Abstract
Objective:
Single segment great saphenous vein (GSV) is the preferred conduit in infrainguinal bypass. Alternative autologous conduits (AAC) and nonautologous biologic conduits (NABC) are thought to be a better alternative to traditional prosthetic conduits (PC) in the absence of GSV. In this study we analyzed the outcomes of these alternative conduits in lower extremity bypasses (LEB) in patients with chronic limb-threatening ischemia.
Methods:
The Vascular Quality Initiative LEB database from 2003 to 2020 was queried for this study, to identify LEB in patients with chronic limb-threatening ischemia. Primary outcomes were graft patency, major adverse limb events (MALE), and MALE-free survival at 1 year. Standard statistical methods were used as appropriate.
Results:
We identified 22,671 LEB procedures (12,810 GSV, 6002 PC, 1907 AAC, and 1952 NABC). Compared with the GSV group, the other conduit patients were significantly older, had more comorbidities, had an increased rate of prior lower extremity interventions, had a higher rate of infrageniculate bypass targets, and were less ambulatory at baseline. The PC, AAC, and NABC groups had significantly higher rates of postoperative morbidity compared with the GSV group. The PC group had a higher 30-day mortality compared with the GSV, AAC, and NABC groups (3% PC vs 2% GSV, 2% AAC, 2% NABC; P = .049). Both PC and NABC had higher 1-year mortality compared with GSV and AAC (13% PC and 13% NABC vs 10% GSV, 10% AAC; P = .02). In an adjusted Cox regression model (stratified by infrageniculate target and adjusted for age, comorbidities, and prior vascular interventions) PC was not significantly different from GSV, but AAC (hazard ratio [HR], 1.41; 95% confidence interval [CI], 1.19–1.67; P < .001) and NABC (HR, 1.9; 95% CI, 1.61–2.25; P < .001) were associated with an increased risk of loss of primary patency. A similar association with MALE was observed: both AAC (HR, 1.35; 95% CI, 1.15–1.58; P < .001) and NABC (HR, 1.8; 95% CI, 1.53–2.11; P < .001) were associated with an increased risk of MALE compared with GSV; PC was not significantly different from GSV.
Conclusions:
In the absence of GSV, alternative conduits (autologous or nonautologous biologic) do not confer a benefit with regard to graft patency or MALE compared with PCs. Increased operating time or costs associated with the use of these conduits is not justified based on this study.
Keywords: Alternative conduit, Loss of patency, Lower extremity bypass, Major adverse limb events, Outcomes
The surgical management of peripheral arterial disease remains one of the greatest challenges facing vascular surgeons today. Open lower extremity bypass (LEB) is the preferred treatment for most patients with peripheral arterial disease after endovascular therapies fail, and single segment great saphenous vein (GSV) is the conduit of choice for LEB below the knee vessels.1 Historically, up to 50% of patients presenting with lower extremity ischemia necessitating a LEB do not have adequate GSV.2 The reasons for inadequate GSV are multifactorial, but include prior harvest for CABG, insufficient vein caliber, and lower extremity venous insufficiency.
In the absence of GSV, numerous alternative conduits have been used to perform LEB, with varying success rates. Prosthetic conduits (PC) have been used as an alternative when GSV is not available.3 GSV grafts have a statistically higher long term patency rate compared with PC in below knee targets.3 Thus, PC use for below-knee popliteal and other infrageniculate targets remains the subject of much controversy and debate.
Alternative autologous conduits (AAC), such as the lesser saphenous vein, multiple segment GSV, and single segment or spliced cephalic/basilic veins, provide an autologous alternative in the absence of GSV. There is no clear evidence for the use of these conduits. Multiple studies have shown no clear benefit to using AAC over PC in the absence of single segment GSV4–7; although other studies have shown that cephalic/basilic and lesser saphenous veins are similar to GSV in terms of primary and secondary patency and limb salvage.8 Nonautologous biologic conduits (NABC), such as cryopreserved vein grafts and arterial homografts, have been used with increasing frequency, in the absence of autologous conduits or in the presence of an infectious process pre-cluding the use of a PC.9 Given the lack of evidence-based guidance regarding the choice of conduit for LEB, we sought to compare the outcomes of LEB using GSV, PC, AAC, and NABC in the Society for Vascular Surgery Vascular Quality Initiative (SVS VQI) database. We hypothesize that PC will have comparable outcomes to AAC and NABC.
METHODS
We queried the VQI infrainguinal bypass dataset for patients who underwent a LEB from 2003 to 2020. Because de-identified registry data were used, institutional review board approval and informed consent were not required for the conduct of this study. We included patients who underwent a LEB from the femoral artery (common femoral, profunda femoral, or superficial femoral artery) to a below knee target vessel (below knee popliteal, tibial-peroneal trunk, posterior tibial, anterior tibial, peroneal, plantar and dorsalis pedal arteries) for chronic limb-threatening ischemia (CLTI; defined as rest pain or tissue loss), patients with bypasses to the above knee popliteal vessel were excluded from this analysis. Patients with interventions for claudication, acute limb ischemia, or aneurysmal disease were also excluded from this analysis. Demographic data and comorbidities, including age, gender, ambulatory status, preoperative living status, smoking history (current smoker or not), obesity (body mass index of >30), congestive heart failure, chronic obstructive pulmonary disease, coronary artery disease, hypertension, end-stage renal disease, diabetes mellitus, a history of prior bypass, a history of prior endovascular intervention, a history of prior major amputation, and indications for intervention were collected. The primary outcomes assessed were primary conduit patency; major adverse limb events (MALE), defined as any above ankle limb amputation of the index limb(s) or reintervention (new bypass graft, jump or interposition graft revision; angioplasty, stent, or stent graft; or thrombectomy/thrombolysis); and MALE-free survival, a composite end point of MALE or death. Secondary outcomes included primary assisted patency, amputation-free survival (AFS), 30-day and 1-year mortality, and 30-day perioperative morbidities (cardiovascular or respiratory).
