Abstract
The presence of an epicardial connection between the left-sided pulmonary vein and left atrium was suggested during catheter ablation of atrial fibrillation because of sustainable unidirectional entrance conduction after complete endocardial ablation, centrifugal breakout deep inside the pulmonary vein, and immediate elimination of the conduction by point ablation. (Level of Difficulty: Advanced.)
Key Words: catheter ablation, entrance block, epicardial connection, exit block, pulmonary vein isolation, unidirectional conduction
Abbreviations and Acronyms: AF, atrial fibrillation; CS, coronary sinus; LA, left atrium; LSPV, left superior pulmonary vein; PV, pulmonary vein; RA, right atrium
Central Illustration

Pulmonary vein (PV) isolation is a standard strategy for the nonpharmacologic treatment of atrial fibrillation (AF). Although optimal ablation lesion sets can be effectively achieved from the endocardial viewpoint thanks to the development of technologies such as 3-dimensional mapping systems and contact force monitoring, first-pass isolation is sometimes difficult to perform, and additional ablation inside the veins is commonly necessary and effective for isolation. Recent studies have suggested the presence of an epicardial connection involving the PVs as one of the mechanisms for failure of isolation by circumferential lesion sets.1, 2, 3, 4
Learning Objectives
-
•
To recognize the presence of epicardial connections as a possible cause of failure of first-pass isolation.
-
•
To recognize an epicardial connection between the pulmonary veins and the left atrium in addition to Marshall bundle and coronary sinus musculature.
-
•
To recognize that an epicardial connection occasionally has a unidirectional conduction property.
History of Presentation
A 73-year-old woman had experienced palpitations because of paroxysmal AF for 8 months and was referred to our institution for catheter ablation.
Past Medical History
The patient had a history of hypertension.
Differential Diagnosis
In cases of failure of first-pass isolation, discrimination of epicardial conduction from gap conduction is important to avoid unnecessary additional ablation on the circumferential ablation line.
Investigations
On admission, physical examination found a respiratory rate of 16 breaths/min, regular pulse rate of 72 beats/min, and blood pressure of 136/76 mm Hg. Her body height was 156 cm and weight was 55 kg. No abnormal heart or breath sounds were noted. Echocardiography revealed normal left ventricular function and an enlarged left atrium (LA) (38 mL/m2).
Management
After written informed consent was obtained, the electrophysiologic study was performed under CARTO guidance (Biosense Webster, Inc). A 6-F 20-pole dual-site mapping catheter (BeeAT, Japan Lifeline Co, Ltd) was inserted through the subclavian vein and positioned in the coronary sinus (CS), right atrium (RA), and superior vena cava. The patient presented to the laboratory in sinus rhythm with incessant paroxysms of AF (Figure 1A).
Figure 1.
Intracardiac Electrograms Before and After Ablation Encircling the Pulmonary Veins
(A) Intracardiac electrograms of the repetitive firing from the LSPV at baseline. (B) Unidirectional entrance conduction from the LA to the LSPV after first-pass radiofrequency ablation encircling the left-sided pulmonary veins. ABL = ablation, APC = atrial premature contraction, B. = block, C. = conduction, CS = coronary sinus, d. = distal, Ent. = entrance, LA = left atrium, LSPV = left superior pulmonary vein, p. = proximal, RA = right atrium, SR = sinus rhythm, SVC = superior vena cava.
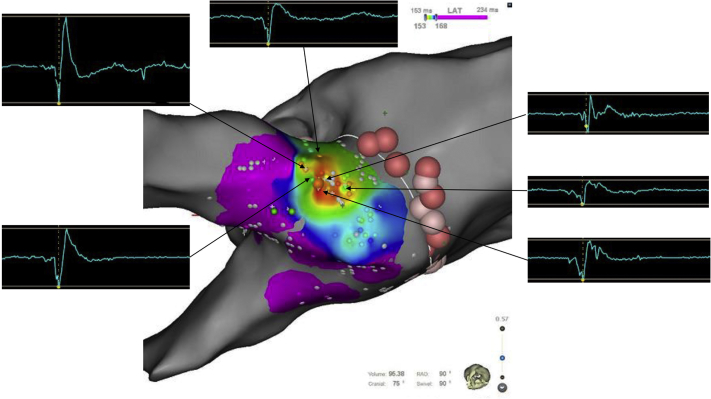
Circumferential ablation encircling the left-sided PVs was performed. The respective mean ablation index and mean interlesion distance were 345 ± 50 mm and 3.5 ± 1.1 mm in the anterior aspect and 300 ± 61 mm and 3.1 ± 1.0 mm in the posterior aspect, respectively. The repetitive paroxysms of AF disappeared despite nonachievement of first-pass isolation. Although we first thought that there might be a conduction gap on the ablation line, close observation reminded us that the left superior pulmonary vein (LSPV) firing did not conduct to the LA (exit block), but the sinus impulse did conduct to the LSPV (unidirectional entrance conduction) (Figure 1B). This unidirectional conduction was not transient but persisted stably for more than 30 minutes. Because we previously experienced that an epicardial connection between the right-sided PVs and RA exhibited a unidirectional conduction property5 and there was no potential in the circumferential ablation line in the present patient, we suspected the contribution of an epicardial connection, but not of a conduction gap, to this sustainable and unidirectional conduction. During CS pacing, activation mapping was performed within the LSPV and revealed a centrifugal breakout pattern with the earliest activation at the posterior aspect of the LSPV, which was 15 mm away from the circumferential ablation line (Figure 2). Ablation at that site with a low power setting of 20 W immediately eliminated the entrance conduction (Figure 3).
