Abstract
Objectives
This retrospective chart review examined real‐world healthcare resource utilization (HRU) in patients with AML ineligible for intensive therapy who received first‐line systemic therapy or best supportive care (BSC).
Methods
Data were collected anonymously on patients with AML who initiated first‐line hypomethylating agents (HMA), low‐dose cytarabine (LDAC), other systemic therapy, or BSC. HRU endpoints included hospitalizations, outpatient consultations, transfusions, and supportive care.
Results
Of 1762 patients included, 46% received HMA, 11% received LDAC, 17% received other systemic therapy, 26% received BSC; median treatment durations were 118, 35, 33, and 57 days, respectively. Most patients were hospitalized, most commonly for treatment administration, transfusion, or infection (HMA 82%, LDAC 93%, other systemic therapy 83%, BSC 83%). A median number of hospitalizations were 2–6 across systemic groups and two for BSC, with median durations of 8–18 days. Transfusion rates and outpatient consultations were highest for HMA (80% and 79%) versus LDAC (57% and 53%), other systemic therapy (57% and 63%), and BSC (71% and 66%). Antivirals/antibiotics and antifungals were used more frequently than growth factors (72–92%, 34–63%, and 7–27%, respectively).
Conclusion
Patients with AML ineligible for intensive therapy have high HRU; novel therapies are needed to alleviate this burden.
Keywords: AML, best supportive care, healthcare resource utilization, hypomethylating agents, low‐dose cytarabine, low‐intensity therapy
What is the NEW aspect of your work?
CURRENT provides a valuable insight into the treatment patterns for patients with AML who were considered ineligible for intensive treatment, and their associated healthcare burdens, providing a comprehensive picture of real‐world scenarios in AML, illuminating areas of particular need.
What is the CENTRAL finding of your work?
Patients with AML ineligible for intensive therapy have high healthcare resource utilization and novel therapies are needed to alleviate this burden.
What is (or could be) the SPECIFIC clinical relevance of your work?
Patients with AML ineligible for intensive therapy have high levels of healthcare resource utilization; novel therapies are needed to alleviate this burden because although the improved long‐term outcomes associated with these agents unavoidably incur additional resource use, the desirable outcome is high HRU in the context of good patient outcomes.
1. INTRODUCTION
Acute myeloid leukemia (AML) is a highly heterogeneous malignancy characterized by the infiltration of abnormally differentiated hematopoietic cells into the blood and bone marrow. 1 AML occurs more commonly in older adults, with the majority of patients older than age 65 years at diagnosis. 2 Although recent developments in understanding the molecular heterogeneity and pathogenesis of AML have led to improvements in outcomes for patients with AML, older age is still associated with a particularly poor prognosis. 3 , 4 The overall estimated 5‐year survival for patients with AML in the United States between 2010 and 2017 has been reported at 28%, but only 5% for patients aged ≥70 years. 5
The current standard of care for patients with AML is induction therapy with cytarabine and an anthracycline followed by post remission (consolidation) therapy, with the possibility of hematopoietic stem cell transplantation (HSCT) for eligible patients. 6 , 7 , 8 However, such intensive therapy is not considered to be suitable for all patients. In particular, older patients and those with preexisting comorbidities or poor performance status are often considered unsuitable due to an increased risk of treatment‐related morbidity and mortality. 1 , 6 , 7
Older patients especially are more likely to experience poor outcomes with intensive therapy. 9 Until recently there were limited alternatives to intensive therapy, 4 , 10 , 11 and these included low‐intensity treatment with hypomethylating agents (HMA), low‐dose cytarabine (LDAC), and best supportive care (BSC), such as hydroxyurea and transfusion support. 8 The outcomes associated with these established therapies are typically poor and median overall survival (OS) estimates in clinical trials of patients who received traditional nonintensive therapy range from 3.6 to 10.4 months. 12 , 13 , 14 , 15 Novel therapies are now emerging, with encouraging efficacy in patients with newly diagnosed AML who are ineligible for intensive therapy. 16 Of note, strategies that combine the B‐cell lymphoma 2 (BCL‐2) inhibitor, venetoclax, or hedgehog pathway inhibitor, glasdegib, with low‐intensity therapy have demonstrated higher response rates and increased OS compared with low‐intensity therapy alone, alongside tolerable safety profiles. 10 , 11 , 17 , 18
Poor outcomes in elderly patients, particularly those with comorbidities, place additional burden on healthcare resources. Although large variations in healthcare resource utilization (HRU) exist between patient populations, hospitalization rates are known to be higher among older patients and those with relapsed or refractory AML, compared with younger patients with newly diagnosed disease, illustrating the aforementioned increased burden on healthcare services. 19 The high HRU of patients with AML has been characterized in several retrospective database or chart review studies. 19 , 20 , 21 , 22 , 23 Furthermore, the high hospitalization and transfusion rates associated with AML management incur particularly high costs, and the financial burden of active treatment has been widely reported. 19 , 22 , 23 However, there are limited data specifically regarding HRU with nonintensive treatments for AML.
