Abstract
Background
Many of the motor symptoms of Parkinson's disease (PD) impact quality of life and are not fully ameliorated by current pharmacological and surgical treatments. A better understanding of the pathophysiology underlying these symptoms is needed. Previous research has suggested that inflammation may play a significant role in PD pathophysiology and progression, but there is limited research exploring how inflammation directly relates to motor symptoms in PD. Thus, the purpose of this study was to evaluate associations between peripheral immune inflammatory markers and motor symptoms of PD, specifically, tremor, bradykinesia, and postural and gait instability. We hypothesized that peripheral inflammatory cytokines would predict the severity of motor symptoms in persons with PD, and that there will be higher levels of peripheral inflammatory cytokine markers in persons with PD when compared to age-matched healthy older adults.
Methods
Twenty-six participants with PD and fourteen healthy older adults completed the study. For participants with PD, the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS) was recorded and scored by two Movement Disorders Neurologists masked to the study. A blood sample was collected from both participants with PD and the healthy older adults. Through the MILLIPLEX® map High Sensitivity Human Cytokine Kit, key inflammation-related markers were analyzed (TNF-α, IFN-γ, IL-1β, IL-8, IL-2, IL-7, IL-5, IL-13, IL, 4, IL-10 IL-12p70, GM-CSF, and IL-6).
Results
Results revealed significantly higher levels of IL-6 in persons with PD when compared to healthy older adults (p = 0.005). Moreover, results revealed that higher levels of IL-4 (p = 0.011) and lower levels of IFNγ (p = 0.003) significantly predicted more severe tremor in persons with PD. No other associations between the peripheral inflammation markers and other motor symptoms were observed.
Conclusions
Overall, these results are consistent with a growing body of literature that implicates inflammatory cytokines in the PD, and further suggests that inflammatory cytokines, or lack thereof, may be associated with tremor in persons with PD.
Keywords: Parkinson's disease, Peripheral inflammation, Inflammatory cytokines, Motor impairments, Movement Disorders Society Unified Parkinson's Disease Rating Scale
Abbreviations: PD, Parkinson's disease; MDS-UPDRS, Movement Disorders Society Unified Parkinson's Disease Rating Scale; MMSE, Mini-Mental State Exam; BDI, Beck Depression Inventory; Th cells, T helper cells; IL, interleukins; TNFα, Tumor necrosis factor-alpha; IFNγ, Interferon gamma
Highlights
-
•
Evaluated associations between peripheral immune-inflammatory markers and motor symptoms of PD.
-
•
For participants with PD, the motor section of UPDRS was recorded and scored by two Movement Disorders Neurologists masked to the study.
-
•
A blood sample was collected from both participants with PD and healthy older adults.
-
•
Results revealed significantly higher levels of IL-6 in persons with PD when compared to healthy older adults.
-
•
Higher levels of IL-4 and lower levels of IFNγ significantly predicted more severe tremor in persons with PD.
1. Introduction
Inflammation has been postulated to be one of the mechanisms underlying the pathophysiology and progression of Parkinson's disease (PD). Evidence for this mechanism includes activated microglia in the substantia nigra (McGeer et al., 1988; Theodore et al., 2008), and alterations in inflammation-related cytokines in the brain and cerebrospinal fluid (CSF) of patients with PD (Boka et al., 1994; Mogi et al., 1994, 1996). Inflammatory cytokines, such as tumor necrosis factor-alpha (TNFα), interleukin-1β (IL-1β), and interleukin-6 (IL-6) amplify and sustain inflammation and immune responses that can exert changes in dopamine neural integrity (Nagatsu et al., 2000) and dopamine-mediated behaviors. Interestingly, levels of TNF-α, IL-1β, and IL-6 are found to be elevated in the postmortem brains of patients with PD (Boka et al., 1994; Mogi et al., 1994). In vivo studies have also demonstrated that the CSF of patients with PD has higher levels of IL-1β, IL-2, IL-4, and IL-6 (Blum-Degen et al., 1995; Mogi et al., 1996). Moreover, there is evidence of peripheral immune dysregulation in persons with PD (Marttila et al., 1984; Tufekci et al., 2012). Particularly, there are alterations in peripheral monocytic and lymphocytic subsets (Grozdanov et al., 2014; Jiang et al., 2017; Kustrimovic et al., 2018; Marttila et al., 1984), and when compared to healthy controls, persons with PD show elevated levels of peripheral cytokines IL-2, IL-4, IL-6, IL-10, and TNF-α (Chen et al., 2008; Kwiatek-Majkusiak et al., 2020; Reale et al., 2009; Stypuła et al., 1996). These findings suggest that central and peripheral inflammation may play a role in PD.
Inflammation has also been implicated in PD symptomatology (Barnum and Tansey, 2012; Lindqvist et al., 2012, 2013; Menza et al., 2010; Rathnayake et al., 2019; Veselý et al., 2018). Specifically, TNF-α has been found to correlate with symptoms of depression, fatigue, and cognitive impairment in persons with PD (Lindqvist et al., 2013; Menza et al., 2010; Scalzo et al., 2010; Veselý et al., 2018). Although some studies have reported negative results (Dufek et al., 2009; Kim et al., 2018), higher levels of peripheral cytokines may also be associated with the motor symptoms of PD. Indeed, clinical studies have shown significant associations between serum cytokines and cytokines/chemokines produced by peripheral blood mononuclear cells (PBMC); IL-1β, IFN-γ, TNF-α, IL-6, IL-13, IL-8, IL-17A, and RANTES and motor symptom progression (Green et al., 2019; Reale et al., 2009; Rentzos et al., 2007; Williams-Gray et al., 2016). However, motor symptoms in PD are varied and there is a lack of literature examining the relationship between peripheral inflammation and specific cardinal motor symptom domains, such as bradykinesia, tremor, and gait instability.
