ABSTRACT
Objective
The aim of this systematic review and meta‐analysis was to evaluate the diagnostic accuracy of the sliding sign on transvaginal ultrasound (TVS) in detecting pouch of Douglas obliteration and bowel involvement in patients with suspected endometriosis, using laparoscopy as the reference standard.
Methods
A search for studies evaluating the role of the sliding sign in the assessment of pouch of Douglas obliteration and/or bowel involvement using laparoscopy as the reference standard published from January 2000 to October 2021 was performed in PubMed/MEDLINE, Web of Science, CINAHL, The Cochrane Library, ClinicalTrials.gov and SCOPUS databases. The Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) was used to evaluate the quality of the studies. Analyses were performed using MIDAS and METANDI commands in STATA.
Results
A total of 334 citations were identified. Eight studies were included in the analysis, resulting in 938 and 963 patients available for analysis of the diagnostic accuracy of the sliding sign for pouch of Douglas obliteration and bowel involvement, respectively. The mean prevalence of pouch of Douglas obliteration was 37% and the mean prevalence of bowel involvement was 23%. The pooled estimated sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio of the sliding sign on TVS for detecting pouch of Douglas obliteration were 88% (95% CI, 81–93%), 94% (95% CI, 91–96%), 15.3 (95% CI, 10.2–22.9), 0.12 (95% CI, 0.07–0.21) and 123 (95% CI, 62–244), respectively. The heterogeneity was moderate for sensitivity and low for specificity for detecting pouch of Douglas obliteration. The pooled estimated sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio of the sliding sign on TVS for detecting bowel involvement were 81% (95% CI, 64–91%), 95% (95% CI, 91–97%), 16.0 (95% CI, 9.0–28.6), 0.20 (95% CI, 0.10–0.40) and 81 (95% CI, 34–191), respectively. The heterogeneity for the meta‐analysis of diagnostic accuracy for bowel involvement was high.
Conclusion
The sliding sign on TVS has good diagnostic performance for predicting pouch of Douglas obliteration and bowel involvement in women with suspected endometriosis. © 2022 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: bowel, endometriosis, pouch of Douglas, sliding sign, sonography
Short abstract
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
RESUMEN
Precisión diagnóstica del signo deslizante para detectar la obliteración del fondo de saco de Douglas y la afectación intestinal en mujeres con sospecha de endometriosis: revisión sistemática y metaanálisis
Objetivo
El objetivo de esta revisión sistemática y metaanálisis fue evaluar la precisión diagnóstica del signo deslizante en la ecografía transvaginal (ETV) para detectar la obliteración del fondo de saco de Douglas y la afectación intestinal en pacientes con sospecha de endometriosis, utilizando la laparoscopia como estándar de referencia.
Métodos
Se realizó una búsqueda de estudios que hubieran evaluado la función del signo deslizante en la valoración de la obliteración del fondo de saco de Douglas y/o la afectación intestinal utilizando la laparoscopia como estándar de referencia, publicados desde enero de 2000 hasta octubre de 2021 en las bases de datos PubMed/MEDLINE, Web of Science, CINAHL, The Cochrane Library, ClinicalTrials.gov y SCOPUS. Para evaluar la calidad de los estudios se utilizó la herramienta de Evaluación de Calidad de los Estudios de Precisión Diagnóstica‐2 (QUADAS‐2, por sus siglas en inglés) Los análisis se realizaron mediante los comandos MIDAS y METANDI de STATA.
Resultados
Se identificaron un total de 334 citas. En el análisis se incluyeron ocho estudios, lo que dio como resultado 938 y 963 pacientes disponibles para el análisis de la precisión diagnóstica del signo deslizante para la obliteración del fondo de saco de Douglas y la afectación intestinal, respectivamente. La prevalencia media de la obliteración del fondo de saco de Douglas fue del 37% y la prevalencia media de la afectación intestinal fue del 23%. La estimación combinada de la sensibilidad, especificidad, cociente de verosimilitud positivo, cociente de verosimilitud negativo y razón de momios del diagnóstico del signo deslizante en la ETV para detectar la obliteración del fondo de saco de Douglas fue del 88% (IC 95%, 81–93%), 94% (IC 95%, 91–96%), 15,3 (IC 95%, 10,2–22,9), 0,12 (IC 95%, 0,07–0,21) y 123 (IC 95%, 62–244), respectivamente. La heterogeneidad fue moderada en cuanto a la sensibilidad y baja en cuanto a la especificidad para detectar la obliteración del fondo de saco de Douglas. La estimación combinada de la sensibilidad, especificidad, cociente de verosimilitud positivo, cociente de verosimilitud negativo y razón de momios del diagnóstico del signo deslizante en la ETV para detectar la afectación intestinal fue del 81% (IC 95%, 64–91%), 95% (IC 95%, 91–97%), 16,0 (IC 95%, 9,0–28,6), 0,20 (IC 95%, 0,10–0,40) y 81 (IC 95%, 34–191), respectivamente. La heterogeneidad del metaanálisis de la precisión diagnóstica de la afectación intestinal fue alta.
Conclusiones
El signo deslizante en la ETV tiene un buen rendimiento diagnóstico para predecir la obliteración del fondo de saco de Douglas y la afectación intestinal en mujeres con sospecha de endometriosis.
摘要
滑动征检测疑似子宫内膜异位症女性道格拉斯小袋闭塞和肠道受累的诊断准确性:系统评价和荟萃分析
目的
本系统评价和荟萃分析的目的是 以腹腔镜作为参考标准用于评估经阴道超声 (TVS) 滑动征在检测疑似道格拉斯小袋闭塞和肠道受累并伴有子宫内膜异位患者中的诊断准确性。
方法
使用2000 年 1 月至 2021 年 10 月出版的《腹腔镜参考标准》,在 PubMed/MEDLINE、Web of Science、CINAHL、Cochrane 图书馆、ClinicalTrials.gov 和 SCOPUS 数据库中搜索滑动征在评估道格拉斯小袋闭塞和/或肠道受累中的作用的研究 。使用诊断准确性研究的质量评价工具‐2(Quality Assessment of Diagnostic Accuracy Studies‐2)来评估这些研究的质量。在STATA 中使用MIDAS 和METANDI 命令进行分析。
结果
共识别出 334 次引用。共有八项研究被纳入分析,分别有 938 名和 963 名患者可用于分析滑动征对道格拉斯小袋闭塞和肠道受累的诊断准确性。道格拉斯小袋闭塞的平均患病率为 37%,肠道受累的平均患病率为 23%。经阴道超声(TVS )滑动征检测道格拉斯闭塞袋的综合估计敏感性、特异性、阳性似然比、阴性似然比和诊断优势比分别为 88%(95% CI,81‐93%)、94%(95% CI , 91–96%), 15.3 (95% CI, 10.2–22.9), 0.12 (95% CI, 0.07–0.21) 和 123 (95% CI, 62–244)。检测道格拉斯小袋闭塞敏感性中的异质性为中等,特异性低。经阴道超声(TVS )滑动征检测肠道受累的汇总估计敏感性、特异性、阳性似然比、阴性似然比和诊断优势比分别为 81%(95% CI,64‐91%)、95%(95% CI,91 –97%)、16.0 (95%CI, 9.0–28.6)、0.20 (95%CI, 0.10–0.40) 和 81 (95% CI, 34–191)。肠道受累诊断准确性的荟萃分析异质性很高。
结论
经阴道超声( TVS) 滑动征对预测疑似患有道格拉斯小袋闭塞和肠道受累的子宫内膜异位女性具有良好的诊断性能。
CONTRIBUTION —
What are the novel findings of this work?