The patient cohort was divided into four groups based on bypass conduit type: single segment GSV, PC, AAC composed of small saphenous/basilic/cephalic veins, and NABC composed of cryopreserved vein grafts and arterial homografts.
Stata SE 15.1 (StataCorp, College Station, TX) was used for statistical analysis. The Student t test (for continuous variables) and the χ2 test (for categorical variables) were used for comparing perioperative factors and outcomes of interest between groups, the rank-sum test and Fisher exact test were used when deemed appropriate for violations of normality and sample size. A Kaplan-Meir survival analysis was then used to analyze primary patency, MALE, and MALE-free survival at 1 year; the log-rank test was used to compare between conduit types. Finally, multivariable Cox proportional hazard models of primary patency, MALE, and MALE-free survival at 1 year were constructed, adjusting for key comorbidities and perioperative and operative factors. Each final multivariable model was stratified by distal bypass target and chosen by a backward-stepwise elimination process, using all univariable predictors with a P values of less than .20 as candidate predictors. A cutoff of a P values of less than .05 was used for all other statistical tests. Our study had 80% power to detect our observed effect size. Model fit was assessed visually using Snell residuals.
RESULTS
A total of 22,671 LEB procedures from a femoral artery origin (common femoral superficial femoral or profunda femoral) to a below knee target vessel (below knee popliteal, tibial-peroneal trunk, posterior tibial, anterior tibial, peroneal, plantar and dorsalis pedal arteries) were identified, after excluding those with suprainguinal bypasses and indications other than CLTI. Of those, 12,810 patients underwent LEB with GSV, 6002 with PC, 1907 with AAC, and 1952 with NABC. The patients differed significantly in terms of demographics and preoperative characteristics. Patients in the PC and NABC cohorts were older, less ambulatory, and had more comorbid conditions compared with the GSV and AAC cohorts (Table I). PC, AAC, and NABC cohorts had more frequent prior LEB, and prior lower extremity endovascular intervention than those in the GSV cohort (Table I).
Table I.
Demographics and operative details
| GSV (n = 12,810) | AAC (n = 1907) | PC (n = 6002) | NABC (n = 1952) | P value | |
|---|---|---|---|---|---|
| Age, years | 67.25±11.12 | 67.11±10.6 | 69.12±10.53a,b | 70.22±10.63a,b,c | .001 |
| Male | 8922 (70) | 1325 (70) | 3739 (62)a,b | 1224 (63)a,b | <.001 |
| Hispanic | 681 (5) | 94 (5) | 377 (6)a | 197 (10)a,b,c | .047 |
| Obese | 3769 (29) | 606 (32) | 1534 (26)a,b | 480 (25)a,b | <.001 |
| Prior/current smoker | 10,654 (83) | 1599 (84) | 5100 (85)a | 1556 (80)a,b,c | .009 |
| Hypertensive | 11,295 (88) | 1718 (90) | 5509 (92)a | 1819 (93)a,b | .002 |
| History of diabetes | 7003 (55) | 1083 (57) | 3320 (55) | 1145 (59)a | .006 |
| History of CAD | 3813 (30) | 682 (36)a | 2172 (36)a | 799 (41)a,b,c | .006 |
| History of CHF | 2333 (18) | 365 (19) | 1356 (23)a,b | 534 (27)a,b,c | .009 |
| History of COPD | 3307 (26) | 485 (25) | 1741 (29)a,b | 562 (29)a | .03 |
| On hemodialysis | 1011 (8) | 97 (5) | 580 (10)a,b | 236 (12)a,b,c | .013 |
| Prior Carotid intervention | 809 (8) | 160 (10)a | 612 (11)a | 208 (12)a | .001 |
| Prior Aneurysm repair | 472 (4) | 97 (5)a | 280 (5)a | 96 (5) | .018 |
| Prior CABG | 1630 (15) | 436 (28)a | 1686 (31)a | 636 (36)a,b,c | .002 |
| Prior coronary PCI | 2220 (21) | 361 (23) | 1270 (23)a | 442 (25)a | .001 |
| Ambulatory preoperatively | 8497 (67) | 1310 (69) | 3646 (61)a,b | 988 (51)a,b | <.001 |
| Preoperative stress testing | .041 | ||||
| None | 8576 (67) | 1219 (64) | 3858 (64) | 1325 (68) | |
| Normal | 2945 (23) | 451 (24) | 1456 (24) | 391 (20) | |
| Ischemia | 707 (6) | 124 (7) | 343 (6) | 108 (6) | |
| MI | 386 (3) | 79 (4) | 217 (4) | 86 (4) | |
| MI + ischemia | 172 (1) | 29 (2) | 110 (2) | 35 (2) | |
| Preoperative living status | .039 | ||||
| Home | 12231 (96) | 1847 (97) | 5710 (96) | 1827 (94) | |
| Nursing home | 495 (4) | 48 (3)a | 252 (4)b | 117 (6)a,b,c | |
| Homeless | 50 (0) | 8 (0) | 16 (0) | 7 (0) | |
| Prior LE bypass | 2948 (23) | 856 (45)a | 2594 (43)a | 1079 (55)a,b,c | <.001 |
| Prior LE endovascular | 5290 (41) | 942 (49)a | 3086 (51)a | 1054 (54)a,b | .024 |
| Prior major amputation | 668 (5) | 117 (6) | 453 (8)a | 195 (10)a,b,c | .004 |
| Laterality, right | 6516 (51) | 953 (50) | 2979 (50) | 981 (50) | >.05 |
| Indication | |||||