Only one study has examined the relationship between peripheral inflammation and mobility and gait in PD. Scalzo et al. (2010) showed that persons with PD with higher serum levels of IL-6 have greater impairment in functional mobility as measured through the Timed Up and Go Test (TUG) and gait. Given that many of the motor symptoms of PD are not fully ameliorated by the pharmacological treatments available (Sethi, 2008), and have a significant negative impact on quality of life (Jankovic, 2008), there remains a need for continued research on other potential underlying mechanisms of PD symptomatology and pathophysiology, such as inflammation. Thus, in this study, we evaluated associations between immune inflammatory markers previously associated with PD and the motor symptoms, specifically, tremor, bradykinesia, and postural and gait instability. We hypothesized that there will be higher levels of peripheral inflammatory cytokine markers in persons with PD when compared to age-matched healthy older adults and that peripheral inflammatory cytokines will predict the severity of motor symptoms.
2. Materials and methods
2.1. Participants
Twenty-six (58% females) persons diagnosed with idiopathic PD were included in the study. Their mean age was 72.76 ± 7.14 years, mean disease duration was 8.04 ± 5.37 years, the mean Total Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) was 68.15 ± 17.05, and the mean Hoehn-Yahr rating was 2.19. ± 0.49. Fourteen (57% females), age and gender-matched volunteers, free of immune- or immune-related disorders, served as controls. Their mean age was 69.21 ± 5.43 years (Table 1.). An independent t-test revealed no significant difference in age between persons with PD and the control group (p = 0.113). The inclusion criteria for all participants with PD included the clinical diagnosis of PD and being on a stable regimen of antiparkinsonian and psychotropic medication for 30 days prior to participation. All participants were excluded from the study if they presented significant cognitive impairment (Mini-Mental State Exam score <24), and major psychiatric disorder (Beck Depression Inventory score >18). Participants with PD were tested on medication. All participants provided written informed consent before admission to the study. The Research Ethics Committee of Iowa State University approved this study.
Table 1.
Participant demographics.
| Control (n = 14) | Patients (n = 26) | |
|---|---|---|
| Sex | F = 8 (61.5%), M = 6 | F = 15 (57.6%), M = 11 |
| Age | 69.21 ± 5.43 | 72.76 ± 7.14 |
| Disease duration (yrs., mean ± SD) | – | 8.04 ± 5.37 |
| On Levodopa (%) | – | 88.40% |
| On specific anti-inflammatory drugs (%) | 28.5% | 34.6% |
| MMSE (mean ± SD) | 29.42 ± 0.85 | 28.84 ± 1.58 |
| DBI (mean ± SD) | – | 9.07 ± 5.32 |
| Hoehn and Yahr (mean ± SD) | – | 2.19 ± 0.49 |
| Total UPDRS (mean ± SD) | – | 68.15 ± 17.05 |
| Total Motor UPDRS (mean ± SD) | – | 38.34 ± 10.12 |
| Total Bradykinesia (mean ± SD) | – | 24.48 ± 6.37 |
| Upper Extremity Bradykinesia (mean ± SD) | – | 12.94 ± 4.01 |
| Lower Extremity Bradykinesia (mean ± SD) | – | 9.46 ± 2.60 |
| Tremor (mean ± SD) | – | 4.78 ± 4.80 |
| postural and gait instability (mean ± SD) | – | 5.53 ± 2.97 |
All values are presented as mean ± standard deviation. F = female; yrs. = years; UPDRS=Unified Parkinson's. Disease Rating Scale; MMSE = Mini-Mental State Exam; BDI=Beck Depression Inventory.
3. Data collection
3.1. Clinical assessment
For participants with PD, clinical symptoms were evaluated with the MDS-UPDRS. The MDS-UPDRS scale we used consists of the following five segments: Section I-mentation, behavior, and mood; Section II-activities of daily living (scored for “on” and “off”); Section III-motor exam; and Section IV-complications. Each subscale has a 0–4 rating, where 0 = normal, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe (Goetz et al., 2008). The total score was obtained by summing the first four segments of the MDS-UPDRS. Scores for motor symptoms were taken from Part III of the MDS-UPDRS. Specifically, scores for Upper Extremity Bradykinesia were calculated by summing items 3.4 (Finger Tapping), 3.5 (Hand Movements), and 3.6 (Pronation-Supination). Scores for Lower Extremity Bradykinesia were calculated by summing items 3.7 (Toe Tapping), and 3.8 (Leg Agility). Scores of Total Bradykinesia were calculated by summing the Upper Extremity Bradykinesia scores, the Lower Extremity Bradykinesia scores, and item 3.14 (Global Spontaneity of Movement). Scores for Postural and Gait Instability were calculated by summing items 3.9 (Arising from the Chair), 3.10 (Gait), 3.11(Freezing of Gait), 3.12 (Postural Stability), and 3.13 (Posture). Scores for tremor were calculated by summing items 3.15 (Postural Tremor), 3.16 (Kinetic Tremor), 3.17 (Rest Tremor), and 3.18 (Constancy of Rest). The MDS-UPDRS was scored by two Movement Disorders Neurologists trained in scoring the MDS-UPDRS and were masked to the study. The average MDS-UPDRS scores from both neurologists were used for statistical analysis. The correlation coefficient between raters was 0.800 (p < 0.001), and an independent t-test revealed that the average scores did not differ between the two raters’ scores (p = 0.58).
3.2. Blood sample collection
For all participants, a blood sample was collected using a 10 ml BD Vacutainer serum tube within 5 min of the clinical assessments. Blood was allowed to clot for at least 30 min before centrifugation for 10 min at 3,000 rpms. Serum was then removed, aliquot, and stored at −20 Co. Through the MILLIPLEX® map Human Cytokine Kit, key inflammation-related markers were analyzed (TNF-α, IFN-γ, IL-1β, IL-8, IL-2, IL-7, IL-5, IL-13, IL, 4, IL-10 IL-12p70, GM-CSF, and IL-6). The Assay was performed according to the manufacturer's instructions. The multiplex immunoassay panel was analyzed on a Bio-Plex (BioRad) 200 System (Luminex® 200TM).