This is the first systematic review and meta‐analysis to focus specifically on studies assessing the diagnostic performance of the sliding sign on transvaginal ultrasound for detecting bowel involvement in women with suspected pelvic endometriosis. This study also provides up‐to‐date evidence regarding the diagnostic performance of the sliding sign for detecting pouch of Douglas obliteration.
What are the clinical implications of this work?
Given its good diagnostic performance, evaluation of the sliding sign using ultrasound should be implemented to assess for pouch of Douglas obliteration and bowel involvement in patients with suspected pelvic endometriosis.
INTRODUCTION
Endometriosis is a gynecological disease, defined as the presence of endometrial‐like tissue outside the uterus, that affects up to 5–10% of premenopausal women, being more frequent in women with symptoms such as dysmenorrhea, chronic pelvic pain, dyspareunia, dyschezia and infertility 1 .
The diagnosis of endometriosis can be difficult and is often delayed 2 . Transvaginal ultrasound (TVS) has been shown to be a highly accurate and reproducible tool for detecting endometriosis. It has been proposed as the primary imaging modality in patients with pelvic pain 3 , 4 , 5 and has shown a high correlation with laparoscopy 6 . The International Deep Endometriosis Analysis (IDEA) group proposed a systematic scanning technique for sonographic evaluation of the pelvis when a patient is suspected to have endometriosis 7 . This technique is based on four steps: evaluation of the uterus and the adnexa to identify and describe signs of adenomyosis and examine for the presence of endometrioma; assessment of ‘soft markers’, such as ‘kissing’ ovaries; assessment of the ‘sliding sign’; and identification of deep endometriotic nodules.
The sliding‐sign diagnostic test, which involves determining whether the anterior rectum glides freely across the posterior aspect of the cervix, posterior vaginal wall (for an anteverted uterus) or uterine fundus (for a retroverted uterus) 7 , has been associated with bowel involvement, namely rectal or sigmoid anterior wall infiltration by endometriotic nodules 8 , and pouch of Douglas obliteration 9 . Pouch of Douglas obliteration is considered to be a sign of severe endometriosis and could result in marked anatomical distortion of the pelvis. Women with pouch of Douglas obliteration are three times more likely to have bowel endometriosis and bowel surgery than are patients with a non‐obliterated pouch of Douglas 10 . A negative sliding sign is considered a ‘hard marker’ for rectal/sigmoid infiltration by deep endometriosis, which may make surgery more complex 11 .
Pouch of Douglas obliteration or bowel involvement during surgery may increase the duration of the procedure and necessitate advanced surgical skills. Consequently, in addition to improving our understanding of pelvic pain symptoms, the ability to evaluate the sliding sign preoperatively may help surgery planning, prompt colorectal surgeon support and allow proper informed consent to be obtained 12 , 13 .
The aim of this systematic review and meta‐analysis was to evaluate the diagnostic performance of the sliding sign assessed by TVS for detecting pouch of Douglas obliteration and bowel involvement in patients with suspected endometriosis, using laparoscopy as the reference standard.
METHODS
Protocol and registration
The systematic review and meta‐analysis was performed according to preferred reporting items for systematic reviews and meta‐analyses (PRISMA) and synthesizing evidence from diagnostic accuracy tests (SEDATE) guidelines 14 , 15 . Inclusion and exclusion criteria and methods for data extraction and quality assessment were specified in advance. The protocol was registered with PROSPERO (CRD42021290671) and is available in Appendix S1. No amendment was made after registration. Institutional review board approval was waived owing to the nature and design of the study.
Data search
Studies published between January 2000 and October 2021 were identified by two authors (E.T. and C.M.) using PubMed/MEDLINE, Web of Science, CINAHL, The Cochrane Library, ClinicalTrials.gov and SCOPUS databases to identify potentially eligible studies. The search terms were as follows: ‘endometriosis’, ‘pouch of Douglas’, ‘bowel’, ‘recto‐sigmoid’, ‘rectal’ and ‘sliding’. Language restriction in the search was set to English, French and Spanish.
Study selection and data collection
Three authors (P.M.E., P.F. and J.L.A.) screened the titles and abstracts of the identified studies to exclude articles that were not relevant to the topic under review, such as those focusing on magnetic resonance imaging instead of ultrasound as the diagnostic method, as well as reviews, letters and case reports. Full‐text articles were obtained to identify eligible studies, and reviewers applied independently the following inclusion criteria: (1) prospective cohort design with at least 20 women included (sample size was set arbitrarily); (2) premenopausal women with a clinical suspicion of endometriosis included as participants; (3) TVS performed by an expert or trained gynecologist used as the index test; (4) laparoscopy (visual inspection with or without histological diagnosis) used as the reference standard; (5) sufficient data reported to construct a 2 × 2 table of diagnostic performance.
The ‘snowball strategy’ was used to identify relevant papers by reviewing the reference lists of the papers selected for full‐text review. In the case of missing relevant data, we sought to contact the authors to ask for more information.
For studies by the same research group, the time period of patient recruitment was examined. If we detected at least two studies from the same group with a clear overlap or a potential risk of overlap of patients, the most recent study was selected for analysis. The Patients, Intervention, Comparator, Outcomes and Setting (PICOS) criteria were used to describe the included studies (Table 1).
Table 1.