CURRENT (Real‐World Treatment Patterns and Clinical Outcomes in Unfit AML Patients Receiving First Line Systemic Treatment or Best Supportive Care) was a retrospective chart review designed to examine real‐world treatment patterns in patients who were ineligible for intensive therapy and who received first‐line systemic therapy or BSC, with a primary endpoint of OS from diagnosis. 24 The primary analysis of CURRENT was consistent with results from earlier clinical trials and demonstrated that real‐world clinical outcomes remain poor for these patients, with median OS of <10 months for all systemic therapy and BSC groups. 10 , 12 , 13 , 14 , 15 , 17 , 24
The continued rise of AML incidence as populations age, having almost doubled between 1990 and 2017, 25 coupled with the high costs of treatment, 26 underscores a critical need to understand the current treatment pathways, their associated clinical outcomes, and the affiliated burden on healthcare systems. The objective of the present analysis was to better understand this healthcare burden by evaluating patterns of real‐world HRU by patients with AML who were considered ineligible for intensive chemotherapy and received first‐line systemic treatment or BSC within the CURRENT study.
2. METHODS
2.1. Study design
This noninterventional, retrospective chart review collected data on patients diagnosed with AML who were ineligible for intensive induction chemotherapy and who initiated first‐line systemic treatment or BSC, as determined by the treating physician between January 1, 2015, and December 31, 2018. The notification/submission to the responsible ethics committees, health institutions, and/or competent authorities was performed as required by local laws and regulations. Data collection was carried out anonymously following ethics committee approval.
2.2. Patient selection and data collection
Patients aged ≥18 years who were diagnosed with primary or secondary AML and deemed ineligible for intensive chemotherapy by the treating physician due to age, performance status, comorbidities, regional guidelines, or institutional practice were enrolled in the study. Patients received first‐line systemic treatment with HMAs (azacitidine or decitabine), LDAC, targeted therapy, or BSC and visited their physician at least three times during the treatment period, including the initial treatment visit (defined as start of systemic treatment or BSC). Patients with unconfirmed AML diagnosis, acute promyelocytic leukemia, and those who received first‐line treatment within a clinical trial were excluded from the study.
Patients with AML were identified from 112 community or hospital medical centers that treated patients with AML between 2015 and 2018 across 22 countries. Each site planned enrollment of approximately 5–35 patients, and the maximum number of patients per site was defined locally. Where sites identified an excess of patients meeting the inclusion criteria, a random sampling method was used to select patients for inclusion; the total number of eligible patients was divided by the maximum number allowed to enroll to determine the selection factor (i.e., every 3rd or 4th patient). Patients were followed until the last recorded contact or death, whichever was applicable at the time of data collection. Anonymized patient data were extracted from patient charts and/or site documentation and recorded via electronic case report forms (eCRFs) completed by each center.
2.3. Endpoints
The primary endpoint was OS from diagnosis of AML (reported separately), alongside secondary efficacy endpoints which included progression‐free survival, time‐to‐treatment failure, measurable residual disease testing rate, and response rate per physician assessment. 24 HRU was an additional secondary endpoint, assessed by receipt of transfusions (red blood cell [RBC] and/or platelet), hospital admissions (including days spent in an intensive care unit [ICU]), outpatient consultations, supportive care (including growth factors), antibiotic use, and other medications, from the initiation of first‐line systemic therapy or BSC until treatment discontinuation.
2.4. Statistical analysis
The overall target sample size was 1600 patients with AML globally. Formal statistical power considerations are not provided due to the descriptive nature of the study. However, the sample size was considered sufficient to provide reasonably precise estimates, whereby the width of a two‐sided 95% confidence interval (CI) for proportion‐based estimates would be within ±2.8% with N = 1200 (using normal approximation for binomial distribution); for treatment subgroups (n = 300), geographic subgroups (n = 200), and combinations of these (n = 50), the widths will be at most ±5.7%, ±6.9%, and ±13.9%, respectively. The final data cutoff were March 31, 2020.
Continuous variables are described with mean, standard deviation, median, and ranges. Categorical variables are reported as counts and proportions. Time‐to‐event data were estimated using the Kaplan–Meier method, with median time and 95% CIs reported.
3. RESULTS
3.1. Patient demographics and clinical characteristics
In total, 1762 patients were enrolled across 22 countries at the time of the final data cutoff on March 31, 2020. Baseline patient demographics and clinical characteristics are presented in Table S1. In brief, the highest recruitment was from the Japan and Asian‐Pacific (JAPAC; 35%) and Western Europe and Canada (WEC; 31%) regions, followed by Eastern Europe, Middle East, Africa (EEMEA; 20%), and Latin America (LATAM; 13%). Approximately, half the patients were aged >75 years at diagnosis (47%) and had intermediate (32%) or poor (25%) cytogenetic risk.
A total of 1310 (74%) patients received first‐line systemic therapy; 809 (62%) who received HMA monotherapy (533 received azacitidine, 276 received decitabine), 199 (15%) who received LDAC monotherapy, and 302 (23%) who received other systemic therapies (including combination regimens including cytarabine, aclarubicin, granulocyte‐colony stimulating factor [CAG], gemtuzumab ozogamicin, FMS‐like tyrosine kinase‐3 [FLT3] inhibitors, venetoclax, and enocitabine). The remaining 452 (26%) patients received BSC only, comprising transfusions (83%), infection management (62%), pain relief (40%), nutritional support (27%), and other supportive measures (21%). Baseline characteristics were generally similar between the systemic therapy and BSC cohorts (Table S1).
