Abstract
Aim:
To evaluate the effectiveness of tele-ophthalmology (TO) versus face-to-face screening for diabetic retinopathy (DR) in diabetes care centers (DCC) across India.
Methods:
This is an observational, multicenter, retrospective, cross-sectional study of DR screening in individuals with diabetes performed across 35 branches of a chain of DCC in 20 cities in India over 1 year. In 30 DCC, DR screening was performed by TO, where retinal images obtained using Fundus on Phone camera were uploaded through the telemedicine network for centralized DR grading by eight retina specialists. In five DCC, DR screening was performed by fundus examination (FE) by the same retina specialists. The rate of detection of sight-threatening DR (STDR) (defined as the presence of proliferative DR and/or diabetic macular edema) through the two modes was compared.
Results:
A total of 58,612 individuals were screened for DR from January 1, 2018 to December 31, 2018: 25,316 by TO and 33,296 by FE. The mean age and mean duration of diabetes of the individuals with diabetes screened by TO was 55.8 ± 11.2 years and 9.5 ± 7.3 years; and in individuals screened by FE, it was 57.5 ± 11.6 years and 11.5 ± 8.0 years respectively. The mean glycated hemoglobin was 8.8% ± 2.1% and 8.5% ± 1.9% in the two groups, respectively. Any DR was detected in 31.7% (95% confidence interval [CI]: 31.0–32.3) by tele-screening and in 38.5% (95% CI: 37.9–39.0) by FE, whereas STDR was detected in 7.3% (95% CI: 7.0–7.7) by TO and in 10.5% (95% CI: 10.2–10.9) by FE. Overall, 11.4% individuals with diabetes in the TO group, including 4.1% with ungradable images, were advised referral to retina specialists for further management.
Conclusion:
Screening for DR at DCC using TO is feasible and effective for STDR detection in India and may be adopted throughout India.
Keywords: Diabetic retinopathy screening, Teleophthalmology, Retinal imaging, Sight-threatening diabetic retinopathy, Diabetes care centers, India
Key Points
Question: Is tele-ophthalmology (TO) at diabetes care centers (DCC) an effective model to establish a wider coverage of diabetic retinopathy (DR) screening in India with large number of individuals with diabetes and the absence of a systematic program?
Findings: TO in DCC in India, with real-time reporting of DR status yielded significant detection of sight-threatening DR.
Meaning: DR screening by TO at DCC is both feasible and effective in India.
Introduction
Diabetes and its complications represent a major public health burden globally, consuming significant resources especially when associated with end-organ damage.1 Early diagnosis and treatment of its complications is key to reducing the burden due to diabetes.2 All individuals with diabetes need to be screened for diabetic retinopathy (DR), which is a potentially sight-threatening complication of diabetes if left untreated.3
The number of people with diabetes requiring systematic DR screening far outweighs the number of ophthalmologists available to provide face-to-face consultation for DR screening, followed by management.4 The current standard of care is opportunistic and not systematic and there is an urgent need to ensure that individuals with sight-threatening DR (STDR) are identified early and treated.
Screening for DR is conventionally done by fundus examination (FE) by ophthalmologists or by obtaining retinal color photography using conventional mydriatic or nonmydriatic fundus cameras or smartphone-based fundus cameras by optometrists or trained eye technicians.5–7 Considerable progress has been made in retinal imaging as well as in the field of telecommunications in the 21st century.8 Tele-ophthalmology (TO) has increasingly been adopted as a cost-effective option for DR screening.9–11
We hypothesize that screening for DR regularly at diabetes care centers (DCC) either by FE by an ophthalmologist or through TO would enable regular as well as targeted screening of individuals with diabetes, especially those with long-term diabetes or uncontrolled diabetes, who are at a higher risk for the development of STDR.
In this study, we compared two DR assessment models that were integrated into a diabetes care package for people with diabetes mellitus seen at DCC. These included DR assessments provided by TO versus face-to-face FE and consultation by ophthalmologists within DCC in India.
The aim of this study was to evaluate the effectiveness of TO versus FE for DR in DCC in India to test whether these models of care within DCC are feasible models that can be translated to provide systematic DR screening in India.
Design, Setting, and Participants
The study is a retrospective analysis of DR screening done at Dr. Mohan's Diabetes Specialities Centre, one of the largest tertiary care diabetes centers in India with a chain of DCC (currently with 50 branches in 32 cities across the country). The center has a Diabetes Electronic Medical Records (DEMR) system that collates the complete details of all patients with diabetes who visit any of its branches.
