ABSTRACT
We utilized a CRISPR interference (CRISPRi) assay to control the gene expressions of two predicted essential peptidoglycan biosynthesis genes, pbpB and cwIM, in Mycobacterium abscessus and to evaluate their contribution to β-lactam susceptibility. Our results showed that CRISPR inhibition of each gene led to a significant 3-log10 reduction in CFU in the presence of imipenem but not for cefoxitin. These results demonstrate that CRISPRi provides an experimental approach to study drug/target interactions in M. abscessus.
KEYWORDS: β-lactam, CRISPRi, Mycobacterium abscessus, peptidoglycan biosynthesis
TEXT
The rise in incidence of Mycobacterium abscessus infections in patients with cystic fibrosis and in transplant recipients highlights an emerging environmental pathogen that is recalcitrant to treatment with most antibiotics and associated with a poor clinical outcome (1–3). Clarithromycin resistance, which is commonly detected in clinical M. abscessus subsp. abscessus isolates, is associated with treatment success rates of only 25 to 40% (4–7). Combination drug regimens, including the use of two different β-lactams, such as imipenem (a carbapenem) and cefoxitin (a cephamycin), have been tried but the clinical efficacy remains inconclusive (8). Interestingly, we and others have shown that other β-lactams, used together, are synergistic in vitro and in vivo (9–11). For example, the combination of ceftaroline or imipenem with ceftazidime showed a 4- to 32-fold reduction in the MIC50 to either drug alone (9). These findings support the hypothesis that optimal β-lactam treatment will require targeting multiple proteins that are part of the complex and highly redundant battery of enzymes involved in peptidoglycan (PG) biosynthesis (8, 10, 12).
We recently created a saturated Himar1 transposon insertion mutant library in M. abscessus ATCC 19977, comparatively assessed its genomic content to similar libraries in Mycobacterium tuberculosis and Mycobacterium avium, and catalogued genetic elements essential for in vitro growth (13). Included in this analysis were 28 genes predicted to be involved in peptidoglycan biosynthesis and remodeling, three of which were deemed essential to M. abscessus as follows: MAB_2000 (penicillin-binding membrane protein PbpB), MAB_3167c (penicillin-binding lipoprotein), and MAB_4942 (N-acetylmuramoyl-l-alanine amidase CwlM). Among them, pbpB and cwIM are essential in both M. tuberculosis and M. abscessus (13, 14).
The interaction of proteins with essential functions and selected antibiotics (e.g., β-lactams, which target PG biosynthesis) in M. abscessus has only recently been explored due to the availability of new tools to genetically control the expression of essential genes. CRISPR interference (CRISPRi), which uses a nuclease-deactivated Cas9 (dCas9) paired with a single-guide RNA (sgRNA) to sterically hinder transcription at the sgRNA base-pairing genomic locus (15) has been developed as a specific and efficient approach for gene knockdown in both essential and nonessential genes. In this study, we utilized the CRISPRi assay (16, 17) to control essential gene expression in M. abscessus, enabling us to experimentally confirm the essentiality of PbpB and CwIM and to evaluate their contribution to β-lactam susceptibility.
The plasmid pLJR962 (Addgene no. 115162) (16), containing an anhydrotetracycline (ATc)-inducible dCas9Sth1 (from Streptococcus thermophilus) and an ATc-inducible sgRNA along with a kanamycin selection marker was used as the vector for CRISPRi in M. abscessus. sgRNA was designed using the method described recently (17) in Geneious 9.1.5 software by identifying Sth1 dCas9 protospacer adjacent motif (PAM) sequences against the reference ATCC 19977 genome. We then extracted ∼26 nucleotide sgRNA targeting sequences upstream of each PAM, and only sgRNA targeting sequences in which the transcription-initiating nucleotide was an “A” or “G” were kept for further processing (17) (Table 1). The sgRNA forward and reverse oligonucleotides were ordered from IDT (Integrated DNA Technologies, Inc., Coralville, Iowa), annealed, and ligated into the pLJR962 backbone (pLJR962-sgRNA) (16, 17). pLJR962-sgRNA constructs were initially created in Escherichia coli DH10B and then isolated and electroporated into M. abscessus ATCC 19977 with selection for kanamycin.
TABLE 1.