The median duration of first‐line systemic treatment was five cycles/118 days in the HMA group, two cycles/35 days in the LDAC group, and two cycles/33 days in other systemic therapies group. The median duration of BSC was 57 days. In total 90%, 92%, 93%, and 93% of patients in the HMA, LDAC, other systemic therapy, and BSC groups discontinued first‐line treatment. The most common reasons for discontinuation of first‐line therapy were disease progression and death in all but the other systemic therapies group where treatment was most commonly discontinued due to treatment toxicity or death (Table 1).
TABLE 1.
Overview of first‐line treatment
| First‐line systemic therapy | BSC n = 452 | |||
|---|---|---|---|---|
| HMA n=809 | LDAC n = 199 | Other a n = 302 | ||
| Duration of treatment | ||||
| Median number of cycles (range) | 5 (0–62) | 2 (0–26) | 2 (0–252) | NR |
| Median number of days (range) | 118 (0–1450) | 35 (1–1132) | 33 (1–1124) | 57 (0–1680) |
| Discontinued first‐line therapy | 730 (90) | 183 (92) | 282 (93) | 421 (93) |
| Reasons for discontinuation b | ||||
| Disease progression | 288 (39) | 56 (31) | 1 (<1) | 79 (19) |
| Death | 231 (32) | 45 (25) | 98 (35) | 310 (74) |
| Decline in performance status | 125 (17) | 27 (15) | 29 (10) | 28 (7) |
| Patient preference | 70 (10) | 16 (9) | 22 (8) | 32 (8) |
| Toxicity | 66 (9) | 35 (19) | 37 (13) | 4 (1) |
| Completed planned treatment | 27 (4) | 27 (15) | 37 (13) | 4 (1) |
| Physician preference | 21 (3) | 16 (9) | 10 (4) | 4 (1) |
| Financial/insurance | 2 (<1) | 0 | 2 (1) | 2 (<1) |
| Other | 65 (9) | 9 (5) | 16 (6) | 29 (7) |
| Unknown | 27 (4) | 10 (5) | 6 (2) | 12 (3) |
Data are n (%) unless otherwise stated.
Abbreviations: BSC, best supportive care; CAG, cytarabine, aclarubicin, G‐CSF; G‐CSF, granulocyte‐colony stimulating factor; HMA, hypomethylating agent; LDAC, low‐dose cytarabine; NR, not reported.
Other includes cytarabine, aclarubicin, G‐CSF (CAG regimen), enocitabine, venetoclax, or combination therapies.
Percentages may sum up to >100% as multi‐selection was permitted.
A total of 230 (18%) patients went on to receive second‐line systemic therapy (Figure 1). Of these, 214 (91%) patients discontinued second‐line systemic therapy, most commonly due to disease progression (44%) and death (30%).
FIGURE 1.
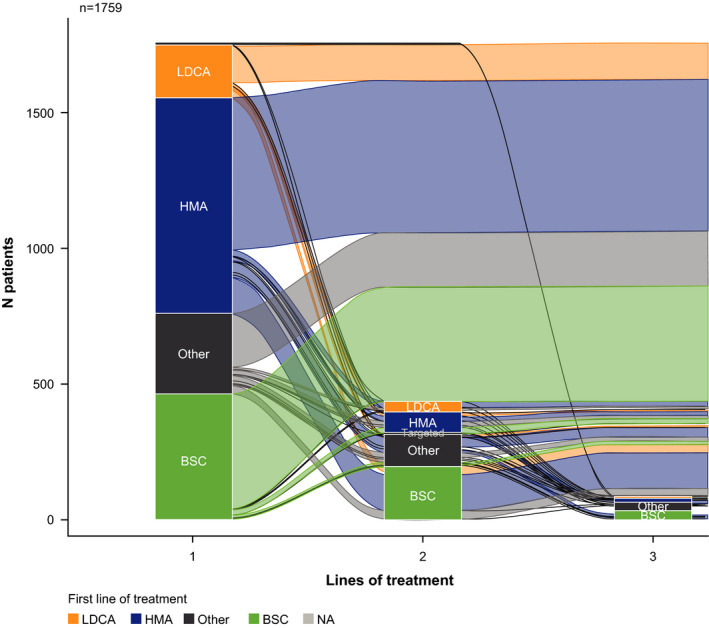
Changes in treatment from first‐line to fourth‐line therapy. BSC, best supportive care; HMA, hypomethylating agent; LDAC, low‐dose cytarabine; N/A, not available
3.2. Healthcare resource utilization
3.2.1. Hospitalizations and outpatient consultations
The rate of hospitalization was high across the treatment groups, with consistent proportions of patients hospitalized in the HMA (82%), other systemic therapy (83%), and BSC (83%) groups, rising to 93% in the LDAC group. The median number of hospitalizations was similar across the systemic therapy groups (HMA, 6 [range 1−50]; LDAC, 5 [1−26]; other systemic therapy, 4 [1−15]) and lower in the BSC group (2 [1−21]). The median number of days hospitalized was lower in the HMA and BSC groups (8 days for both) than the LDAC (16 days) and other systemic therapy (18 days) groups. Most patients did not require treatment within an ICU (median of 0 days in ICU across all groups; Table 2).
TABLE 2.
