Abstract
Background:
Secondary hyperparathyroidism (SHPT) impacts nearly all patients with renal failure on dialysis. Medical treatment of SHPT has considerably evolved over the past two decades with parathyroidectomy reserved for severe cases. The primary objective of our study was to understand how trends in medical treatments affected parathyroidectomy rates in SHPT patients on dialysis.
Methods:
We used the United States Renal Data System (USRDS) to identify 379,835 adult (age≥18) patients on who were on maintenance dialysis in the United States between 2006–2016 with Medicare as the primary payor and ascertained treatment for SHPT. Adjusted rate ratios for rates of parathyroidectomy were calculated using multivariable adjusted Poisson regression.
Results:
Of 379,835 SHPT patients, 4,118 (1.1%) underwent parathyroidectomy, 39,835 (10.5%) received cinacalcet, 243,522 (64.1%) received phosphate binders, 17,571 (4.6%) received vitamin D analogs, and 86,899 (22.9%) received no treatment during the 10 years of follow-up. Over the entire study period, there was a 3.5-fold increase in the use of calcimimetics and a 3.4-fold increase in rates of parathyroidectomy. Compared to 2006–2009, utilization of parathyroidectomy increased 52% (aRR=1.52, 95% CI: 1.39–1.65) between 2010–2013 and by 106% (aRR=2.06, 95% CI: 1.90–2.24) between 2014–2016. The greatest increase in parathyroidectomy utilization occurred in younger patients (age 18–64 years), black patients, female patients, those living in higher poverty neighborhoods, those listed for kidney transplant, and those who live in the Southern region of the United States.
Conclusion:
Despite the evolution of medical treatments and increase in use of calcimimetics to treat SHPT, parathyroidectomy rates have been steadily increasing among dialysis patients with Medicare coverage.
Graphical Abstract

Increasing Rates of Parathyroidectomy to Treat Secondary Hyperparathyroidism (SHPT) Among Dialysis Patients with Medicare
Despite the considerable evolution of medical treatments for secondary hyperparathyroidism (SHPT), parathyroidectomy rates in the United States have increased 3.4-fold with dramatic increases in specific subgroups of patients. Our work reflects a changing landscape in the treatment that surgeons should be aware of.
INTRODUCTION
Secondary hyperparathyroidism (SHPT), or excess production of parathyroid hormone (PTH) by the parathyroid glands, affects nearly all patients with kidney failure on maintenance dialysis therapy (2). While modest increases in parathyroid hormone levels may reflect an appropriate compensatory response, levels above 600pg/mL have been independently associated with increased mortality, cardiovascular morbidity, bone loss, fractures, and decreased health-related quality of life (2–6).
Therefore, guidelines by Kidney Diseases Improving Global Outcomes (KDIGO) recommend treatment of SHPT to maintain a parathyroid hormone (PTH) level at 2–9 times the upper limit of the normal range (7). Over the past two decades, medical treatment for SHPT has advanced considerably with the introduction of synthetic active vitamin D analogs, non-calcium containing phosphate binders, and FDA approval of the first oral calcimimetic agent, cinacalcet, in 2004. However, Cinacalcet is only FDA approved for the treatment of secondary hyperparathyroidism in adults with ESRD on dialysis. Thus, current treatment options for ESRD patients with SHPT include Vitamin D analogs, phosphate binders, calcimimetics, or parathyroidectomy. Although parathyroidectomy is typically reserved for patients with refractory SHPT or those unable to tolerate calcimimetics, there is lack of consensus in recommending calcimimetics as first line treatment (1, 8). Even though rates of parathyroidectomy initially, declined after its approval in 2004, subsequent studies demonstrated significant variation in cinacalcet prescriptions among U.S. hemodialysis facilities (9, 10).
Despite the increasing availability of cinacalcet for ESRD patients with SHPT and guidelines outlining ideal PTH ranges for this patient population, the mean PTH levels of patients on hemodialysis in the U.S. have gradually risen, with 24% of patients living with PTH levels >600 pg/ml in 2017 compared to 13% in 2002 (10). The combination of rising PTH levels, variability of cinacalcet prescriptions, evolution of guidelines, and advances in pharmacotherapy suggest that the treatment paradigm of SHPT has likely shifted over the past decade, but it is unclear how it has changed over time with availability of improved medical treatments.
The primary objective of our study was to understand how trends in medical treatments affected parathyroidectomy rates in patients on dialysis and how they have differed by subgroups. This work will be instrumental to understand the evolution of and current landscape of utilization of parathyroidectomy as treatment for ESRD patients with SHPT in light of increasingly available medical therapies.
MATERIALS AND METHODS
Study Population
Using the United States Renal Data System (USRDS) with IRB approval, we identified 379,835 adults (age≥18) who initiated dialysis between January 1, 2004 and December 31, 2016 who had Medicare as primary payor and were enrolled in Part D. A 4-month gap was allowed for Medicare enrollment since the date of first end-stage renal disease (ESRD) service. All dialysis patients in the U.S. are eligible for Medicare when they start dialysis. Because Medicare is the largest single insurer of ESRD patients of any age, Medicare coverage is often used as inclusion criteria for studies of this population (11–13).
We chose ESRD patients on dialysis as our study population but not those with chronic kidney disease (CKD) stage 3 and 4, for several reasons. First, Cinacalcet is only Food and Drug Administration (FDA) approved for the treatment of SHPT in adults with ESRD on dialysis. Second, nearly all patients with ESRD are affected by SHPT and are eligible for medical and surgical treatments allowing us to compare trends in all SHPT treatments. Third, PTH thresholds are clearly outlined by the Kidney Disease: Improving Global Outcomes (KDIGO) Guidelines on CKD-MBD allowing us to compare how various treatments or lack thereof are being utilized (7).
Patient demographic and clinical information was obtained at the time of dialysis initiation because this was the start of follow-up for SHPT medical and surgical treatment. We followed the study population from January 1, 2006 allowing for at least two years on maintenance dialysis until one of the events whichever occurred first: death, transplant, end of Medicare coverage, or end of follow-up (12/31/2016).
SHPT treatments
Using Medicare claims data, parathyroidectomy was ascertained by using a list of Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision, Procedure Coding System (ICD-9-PCS), and International Classification of Diseases, Tenth Revision, Procedure Coding System (ICD-10-PCS) codes, which were converted to approximate correspondences of ICD-9-PCS codes (Supplement). We used Medicare Part D data to obtain if patients were dispensed a prescription for cinacalcet, vitamin D analogs (calcitriol, doxercalciferol, or paricalcitol), or phosphate binders (calcium acetate, calcium carbonate, sevelamer hydrochloride/carbonate, lanthanum carbonate).
