Summary
Adjuvant chemotherapy is the standard treatment for patients with resectable pancreatic ductal carcinoma. Perioperative chemotherapy has been given in less than 50% of patients with potentially resectable pancreatic cancer in Japan. A modified combination regimen of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX; oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 150 mg/m2 on day 1, and 5-fluorouracil 2,400 mg/m2 over 46 hours every 14 days for 12 cycles) is now preferred worldwide because it mitigates concerns regarding toxicity and tolerance. Adjuvant chemotherapeutic regimens employ S-1 in East Asia, whereas other areas use FOLFIRINOX, capecitabine plus gemcitabine, or gemcitabine monotherapy. Adjuvant chemoradiotherapy is not recommended because randomized controlled trials and meta-analyses revealed no survival benefit compared with chemotherapy. Preoperative chemotherapy with S-1 and gemcitabine combination chemotherapy for patients with resectable/borderline resectable pancreatic cancer significantly increased survival compared to upfront surgery in a recent clinical trial. Perioperative outcomes, including R0 resection rate and post-operative morbidity, were not significantly different between groups. When compared to upfront surgery, neoadjuvant S-1 and gemcitabine treatment significantly reduced the number of pathological nodal metastases in patients who underwent resection. Japanese guidelines therefore recommend neoadjuvant chemotherapy for patients with resectable pancreatic cancer. Preoperative chemotherapy can increase R0 cases by down-staging with higher relative dose intensity of chemotherapy. In contrast, patients who do not respond to chemotherapy may miss resection opportunities and would therefore be at a disadvantage. Therefore, it is critical for both patients and doctors that predictive markers for the response to chemotherapy are identified.
Keywords: FOLFIRINOX, gemcitabine, S-1, oxaliplatin, excision repair cross-complementing gene 1 (ERCC1)
Introduction
Pancreatic cancer is the seventh leading cause of cancer-related deaths worldwide; in 2020, there were 496,000 new cases of pancreatic cancer and 466,000 deaths due to the disease (1). The number of cancer deaths in 2019 in Japan was approximately 370,000 (2). The number of male cancer deaths was 1.5 times greater than that of female cancer deaths. Lung was the leading site (24.2%) for males in mortality, followed by stomach (12.7%), colon/rectum (12.4%), pancreas (8.2%), and liver (7.6%). The leading site for females was colon/ rectum (15.4%), followed by lung (14.1%), pancreas (11.7%), stomach (9.5%), and breast (9.5%). In Japan, 18,124 males and 18,232 females died of pancreatic cancer in 2019, making this malignancy the fourth leading cause of cancer-related deaths in the country (2). The proportions of patients in Japan with clinical stage I, II, III, and IV disease in 2018 were 24.5%, 11.9%, 13.1%, and 44.3%, respectively (2). The 5-year overall survival rates of patients with pathological stage I, II, III, and IV disease were 39.9%, 16.4%, 5.8%, and 1.3%, respectively. The number of patients with pancreatic cancer has increased steadily since 1955 (3). Surgery alone, chemotherapy alone, surgery plus chemotherapy, and no therapy underwent: 25.9%, 10.0%, 43.7%, 14.8% in preoperative clinical stage I, 18.9%, 20.9%, 36.0%, 18.4% in stage II, and 1.8%, 59.6%, 7.0%, 20.8% in stage III in Japan. Regardless of disease stage, fewer than 50% of patients with resectable pancreatic cancer in Japan receive adjuvant chemotherapy (2). Perioperative chemotherapy has been given in less than 50% of patients with potentially resectable pancreatic cancer.
In this review, present status and perspective of perioperative chemotherapy in Japan and overseas for pancreatic cancer are described.
Prognosis in resectable pancreatic cancer
Based on the results from several phase III trials (4,5), adjuvant chemotherapy has become the standard treatment for patients with resectable pancreatic ductal carcinoma (Figure 1). However, data from the Medicare database shows that only 7% of 2,440 patients who underwent upfront resection for pancreatic cancer completed adjuvant chemotherapy; 65% of the patients received no adjuvant chemotherapy and 28% received incomplete therapy. Factors that were significantly associated with chemotherapy completion were nodal metastases, comorbidities, and treatment at a National Cancer Institute-designated cancer center (6). The median overall survival (OS) was 14 months for patients who received no adjuvant chemotherapy, 17 months for those receiving incomplete chemotherapy, and 22 months for those who completed the chemotherapy regimen. Therefore, completion of adjuvant chemotherapy should be the goal after upfront resection, and neoadjuvant chemotherapy may ensure that patients then receive systemic chemotherapy (6).