| Rest pain | 4772 (23) | 782 (28)a,d | 2520 (26)a,d | 560 (22) | |
| Tissue loss | 8038 (40) | 1125 (40) | 3482 (36) | 1392 (56)a,b,c | |
| Infrageniculate bypass target | 7548 (59) | 1506 (79)a | 2684 (45)a,b | 1613 (83)a,b,c | .026 |
| Infrageniculate bypass target | .001 | ||||
| BK popliteal | 5243 (41) | 400 (21) | 3300 (55) | 339 (17) | |
| Tibial | 6875 (54) | 1364 (72) | 2595 (43)a,b | 1459 (75)a,c | |
| Tibial at ankle/pedal | 673 (5) | 142 (7) | 89 (1)a,b | 154 (8)a,b,c | |
| Timing of surgery | .042 | ||||
| Elective | 9877 (77) | 1506 (79) | 4624 (77) | 1446 (74) | |
| Urgent | 2788 (22) | 383 (20) | 1271 (21) | 476 (24)b,c | |
| Emergent | 139 (1) | 18 (1) | 102 (2) | 29 (1) | |
| Type of anesthesia | .004 | ||||
| Spinal | 402 (3) | 33 (2) | 93 (2) | 32 (2) | |
| Epidural | 262 (2) | 24 (1) | 56 (1) | 16 (1) | |
| General | 12,137 (95) | 1850 (97)a | 5848 (98)a | 1903 (98)a | |
| No. of vein segments | NA | ||||
| 1 | NA | 420 (22) | NA | NA | |
| 2 | NA | 1300 (68) | NA | NA | |
| ≥3 | 151 (8) | ||||
| Distal venous cuff | 140 (1) | 22 (1) | 927 (16)a,b,d | 30 (2) | <.001 |
| Concomitant PVI | 866 (7) | 107 (6) | 577 (10)a,b,d | 122 (6) | <.001 |
| Concomitant endarterectomy | 3741 (29) | 475 (25) | 2064 (34)a,b,d | 535 (27) | .005 |
AAC, alternative autologous conduits; BK, below the knee; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; GSV, great saphenous vein; LE, lower extremity; MI, myocardial infarction; NA, not applicable; NABC, nonautologous biologic conduits; PC, prosthetic conduits; PCI, percutaneous coronary intervention; PVI, peripheral vascular intervention.
Values are mean ± standard deviation or number (%).
Significant vs GSV.
Significant vs AAC.
Significant vs PC.
Significant vs NABC.
The AAC and NABC cohorts had a higher percentage of infrageniculate bypass targets (79% AAC, 83% NABC vs 59% GSV, 45% PC; P = .026) compared with GSV and PC. LEB in the PC cohort more frequently included concomitant endovascular intervention or endarterectomy compared with GSV, AAC, and NABC cohorts (Table I). Of GSV bypasses, 27% received postoperative anticoagulation, compared with 40% in AAC, 40% in PC, and 43% in NABC. There was no statistically significant difference between AAC, PC, and NABC, but all three groups had a significantly higher percentage of postoperative anticoagulation compared with GSV. Postoperative complications and mortality were infrequent in this analysis; however, they were occurred more frequently in the alternative conduit cohorts compared with the GSV cohort (Table II).
Table II.
Postoperative outcomes
| GSV (n = 12,810) | AAC (n = 1907) | PC (n = 6002) | NABC (n = 1952) | P value | |
|---|---|---|---|---|---|
| Surgical site infection | 459 (4) | 65 (3) | 189 (3) | 64 (3) | >.05 |
| Stroke | 88 (1) | 18 (1) | 43 (1) | 8 (0) | >.05 |
| Return to operating room | 1800 (14) | 311 (16)a | 876 (15) | 351 (18)a,b | .049 |
| MI | 434 (3) | 92 (5)a | 253 (4)a | 73 (4) | .028 |
| Dysrhythmia | 527 (4) | 103 (5) | 272 (5) | 96 (5) | >.05 |
| CHF | 257 (2) | 48 (3) | 147 (2) | 67 (3)a | <.001 |
| Respiratory complication | 276 (2) | 48 (3) | 198 (3)a | 64 (3)a | .012 |
| 30-Day mortality | 235 (2) | 40 (2) | 197 (3)a,c | 46 (2) | .049 |
| 1-Year mortality | 1232 (10) | 189 (10) | 768 (13)a,c | 252 (13)a,c | .02 |
AAC, Alternative autologous conduits; CHF, congestive heart failure; GSV, great saphenous vein; MI, myocardial infarction; NABC, nonautologous biologic conduits; PC, prosthetic conduits.
Values are number (%).
Significant vs GSV.
Significant vs PC.
Significant vs AAC.
Primary outcomes were primary graft patency, MALE, and MALE-free survival. Using Kaplan-Meier analysis, we observed that the AAC and NABC cohorts had a significantly lower primary graft patency (45.73% ± 3.37% AAC and 35.98% ± 3.11% NABC vs 61.36% ± 1.34% GSV and 61.36% ± 1.34% PC; P < .001, log-rank test) at 1 year compared with GSV and PC. There was no statistical difference between the GSV and PC cohorts (Fig 1, A). AAC and NABC had significantly higher rates of MALE at 1 year compared with GSV and PC (58.7% ± 3.04% AAC and 68.07% ± 3.01% NABC vs 43.75% ± 1.28% GSV and 43.69% ± 2.27% PC; P < .001, log-rank test), with no statistical difference between PC and GSV (Fig 1, B). PC, AAC, and NABC all had worse MALE-free survival when compared with GSV at 1 year (47.25% ± 1.09% PC, 46.03% ± 1.82% AAC, 34.84% ± 1.83% NABC vs 53.13% ± 0.74%; P < .001, log-rank test). There was no difference in MALE-free survival between PC and AAC cohorts, whereas both were significantly higher than NABC (Fig 1, C).