Statistical Analysis
We used Poisson regression to estimate the rate of ever receiving each treatment per 10,000 person-years during each calendar year. Patients who received parathyroidectomy were censored at the date of the operation for the parathyroidectomy risk set, but still contributed to the risk set for medications. This was done so that a patient who underwent a parathyroidectomy would not contribute to the denominator for subsequent parathyroidectomy rates. We conducted stratified analysis by patient age (18–64 versus ≥65 years), sex (male versus female), race (White, Black, and others), neighborhood poverty level (low versus high), being on waitlist (not listed versus listed), and by region (Northeast, South, Midwest, and West). USRDS data contains Zip codes at time of dialysis initiation and American Community Survey (ACS) data contains only Zip Code Tabulation Area (ZCTA). Thus we obtained the percent of population below poverty level by county by linking American Community Survey 2014 data(14) using the Zip Code to Zip Code Tabulation Area (ZCTA) Crosswalk(15). The entire study period was divided into three eras as follows: 2006–2009, 2010–2013, and 2014–2016. We then calculated rate ratios for parathyroidectomy by each era adjusting for age, sex, race, poverty, listing for kidney transplant, geographic region, and time to the initiation of SHPT treatment, a surrogate for SHPT disease severity, and comorbidities including diabetes mellitus, hypertension, heart failure, coronary artery disease, cardiovascular disease, peripheral vascular disease, cancer, chronic obstructive pulmonary disease, and functional impairment. Date of listing status for kidney transplant was obtained from USRDS. Because discharge date was only available from 2013, patients who were discharged the same day were not excluded. A p-value less than 0.05 was considered statistically significant. All analyses were performed using Stata 16.0/MP for Linux (College Station, Texas) and R version 3.6.2.
RESULTS
Characteristics of dialysis patients with SHPT
For the entire study population, the median age at dialysis initiation was 67 years and 48.3% of the patients were female. The majority of patients were white (66.2%), unemployed (96.3%), and above poverty level (82.1%). The most common dialysis modality was hemodialysis (93.7%). Most patients had hypertension (88.4%) and over half had diabetes (56.4%). Median age at time of parathyroidectomy was 51.0 (IQR: 40.2, 61.8). When stratified by three different eras, although statistically significant differences were noted for all patient characteristics across eras, clinically meaningful differences were not noted (Table 1).
Table 1.
Characteristics of dialysis patients at the start of dialysis during the study (2006–2016) (n = 379,835) and over each study era: in 2006–2009 (n=70,851), 2010–2013 (n=101,034), and 2014–2016 (n=207,950) whose first end-stage kidney disease service started between 2004–2016. Statistically significant differences for noted for all characteristics across eras (p<0.001).
| 2006–2016 (n=379,835) | 2006–2009 (n=70,851) | 2010–2013 (n=101,034) | 2014–2016 (n=207,950) | |
|---|---|---|---|---|
| Age at dialysis initiation | 67 (56, 76) | 68 (57, 77) | 68 (56, 76) | 66 (55, 75) |
| Age at time of parathyroidectomy | 51 (40, 62) | 50 (39, 62) | 51 (50, 61) | 52 (41, 62) |
| Female | 48.3% | 50.4% | 48.8% | 47.4% |
| Race | ||||
| White | 66.2% | 66.8% | 67.9% | 65.1% |
| Black | 28.1% | 28.0% | 26.6% | 28.9% |
| Others | 5.7% | 5.2% | 5.5% | 6.0% |
| Currently employed % below poverty level | 3.7% 17.9 (11.2, 25.7) | 3.2% 18.2 (11.5, 25.9) | 3.5% 17.8 (11.1, 25.6) | 3.9% 17.8 (11.1, 25.6) |
| Geographic region | ||||
| Northeast | 17.0% | 17.2% | 16.7% | 17.0% |
| South | 41.8% | 41.6% | 41.2% | 42.1% |
| Midwest | 23.4% | 24.8% | 24.6% | 22.3% |
| West | 17.9% | 16.3% | 17.5% | 18.6% |
| Body mass index, kg/m2 | 27.8 (23.7, 33.4) | 26.6 (22.8, 31.7) | 27.4 (23.4, 32.9) | 28.4 (24.2, 34.2) |
| Hemodialysis (%) | 93.7% | 95.1% | 94.7% | 92.7% |
| Comorbidity | ||||
| Hypertension | 88.4% | 86.7% | 87.7% | 89.4% |
| Diabetes Mellitus | 56.4% | 49.5% | 56.6% | 58.6% |
| Heart failure | 34.3% | 40.8% | 37.3% | 30.6% |
| Coronary artery disease | 18.9% | 19.7% | 22.5% | 16.8% |
| Cardiovascular disease | 10.7% | 12.5% | 11.4% | 9.7% |
| Peripheral vascular disease | 14.1% | 18.1% | 16.1% | 11.7% |
| Cancer | 6.8% | 7.3% | 7.3% | 6.4% |
| Chronic obstructive | ||||
| Pulmonary disease | 10.5% | 11.8% | 11.6% | 9.6% |
| Functional impairment | 15.7% | 16.3% | 17.4% | 14.8% |
Median and interquartile ranges presented for continuous variables
Treatment for SHPT
Of the entire cohort, 4,118 (1.1%) underwent parathyroidectomy, 39,835 (10.5%) received cinacalcet, 243,522 (64.1%) received phosphate binders, 17,571 (4.6%) received vitamin D analogs, and 86,899 (22.9%) received no treatment for SHPT during the 10 years of follow-up. A majority of patients took a single kind of oral medication: 61.4% for phosphate binders, 8.5% for cinacalcet, and 4.0% for vitamin D analogs. The remaining 26.1% of patients required multiple treatments. Among patients who received parathyroidectomy, 2,508 (60.9%) were ever prescribed phosphate binders, 1,304 (31.7%) were prescribed cinacalcet, and 377 (9.2%) were prescribed vitamin D analogs (Figure 1). Of the 4,118 patients who underwent parathyroidectomy during the study period, 3,654 (88.7%) were prescribed medications at least once after surgery. The median time to first prescription since parathyroidectomy was 13 days (IQR: 5, 57). Vitamin D was prescribed in 1,479 (35.9%) patients, Phosphate Binder in 1,778 (43.2%) patients, and cinacalcet in 397 patients (9.6%). Among all patients who underwent parathyroidectomy, 18.3% received at least one prescription for cinacalcet within the first post-operative year.
Figure 1.