The data of individuals with diabetes (type 1 or 2 diabetes) aged older than 18 years who visited the DCC for their diabetes care and had undergone DR screening as a part of their complete assessment either through TO or by face-to-face FE by a retina specialist were retrieved from the DEMR. Individuals excluded from the analysis included those who did not have DR screening during this study period, those without diabetes, and those who opted out of use of their data for research. This study includes 1 year DR screening data from the DEMR of 35 branches (in the chain of DCC [Appendix Table A1] across 20 cities in 2018); 5 larger branches where a medical retina specialist was available; and 30 small branches where there was no ophthalmologist available.
The study of the retrospective data analysis from DEMR was approved by the Institutional Ethics Committee of the Madras Diabetes Research Foundation. Included patients provided written informed consent to use their anonymized medical data for research.
Data collection
Individuals with diabetes during the visit to the DCC underwent detailed clinical and biochemical assessments and ophthalmic assessment by direct FE or by retinal color photography (TO). The data retrieved from DEMR for this study included age, gender, type of diabetes, duration of diabetes, treatment for diabetes,12 history of hypertension,13 blood pressure, serum glycated hemoglobin (HbA1c), presence of micro-albuminuria, and the complete details of DR screening—grading and diagnosis.
Blood pressure was measured by using the standardized technique. HbA1c was estimated by high-performance liquid chromatography using the Variant machine (Bio-Rad, Hercules, CA). Urinary albumin concentration was measured in a fasting urine sample by using an immunoturbidimetric assay (Beckman Coulter AU2700 biochemistry analyzer).
Screening for DR
Model 1: Direct face-to-face assessment
Patients at five larger DCC had the services of in-house consultant ophthalmologists (medical retina specialists) available, and they underwent a complete ophthalmic assessment. The pupils were dilated with tropicamide eye drops after visual acuity assessment, intraocular pressure measurement, and slit-lamp examination of the anterior segment. After dilatation, FE using direct and indirect ophthalmoscopy and slit-lamp biomicroscopy (with +90D lens) was performed by the medical retina specialists.
The DR grading was provided after the FE by using the International Clinical Diabetic Retinopathy (ICDR) severity scale grading system.14 The ICDR severity scales provides a classification of five stages of DR as follows: (1) No apparent retinopathy; (2) Mild non-proliferative DR (NPDR); (3) Moderate NPDR; (4) Severe NPDR; and (5) Proliferative DR (PDR).14 Any-DR was defined by the presence of at least one definite microaneurysm in one or both eyes, and STDR was defined by the presence of PDR and/or diabetic macular edema (DME).2 DME was defined as retinal thickening and/or the presence of definite hard exudates at or within one disk diameter of the center of the macula.
Model 2: TO
At 30 branches of the chain of DCC where ophthalmologists were not available, TO services were utilized. After mydriasis, digital retinal (fundus) color photography was performed by trained optometrists/trained eye technicians using Fundus on Phone (FOP) (Remidio Innovative Solutions Pvt. Ltd., Bangalore, India), a smartphone-based fundus camera.7 The TO program uses an anterior segment photograph and four field mydriatic 45° field of view fundus images of both eyes.5 The retinal images were immediately transferred through local area networks and wide area networks to enable remote DR grading.
The retinal images were graded by a team of eight medical retina specialists (who are certified graders) by using the ICDR grading system.14 The graded retinal images were assigned a retinopathy level for each eye. If in one of the eyes, the retinal images were not clearly gradable/ungradable, the DR diagnosis of the gradable retinal image of the other eye was provided in the eye report along with advice for referral to the ophthalmologist for fundus assessment of the other eye. If the retinal images of both eyes were ungradable due to media opacities, immediate referral to the ophthalmologist was advised for further assessment and management. Eye reports with DR severity diagnosis and appropriate advice were sent back in real time to the DCC usually within half an hour. The DR reports were made available to the consultant diabetologist for providing consultation advice to the patients at the DCCs in real time. The DR grading of the FE and the TO DR grading were provided by the same medical retina specialists.
Statistical analysis
SPSS for Windows version 24.0 was used for data analysis (SPSS, Inc., Chicago, IL). Continuous data were expressed as mean ± standard deviation, whereas categorical data were presented as proportions. Descriptive analysis was used for continuous variables, and chi-square test was used for categorical variables. The final diagnosis for each individual was determined from the level of DR of the worse eye by using ICDR severity scale, and these were compared between the two care models. The adjusted prevalence of DR and STDR [with 95% confidence interval (CI)] for both models was calculated by using logistic regression analysis (after adjusting for age, duration of diabetes, and HbA1c).