Figure 1.
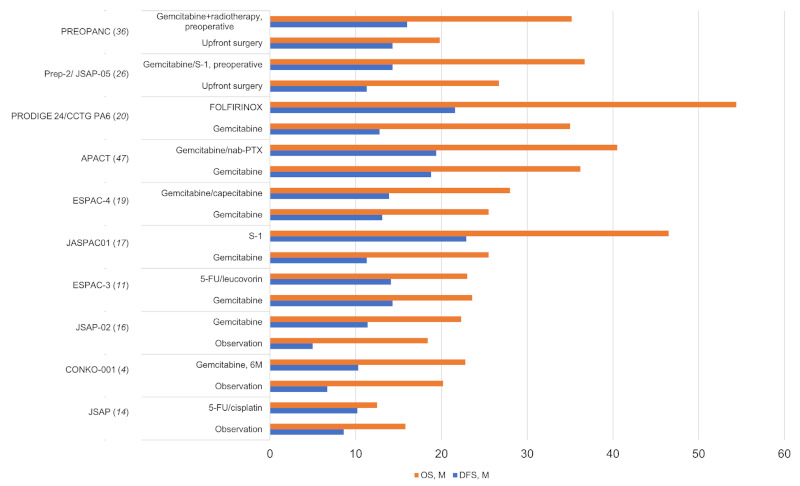
Overall survival (OS) and disease-free survival (DFS) of resectable pancreatic cancer in clinical trials of adjuvant and neoadjuvant chemotherapy. 5-FU, 5-fluorouracil; nab-PTX, nab paclitaxel; FOLFOXIRI, 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan; M, months.
Meta-analysis of 27 studies suggested neoadjuvant chemotherapy prolonged survival compared with an upfront surgery approach [Hazard ratio 0.72 (95% CI, 0.69-0.76)]. In addition, R0 resection rates were significantly higher in patients who received neoadjuvant chemotherapy (7). Of 35,599 patients with stage I to III pancreatic adenocarcinoma in the National Cancer Database, 3,395 (9%) underwent neoadjuvant chemotherapy, 19,865 (56%) received adjuvant chemotherapy, and 12,299 (35%) underwent surgery alone. Cox-regression analysis showed superior OS in the neoadjuvant chemotherapy group compared with patients receiving adjuvant chemotherapy or surgery alone (26 vs. 23 vs. 14 months, p < 0.001) (8). Analysis of data in the National Cancer Data Base (1998-2011) from 18,243 patients with Stage I or II pancreatic adenocarcinoma who underwent pancreaticoduodenectomy revealed that 1,375 (7.5%) received neoadjuvant therapy. Over this time frame, the use of neoadjuvant therapy increased from 4.3% to 17.0%. Patients receiving neoadjuvant therapy were more likely to receive treatment at an academic facility (64.4% vs. 51.4%, p < 0.001). Patients who received neoadjuvant therapy were more likely to have negative margins (77.8% vs. 85.5%, p < 0.001) and negative lymph nodes (42.9% vs. 59.3%, p < 0.001) (9).