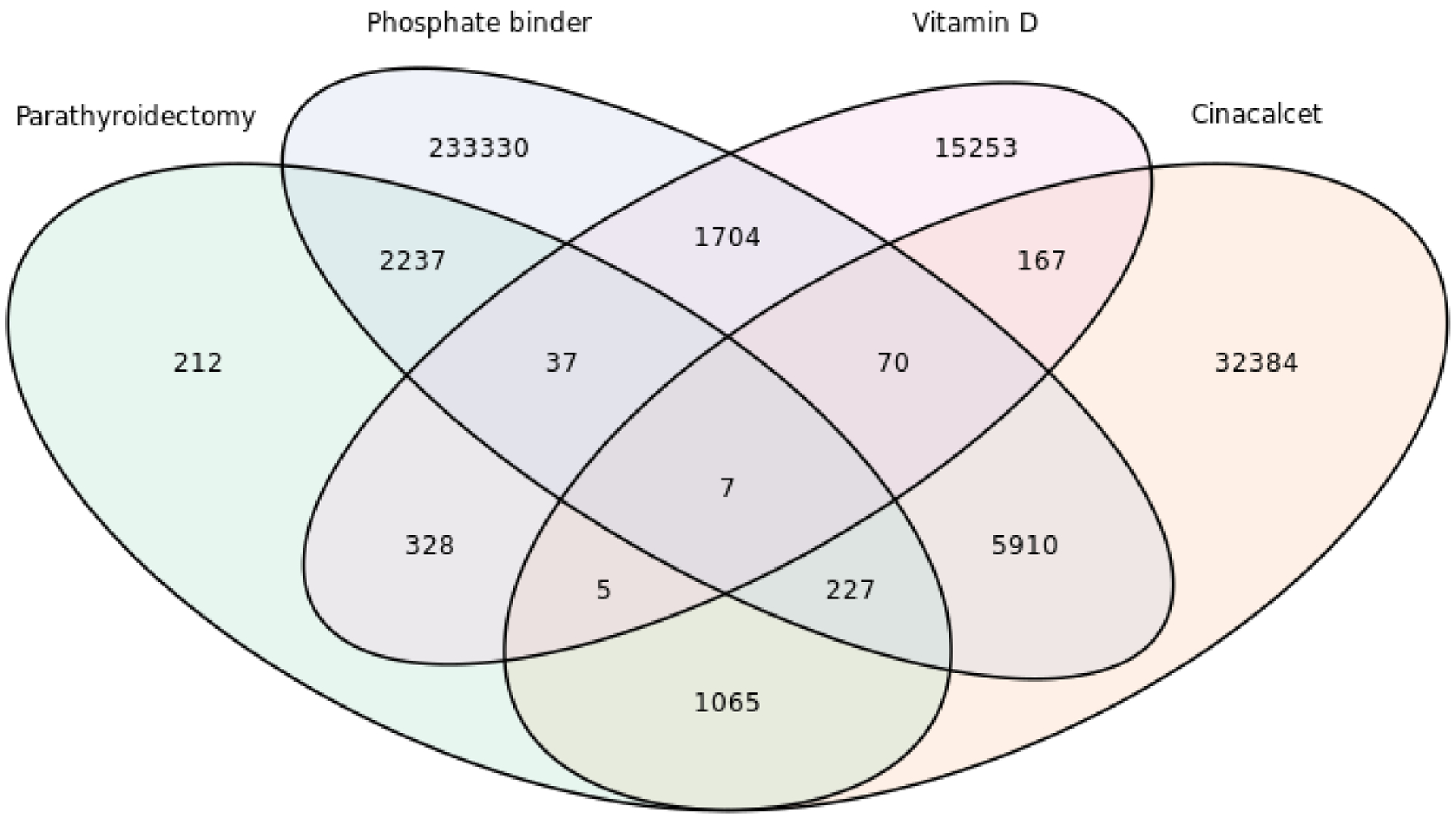
Overlap of treatments for secondary hyperparathyroidism (SHPT) among patients who initiated dialysis between 2004–2016 (n=379,835). Of those, 86,899 (22.9%) received no treatment. Treatments include: Parathyroidectomy (PTDx), Phosphate Binders, Vitamin D analogs, and Cinacalcet.
Trends in Treatment for SHPT
The annual rate of parathyroidectomy increased substantially over time from 2.9 (95% CI: 2.4–3.4) per 10,000 person-years in 2006 to 9.8 (95% CI: 9.0–10.6) per 10,000 person-years in 2016 (Figure 2). Similarly, the rate of at least one cinacalcet prescription and phosphate binders increased sharply: 366 (95% CI: 361–367) per 10,000 person-years in 2009, 539 (95% CI: 533–545) per 10,000 person-years in 2013, and 652 (95% CI: 646–659) per 10,000 person-years in 2016 for cinacalcet; 1469 (95% CI: 1459–1479) per 10,000 person-years in 2009, 1726 (95% CI: 1716–1737) per 10,000 person-years in 2013, and 1715 (95% CI: 1704–1725) per 10,000 person-years in 2016 for phosphate binders. Thus, the rate of parathyroidectomy increased 3.4-fold, the rate of cinacalcet use increased 3.5-fold, and the rate of phosphate binders increased 1.7-fold over the study period (p<0.001). There was no clear trend for rate of prescribing vitamin D analogs (Figure 2).
Figure 2. Trends of (a) parathyroidectomy, (b) cinacalcet, (c) phosphate binders, and (d) vitamin D among patients on dialysis in 2006–2016 (n=379,835).
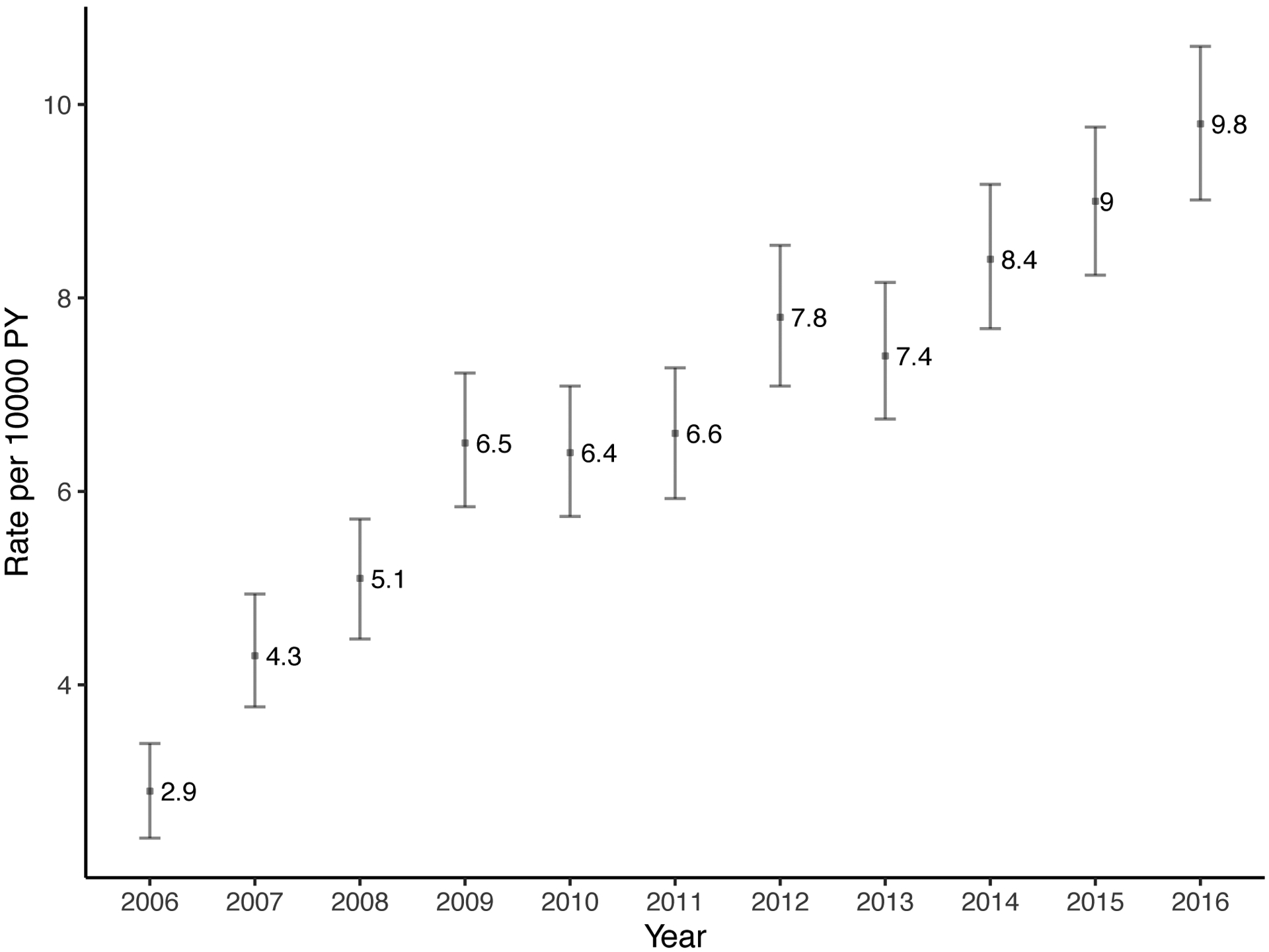
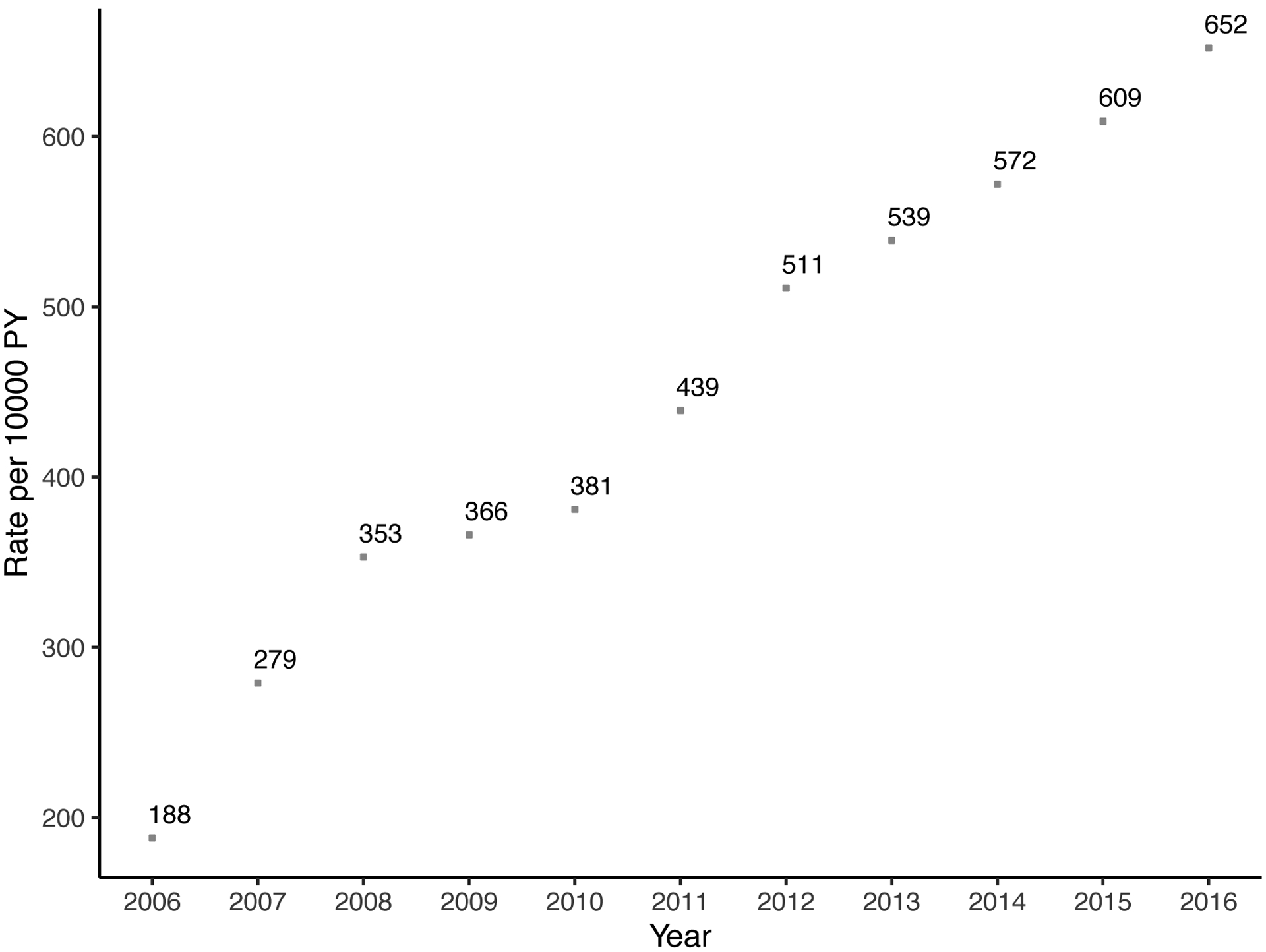
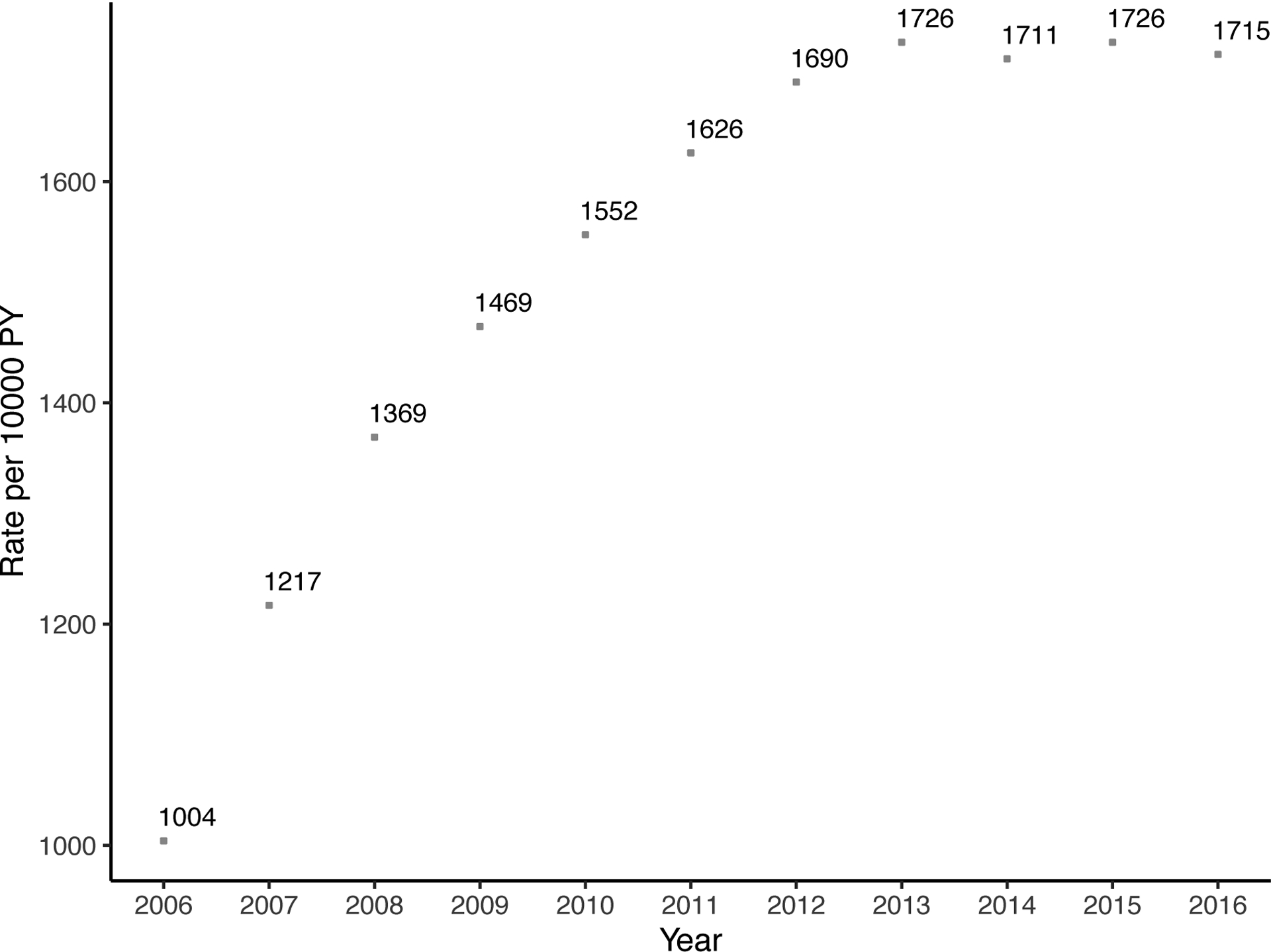
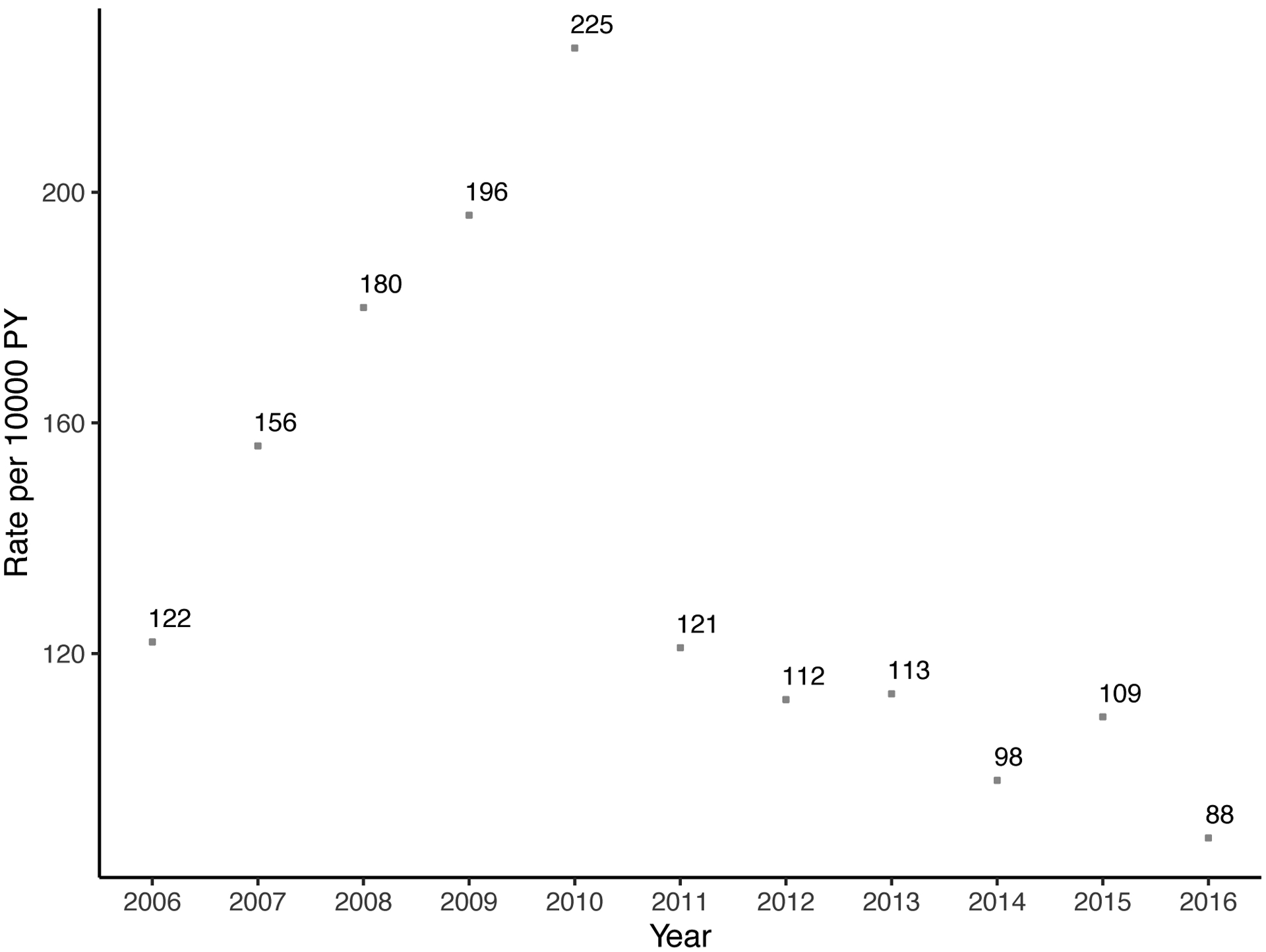
In 2016, 9.8 parathyroidectomies were performed per 10,000 person-years and 652 patients received at least one cinacalcet prescription per 10,000 person-years. Patients who received parathyroidectomy were censored at the date of operation. Rates per 10,000 person-year and 95% confidence interval are presented for parathyroidectomy and 95% confidence intervals for medications are narrow to be presented.