To assess the risk factor association and calculate the odds ratio, multiple logistic regression analysis was performed by using with STDR as a dependent variable. For all statistical tests, P-value <0.05 was considered significant.
Results
Overall, 58,612 individuals with diabetes were screened for DR across various DCCs in 1 year; 25,316 individuals were screened through TO mode; and 33,296 underwent DR screening through FE by medical retina specialists. The mean age of those who were underwent DR screening was 56.7 ± 11.4 years, the mean duration of diabetes was 10.6 ± 7.8 years, and the mean HbA1c was 8.6% ± 2.0% (Table 1). Overall, DR was detected in 21,746 (37.1%) and STDR was detected in 6483 (11.1%) individuals across the 35 DCC.
Table 1.
Characteristics of People with Diabetes Assessed for Diabetic Retinopathy at the Diabetes Care Centers
| Variables | Overall DR screening (n = 58,612) | TO (n = 25,316) | FE (n = 33,296) | P |
|---|---|---|---|---|
| Age (in years) | 56.7 ± 11.4 | 55.8 ± 11.2 | 57.5 ± 11.6 | <0.001 |
| Duration of diabetes (in years) | 10.6 ± 7.8 | 9.5 ± 7.3 | 11.5 ± 8.0 | <0.001 |
| Age at onset of diabetes (in years) | 44.0 ± 10.3 | 44.4 ± 10.1 | 43.7 ± 10.5 | <0.001 |
| Gender (males), n (%) | 38,270 (65.2) | 16,214 (64.0) | 22,056 (66.2) | <0.001 |
| Type of diabetes | ||||
| Type 1 diabetes, n (%) | 1136 (1.9) | 306 (1.2) | 830 (2.5) | |
| Type 2 diabetes, n (%) | 57,476 (98.1) | 25,010 (98.8) | 32,466 (97.5) | <0.001 |
| HbA1c (%) | 8.6 ± 2.0 | 8.8 ± 2.1 | 8.5 ± 1.9 | <0.001 |
| Systolic blood pressure (mm Hg) | 128 ± 16 | 129 ± 15 | 128 ± 16 | <0.001 |
| Diastolic blood pressure (mm Hg) | 79 ± 8 | 80 ± 7 | 79 ± 9 | <0.001 |
| History of hypertension % | 32.6 | 32.1 | 32.9 | 0.010 |
| Microalbuminuria % | 29.5 | 31.0 | 28.5 | <0.001 |
DR, diabetic retinopathy; HbA1c, glycated hemoglobin; TO, tele-ophthalmology.
Figure 1 shows the proportion of individuals with diabetes detected with DR by the two modes of screening. Any DR was detected in 31.7% (95% CI: 31.0–32.3) by tele-screening and in 38.5% (95% CI: 37.9–39.0) by FE, whereas STDR was detected in 7.3% (95% CI: 7.0–7.7) by TO and in 10.5% (95% CI: 10.2–10.9) individuals by FE. The gender distribution of STDR is shown in Supplementary Table S1.
FIG. 1.
DR detection by tele-ophthalmology and by fundus examination at diabetes care centers across India. DR, diabetic retinopathy. Color images are available online.
Table 1 shows the general clinical and biochemical characteristics of the individuals who underwent DR screening through the two pathways. The mean duration of diabetes of the individuals screened by FE was higher than those screened through TO. The mean HbA1c was slightly higher (8.8% ± 2.1%) in the TO group when compared with the FE group (8.5% ± 1.9%). History of co-existent hypertension was present in 32.6% of the individuals with diabetes across DCCs. Microalbuminuria was detected in 31% of the individuals in the TO screening group and in 28.5% of the individuals in the FE assessment group.