Fig 1.
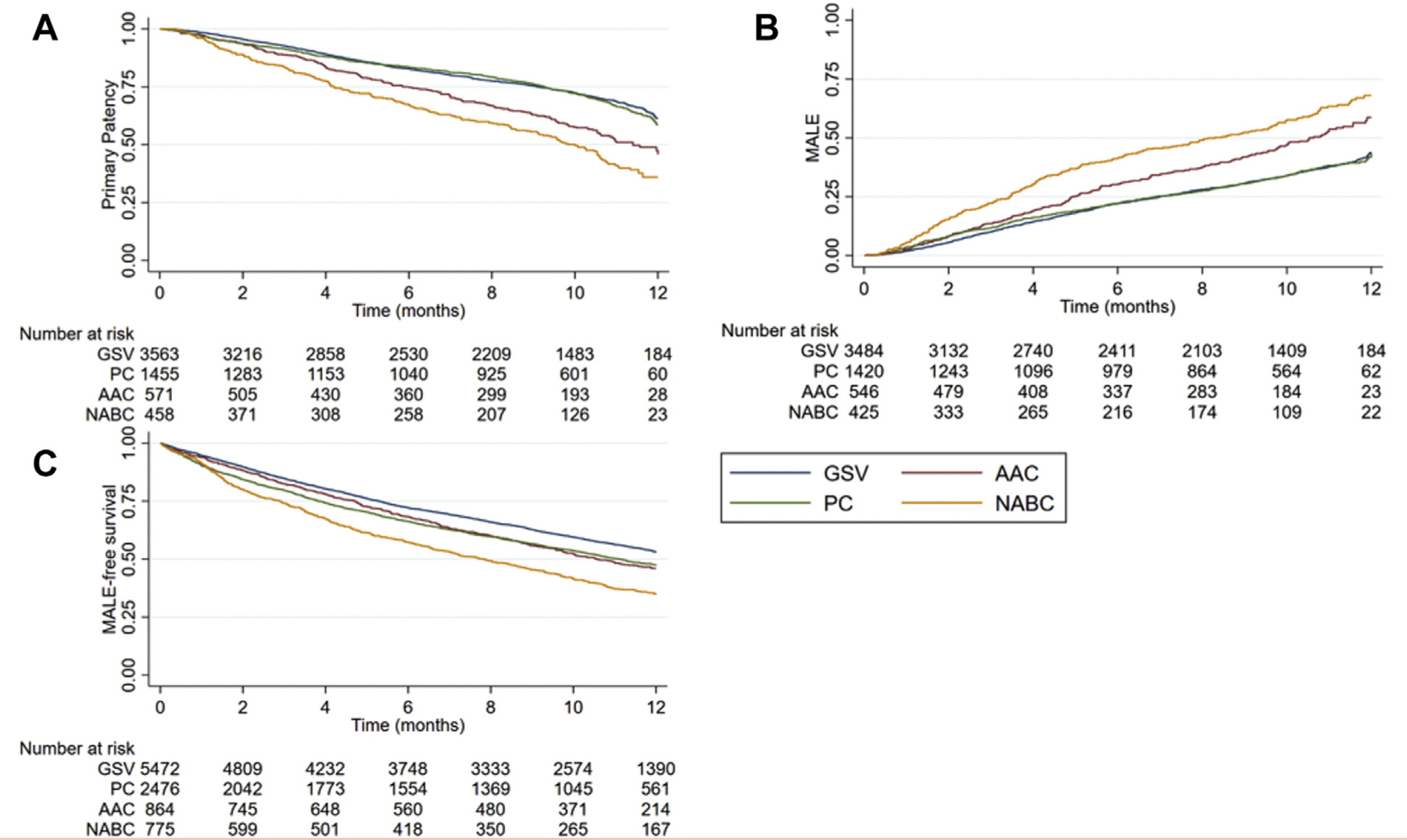
(A) Unadjusted Kaplan-Meier (KM) estimates of primary patency at 1 year. (B) Unadjusted KM estimates of major adverse limb events (MALE) at 1 year. (C) Unadjusted KM estimates of MALE-free survival at 1 year. AAC, alternative autologous conduits; GSV, great saphenous vein; NABC, nonautologous biologic conduits.
Secondary outcomes included primary assisted patency and amputation free survival. There was no difference in primary assisted patency between GSV and PC groups at 1 year by unadjusted Kaplan-Meier analysis (78.7% ± 1.62% for GSV vs 85.86% ± 1.84% for PC; P = .1272, log-rank test). PC did have a higher primary assisted patency at 1 year compared with AAC (85.86% ± 1.84% for PC vs 62.9% ± 5.4% for AAC; P < .001, log-rank test) and NABC (85.86% ± 1.84% for PC vs 70.4% ± 4.5% for NABC; P < .001, log-rank test) (Fig 2, A). In terms of AFS, GSV had higher AFS compared with PC, AAC, and NABC (86.45% ± 0.55% for GSV vs 79.46% ± 1% PC, 80.23% ± 1.6% AAC, and 68.4% ±2% for NABC; P ≤ .001, log-rank test). There was no difference in AFS between PC and AAC (P = .5335, log-rank test), both PC and AAC had higher AFS compared with NABC (P < .001, log-rank test) (Fig 2, B). We then examined potential differences between single segment and spliced alternative conduits, and found that there was no difference in primary patency (P = .6783) or freedom from MALE (P = .2128) between single segment and spliced segment alternative conduits, with single segment alternative conduits having better MALE-free survival (P = .0111) (Supplementary Fig 1, online only).