When the study period was divided into three eras, rates of parathyroidectomy were 52% higher in 2010–2013 (adjusted rate ratio [aRR] = 1.52, 95% CI: 1.39–1.65) and 106% higher in 2014–2016 (aRR = 2.06, 95% CI: 1.90–2.24) when compared to the 2006–2009 era after adjusting for confounding (p<0.001) (Table 2).
Table 2.
Rate ratio of parathyroidectomy by era (2006–2009, 2010–2013, and 2014–2016) after adjusting for age, sex, race, poverty, being listed, region, time to initiation of treatment, and comorbidities (diabetes mellitus, hypertension, heart failure, coronary artery disease, cardiovascular disease, peripheral vascular disease, cancer, chronic obstructive pulmonary disease, and functional impairment) for Secondary Hyperparathyroidism (SHPT).
| Era | Rate ratio | P-value |
|---|---|---|
| 2006–2009 | Reference | |
| 2010–2013 | 1.52 (1.39–1.65) | < 0.001 |
| 2014–2016 | 2.06 (1.90–2.24) | < 0.001 |
Rates of Parathyroidectomy Rates by Subgroups
Parathyroidectomy rates to treat SHPT increased disproportionately in patients who were younger, female gender black race, living in a higher poverty level, and in those listed for kidney transplant compared to those not listed (Figure 3). Annual rates of parathyroidectomy in younger (age 18–64 years) patients increased from 5.4 (95% CI: 4.5–6.5) per 10,000 person-years in 2006 to 19.0 (95% CI: 17.4–20.7) per 10,000 person-years in 2016 (p<0.001), which represents a 3.5-fold increase. In contrast, rates of parathyroidectomy for older (≥65 years) patients only increased from 0.9 (95%CI: 0.6–1.4) per 10,000 person-years to 2.7 (95% CI: 2.2–3.3) per 10,000 person years (p<0.001), representing a 3-fold increase (Figure 3A). Females underwent parathyroidectomy more frequently throughout the study period, with rates of 4.0 (95% CI: 3.2–4.9) per 10,000 person-years in 2006, which increased to 10.7 (95%CI: 9.7–12.1) per 10,000 person-years in 2016, which represents a 2.7-fold increase (p<0.001). However, parathyroidectomy rates in males increased 4.9-fold in men, with rates of 1.8 (95% CI: 1.3–2.4) per 10,000 person-years in 2006, which increased to 8.8 (95%CI: 7.9–9.9) per 10,000 person-years in 2016 (p<0.001). Patients of black race underwent parathyroidectomy more frequently than other races with annual rates increasing from 3.9 (95% CI: 3.0–5.1) per 10,000 person-years in 2006 to 17.1 (95% CI: 15.3–19.2) per 10,000 person-years in 2016, representing a 4.4-fold increase (p<0.001). Rates of those who lived in high-poverty neighborhoods increased from 3.1 (95% CI: 2.4–3.9) per 10,000 person-years in 2006 to 10.7 (95% CI: 9.6–11.0) per 10,000 person-years in 2016 (p<0.001), representing a 3.5-fold increase. Patients listed for kidney transplant underwent parathyroidectomy 3.2–3.5 times more frequently than those not listed throughout the study period. Among patients listed for kidney transplant, annual rates increased from 7.8 (95%CI: 5.7–10.5) per 10,000 person-years in 2006 to 25.2 (95%CI: 21.6–29.3) (p<0.001). Among patients who received parathyroidectomy during the study period (n=4,118), 30.1% were listed prior to surgery, 8% were listed on the date of surgery or later, and 62% were never listed Patients in the Southern part of the United States underwent parathyroidectomy more frequently than in the Northeast, West, or Midwest. Annual rates for patients in the Southern part of the US increased from 3.0 (95% CI: 2.3–3.9) per 10,000 person-years in 2006 to 13.2 (95% CI: 11.9–14.7) per 10,000 person-years in 2016 (p<0.001).
