localized distribution of mycorrhizal fungi in the soil may contribute to co-existence of orchid species. Fungal Ecology 20: 155–165. [Google Scholar]
- White TJ, Bruns TD, Taylor LS. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: McInnes MA, Gelfand DH, Snisky JJ, White TJ, eds. PCR protocols – a guide to method and application. San Diego: Academic Press. [Google Scholar]
- Witkowski ETF, Mitchell DT. 1987. Variations in soil phosphorus in the fynbos biome, South Africa. Journal of Ecology 75: 1159–1171. [Google Scholar]
- Zemunik G, Turner BL, Lambers H, Laliberté E. 2015. Diversity of plant nutrient-acquisition strategies increases during long term ecosystem development. Nature Plants 1: 15050. [Google Scholar]
- Zemunik G, Turner BL, Lambers H, Laliberté E. 2016. Increasing plant species diversity and extreme species turnover accompany declining soil fertility along a long-term chronosequence in a biodiversity hotspot. Journal of Ecology 104: 792–805. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
GenBank accession numbers for the fungal isolates identified in this study: Disa bracteata (KT601562); Diuris magnifica (KT601561); Microtis media subsp. media (KT601563); Pterostylis sanguinea (KT601560).