Abstract
Objectives
To identify individual characteristics associated with serological COVID-19 vaccine responsiveness and the durability of vaccine-induced antibodies.
Methods
Adults without history of SARS-CoV-2 infection from the Danish population scheduled for SARS-CoV-2 vaccination were enrolled in this parallel group, phase 4 study. SARS-CoV-2 Spike IgG and Spike-ACE2-receptor-blocking antibodies were measured at days 0, 21, 90, and 180. Vaccine responsiveness was categorized according to Spike IgG and Spike-ACE2-receptor-blocking levels at day 90 after first vaccination. Nondurable vaccine response was defined as day-90 responders who no longer had significant responses by day 180.
Results
Of 6544 participants completing two vaccine doses (median age 64 years; interquartile range: 54–75), 3654 (55.8%) received BTN162b2, 2472 (37.8%) mRNA-1273, and 418 (6.4%) ChAdOx1 followed by an mRNA vaccine. Levels of both types of antibodies increased from baseline to day 90 and then decreased to day 180. The decrease was more pronounced for levels of Spike-ACE2-receptor-blocking antibodies than for Spike IgG. Proportions with vaccine hyporesponsiveness and lack of durable response were 5.0% and 12.1% for Spike IgG and 12.7% and 39.6% for Spike-ACE2-receptor-blocking antibody levels, respectively. Male sex, vaccine type, and number of comorbidities were associated with all four outcomes. Additionally, age ≥75 years was associated with hyporesponsiveness for Spike-ACE2-receptor-blocking antibodies (adjusted odds ratio: 1.59; 95% confidence interval: 1.25–2.01) but not for Spike IgG.
Discussion
Comorbidity, male sex, and vaccine type were risk factors for hyporesponsiveness and nondurable response to COVID-19 vaccination. The functional activity of vaccine-induced antibodies declined with increasing age and had waned to pre-second-vaccination levels for most individuals after 6 months.
Keywords: COVID-19, Immunity, Vaccination, Antibody, SARS-CoV-2
Introduction
Early reports on effects of COVID-19 booster vaccination against symptomatic and severe COVID-19 suggested dramatic drops in the risk of infection after administration of a third vaccine dose [1]. Out of concern of waning immunity and nonprotective levels of antibodies, health authorities in many countries now recommend booster vaccination to specific risk groups based on comorbidities and/or age [2,3], or to the entire adult population in some countries [4,5]. The emergence of the SARS-CoV-2 Omicron variant has widely stressed national vaccine booster initiatives [6,7]. However, only sparse information on predictors associated with serological COVID-19 vaccine responsiveness and durability exist from clinical phase 2 and 3 trials, as most individuals with significant immunodeficiency were excluded from these trials [[8], [9], [10], [11]].
In the present study, we prospectively performed comprehensive SARS-CoV-2 serological profiling of more than 6500 individuals enrolled in the Danish National Cohort Study of Effectiveness and Safety of SARS-CoV-2/COVID-19 vaccines (ENFORCE). We used this dataset to discriminate COVID-19 vaccine responders from nonresponders and to determine risk factors for vaccine hyporesponsiveness and reduced durability of vaccine-induced antibodies that may increase the risk of breakthrough infection.
Methods
ENFORCE is an open-label, nonrandomized, parallel group, phase 4 study that enrolled Danish citizens before their first COVID-19 vaccination (clinicaltrials.gov identifier: NCT04760132). The study has seven study sites across the country covering all five Danish regions.
Study population
The study enrolled Danish citizens 18 years or older accepting a SARS-CoV-2 vaccine. Inclusion criteria were (a) written informed consent provided before any trial-related procedures were performed and (b) Danish citizens eligible for SARS-CoV-2 immunization. Exclusion criteria were (a) age <18 years, (b) contraindications for vaccination, and (c) Previous COVID-19 vaccination (for study recruitment strategy, see supplementary appendix). We excluded individuals with prior infection at baseline, defined as either positive SARS-CoV-2 PCR or Spike-Ig positive.
Data collection
Baseline information on age, sex, focused medical history, concomitant medication, vaccine priority group as defined by the Danish COVID-19 Vaccination Program [12], date, and vaccine type (BTN162b2, Pfizer-BioNTech; mRNA-1273, Moderna; ChAdOx1, AstraZeneca) were collected. Concomitant diseases and vaccine type were confirmed by cross-referencing data from the Danish National Patient Registry and the Danish Vaccination Registry using participants' unique civil registration number. Data on any previous positive SARS-CoV-2 PCR tests were extracted from Danish national microbiology database MiBa (Statens Serum Institut, Copenhagen, Denmark). The study protocol was approved by the Danish Medicines Agency (#2020-006003-42) and the National Committee on Health Research Ethics (#1-10-72-337-20).