3.3. Statistical analysis
Following the removal of outliers that were three standard deviations above and below the mean (7 outliers in total out of 520 total data points), the data was inspected for normality. Normality test revealed a non-normal distribution. Therefore, mean peripheral immune marker levels were compared between PD and control groups using the Mann-Whitney U tests. Significance was set at p < 0.05. Effect sizes were calculated by using Cohen's d. Stepwise regression linear regressions were used to identify factors that predicted clinical motor scores in persons with PD. In particular, the regression was used to examine the contribution of each cytokine while controlling for confounding variables of age, disease duration, and disease severity (Lindqvist et al., 2013; Menza et al., 2010). Therefore, age, disease duration, and Hoehn and Yahr scores were entered as control variables in step one. The Hoehn and Yahr Scale is used to measure how Parkinson's symptoms progress and the level of disability. The scale includes stages 0 (No signs of disease) to 5 (needing a wheelchair or bedridden unless assisted). In step two, TNF-α, IFN-γ, IL-1β, IL-8, IL-2, IL-7, IL-5, IL-13, IL, 4, IL-10 IL-12p70, GM-CSF, and IL-6 were entered as predictors, with forward stepwise entry. The Total MDS-UPDRS Scores, Total Motor MDS-UPDRS Score, Total Bradykinesia Score, Upper-Bradykinesia Score, Lower-Bradykinesia Score, Posture and Gait Score, and Tremor were entered in the model as dependent variables, respectively, meaning separate analyses were conducted for each independent variable. To control for health status, additional exploratory analyses were completed, which used medication categories to group health status. In the first stepwise regression exploratory analysis, three individuals with PD that were taking steroidal anti-inflammatory medication were removed as these medications would be expected to significantly impact cytokine levels. In the second stepwise regression exploratory analysis, participants with PD taking steroidal anti-inflammatory medications or treated with NSAIDs, including low doses of aspirin were removed. Statistical analysis was performed with IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, New York, USA).
4. Results
4.1. Comparison between PD and control groups
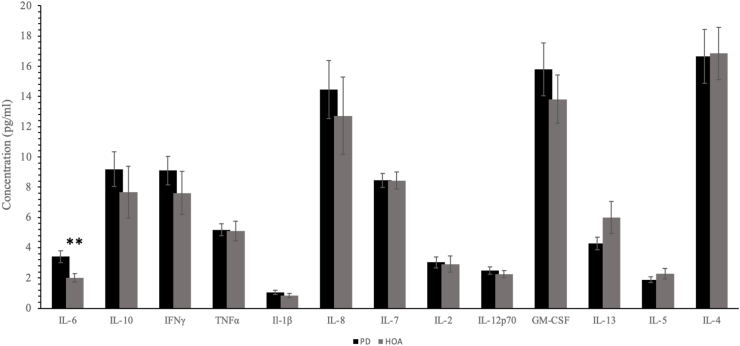
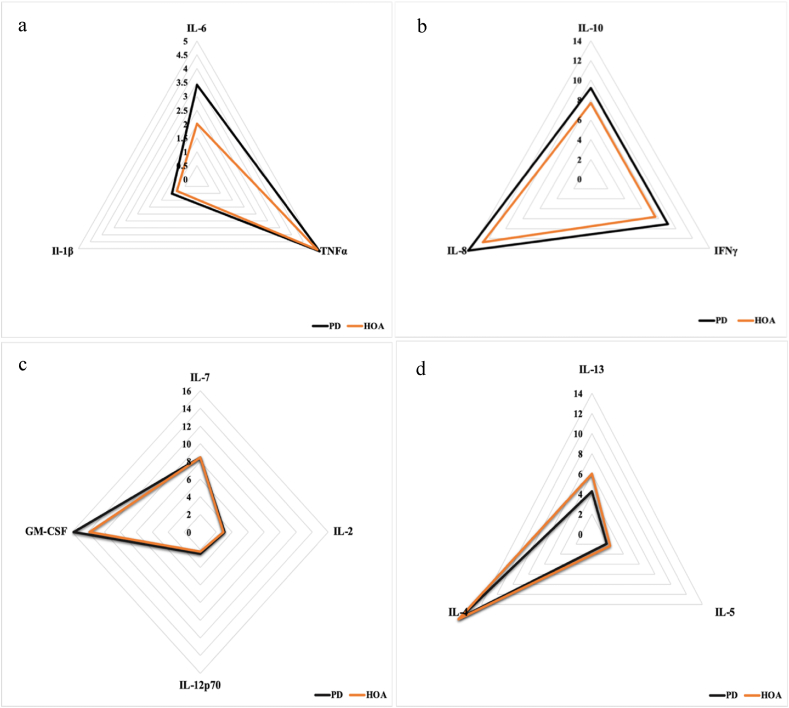
Fig. 1 shows the inflammatory marker concentrations in the PD and control groups. When compared to healthy controls, levels of IL-6 were found to be significantly higher in persons with PD (p = 0.005, d = 0.81). Fig. 2 shows the pattern for peripheral inflammatory cytokines in a radial plot for persons with PD and healthy older adults. Specifically, Fig. 2a and b represent inflammation-related cytokines, Fig. 2c are the mixed-function cytokines, and Fig. 2d are T helper type 2 (Th2) related cytokines. Levels of TNF-α, IFN-γ, IL-1β, IL-8, IL-2, IL-7, IL-10, IL-12p70, and GM-CSF (p > 0.05), albeit not significant, were higher in persons with PD (Fig. 2a, b, and 2c). Finally, levels of IL-4 (p = 0.71, d = −0.024), IL-5 (p = 0.46, d = −0.36) and IL-13 (p = 0.44, d = 0.55), albeit not significant, were higher in the healthy controls (Fig. 2d).
Fig. 1.