In the European Study Group for Pancreatic Cancer (ESPAC)-3 randomized controlled trial, the median OS was 24.9 (22.9-27.2) months for 646 (56.1%) patients with resection margin negative (R0 > 1 mm) tumors, 25.4 (21.6-30.4) months for 146 (12.7%) patients with R1 < 1 mm positive resection margins, and 18.7 (17.2- 21.1) months for 359 (31.2%) patients with R1-direct positive margins (p < 0.001) (10,11). Multivariate analysis indicated that overall R1-direct tumor margins, poor tumor differentiation, and positive lymph node status were all independently and significantly associated with reduced OS and recurrence-free survival (RFS). Resection margin involvement was also associated with an increased risk for local recurrence (10). Patients with borderline resectable/ locally advanced pancreatic ductal adenocarcinoma are often treated with 5-FU, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) to obtain a margin-negative resection, yet selection of patients for resection remains challenging. One hundred and forty-one patients underwent exploratory surgery (borderline, 49%; locally advanced, 51%) and 110 (78%) underwent tumor resection in Massachusetts General Hospital. Although resected patients had lower preoperative CA 19-9 levels (21 vs. 40 U/mL, p = 0.03) and smaller tumors on preoperative computed tomography scan (2.3 vs. 3.0 cm, p = 0.03), no predictors of resectability were identified. Disease-free survival (DFS) and OS were significantly better for borderline resectable/ locally advanced pancreatic cancer patients treated with neoadjuvant FOLFIRINOX compared with upfront resected patients (DFS, 29.1 vs. 13.7, p < 0.001; OS, 37.7 vs. 25.1 months from diagnosis, p = 0.01) (12).
The updated American Society of Clinical Oncology (ASCO) clinical practice guidelines state that all patients with resected pancreatic adenocarcinoma who did not receive preoperative therapy should be offered 6 months of adjuvant chemotherapy in the absence of medical or surgical contraindications (13). The modified combination regimen of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX; oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 150 mg/ m2 on day 1, and 5-fluorouracil 2,400 mg/m2 over 46 hours every 14 days for 12 cycles) is now preferred to mitigate concerns regarding toxicity or tolerance; alternatively, doublet therapy with gemcitabine and capecitabine or monotherapy with gemcitabine alone or fluorouracil plus leucovorin alone can be offered (13).
Clinical trials of adjuvant chemotherapy
The Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer (JSAP) conducted a randomized controlled trial in patients who underwent surgical resection of pancreatic cancer with clear histological margin between 1992 and 2000. The aim was to evaluate the efficacy of adjuvant chemotherapy with 5-fluorouracil plus cisplatin compared to observation alone (14). Adjuvant 5-fluorouracil plus cisplatin provided no survival benefit, and lymph node involvement and moderately or poorly differentiated tubular adenocarcinoma versus well-differentiated tubular or papillary adenocarcinoma were factors associated with significantly worse prognosis (14). However, a meta-analysis suggested that adjuvant fluorouracil-based chemotherapy provided some survival benefit (15).
S-1 is considered a standard adjuvant therapy in Japan based on the results of a randomized trial (16) that showed oral S-1 (a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine drug) with gemcitabine was superior (in terms of OS) to observation alone in the Charité Onkologie (CONKO)-001 and JSAP-02 trials (4,17) . While S-1 can be used in Caucasian populations, it has only been approved in a limited number of western countries (18). While direct comparisons of S-1 efficacy in combination with gemcitabine in western populations are therefore not possible, the European ESPAC-3 trial demonstrated that gemcitabine was not superior to a combination of leucovorin with fluorouracil, a fluoropyrimidine (11). Capecitabine plus gemcitabine showed superiority to gemcitabine monotherapy in the ESPAC-4 study (19). Furthermore, FOLFIRINOX showed significantly superior activities to gemcitabine in phase III trials (PRODIGE [Partenariat de Recherche en Oncologie Digestive] 24-ACCORD [Actions Concertées dans les Cancers Colorectaux et Digestifs] 24 and CCTG PA [Canadian Cancer Trials Group Pancreatic Adenocarcinoma] 6) that were conducted in France and Canada (20). The modified FOLFIRINOX regimen consisted of oxaliplatin (85 mg/m2) delivered as a 2-hour intravenous infusion, followed by leucovorin (400 mg/m2) given as a 2-hour intravenous infusion, and after 30 minutes, the addition of irinotecan (180 mg/ m2) administered as a 90-minute intravenous infusion, immediately followed by fluorouracil (2400 mg/m2) administered by continuous intravenous infusion over a period of 46 hours, every 14 days for 24 weeks (12 cycles).