Data on comorbidity
We assessed levels of comorbidity based on hospital diagnoses within the 5 years prior to vaccination obtained from the Danish National Patient Registry as of each person's study entry date. The Charlson Comorbidity Index (CCI) score assigns a weight (1, 2, 3, or 6) to each of 19 major disease categories and is a validated measure of comorbidity [11]. With the CCI score, we defined three levels of comorbidity: low (CCI = 0), medium (CCI = 1–2), and high (CCI ≥3).
Follow-up
The second study visit occurred before (0–7 days) the second vaccination (usually 21 days for BTN162b2 and 28 days for mRNA-1273 after the first vaccination); the third study visit happened 90 days (±14 days) after the first vaccination, and the fourth visit was 180 days (±14 days) after the first vaccination. At each study visit, blood samples for measuring SARS-CoV-2 serology were obtained.
SARS-CoV-2 antibody profiling
Serum levels of SARS-CoV-2 Spike and receptor-binding domain (RBD) antibodies were measured at all visits using a diagnostic multiantigen serology assay (Meso Scale Diagnostics LLC, Rockville, MD) at the Department of Infectious Diseases, Aarhus University Hospital. Total serum Spike-RBD SARS-CoV-2 Ig levels were measured by WANTAI ELISA (Beijing Wantai BPE) and performed at Statens Serum Institut. An ACE2 competition assay was used to evaluate the functional potential of serum antibodies for blocking SARS-CoV-2 Spike and RBD binding to the ACE2 receptor using the ACE2 competition assay (Meso Scale Diagnostics). Results obtained by the ACE2 competition assay closely correlate with results obtained in pseudovirus neutralization assays [13].
Statistical analysis
Baseline data were tabulated according to vaccine type as percentages or means with interquartile range (IQR) and p-values calculated with the χ2 test. Serology data are presented as geometric mean titres (GMT) with 95% CIs. Three levels of vaccine response were defined based on the participant's change in Spike-IgG at day 90 relative to pre-vaccine (baseline) levels: Vaccine hyporesponders were defined as individuals who had a <2 log10 increase in Spike-IgG, moderate responders had 2 to 3 log10 increase, and high responders had a >3 log10 increase in Spike-IgG. Corresponding categories of vaccine responsiveness were also made based on absolute levels of Spike-ACE-2-receptor-blocking antibodies (<1, 1–4, >4 AU/mL) at day 90. Participant characteristics were compared across the three groups, and logistic regression was used to evaluate risk factors for vaccine hyporesponsiveness. Variables selected a priori included age at enrolment, sex, vaccine type, CCI score, and individual comorbidities and were evaluated in univariable and multivariable analysis. Finally, the durability of COVID-19 vaccine responses was evaluated by comparing vaccine-induced antibodies at day 180 to the levels observed at previous visits.
Results
A total of 6544 individuals who were Spike-Ig negative and had no prior positive SARS-CoV-2 PCR at baseline were included. Of these, 3654 (55.8%) received BTN162b2, 2472 (37.8%) mRNA-1273, and 418 (6.4%) one dose of ChAdOx1 followed by a second dose of one of the mRNA vaccines. Baseline demographics of the study participants are shown in Table 1 . The median age was 64 years, and 3683 (56.3%) were female. BTN162b2 vaccinees were generally older, had more comorbidity, and were more likely to be categorized as high-risk individuals in the Danish vaccination program than the two other groups. In contrast, the ChAdOx1/mRNA group mainly consisted of younger individuals, with a large representation of female health-care workers.
Table 1.