Levels inflammatory markers in patients with Parkinson's disease (black bars) and controls (gray bars). Bars represent mean values, and T-bars indicate standard errors; ∗P < 0.05.
Fig. 2.
Peripheral inflammatory cytokines in persons with PD and healthy older adults. a) and b) inflammation-related cytokines, c) mixed-function cytokines, and d) T helper type 2 (Th2) related cytokines. Data were obtained from the blood of persons with PD and healthy older adults.
4.2. Regression analysis
4.2.1. Total MDS-UPDRS score
In the regression analysis for total MDS-UPDRS, multiple R2 was 0.457 (p = 0.014). Age, disease duration, and Hoehn and Yahr scores explained all variation in the in total UPDRS scores. Upon further inspection, the p-value of the beta weight for disease duration (B = 0.394; p < 0.05), but not age (B = 0.324; p > 0.05), and Hoehn and Yahr scores (B = 0.325; p > 0.05) was statistically significant.
4.2.2. Total motor UPDRS
In the regression analysis for total Motor UPDRS, multiple R2 was 0.523 (p = 0.005). Age, disease duration, and Hoehn and Yahr scores explained all variations in total motor UPDRS scores. Upon further inspection, the p-value for beta weights of age (B = 0.518; p < 0.05) and disease duration (B = 0.43; p < 0.05), but not Hoehn and Yahr scores (B = 0.08; p > 0.05) were statistically significant.
4.2.3. Total Bradykinesia
In the regression analysis for total bradykinesia, multiple R2 was 0.404 (p = 0.029). Similarly, to the total MDS-UPDRS scores, age, disease duration, and Hoehn and Yahr score explained all variations in total bradykinesia scores. Upon further inspection, the p level for the beta weight of age (B = 0.553; p < 0.05), but not disease duration (B = 0.177; p > 0.05), and Hoehn and Yahr scores (B = 0.096; p > 0.05) was statistically significant.
4.2.4. Upper Extremity Bradykinesia
In the regression analysis for Upper Extremity Bradykinesia, multiple R2 was 0.39(p = 0.035). Similarly, to the total MDS-UPDRS scores, age and disease duration explained all variations in upper bradykinesia scores. However, upon further inspection, the p level for beta weight of age (B = 0.596; p < 0.05), but not disease duration (B = 0.08; p > 0.05), and Hoehn and Yahr scores (B = 0.038; p > 0.05) was statistically significant.
4.2.5. Lower Extremity Bradykinesia
In the regression analysis for Lower Extremity Bradykinesia, multiple R2 was 0.239 (p = 0.189). Given this result, regression weights were not examined further.
4.2.6. Postural and gait instability
In the regression analysis for posture, multiple R2 was 0.434(p = 0.019). Similarly, to the total MDS-UPDRS scores, Age, disease duration, and Hoehn and Yahr score explained all variations in Postural and gait instability scores. However, upon further inspection, the p level for beta weight of the Hoehn and Yahr score (B = 0.559; p < 0.05), but not age (B = 0.162; p > 0.05), and disease duration (B = 158; p > 0.05) was statistically significant.
4.2.7. Tremor
In the stepwise regression analysis for tremor, multiple R2 was 0.734(p = 0.011). Here, age, disease duration, and Hoehn and Yahr score accounted for 26% (p = 0.153) of the variance in tremor scores. However, upon further inspection, the p level for beta weight of disease duration (B = 0.45; p = 0.05), but not age (B = 0.06; p > 0.05), and Hoehn and Yahr scores (B = −0.22; p > 0.05) was statistically significant. After controlling for age and disease duration, and Hoehn and Yahr score in the stepwise regression model, cytokines IFN-γ and IL-4 further contributed to the changes in tremor score. When IFN-γ was entered into the model, IFN-γ accounted for 32.5% of the variance in tremor scores, and the standardized regression weight associated with IFN-γ was −0.67 (p = 0.003). Moreover, when IL-4 was entered into the regression model, IL-4 accounted for an additional 14.9% of the variance in tremor scores, and the standardized regression weight associated with IL-4 was 0.52 (p = 0.011) (Table 2.)
Table 2.
Results from stepwise regression examining peripheral serum cytokines that predicted clinical motor scores (i.e., tremor scores) in persons with PD, while controlling for age, disease duration, disease duration.
| Variables entered | Full Data Set (N = 26) |
Exploratory Analysis 1 (N = 23) |
Exploratory Analysis 2 (N = 16) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | t | p | β | t | p | β | t | p | |
| Step one | |||||||||
| Age | .06 | .25 | .80 | −.09 | −.31 | .75 | .077 | .22 | .83 |
| Disease duration | .45 | 2.12 | .05 | .36 | 1.48 | .16 | .33 | 1.08 | .31 |
| Hoehn and Yahr | −.22 | −.96 | .35 | −.12 | −.45 | .65 | −.11 | −.34 | .74 |
| Step two | |||||||||
| Age | −.27 | −1.36 | .19 | −.473 | −2.07 | .05 | −.36 | −1.43 | .19 |
| Disease duration | .46 | 2.80 | .013 | .41 | 2.38 | .03 | .38 | 1.86 | .10 |
| Hoehn and Yahr IFN-γ |
−.26 −.67 |
−1.47 −3.54 |
.16 .003 |
−.13 −.76 |
−.66 −3.74 |
.52 .002 |
−.19 −.86 |
−.86 −3.66 |
.42 .006 |
| Step three | |||||||||
| Age | −0.34 | −1.99 | 0.065 | −0.51 | −2.97 | .01 | −.45 | −2.36 | .05 |
| Disease duration Hoehn and Yahr |
0.49 −0.30 |
3.63 −1.98 |
0.002 0.07 |
0.39 −0.22 |
3.00 −1.43 |
.01 .17 |
.39 −.16 |
2.58 −.99 |
.04 .35 |
| IFN-γ IL-4 |
−1.05 0.52 |
−5.14 2.89 |
0 .011 |
−1.09 0.53 |
−5.99 3.33 |
0 .006 |
−1.24 0.52 |
−5.62 2.76 |
0.001 0.02 |
In the exploratory analysis 1, which only included PD participants not taking steroidal anti-inflammatory medication, associations between tremor scores, IFNγ and IL-4 were observed. The R2 for the regression model was 0.794 (p = 0.006). Here, age, disease duration, and Hoehn and Yahr score accounted for 17.7% (p = 0.42) of the variance in tremor scores. After controlling for age and disease duration, and Hoehn and Yahr score in the stepwise regression model, IFN-γ accounted for 42.7% of the variance in tremor scores, and the standardized regression weight associated with IFN-γ was −0.762 (p = 0.002). Moreover, when IL-4 was entered into the regression model, IL-4 accounted for an additional 19% of the variance in tremor scores, and the standardized regression weight associated with IL-4 was 0.53 (p = 0.006) (Table 2.)