Characteristics of studies included in systematic review and meta‐analysis, according to Patients, Intervention, Comparator, Outcomes and Setting (PICOS) criteria
| Study | Country | Study design | Multicenter | Consecutive recruitment | Mean patient age (years) | Total (n) | PoD obliteration (n) | Bowel involvement (n) | Index test | TVS examiners (n) | Reference standard |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Venkatesh (2020) 24 | India | Prosp | No | NS | NS | 136 | 89 | 43 | TVS | NS | LPS |
| Arion (2019) 23 | Canada | Prosp | No | NS | 34.4 | 269 | 41 | — | TVS | 1 | LPS |
| Reid (2018) 22 | Australia | Prosp | Yes | Yes | NS | 376 | — | 76 | TVS | > 2 | LPS with/without histology |
| Menakaya (2016) 21 | Australia | Prosp | Yes | Yes | 32.1 | 199 | 51 | — | TVS | > 2 | LPS with/without histology |
| Piessens (2014) 20 | Australia | Prosp | No | NS | NS | 85 | 34 | 25 | TVS | 1 | LPS with/without histology |
| Leon (2014) 19 | Chile | Prosp | No | No | 32.6 | 51 | 24 | 13 | TVS | 1 | LPS with/without histology |
| Hudelist (2013) 8 | Austria | Prosp | No | NS | 31.6 | 117 | — | 34 | TVS | 1 | LPS with/without histology |
| Holland (2013) 18 | UK | Prosp | Yes | Yes | 35.0 | 198 | 54 | 9 | TVS | 2 | LPS with histology |
Only first author of each study is given.
LPS, laparoscopy; NS, not stated; PoD, pouch of Douglas; Prosp, prospective; TVS, transvaginal ultrasound.
Diagnostic accuracy results and additional useful information about patients and procedures were retrieved from selected primary studies independently by three authors (P.M.E., P.F. and J.L.A). Any disagreement regarding study selection and data collection was resolved by consensus among the three authors.
Risk of bias in individual studies
Quality assessment was conducted using the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool adapted to this systematic review. The QUADAS‐2 tool includes four domains: patient selection, index test, reference standard and flow and timing. For each domain, the risk of bias and concerns regarding applicability were classified as high, low or unclear. The results of the quality assessment were used to evaluate the overall quality of included studies and investigate potential sources of heterogeneity. Three authors (P.M.E., P.F. and J.L.A) studied independently the methodological quality using a standard form with quality assessment criteria and a flow diagram. Disagreements were resolved by discussion among the three authors until a consensus was reached.
The risk of bias in the patient‐selection domain was determined based on the description of inclusion and exclusion criteria of the studies. Patient selection was considered to be at high risk of bias if studies included a non‐consecutive or non‐random series of patients and performed inappropriate exclusions (for example, excluding patients with poor imaging).
The index‐test domain was assessed based on the description of the technique of the sliding‐sign assessment. The risk of bias was considered low when the sliding‐sign technique was described in detail.
The reference‐standard domain was evaluated based on the method used in the study to diagnose obliteration of the pouch of Douglas and/or bowel involvement. The correct reference standard was considered to be laparoscopic surgical and/or histological findings. A lack of blinding of surgeons to ultrasound findings was not considered to indicate a high risk of bias.
For the flow‐and‐timing domain, a description of the time elapsed from the index test to the reference‐standard assessment was evaluated. An interval of more than 3 months was considered to indicate a high risk of bias.
Statistical analysis
Data on the diagnostic performance of the sliding‐sign test performed during TVS were extracted or derived. A positive test (negative sliding sign) was defined as the absence of sliding between the anterior rectum and the serosa on the posterior surface of the cervix, posterior vaginal wall (for an anteverted uterus) or uterine fundus (for a retroverted uterus); the test was considered negative (positive sliding sign) when those structures were completely free of one another. The reference standard was obliteration of the pouch of Douglas and/or bowel involvement demonstrated on laparoscopy, either by visual inspection or histological confirmation.
The primary outcome was pooled sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR–) and diagnostic odds ratio (OR) of the sliding sign. The numbers of true‐positive, true‐negative, false‐positive and false‐negative cases were obtained from each included study. Post‐test probabilities were calculated and plotted on Fagan nomograms, using the mean prevalence of pouch of Douglas obliteration and bowel involvement as the pretest probability.
The presence of heterogeneity for sensitivity and specificity was assessed graphically by drawing forest plots of sensitivity and specificity, and then using Cochran's Q and the I 2 statistic. A test for heterogeneity examines the null hypothesis that all studies are evaluating the same effect; P < 0.1 was considered to indicate heterogeneity. According to Higgins et al. 16 , I 2 values of 25%, 50% and 75% are considered to indicate low, moderate and high heterogeneity, respectively. In cases of moderate or high heterogeneity, meta‐regression was used. The covariates analyzed in meta‐regression were year of publication, sample size and prevalence of pouch of Douglas obliteration or bowel involvement.
Summary receiver‐operating‐characteristics (sROC) curves for each condition were plotted to illustrate the relationship between sensitivity and specificity, and the area under the curve was calculated.
Analyses were performed using Meta‐analytical Integration of Diagnostic Accuracy Studies (MIDAS) and METANDI commands in STATA version 12 for Windows (Stata Corp., College Station, TX, USA); P < 0.05 was considered to indicate statistical significance.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to assess the quality of the retrieved evidence 17 . The online GRADE tool was adopted (http://GRADEPro.org, accessed in December 2021). The assessment was performed by three authors (J.L.A., S.G., M.A.P.) by consensus.
RESULTS
Search results
The electronic search provided a total of 334 citations. After removal of 162 duplicate records, 172 citations remained. Of these 172 citations, 123 were excluded after screening by title and abstract, including reviews (n = 6), case reports (n = 3), letters to the editor (n = 1), opinions (n = 1) and studies not related to the addressed topic (n = 112).
We reviewed the full text of the remaining 49 articles. Forty‐three studies were excluded for the following reasons: study not relevant to the topic addressed (n = 8), data for 2 × 2 table not available (n = 6), retrospective study design (n = 2), surgery not used as the reference standard (n = 4), overlapping series (n = 6), no specific evaluation of the sliding sign (n = 5), review/opinion paper (n = 7) and reproducibility/learning curve rather than diagnostic type of study (n = 5) (Appendix S2). Two additional relevant studies were found in the reference lists of the studies included in the review (snowball technique).
A flowchart summarizing study identification and selection is presented in Figure 1. There was no need to contact the authors, as all relevant data needed to perform the meta‐analysis were available.
Figure 1.