Participant demographics at study enrolment by vaccine type
| Vaccine type |
|||||
|---|---|---|---|---|---|
| Total (N = 6544) |
BTN162b2 (n = 3654) |
mRNA-1273 (n = 2472) |
ChAdOx1/mRNA (n = 418) |
p-value | |
| Age at enrolment (y), median (IQR) | 64 (54–75) | 71 (55–78) | 61 (54–69) | 45 (31–56) | . |
| Age group, n (%) | <.001 | ||||
| 18–25 y | 139 (2.1) | 52 (1.4) | 57 (2.3) | 30 (7.2) | . |
| 25–39 y | 341 (5.2) | 148 (4.1) | 60 (2.4) | 133 (31.8) | . |
| 40–64 y | 2967 (45.3) | 1243 (34.0) | 1474 (59.6) | 250 (59.8) | . |
| 65–79 y | 2228 (34.0) | 1503 (41.1) | 721 (29.2) | 4 (1.0) | . |
| ≥80 y | 869 (13.3) | 708 (19.4) | 160 (6.5) | 1 (0.2) | . |
| Sex, n (%) | <.001 | ||||
| Male | 2861 (43.7) | 1772 (48.5) | 1024 (41.4) | 65 (15.6) | . |
| Female | 3683 (56.3) | 1882 (51.5) | 1448 (58.6) | 353 (84.4) | . |
| Vaccine priority group | <.001 | ||||
| 1. Individuals at increased risk, n (%) | 1539 (23.5) | 1386 (37.9) | 145 (5.9) | 8 (1.9) | . |
| 2. Health-care workers | 525 (8.0) | 100 (2.7) | 25 (1.0) | 400 (95.7) | . |
| 3. General population | 4480 (68.5) | 2168 (59.3) | 2302 (93.1) | 10 (2.4) | . |
| CCI score categories, n (%) | <.001 | ||||
| 0 | 4797 (73.3) | 2305 (63.1) | 2099 (84.9) | 393 (94.0) | . |
| 1–2 | 1468 (22.4) | 1112 (30.4) | 332 (13.4) | 24 (5.7) | . |
| >2 | 279 (4.3) | 237 (6.5) | 41 (1.7) | 1 (0.2) | . |
| Comorbidities in the previous 5 y, n (%) | . | ||||
| Myocardial infarction | 115 (1.8) | 87 (2.4) | 27 (1.1) | 1 (0.2) | . |
| Congestive heart failure | 161 (2.5) | 136 (3.7) | 24 (1.0) | 1 (0.2) | . |
| Peripheral vascular disease | 66 (1.0) | 52 (1.4) | 13 (0.5) | 1 (0.2) | . |
| Cerebrovascular disease | 219 (3.3) | 157 (4.3) | 57 (2.3) | 5 (1.2) | . |
| Dementia | 11 (0.2) | 10 (0.3) | 1 (0.0) | 0 | . |
| Chronic pulmonary disease | 318 (4.9) | 253 (6.9) | 60 (2.4) | 5 (1.2) | . |
| Rheumatic disease | 147 (2.2) | 118 (3.2) | 25 (1.0) | 4 (1.0) | . |
| Peptic ulcer disease | 24 (0.4) | 18 (0.5) | 6 (0.2) | 0 | . |
| Liver disease | 104 (1.6) | 82 (2.2) | 22 (0.9) | 0 | . |
| Diabetes | 248 (3.8) | 190 (5.2) | 56 (2.3) | 2 (0.5) | . |
| Hemiplegia or paraplegia | 5 (0.1) | 5 (0.1) | 0 | 0 | . |
| Renal disease | 99 (1.5) | 92 (2.5) | 7 (0.3) | 0 | . |
| Any malignancy | 612 (9.4) | 468 (12.8) | 136 (5.5) | 8 (1.9) | . |
| Metastatic solid tumour | 31 (0.5) | 22 (0.6) | 9 (0.4) | 0 | . |
| HIV | 45 (0.7) | 39 (1.1) | 6 (0.2) | 0 | . |
| Organ transplantation | 136 (2.1) | 127 (3.5) | 9 (0.4) | 0 | . |
Follow-up
Of the 6544 participants, 6036 (92%) had complete SARS-CoV-2 serology measurements available for the study visit prior to the second planned vaccine dose (day 21); 5662 (86.5%) and 4096 (62.6%) had complete SARS-CoV-2 serology at the study visits 90 and 180 days, respectively.
Levels of SARS-CoV-2 antibodies before and after vaccination
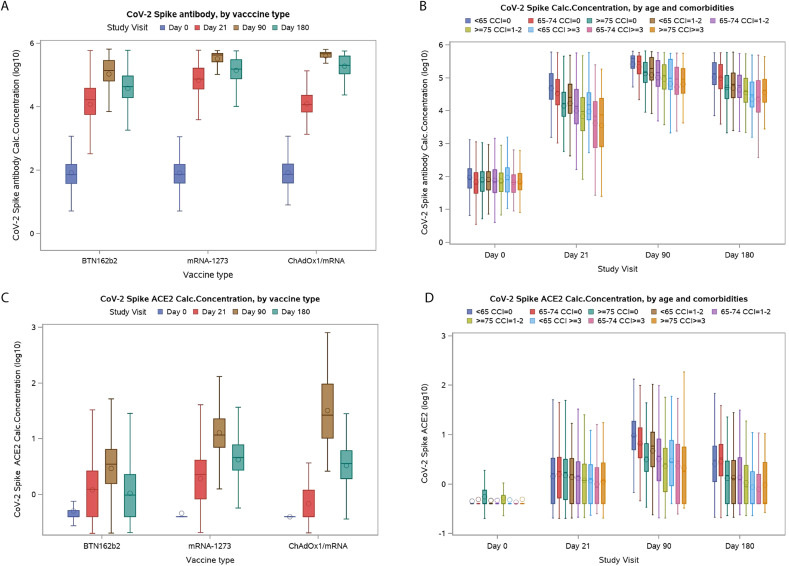
The GMT of Spike-IgG increased from 82 (95% CI: 102−110) at baseline to 23 572 (95% CI: 22 503−24 692) before second vaccination and to 167 137 (95% CI: 161 366−173 114) at day 90, and then decreased at day 180 (61 904; 95% CI: 59 225−64 704) (Fig. 1 A, Table S1). Similar patterns were seen in GMT for RBD-specific IgG levels (Fig. S1, Table S1). Stratifying participants according to age and CCI score showed that postvaccination levels of Spike-IgG were progressively lower with increasing age and number of comorbidities (Fig. 1B). Similar associations with age and comorbidity were also observed for RBD-IgG (Fig. S2). Total Spike Ig was detected in 0% at baseline and increased to 82.3% after the first vaccination and 97.8% at day 90 within the cohort (Fig. S3, Table S2).