Figure 3. Trends of parathyroidectomy by (a) age, (b) sex, (c) race, (d) neighborhood poverty level, (e) being listed, and (f) region (Northeast, South, Midwest, and West).
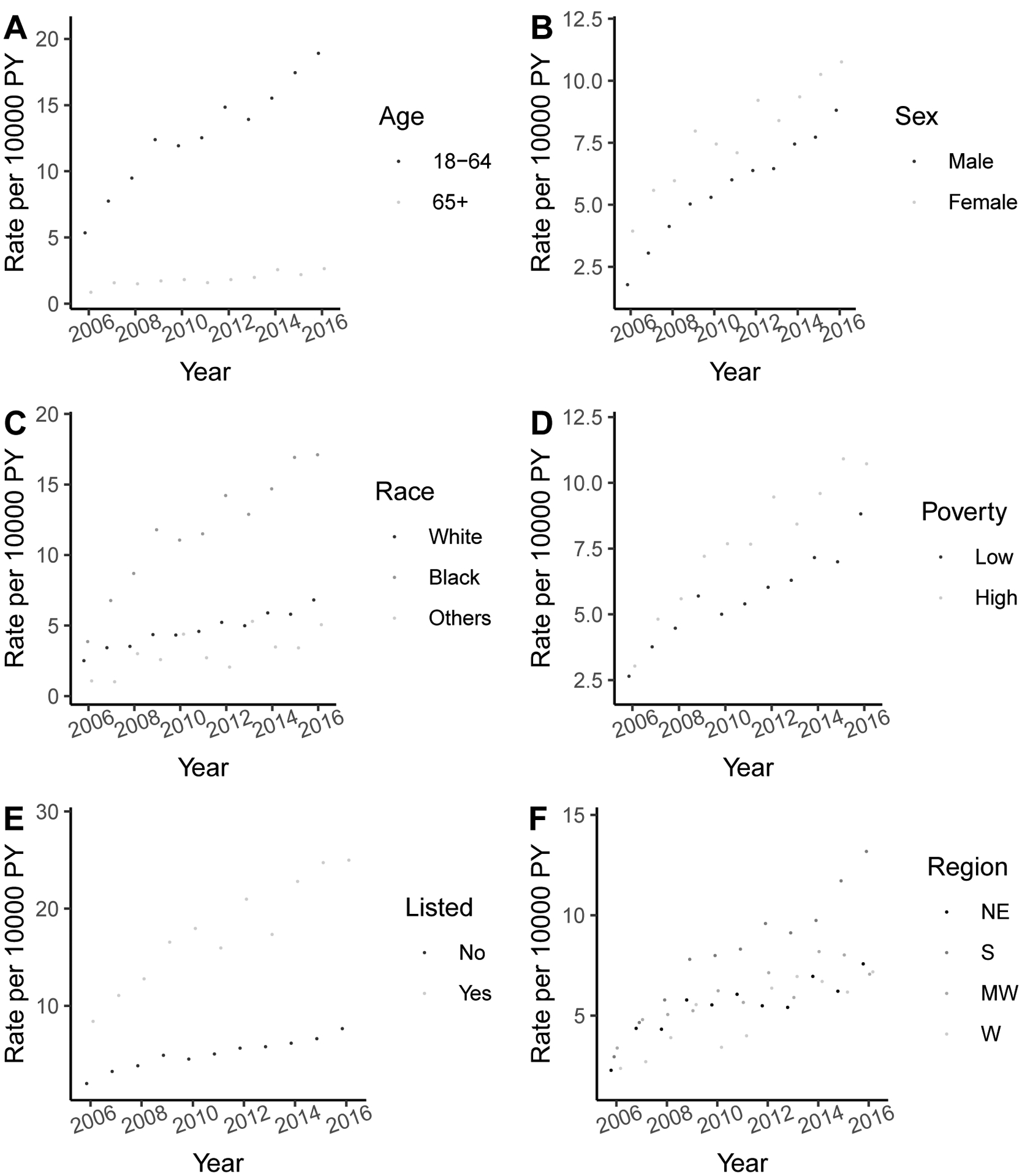
Rates per 10,000 person-year and 95% confidence interval are presented. Listing status was assessed at each follow up time period.
Prior to parathyroidectomy, there were no significant differences in number of medical therapies attempted prior to parathyroidectomy. However, approximately 20% of patients were prescribed a combination of all medical therapies including Vitamin D analogs, phosphate binders, and cinacalcet (Supplementary Table 1).For the entire cohort, time to parathyroidectomy since dialysis initiation was 0.1% at 1-year, 1.9% at 5-years, and 6.5% at 10-years (Supplementary Table 2). Incidence of parathyroidectomy did not differ by subgroups at 1-year after dialysis initiation. However, at 5-years and 10-years after dialysis initiation, incidence of parathyroidectomy differed by age, race, and listing status (Supplementary Table 2).
DISCUSSION
In this national study of 379,835 Medicare beneficiaries with SHPT on maintenance dialysis therapy, we have identified an evolving landscape in the management of SHPT, with 22.9% of patients on no SHPT treatment, a dramatic 3.5-fold increase in the use of calcimimetics, and a 3.4-fold increase in rates of parathyroidectomy to treat SHPT between 2006 and 2016. The greatest increase in rate of parathyroidectomy was observed across various subgroups including younger patients (age 18–64), Black patients, male gender, lower socioeconomic status, and those listed for kidney transplant. After adjusting for confounding, rates of parathyroidectomy were 52% higher in 2010–2013 and 106% higher in 2014–2016 as compared to rates in 2006–2009.
Similar to other studies, we found a significant increase in the use of cinacalcet to treat SHPT since its approval in 2004 (4, 10, 16, 17). However, these studies were only conducted through 2011 and also demonstrated an initial decline and eventual stabilization in the rate of parathyroidectomies performed as cinacalcet utilization increased. Our study built upon those findings and demonstrated a progressive increase in the utilization and annual rates of both cinacalcet and parathyroidectomy to treat SHPT between 2006–2016.