Fig 2.
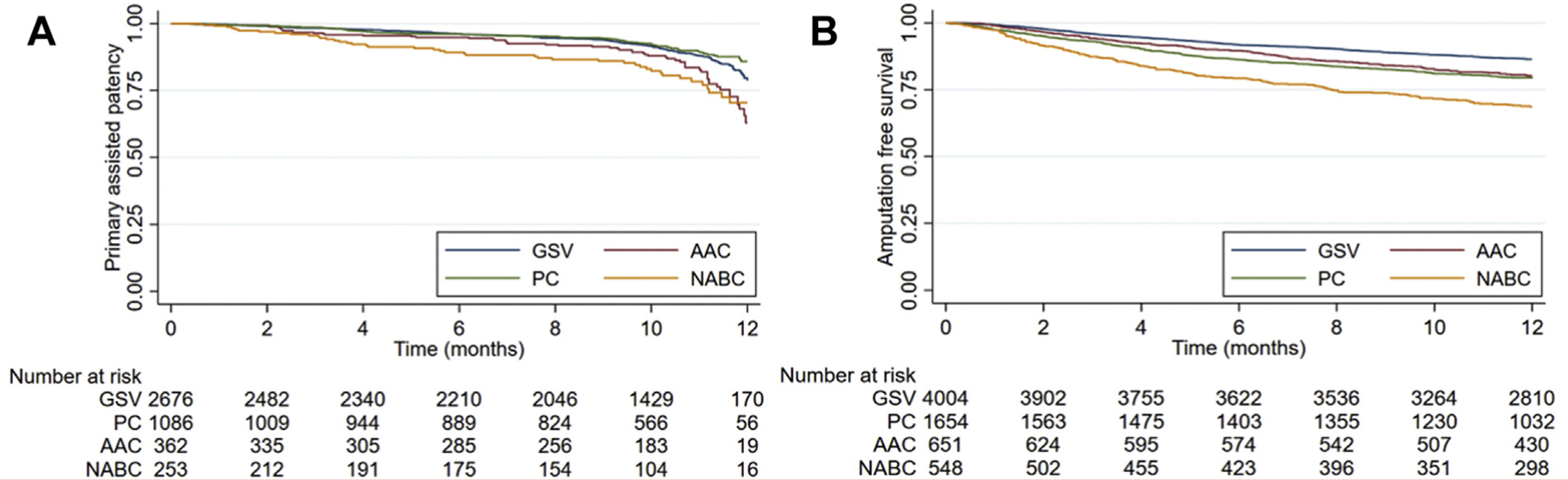
(A) Unadjusted Kaplan-Meier (KM) estimates of primary patency at 1 year. (B). Unadjusted KM estimates of amputation free survival at 1 year. AAC, alternative autologous conduits; GSV, great saphenous vein; NABC, non-autologous biologic conduits
We then constructed a multivariable Cox regression model that was adjusted for age, comorbidities, number of vein segments used for the bypass, use of venous cuff, and prior vascular interventions, and then stratified by infrageniculate target. Table III summarizes these adjusted analyses comparing conduits for different outcomes. Comparing GSV with PC, the adjusted analysis suggests statistically similar results in terms of primary patency and MALE, with GSV having better MALE-free survival. Comparing alternative conduits, we noted that AAC had a significantly higher risk of loss of patency and MALE compared with PC, but no difference in MALE-free survival. NABC had higher risk of adverse outcomes than PC and AAC in all categories (Fig 3) (Table III). We then performed a sensitivity analysis looking at below-knee targets and tibial/pedal targets separately. In below-knee popliteal targets, GSV had a higher MALE-free survival at 1 year in both unadjusted (Supplementary Fig 2, online only) and adjusted (Supplementary Table I, online only) analyses compared with PC. We observed no statistically significant differences in primary patency and MALE between GSV and PC in both unadjusted (Supplementary Fig 2, online only) and adjusted analysis (Supplementary Table I, online only). PC was superior to AAC and NABC in terms of primary patency and MALE in both unadjusted (Supplementary Fig 2, online only) and adjusted (Supplementary Table I, online only) analysis. For tibial targets, GSV was superior to PC in primary patency on unadjusted analysis (Supplementary Fig 3, online only) and MALE-free survival in both unadjusted (Supplementary Fig 3, online only) and adjusted (Supplementary Table I, online only) analysis. Comparing PC to other conduits in tibial targets, PC again was superior to both of those conduits in terms of primary patency and MALE in both unadjusted (Supplementary Fig 3, online only) and adjusted (Supplementary Table I, online only) analysis.
Table III.
Adjusted Cox regression model of risk of loss primary patency, risk of major adverse limb events (MALE), and MALE-free survival at 1 year stratified by bypass target
| HR | 95% CI | P value | |
|---|---|---|---|
| Risk of loss of primary patency | |||
| GSV vs PC | 0.97 | 0.86–1.09 | .596 |
| AAC vs PC | 1.41 | 1.19–1.67 | <.001 |
| NABC vs PC | 1.9 | 1.61–2.25 | <.001 |
| NABC vs AAC | 1.35 | 1.12–1.63 | .002 |
| Risk of MALE | |||
| GSV vs PC | 0.99 | 0.89–1.11 | .861 |
| AAC vs PC | 1.35 | 1.15–1.58 | <.001 |
| NABC vs PC | 1.8 | 1.53–2.11 | <.001 |
| NABC vs AAC | 1.34 | 1.11–1.6 | .002 |
| MALE-free survival | |||
| GSV vs PC | 0.85 | 0.8–0.9 | <.001 |
| AAC vs PC | 1.016 | 0.91–1.13 | .779 |
| NABC vs PC | 1.21 | 1.1–1.4 | <.001 |
| NABC vs AAC | 1.19 | 1.04–1.36 | .009 |
AAC, Alternative autologous conduits; CI, confidence interval; GSV, great saphenous vein; HR, hazard ratio; NABC, nonautologous biologic conduits; PC, prosthetic conduits.