Figure 2.
Activation Map of the Left Superior Pulmonary Vein During Coronary Sinus Pacing
The centrifugal breakout 15 mm away from the antral ablation line was identified. Around the earliest activation site, electrograms exhibited a long-duration and multiphasic fusion morphology of the high- and low-frequency components. LAT = local activation time.
Figure 3.
Intracardiac Electrograms During Ablation Deep Inside the LSPV
Ablation at the yellow tag in Figure 2 eliminated the entrance conduction in 3 seconds. The red arrows indicate PV potentials, and the blue dashed arrow indicates a dissociated activity. RF = radiofrequency ablation. Abbreviations as in Figure 1.
Discussion
In this case, it was possible that the unidirectional conduction from the LA to LSPV (entrance conduction) after circumferential ablation was stably maintained not by a gap in the ablation line but by an epicardial connection.
Unidirectional conduction from the PV to the LA (exit conduction) has rarely been observed during PV isolation. Chen et al6 reported that such a unidirectional exit conduction was confirmed in 11 (4.8%) of 231 patients with spontaneous activities after PV isolation. Half of such a unidirectional conduction were transient, suggesting local effects of ablation such as inflammation and edema on the ablation line as a mechanism of the characteristic conduction property. To the best of our knowledge, however, stable unidirectional conduction from the LA to the PV (ie, entrance conduction) has been reported only once in the literature. In this single case, unstable rate-dependent exit block was observed under the presence of an entrance conduction.7
A recent comprehensive study evaluated epicardial connections in 534 patients undergoing radiofrequency catheter ablation of AF.1 All epicardial connections involving the left-sided PVs were associated with the CS or Marshall bundle (8.1%; 43 patients) and therefore were totally different from the epicardial connection between the LA and LSPV, as suggested in the present case.
Moreover, we recently experienced an interesting case of AF in which the dissociated PV activities after isolation were unidirectionally conducted to the RA through an interatrial epicardial connection, resulting in RA parasystole.5 Sink-source mismatch may contribute to this phenomenon in this fine fiber. On the basis of our experience, activation mapping within the LSPV was performed early after the observation of the unidirectional conduction in the present patient. Because the earliest activation site in the centrifugal breakout was 15 mm away from the antral ablation line, gap conduction was unlikely. Pambrun et al8 recently reported that epicardial electrograms derived from the septopulmonary bundle can be recorded from the endocardium as a low-frequency/dull potential during roof line ablation. The morphologies of the electrograms recorded around the earliest activation site in the present case (Figure 2) might be a fusion of the high- and low-frequency components, suggesting near-field endocardial and far-field epicardial potentials, respectively. Of note, ablation at that site with a low power setting abolished the entrance conduction and achieved bidirectional conduction block within 3 seconds.
Follow-Up
The patient has remained free from any atrial tachyarrhythmias without any antiarrhythmic drugs for 7 months.
Conclusions
A sustainable unidirectional entrance conduction after complete endocardial ablation, centrifugal breakout deep inside the LSPV during CS pacing, and the immediate elimination of the conduction by point ablation suggested the presence of an epicardial connection between the LSPV and LA.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Barrio-Lopez M.T., Sanchez-Quintana D., Garcia-Martinez J., et al. Epicardial connections involving pulmonary veins: the prevalence, predictors, and implications for ablation outcome. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007544. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K., Baba M., Shinoda Y., et al. Epicardial connection between the right-sided pulmonary venous carina and the right atrium in patients with atrial fibrillation: a possible mechanism for preclusion of pulmonary vein isolation without carina ablation. Heart Rhythm. 2019;16:671–678. doi: 10.1016/j.hrthm.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Hasebe H., Furuyashiki Y., Yoshida K. Temporal elimination of an interatrial epicardial connection by ablation encircling the right-sided pulmonary veins. HeartRhythm Case Rep. 2020;6:841–844. doi: 10.1016/j.hrcr.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasebe H., Yoshida K., Nogami A., et al. A simple pacing maneuver to unmask an epicardial connection involving the right-sided pulmonary veins. J Cardiovasc Electrophysiol. 2021;32:287–296. doi: 10.1111/jce.14835. [DOI] [PubMed] [Google Scholar]
- 5.Hanaki Y., Hasebe H., Baba M., Yoshida K. Right atrial parasystole originating from isolated activities in the right inferior pulmonary vein with an epicardial connection. HeartRhythm Case Rep. 2020;6:437–440. doi: 10.1016/j.hrcr.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S., Meng W., Sheng He D., et al. Blocking the pulmonary vein to left atrium conduction in addition to the entrance block enhances clinical efficacy in atrial fibrillation ablation. Pacing Clin Electrophysiol. 2012;35:524–531. doi: 10.1111/j.1540-8159.2012.03343.x. [DOI] [PubMed] [Google Scholar]
- 7.Oi M., Nomura S., Miho M., et al. Rate-dependent and unidirectional conduction block between the left pulmonary vein and left atrium after catheter ablation for atrial fibrillation. J Arrhythm. 2020;36:1096–1099. doi: 10.1002/joa3.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pambrun T., Duchateau J., Delgove A., et al. Epicardial course of the septopulmonary bundle: anatomical considerations and clinical implications for roof line completion. Heart Rhythm. 2021;18:349–357. doi: 10.1016/j.hrthm.2020.11.008. [DOI] [PubMed] [Google Scholar]