Fig. 1.
Plasma levels of SARS-CoV-2 Spike IgG and Spike-ACE2-receptor-blocking antibody levels at each study visit. (A) Plasma levels of SARS-CoV-2 Spike IgG at each study visit by vaccine type. (B) Plasma levels of SARS-CoV-2 Spike IgG at each study visit by age group and level of comorbidity. (C) Plasma concentration of Spike-ACEII-receptor blocking antibodies according to vaccine type. (D) Plasma concentration of Spike-ACEII-receptor-blocking antibodies according to age group and level of comorbidity. CCI, Charlson Comorbidity Index.
Blocking of Spike-ACE2-receptor binding
The median serum concentration of Spike-ACE2-receptor blocking antibodies was zero at baseline, increased slightly before the second dose (median 1 AU/mL, IQR: 1–1), and then increased substantially at day 90 (median 5 AU/mL, IQR: 5–6; Fig. 1C, Table S3). Median level at day 90 differed depending on vaccine type and was 3 AU/mL (IQR: 3–3) for BTN162b2, 13 AU/mL (IQR: 12–13) for mRNA-1273, and 31 (IQR: 22–44) for those who received first a single ChAdOx1 dose and then either mRNA vaccine as their second dose. Of note, this latter group received their second dose closer to the day 90 visit than the two first groups.
At day 180, levels of Spike-ACE2-receptor blocking antibodies had decreased significantly (median 2, IQR: 2–2). Stratifying participants according to age and CCI score revealed that after vaccination, antibody levels were gradually lower with increasing age and number of comorbidities (Fig. 1D). Among participants with comorbidity or age >75 years, levels of Spike-ACE2-receptor blocking antibodies had declined to pre–second vaccination levels at day 180 (Table S3). Similar associations with vaccine type, age, and comorbidity were also observed for RBD-ACE2-receptor-neutralizing antibodies (Figs. S3 and S4).
Responsiveness to COVID-19 vaccination
We first categorized participants who had complete serology measurements from baseline to day 90 after first vaccination according to the relative increase in Spike-IgG antibodies and Spike ACE-2-receptor-blocking antibodies from baseline. Vaccine hyporesponsiveness, defined as having a <2-fold log10 increase in Spike IgG or Spike-ACE2-receptor-blocking antibodies <1 AU/mL at day 90, was seen in 5.0% and 12.7% of the cohort, respectively (Table 2 ). The corresponding proportion of individuals with vaccine hyporesponsiveness at day 180 was 12.1% for Spike IgG and 39.6% for Spike-ACE2-receptor-blocking antibodies. Thereafter, and to evaluate the durability of the vaccine response, among those defined as responders at day 90 and with follow-up at day 180 (n = 3474), we assessed how many became hyporesponsive at day 180. According to Spike-IgG and ACE2-receptor-blocking antibodies, 12.1% and 28% were hyporesponsive at day 180, whereas 45% and 48% had a moderate and 43% and 24% had a strong response, respectively.
Table 2.