Because of the morbidity and vulnerability often observed in patients after pancreatectomy, bolus fluorouracil was not administered; this allowed the maintenance of FOLFIRINOX dose intensity and avoided severe or prolonged neutropenia. After the enrollment of 162 patients, however, the dose of irinotecan was reduced to 150 mg/m2 due to incidences of neutropenia, in accordance with protocol-specified interim safety analysis. In cases of febrile neutropenia or delays in treatment administration due to neutropenia, the use of granulocyte colony-stimulating factor, G-CSF, was advised for the following cycles. Irinotecan-induced severe neutropenia is associated with homozygosity at the UGT1A1*28 or UGT1A1*6 alleles. The allele frequency of UGT1A1*28 is lower in Asians than in Caucasians, and grade 3 or more neutropenia is associated with UGT1A1*6 polymorphisms in Asians (21). The homozygotes and double heterozygotes of UGT1A1*6 and *28 (*6/*6, *28/*28 and *6/*28) were significantly associated with severe neutropenia in patients who received irinotecan monotherapy (21,22). Additional dose reduction of irinotecan in the adjuvant FOLFIRINOX setting is required in patients with UGT1A1 alleles that are associated with poor metabolism of the drug. The most highly quoted adjuvant trials (PRODIGE 24-ACCORD 24-CCTG PA 6, CONKO-001, and ESPAC-4) enrolled between 0.6 and 1.5 patients per center per year, and the number of eligible patients (at each center) should be given the most attention (23).
Adjuvant chemotherapeutic regimens have commonly used S-1 in East Asia whereas FOLFIRINOX, capecitabine plus gemcitabine, or gemcitabine monotherapy are used in Western countries. Adjuvant chemoradiotherapy is not recommended because randomized controlled trials and meta-analyses indicated that they provided no additional survival benefit compared with chemotherapy (15,24).
Neoadjuvant chemotherapy
Many patients fail to complete courses of postoperative adjuvant chemotherapy due to postoperative complications, poor oral intake, or poor PS. In regard to tumor excision, pancreatic cancer is classified into three groups; resectable, borderline resectable, and unresectable. Even clinically localized pancreatic cancer is associated with the highest probability of harboring radiographically occult metastatic disease. Preoperative chemotherapy with S-1 and gemcitabine (NAC-GS) for patients with resectable and borderline resectable pancreatic cancer significantly increased survival compared to upfront surgery in the Prep-02/ JSAP-05 phase II/III trial (25-28). The median OS was 36.7 months in the NAC-GS group and 26.6 months in patients who received upfront surgery (HR 0.72, 95% CI 0.55-0.94, p = 0.015). Although grade 3 or 4 adverse events of leucopenia and neutropenia were frequently (73%) observed in the NAC-GS group, there was no significant difference between groups with respect to perioperative outcomes including R0 resection rate and post-operative morbidity (26,29). A significant decrease in pathological nodal metastases in the NAC-GS group (60%) was noted compared to upfront surgery (82%) for resected patients (p < 0.01). The frequency of hepatic metastasis after surgery was significantly reduced in the NAC-GS group (30%) compared to upfront surgery (48%) (28). Hence, Japanese guidelines recommend neoadjuvant chemotherapy for patients with resectable pancreatic cancer (30).
Preoperative chemotherapy can increase R0 cases by down-staging with higher relative dose intensity of chemotherapy, while non-responders are disadvantaged since they miss resection opportunities during chemotherapy. Outside Japan, many clinical trials of preoperative chemotherapy have been conducted but not completed due to low accrual rates. The low enrollment is due to the reluctance of surgeons to risk disease progression while patients are receiving chemotherapy, as progression can render the patients ineligible for tumor resection. The Southwest Oncology Group (SWOG) S1505 was a randomized phase II trial of perioperative mFOLFIRINOX compared with gemcitabine plus nab-paclitaxel in patients with either resectable or borderline resectable pancreatic cancer. Eighty-two percent of patients completed all intended neoadjuvant chemotherapy and surgery; the resectability rate in the group with resectable disease was 92%, and R0 resection rates were 47% and 48% in the FOLFIRINOX and gemcitabine plus nab-paclitaxel arms, respectively. The median OS and DFS were 23.2 and 10.9 months in the FOLFIRINOX arm, and 23.6 and 14.2 months in the gemcitabine plus nab-paclitaxel arm; there was thus no significant difference in clinical outcomes between the two treatment regimens (31). In total, 11 out of 68 patients had postoperative grade 3 or 4 adverse events. The most common postoperative adverse events included anemia (n = 6), abnormal liver function tests (n = 5), anorexia/nausea/vomiting (n = 5), and dehydration/diarrhea (n = 3). A total of 61 patients started postoperative adjuvant therapy and 46 completed adjuvant therapy in SWOG S1505. A021806, a phase III trial of perioperative vs. postoperative FOLFIRINOX, and NEPAFOX, a phase II/III trial of perioperative FOLFIRINOX vs. postoperative gemcitabine, and are ongoing (32,33).