Primers used in this study
| Oligonucleotide name | Sequences 5′–3′ | Purpose |
|---|---|---|
| sth-cwlM-gRNA-F2 | GGGAGGAAGTTCCCGTCGACCAGACCGGT | cwlM sgRNA |
| sth-cwlM-gRNA-R2 | AAACACCGGTCTGGTCGACGGGAACTTCC | |
| sth-pbpb-gRNA-F2 | GGGAGGCTGAATGGCCGCGATGGCCAAGGT | pbpB sgRNA |
| sth-pbpb-gRNA-R2 | AAACACCTTGGCCATCGCGGCCATTCAGCC | |
| cwlM_RT-F(n) | CTACCACTTCGGCAACCTAC | cwlM RT PCR |
| cwlM_RT-R(n) | GCCACTTCTCGCTGAATGA | |
| pbpb_RT-F(n) | GATTCATGGACCTGGTGGAC | pbpB RT PCR |
| pbpb_RT-R(n) | TCGATCGGTGGCACAATAC | |
| MABrpo-F1(RT) | ATCTCGGTGGTCAGGAAGTA | control RT PCR |
| MABrpo-R1(RT) | TGCTGTCCTCGAACAACATC |
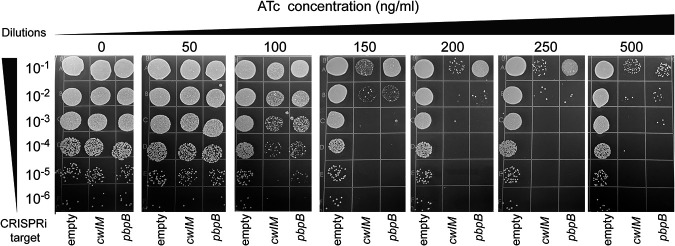
We firstly examined the essentiality of pbpB and cwIM under different degrees of ATc-induced gene inhibition. In brief, overnight cultures of pLJR962 ATCC 19977 transformants with or without target sgRNA were diluted to an optical density at 600 nm (OD600) of 0.2 and then serially diluted and plated (10 μl) on 7H10 agar plates supplemented with ATc at concentrations ranging from 0 to 500 ng/mL. As shown in Fig. 1, we identified sgRNAs targeted to each gene that successfully suppressed the growth of M. abscessus at ATc concentrations of ≥100 ng/mL. Plating of the dilutions provided a direct method to quantify the CFU and the effects of CRISPRi. For either construct targeting cwIM or pbpB, ATc concentrations of 100 and ≥150 ng/mL reduced the CFU counts by ≥1 and ≥4 log10, respectively, compared to the empty vector control, confirming the essentiality of both genes in M. abscessus under these in vitro conditions.
FIG 1.
The growth of M. abscessus ATCC 19977 CRISPRi constructs exposed to different ATc concentrations.
The same CRISPR plasmids were electroporated into a Mycobacterium bolletii clinical isolate and evaluated for growth in the presence of differing concentrations of ATc. The results mimicked the findings that we observed with M. abscessus ATCC 19977, supporting the finding that cwlM and pbpB are also essential in M. bolletii (data not shown).
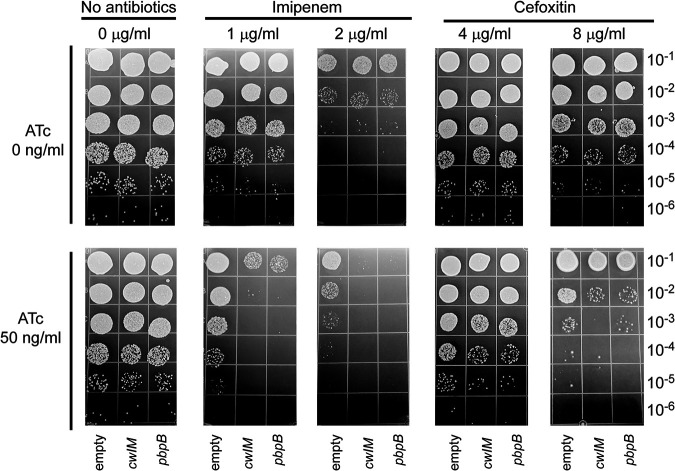
With the knowledge that cwlM and pbpB are predicted to be involved in peptidoglycan synthesis in M. abscessus (13, 18), we investigated whether reducing their gene expression would impact their susceptibility to β-lactam antibiotics. We determined the imipenem and cefoxitin MICs against M. abscessus ATCC 19977 to be 2 μg/mL and 8 μg/mL, respectively, using the microdilution method in 7H9 broth (19, 20). In the absence of ATc, MIC values remained constant with and without the CRISPRi plasmids. To test whether inhibiting the gene expression of cwIM and pbpB affects susceptibility to β-lactams, we plated each strain on agar containing imipenem or cefoxitin, with or without ATc at 50 ng/mL, a concentration that does not reduce the growth of cwlM and pbpB CRISPRi constructs in the absence of other drugs (Fig. 1). Imipenem and cefoxitin were tested at one-half and 1× MIC. This plating approach provided a direct method to evaluate any synergistic drug/protein interactions. Synergy was defined as ≥2-log10 reduction in CFU at 72 h with the combination of antibiotic and ATc compared with the antibiotic alone. As shown in Fig. 2, one-half of the MIC of imipenem (1 μg/mL) reduced CFU of both cwlM and pbpB CRISPRi constructs by approximately 1 log10 compared to the no-drug control in the absence of ATc induction, while imipenem 2 μg/mL caused a >2-log10 reduction of CFU, consistent with its MIC. The addition of ATc at 50 ng/mL caused