Vaccine-related response for two anti-Spike antibodies at day 90 after start of vaccination by demographic characteristics
| Day 90 Spike IgG fold change from baseline (log10) (N = 5625) |
Day 90 Spike-ACE2r blocking antibody (AU/mL) (N = 5662) |
|||||
|---|---|---|---|---|---|---|
| <2-fold change | 2- to 3-fold change | >3-fold change | <1 | 1–4 | >4 | |
| Participants, n (% of total) | 281 (5.0) | 1239 (22.0) | 4105 (73.0) | 718 (12.7) | 1380 (24.4) | 3564 (62.9) |
| Age (y), median (IQR) | 66 (55–75) | 69 (56–78) | 64 (54–74) | 73 (60–79) | 73 (59–79) | 62 (53–69) |
| Age group, n (%) | ||||||
| <55 y | 63 (22.4) | 266 (21.5) | 1050 (25.6) | 120 (16.7) | 227 (16.4) | 1038 (29.1) |
| 55–64 y | 69 (24.6) | 223 (18.0) | 1127 (27.5) | 106 (14.8) | 229 (16.6) | 1095 (30.7) |
| 65–74 y | 71 (25.3) | 291 (23.5) | 936 (22.8) | 181 (25.2) | 335 (24.3) | 800 (22.4) |
| ≥75 y | 78 (27.8) | 459 (37.0) | 992 (24.2) | 311 (43.3) | 589 (42.7) | 631 (17.7) |
| Sex, n (%) | ||||||
| Male | 153 (54.4) | 665 (53.7) | 1732 (42.2) | 421 (58.6) | 739 (53.6) | 1400 (39.3) |
| Female | 128 (45.6) | 574 (46.3) | 2373 (57.8) | 297 (41.4) | 641 (46.4) | 2164 (60.7) |
| Vaccine priority group, n (%) | ||||||
| 1. Individuals at increased risk | 190 (67.6) | 401 (32.4) | 760 (18.5) | 369 (51.4) | 432 (31.3) | 560 (15.7) |
| 2. Health-care workers | 5 (1.8) | 28 (2.3) | 131 (3.2) | 8 (1.1) | 26 (1.9) | 132 (3.7) |
| 3. General population | 86 (30.6) | 810 (65.4) | 3214 (78.3) | 341 (47.5) | 922 (66.8) | 2872 (80.6) |
| Vaccine received, n (%) | ||||||
| BTN162b2 | 255 (90.7) | 975 (78.7) | 2020 (49.2) | 667 (92.9) | 1210 (87.7) | 1389 (39.0) |
| mRNA-1273 | 26 (9.3) | 256 (20.7) | 2022 (49.3) | 51 (7.1) | 166 (12.0) | 2106 (59.1) |
| ChAdOx1/mRNA | 0 | 8 (0.6) | 63 (1.5) | 0 | 4 (0.3) | 69 (1.9) |
| Time between first and second dose (d), median (IQR) | 22 (21–27) | 24 (21–35) | 35 (24–35) | 22 (21–26) | 24 (21–28) | 35 (27–35) |
| Time from first vaccine to third study visit (d), median (IQR) | 91 (89–95) | 92 (89–96) | 91 (89–96) | 92 (90–95) | 92 (89–96) | 91 (89–96) |
| Enrolment month in 2021, median (IQR) | March (March–April) | April (March–April) | April (March–May) | March (March–April) | April (March–April) | May (April–May) |
| CCI score categories, n (%) | ||||||
| 0 | 130 (46.3) | 833 (67.2) | 3137 (76.4) | 366 (51.0) | 914 (66.2) | 2843 (79.8) |
| 1–2 | 117 (41.6) | 340 (27.4) | 833 (20.3) | 281 (39.1) | 391 (28.3) | 631 (17.7) |
| >2 | 34 (12.1) | 66 (5.3) | 135 (3.3) | 71 (9.9) | 75 (5.4) | 90 (2.5) |
| Comorbidities, n (%) | ||||||
| Myocardial infarction | 7 (2.5) | 32 (2.6) | 64 (1.6) | 20 (2.8) | 37 (2.7) | 47 (1.3) |
| Congestive heart failure | 13 (4.6) | 49 (4.0) | 75 (1.8) | 37 (5.2) | 46 (3.3) | 54 (1.5) |
| Peripheral vascular disease | 6 (2.1) | 16 (1.3) | 37 (0.9) | 17 (2.4) | 20 (1.4) | 22 (0.6) |
| Cerebrovascular disease Dementia | 14 (5.0) | 53 (4.3) | 123 (3.0) | 44 (6.1) | 62 (4.5) | 86 (2.4) |
| Chronic pulmonary disease | 21 (7.5) | 74 (6.0) | 178 (4.3) | 52 (7.2) | 85 (6.2) | 139 (3.9) |
| Rheumatic disease | 10 (3.6) | 36 (2.9) | 82 (2.0) | 31 (4.3) | 42 (3.0) | 55 (1.5) |
| Peptic ulcer disease | 0 | 7 (0.6) | 15 (0.4) | 6 (0.8) | 7 (0.5) | 9 (0.3) |
| Liver disease | 23 (8.2) | 18 (1.5) | 49 (1.2) | 27 (3.8) | 28 (2.0) | 36 (1.0) |
| Diabetes | 23 (8.2) | 56 (4.5) | 131 (3.2) | 57 (7.9) | 58 (4.2) | 97 (2.7) |
| Hemiplegia or paraplegia | 0 | 2 (0.2) | 3 (0.1) | 1 (0.1) | 1 (0.1) | 3 (0.1) |
| Renal disease | 27 (9.6) | 19 (1.5) | 36 (0.9) | 35 (4.9) | 25 (1.8) | 22 (0.6) |
| Any malignancy | 57 (20.3) | 138 (11.1) | 343 (8.4) | 127 (17.7) | 158 (11.4) | 259 (7.3) |
| Metastatic solid tumour | 3 (1.1) | 7 (0.6) | 16 (0.4) | 5 (0.7) | 7 (0.5) | 14 (0.4) |
| HIV | 2 (0.7) | 12 (1.0) | 22 (0.5) | 8 (1.1) | 12 (0.9) | 16 (0.4) |
| Organ transplantation | 49 (17.4) | 20 (1.6) | 49 (1.2) | 61 (8.5) | 27 (2.0) | 31 (0.9) |
Those who were SARS-CoV-2 PCR or plasma Spike Ig positive at baseline were excluded.