Fig 3.
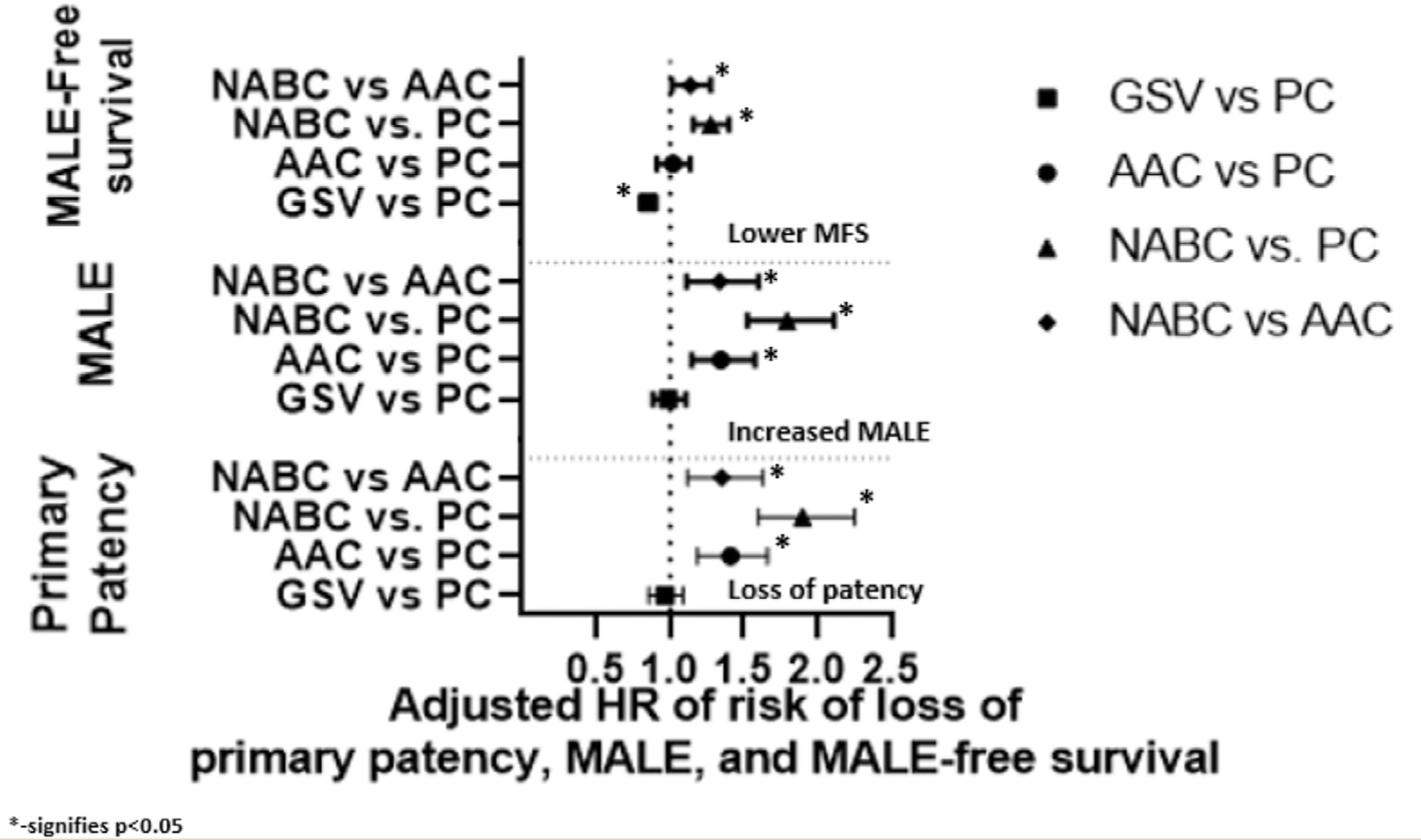
Adjusted Cox regression models of primary patency, major adverse limb events (MALE), and MALE-free survival at one year stratified by infrageniculate target. AAC, alternative autologous conduits; GSV, great saphenous vein; HR, hazard ratio; NABC, nonautologous biologic conduits; PC, prosthetic conduits.
DISCUSSION
In our retrospective analysis of the SVS VQI database primary patency rates of GSV at 1 year were similar to published patency rates across multiple studies.5,7,10 We showed that the PC cohort was similar to the GSV cohort in terms of primary patency and MALE at 1 year; however, GSV had a statistically significantly higher MALE-free survival at 1 year compared with PC. We also demonstrated that AAC and NABC had lower primary patency and increased MALE at 1 year compared with PC. NABC had lower primary patency rates, increased MALE, and decreased MALE-free survival at 1 year compared with AAC.