a 3-log10 reduction in CFU of the cwlM and pbpB CRISPRi constructs compared to the empty vector control in the presence of imipenem. Addition of ATc caused a smaller, ≤1-log10 reduction in CFU of the cwlM and pbpB CRISPRi constructs compared to the empty vector control in the presence of cefoxitin, which did not meet our definition of synergy. We also tested the synergistic activity between imipenem and ATc against the M. bolletii strain with cwlM and pbpB CRISPRi constructs, and a similar synergy was observed in the presence of imipenem 2 μg/mL (one-half MIC) and 50 ng/mL ATc (see Fig. S1 in the supplemental material).
FIG 2.
The interaction of ATc and either imipenem or cefoxitin and their effects on the growth of M. abscessus ATCC 19977 CRISPRi constructs.
In addition, we tested the cwIM and pbpB transcriptional response in the presence of CRISPRi constructs with 50 ng/mL ATc treatment. In brief, overnight cultures of cwIM and pbpB CRISPRi ATCC 19977 strains were inoculated onto 7H10 agar plates with and without 50 ng/mL ATc and incubated for 72 h at 37°C. Total RNAs were extracted with FastPrep (MP Biomedicals) and RNeasy minikit (Qiagen), followed by on-column DNase digestion using RNase-Free DNase set (Qiagen) with an additional TURBO DNase treatment (ThermoFisher). Reverse transcription-quantitative PCR (qRT-PCR) was run in an Mx3005 real-time PCR system using qRT-PCR Brilliant II SYBR kit (Agilent). In the presence of ATc, transcription of cwIM and pbpB was reduced by 28% ± 14% (mean ± standard deviation [SD]) and 36% ± 10%, respectively.
Essential bacterial proteins can be important therapeutic targets. Our recent sequencing of a saturated transposon library created in the M. abscessus ATCC 19977 clinical isolate (13) provided the first comprehensive prediction of essential genes in the genome of this emerging pathogen. However, such essentiality predictions require experimental confirmation. Genetic constructs enabling conditional knockdown of gene expression are an important means of confirming essentiality predictions and investigating whether inhibiting essential proteins can provide therapeutic benefit. With a focus on peptidoglycan biosynthesis enzymes and their importance as β-lactam drug targets, we identified two genes predicted to be essential in both M. abscessus and M. tuberculosis and targeted them for CRISPRi to confirm their essentiality and to study the consequences of suppressing their expression in combination with each of the first-line β-lactams used in the treatment of M. abscessus infections.
Although both cwIM and pbpB have been annotated, there is currently no experimental data to directly dissect their biology in M. abscessus. However, their homologues in M. tuberculosis, both shown to be essential, have been studied to elucidate their roles in peptidoglycan biosynthesis and remodeling. The M. tuberculosis CwlM protein (Rv3915) is a unique cytoplasmic regulatory protein that, when phosphorylated, interacts with and dramatically increases the activity of MurA, the first enzyme in peptidoglycan precursor synthesis (21–23). Under nutrient starvation, CwlM is dephosphorylated, and peptidoglycan synthesis is arrested (23). We hypothesized that the M. abscessus CwlM protein has a similar function in peptidoglycan synthesis. The MAB_2000 (pbpB) gene homologue in M. tuberculosis (Rv2163c) encodes PbpB, also known as PBP-3 or FtsI, an essential d,d-transpeptidase required for cell division and targeted in drug discovery programs (24, 25).