In contrast to our study of, two prior studies including CKD patients, found steady or decreasing rates of parathyroidectomy (4, 18). However, these studies differed from the current study as they included a different study population, methodology for estimating parathyroidectomy rates, and treatments for SHPT. Kim et al. and Fligor et al. included a broader patient population consisting of CKD patients not on dialysis and kidney transplant recipients. Therefore, Kim et al. included patients with primary, secondary, and tertiary hyperparathyroidism, and Fligor et al, included patients with secondary and tertiary hyperparathyroidism. Both of these studies utilized the National Inpatient Sample (NIS) database of hospital inpatient stays in the United States to capture a wider spectrum of patients including varied payor mix. By database design, parathyroidectomy rates estimated using NIS data were based on projections and sampling weights. NIS approximates a 20% stratified sample of all United States hospitals and uses weights to provide estimates of the numerator (# of parathyroidectomies) without providing a clear denominator (# of patients SHPT). In contrast, we used USRDS linked to Medicare, which allowed us to ensure that every participant who receives an SHPT treatment is identifiable and thus able to be included in the denominator when calculating person-years for rates rather than using projections from other data sources reducing potential biases in the rates. Our study built upon the prior studies as it allowed calculation of person-years for rates of parathyroidectomy and multiple pharmacotherapies simultaneously over time while also capturing suboptimal performed parathyroidectomy procedures (only removal of 1 or 2 glands) that could have been done as an outpatient procedure in a very specific patient population or would require cinacalcet for continued treatment of hyperparathyroidism after surgery.
Our study also uniquely demonstrated the significant variation and overlap of treatments utilized for management of SHPT. The reason for this overlap is likely multifactorial. Proposed PTH targets for those on maintenance dialysis therapy have varied globally over time, mostly due to the paucity of randomized-controlled trials to define an ideal PTH range. For example, the Japanese Society for Dialysis Therapy recommends maintaining a PTH range between 60–240 pg/mL, the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend target PTH levels between 150–300 pg/mL, whereas the KDIGO guidelines recommend maintaining a broader range of 2–9 times the upper limit of normal (PTH <600 pg/mL) (7, 19, 20). These guidelines are also unable to provide recommendations for the first line of treatment for SHPT patients. While many patients in our cohort received a single oral medication (either a phosphate binder, Vitamin D analog, or calcimimetic) for treatment, the majority of patients who underwent parathyroidectomy had also received oral medications during their treatment course, likely reflecting the common practice of reserving surgical management for those with refractory SHPT.
An alternative explanation may be that increasing severity of SHPT is not amenable to medical treatment among specific subgroups of patients. Similar to previous studies, those who underwent parathyroidectomy for SHPT were more likely to be younger and African American, likely reflective of a select group of patients with medically refractory SHPT (17, 21, 22). Our study further demonstrated that the utilization rate of parathyroidectomy has disproportionately continued to increase in younger patients and in African American patients since 2006. Prior investigations found that African Americans and younger adults have higher parathyroid gland mass and circulating PTH levels at baseline (23, 24). Additionally, in 2018, 32% of African American dialysis patients, compared to 20% of non-Black patients, were living with PTH levels >600 pg/mL despite a higher initiation of cinacalcet in this population (10, 17). These data coupled with the continuous rise in the utilization of parathyroidectomy to adequately control SHPT in the younger and African American subgroups suggest that starting with medical treatment options could be delaying necessary and definitive surgical management for select patients with these characteristics.
Alternatively, it is possible that maybe nephrologists are becoming more aggressive at referring patients for parathyroidectomy as an increasing number of studies have demonstrated improvement in symptoms from hyperparathyroidism and decreased rates post-transplant graft-failure (25–27). The combination in differences in PTH targets, lack of consensus in treatment strategies among guidelines, and practice variation all likely contribute to the variation and overlap of treatments and the rise in parathyroidectomy rates that surgeons performing these procedures should be aware of (10, 28).
We additionally found a greater increase in parathyroidectomy rates in patients of lower socioeconomic status, possibly attributable to a combination of more severe SHPT and lack of access to prescription drug coverage and KT (21, 29, 30). However, this seems less likely as all patients had Medicare coverage. Finally, a disproportionate increase was seen in those listed for KT. This finding may be due to the perception that patients would be healthy enough to undergo parathyroidectomy if they are KT candidates. Additionally, prior studies have demonstrated an association between pre-KT hypercalcemia or elevated PTH level and the development of delayed graft function and graft failure post-transplant (25, 26, 31, 32). Therefore, the KDIGO guidelines recommend delaying KT in patients with severe SHPT until their disease is adequately managed, which has likely led to the increased rate of parathyroidectomy in this group (33).
The main strengths of our study are large population size, patient-specific follow-up, utilizing data from USRDS and Medicare claims, and the availability of diagnostic and procedure codes for parathyroid-related disease. The strength of using of Medicare claims data is that it allowed us to simultaneously evaluate cinacalcet, phosphate binder, and vitamin D utilization by year and go beyond just the trends in parathyroidectomy. This allowed us to calculate rates of not just medical and surgical treatments, but also lack of treatment for SHPT. Because this Medicare database, is not reliant on projections or sampling weights of the “denominator” estimates for the rates, it allowed for an unbiased estimate of treatment rates. Finally, utilizing the Medicare database allowed us to capture patients who may have had a suboptimal parathyroidectomy procedure, as cinacalcet is typically not prescribed within the first post-operative year. Additionally, if we assume that dialysis patients are often admitted after undergoing subtotal or total parathyroidectomy as opposed to patients undergoing a single or even 2 gland parathyroidectomy as an outpatient, additional suboptimal procedures could be captured. This is the largest study to evaluate the evolution of treatment patterns for SHPT in patients on dialysis.
There are several limitations to this study in addition to those inherent to retrospective database studies including coding errors, missing values, confounding by indication, and selection bias. Although patients with CKD, not yet on dialysis, may also have SHPT, the results of our study do not reflect treatment trends in this patient population. Because Medicare is not the primary health care payer for all patients on hemodialysis in the United States, results of this study may not be generalizable to the entire population of hemodialysis patients. However, Medicare is the largest single insurer for patients requiring hemodialysis, and therefore Medicare coverage is often used as an inclusion criterion for studies of this population (34, 35). It is possible that some patients who were classified as receiving no treatment actually did receive treatment that was either over-the-counter, intravenously during dialysis, or part of a bundled payment. This may explain the lack of trend in Vitamin D utilization, as it is covered in a bundled payment. While it is possible that some patients may have been on some form of treatment for SHPT that was covered by a second payer, all patients included in this study had Medicare claims for other medications, and therefore it was presumed that they would receive all of their prescriptions through Medicare. The median age of our patients also reflects an older population. However, this likely reflects the age distribution of all ESRD patients. The age distribution among all incident ESRD patients in USRDS between 2004–2016 regardless of their Medicare coverage is 64 years (IQR: 53–74) and 67 years (IQR: 56–76) when restricted to those with Medicare.
Finally, PTH values corresponding to each patient were not available in the database. Therefore, severity of SHPT, adherence to or effectiveness of treatment modalities could not be factored into our analysis. However, time to SHPT treatment was used as a surrogate to SHPT severity.
As the prevalence and severity of SHPT in dialysis-dependent patients in the United States continue to rise, the use of both calcimimetics and parathyroidectomy for treatment have increased since 2006. While the rates of parathyroidectomy increased for all patients, there has been a disproportionate rise in surgical management for younger patients, African Americans, and those with lower socioeconomic status, which may represent a subset of patients with SHPT that is less amenable to medical treatment. Surgeons performing parathyroid procedures should be aware of this trend. Future prospective studies should build upon these findings and evaluate the optimal treatment algorithms and ideal PTH ranges based on patient and clinical characteristics.
Supplementary Material
Funding/Financial Support
Funding for this study was provided in part by the National Cancer Institute, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and the National Institute on Aging (NIA); grant numbers T32CA126607 (Whitney Sutton), K23AG053429 (PI: Aarti Mathur), R01DK120518 (PI: Mara McAdams-DeMarco), and R01AG055781 (PI: Mara McAdams-DeMarco), and K24AI144954 (PI: Dorry Segev). Additional support came from other grants.