Despite the robust evidence concerning the superiority of GSV in below-knee popliteal bypasses, some investigators would suggest clinical equipoise regarding the ideal conduit for these bypasses remains. The literature regarding the patency and limb salvage rates of alternative venous conduits compared with other conduits (prosthetic, biologic) is conflicting. Kreienberg et al11 in 2002 compared spliced vein conduits with PCs with a vein cuff in a prospective randomized control trial of below knee popliteal targets (including below-knee popliteal and tibial arteries). They found no difference in rates of primary patency or limb salvage rates between the cohorts.11 Several small retrospective series found similar outcomes between AAC and PC. Mcphee et al12 in 2012 found that PC (with or without vein cuff adjuncts) performed comparably to alternative veins in below knee targets (below-knee popliteal and tibial targets) in terms of patency and limb salvage. Avgerinos et al5 found that PC had better rates of primary patency than AAC in a cohort of LEB bypasses to below knee-popliteal and tibial target; however, that did not affect the rates of limb salvage, which was similar among the groups. A recent study compared the outcomes of contralateral GSV, small saphenous vein, and arm vein bypasses in a cohort of 2642 LEB to below-knee popliteal, tibial, and pedal targets.8 They reported equivalent outcomes in terms of rates of primary and secondary patency between contralateral GSV, small saphenous vein, and spliced arm vein. Their outcomes deviated from the existing literature comparing GSV and alternative venous conduits. Our study corroborates the majority of other observations that suggest that AAC offer no major benefit in terms of primary patency or MALE compared with PC. This observation was noted on univariate and unadjusted analysis, and after adjusting for differences in comorbidities, prior interventions, and bypass target; PC continued to outperform AAC in terms of both primary patency and MALE at 1 year.
NABC have been proposed as useful conduit options in the absence of autologous conduits. In our study, NABC had primary patency rates of 37% at 1 year, which is similar to previously published data that estimate the patency of cryopreserved allografts to be between 30% and 50% among different series.13–15 Despite the poor rate of primary patency, NABC are often presented in the literature as viable options to optimize wound healing and for limb salvage in the absence of GSV. No prior studies have explicitly compared outcomes between alternative venous conduits (spliced arm veins or small saphenous veins), PCs, and cryopreserved allografts. A 2016 study by Moreira et al7 using the Vascular Study Group of New England database, retrospectively analyzed outcomes after LEB using GSV, PC/NABC, and AAC. They demonstrated superiority of the PC/NABC group compared with AAC in BK popliteal targets with respect to primary patency and MALE, whereas AAC out-performed PC and NABC in infrageniculate targets. However, this study was underpowered to detect differences between PC and NABC. In our study, both PC and AAC outperform NABC in primary patency, MALE and MALE-free survival at 1 year when controlling for differences in comorbidities, prior interventions, and bypass target.
A possible explanation for the superiority of PC compared with AAC and NABC could be the advancements in the quality of PCs used. Given the time period of our patient cohort (2003–2020) from the SVS VQI it is more than likely that the majority of PC are heparin-bonded polytetrafluoroethylene. Heparin-bonded prosthetic grafts have been shown to have outcomes that approach that of GSV in smaller nonrandomized series16 for femoral-popliteal (including above-knee and below-knee popliteal targets) and in some cases tibial vessel disease.17 Other conduit-specific factors could be possible mechanisms to explain the observed differences. AAC are known to suffer from intraluminal irregularities due to frequent phlebotomy/intravenous lines that go unnoticed during the harvest and evaluation of these conduits. They are also more time intensive to harvest, thin walled, and delicate compared with the GSV. They often require splicing of two or more segments to achieve the necessary conduit length, and the resulting venovenostomy is a frequent site of restenosis.18–21 In contrast, the worse outcomes observed with the use of NABC could be secondary to the inherent limitations of cryopreserved allografts such as allograft rejection and aneurysmal degeneration.22
Although our study is the largest described cohort comparing different conduits of LEB, the retrospective and observational nature of the analysis remains an important limitation. The similarity in patency rates between PC and GSV at one year should be interpreted with caution, because GSV have previously been shown to be superior to PC in long-term follow-up (≥5 years). However, because the VQI collects follow-up at an average of 1 year, longer term outcomes are not available uniformly in this dataset, and some of the comparisons, therefore, may lose power beyond 1 year. Conduit-specific factors are lacking in the VQI database; therefore, we assume that every patient treated with an alternative conduit is truly lacking an adequate GSV. Operator-specific factors such as familiarity with harvesting and constructing AACs is also not available in this dataset. Variables such as TransAtlantic InterSociety Consensus classifications are not uniformly available and often missing; however, we attempted to mitigate this by using CLTI as an inclusion criteria to create a more homogenous patient population. The study also does not explicitly address the role of alternative biologic conduits in the setting of infection, where this conduit may be an attractive alternative to an amputation and may allow for wound healing.
CONCLUSIONS
Our study is the largest retrospective series to examine conduits for LEB in patients with CLTI, and among the first to compare prosthetic grafts versus cryopreserved allografts and alternative venous conduits versus cryopreserved allografts. Our data suggest that there is no advantage to the use of AAC or NABC over PC in patients with critical limb ischemia requiring infrageniculate reconstruction. Given the time and expense associated with these alternatives, their use should be reconsidered. Further studies are needed to compare the usefulness of PC over AAC in extremely distal targets, to compare longer term outcomes between PC and AAC, and to compare PC vs GSV as PC technology continues to improve and evolve.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research:
Retrospective review of prospectively collected Vascular Quality Initiative data
Key Findings:
In a study of 22,671 lower extremity bypasses, the use of alternative autologous conduits or nonautologous biologic conduits were associated with increased risk of loss of primary patency (hazard ratio [HR], 1.41 and HR, 1.9, respectively) and increased major adverse limb events (HR, 1.35 and HR, 1.8, respectively) compared with prosthetic conduits.