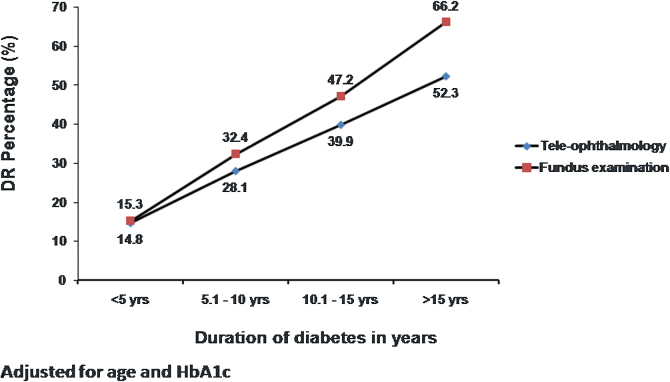
Figure 2 shows the frequency distribution of DR detected by both models of screening based on the duration of diabetes, after adjusting for age and HbA1c. In both models, the prevalence of DR increased proportionately with increasing duration of diabetes. In the FE group, there was a steep increase in the prevalence of DR in individuals with a duration of diabetes over 15 years.
FIG. 2.
Frequency distribution of any DR through the two models of DR screening based on duration of diabetes. Color images are available online.
The varying grades of severity of DR detected by both modes of DR screening are shown in Table 2. The detection of moderate NPDR, severe NPDR and PDR was significantly higher in the FE model when compared with the TO model. There was no significant difference in the severity of DR in type 1 and type 2 diabetes in both modes of screening (Supplementary Table S2).
Table 2.
Severity of Diabetic Retinopathy in the Two Modes of Diabetic Retinopathy Screening in the Diabetes Care Centers
| DR severitya | TO (n = 25,316) % (95% CI) | FE (n = 33,296) % (95% CI) | P |
|---|---|---|---|
| Mild NPDR (%) | 68.5 (67.4–69.5) | 60.3 (59.5–61.2) | <0.001 |
| Moderate NPDR (%) | 24.5 (23.6–25.5) | 30.3 (29.5–31.1) | <0.001 |
| Severe NPDR (%) | 6.8 (6.2–7.4) | 8.9 (8.4–9.4) | <0.001 |
| PDR (%) | 1.0 (0.9–1.2) | 2.3 (2.1–2.4) | <0.001 |
Adjusted for age, HbA1c, and duration of diabetes.
CI, confidence interval; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Fundus was not gradable in some in both models; fundus of 539 (1.6%) individuals were ungradable (media opacities such as dense cataract, total vitreous hemorrhage) in the FE mode, whereas 1047 (4.1%) individuals with diabetes screened by TO had ungradable images (cataract, poor mydriasis, vitreous hemorrhage). Individuals with STDR as well as those with ungradable images screened by TO were advised referral to the retina specialist for further management. Overall, 11.4% in the TO group, including 4.1% individuals with ungradable images, were advised referral to retina specialists for further management.
We also performed a multiple logistic regression analysis with STDR as a dependent variable and age, duration of diabetes, HbA1c, hypertension, and microalbuminuria as independent variables to assess the association with systemic risk factors (Table 3). Longer duration of diabetes, poor glycemic control (especially HbA1c value >10%), hypertension, presence of microalbuminuria, and male gender showed a significant association with STDR in both modes. There were no differences in the systemic risk factors associated with STDR between those screened by the two screening models except the duration of diabetes, which showed a significantly greater association with STDR in the FE group. The odds for STDR in individuals with a duration of diabetes over 15 years was 4.93 (95% CI: [4.00–6.07] P < 0.001) in the TO group and in the FE group, it was 6.92 (95% CI: [5.84–8.21] P < 0.001).
Table 3.
Systemic Risk Factors Associated with Sight-Threatening Diabetic Retinopathy in the Two Pathways
| Variables | Tele-ophthalmology |
Face-to-face consultation |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Duration of diabetes (<5 years)-reference | 1.00 | 1.00 | ||
| Duration of diabetes (5.1–10 years) | 2.20 (1.80–2.70) | <0.001 | 2.24 (1.87–2.68) | <0.001 |
| Duration of diabetes (10.1–15 years) | 3.92 (3.20–4.81) | <0.001 | 4.34 (3.65–5.16) | <0.001 |
| Duration of diabetes >15 years | 4.93 (4.00–6.07) | <0.001 | 6.92 (5.84–8.21) | <0.001 |
| HbA1c—<7.0%-reference | 1.00 | 1.00 | ||
| HbA1c—7.1–8.0% | 1.362 (1.05–1.76) | <0.001 | 1.36 (1.17–1.59) | <0.001 |
| HbA1c—8–10% | 2.27 (1.81–2.84) | <0.001 | 1.96 (1.70–2.25) | <0.001 |
| HbA1c—>10% | 3.56 (2.85–4.45) | <0.001 | 3.37 (2.92–3.90) | <0.001 |
| Hypertension | 1.41 (1.24–1.59) | <0.001 | 1.37 (1.25–1.50) | <0.001 |
| Microalbuminuria | 2.44 (2.15–2.76) | <0.001 | 2.75 (2.51–3.01) | <0.001 |
| Age | 0.99 (0.98–0.99) | <0.001 | 0.98 (0.98–0.98) | <0.001 |
| Gender (male) | 1.36 (1.19–1.54) | <0.001 | 1.35 (1.23–1.48) | <0.001 |
After adjusting for one another and other covariates.