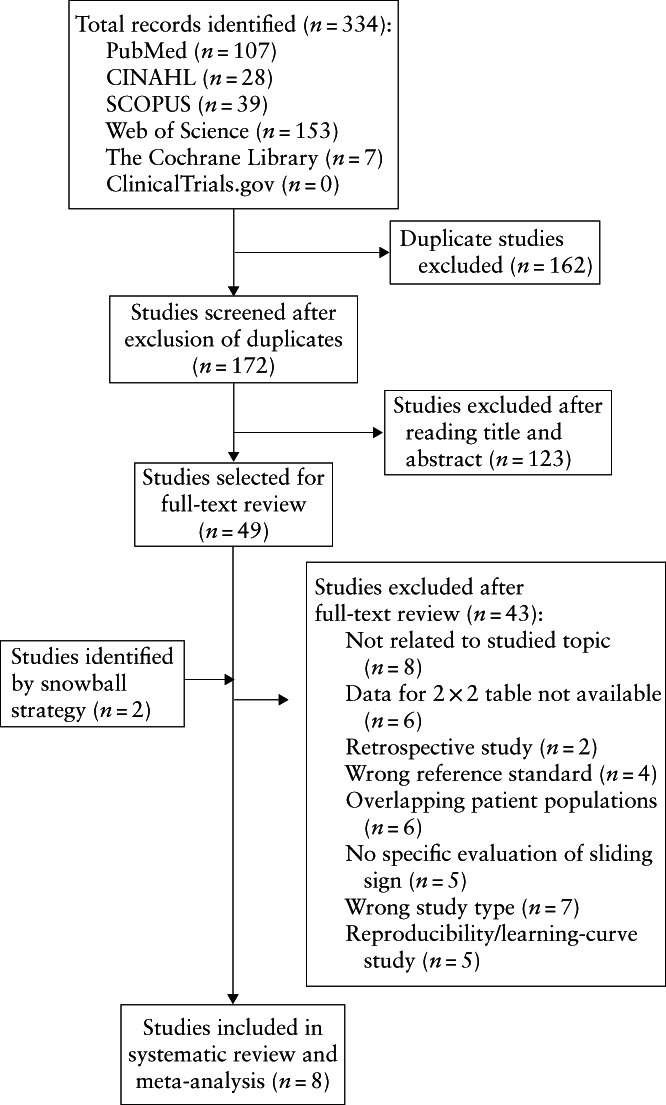
Flowchart summarizing inclusion in systematic review and meta‐analysis of studies evaluating the diagnostic accuracy of the sliding sign on transvaginal ultrasound for pouch of Douglas obliteration and/or bowel involvement in women with suspected endometriosis.
Characteristics of included studies
Eight studies published between October 2013 and January 2020 were included in the final analysis 8 , 18 , 19 , 20 , 21 , 22 , 23 , 24 . Five studies analyzed the accuracy of the preoperative sliding sign for the prediction of both pouch of Douglas obliteration and bowel involvement in women with suspected deep infiltrating endometriosis 18 , 19 , 20 , 21 , 24 . One study 23 analyzed the accuracy of the preoperative sliding sign for the prediction of pouch of Douglas obliteration only, and two studies 8 , 22 analyzed the accuracy of the preoperative sliding sign for detecting bowel involvement only.
Two studies from the same research group were included 21 , 22 . The study by Menakaya et al. 21 provided data on diagnostic accuracy of the sliding sign to detect pouch of Douglas obliteration and bowel involvement, whereas the study by Reid et al. 22 reported data on bowel involvement only. It was concluded that patients with bowel involvement in the study by Menakaya et al. 21 were also included in the study by Reid et al. 22 . Therefore, data on bowel involvement from Menakaya et al. were excluded from the analysis. Thus, we analyzed data from six studies to assess the diagnostic performance of the sliding sign for detecting pouch of Douglas obliteration 18 , 19 , 20 , 21 , 23 , 24 and from six studies to assess the diagnostic performance of the sliding sign for detecting bowel involvement 8 , 18 , 19 , 20 , 22 , 24 .
Nine hundred and thirty‐eight women were assessed for detecting obliteration of the pouch of Douglas. Of these 938 patients, 293 had pouch of Douglas obliteration on laparoscopy. The mean prevalence of pouch of Douglas obliteration was 37%, ranging from 15% to 65%.
Nine hundred and sixty‐three women were assessed for detecting bowel involvement. Of these 963 patients, 200 had bowel involvement on laparoscopy. The mean prevalence of bowel involvement was 23%, ranging from 5% to 32%.
The mean age of patients was reported in five of the eight included studies 8 , 18 , 19 , 21 , 23 . All studies were observational prospective studies. Three of them were multicenter studies 18 , 21 , 22 . Only three studies specified that patient recruitment was consecutive 18 , 21 , 22 ; in one study, recruitment was non‐consecutive 19 . Four studies did not specify the type of recruitment 8 , 20 , 23 , 24 .
In all studies, TVS was performed by an expert or trained examiner. Most studies did not report whether the sonographer was blinded to the patient's medical history. In all studies, surgery was performed by an expert surgeon. One study reported that the surgeon was blinded to TVS findings 18 , and one reported that the surgeon was not blinded to TVS findings 22 . In the remaining studies, this information was not provided. The interval between TVS and surgery was not specified in four studies 19 , 20 , 23 , 24 . In two studies 21 , 22 , surgery was performed within 6 months after TVS, and in two other studies 8 , 18 , the interval elapsed between TVS and surgery was less than 3 months. Table 1 shows PICOS characteristics of the included studies.
Quality of included studies
Evaluation of the risk of bias and concerns regarding applicability of the selected studies is shown in Table 2.
Table 2.
Quality assessment of studies included in systematic review and meta‐analysis, according to Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool
| Risk of bias | Applicability concerns | ||||||
|---|---|---|---|---|---|---|---|
| Study | Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard |
| Venkatesh (2020) 24 | Low | Low | Unclear | Unclear | Low | Low | Low |
| Arion (2019) 23 | Low | Low | Low | Unclear | Low | Low | Low |
| Reid (2018) 22 | Low | Low | Low | High | Low | Low | Low |
| Menakaya (2016) 21 | Low | Low | Low | High | Low | Low | Low |
| Piessens (2014) 20 | Unclear | Low | Low | Unclear | Low | Low | Low |
| Leon (2014) 19 | High | Low | Low | Unclear | Low | Low | Low |
| Hudelist (2013) 8 | Low | Low | Low | Low | Low | Low | Low |
| Holland (2013) 18 | Low | Low | Low | Low | Low | Low | Low |
Only first author of each study is given.
Risk of bias
For the patient‐selection domain, all studies included patients with clinical suspicion of endometriosis. Most studies were considered to be at low risk of bias for patient selection, as there was a clear explanation of inclusion and exclusion criteria. One study was considered to be high risk because it used non‐consecutive recruitment and included patients with previous pelvic surgery 19 .
For the index‐test domain, all studies were considered to be low risk because they provided an adequate description of the method for the sliding‐sign assessment on TVS, as well as how it was interpreted. For the reference‐standard domain, all but one studies were likely to classify correctly the target condition using the reference standard, and one study did not describe in detail the surgical procedure performed 24 . For the flow‐and‐timing domain, the time elapsed between the index test and reference standard indicated a low risk of bias in two studies 8 , 18 and a high risk in two studies 21 , 22 . The risk for this domain was unclear in four studies 19 , 20 , 23 , 24 .
Applicability
In terms of applicability, all studies were deemed to include patients who were relevant to the review question. For the index‐test and reference‐standard domains, all studies presented low concerns regarding applicability.