Risk factors for COVID-19 vaccine hyporesponsiveness at day 90
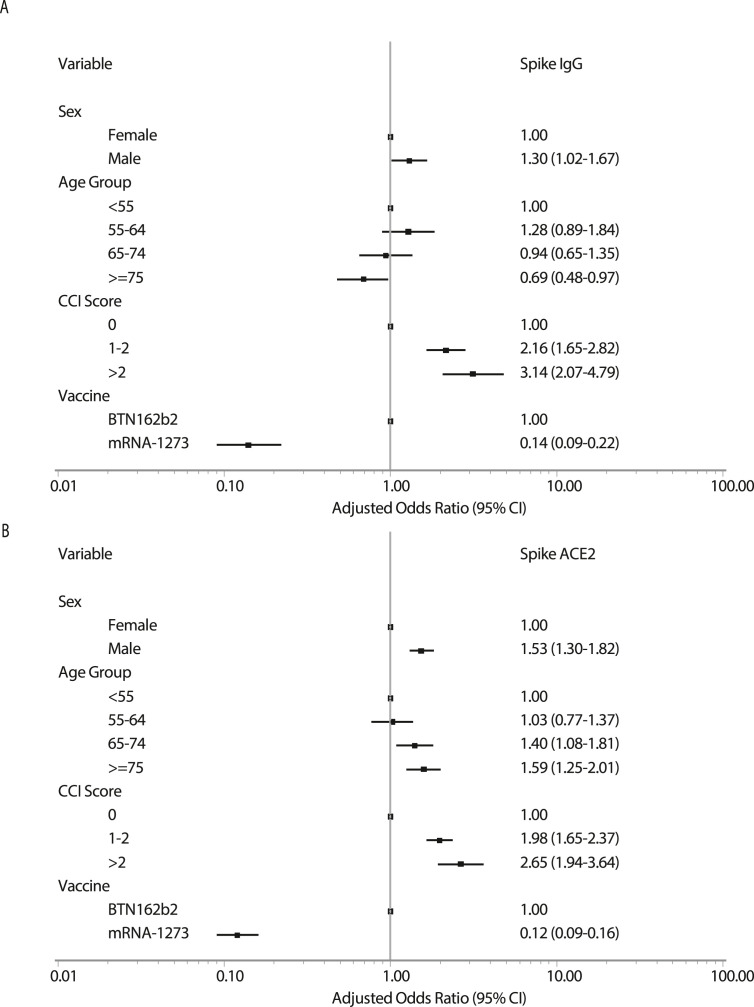
Responsiveness differed by several vaccine, demographics, and comorbidity factors (Table 2). Independent risk factors for vaccine Spike-IgG and Spike-ACE2-receptor-blocking antibody hyporesponders vs. moderate and high responders were first evaluated in multivariable logistic regression models including sex, age group, CCI score category, and vaccine type, excluding ChAdOx1 vaccinees due to low numbers (Fig. 2 A and B). For both antibodies, hyporesponsiveness was associated with male sex, increasing number of comorbidities, and vaccine type. Conversely, whereas older age was associated with elevated risk of being hyporesponsive, as determined by low levels of Spike-ACE2-receptor-blocking antibodies, this was not observed when assessing Spike IgG.
Fig. 2.
Risk factors for COVID-19 vaccine hyporesponsiveness in a multivariate logistical regression model according to (A) Spike IgG and (B) Spike-ACE2-receptor-blocking antibody levels at day 90.
The association with number of comorbidities was further assessed by assessing each type of comorbidities (Fig. S5). Hyporesponders were found consistently more often among transplant recipients and patients with renal disease and malignancies. Patients with diabetes and autoimmune disease had an increase chance of being hyporesponsive when assessing response by change in Spike-ACE2-receptor antibody only, whereas patients with liver disease had an elevated chance when assessing Spike-IgG antibodies only.