OR, odds ratio.
Discussion
In the absence of a systematic DR screening program in India,4 STDR remains a common cause of preventable blindness in adults.3 The most common point of care for individuals with diabetes is the diabetologist/endocrinologist who manages the systemic control of hyperglycemia, the comorbidities, and the other complications of diabetes. Ideally, adding DR screening to the diabetes care package in DCC would result in more individuals with diabetes being screened for STDR in India. This study shows that DR screening can be successfully implemented in DCC as either a TO or FE model. We have shown that it has been possible to screen more than 50,000 individuals with diabetes for DR within a single calendar year through two modes of DR screening across the DCC.
Although TO allows a large population to be screened, individuals identified with STDR need to be referred to an ophthalmologist.15 Our study shows that the DR screening report can be provided to the patient in real time during the same hospital visit, allowing the diabetologists to explain to the patients with STDR directly about the need for urgent referral and treatment. Therefore, the uptake for retinal consultations is likely to be better. The diabetologists also have the added advantage of holistically managing individuals with diabetes having access to records on all systemic parameters and complications of diabetes.
Our study was conducted in DCC with EMR and both care models were able to identify a significant number of people with DR through regular annual screening, which is not possible with opportunistic DR screening that is currently practiced in many places in India. The prevalence of DR and STDR is higher in our study than what has been reported earlier in the other earlier population-based studies from South India,16,17 possibly because of referral bias in a clinic-based study.
Another explanation for the higher prevalence was the longer duration of diabetes in both care models in this study of ∼10 years. However, the care pathways are compared in the same settings and the results also indicate that screening in DCC results in an enriched cohort of individuals with diabetes, resulting in DR detection in more than 30% of the individuals compared with community screening, where the prevalence of DR is less than half.17
Although more than 30% of individuals with diabetes screened by TO had some form of DR, only 7.3% who had STDR and 4.1% who had ungradable images required further referral to retina specialists for further evaluation and management. The rest of the individuals with diabetes did not have to consult the ophthalmologist and were advised annual follow-up with good glycemic control. We also found that the detection of any DR and STDR was higher in the FE group, despite the same retinal specialists involved in grading the TO images and completing the FE in the two groups.
The five centers that have in-house retina specialists are more established DCC. This could reflect referral bias, as individuals with long-term or uncontrolled diabetes or those with other microvascular complications tend to get referred to the retina specialists for FE. It is also likely that FE by indirect ophthalmoscopy enabled the identification of peripheral DR lesions. Although use of ultra-wide field imaging with wide-angle fundus cameras can allow simultaneous evaluation of the peripheral and central retina,18 it is currently an expensive modality for consideration for DR screening in India.7
The quality of the images plays a key role in DR detection and is influenced by various factors. Single-field imaging without mydriasis can reduce the sensitivity of DR detection in TO.19 Raman et al.20 have shown, after pupillary dilatation, that the nongradability of fundus images was reduced from 29.1% to 8.6% in their study with a single field tele-screening model. In our study, the number of ungradable images was even lower (4.1%), possibly on account of the use of mydriasis as well as multiple retinal fields. Also, the FOP camera used in this study has been validated for use for DR screening and is being used regularly in TO.21
Although national and international guidelines recommend annual retinal screening, screening for DR in India is often opportunistic in nature.4,22 Telemedicine for DR screening is practiced at some large eye care centers in India through their vision centers.23 Visually asymptomatic individuals with diabetes regularly meet the physicians/diabetologists for long-term care. Hence, tele-screening in DCC with links to an ophthalmologist may enable regular repetitive DR screening covering a wider population.
The assessment of systemic risk factors associated with STDR requiring referral was also possible in our study, done at DCCs.24 Our TO screening program has increased the access to care in remote as well as rural areas, has saved travel costs of individuals with diabetes who otherwise have to come to the centers where the retina specialists are available to perform FE and provide face-to-face consultation. It has enabled virtual counseling through video calls from the concerned retina specialist for those who need further investigations and treatment. It has helped to identify those who have an immediate need for further retinal assessment and management versus those who just require screening again after 1 year.