In the exploratory analysis 2, which only included PD participants not taking steroidal anti-inflammatory medications or treated with NSAIDs, including low doses of aspirin, associations between tremor scores, IFNγ and IL-4 were still observed. The R2 for the regression model was 0.845 (p = 0.028). Here, age, disease duration, and Hoehn and Yahr score accounted for 17.7% (p = 0.42) of the variance in tremor scores. After controlling for age and disease duration, and Hoehn and Yahr score in the stepwise regression model, IFN-γ accounted for 54.3% of the variance in tremor scores, and the standardized regression weight associated with IFN-γ was −0.86 (p = 0.006). Moreover, when IL-4 was entered into the regression model, IL-4 accounted for an additional 16.9% of the variance in tremor scores, and the standardized regression weight associated with IL-4 was 0.52 (p = 0.02) (Table 2.)
Exploratory Analysis 1 included participants with PD not taking steroidal anti-inflammatory medication. Exploratory Analysis 2 included participants with PD not taking steroidal anti-inflammatory medication or treated with NSAIDs, including low doses of aspirin.
5. Discussion
The present study assessed associations between peripheral inflammation and PD symptomatology. Inflammatory markers, specifically TNF-α, IFN-γ, IL-1β, IL-8, IL-2, IL-7, IL-5, IL-13, IL, 4, IL-10 IL-12p70, GM-CSF, and IL-6 and cardinal motor symptoms of PD, specifically, tremor, bradykinesia, and postural and gait instability, were assessed. Our results are in keeping with our hypothesis that when compared to healthy older adults, peripheral inflammatory cytokines were higher in persons with PD. Additionally, regression analyses showed that peripheral inflammation markers predict the severity of tremor, but not bradykinesia, posture, and gait instability in persons with PD. Moreover, even when controlling for medications that impact immune response, the same serum cytokines predict the severity of tremor.
The finding that participants with PD have significantly higher levels of IL-6 than healthy older adults substantiate earlier observations of IL-6 alterations in the peripheral nervous system (Chen et al., 2008; Dobbs et al., 1999; Kwiatek-Majkusiak et al., 2020; Lindqvist et al., 2012; Reale et al., 2009; Scalzo et al., 2010), and CSF (Blum-Degen et al., 1995; Mogi et al., 1994; Müller et al., 1998) of persons with PD. The role of inflammation in PD has led researchers to examine the effects that peripheral inflammation has on the central nervous system (CNS) of persons with PD. Specifically, the effects that leukocytes have on the CNS inflammatory response, as T-lymphocytes and monocytes have been reported to cross from the periphery to initiate or participate in the CNS immune response (Brochard et al., 2009; Garré and Yang, 2018; Hemmer et al., 2004; Wijeyekoon et al., 2018). In animal models, both protective and detrimental effects of IL-6 have been reported. Conroy et al. (2004) showed that chronic exposure to IL-6 during neuronal development can lead to cell damage and death in a subpopulation of developing granule neurons (Conroy et al., 2004). On the other hand, a neuroprotective effect of IL-6 was observed in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–induced parkinsonian mice. According to Bolin et al. (2002), in the absence of IL-6 (IL-6 (−/−) mice), a single injection of MPTP resulted in striatal dopamine depletion. Per the authors, this result suggests that IL-6 may be involved in neuroprotective mechanisms in the MPTP-lesioned nigrostriatal system (Bolin et al., 2002). Thus, enhanced circulating levels of IL-6 may be pro-inflammatory, leading to the progression of PD pathophysiology, or anti-inflammatory providing protection against other pro-inflammatory mechanisms.
Likewise, cytokines IL-4 and IFNγ which in our study predicted a substantial part of the variance in tremor scores may have dual functions in the CNS, even when controlling for medications that impact immune response. For example, IL-4 has been linked to the death of activated microglia and neuronal survival (Park et al., 2005). On the other hand, IL-4 was also shown to promote neurodegeneration in LPS treated rats (pro-inflammatory) by contributing to microglial activation, production of IL-1β, and disruption of the blood-brain barrier (Bok et al., 2018). Although IFN-γ has been associated with the death of dopaminergic neurons in models of PD (Barcia et al., 2011; Chakrabarty et al., 2011; Wang et al., 2015), in other neurodegenerative disease models, such as AD, IFNγ can increase the proliferation of neural precursor cells or enhance neurogenesis (Baron et al., 2008; Kulkarni et al., 2016; Mastrangelo et al., 2009). Thus, in order to establish a specific mechanism, additional experiments need to be conducted. Nonetheless, our findings support the view that there is an abnormal peripheral inflammatory immune response in persons with PD that predict specific motor symptoms.