The benefit of adjuvant chemotherapy after resection of pancreatic cancer following neoadjuvant combination treatment with FOLFIRINOX is unclear. A retrospective cohort study showed no survival difference for patients who received adjuvant chemotherapy vs. those who did not (median OS, 29 vs. 29 months; HR 0.99, p = 0.93) (34). In patients with pathologically node-positive disease, adjuvant chemotherapy was associated with improved survival (median OS, 26 vs. 13 months; multivariable HR 0.41, p = 0.004).
Neoadjuvant chemoradiotherapy
In the National Cancer Database, data regarding use of neoadjuvant and adjuvant therapies was available for 8,472 of 9,795 patients (86%) who underwent surgery for clinical T1 or T2 pancreatic head adenocarcinoma. Seven hundred and seventy-four (9.1%) received neoadjuvant and 435 (5.1%) received chemoradiotherapy. Neoadjuvant chemotherapy was found to lower positive margin rates from 21.8 to 15.5% (p < 0.0001), and when radiotherapy was added this rate dropped to 13.4%. Positive margins were associated with worse overall survival (14.9 vs. 23.9 months; HR 1.702, p < 0.0001) (35).
In the recent PREOPANC trial, neoadjuvant chemoradiotherapy with gemcitabine did not prolong overall survival compared with upfront surgery for patients with resectable or borderline resectable pancreatic cancer. Patients were randomly assigned to one of two groups. In the first group, patients received preoperative chemoradiotherapy consisting of 3 courses of gemcitabine (the second course was combined with 15 × 2.4 Gy radiotherapy) followed by surgery, then received 4 courses of gemcitabine as adjuvant setting. In the second group, patients received 6 courses of adjuvant gemcitabine after upfront surgery. The median OS was 16.0 months with neoadjuvant chemoradiotherapy and 14.3 months with upfront surgery (HR 0.78, 95% CI 0.58-1.05, p = 0.096). The R0 resection rate was 71% (51/72) in patients who received preoperative chemoradiotherapy and 40% (37/92) in patients assigned to upfront surgery (p < 0.001) (36).
Future perspectives
Neoadjuvant chemotherapy will become standard therapy for resectable pancreatic cancer. Mutations in BRCA1/2 and PALB2 genes are present in approximately 5% to 10% of patients with pancreatic cancer (37). The presence of DNA damage repair gene mutations such as ATM, BRCA1/2, CHEK2, PALB are associated with improved OS in metastatic pancreatic cancer patients treated with FOLFIRINOX (38-40). Cisplatin and gemcitabine combination therapy is an active regimen in advanced germline BRCA1/2 and PALB2 pancreatic cancer. The addition of veliparib to cisplatin and gemcitabine was not superior to cisplatin and gemcitabine, and the triplet combination was notable for increased hematologic toxicity relative to the doublet. The median OS was 15.5 months for the triplet therapy and 16.4 months for the doublet. The response rate for the triplet was 74% and 65% for the doublet (p = 0.55). The small increase in response rate with triplet therapy does not offset the negative effects of increased hematotoxicity, and therefore the triplet regimen may not be the optimal choice in the current neoadjuvant setting.