Tele-retinal DR screening projects by government bodies or private institutions integrated with additional simple investigations to screen for other complications will enable holistic screening and will increase compliance and help identify and manage other diabetes complications as well.24,25 The use of artificial intelligence (AI)/deep learning for automated detection of DR in TO will reduce the burden on the ophthalmologists.26,27
The goal of TO is to ensure that more individuals get screened regularly and to bridge the gaps in time, distance, and availability of trained ophthalmologists for DR screening.5,9 Some of the challenges in routine TO DR screening include good network connection for immediate transfer of retinal images for real-time reporting, management of the workflow, the image quality, image data storage, ensuring data security especially with the use of AI, and the perception and attitude of the people toward TO.9,23
Improving awareness among individuals with diabetes regarding the importance of regular repetitive DR screening,5 use of secure servers for image storage, and use of offline AI for DR detection26 are options to overcome some of these challenges. Targeted screening in those with a longer duration of diabetes can ensure even more cost-effective DR/STDR detection. The TO service can be implemented at any center that caters to individuals with diabetes, such as primary care9,28 and private care providers, as long as a systematic approach of re-call is implemented.
Telemedicine/TO has developed significantly globally during the coronavirus disease 2019 (COVID-19) lockdown times,29,30 reducing the need for the patient to travel and facilitating easier access to virtual health care. In our study, the FE as well as TO DR grading was performed by the same team of certified medical retina specialists and, hence, comparable.31 However, improvements in DR screening do not necessarily lead to the prevention of blindness from STDR unless the loop of appropriate immediate referral and further treatment for STDR is closed.32 Some studies have shown that as many as 80% of people with STDR identified through retinal screening did not complete follow-up ophthalmological evaluation and suggested treatment.15
Our TO model facilitates individuals with diabetes and those with STDR to consult their diabetologists in real time and onward referral to the ophthalmologists for management of STDR. Further studies are needed to assess the actual percentage of individuals detected with STDR who consulted the ophthalmologists and underwent further treatment in a TO model in DCC.
The strengths of the study are as follows: To our knowledge, this is the first study from a developing country that has provided a detailed analysis comparing two models of DR screening across a chain of DCC. Second, the sample size is large and represents multiple regions in India. Given the advantages of this system compared with opportunistic screening, we recommend that DR screening in DCC be considered by policy makers to enable better screening coverage of individuals with diabetes. We believe that this is an excellent model that can be followed across the country, and it can be scaled up easily.
Our study also has a few limitations: This is a clinic-based study and hence the findings with respect to prevalence of STDR cannot be extrapolated to the population at large as referral bias could have influenced the results. However, the study shows the efficiency of both care models in detecting STDR. There were some individuals with diabetes who visited the DCC but refused DR screening due to lack of awareness or if they were already under care in an ophthalmic department. The DR results of these individuals were not available. It is a retrospective cross-sectional observational study, and causal inferences cannot therefore be drawn.
To conclude, targeted screening using TO in DCC is a valuable strategy for DR detection in India. This study provides evidence that adding tele-ophthalmology to the diabetes care model should be scaled up throughout India to enable rapid coverage of DR screening in any setting (government, private, and charitable institutions) treating diabetes and the comorbidites.
Supplementary Material
Acknowledgment
The authors wish to thank all the ophthalmologists/medical retina specialists who performed the DR grading, the eye technicians who were involved in the fundus imaging, and the diabetologists at the various diabetes care centers.
Appendix Table A1.