Sliding sign on TVS for detection of pouch of Douglas obliteration
Pooled sensitivity, specificity, LR+, LR– and OR of the sliding sign on TVS for detecting pouch of Douglas obliteration were 88% (95% CI, 81–93%), 94% (95% CI, 91–96%), 15.3 (95% CI, 10.2–22.9), 0.12 (95% CI, 0.07–0.21) and 123 (95% CI, 62–244), respectively. Heterogeneity was moderate for sensitivity (Cochran's Q, 16.20; P = 0.01, I 2 = 69.1%) and low for specificity (Cochran's Q, 8.96; P = 0.11, I 2 = 44.2%) (Figure 2a). As heterogeneity was moderate, metaregression was performed. We observed that the differences in the prevalence of pouch of Douglas obliteration across studies could explain this heterogeneity (P < 0.01).
Figure 2.
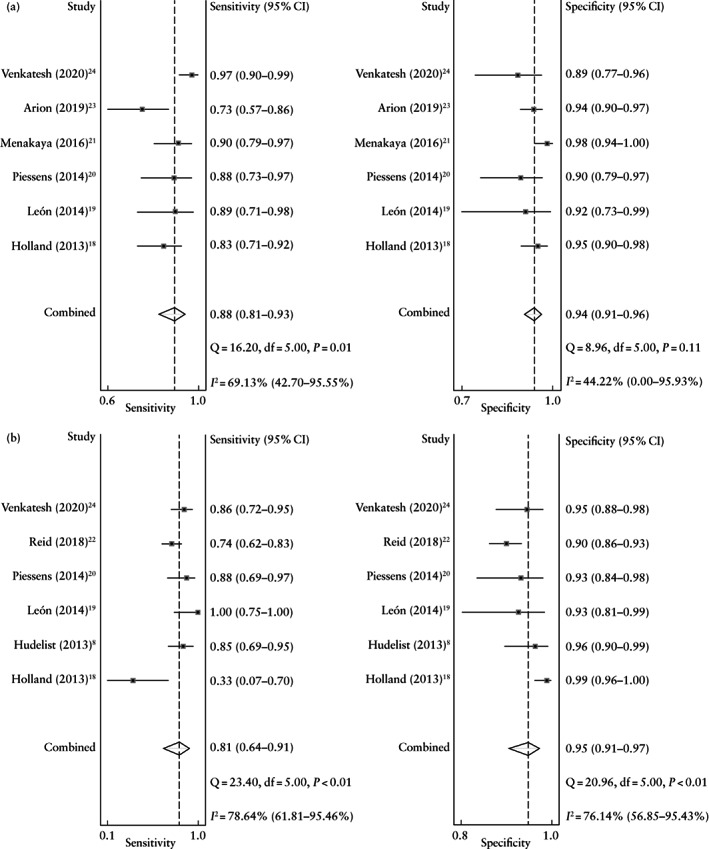
Forest plots showing pooled sensitivity and specificity of the sliding sign on transvaginal ultrasound in the detection of pouch of Douglas obliteration (a) and bowel involvement (b) in women with suspected endometriosis. Only first author of each study is given.
The area under the sROC curve was 0.97 (95% CI, 0.95–0.98) (Figure 3a). As shown in the Fagan nomogram (Figure 4a), a positive test on TVS (negative sliding sign) in women with suspected deep endometriosis significantly increased the pretest probability of pouch of Douglas obliteration on laparoscopy, from 37% to 90%, while a negative test (positive sliding sign) significantly decreased the pretest probability, from 37% to 7%. No publication bias was observed (P = 0.64).
Figure 3.
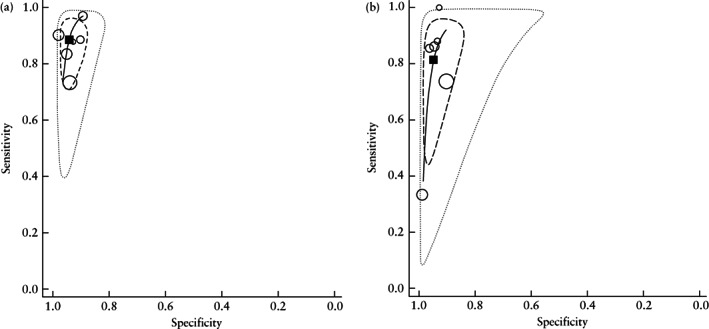
Summary receiver‐operating‐characteristics curves ( ) showing performance of the sliding sign on transvaginal ultrasound in the detection of pouch of Douglas obliteration (a) and bowel involvement (b) in women with suspected endometriosis.
) showing performance of the sliding sign on transvaginal ultrasound in the detection of pouch of Douglas obliteration (a) and bowel involvement (b) in women with suspected endometriosis.  , Study estimate;
, Study estimate;  , summary point;
, summary point;  , 95% confidence region;
, 95% confidence region;  , 95% prediction region.
, 95% prediction region.
Figure 4.
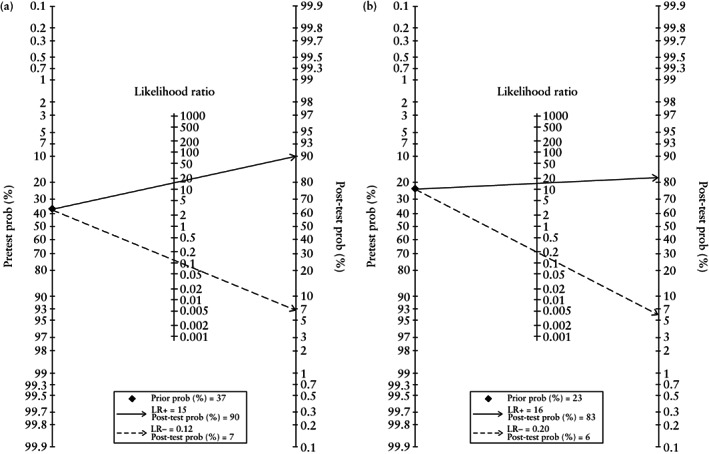
Fagan nomograms for detecting pouch of Douglas obliteration (a) and bowel involvement (b) based on negative ( ) and positive (
) and positive ( ) sliding sign on transvaginal ultrasound in women with suspected endometriosis. LR−, negative likelihood ratio; LR+, positive likelihood ratio; prob, probability.
) sliding sign on transvaginal ultrasound in women with suspected endometriosis. LR−, negative likelihood ratio; LR+, positive likelihood ratio; prob, probability.
Sliding sign on TVS for detection of bowel involvement
Pooled sensitivity, specificity, LR+, LR– and OR of the sliding sign on TVS for detecting bowel involvement were 81% (95% CI, 64–91%), 95% (95% CI, 91–97%), 16.0 (95% CI, 9.0–28.6), 0.20 (95% CI, 0.10–0.40) and 81 (95% CI, 34–191), respectively. Heterogeneity was high for both sensitivity (Cochran's Q, 23.40; P < 0.01, I 2 = 78.6%) and specificity (Cochran's Q, 20.96; P < 0.01, I 2 = 76.1%) (Figure 2b). As heterogeneity was high, meta‐regression was performed. We observed that the differences in the prevalence of bowel involvement across studies could explain this heterogeneity (P < 0.01).