Durability of Covid-19 vaccine responses at day 180
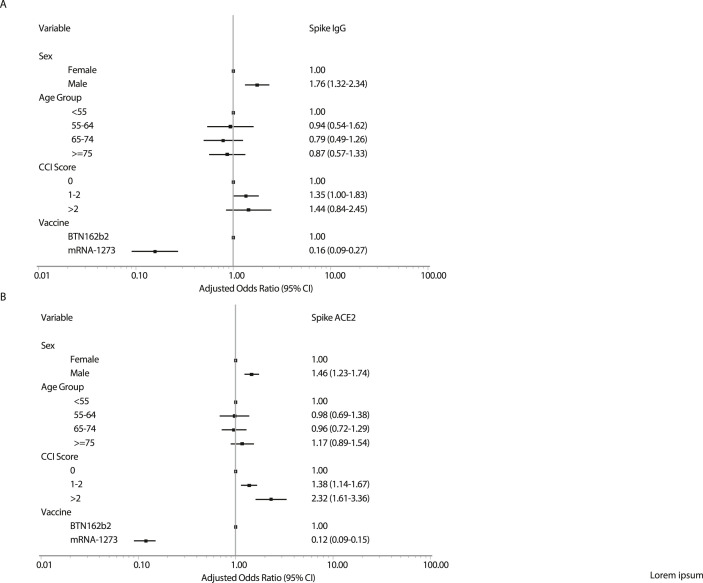
Among initial responders at day 90, independent risk factors for vaccine hyporesponsiveness at day 180 are presented in Fig. 3 A (for Spike IgG) and 3B (for Spike-ACE2-receptor antibody). For both antibodies assessed, risk factors for this outcome were male sex and vaccine type (mRNA-1273 compared to BTN162b2 adjusted OR = 0.16, 95% CI: 0.09−0.27) but not age. Multiple comorbidities were associated with excess risk of hyporesponsiveness for Spike-ACE2-receptor antibody but not clearly so for Spike IgG (Table S6).
Fig. 3.
Risk factors for COVID-19 vaccine hyporesponsiveness in a multivariate logistical regression model according to (A) Spike IgG and (B) Spike-ACE2-receptor-blocking antibody levels at day 180.
Discussion
The present study adds significant insight into the potential causes of the observed reduction in vaccine effectiveness over time across different populations [3,14,15]. First, we document that in this large cohort of mainly middle-aged and older individuals with a substantial burden of comorbidities, initial peak antibody responses to all vaccine regimens were robust in 95% of vaccinated individuals. Increasing age was associated with lower levels of neutralizing antibodies, but interestingly, this association was not present when we analyzed relative increases in quantitative SARS-CoV-2 IgG levels. Others have also reported an association between age and neutralizing antibody titres after BTN-162b2 vaccination [16]. Elderly individuals are known to have reduced ability to mount robust and sustained responses to new antigens, and age-related decrease in the production of new B cells from their precursors may lead to production of antibodies with lower avidity and affinity [17]. Thus, it may be important to consider both functional and quantitative measures of antibody levels in the evaluation of vaccine response and protection against COVID-19.
The efficacy of the two mRNA vaccines, BNT162b2 and mRNA-1273, against COVID-19 was almost identical in the respective phase 3 trials, but subsequent studies have indicated that total antibody levels and neutralization activity may be higher after two doses of mRNA-1273 compared to BNT162b2. In one report, recipients of the BNT162b2 vaccine had a 27% higher risk of documented SARS-CoV-2 infection and a 70% higher risk of hospitalization for COVID-19 than recipients of the mRNA-1273 vaccine over 24 weeks of follow-up in a period marked by Alpha-variant predominance [14]. The authors also reported a trend towards higher risk of documented infection among BNT162b2 vaccinees than among mRNA-1273 vaccinees over 12 weeks of follow-up in a period marked by Delta-variant predominance [14]. Others have reported higher SARS-CoV-2–binding antibody response after mRNA-1273 vaccination compared to BNT162b2 vaccination [18,19]. In contrast to our study, these two studies did not measure functional or neutralizing antibodies. Indeed, we not only observed higher levels of total Spike and RBD IgG but also higher levels of functional Spike and RBD-ACE2-blocking antibodies in mRNA-1273 compared to BNT162b2 vaccinees. Differences in immunogenicity and effectiveness between the BNT162b2 and mRNA-1273 vaccines could be due to several factors, including variation in mRNA content of the vaccines (100 vs. 30 μg for mRNA-1273 vs. BNT162b2), differences in the recommended interval between the first and second dose (4 weeks for mRNA-1273 vs. 3 weeks for BNT162b2), or other factors, such as the lipid composition of the nanoparticles used for packaging the mRNA content [8,9,20].
Our study also had some limitations. Due to the temporal variations in the availability of specific COVID-19 vaccines in Denmark, most of the individuals who were categorized as high risk in the national vaccination program received BTN162b2. This may bias the antibody responses in the BTN162b2 to be lower compared to the other groups. Also, the ChAdOx1/mRNA group mainly consisted of female health-care workers, and the timing of their second vaccine was closer to the 3- and 6-month visit. We have used different strategies to balance out the inherent differences between vaccine groups, such as stratification and multivariate adjustment in the analyses, but some residual confounding may persist; therefore, direct comparison of vaccine responses between the groups should be made with caution.