Diabetes Care Centers Where Diabetic Retinopathy Screening Was Carried Out
| DMDSC branchesa (DCC) | Location (in alphabetical order) | City/district | State in India |
|---|---|---|---|
| 1 | Adyarb | Chennai | Tamilnadu |
| 2 | Annanagarc | Chennai | Tamilnadu |
| 3 | AS Rao Nagarb | Hyderabad | Telangana |
| 4 | Avadib | Chennai | Tamilnadu |
| 5 | Bhubhaneshwar | Bhubhaneshwar | Orissa |
| 6 | Chunampetb | Chengalpattu | Tamilnadu |
| 7 | Cochinb | Kochi | Kerala |
| 8 | Coimbatorec | Coimbatore | Tamilnadu |
| 9 | Domalgudac | Hyderabad | Telangana |
| 10 | East Maredpallyb | Hyderabad | Telangana |
| 11 | Gopalapuramc | Chennai | Tamilnadu |
| 12 | Indiranagarc | Bengaluru | Karnataka |
| 13 | JP Nagarb | Bengaluru | Karnataka |
| 14 | Jubileehillsb | Hyderabad | Telangana |
| 15 | Kukatpallyb | Hyderabad | Telangana |
| 16 | Lucknowb | Lucknow | Uttar Pradesh |
| 17 | Maduraib | Madurai | Tamilnadu |
| 18 | Malleswaramb | Bengaluru | Karnataka |
| 19 | Mangaloreb | Mangalore | Karnataka |
| 20 | Mysoreb | Mysuru | Karnataka |
| 21 | New Delhib | New Delhi | New Delhi |
| 22 | Omrb | Chengalpattu | Tamilnadu |
| 23 | Pondib | Pondicherry | Pondicherry |
| 24 | Porurb | Tiruvallur | Tamilnadu |
| 25 | Tambaramb | Chennai | Tamilnadu |
| 26 | Thanjavurb | Thanjavur | Tamilnadu |
| 27 | Tolichowkib | Hyderabd | Telangana |
| 28 | Trichyb | Tiruchirapalli | Tamilnadu |
| 29 | Trivandrumb | Trivandrum | Kerala |
| 30 | Tuticorinb | Tuticorin | Tamilnadu |
| 31 | Vadapalanib | Chennai | Tamilnadu |
| 32 | Velloreb | Vellore | Tamilnadu |
| 33 | Vijayawadab | Vijayawada | Andhra Pradhesh |
| 34 | Visakhapatnamb | Visakhapatnam | Andhra Pradhesh |
| 35 | White Fieldb | Bangalore | Karnataka |
As of 2018.
DR screening by Tele-ophthalmology.
DR screening by FE by retina specialist.
DCC, diabetes care centers; DR, diabetic retinopathy; FE, fundus examination; TO, tele-ophthalmology.
Authors' Contributions
Conceived and designed the study: R.R., R.M.A., and S.S.; Wrote the article: R.R.; Data Retrieval: S.J.; Data cleansing and Analysis: G.U., C.S. Statistical Analysis: G.U., U.V.; Helped revising the article for important intellectual content: V.P., V.M., R.U., R.M.A., and S.S. Read and approved the final article: R.R., G.U., V.P., S.J., C.S., R.U., U.V., R.M.A., S.S., and V.M.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This analysis, within the ORNATE India project, was funded by the Global Challenges Research Fund (GCRF) UK Research and Innovation (UKRI) (MR/P207881/1).
Supplementary Material
References
- 1. India State-Level Disease Burden Initiative Diabetes Collaborators: The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health 2018;6:e1352–e1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajalakshmi R, Shanthi Rani CS, Venkatesan U, et al. : Correlation between markers of renal function and sight-threatening diabetic retinopathy in type 2 diabetes: a longitudinal study in an Indian clinic population. BMJ Open Diabetes Res Care 2020;8:e001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leasher JL, Bourne RR, Flaxman SR, et al. : Vision Loss Expert Group of the Global Burden of Disease Study. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care 2016;39:1643–1649. [DOI] [PubMed] [Google Scholar]
- 4. Wong TY, Sun J, Kawasaki R, et al. : Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018;125:1608–1622. [DOI] [PubMed] [Google Scholar]
- 5. Rajalakshmi R, Prathiba V, Rani PK, et al. : Various models for diabetic retinopathy screening that can be applied to India. Indian J Ophthalmol 2021;69:2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hutchinson A, McIntosh A, Peters J, et al. : Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000;17:495–506. [DOI] [PubMed] [Google Scholar]
- 7. Rajalakshmi R, Prathiba V, Arulmalar S, et al. : Review of retinal cameras for global coverage of diabetic retinopathy screening. Eye (Lond) 2021;35:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Das T, Raman R, Ramasamy K, et al. : Telemedicine in diabetic retinopathy: current status and future directions. Middle East Afr J Ophthalmol 2015;22:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pradeepa R, Rajalakshmi R, Mohan V: Use of telemedicine technologies in diabetes prevention and control in resource-constrained settings: lessons learned from emerging economies. Diabetes Technol Ther 2019;21(S2):S29–S216. [DOI] [PubMed] [Google Scholar]
- 10. Rachapelle S, Legood R, Alavi Y, et al. : The cost-utility of telemedicine to screen for diabetic retinopathy in India. Ophthalmology 2013;120:566–573. [DOI] [PubMed] [Google Scholar]