Levels of IL-4, IL-5, and IL-13, albeit not significant, were higher in healthy older adults than in persons with PD (Fig. 2C). However, effect sizes for IL-5 and IL-13 were moderate (small effect size = 0.10, medium effect size = 0.30, and large effect size = 0.50). In the peripheral blood of persons with PD, irregularities in T-cell subsets are abundant. In particular, numbers of CD4+ T-cells have been shown to be reduced (Jiang et al., 2017), whereas numbers of CD8+ T-cells are unchanged. CD4+ T-cells differentiate into effector subtypes corresponding to different inflammatory states. Generally, CD4+ T-cells are divided into pro-inflammatory (Th1 and Th17) and anti-inflammatory (Th2 and Tregs), each producing a unique set of pro-and anti-inflammatory cytokines (Supplemental Table 1.). Kustrimovic and collaborators have recently indicated that there is a Th1 bias in the blood of patients with PD, with a reduction in the number of Th17, Th2, and Tregs (Kustrimovic et al., 2018). This Th1-bias combined with lower levels of IL-4, IL-5, and IL-13 reported in our study may contribute to the pro-inflammatory environment seen in persons with PD.
In this study, higher levels of IL-4 and lower levels of IFNγ predicted more severe tremors scores. This finding is further supported by the work conducted by Lian et al. (2019). In their study, Lian and colleagues reveal that inflammation, especially IL-6-induced, in both the peripheral and central nervous system may play a critical role on tremor-dominant persons with PD (Lian et al., 2019). However, there were no predictors of other motor symptoms. According to the literature, tremor does not progress at the same rate (Louis et al., 1999) or correlate with other PD motor symptoms (Louis et al., 2001). Moreover, tremor does not respond well to dopaminergic treatment (Fishman, 2008; Koller et al., 1994). Thus, tremor may be considered an independent symptom (Helmich et al., 2012), and inflammatory cytokines, or lack thereof, may be involved in the production and/or progression of select motor symptoms of PD (i.e., tremor). Nonetheless, our data suggest that peripheral inflammatory cytokines are associated with clinical motor symptoms in PD, thus corroborating a growing body of literature that implicates inflammatory cytokines in the progression of PD and symptomatology (Barcia et al., 2011; Reale et al., 2009; Scalzo et al., 2010; Williams-Gray et al., 2016).
In summary, peripheral immune cytokines have been implicated in PD pathophysiology and symptomology. In this study, we found that participants with PD have significantly higher levels of IL-6 than healthy older adults. We also found significant associations between IL-4, IFNγ, and tremor in persons with PD, even when controlling for health status by removing persons that were on medications that impact immune response from our exploratory regression analysis. The relationship between those cytokines and tremor scores was particularly strong. These data, therefore, suggest that peripheral inflammatory cytokines, or lack thereof, may be involved in the neurobiology behind the initiation and/or maintenance of tremor, an important motor symptom in PD. Further study of these relationships could yield important clues to the pathophysiology, course, and treatment of motor symptoms in persons with PD.
5.1. Limitations
Despite some limitations, such as being subjective, the MDS-UPDRS scale has several strengths and is considered the reference standard for disability and impairment measures amongst persons with PD. Several of the individuals comprising the control group were spouses of the participants with PD. One may speculate that some of the individuals in the control group may have been subjected to caregiver stress that could potentially have influenced their cytokine levels. In our study, 64.40% of healthy older adults, and 73.07% of persons with PD were on anti-inflammatory medications, potentially influencing cytokines levels. Nevertheless, differences were found between the two groups. Additionally, by using a stepwise regression procedure to identify factors that predicted clinical motor scores in persons with PD, there is the possibility of capitalizing on the chance characteristics of this sample. Nonetheless, given the evidence implicating inflammatory cytokines in PD symptomology, the results are intriguing and clearly warrant replication as they could point to pathophysiologic processes that are involved in the production of these symptoms. All participants were tested on antiparkinsonian medication. The effect of dopaminergic medication on inflammatory cytokines in persons with PD remains unknown. Thus, studies should examine the possible mechanism of L- DOPA involving cytokines in persons with PD. Future studies are needed to identify the cell source of the significant cytokines revealed in this study and to verify serum cytokine concentration with multiple approaches such as RT-PCR or flow cytometry to determine the contribution of specific immune cell populations. Nonetheless, these preliminary results provide additional information regarding the association between peripheral inflammatory cytokines and clinical motor symptoms in persons with PD.
6. Conclusion
Delineating the relationship between inflammation and clinical motor symptoms of PD will increase our understanding of the mechanisms underlying PD pathophysiology and symptomatology and may provide a stepping-stone to allow for the use of anti-inflammatory medications currently available to aid in the alleviation of PD specific motor symptoms.
Ethics approval and consent to participate
The Research Ethics Committee of Iowa State University approved this study.
Consent for publication
Not applicable.
Availability of data and materials
The article's supporting data and materials can be accessed by contacting the corresponding author.
Competing interests
The authors declare that they have no competing interests.