Although we report on plasma levels of SARS-CoV-2 Spike and RBD antibodies, which have been shown to correlate with protection against COVID-19, data on cellular immunity were not available for this study. It should be noted that T-cell responses may be of particular importance in preventing severe COVID-19 among those who become infected with SARS-CoV-2 [13,21]. Of note, there is no universal definition of vaccine hyporesponsiveness for COVID-19 vaccines. In the present study, we defined hyporesponsiveness as a 2-fold or smaller increase in vaccine-induced antibodies, similar to the threshold that has been applied in the pneumococcal vaccine literature [22]. Finally, it was beyond the scope of this study to analyze any association between adverse events and vaccine responsiveness, but this would be of interest in future studies.
In conclusion, most individuals, even those at high risk, mounted robust immune responses to the two-dose vaccine regimens. However, certain comorbidities were strongly associated with hyporesponsiveness to COVID-19 vaccination. In addition, male sex and vaccine type were associated with decreased durability of vaccine-induced antibodies. On a population level, the functional activity of vaccine-induced antibodies appeared to wane quickly during the 6 months of follow-up, demonstrating that booster vaccination is required to maintain high levels of protective antibodies. These findings have important implications for the roll-out of booster vaccines.
Transparency declaration
This work was supported by the Danish Ministry of Health (document 150, parliamentary year 2020/2021, the Danish Parliament). HN declares participation on advisory board meetings with GSK and MSD. TB declares receipt of unrestricted research or travel grants from GSK, Pfizer, Gilead Sciences, and MSD; being principal investigator on trials conducted by Boehringer Ingelheim, Roche, Novartis, and Kancera; being a board member for Pentabase and an advisory board member for Janssen and AstraZeneca; receiving consulting fees from GSK and Pfizer; receiving donation of study drug from Eli Lilly; and receiving honoraria for lectures from GSK, Pfizer, Gilead Sciences, Boehringer Ingelheim, Abbvie, and AstraZeneca. All other authors declare no conflicts of interest.
Author contributions
OSS, JR, ISJ, HN, TB, LW, NBS, KI, CM, DR, MT, LØ, and JL developed the design. ISL, HN, TB, LW, NBS, KI, KF, JB, MI, LSK, VK, FDL, LWM, SOL, AØ, and CA did the clinical visits and data collection. SDA, AKH, SRA, and MA did the laboratory assays. JR, OSS, SRO, TOJ, CE, TKF, MT, LØ, and JL analyzed data. OSS drafted the manuscript. All authors critically revised the manuscript for important intellectual content.
Acknowledgements
The ENFORCE study group members all contributed substantially to the study. A full list of members of the ENFORCE study group is provided as supplementary material.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.03.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. The Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjan S., Natori Y., Fernandez Betances A.A., Agritelley M.S., Mattiazzi A., Arosemena L., et al. Breakthrough COVID-19 infections after mRNA vaccination in solid organ transplant recipients in Miami, Florida. Transplantation. 2021;105:e139–e141. doi: 10.1097/TP.0000000000003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook C., Patel N.J., D'Silva K.M., Hsu T.Y., DiIorio M., Prisco L., et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:289–291. doi: 10.1136/annrheumdis-2021-221326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ducloux D., Colladant M., Chabannes M., Yannaraki M., Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100:702–704. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mbaeyi S., Oliver S.E., Collins J.P., Godfrey M., Goswami N.D., Hadler S.C., et al. The Advisory Committee on Immunization Practices' Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines - United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545–1552. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen E., Ntoumi F., Hui D.S., Abubakar A., Kramer L.D., Obiero C., et al. Emergence of new SARS-CoV-2 Variant of Concern Omicron (B.1.1.529) - highlights Africa's research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int J Infect Dis. 2021;114:268–272. doi: 10.1016/j.ijid.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danish Health Authority . 2021. Vaccination calendar.https://www.sst.dk/en/english/corona-eng/vaccination-against-covid-19/how-you-will-get-vaccinated Available from: [Google Scholar]
- 13.Nielsen S.S., Vibholm L.K., Monrad I., Olesen R., Frattari G.S., Pahus M.H., et al. SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity. EBioMedicine. 2021;68:103410. doi: 10.1016/j.ebiom.2021.103410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickerman B.A., Gerlovin H., Madenci A.L., Kurgansky K.E., Ferolito B.R., Figueroa Muñiz M.J., et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386:105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates T.A., Leier H.C., Lyski Z.L., Goodman J.R., Curlin M.E., Messer W.B., et al. Age-dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. JAMA. 2021;326:868–869. doi: 10.1001/jama.2021.11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegrist C.A., Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 18.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards N.E., Keshavarz B., Workman L.J., Nelson M.R., Platts-Mills T.A.E., Wilson J.M. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenmaker L., Witzigmann D., Kulkarni J.A., Verbeke R., Kersten G., Jiskoot W., et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sogaard O.S., Lohse N., Harboe Z.B., Offersen R., Bukh A.R., Davis H.L., et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis. 2010;51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.