- 11. Mohan V, Prathiba V, Pradeepa R: Tele-diabetology to screen for diabetes and associated complications in rural India: the Chunampet Rural Diabetes Prevention Project Model. J Diabetes Sci Technol 2014;8:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Supplement 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 13. Chobanian AV: National heart, lung, and blood institute joint national committee on prevention, detection, evaluation, and treatment of high blood pressure; national high blood pressure education program coordinating committee: the seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 14. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. ; Global Diabetic Retinopathy Project Group: Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682. [DOI] [PubMed] [Google Scholar]
- 15. Pieczynski J, Grzybowski A: Review of diabetic retinopathy screening methods and programmes adopted in different parts of the world. Eur Ophthal Rev 2015;9:49–55. [Google Scholar]
- 16. Rema M, Premkumar S, Anitha B, et al. : Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest Ophthalmol Vis Sci 2005;46:2328–2333. [DOI] [PubMed] [Google Scholar]
- 17. Namperumalsamy P, Kim R, Vignesh TP, et al. : Prevalence and risk factors for diabetic retinopathy: a population-based assessment from Theni District, South India. Br J Ophthalmol 2009;93:429–434. [DOI] [PubMed] [Google Scholar]
- 18. Silva PS, Cavallerano JD, Tolls D, et al. : Potential efficiency benefits of nonmydriatic ultrawide field retinal imaging in an ocular telehealth diabetic retinopathy program. Diabetes Care 2014;37:50–55. [DOI] [PubMed] [Google Scholar]
- 19. Gupta V, Bansal R, Gupta A, et al. : Sensitivity and specificity of nonmydriatic digital imaging inscreening diabetic retinopathy in Indian eyes. Indian J Ophthalmol 2014;62:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raman R, Rani PK, Mahajan S, et al. : The tele-screening model for diabetic retinopathy: evaluating the influence of mydriasis on the gradability of a single-field 45 degrees digital fundus image. Telemed J E Health 2007;13:597–602. [DOI] [PubMed] [Google Scholar]
- 21. Rajalakshmi R, Arulmalar S, Usha M, et al. : Validation of smartphone based retinal photography for diabetic retinopathy screening. PLoS One 2015;10:e0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raman R, Ramasamy K, Rajalakshmi R, et al. : Diabetic retinopathy screening guidelines in India: all India Ophthalmological Society diabetic retinopathy task force and Vitreoretinal Society of India Consensus Statement. Indian J Ophthalmol 2021;69:678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramasamy K, Mishra C, Kannan NB, et al. : Telemedicine in diabetic retinopathy screening in India. Indian J Ophthalmol 2021;69:2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jani PD, Forbes L, Choudhury A, et al. : Evaluation of diabetic retinal screening and factors for ophthalmology referral in a telemedicine network. JAMA Ophthalmol 2017;135:706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pearce I, Simó R, Lövestam-Adrian M, et al. : Association between diabetic eye disease and other complications of diabetes: implications for care. A systematic review. Diabetes Obes Metab 2019;21:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dutt S, Sivaraman A, Savoy F, et al. : Insights into the growing popularity of artificial intelligence in ophthalmology. Indian J Ophthalmol 2020;68:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajalakshmi R: The impact of artificial intelligence in screening for diabetic retinopathy in India. Eye 2020;34:420–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sivaprasad S, Netuveli G, Wittenberg R, et al. : Nayanamritham Project Collaborators. Complex interventions to implement a diabetic retinopathy care pathway in the public health system in Kerala: the Nayanamritham study protocol. BMJ Open 2021;11:e040577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chong JC, Tan CHN, Chen DZ: Teleophthalmology and its evolving role in a COVID-19 pandemic: a scoping review. Ann Acad Med Singap 2021;50:61–76. [PubMed] [Google Scholar]
- 30. Raman R, Rajalakshmi R, Surya J, et al. : Impact on health and provision of healthcare services during the COVID-19 lockdown in India: a multicentre cross-sectional study. BMJ Open 2021;11:e043590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan IJ, Dobson LP, Bartnik S, et al. : Real-time teleophthalmology versus face-to-face consultation: a systematic review. J Telemed Telecare 2017;23:629–638. [DOI] [PubMed] [Google Scholar]
- 32. Das T, Murthy GVS: Commentary: a health policy change would benefit a protocol-based screening for diabetic retinopathy in India. Indian J Ophthalmol 2021;69:689–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.