Patients and doctors would greatly benefit from a panel of factors that predict the response to chemotherapy. DNA repair systems allow cells to overcome the DNA damage induced by chemotherapy. DNA interstrand, intrastrand, and DNA-protein crosslinks caused by cisplatin and oxaliplatin are repaired by the nuclear excision repair pathway, of which excision repair cross-complementation group 1 (ERCC1) is an essential part. In the JCOG9912 trial involving patients with advanced gastric cancer, low ERCC1 expression was a significant independent favorable prognostic factor in those who received first-line chemotherapy, regardless of treatment regimen (41). FOLFIRINOX was more effective in metastatic pancreatic cancer patients with lower expression of ERCC1 mRNA than in those with higher expression (42). The median OS in "ERCC1 low" vs. "ERCC1 high" patients was 16 vs. 8 months (HR 0.23, 95% CI 0.12-0.46, p < 0.0001), and disease control rate was 93% vs. 50% (p = 0.00006). These data indicate that ERCC1 could therefore be an effective predictor of response to FOLFIRINOX also in pancreatic cancer. In an animal model, high ERCC1 expression led to cisplatin resistance and restored the ability of cells to displace cisplatin from DNA. Fluoropyrimidines can induce a variety of DNA damage in human cancer cell lines due to its functional interaction with enzymes involved in DNA repair, leading to the activation of downstream factors such as p53. The expression of wild-type p53 was a strong predictor of sensitivity to 5-FU in cell lines of the National Cancer Institute's Anticancer Drug Screen panel in vitro (43).
Positive circulating tumor (ct) DNA indicated significantly poorer OS in patients with resectable pancreatic cancer (at baseline, HR 2.27, 95%CI 1.13- 4.56; postoperative, HR 3.66, 95% CI 1.45-9.28). Patients with detectable ctDNA tended to have a higher risk for disease recurrence than those without detectable ctDNA (at baseline, HR 1.96, 95% CI 0.65- 5.87; postoperative, HR 2.20, 95% CI 0.99-4.87). The results were consistent regardless of whether ctDNA was detected pre- or post-operation. Intensive chemotherapy is required for ctDNA positive resectable pancreatic cancer, however, the number of patients who can complete a full course of FOLFIRINOX is limited (44-46).
In summary, the current strategies used against pancreatic cancer need to be modified with regard to innovative treatments with current drugs and/or novel patient selection strategies. Such approaches will be facilitated by correlating "omic" data from clinical samples with patient clinical characteristics and drug responses, and will lead to improved survival and quality of life.
Funding: None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Center Japan. Cancer Statistics in Japan. https://ganjoho.jp/en/professional/statistics/table_download.html (accessed September 7, 2021).
- 3. National Cancer Center Japan. Cancer Statistics in Japan. Hospital Cancer Registry Survival Rate Summary. https://ganjoho.jp/reg_stat/statistics/brochure/hosp_c_reg_surv.html (accessed September 7, 2021). (in Japanese) .
- 4. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007; 297:267-277. [DOI] [PubMed] [Google Scholar]
- 5. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013; 310:1473-1481. [DOI] [PubMed] [Google Scholar]
- 6. Altman AM, Wirth K, Marmor S, Lou E, Chang K, Hui JYC, Tuttle TM, Jensen EH, Denbo JW. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2019; 26:4108-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rangarajan K, Pucher PH, Armstrong T, Bateman A, Hamady Z. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta-analysis. Ann R Coll Surg Engl. 2019; 101:453-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macedo FI, Picado O, Hosein PJ, Dudeja V, Franceschi D, Mesquita-Neto JW, Yakoub D, Merchant NB. Does neoadjuvant chemotherapy change the role of regional lymphadenectomy in pancreatic cancer survival? Pancreas. 2019; 48:823-831. [DOI] [PubMed] [Google Scholar]
- 9. Youngwirth LM, Nussbaum DP, Thomas S, Adam MA, Blazer DG, 3rd, Roman SA, Sosa JA. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18 243 patients. J Surg Oncol. 2017; 116:127-132. [DOI] [PubMed] [Google Scholar]
- 10. Ghaneh P, Kleeff J, Halloran CM, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg. 2019; 269:520-529. [DOI] [PubMed] [Google Scholar]