Abbreviations
- aRR
adjusted Rate Ratio
- CKD
Chronic Kidney Disease
- ESRD
End-stage Renal Disease
- KDIGO
Kidney Diseases Improving Global Outcomes
- KDOQI
Kidney Disease Outcomes Quality Initiative
- NIS
Nationwide Inpatient Sample
- PTH
Parathyroid Hormone
- SHPT
Secondary Hyperparathyroidism
- USRDS
United States Renal Data System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosures
The authors’ have no conflicts of interest to declare.
REFERENCES
- 1.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB 2017. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 92:26–36. [DOI] [PubMed] [Google Scholar]
- 2.Lau WL, Obi Y, Kalantar-Zadeh K 2018. Parathyroidectomy in the Management of Secondary Hyperparathyroidism. Clin J Am Soc Nephrol 13:952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD 2006. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70:771–780. [DOI] [PubMed] [Google Scholar]
- 4.Kim SM, Long J, Montez-Rath ME, Leonard MB, Norton JA, Chertow GM 2016. Rates and Outcomes of Parathyroidectomy for Secondary Hyperparathyroidism in the United States. Clin J Am Soc Nephrol 11:1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Investigators ET, Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS 2012. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367:2482–2494. [DOI] [PubMed] [Google Scholar]
- 6.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK 2008. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52:519–530. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes CKDMBDUWG 2017. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011) 7:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rroji M, Spasovski G 2018. Calcimimetics versus parathyroidectomy: What is preferable? Int Urol Nephrol 50:1271–1275. [DOI] [PubMed] [Google Scholar]
- 9.Seethapathy H, Nigwekar SU 2019. Medication Prescription Patterns for Secondary Hyperparathyroidism: More Questions than Answers. Clin J Am Soc Nephrol 14:178–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller DS, Xing S, Belozeroff V, Yehoshua A, Morgenstern H, Robinson BM, Rubin RJ, Bhatt N, Pisoni RL 2019. Variability in Cinacalcet Prescription across US Hemodialysis Facilities. Clin J Am Soc Nephrol 14:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW 2017. Opioid Prescription, Morbidity, and Mortality in United States Dialysis Patients. J Am Soc Nephrol 28:3658–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA, Daubresse M, Bae S, Gross AL, Carlson MC, Segev DL 2018. Dementia, Alzheimer’s Disease, and Mortality after Hemodialysis Initiation. Clin J Am Soc Nephrol 13:1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, Balkrishnan R, Hirth RA, Hutton DW, He K, Steffick DE, Saran R 2020. Assessment of Prescription Analgesic Use in Older Adults With and Without Chronic Kidney Disease and Outcomes. JAMA Netw Open 3:e2016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bureau USC. Poverty Status in the Past 12 Months: American Community Survey 2014. https://data.census.gov/cedsci/. (Last accessed on October 9, 2020).
- 15.Physicians AAoF. UDS Mapper: Zip Code to ZCTA Crosswalk. https://udsmapper.org/zip-code-to-zcta-crosswalk/).
- 16.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, Andreucci VE, Fukagawa M, Frimat L, Mendelssohn DC, Port FK, Pisoni RL, Robinson BM 2015. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 10:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilpatrick RD, Newsome BB, Zaun D, Liu J, Solid CA, Nieman K, St Peter WL 2013. Evaluating real-world use of cinacalcet and biochemical response to therapy in US hemodialysis patients. Am J Nephrol 37:389–398. [DOI] [PubMed] [Google Scholar]
- 18.Fligor SC, Li C, Hamaguchi R, William J, James BC 2021. Decreasing Surgical Management of Secondary Hyperparathyroidism in the United States. J Surg Res 264:444–453. [DOI] [PubMed] [Google Scholar]
- 19.National Kidney F 2003. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42:S1–201. [PubMed] [Google Scholar]
- 20.Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T, Group C-MGW, Japanese Society for Dialysis T 2013. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 17:247 288. [DOI] [PubMed] [Google Scholar]
- 21.Greene B, Kim SJ, McCarthy EP, Pasternak JD 2020. Effects of Social Disparities on Management and Surgical Outcomes for Patients with Secondary Hyperparathyroidism. World J Surg 44:537–543. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Chen YW, Peng Y, Foley RN, St Peter WL 2011. Trends in parathyroidectomy rates in US hemodialysis patients from 1992 to 2007. Am J Kidney Dis 57:602–611. [DOI] [PubMed] [Google Scholar]
- 23.Owda A, Elhwairis H, Narra S, Towery H, Osama S 2003. Secondary hyperparathyroidism in chronic hemodialysis patients: prevalence and race. Ren Fail 25:595–602. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Kallenbach LR, Zasuwa G, Divine GW 2000. Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol 11:330–334. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadi F, Ali-Madadi A, Lessan-Pezeshki M, Khatami M, Mahdavi-Mazdeh M, Razeghi E, Maziar S, Seifi S, Abbasi M 2008. Pre-transplant calcium-phosphate-parathormone homeostasis as a risk factor for early graft dysfunction. Saudi J Kidney Dis Transpl 19:54–58. [PubMed] [Google Scholar]
- 26.Boom H, Mallat MJ, de Fijter JW, Paul LC, Bruijn JA, van Es LA 2004. Calcium levels as a risk factor for delayed graft function. Transplantation 77:868–873. [DOI] [PubMed] [Google Scholar]
- 27.Mathur A, Sutton W, Ahn JB, Prescott JD, Zeiger MA, Segev DL, McAdams-DeMarco M 2021. Association Between Treatment of Secondary Hyperparathyroidism and Posttransplant Outcomes. Transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Health ARCf. The DOPPS Practice Monitor. https://www.dopps.org/dpm/default.aspx. (Last accessed on 6/1/2020, 2020).
- 29.Purnell TS, Luo X, Cooper LA, Massie AB, Kucirka LM, Henderson ML, Gordon EJ, Crews DC, Boulware LE, Segev DL 2018. Association of Race and Ethnicity With Live Donor Kidney Transplantation in the United States From 1995 to 2014. JAMA 319:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axelrod DA, Dzebisashvili N, Schnitzler MA, Salvalaggio PR, Segev DL, Gentry SE, Tuttle-Newhall J, Lentine KL 2010. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol 5:2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roodnat JI, van Gurp EA, Mulder PG, van Gelder T, de Rijke YB, de Herder WW, Kal-van Gestel JA, Pols HA, Ijzermans JN, Weimar W 2006. High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation 82:362–367. [DOI] [PubMed] [Google Scholar]
- 32.Callender GG, Malinowski J, Javid M, Zhang Y, Huang H, Quinn CE, Carling T, Tomlin R, Smith JD, Kulkarni S 2017. Parathyroidectomy prior to kidney transplant decreases graft failure. Surgery 161:44–50. [DOI] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes CKDMBDWG 2009. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl:S1–130. [DOI] [PubMed] [Google Scholar]
- 34.Jackson KR, Motter JD, Bae S, Kernodle A, Long JJ, Werbel W, Avery R, Durand C, Massie AB, Desai N, Garonzik-Wang J, Segev DL 2020. Characterizing the landscape and impact of infections following kidney transplantation. Am J Transplant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL 2014. Sequelae of early hospital readmission after kidney transplantation. Am J Transplant 14:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