Author contributions
DK and SEL aided in the conceptualization, study design, acquisition of the data, and in the analysis, and interpretation of the data, and in drafting and revision of the original manuscript. KLM and EWD aided in the analysis and interpretation of the data, reviewing, and editing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Maria A. Ortiz for her helpful review and proofreading the article. Our study was funded in part by the GRAMMY Museum Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100442.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Barcia C., Ros C.M., Annese V., Gómez A., Ros-Bernal F., Aguado-Yera D., Martínez-Pagán M.E., de Pablos V., Fernandez-Villalba E., Herrero M.T. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death Dis. 2011;2:e142. doi: 10.1038/cddis.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnum C.J., Tansey M.G. Neuroinflammation and non-motor symptoms: the dark passenger of Parkinson's disease? Curr. Neurol. Neurosci. Rep. 2012;12:350–358. doi: 10.1007/s11910-012-0283-6. [DOI] [PubMed] [Google Scholar]
- Baron R., Nemirovsky A., Harpaz I., Cohen H., Owens T., Monsonego A. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer's disease. Faseb. J. 2008;22:2843–2852. doi: 10.1096/fj.08-105866. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D., Müller T., Kuhn W., Gerlach M., Przuntek H., Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci. Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Bok E., Cho E.J., Chung E.S., Shin W.H., Jin B.K. Interleukin-4 contributes to degeneration of dopamine neurons in the lipopolysaccharide-treated Substantia nigra. Exp. Neurobiol. 2018;27:309–319. doi: 10.5607/en.2018.27.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boka G., Anglade P., Wallach D., Javoy-Agid F., Agid Y., Hirsch E.C. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci. Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Bolin L.M., Strycharska-Orczyk I., Murray R., Langston J.W., Di Monte D. Increased vulnerability of dopaminergic neurons in MPTP-lesioned interleukin-6 deficient mice. J. Neurochem. 2002;83:167–175. doi: 10.1046/j.1471-4159.2002.01131.x. [DOI] [PubMed] [Google Scholar]
- Brochard V., Combadière B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J.M., Duyckaerts C., Flavell R.A., Hirsch E.C., Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P., Ceballos-Diaz C., Lin W.L., Beccard A., Jansen-West K., McFarland N.R., Janus C., Dickson D., Das P., Golde T.E. Interferon-γ induces progressive nigrostriatal degeneration and basal ganglia calcification. Nat. Neurosci. 2011;14:694–696. doi: 10.1038/nn.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., O'Reilly E.J., Schwarzschild M.A., Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am. J. Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- Conroy S.M., Nguyen V., Quina L.A., Blakely-Gonzales P., Ur C., Netzeband J.G., Prieto A.L., Gruol D.L. Interleukin-6 produces neuronal loss in developing cerebellar granule neuron cultures. J. Neuroimmunol. 2004;155:43–54. doi: 10.1016/j.jneuroim.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Dobbs R.J., Charlett A., Purkiss A.G., Dobbs S.M., Weller C., Peterson D.W. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta Neurol. Scand. 1999;100:34–41. doi: 10.1111/j.1600-0404.1999.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Dufek M., Hamanová M., Lokaj J., Goldemund D., Rektorová I., Michálková Z., Sheardová K., Rektor I. Serum inflammatory biomarkers in Parkinson's disease. Park. Relat. Disord. 2009;15:318–320. doi: 10.1016/j.parkreldis.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Fishman P.S. Paradoxical aspects of parkinsonian tremor. Mov. Disord. 2008;23:168–173. doi: 10.1002/mds.21736. [DOI] [PubMed] [Google Scholar]
- Garré J.M., Yang G. Contributions of monocytes to nervous system disorders. J. Mol. Med. (Berl.) 2018;96:873–883. doi: 10.1007/s00109-018-1672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N. Movement disorder society-sponsored revision of the Unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- Green H.F., Khosousi S., Svenningsson P. Plasma IL-6 and IL-17A correlate with severity of motor and non-motor symptoms in Parkinson's disease. J. Parkinsons Dis. 2019;9:705–709. doi: 10.3233/JPD-191699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov V., Bliederhaeuser C., Ruf W.P., Roth V., Fundel-Clemens K., Zondler L., Brenner D., Martin-Villalba A., Hengerer B., Kassubek J., Ludolph A.C., Weishaupt J.H., Danzer K.M. Inflammatory dysregulation of blood monocytes in Parkinson's disease patients. Acta Neuropathol. 2014;128:651–663. doi: 10.1007/s00401-014-1345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich R.C., Hallett M., Deuschl G., Toni I., Bloem B.R. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206–3226. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer B., Cepok S., Zhou D., Sommer N. Multiple sclerosis -- a coordinated immune attack across the blood brain barrier. Curr. Neurovascular Res. 2004;1:141–150. doi: 10.2174/1567202043480152. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jiang S., Gao H., Luo Q., Wang P., Yang X. The correlation of lymphocyte subsets, natural killer cell, and Parkinson's disease: a meta-analysis. Neurol. Sci. 2017;38:1373–1380. doi: 10.1007/s10072-017-2988-4. [DOI] [PubMed] [Google Scholar]
- Kim R., Kim H.J., Kim A., Jang M., Kim Y., Yoo D., Im J.H., Choi J.H., Jeon B. Peripheral blood inflammatory markers in early Parkinson's disease. J. Clin. Neurosci. 2018;58:30–33. doi: 10.1016/j.jocn.2018.10.079. [DOI] [PubMed] [Google Scholar]
- Koller W.C., Busenbark K., Miner K. The relationship of essential tremor to other movement disorders: report on 678 patients. Essential Tremor Study Group. Ann. Neurol. 1994;35:717–723. doi: 10.1002/ana.410350613. [DOI] [PubMed] [Google Scholar]
- Kulkarni A., Ganesan P., O'Donnell L.A. Interferon gamma: influence on neural stem cell function in neurodegenerative and neuroinflammatory disease. Clin. Med. Insights Pathol. 2016;9:9–19. doi: 10.4137/CPath.S40497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustrimovic N., Comi C., Magistrelli L., Rasini E., Legnaro M., Bombelli R., Aleksic I., Blandini F., Minafra B., Riboldazzi G., Sturchio A., Mauri M., Bono G., Marino F., Cosentino M. Parkinson's disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J. Neuroinflammation. 2018;15:205. doi: 10.1186/s12974-018-1248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatek-Majkusiak J., Geremek M., Koziorowski D., Tomasiuk R., Szlufik S., Friedman A. Serum levels of hepcidin and interleukin 6 in Parkinson's disease. Acta Neurobiol. Exp. 2020;80:297–304. [PubMed] [Google Scholar]