Overview of hospitalizations and outpatient consultations during first‐line systemic therapy or BSC
| First‐line systemic therapy | BSC n = 452 | |||
|---|---|---|---|---|
| HMA n = 809 | LDAC n = 199 | Other a n = 302 | ||
| Patients with hospitalization | ||||
| Yes | 664 (82) | 186 (93) | 251 (83) | 376 (83) |
| No | 137 (17) | 13 (7) | 50 (17) | 66 (15) |
| Unknown | 8 (1) | 0 | 1 (<1) | 10 (2) |
| Number of hospitalization events, n | ||||
| Median (range) | 6 (1–50) | 5 (1–26) | 4 (1–15) | 2 (1–21) |
| 1 hospitalization | 302 (45) | 70 (38) | 138 (55) | 245 (65) |
| 2 hospitalizations | 99 (15) | 28 (15) | 35 (14) | 57 (15) |
| 3+ hospitalizations | 263 (40) | 88 (47) | 77 (31) | 74 (20) |
| Total number of days hospitalized b | 2262 | 658 | 601 | 667 |
| Median (range) c | 8 (1–546) | 16 (1–696) | 18 (0–933) | 8 (1–157) |
| Total number of days in ICU | 2223 | 639 | 490 | 646 |
| Median (range) | 0 (0–69) | 0 (0–33) | 0 (0–42) | 0 (0–83) |
| Outpatient consultation | ||||
| Yes | 639 (79) | 105 (53) | 191 (63) | 300 (66) |
| No | 137 (17) | 82 (41) | 99 (33) | 142 (31) |
| Unknown | 33 (4) | 12 (6) | 12 (4) | 10 (2) |
| Number of outpatient consultations | 603 | 94 | 183 | 279 |
| Median (range) | 13 (1–202) | 6 (1–90) | 11 (1–296) | 6 (1–250) |
Data are n (%) unless otherwise stated.
Abbreviations: BSC, best supportive care; CAG, cytarabine, aclarubicin, G‐CSF; G‐CSF, granulocyte‐colony stimulating factor; HMA, hypomethylating agent; ICU, intensive care unit; LDAC, low‐dose cytarabine.
Other includes cytarabine, aclarubicin, G‐CSF (CAG regimen), enocitabine, venetoclax, or combination therapies.
Number refers to individualized hospitalizations.
The end of study date was entered whenever the date of discharge was not recorded; for this reason, the max range misrepresents the true range.
In the HMA, LDAC, and other systemic therapy groups, the most common reason for hospitalization was related to treatment administration (61%, 72%, and 61%, respectively). This was followed by transfusion‐related (26%, 52%, and 25%) and infection‐related (36%, 29%, and 39%) reasons. In the BSC group, infection was the most common reason for hospitalization (49%), followed by transfusion (36%) and progression/relapse (23%) (Figure 2).
FIGURE 2.
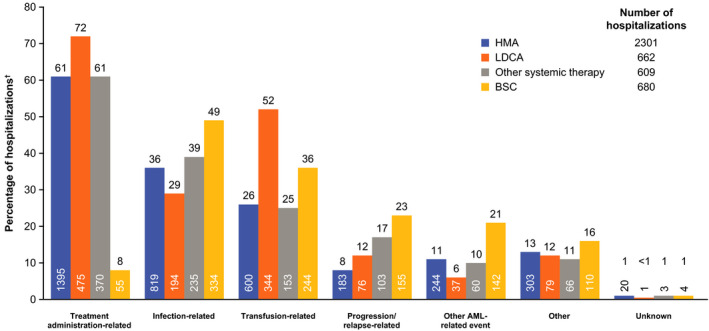
Reasons for hospitalizations during first‐line systemic therapy or BSC. †Percentages may sum up to >100% as multi‐selection was permitted. AML, acute myeloid leukemia; BSC, best supportive care; HMA, hypomethylating agent; LDAC, low‐dose cytarabine
Regionally, rates of hospitalization were highest among patients from EEMEA (93% and 81% for patients who received first‐line systemic therapy and BSC, respectively) and JAPAC (91% and 89%) and lowest among patients from LATAM (80% and 81%) and WEC (73% and 77%). The median number of hospitalizations was also higher for patients from EEMEA (8 and 4 for patients who received first‐line systemic therapy and BSC, respectively) and JAPAC (5 and 3) versus those from WEC (3 and 1) and LATAM (2 and 1). The median length of hospital admissions was consistent across the regions, ranging from 8 to 11 days for those who received first‐line systemic therapy and 7–12 days for those who received BSC (Table S2). The treatment administration was the most common reason for hospitalization among patients who received first‐line systemic therapy in all regions (74%, 61%, 49%, and 48% in EEMEA, JAPAC, WEC, and LATAM, respectively). Among patients who received BSC, the most common reason for hospitalization in JAPAC, WEC, and LATAM was infection‐related (51%, 52%, 42%, respectively). In EEMEA, the most common reason for hospitalization was related to transfusion (76%) (Figure S1).
Patients in the HMA group were most likely to receive outpatient consultation during first‐line treatment (79% vs 53% LDAC, 63% other systemic therapy, and 66% BSC). The median (range) number of outpatient consultations was 13 (1–202) for the HMA group, 6 (1–90) for the LDAC group, 11 (1–296) for the other systemic therapy group, and 6 (1–250) for BSC (Table 2).