The area under the sROC curve was 0.96 (95% CI, 0.94–0.98) (Figure 3b). As shown in the Fagan nomogram (Figure 4b), a positive test on TVS (negative sliding sign) in women with suspected deep endometriosis significantly increased the pretest probability of bowel involvement on laparoscopy, from 23% to 83%, while a negative test (positive sliding sign) significantly decreased the pretest probability, from 23% to 6%. No publication bias was observed (P = 0.14).
GRADE assessment and recommendation
Regarding GRADE assessment, evidence of high quality showed that the sliding sign as assessed by TVS has a high accuracy for detecting pouch of Douglas obliteration and bowel involvement in women with endometriosis (Tables 3 and 4). This assessment should be recommended for all women evaluated for this clinical entity.
Table 3.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment of the quality of evidence regarding the diagnostic accuracy of the sliding sign on transvaginal ultrasound for pouch of Douglas obliteration in women with suspected endometriosis
| Factors that may decrease CoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Studies (n)/ patients (n) | Study design | Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Effect per 1000 patients tested (95% CI) (n)* | Test accuracy CoE |
| True positive | Six studies/293 patients | Cohort diagnostic accuracy study | Not serious | Not serious | Not serious | Not serious | None | 326 (300–344) | ⨁⨁⨁⨁ High |
| False negative | 44 (26–70) | ||||||||
| True negative | Six studies/645 patients | Cohort diagnostic accuracy study | Not serious | Not serious | Not serious | Not serious | None | 592 (573–605) | ⨁⨁⨁⨁ High |
| False positive | 38 (25–57) | ||||||||
Pretest probability of 37%.
CoE, class of evidence.
Table 4.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment of the quality of evidence regarding the diagnostic accuracy of the sliding sign on transvaginal ultrasound for bowel involvement in women with suspected endometriosis
| Factors that may decrease CoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Studies (n)/ patients (n) | Study design | Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Effect per 1000 patients tested (95% CI) (n)* | Test accuracy CoE |
| True positive | Six studies/200 patients | Cohort diagnostic accuracy study | Not serious | Not serious | Not serious | Not serious | None | 186 (147–209) | ⨁⨁⨁⨁ High |
| False negative | 44 (21–83) | ||||||||
| True negative | Six studies/763 patients | Cohort diagnostic accuracy study | Not serious | Not serious | Not serious | Not serious | None | 731 (701–747) | ⨁⨁⨁⨁ High |
| False positive | 39 (23–69) | ||||||||
Pretest probability of 23%.
CoE, class of evidence.
DISCUSSION
Summary of evidence
In this meta‐analysis, we observed that the diagnostic performance of the TVS sliding sign for detecting pouch of Douglas obliteration and bowel involvement in women with suspected endometriosis was high, with pooled sensitivity of 88% and 81% and pooled specificity of 94% and 95%, respectively.
The studies included had mostly low risk of bias and low concerns regarding applicability. However, it should be borne in mind for the flow‐and‐timing domain that the time elapsed from TVS to laparoscopy was not reported in four studies. We cannot assume that the time elapsed between the two procedures was long; however, we believe that, if the time elapsed was long, it could be a potential confounding factor because the condition of the pelvis could become worse.
Interpretation of results
Our findings demonstrate that the sliding sign is an excellent ultrasound sign for detecting pouch of Douglas obliteration and bowel involvement in women undergoing surgery for suspected endometriosis. The heterogeneity observed across the studies analyzing pouch of Douglas obliteration was low for specificity and moderate for sensitivity, demonstrating comparability of the studies. However, the heterogeneity of the studies assessing bowel involvement was high. Therefore, in the latter case, our findings should be interpreted with caution.
These findings might be clinically relevant, as pouch of Douglas obliteration may increase the duration and complexity of surgery 25 . Having this information prior to surgery may be helpful to surgeons, as it may influence the choice of surgical technique, lead to involvement of a multidisciplinary surgical team and allow referral to the most appropriate practice 26 . Additionally, a negative sliding sign alone may be useful for identifying women with clinical suspicion of deep endometriosis who require further evaluation, for example, an examination by an expert sonologist to assess for the presence of classic signs of rectal infiltration. Furthermore, a negative sliding sign may be associated with sigmoid involvement. This may also constitute a reason for referring the patient for expert examination. However, five of the six studies on bowel involvement included in this meta‐analysis did not provide separate information on rectal and sigmoid involvement; therefore, a subgroup analysis could not be performed.
Some studies have shown that assessment of the sliding sign may have a short learning curve and be reproducible among expert examiners 4 , 27 , 28 , 29 . However, it is important to bear in mind that diagnostic performance depends on expertise and that not all trainees may reach competence 28 , 29 , 30 . Reproducibility should be tested in larger prospective studies.
We should also consider the fact that a negative sliding sign may be produced by inflammatory changes, for example due to pelvic surgery or pelvic inflammatory disease. This might be a confounding factor. In patients with such a medical history, it would be difficult to ascertain whether a negative sliding sign is related to endometriosis or to postsurgical/disease‐related inflammatory processes.
Strengths and limitations
Some limitations of this meta‐analysis should be considered. We believe that the main limitation is the small number of studies and patients included. Additionally, the reported prevalence of pouch of Douglas obliteration on laparoscopy may vary depending on the surgeon's skills. Most of the included studies did not provide details regarding this aspect, and we should not assume that inspection of the abdominal cavity was made by properly trained experienced surgeons. In fact, one study did not describe at all the surgical procedures performed 24 . However, we do not consider that this had a significant effect on the results of the quantitative synthesis.
The strengths of our study are that, to the best of our knowledge, it is the first meta‐analysis to analyze specifically the performance of the TVS sliding sign in detecting bowel involvement and that it provides up‐to‐date evidence on the diagnostic performance of this sign for obliteration of the pouch of Douglas in women with clinical suspicion of endometriosis.
Two previous meta‐analyses have assessed the diagnostic performance of TVS for detecting pouch of Douglas obliteration 31 , 32 . However, neither of them assessed specifically the sliding sign for detecting bowel involvement. Nisenblat et al. 31 assessed six studies that evaluated the diagnostic performance of TVS for detecting pouch of Douglas obliteration. Two of those studies have been included in our meta‐analysis 19 , 20 , but four of them were not9,33–35. We did not include these four studies because they reported data from series that overlapped with more recent studies from the same group 9 , 35 or did not report specifically on the sliding sign as a marker for diagnosing pouch of Douglas obliteration 33 , 34 . The pooled sensitivity and specificity reported by Nisenblat et al. (83% and 97%, respectively) were similar to those in our study.