- Lian T.H., Guo P., Zuo L.J., Hu Y., Yu S.Y., Yu Q.J., Jin Z., Wang R.D., Li L.X., Zhang W. Tremor-dominant in Parkinson disease: the relevance to iron metabolism and inflammation. Front. Neurosci. 2019;13:255. doi: 10.3389/fnins.2019.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D., Hall S., Surova Y., Nielsen H.M., Janelidze S., Brundin L., Hansson O. Cerebrospinal fluid inflammatory markers in Parkinson's disease--associations with depression, fatigue, and cognitive impairment. Brain Behav. Immun. 2013;33:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Lindqvist D., Kaufman E., Brundin L., Hall S., Surova Y., Hansson O. Non-motor symptoms in patients with Parkinson's disease - correlations with inflammatory cytokines in serum. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E.D., Levy G., Côte L.J., Mejia H., Fahn S., Marder K. Clinical correlates of action tremor in Parkinson disease. Arch. Neurol. 2001;58:1630–1634. doi: 10.1001/archneur.58.10.1630. [DOI] [PubMed] [Google Scholar]
- Louis E.D., Tang M.X., Cote L., Alfaro B., Mejia H., Marder K. Progression of parkinsonian signs in Parkinson disease. Arch. Neurol. 1999;56:334–337. doi: 10.1001/archneur.56.3.334. [DOI] [PubMed] [Google Scholar]
- Marttila R.J., Eskola J., Päivärinta M., Rinne U.K. Immune functions in Parkinson's disease. Adv. Neurol. 1984;40:315–323. [PubMed] [Google Scholar]
- Mastrangelo M.A., Sudol K.L., Narrow W.C., Bowers W.J. Interferon-{gamma} differentially affects Alzheimer's disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am. J. Pathol. 2009;175:2076–2088. doi: 10.2353/ajpath.2009.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer P.L., Itagaki S., Boyes B.E., McGeer E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Menza M., Dobkin R.D., Marin H., Mark M.H., Gara M., Bienfait K., Dicke A., Kusnekov A. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson's disease. Psychosomatics. 2010;51:474–479. doi: 10.1176/appi.psy.51.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Harada M., Kondo T., Riederer P., Inagaki H., Minami M., Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M., Harada M., Narabayashi H., Inagaki H., Minami M., Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci. Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Müller T., Blum-Degen D., Przuntek H., Kuhn W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson's disease. Acta Neurol. Scand. 1998;98:142–144. doi: 10.1111/j.1600-0404.1998.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Mogi M., Ichinose H., Togari A. Cytokines in Parkinson's disease. J. Neural. Transm. 2000;Suppl:143–151. [PubMed] [Google Scholar]
- Park K.W., Lee D.Y., Joe E.H., Kim S.U., Jin B.K. Neuroprotective role of microglia expressing interleukin-4. J. Neurosci. Res. 2005;81:397–402. doi: 10.1002/jnr.20483. [DOI] [PubMed] [Google Scholar]
- Rathnayake D., Chang T., Udagama P. Selected serum cytokines and nitric oxide as potential multi-marker biosignature panels for Parkinson disease of varying durations: a case-control study. BMC Neurol. 2019;19:56. doi: 10.1186/s12883-019-1286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M., Iarlori C., Thomas A., Gambi D., Perfetti B., Di Nicola M., Onofrj M. Peripheral cytokines profile in Parkinson's disease. Brain Behav. Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Rentzos M., Nikolaou C., Andreadou E., Paraskevas G.P., Rombos A., Zoga M., Tsoutsou A., Boufidou F., Kapaki E., Vassilopoulos D. Circulating interleukin-15 and RANTES chemokine in Parkinson's disease. Acta Neurol. Scand. 2007;116:374–379. doi: 10.1111/j.1600-0404.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- Scalzo P., Kümmer A., Cardoso F., Teixeira A.L. Serum levels of interleukin-6 are elevated in patients with Parkinson's disease and correlate with physical performance. Neurosci. Lett. 2010;468:56–58. doi: 10.1016/j.neulet.2009.10.062. [DOI] [PubMed] [Google Scholar]
- Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Mov. Disord. 2008;23(Suppl. 3):S521–S533. doi: 10.1002/mds.22049. [DOI] [PubMed] [Google Scholar]
- Stypuła G., Kunert-Radek J., Stepień H., Zylińska K., Pawlikowski M. Evaluation of interleukins, ACTH, cortisol and prolactin concentrations in the blood of patients with Parkinson's disease. Neuroimmunomodulation. 1996;3:131–134. doi: 10.1159/000097237. [DOI] [PubMed] [Google Scholar]
- Theodore S., Cao S., McLean P.J., Standaert D.G. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufekci K.U., Meuwissen R., Genc S., Genc K. Inflammation in Parkinson's disease. Adv. Protein Chem. Struct. Biol. 2012;88:69–132. doi: 10.1016/B978-0-12-398314-5.00004-0. [DOI] [PubMed] [Google Scholar]
- Veselý B., Dufek M., Thon V., Brozman M., Királová S., Halászová T., Koriťáková E., Rektor I. Interleukin 6 and complement serum level study in Parkinson's disease. J. Neural. Transm. 2018;125:875–881. doi: 10.1007/s00702-018-1857-5. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang Q.X., Sun X., Vesek J., Mosher Z., Vasavada M., Chu J., Kanekar S., Shivkumar V., Venkiteswaran K., Subramanian T. MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson's disease. Park. Relat. Disord. 2015;21:590–596. doi: 10.1016/j.parkreldis.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Wijeyekoon R.S., Kronenberg-Versteeg D., Scott K.M., Hayat S., Jones J.L., Clatworthy M.R., Floto R.A., Barker R.A., Williams-Gray C.H. Monocyte function in Parkinson's disease and the impact of autologous serum on phagocytosis. Front. Neurol. 2018;9:870. doi: 10.3389/fneur.2018.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray C.H., Wijeyekoon R., Yarnall A.J., Lawson R.A., Breen D.P., Evans J.R., Cummins G.A., Duncan G.W., Khoo T.K., Burn D.J., Barker R.A., I.-P.s. group Serum immune markers and disease progression in an incident Parkinson's disease cohort (ICICLE-PD) Mov. Disord. 2016;31:995–1003. doi: 10.1002/mds.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The article's supporting data and materials can be accessed by contacting the corresponding author.