3.2.2. Transfusions
RBC or platelet transfusions were received by 80% of patients who received HMA (median treatment duration 118 days), 57% of patients who received LDAC (median treatment duration 35 days), 57% of patients who received other systemic therapy (median treatment duration 33 days), and 71% of patients who received BSC (median treatment duration 57 days). The median number of times RBC/platelet transfusions were received was 10/4 for HMA, 13/11 for LDAC, 8/4 for other systemic therapy, and 7/2 for BSC (Table 3).
TABLE 3.
Overview of RBC and platelet transfusions during first‐line systemic therapy or BSC
| First‐line systemic therapy | BSC n = 452 | |||
|---|---|---|---|---|
| HMA n = 809 | LDAC n = 199 | Other a n = 302 | ||
| Patients receiving RBC and/or platelet transfusions | ||||
| Yes | 646 (80) | 114 (57) | 173 (57) | 321 (71) |
| No | 141 (17) | 75 (38) | 114 (38) | 118 (26) |
| Unknown | 22 (3) | 10 (5) | 15 (5) | 13 (3) |
| Patients receiving RBC transfusions | 543 | 105 | 157 | 298 |
| Median (range) number of RBC transfusions b | 10 (0–357) | 13 (1–9360) | 8 (0–111) | 7 (0–164) |
| Patients receiving platelet transfusions | 535 | 106 | 153 | 294 |
| Median (range) number of platelet transfusions b | 4 (0–727) | 11 (0–8330) | 4 (0–232) | 2 (0–200) |
Data are n (%) unless otherwise stated.
Abbreviations: BSC, best supportive care; CAG, cytarabine, aclarubicin, G‐CSF; eCRF; electronic case report form; G‐CSF, granulocyte‐colony stimulating factor; HMA, hypomethylating agent; LDAC, low‐dose cytarabine; RBC, red blood cell.
Other includes cytarabine, aclarubicin, G‐CSF (CAG regimen), enocitabine, venetoclax, or combination therapies.
Data presented as recorded in the eCRF; inter‐site variances in recording these values may have impacted the max values reported.
3.2.3. Supportive care and additional medications
Most patients received antibiotics or antivirals during first‐line treatment (80%, 92%, 87%, and 72% in the HMA, LDAC, other systemic therapy, and BSC groups, respectively) (Table 4). Antibiotics and antivirals were most often used in response to infection rather than prophylactically, regardless of treatment group. Antifungals were utilized by 43%, 63%, 57%, and 34% of patients in the HMA, LDAC, other systemic therapy, and BSC groups, respectively, during first‐line treatment (Table 4). Across all treatment groups, antifungal use tended to be prophylactic rather than in response to infection. Growth factors were not frequently used during first‐line treatment (18%, 18%, 27%, and 7% in the HMA, LDAC, other systemic therapy, and BSC groups, respectively) (Table 4). Growth factors were more frequently utilized with curative intent than for prophylaxis across all treatment groups. Of all the treatment groups, patients receiving HMA had the longest mean time on antibiotics/antivirals, antifungals, and growth factors, which is likely reflective of the longer median duration of first‐line HMA compared with other systemic treatments.
TABLE 4.
Overview of antibiotics or antiviral, antifungal, and growth factor use during first‐line systemic therapy or BSC
| First‐line systemic therapy | BSC n = 452 | |||
|---|---|---|---|---|
| HMA n = 809 | LDAC n = 199 | Other a n = 302 | ||
| Antibiotic or antiviral use | ||||
| Yes | 648 (80) | 183 (92) | 264 (87) | 324 (72) |
| No | 145 (18) | 15 (8) | 35 (12) | 102 (23) |
| Unknown | 16 (2) | 1 (1) | 3 (1) | 26 (6) |
| Reason for use b | ||||
| Prophylaxis | 376 (58) | 89 (49) | 132 (50) | 144 (44) |
| Curative | 499 (77) | 157 (86) | 203 (77) | 272 (84) |
| Unknown | 4 (1) | 3 (2) | 8 (3) | 8 (2) |
| Number of days on antibiotic/antiviral | ||||
| Mean (SD) | 98 (155) | 37 (52) | 39 (50) | 42 (77) |
| Median (range) c | 40 (1–1450) | 26 (1–430) | 20 (1–300) | 16 (2–740) |
| Antifungal use | ||||
| Yes | 347 (43) | 125 (63) | 172 (57) | 152 (34) |
| No | 440 (54) | 71 (36) | 121 (40) | 270 (60) |
| Unknown | 22 (3) | 3 (2) | 9 (3) | 30 (7) |
| Reason for use b | ||||
| Prophylaxis | 257 (74) | 84 (67) | 131 (76) | 105 (69) |
| Curative | 145 (42) | 46 (37) | 53 (31) | 60 (39) |
| Unknown | 5 (1) | 3 (2) | 5 (3) | 8 (5) |
| Number of days on antifungals d | ||||
| Mean (SD) | 105 (151) | 35 (55) | 61 (100) | 56 (102) |
| Median (range) c | 41 (1–805) | 22 (1–430) | 27 (1–584) | 22 (1–724) |
| Growth factor use | ||||
| Yes | 143 (18) | 36 (18) | 83 (27) | 32 (7) |
| No | 642 (79) | 159 (80) | 209 (69) | 398 (88) |
| Unknown | 24 (3) | 4 (2) | 10 (3) | 22 (5) |
| Reason for use b | ||||
| Prophylaxis | 72 (50) | 14 (39) | 40 (48) | 15 (47) |
| Curative | 91 (64) | 22 (61) | 46 (55) | 23 (72) |
| Unknown | 3 (2) | 1 (3) | 3 (4) | 0 |
| Number of days on growth factors c | ||||
| Mean (SD) | 31 (58) | 15 (15) | 13 (15) | 19 (24) |
| Median (range) c | 14 (1–417) | 12 (1–78) | 6 (1–67) | 12 (2–110) |
Data are n (%) unless otherwise stated.