The CRISPRi approach provided the opportunity to control gene expression with ATc titration, repress essential gene expression, and evaluate the consequences. Given the likelihood that both cwIM and pbpB encode for proteins vital for peptidoglycan synthesis in M. abscessus, we investigated their interaction with β-lactams to determine whether suppressing their gene expression would alter susceptibility to these important antibiotics. Although we did not observe reductions in CFU when cwIM or pbpB expression was inhibited approximately 25 to 35% by 50 ng/mL ATc, we did observe a significant 3-log10 reduction in CFU in the presence of imipenem for both genes and a modest effect in the presence of cefoxitin. One hypothesis to explain the potentiation of imipenem is that it targets one or both essential M. abscessus proteins, and the result of CRISPRi inhibiting gene expression is to create a “hypomorph” that is inhibited by lower drug concentrations. Indeed, meropenem and faropenem inactivate PbpB of M. tuberculosis (26). A related possibility is that imipenem inhibits one or more proteins in the same peptidoglycan synthesis and remodeling pathway(s) as PbpB or CwlM, and it is potentiated by reduced activity of the pathway resulting from cwIM or pbpB silencing. For example, reduced expression of cwIM may globally reduce PG synthesis by inhibiting precursor synthesis, thus causing additive effects with subinhibitory imipenem concentrations. One hypothesis for the greater potentiation of imipenem compared to cefoxitin is that imipenem is a more effective inhibitor of other enzymes or pathways that are able to compensate for the reduced activity of PbpB or CwlM. This is supported by the observation that carbapenems inhibit l,d-transpeptidases more than cephalosporins (8) and that imipenem is known to target multiple PG synthesis enzymes in comparison to cefoxitin, including l,d-transpeptidases (LdtMab1, LdtMab2, LdtMab4, LdtMab5) and d,d-carboxypeptidase (12).
Although further studies are needed to investigate these and other hypotheses, the results presented here demonstrate that CRISPRi provides an experimental approach to catalogue drug/target interactions, and this strategy can be extended to target these and other peptidoglycan remodeling proteins and investigate the consequences of inhibiting multiple genes versus different drug combinations in the same assay.
ACKNOWLEDGMENT
This study is supported by a grant from NIH (5R01AI141805) to B.N.K.
Footnotes
Supplemental material is available online only.
Contributor Information
Liang Chen, Email: liang.chen@hmh-cdi.org.
Barry N. Kreiswirth, Email: barry.kreiswirth@hmh-cdi.org.
REFERENCES
- 1.Cowman S, van Ingen J, Griffith DE, Loebinger MR. 2019. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J 54:1900250. doi: 10.1183/13993003.00250-2019. [DOI] [PubMed] [Google Scholar]
- 2.Johansen MD, Herrmann JL, Kremer L. 2020. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 18:392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 3.Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. 2015. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 21:1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis 64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 5.Koh WJ, Stout JE, Yew WW. 2014. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. Int J Tuber Lung Dis 18:1141–1148. doi: 10.5588/ijtld.14.0134. [DOI] [PubMed] [Google Scholar]
- 6.Smibert O, Snell GI, Bills H, Westall GP, Morrissey CO. 2016. Mycobacterium abscessus complex - a particular challenge in the setting of lung transplantation. Expert Rev Anti Infect Ther 14:325–333. doi: 10.1586/14787210.2016.1138856. [DOI] [PubMed] [Google Scholar]
- 7.Guo Q, Chu H, Ye M, Zhang Z, Li B, Yang S, Ma W, Yu F. 2018. The clarithromycin susceptibility genotype affects the treatment outcome of patients with Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 62:e02360-17. doi: 10.1128/AAC.02360-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Story-Roller E, Maggioncalda EC, Cohen KA, Lamichhane G. 2018. Mycobacterium abscessus and β-lactams: emerging insights and potential opportunities. Front Microbiol 9:2273. doi: 10.3389/fmicb.2018.02273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey R, Chen L, Manca C, Jenkins S, Glaser L, Vinnard C, Stone G, Lee J, Mathema B, Nuermberger EL, Bonomo RA, Kreiswirth BN. 2019. Dual beta-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 10:e02895-18. doi: 10.1128/mBio.02895-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Select