- 11. Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010; 304:1073-1081. [DOI] [PubMed] [Google Scholar]
- 12. Michelakos T, Pergolini I, Castillo CF, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment With FOLFIRINOX. Ann Surg. 2019; 269:733-740. [DOI] [PubMed] [Google Scholar]
- 13. Khorana AA, McKernin SE, Berlin J, Hong TS, Maitra A, Moravek C, Mumber M, Schulick R, Zeh HJ, Katz MHG. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019; 37:2082-2088. [DOI] [PubMed] [Google Scholar]
- 14. Kosuge T, Kiuchi T, Mukai K, Kakizoe T; Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer (JSAP). A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol. 2006; 36:159-165. [DOI] [PubMed] [Google Scholar]
- 15. Stocken DD, Buchler MW, Dervenis C, Bassi C, Jeekel H, Klinkenbijl JH, Bakkevold KE, Takada T, Amano H, Neoptolemos JP; Pancreatic Cancer Meta-analysis Group. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005; 92:1372-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016; 388:248-257. [DOI] [PubMed] [Google Scholar]
- 17. Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M, Shimada M, Kanemitsu K. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009; 101:908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winther SB, Bjerregaard JK, Schonnemann KR, Ejlsmark MW, Krogh M, Jensen HA, Pfeiffer P. S-1 (Teysuno) and gemcitabine in Caucasian patients with unresectable pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2018; 81:573-578. [DOI] [PubMed] [Google Scholar]
- 19. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017; 389:1011-1024. [DOI] [PubMed] [Google Scholar]
- 20. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018; 379:2395-2406. [DOI] [PubMed] [Google Scholar]
- 21. Minami H, Sai K, Saeki M, et a l. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics. 2007; 17:497-504. [DOI] [PubMed] [Google Scholar]
- 22. Satoh T, Ura T, Yamada Y, Yamazaki K, Tsujinaka T, Munakata M, Nishina T, Okamura S, Esaki T, Sasaki Y, Koizumi W, Kakeji Y, Ishizuka N, Hyodo I, Sakata Y. Genotype-directed, dose-finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphisms. Cancer Sci. 2011; 102:1868-1873. [DOI] [PubMed] [Google Scholar]
- 23. Evans DB. The complexity of neoadjuvant therapy for operable pancreatic cancer: lessons learned from SWOG S1505. Ann Surg. 2020. 272:487. [DOI] [PubMed] [Google Scholar]
- 24. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004; 350:1200-1210. [DOI] [PubMed] [Google Scholar]
- 25. Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, Matsuyama Y, Unno M; Study Group of Preoperative Therapy for Pancreatic Cancer (Prep) and Japanese Study Group of Adjuvant Therapy for Pancreatic cancer (JSAP). Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/ JSAP05). Jpn J Clin Oncol. 2019; 49:190-194. [DOI] [PubMed] [Google Scholar]
- 26. Unno M, Motoi F, Matsuyama Y, Satoi S, Matsumoto I, Aosasa S, Shirakawa H, Wada K, Fujii T, Yoshitomi H, Takahashi S, Sho M, Ueno H, Kosuge T. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. 2019; 37:suppl (February 01) 189. DOI: 101200/ JCO2019374_suppl189 [DOI] [PubMed] [Google Scholar]
- 27. Motoi F, Ishida K, Fujishima F, Ottomo S, Oikawa M, Okada T, Shimamura H, Takemura S, Ono F, Akada M, Nakagawa K, Katayose Y, Egawa S, Unno M. Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreatic ductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol. 2013; 20:3794-3801. [DOI] [PubMed] [Google Scholar]
- 28. Satoi S, Unno M, Motoi F, Matsuyama Y, Matsumoto I, Aosasa S, Shirakawa H, Wada K, Fujii T, Yoshitomi H, Takahashi S, Sho M, Ueno H, Yamamoto T, Kosuge T. The effect of neoadjuvant chemotherapy with gemcitabine and S-1 for resectable pancreatic cancer (randomized phase II/III trial; Prep-02/JSAP-05). J Clin Oncol. 2019; 37:suppl (May 20) 4126. DOI: 101200/JCO20193715_ suppl4126 [Google Scholar]
- 29. Ueno H, Okusaka T, Furuse J, Yamao K, Funakoshi A, Boku N, Ohkawa S, Yokosuka O, Tanaka K, Moriyasu F, Nakamori S, Sato T. Multicenter phase II study of gemcitabine and S-1 combination therapy (GS Therapy) in patients with metastatic pancreatic cancer. Jpn J Clin Oncol. 2011; 41:953-958. [DOI] [PubMed] [Google Scholar]
- 30. Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer 2019. http://www.suizou.org/pdf/guide2019_P176-179.pdf (accessed September 7, 2021). (in Japanese) .