Noventa et al. 32 reported data from eight studies that assessed the role of TVS in detecting pouch of Douglas obliteration. Two of these studies have been included in our meta‐analysis 18 , 19 . Six studies were excluded for one of the following reasons: overlapping data with more recent studies from the same group 9 , 34 , not describing the sliding sign 36 , 37 , data for constructing 2 × 2 table could not be extracted 38 or the study focused on transrectal ultrasound 39 . The pooled sensitivity and specificity reported by Noventa et al. were 80% and 95%, respectively.
Our meta‐analysis reports data from more recent studies and represents a larger series.
Conclusions
In conclusion, the TVS sliding sign seems to be an accurate method for the diagnosis of pouch of Douglas obliteration and bowel involvement in women with a clinical suspicion of pelvic endometriosis who undergo surgery when expert examiners perform the ultrasound examination. It remains to be seen whether the TVS sliding sign test performs equally well and is reproducible in the hands of less experienced examiners. The findings of this meta‐analysis confirm the fundamental role of TVS as a diagnostic tool in women with suspected endometriosis, suggested by previous studies 40 , 41 , 42 .
Supporting information
Appendix S1 Protocol for the systematic review and meta‐analysis
Appendix S2 Studies excluded after full‐text review
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Piessens S, Edwards A. Sonographic Evaluation for Endometriosis in Routine Pelvic Ultrasound. J Minim Invasive Gynecol 2020; 27: 265–266. [DOI] [PubMed] [Google Scholar]
- 2. Ghai V, Jan H, Shakir F, Haines P, Kent A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J Obstet Gynaecol 2020; 40: 83–89. [DOI] [PubMed] [Google Scholar]
- 3. Piketty M, Chopin N, Dousset B, Millischer‐Bellaische AE, Roseau G, Leconte M, Borghese B, Chapron C. Preoperative work‐up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first‐line imaging examination. Hum Reprod 2009; 24: 602–607. [DOI] [PubMed] [Google Scholar]
- 4. Holland TK, Hoo WL, Mavrelos D, Saridogan E, Cutner A, Jurkovic D. Reproducibility of assessment of severity of pelvic endometriosis using transvaginal ultrasound. Ultrasound Obstet Gynecol 2013; 41: 210–215. [DOI] [PubMed] [Google Scholar]
- 5. Bean E, Chaggar P, Thanatsis N, Dooley W, Bottomley C, Jurkovic D. Intra‐ and interobserver reproducibility of pelvic ultrasound for the detection and measurement of endometriotic lesions. Hum Reprod Open 2020; 2020: hoaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leonardi M, Espada M, Choi S, Chou D, Chang T, Smith C, Rowan K, Condous G. Transvaginal Ultrasound Can Accurately Predict the American Society of Reproductive Medicine Stage of Endometriosis Assigned at Laparoscopy. J Minim Invasive Gynecol 2020; 27: 1581–1587.e1. [DOI] [PubMed] [Google Scholar]
- 7. Guerriero S, Condous G, van den Bosch T, Valentin L, Leone FP, Van Schoubroeck D, Exacoustos C, Installé AJF, Martins WP, Abrao MS, Hudelist G, Bazot M, Alcazar JL, Gonçalves MO, Pascual MA, Ajossa S, Savelli L, Dunham R, Reid S, Menakaya U, Bourne T, Ferrero S, Leon M, Bignardi T, Holland T, Jurkovic D, Benacerraf B, Osuga Y, Somigliana E, Timmerman D. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol 2016; 48: 318–332. [DOI] [PubMed] [Google Scholar]
- 8. Hudelist G, Fritzer N, Staettner S, Tammaa A, Tinelli A, Sparic R, Keckstein J. Uterine sliding sign: a simple sonographic predictor for presence of deep infiltrating endometriosis of the rectum. Ultrasound Obstet Gynecol 2013; 41: 692–695. [DOI] [PubMed] [Google Scholar]
- 9. Reid S, Lu C, Casikar I, Reid G, Abbott J, Cario G, Chou D, Kowalski D, Cooper M, Condous G. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real‐time dynamic transvaginal ultrasound technique: the sliding sign. Ultrasound Obstet Gynecol 2013; 41: 685–691. [DOI] [PubMed] [Google Scholar]
- 10. Khong SY, Bignardi T, Luscombe G, Lam A. Is pouch of Douglas obliteration a marker of bowel endometriosis? J Minim Invasive Gynecol 2011; 18: 333–337. [DOI] [PubMed] [Google Scholar]
- 11. Bendifallah S, Puchar A, Vesale E, Moawad G, Daraï E, Roman H. Surgical Outcomes after Colorectal Surgery for Endometriosis: A Systematic Review and Meta‐analysis. J Minim Invasive Gynecol 2021; 28: 453–466. [DOI] [PubMed] [Google Scholar]
- 12. Working group of ESGE, ESHRE, and WES ; Keckstein J, Becker CM, Canis M, Feki A, Grimbizis GF, Hummelshoj L, Nisolle M, Roman H, Saridogan E, Tanos V, Tomassetti C, Ulrich UA, Vermeulen N, De Wilde RL. Recommendations for the surgical treatment of endometriosis. Part 2: deep endometriosis. Hum Reprod Open 2020; 2020: hoaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malzoni M, Casarella L, Coppola M, Falcone F, Iuzzolino D, Rasile M, Di Giovanni A. Preoperative Ultrasound Indications Determine Excision Technique for Bowel Surgery for Deep Infiltrating Endometriosis: A Single, High‐Volume Center. J Minim Invasive Gynecol 2020; 27: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analysis: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sotiriadis A, Papatheodorou SI, Martins WP. Synthesizing Evidence from Diagnostic Accuracy Tests: the SEDATE guideline. Ultrasound Obstet Gynecol 2016; 47: 386–395. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. Evid Based Med 2008; 13: 162–163. [DOI] [PubMed] [Google Scholar]
- 18. Holland TK, Cutner A, Saridogan E, Mavrelos D, Pateman K, Jurkovic D. Ultrasound mapping of pelvic endometriosis: does the location and number of lesions affect the diagnostic accuracy? A multicentre diagnostic accuracy study. BMC Womens Health 2013; 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. León M, Vaccaro H, Alcázar JL, Martinez J, Gutierrez J, Amor F, Iturra A, Sovino H. Extended transvaginal sonography in deep infiltrating endometriosis: use of bowel preparation and an acoustic window with intravaginal gel: preliminary results. J Ultrasound Med 2014; 33: 315–321. [DOI] [PubMed] [Google Scholar]