Abbreviations: BSC, best supportive care; CAG, cytarabine, aclarubicin, G‐CSF; eCRF; electronic case report form; G‐CSF, granulocyte‐colony stimulating factor; HMA, hypomethylating agent; LDAC, low‐dose cytarabine; SD, standard deviation.
Other includes cytarabine, aclarubicin, G‐CSF (CAG regimen), enocitabine, venetoclax, or combination therapies.
Percentages may sum up to more than 100% as multi‐selection is allowed.
Data presented as recorded in the eCRF; inter‐site variances in recording these values may have impacted the max values reported.
Data represents the total days on treatment during first‐line therapy, irrespective of whether treatment was stopped and re‐started one or more times.
4. DISCUSSION
Rates of HRU were high across all treatment groups in this noninterventional, retrospective chart review in which almost three quarters of patients received systemic therapy (most commonly HMAs), followed by other systemic therapies, and the remaining quarter received BSC. More than 80% of patients were hospitalized during first‐line therapy. The highest rate was documented in the LDAC group (93%), which also had the highest rate of hospitalizations related to treatment administration (72% vs 61% in the HMA and other systemic therapy groups). Most patients also received outpatient consultations during first‐line therapy. While this rate was lower for the LDAC group (53%), it was notably higher in the HMA group (79%), which also experienced a high rate of hospitalizations (82%) and had the highest median number of hospitalizations. 6 Furthermore, rates of transfusion were highest among patients who received HMA (80%). The primary analysis of CURRENT, presented at the American Society of Hematology, 2020, reported the longest median OS for the HMA group (9.9 months), followed by the LDAC group (7.9 months), other systemic therapy group (5.4 months), and finally the BSC group (2.5 months). 24 Perhaps unsurprisingly, this corresponds with the analysis of clinical outcomes in the primary analysis of CURRENT whereby treatment groups with longer median OS required a higher utilization of healthcare resources. Patients who received BSC had the shortest median OS and higher rates of hospitalization for infection and progression/relapse than those who received systemic therapies. The relatively high rate of BSC utilization reflects an unmet clinical need for alternative therapies in this patient population and our data suggest that improving outcomes in this group may also serve to lower hospitalization rates. Emerging therapies, including low‐intensity combination strategies, may provide a viable alternative for patients currently selected to receive BSC, with improved outcomes versus low‐intensive therapy alone. 10 , 11 , 17
The extensive variation across HRU studies in AML, including differences in patient populations, study types, and HRU parameters, prevents any meaningful comparisons. However, high HRU is consistently documented among patients with AML, regardless of treatment received and across a variety of cohorts, including younger patients, 27 those with newly diagnosed disease, 20 , 21 , 23 , 27 relapsed/refractory disease, 21 , 22 and the presence of FLT3‐mutated disease. 19 This is paralleled by the data reported here in a cohort of patients with AML who were ineligible for intensive therapy. Despite more frequently receiving nonintensive therapies, 28 older patients with AML have been reported to more often utilize healthcare resources than their younger counterparts. 19 Furthermore, it has been shown that the majority of healthcare costs associated with AML are incurred prior to remission/relapse and that costs are driven by inpatient care, which potentially highlights an area of particular need for alleviating the healthcare burden of AML. 20 , 23 , 29 Interventions to reduce the burden on inpatient care, such as a shift toward oral prophylactic antibiotics and routine outpatient transfusions, could reduce associated healthcare costs. 30
While HRU is widely reported among patient populations with AML, only limited data exist regarding HRU associated with specific nonintensive treatments. The burden associated with HMA received by patients ineligible for intensive therapy has been documented in real‐world studies and is consistent with that of the HMA treatment group of the CURRENT study. 31 , 32 A retrospective US community study of 378 patients who received first‐line treatment for AML (median age 79 years) found that most patients received a HMA (58% azacitidine and 26% decitabine). Consistent with the findings reported here, hospitalization rates were high (80% and 84% in the azacitidine and decitabine groups), with a median duration of 7 days, and 85% of patients received ≥1 transfusion. 32 HRU was consistently high across the regions reported within CURRENT. Any differences in trends and reasons for hospitalizations may reflect differences in local practices, labeling, healthcare systems, and reimbursements. It should also be noted that, while HRU was high in patients treated with HMAs, the improved long‐term outcomes associated with these agents necessarily and unavoidably incur additional resource use; however, the associated hospitalizations are less frequently due to complications such as infection, transfusion, and progression. The result is a high HRU in the context of desirable patient outcomes.