- 31. Ahmad SA, Duong M, Sohal DPS, Gandhi NS, Beg MS, Wang-Gillam A, Wade JL 3rd, Chiorean EG, Guthrie KA, Lowy AM, Philip PA, Hochster HS. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann Surg. 2020. 272:481-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hozaeel W, Pauligk C, Homann N, Luley K, Kraus TW, Bechstein JTO, Grimm K, Heise B, Schmiegel W, Pink D, Al-Batran SE. Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX in resectable pancreatic cancer: The NEPAFOX trial. J Clin Oncol. 2015; 33:suppl tps4152. DOI: 101200/jco20153315_suppltps4152 [Google Scholar]
- 33. ClinicalTrials. gov. Testing the Use of the Usual Chemotherapy Before and After Surgery for Removable Pancreatic Cancer. https://www.clinicaltrials.gov/ct2/show/NCT04340141?term=FOLFIRINOX&type=Intr&cond=Pancreas+Cancer&cntry=US&phase=2&draw=2&rank=7 (accessed September 7, 2021).
- 34. van Roessel S, van Veldhuisen E, Klompmaker S, et al. Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020; 6:1733-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greco SH, August DA, Shah MM, Chen C, Moore DF, Masanam M, Turner AL, Jabbour SK, Javidian P, Grandhi MS, Kennedy TJ, Alexander HR, Carpizo DR, Langan RC. Neoadjuvant therapy is associated with lower margin positivity rates after Pancreaticoduodenectomy in T1 and T2 pancreatic head cancers: An analysis of the National Cancer Database. Surg Open Sci. 2020; 3:22-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020; 38:1763-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lynch HT, Deters CA, Snyder CL, Lynch JF, Villeneuve P, Silberstein J, Martin H, Narod SA, Brand RE. BRCA1 and pancreatic cancer: pedigree findings and their causal relationships. Cancer Genet Cytogenet. 2005; 158:119-125. [DOI] [PubMed] [Google Scholar]
- 38. Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014; 111:1132-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sehdev A, Gbolahan O, Hancock BA, Stanley M, Shahda S, Wan J, Wu HH, Radovich M, O'Neil BH. Germline and Somatic DNA Damage Repair Gene Mutations and Overall Survival in Metastatic Pancreatic Adenocarcinoma Patients Treated with FOLFIRINOX. Clin Cancer Res. 2018; 24:6204-6211. [DOI] [PubMed] [Google Scholar]
- 40. Goldstein JB, Zhao L, Wang X, Ghelman Y, Overman MJ, Javle MM, Shroff RT, Varadhachary GR, Wolff RA, McAllister F, Futreal A, Fogelman DR. Germline DNA sequencing reveals novel mutations predictive of overall survival in a cohort of patients with pancreatic cancer. Clin Cancer Res. 2020; 26:1385-1394. [DOI] [PubMed] [Google Scholar]
- 41. Yamada Y, Boku N, Nishina T, et al. Impact of excision repair cross-complementing gene 1 (ERCC1) on the outcomes of patients with advanced gastric cancer: correlative study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol. 2013; 24:2560-2565. [DOI] [PubMed] [Google Scholar]
- 42. Strippoli A, Rossi S, Martini M, Basso M, D'Argento E, Schinzari G, Barile R, Cassano A, Barone C. ERCC1 expression affects outcome in metastatic pancreatic carcinoma treated with FOLFIRINOX: a single institution analysis. Oncotarget. 2016; 7:35159-35168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grem JL, Danenberg KD, Behan K, Parr A, Young L, Danenberg PV, Nguyen D, Drake J, Monks A, Allegra CJ. Thymidine kinase, thymidylate synthase, and dihydropyrimidine dehydrogenase profiles of cell lines of the National Cancer Institute's Anticancer Drug Screen. Clin Cancer Res. 2001; 7:999-1009. [PubMed] [Google Scholar]
- 44. Lee B, Lipton L, Cohen J, et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann Oncol. 2019; 30:1472-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takai E, Totoki Y, Nakamura H, et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci Rep. 2015; 5:18425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee JS, Rhee TM, Pietrasz D, et al. Circulating tumor DNA as a prognostic indicator in resectable pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Sci Rep. 2019; 9:16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tempero M, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019; 37:suppl (May 20) 4000. DOI: 101200/JCO20193715_suppl4000 [Google Scholar]