Take Home Message:
Alternative conduits in lower extremity bypass are associated with worse 1 year outcomes compared with prosthetic grafts.
Footnotes
Author conflict of interest: none.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Pereira CE, Albers M, Romiti M, Brochado-Neto FC, Pereira CAB. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg 2006;44:510–7. [DOI] [PubMed] [Google Scholar]
- 2.Taylor LM, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg 1990;11:193–205. [DOI] [PubMed] [Google Scholar]
- 3.Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg 1986;3: 104–14. [DOI] [PubMed] [Google Scholar]
- 4.Faries PL, LoGerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg 2000;32:1080–90. [DOI] [PubMed] [Google Scholar]
- 5.Avgerinos ED, Sachdev U, Naddaf A, Doucet DR, Mohapatra A, Leers SA, et al. Autologous alternative veins may not provide better outcomes than prosthetic conduits for below-knee bypass when great saphenous vein is unavailable. J Vasc Surg 2015;62:385–91. [DOI] [PubMed] [Google Scholar]
- 6.McPhee JT, Barshes NR, Ozaki CK, Nguyen LL, Belkin M. Optimal conduit choice in the absence of single-segment great saphenous vein for below-knee popliteal bypass. J Vasc Surg 2012;55:1008–14. [DOI] [PubMed] [Google Scholar]
- 7.Moreira CC, Leung AD, Farber A, Rybin D, Doros G, Siracuse JJ, et al. Alternative conduit for infrageniculate bypass in patients with critical limb ischemia. J Vasc Surg 2016;64:131–9. [DOI] [PubMed] [Google Scholar]
- 8.Nierlich P, Enzmann FK, Metzger P, Dabernig W, Aspalter M, Akhavan F, et al. Alternative venous conduits for below knee bypass in the absence of ipsilateral great saphenous vein. Eur J Vasc Endovasc Surg 2020;60:403–9. [DOI] [PubMed] [Google Scholar]
- 9.Neufang A, Dorweiler B, Espinola-Klein C, Savvidis S, Doemland M, Schotten S, et al. Outcomes of complex femorodistal sequential autologous vein and biologic prosthesis composite bypass grafts. J Vasc Surg 2014;60:1543–53. [DOI] [PubMed] [Google Scholar]
- 10.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006;43:742–51. [DOI] [PubMed] [Google Scholar]
- 11.Kreienberg PB, Darling RC, Chang BB, Champagne BJ, Paty PSK, Roddy SP, et al. Early results of a prospective randomized trial of spliced vein versus polytetrafluoroethylene graft with a distal vein cuff for limb-threatening ischemia. J Vasc Surg 2002;35:299–306. [DOI] [PubMed] [Google Scholar]
- 12.McPhee JT, Goodney PP, Schanzer A, Shaykevich S, Belkin M, Menard MT. Distal anastomotic vein adjunct usage in infrainguinal prosthetic bypasses. J Vasc Surg 2013;57:982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris L, O’Brien-Irr M, Ricotta JJ. Long-term assessment of cry-opreserved vein bypass grafting success. J Vasc Surg 2001;33:528–32. [DOI] [PubMed] [Google Scholar]
- 14.Masmejan S, Deslarzes-Dubuis C, Petitprez S, Longchamp A, Haller C, Saucy F, et al. Ten year experience of using cryopreserved arterial allografts for distal bypass in critical limb ischaemia. Eur J Vasc Endovasc Surg 2019;57:823–31. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med 2008;13:209–15. [DOI] [PubMed] [Google Scholar]
- 16.Daenens K, Schepers S, Fourneau I, Houthoofd S, Nevelsteen A. Heparin-bonded ePTFE grafts compared with vein grafts in femoropopliteal and femorocrural bypasses: 1- and 2-year results. J Vasc Surg 2009;49:1210–6. [DOI] [PubMed] [Google Scholar]
- 17.Neville RF, Capone A, Amdur R, Lidsky M, Babrowicz J, Sidawy AN. A comparison of tibial artery bypass performed with heparin-bonded expanded polytetrafluoroethylene and great saphenous vein to treat critical limb ischemia. J Vasc Surg 2012;56:1008–14. [DOI] [PubMed] [Google Scholar]
- 18.Arvela E, Sderstrm M, Albck A, Aho PS, Venermo M, Lepntalo M. Arm vein conduit vs prosthetic graft in infrainguinal revascularization for critical leg ischemia. J Vasc Surg 2010;52:616–23. [DOI] [PubMed] [Google Scholar]
- 19.Arvela E, Venermo M, Söderström M, Albäck A, Lepäntalo M. Outcome of infrainguinal single-segment great saphenous vein bypass for critical limb ischemia is superior to alternative autologous vein bypass, especially in patients with high operative risk. Ann Vasc Surg 2012;26:396–403. [DOI] [PubMed] [Google Scholar]
- 20.Marcaccio EJ, Miller A, Tannenbaum GA, Lavin PT, Gibbons GW, Pomposelli FB, et al. Angioscopically directed interventions improve arm vein bypass grafts. J Vasc Surg 1993;17:994–1002. [DOI] [PubMed] [Google Scholar]
- 21.Stonebridge PA, Miller A, Tsoukas AL, Brophy CM, Gibbons GW, Freeman DV, et al. Arigioscopy of arm vein infrainguinal bypass grafts. Ann Vasc Surg 1991;5:170–5. [DOI] [PubMed] [Google Scholar]
- 22.Guevara-Noriega KA, Lucar-Lopez GA, Pomar JL. Cryopreserved allografts for treatment of chronic limb-threatening ischemia in patients without autologous saphenous veins. Ann Vasc Surg 2019;60: 379–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