The results of CURRENT are limited by several factors common to retrospective chart reviews, and these should be taken into account when considering the data. As a real‐world, retrospective study, it should be noted that the data used were originally collected for record keeping and financial purposes, rather than for research objectives, and therefore there was a lack of consistent recording. Although attempts were made to capture all data through optimization of the eCRF, missing data confound any conclusions. Furthermore, variability within and between sites and regions may influence how data were reported, and HRU results may be more informative when viewed by region, rather than as a whole. The types and combinations of treatment administered within the other systemic therapies group was extensive. Although this was consistent with previous real‐world studies, 19 , 28 it precludes meaningful interpretation of this treatment group and comparisons with other groups. The limited use of targeted therapies within CURRENT (17%) reflects that many of these agents were not widely available for use during the recruitment period. Furthermore, the heterogeneity of this group means further studies are warranted to fully examine the patterns associated with promising emerging therapies. In particular, clinical trials have demonstrated the clinical value of combining low‐intensity therapies with targeted therapies 10 , 11 , 17 but the impact of such strategies on associated HRU remains to be determined. Indeed, with the universally improved outcomes associated with targeted therapies, factors such as burden of administration may become increasingly important when making treatment decisions. 33
The AML treatment landscape has evolved in recent years alongside our enhanced understanding of the molecular pathogenesis of the disease and the availability of genetic testing. 4 , 6 , 7 Although molecular testing was not widely adopted in this study (53% of patients had molecular profiling available), this may reflect the availability of targeted therapies at the time of treatment initiation. The array of targeted therapies emerging onto the landscape, together with the relatively high use of BSC, highlights a need for consensus and optimization of treatment selection for patients with AML considered ineligible for intensive therapy. As the data presented here reflect, the HRU by this patient population is high and there is a parallel need to understand the impact novel therapies may have on this burden, particularly with the current lack of consensus guidance in this population. This may be particularly relevant with regard to the rising costs associated with emerging therapies. 26
CURRENT is one of the largest real‐world studies to have been carried out on patients with AML who were considered ineligible for intensive treatment, with the additional value of analyses by the type of treatment received. This study provides a valuable insight into the treatment patterns currently in use for these patients and their associated healthcare burdens. When coupled with clinical outcomes, 24 this provides a comprehensive picture of real‐world scenarios in AML, illuminating areas of particular need. Overall, patients with AML who receive low‐intensity therapy require substantial healthcare resources. Novel therapies have their own HRU‐associated needs, whether in outpatient or inpatient settings, and it is likely that HRU will remain high as novel strategies become increasingly adopted in clinical practice. However, high HRU may be increasingly balanced against the improved long‐term outcomes associated with these agents, the longevity of which incur additional resource use, but with less requirement for the management of disease progression and indirect complications such as infection.
CONFLICT OF INTEREST
T. Ito: Advisory role for AbbVie and speaker honoraria for AbbVie, BMS, Novartis, Sanofi, Takeda. D. Sanford: Advisory role for AbbVie, Astellas, Novartis, Pfizer. C. Tomuleasa: No potential conflicts of interest are reported. H.‐H. Hsiao: Advisory role for AbbVie, Amgen, Janssen, Novartis, Pfizer. L. J. Enciso Olivera: No potential conflicts of interest are reported. A. K. Enjeti: Advisory role for AbbVie, Astellas, Novartis, Alexion, and Jazz Pharmaceuticals. Speaker for Alexion, Bayer, Sanofi. A. Gimenez Conca: No potential conflicts of interest are reported. T. Bernal del Castillo: No potential conflicts of interest are reported. L. Girshova: No potential conflicts of interest are reported. M. P. Martelli: Advisory role for AbbVie, Amgen, Celgene, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer. Speaker honoraria for Amgen, Celgene, Janssen, Novartis. B. Guvenc: Advisory role for AbbVie. C. Bui, A. Delgado, Y. Duan, B. Garbayo Guijarro, C. Llamas: Employees of AbbVie and may hold stock or options. J.‐H. Lee: Advisory role for AbbVie, Astellas, Celgene, Janssen, Novartis.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The medical writing support was provided by Hayley Ellis, PhD, of Fishawack Communications Ltd, and funded by AbbVie.
Ito T, Sanford D, Tomuleasa C, et al. Healthcare resource utilization trends in patients with acute myeloid leukemia ineligible for intensive chemotherapy receiving first‐line systemic treatment or best supportive care: A multicenter international study. Eur J Haematol. 2022;109:58–68. 10.1111/ejh.13769
Funding information
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
DATA AVAILABILITY STATEMENT
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.