β-lactam combinations exhibit synergy against Mycobacterium abscessus in vitro. Antimicrob Agents Chemother 63:e02613-18. doi: 10.1128/AAC.02613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Synergistic efficacy of β-lactam combinations against Mycobacterium abscessus pulmonary infection in mice. Antimicrob Agents Chemother 63:e00614-19. doi: 10.1128/AAC.00614-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen DC, Dousa KM, Kurz SG, Brown ST, Drusano G, Holland SM, Kreiswirth BN, Boom WH, Daley CL, Bonomo RA. 2021. “One-two punch”: synergistic ß-lactam combinations for Mycobacterium abscessus and target redundancy in the inhibition of peptidoglycan synthesis enzymes. Clin Infect Dis 73:1532–1536. doi: 10.1093/cid/ciab535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rifat D, Chen L, Kreiswirth BN, Nuermberger EL. 2021. Genome-wide essentiality analysis of Mycobacterium abscessus by saturated transposon mutagenesis and deep sequencing. mBio 12:e01049-21. doi: 10.1128/mBio.01049-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeJesus MA, Gerrick ER, Xu W, Park SW, Long JE, Boutte CC, Rubin EJ, Schnappinger D, Ehrt S, Fortune SM, Sassetti CM, Ioerger TR. 2017. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8:e02133-16. doi: 10.1128/mBio.02133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR, Church GM, Rubin EJ, Sassetti CM, Schnappinger D, Fortune SM. 2017. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2:16274. doi: 10.1038/nmicrobiol.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch B, DeJesus MA, Poulton NC, Zhang W, Engelhart CA, Zaveri A, Lavalette S, Ruecker N, Trujillo C, Wallach JB, Li S, Ehrt S, Chait BT, Schnappinger D, Rock JM. 2021. Genome-wide gene expression tuning reveals diverse vulnerabilities of M. tuberculosis. Cell 184:4579–4592. doi: 10.1016/j.cell.2021.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machowski EE, Senzani S, Ealand C, Kana BD. 2014. Comparative genomics for mycobacterial peptidoglycan remodelling enzymes reveals extensive genetic multiplicity. BMC Microbiol 14:75. doi: 10.1186/1471-2180-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2018. Performance standards for susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes, 1st ed. CLSI M62. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2018. Susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes, 3rd ed. CLSI M24. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 21.Turapov O, Forti F, Kadhim B, Ghisotti D, Sassine J, Straatman-Iwanowska A, Bottrill AR, Moynihan PJ, Wallis R, Barthe P, Cohen-Gonsaud M, Ajuh P, Vollmer W, Mukamolova GV. 2018. Two faces of CwlM, an essential PknB substrate, in Mycobacterium tuberculosis. Cell Rep 25:57–67. doi: 10.1016/j.celrep.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng LL, Humphries DE, Arbeit RD, Carlton LE, Smole SC, Carroll JD. 2005. Identification of a novel peptidoglycan hydrolase CwlM in Mycobacterium tuberculosis. Biochim Biophys Acta 1747:57–66. doi: 10.1016/j.bbapap.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Boutte CC, Baer CE, Papavinasasundaram K, Liu W, Chase MR, Meniche X, Fortune SM, Sassetti CM, Ioerger TR, Rubin EJ. 2016. A cytoplasmic peptidoglycan amidase homologue controls mycobacterial cell wall synthesis. Elife 5:e14590. doi: 10.7554/eLife.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datta P, Dasgupta A, Singh AK, Mukherjee P, Kundu M, Basu J. 2006. Interaction between FtsW and penicillin-binding protein 3 (PBP3) directs PBP3 to mid-cell, controls cell septation and mediates the formation of a trimeric complex involving FtsZ, FtsW and PBP3 in mycobacteria. Mol Microbiol 62:1655–1673. doi: 10.1111/j.1365-2958.2006.05491.x. [DOI] [PubMed] [Google Scholar]
- 25.Kieser KJ, Baranowski C, Chao MC, Long JE, Sassetti CM, Waldor MK, Sacchettini JC, Ioerger TR, Rubin EJ. 2015. Peptidoglycan synthesis in Mycobacterium tuberculosis is organized into networks with varying drug susceptibility. Proc Natl Acad Sci USA 112:13087–13092. doi: 10.1073/pnas.1514135112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Z, Wang H, Zhang A, Liu X, Zhou W, Yang C, Guddat L, Yang H, Schofield CJ, Rao Z. 2020. Structures of Mycobacterium tuberculosis penicillin-binding protein 3 in complex with five β-lactam antibiotics reveal mechanism of inactivation. Mol Pharmacol 97:287–294. doi: 10.1124/mol.119.118042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Download aac.00093-22-s0001.pdf, PDF file, 0.4 MB (422.7KB, pdf)