- 20. Piessens S, Healey M, Maher P, Tsaltas J, Rombauts L. Can anyone screen for deep infiltrating endometriosis with transvaginal ultrasound? Aust N Z J Obstet Gynaecol 2014; 54: 462–468. [DOI] [PubMed] [Google Scholar]
- 21. Menakaya U, Reid S, Lu C, Gerges B, Infante F, Condous G. Performance of ultrasound‐based endometriosis staging system (UBESS) for predicting level of complexity of laparoscopic surgery for endometriosis. Ultrasound Obstet Gynecol 2016; 48: 786–795. [DOI] [PubMed] [Google Scholar]
- 22. Reid S, Espada M, Lu C, Condous G. To determine the optimal ultrasonographic screening method for rectal/rectosigmoid deep endometriosis: Ultrasound “sliding sign”, transvaginal ultrasound direct visualization or both? Acta Obstet Gynecol Scand 2018; 97: 1287–1292. [DOI] [PubMed] [Google Scholar]
- 23. Arion K, Aksoy T, Allaire C, Noga H, Williams C, Bedaiwy MA, Yong PJ. Prediction of pouch of Douglas obliteration: point‐of‐care ultrasound versus pelvic examination. J Minim Invasive Gynecol 2019; 26: 928–934. [DOI] [PubMed] [Google Scholar]
- 24. Venkatesh S, Anjali M, Vasudeva A, Kumar P. Sliding Sign and Gel Sonovaginography: A Sneak Peek Prior to Laparoscopy in Patients with Endometriosis. J Hum Reprod Sci 2020; 13: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonardi M, Gibbons T, Armour M, Wang R, Glanville E, Hodgson R, Cave AE, Ong J, Tong YYF, Jacobson TZ, Mol BW, Johnson NP, Condous G. When to Do Surgery and When Not to Do Surgery for Endometriosis: A Systematic Review and Meta‐analysis. J Minim Invasive Gynecol 2020; 27: 390–407.e3. [DOI] [PubMed] [Google Scholar]
- 26. Espada M, Leonardi M, Aas‐Eng K, Lu C, Reyftmann L, Tetstall E, Slusarczyk B, Ludlow J, Hudelist G, Reid S, Condous G. A Multicenter International Temporal and External Validation Study of the Ultrasound‐based Endometriosis Staging System. J Minim Invasive Gynecol 2021; 28: 57–62. [DOI] [PubMed] [Google Scholar]
- 27. Tammaa A, Fritzer N, Strunk G, Krell A, Salzer H, Hudelist G. Learning curve for the detection of pouch of Douglas obliteration and deep infiltrating endometriosis of the rectum. Hum Reprod 2014; 29: 1199–1204. [DOI] [PubMed] [Google Scholar]
- 28. Menakaya U, Infante F, Lu C, Phua C, Model A, Messyne F, Brainwood M, Reid S, Condous G. Interpreting the real‐time dynamic ‘sliding sign’ and predicting pouch of Douglas obliteration: an interobserver, intraobserver, diagnostic‐accuracy and learning‐curve study. Ultrasound Obstet Gynecol 2016; 48: 113–120. [DOI] [PubMed] [Google Scholar]
- 29. Leonardi M, Ong J, Espada M, Stamatopoulos N, Georgousopoulou E, Hudelist G, Condous G. One‐Size‐Fits‐All Approach Does Not Work for Gynecology Trainees Learning Endometriosis Ultrasound Skills. J Ultrasound Med 2020; 39: 2295–2303. [DOI] [PubMed] [Google Scholar]
- 30. Reid S, Lu C, Casikar I, Mein B, Magotti R, Ludlow J, Benzie R, Condous G. The prediction of pouch of Douglas obliteration using offline analysis of the transvaginal ultrasound ‘sliding sign’ technique: inter‐ and intra‐observer reproducibility. Hum Reprod 2013; 28: 1237–1246. [DOI] [PubMed] [Google Scholar]
- 31. Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non‐invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016, 2: CD009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noventa M, Saccardi C, Litta P, Vitagliano A, D'Antona D, Abdulrahim B, Duncan A, Alexander‐Sefre F, Aldrich CJ, Quaranta M, Gizzo S. Ultrasound techniques in the diagnosis of deep pelvic endometriosis: algorithm based on a systematic review and meta‐analysis. Fertil Steril 2015; 104: 366–383.e2. [DOI] [PubMed] [Google Scholar]
- 33. Holland TK, Yazbek J, Cutner A, Saridogan E, Hoo WL, Jurkovic D. Value of transvaginal ultrasound in assessing severity of pelvic endometriosis. Ultrasound Obstet Gynecol 2010; 36: 241–248. [DOI] [PubMed] [Google Scholar]
- 34. Hudelist G, Ballard K, English J, Wright J, Banerjee S, Mastoroudes H, Thomas A, Singer CF, Keckstein J. Transvaginal sonography vs clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2011; 37: 480–487. [DOI] [PubMed] [Google Scholar]
- 35. Reid S, Lu C, Hardy N, Casikar I, Reid G, Cario G, Chou D, Almashat D, Condous G. Office gel sonovaginography for the prediction of posterior deep infiltrating endometriosis: a multicenter prospective observational study. Ultrasound Obstet Gynecol 2014; 44: 710–718. [DOI] [PubMed] [Google Scholar]
- 36. Abrao MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod 2007; 22: 3092–3097. [DOI] [PubMed] [Google Scholar]
- 37. Carbognin G, Girardi V, Pinali L, Raffaelli R, Bergamini V, Pozzi Mucelli R. Assessment of pelvic endometriosis: correlation of US and MRI with laparoscopic findings. Radiol Med 2006; 111: 687–701. [DOI] [PubMed] [Google Scholar]
- 38. Fratelli N, Scioscia M, Bassi E, Musola M, Minelli L, Trivella G. Transvaginal sonography for preoperative assessment of deep endometriosis. J Clin Ultrasound 2013; 41: 69–75. [DOI] [PubMed] [Google Scholar]
- 39. Bazot M, Detchev R, Cortez A, Amouyal P, Uzan S, Daraï E. Transvaginal sonography and rectal endoscopic sonography for the assessment of pelvic endometriosis: a preliminary comparison. Hum Reprod 2003; 18: 1686–1692. [DOI] [PubMed] [Google Scholar]
- 40. Guerriero S, Ajossa S, Minguez JA, Jurado M, Mais V, Melis GB, Alcazar JL. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2015; 46: 534–545. [DOI] [PubMed] [Google Scholar]
- 41. Guerriero S, Ajossa S, Orozco R, Perniciano M, Jurado M, Melis GB, Alcazar JL. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in the rectosigmoid: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2016; 47: 281–289. [DOI] [PubMed] [Google Scholar]
- 42. Guerriero S, Martinez L, Gomez I, Pascual MA, Ajossa S, Pagliuca M, Alcázar JL. Diagnostic accuracy of transvaginal sonography for detecting parametrial involvement in women with deep endometriosis: systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2021; 58: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Protocol for the systematic review and meta‐analysis
Appendix S2 Studies excluded after full‐text review
Data Availability Statement
Data are available upon reasonable request.