REFERENCES
- 1. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136‐1152. [DOI] [PubMed] [Google Scholar]
- 2. Institute NC . Cancer Stat Facts: Leukemia ‐ Acute Myeloid Leukemia. Accessed February 5, 2021. https://seer.cancer.gov/statfacts/html/amyl.html
- 3. Palmieri R, Paterno G, De Bellis E, et al. Therapeutic choice in older patients with acute myeloid leukemia: a matter of fitness. Cancers. 2020;12:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J Hematol Oncol. 2019;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sasaki K, Ravandi F, Kadia TM, et al. De novo acute myeloid leukemia: a population‐ based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer. 2021;127:2049‐2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heuser M, Ofran Y, Boissel N, et al. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2020;31:697‐712. [DOI] [PubMed] [Google Scholar]
- 7. Network NCC . Clinical practice guidelines in oncology. Acute Myeloid Leukemia. 2021;2020:2. [Google Scholar]
- 8. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walter RB, Othus M, Borthakur G, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29:4417‐4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617‐629. [DOI] [PubMed] [Google Scholar]
- 11. Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high‐risk myelodysplastic syndrome. Leukemia. 2019;33:379‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC‐GIMEMA AML‐19 trial. J Clin Oncol. 2016;34:972‐979. [DOI] [PubMed] [Google Scholar]
- 14. Seymour JF, Dohner H, Butrym A, et al. Azacitidine improves clinical outcomes in older patients with acute myeloid leukaemia with myelodysplasia‐related changes compared with conventional care regimens. BMC Cancer. 2017;17:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open‐label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low‐dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670‐2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Network NCC. Clinical practice guidelines in oncology. Acute Myeloid Leukemia. 2021;2021:3. [Google Scholar]
- 17. Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo‐controlled trial. Blood. 2020;135:2137‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow‐up from a phase 1b study. Am J Hematol. 2021;96:208‐217. [DOI] [PubMed] [Google Scholar]
- 19. Griffin JD, Yang H, Song Y, Kinrich D, Shah MV, Bui CN. Treatment patterns and healthcare resource utilization in patients with FLT3‐mutated and wild‐type acute myeloid leukemia: a medical chart study. Eur J Haematol. 2019;102:341‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagiwara M, Sharma A, Chung KC, Delea TE. Healthcare resource utilization and costs in patients with newly diagnosed acute myeloid leukemia. J Med Econ. 2018;21:1119‐1130. [DOI] [PubMed] [Google Scholar]
- 21. Irish W, Ryan M, Gache L, Gunnarsson C, Bell T, Shapiro M. Acute myeloid leukemia: a retrospective claims analysis of resource utilization and expenditures for newly diagnosed patients from first‐line induction to remission and relapse. Curr Med Res Opin. 2017;33:519‐527. [DOI] [PubMed] [Google Scholar]
- 22. Pandya BJ, Chen CC, Medeiros BC, et al. Economic and clinical burden of relapsed and/or refractory active treatment episodes in patients with Acute Myeloid Leukemia (AML) in the USA: a retrospective analysis of a commercial payer database. Adv Ther. 2019;36:1922‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stein EM, Bonifacio G, Latremouille‐Viau D, et al. Treatment patterns, healthcare resource utilization, and costs in patients with acute myeloid leukemia in commercially insured and Medicare populations. J Med Econ. 2018;21:556‐563. [DOI] [PubMed] [Google Scholar]
- 24. Kondo T, Sanford D, Tomuleasa C, et al. Real‐World Treatment Patterns and Clinical Outcomes in Unfit Patients with AML Receiving First‐Line Systemic Treatment or Best Supportive Care (CURRENT): Final Analysis. American Society of Hematology. 2020. [Google Scholar]
- 25. Yi M, Li A, Zhou L, Chu Q, Song Y, Wu K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. 2020;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaughn JE, Shankaran V, Walter RB. Trends in clinical benefits and costs of novel therapeutics in AML: at what price does progress come? Curr Hematol Malig Rep. 2019;14:171‐178. [DOI] [PubMed] [Google Scholar]
- 27. Preussler JM, Meyer CL, Mau LW, et al. Healthcare costs and utilization for patients age 50 to 64 years with acute myeloid leukemia treated with chemotherapy or with chemotherapy and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23:1021‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma E, Bonthapally V, Chawla A, et al. An evaluation of treatment patterns and outcomes in elderly patients newly diagnosed with acute myeloid leukemia: a retrospective analysis of electronic medical records from us community oncology practices. Clin Lymphoma Myeloma Leuk. 2016;16(625–36):e3. [DOI] [PubMed] [Google Scholar]
- 29. LeBlanc T, Seetasith A, Choi M, et al. Healthcare resource utilization and total cost of care in older patients with acute myeloid leukemia ineligible for intensive chemotherapy. J Clin Oncol. 2020;38:S29. [Google Scholar]
- 30. Vaughn JE, Othus M, Powell MA, et al. Resource utilization and safety of outpatient management following intensive induction or salvage chemotherapy for acute myeloid leukemia or myelodysplastic syndrome: a nonrandomized clinical comparative analysis. JAMA Oncol. 2015;1:1120‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mozessohn L, Cheung MC, Mittmann N, Earle CC, Liu N, Buckstein R. Healthcare utilization in patients with higher‐risk MDS/low‐blast count AML treated with azacitidine in the ‘real‐world'. Leuk Lymphoma. 2020;61:1445‐1454. [DOI] [PubMed] [Google Scholar]
- 32. Lyons R, Potluri J, Bui C, et al. Treatment outcomes and healthcare ResourceUtilization (HRU) among patients with acute myeloid leukemia (AML) ineligible for intensive chemotherapy in US CommunityOncology setting. Clin Lymphoma Myeloma Leukemia. 2020;20:S177‐S178. [Google Scholar]
- 33. Tremblay G, Daniele P, Bell T, Chan G, Brown A, Cappelleri JC. Comparative effectiveness of glasdegib versus venetoclax combined with low‐dose cytarabine in acute myeloid leukemia. J Comp Eff Res. 2021;10:603‐612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.


